Abstract
Phosphoinositide kinase (PI3K) is activated by various receptors on lymphocytes and regulates development, activation, and tolerance. Genetic ablation of PI3K function in T cells leads to the appearance of autoimmune disorders. In B cells, loss of the class IA regulatory subunit p85α causes a partial defect in B cell development and proliferation, whereas loss of p85β alone causes no apparent changes in B cell function. Here we investigate further the consequences of p85β deletion in B cells, in the presence or absence of p85α. We demonstrate that p85β partially compensates for loss of p85α in B cell development and peripheral survival, with greater defects observed when both isoforms are absent. BCR-mediated AKT phosphorylation is partially reduced in p85α-deficient B cells and further diminished with concomitant loss of p85β. Unexpectedly, loss of p85β results in increased BCR-mediated proliferation and ERK phosphorylation. These results indicate that the p85β regulatory isoform has partially overlapping functions with p85α in B cells as well as a unique role in opposing BCR responses.
Keywords: B cells, cell activation, cell proliferation, lymphocyte development, signal transduction
Introduction
Signaling through lymphocyte antigen receptors controls cell fate decisions during development and upon antigen recognition in the periphery. Antigen receptor signaling requires complex assemblies of signaling proteins and second messengers at the membrane, including the lipid products of PI3K enzymes [1,2]. Class I PI3Ks generate the phosphatidylinositol (PtdIns) derivatives PtdIns-3,4,5-P3 and PtdIns-3,4-P2, which recruit proteins containing a pleckstrin-homology domain to the cell membrane. Inhibitors of PI3K enzyme activity block lymphocyte proliferation [3–5]; however, the unique and overlapping functions of individual PI3K family members remain incompletely understood.
Class IA PI3Ks exist as a tightly associated heterodimer consisting of a catalytic subunit of approximately 110 kDa and a regulatory subunit [6,7]. The regulatory subunit isoforms have two SH2 domains that bind to the consensus sequence YXXM upon phosphorylation of the tyrosine residue, and this interaction increases activity and substrate access of the associated p110 catalytic subunit. Other protein:protein interactions involving modular domains within catalytic and regulatory subunits also regulate signaling responses [8–10]. There are three catalytic and five regulatory isoforms of class IA PI3K in mammals. In mice, Pik3ca, Pik3cb, and Pik3cd code for the catalytic isoforms p110α, p110β, and p110δ, respectively. The Pik3r1 gene encodes the regulatory isoforms p85α, p55α and p50α through alternative promoter usage. The Pik3r2 and Pik3r3 genes encode p85β and p55γ, respectively. Gene targeting in mice has shown that PI3K activity in T cells must be properly regulated to maintain both antigen responsiveness and self-tolerance. Loss of p110δ limits antigen-specific CD4 T cell expansion, but is also associated with reduced Treg function and mild colitis [11,12]. Similarly, loss of the regulatory subunits p85α/p55α/p50α/p85β diminishes proliferation but leads to development of lacrimal gland destruction resembling Sjögren’s Syndrome [13,14].
PI3K is activated downstream of many receptors that mediate B cell responses including the antigen receptor (BCR), the CD19 coreceptor, toll-like receptors and cytokine receptors [1,15]. Pharmacological and genetic studies have revealed that the class IA catalytic isoform p110δ is crucial for B cell development, proliferation, and function [4,16]. The class IA regulatory subunit p85α is also essential for normal B cell development and function; Pik3r1-null mice exhibit a partial block at the pre-B cell stage with an overall reduction in B cell populations in the spleen, bone marrow, and peritoneum, as well as a complete loss of BCR-mediated proliferation [3]. Similar defects occur in mice lacking only p85α but retaining p55α/p50α (termed p85αKO herein), which fits with the very low expression of the smaller isoforms in B cells [17–19].
Importantly, some mature B cells do develop in p85αKO mice and these retain partial signaling and function [20–22]. The incomplete block in development and signaling in Pik3r1-null and p85αKO mice suggests the possibility of functional compensation by other regulatory isoforms. Our initial analysis of Pik3r2-null (p85βKO) mice revealed apparently normal B cell development and function [23]. To study further the redundant and unique functions of p85α and p85β, we generated conditional knockout mice in which B cells lack both Pik3r1 and Pik3r2. We report that p85β has a required function in B cell development and survival that is revealed upon concurrent loss of p85α/p55α/p50α. In addition, we uncover a negative regulatory role for p85β in BCR-mediated signaling and proliferation.
Results
Pik3r1 deletion is variable using CD19Cre but complete using CD21Cre
Assessing the phenotypes of B cells lacking both Pik3r1 and Pik3r2 required a conditional gene targeting approach, as combined deletion in all tissues causes embryonic lethality [24]. In order to create a B cell specific knockout of Pik3r1 encoding p85α/p55α/p50α, we bred Pik3r1-flox mice [25] with CD19-Cre mice in which the Cre recombinase is knocked into the CD19 locus [26]. Intercrosses with Pik3r2-null mice produced four genotypes: Pik3r1 -flox (considered the wild-type (WT) control), Pik3r1-flox/Pik3r2-null (p85βKO), Pik3r1-flox/CD19-Cre (referred to as BsKO19, for B cell single knockout with CD19-Cre), and Pik3r1-flox/Pik3r2-null/CD19-Cre (BdKO19, B cell double knockout with CD19-Cre). As previously seen in germline knockouts of Pik3r1 or p85α, BsKO19 mice had a partial block in the proB/preB transition in the bone marrow as well as reduced populations of mature subsets in the bone marrow, peritoneum, and spleen (see Supporting Information; Figure S1).
BdKO19 mice exhibited nearly identical developmental defects as BsKO19 mice. This does not rule out a role for p85β in B cell development, since deletion of floxed alleles in CD19-Cre mice is not always efficient. Indeed, BdKO19 splenic B cells had comparable levels of p85 as wildtype (WT) and p85β−/− (p85βKO) B cells while BsKO19 B cells showed variable deletion (Figure S2). The reduced deletion efficiency in BdKO19 vs. BsKO19 supports the conclusion that the loss of p85β together with p85α/p55α/p50α produces a greater selective disadvantage during development than loss of p85α/p55α/p50α alone. However, the variable deletion in BsKO19 mice complicated our attempts to further pinpoint crucial selection checkpoints.
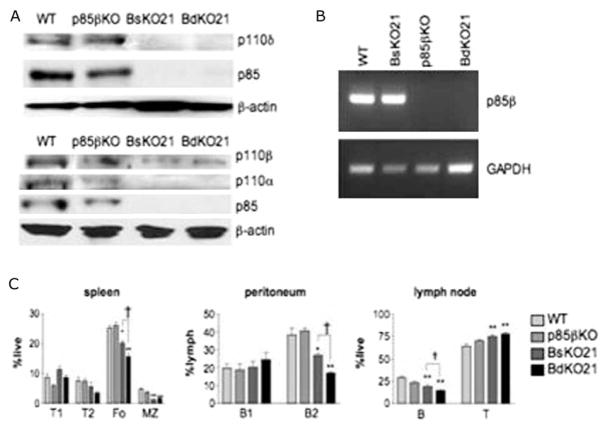
In order to reduce the selective advantage of nondeleters during development, we crossed Pik3r1-flox/Pik3r2-null mice with CD21-Cre transgenic mice [27] to generate BsKO21 and BdKO21 strains. Driving Cre expression with CD21 regulatory elements limits Pik3r1 deletion to late transitional and mature B cells, and follicular dendritic cells (FDC). Analysis of follicular B cells from the lymph nodes of BsKO21 and BdKO21 mice showed undetectable levels of p85α (Figure 1A), confirming that deletion was efficient. Although the anti-pan-p85 antibody can detect p85β in some cell types [3,23,24], no signal was observed in BsKO21 cells suggesting low p85β expression in mature B cells. However, p85β mRNA was readily detected in purified B cells from WT and BsKO21 mice but not in p85βKO or BdKO21 (Figure 1B). Because class IA PI3Ks exist as a stable heterodimer, reduced regulatory subunit expression results in destabilization and downregulation of catalytic subunits [24,28]. Consequently, p110α and p110δ were undetectable and expression of p110β was reduced in BsKO21and BdKO21 B cells (Figure 1A).
Figure 1.
Efficient deletion and development defects in BdKO21 mice, (A) Immunoblot analysis of purified lymph node B cells from WT, p85βKO, BsKO21, and BdKO21 mice reveals the absence of p85α and downregulation of catalytic subunits in BsKO21 and BdKO21 B cells. The absence of a detectable p85 band in BsKO21 indicates that p85β is not greatly upregulated. Other than the anti-pan-p85 antiserum used here, no other antibodies are available that detect p85β selectively and sensitively in murine cells. (B) RT-PCR analysis of RNA from purified lymph node B cells confirms that the Pik3r2 gene encoding p85β is transcribed in FO B cells. (C) Quantitation of FACS data from multiple mice (example in Figure S3) shows that BsKO21 and BdKO21 mice have graded defect in mature B cell subsets in the spleen and periphery. p < 0.05, ★★p < 0.001 vs. WT; †p < 0.05 BsKO21 vs. BdKO21, using one-way ANOVA.
Loss of p85β exacerbates developmental and survival defects in the absence of p85α
In accord with previous reports [20,23], follicular (FO) and marginal zone (MZ) B cell populations were reduced in BsKO21 but not in p85βKO mice (Figure 1C, Supplementary Figure 3; Table I). Combined loss of p85 α and p85 β in BdKO21 mice resulted in greater decreases in the FO populations in the spleen, lymph nodes, and peritoneum compared to BsKO21 mice (Figure 1C, Supplementary Figure 3; Table I). MZ populations were similarly decreased in BsKO21 and BdKO21 mice, and the percentages and numbers of transitional subsets were unchanged. Peritoneal B1a populations, which do not express CD21, remained unaffected.
Table I.
Total numbers of splenic B cell subsets.
| B cell subsets (× 106 cells) (mean ± SEM) |
||||
|---|---|---|---|---|
| T1 | T2 | FO | MZ | |
| WT | 10.8 ± 2.1 | 7.4 ± 2.1 | 27.6 ± 2.6 | 4.8 ± 0.5 |
| P85βKO | 7.3 ± 0.8 | 6.1 ± 0.9 | 26.0 ± 2.0 | 3.9 ± 0.7 |
| BsKO21 | 15.3 ± 3.4 | 7.0 ± 2.3 | 18.3 ± 2.0★ | 1.3 ± 0.2★★ |
| BdKO21 | 9.2 ± 1.7 | 4.1 ± 1.3 | 10.3 ± 1.0†# | 1.6 ± 0.7★ |
p < 0.05,
p < 0.01,
p < 0.001 vs. WT,
p < 0.05 vs. BsKO21, using one-way ANOVA.
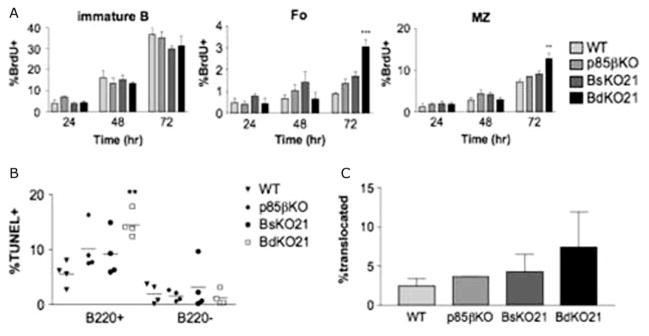
The defects seen in BsKO21 and BdKO21 mice suggested that there was decreased production or survival of mature B cells. In order to distinguish between these possibilities, we injected mice with BrdU for 24–72 h and analyzed BrdU incorporation in different B cell subsets by AA4.1/CD23/IgM staining (Figure 2A). Immature B cells from all four genotypes had comparable percentages of BrdU+ cells. This is consistent with the normal developmental profile of p85βKO B cells and with CD21-Cre causing deletion of Pik3r1 at a late transitional stage. However, BdKO21 mice had a higher percentage of BrdU incorporation in both FO and MZ B cell subsets after 72 h of treatment, whereas BsKO21 mice did not show any significant changes.
Figure 2.
(A) BdKO21 mice have impaired mature B cell maintenance. Mice were injected i.p. with 600 μg of BrdU every 12 h for 24, 48, or 72h. Splenocytes were then harvested, surface stained with anti-CD23/AA4.1/IgM, and analyzed for BrdU incorporation. The graphs show the averages ± SEM with three or four mice per condition. ★p < 0.05 using one-way ANOVA (B) Scatter plot diagram showing the percentage of TUNEL+ cells B220+ and B220− populations in freshly isolated splenocytes. Each point represents an individual mouse. ★p < 0.05 (C) Splenic mature B cells were stained for p65 subunit of NFκB1 and DAPI to determine the percentage showing nuclear localization of NFκB by immunofluorescence. Data are an average of three different experiments ± SEM where at least 100 cells were counted per point by a blinded observer. ★p < 0.05.
The absolute number of BrdU + mature B cells was not increased in BdKO21 (not shown), since there were fewer cells overall. One interpretation of these data is that BdKO21 mature B cells have decreased survival so that newly formed B cells represent a higher fraction of the mature B cell pool. To address this possibility, we carried out TUNEL staining of freshly isolated splenocytes, which revealed that BdKO21 B cells had an increased rate of apoptosis (Figure 2B). We then measured basal activity of the canonical NFκB pathway, which is required for maintenance of mature B cells [29]. The percentage of cells showing nuclear translocation of the p65 subunit of NFκB1 was low and comparable among freshly isolated splenic mature B cells from WT, p85βKO, BsKO21, and BdKO21 mice (Figure 2C).
B cells lacking p85β exhibit an increased proliferative response to BCR cross-linking
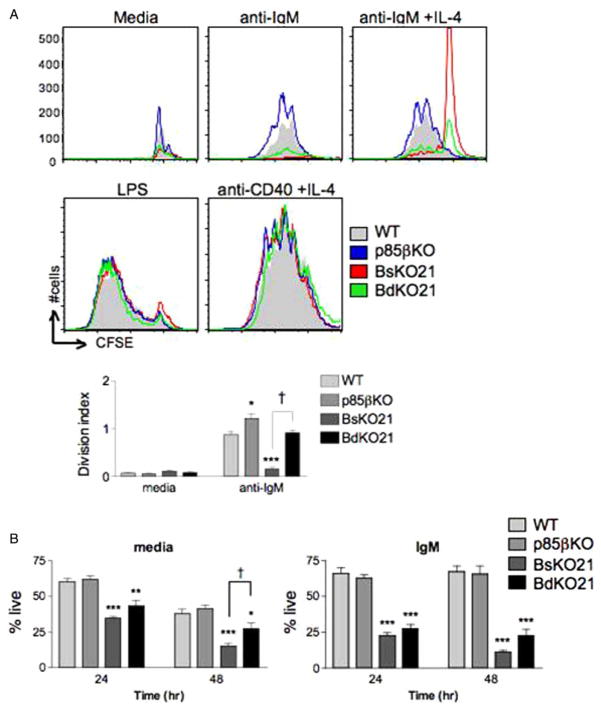
To assess the functional defects in mature B cells, CFSE-labeled lymph node B cells from all four genotypes were stimulated with anti-IgM ± IL-4, anti-CD40 with IL-4, or LPS for 72 h (Figure 3A). In accord with previous studies of Pik3r1 -null and p85αKO B cells, BsKO21 B cells showed a complete block in response to anti-IgM that was only weakly restored by IL-4, yet proliferated normally in response to anti-CD40/IL-4. Surprisingly, BdKO21 B cells consistently showed low but detectable proliferation with anti-IgM treatment. The percentage of cells divided and the average number of divisions of the cycling cells (the division index) were significantly higher in BdKO21 compared to BsKO21 (Figure 3A, lower, and figure legend). Additionally, p85βKO B cells from the lymph node also exhibited enhanced anti-IgM-induced proliferation compared to WT controls. Again, both the percent divided and the division indices were increased in p85βKO vs. WT (Figure 3A, lower, and figure legend).
Figure 3.
(A) Loss of p85β results in increased proliferation upon BCR stimulation. Lymph node B cells from WT, p85βKO, BsKO21, and BdKO21 mice were stimulated with the indicated mitogens for 72 h. Division was determined by CFSE dilution, gating on live cells among 40,000 total events. Data are representative of three experiments. Quantitative analysis of the CFSE dilution data revealed that loss of p85β leads to a higher division index upon anti-IgM treatment (lower panel; mean ± SEM, n = 3). The percentages of live, divided cells among different genotypes were 13.8 ± 1.1 (WT), 26.8 ± 3.6 (p85bKO), 0.002 ± 0.001 (BsKO21) and 2.4 ± 0.3 (BdKO21). ★p < 0.05 compared to WT, ★★★p < 0.001 vs. WT, †p < 0.001 vs. BsKO21 (B) Splenic mature B cells were cultured in media alone or with 20 μg/mL anti-IgM and analyzed for cell viability by PI exclusion at the time points indicated. ★p < 0.05, ★★p < 0.01, ★★★p < 0.001 vs. WT; †p < 0.05 BsKO21 vs. BdKO21, using one-way ANOVA.
Proliferative responses to LPS (10 μg/ml) were comparable among genotypes (Figure 3A), although at lower LPS concentrations the BsKO21 and BdKO21 showed slightly fewer divided cells (data not shown). The increased division in anti-IgM cultures was not due to significantly greater survival, as judged by propidium iodide exclusion at 48 h (Figure 3(B); compare p85βKO vs. WT and BdKO21 vs. BsKO21) and Annexin-V + divided cells at 72 h (data not shown). We did observe an unexpected increase in survival ofBdKO21 vs. BsKO21 B cells in culture medium alone (Figure 3B), contrary to the in vivo survival data (Figure 2B). This difference most likely results from distinct survival factors present in culture medium compared to intact lymphoid organs.
Altered BCR signaling in B cells lacking p85 isoforms
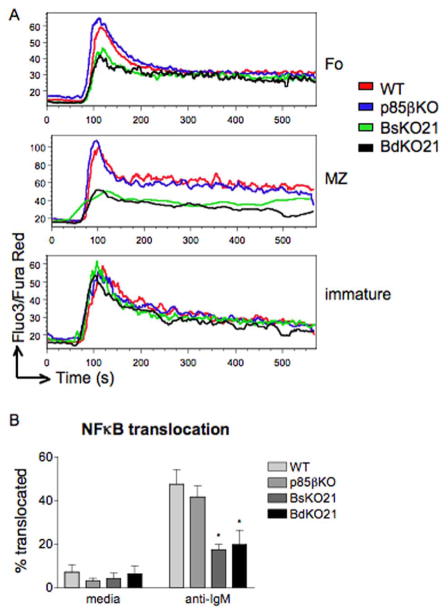
To investigate molecular mechanisms for altered function of B cells with different genotypes, we evaluated various signaling responses. Due to the low number of mature B cells that could be obtained from BdKO21 mice, we used the single cell methods of flow cytometry [22,30] or fluorescence microscopy. First we measured BCR-mediated Ca2+ mobilization. Generation of PtdIns(3,4,5)P3 promotes optimal assembly of the BCR signalosome and PLCγ-mediated Ca2+ elevation [1,2]. Splenic B cells from p85αKO cells display partially impaired Ca2+ flux whereas p110δKI B cells have similar defects as cells treated with the PI3K inhibitor LY294002 [4,22]. In order to determine whether p85β contributes to BCR-mediated Ca2+ flux in the presence or absence of p85α, we measured Ca2+ responses of immature, follicular, and MZ B cell subsets from WT, p85βKO, BsKO21, and BdKO21 splenocytes (Figure 4A). Immature B cells from all four genotypes, which all retain Pik3r1 at this stage, showed similar responses upon stimulation with anti-IgM.
Figure 4.
(A) p85α mediates calcium flux upon BCR crosslinking. Splenocytes were stained with CD1d and CD24 to differentiate immature, FO, and MZ populations. Calcium influx defect in BsKO21 and BdKO21 B cells is seen in the FO and MZ populations (which lack p85α) but not in immature B cells (which still express p85α). (B) NFκB activation in LN B cells was determined by p65 translocation to the nucleus. Data shown is the average of three experiments ± SEM. ★p < 0.05, using one-way ANOVA.
This suggests that loss of p85β does not impair Ca2+ signaling in transitional B cells. p85β was also dispensable for Ca2+ mobilization in mature FO and MZ B cells; the responses of WT and p85βKO were equivalent and BdKO21 cells did not display greater defects than BsKO21. Similar results were obtained from Ca2+ mobilization assays done using lymph node B cells (data not shown). The elevation of intracellular Ca2+ and diacylglycerol downstream of PLCγ initiates a pathway leading to nuclear entry of NFκB transcription factors [1]. Thus, anti-IgM stimulation caused a large increase in the fraction of lymph node B cells exhibiting nuclear translocation of p65 (Figure 4B). Consistent with the Ca2+ data, the NFκB response of p85βKO cells was unaltered and BsKO21 and BdKO21 B cells had a similar reduction in NFkB translocation. These results suggest that PI3K-dependent Ca2+ signaling and NFκB activation are unique functions of p85α with no redundant or compensatory role for p85β.
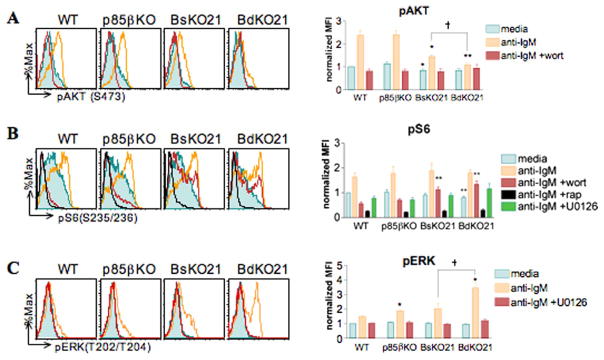
The serine/threonine kinase AKT is a well-characterized downstream effector of PI3K that becomes activated upon recruitment to the cell membrane. p110δKI B cells have > 95% reduction in pAKT levels upon BCR stimulation whereas p85αKO B cells show greater residual AKT phosphorylation [4,22]. In order to determine whether p85β mediates the residual AKT activation, we stimulated lymph node B cells with anti-IgM and determined the level of phosphorylation of AKT on residue S473. The shift in fluorescence detection following BCR engagement was completely wortmannin-sensitive, demonstrating the specificity of detecting PI3K-dependent phosphorylation (Figure 5A; [30]). In accord with previous studies, p85βKO B cells exhibited a normal response whereas BsKO21 B cells showed a partial defect similar to p85αKO B cells. BdKO21 B cells displayed near complete abrogation of AKT phosphorylation, suggesting that p85β partially compensates for the absence of p85α in this arm of the PI3K signaling pathway. The AKT phosphorylation response of splenic FO B cells of different genotypes was comparable to the results from lymph node B cells (data not shown).
Figure 5.
Differential functions of p85β in BCR-mediated phosphorylation of AKT and ERK. Lymph node cells were stimulated with 10 μg/mL anti-IgM to measure levels of pAKT (A), pS6 (B), and pERK (C). Cells were pretreated with 50 nM wortmannin, 10 ng/mL rapamycin, or 10 μM U0126 for 15 min where indicated. Left panels show representative histogram overlays of three to five independent experiments. In panel B, left, the U0126 histograms are omitted for clarity. Right panels show the averages of normalized mean fluorescence intensity (MFI) values ± SEM. MFIs of each experiment were normalized using the value of the unstimulated WT cells. ★p < 0.05, ★★p < 0.01 vs. WT; †p < 0.05 BsKO21 vs BdKO21 with Student’s paired two-tailed t test.
Activation of AKT triggers increases in metabolism and protein synthesis as cells prepare to undergo rapid rounds of cell division. These effects are partially due to indirect activation of the mammalian target of rapamycin (mTOR), a Ser/Thr kinase that phosphorylates proteins required for cell growth and protein synthesis such as 4E-BP1 and S6K [31,32]. In B cells, BCR-mediated mTOR activity is largely PI3K-dependent, as treatment with LY294002 or wortmannin blocks phosphorylation of S6K and its downstream effector S6 [22,33]. However, wildtype and p85αKO B cells showed comparable S6 phosphorylation [22,33]. Similarly, both BsKO21 and BdKO21 B cells showed unimpaired S6 phosphorylation (pS6) upon stimulation with anti-IgM (Figure 5B). The direct mTOR inhibitor rapamycin blocked all pS6 detection, demonstrating specificity in this assay.
Inputs to S6 phosphorylation in BsKO21 and BdKO21 B cells were largely PI3K-independent, as indicated by wortmannin resistance of BCR-induced pS6 in these two genotypes. This suggests compensatory changes in the wiring of other signals affecting S6 kinases. We assessed the possible role of the RAF/MEK/ERK kinase cascade, which can promote S6K activity directly though phosphorylation of residues in the autoinhibitory domain, or through phosphorylation of TSC2 upstream of mTOR [32]. BsKO21 and BdKO21 B cells did not exhibit increased sensitivity to the MEK inhibitor U0126 (Figure 5B), indicating that this pathway does not play a major compensatory role in PI3K-independent S6 phosphorylation. Interestingly, however, BCR-mediated phosphorylation of ERK was greatly enhanced in BdKO21 and to a lesser extent in p85βKO B cells (Figure 5C). This effect was consistently observed at 5 min post-BCR stimulation (Figure 5C) as well as 1 and 15 min (data not shown).
Discussion
Defining specificity in the function of related signaling proteins increases our understanding of lymphocyte biology and can reveal new molecular targets for modulating immune responses and autoimmunity. Applying this approach to the PI3K catalytic isoforms has defined crucial roles for the p110δ (class IA) and p110γ (class IB) enzymes in various aspects of innate and adaptive immunity [34–36]. Our previous work showed that p85β has a unique negative regulatory role in TCR survival, together with a positive role in T cell proliferation that is redundant with other regulatory isoforms [13,23]. Here, we have carried out a detailed comparison of the shared and redundant functions of p85α and p85β in B cell development, proliferation and signaling. Our results confirm that p85α has a B cell intrinsic function as the predominant class IA regulatory isoform for many responses, while revealing previously unknown roles for p85β.
Comparison of BsKO19 and BdKO19 mice suggests a strong selection pressure to maintain PI3K signaling during development. Although BdKO19 B cells do not have a more severe developmental defect than BsKO19 B cells they also have lower deletion efficiency, which suggests that loss of p85β further decreases fitness in the absence of p85α. One possible mechanism could be decreased survival mediated by c-Kit and other cytokine receptors during B cell development in the bone marrow. In mast cells, loss of p85α or p85α/p55α/p50α causes only partially impaired responses to c-Kit ligand (stem cell factor) [37,38]. The cytoplasmic tail of c-Kit contains two tyrosines that when phosphorylated can recruit class IA PI3K regulatory subunits, with p85β possibly able to compensate in the absence of p85α.
Using the CD21Cre system we observed that deleting p85α/p55α/p50α reduces mature B cell numbers without increasing turnover or apoptosis in vivo. This supports our previous finding that the primary role of p85α is to promote developmental progression to mature FO and MZ subsets, rather than to maintain survival [20]. Deleting p85α/p55α/p50α together with p85β further diminishes mature B cell numbers while also increasing turnover and apoptosis. This suggests that Pik3r1 and Pik3r2 gene products have shared but required functions in mature B cell maintenance. The decreased survival might result from impaired tonic BCR signaling and/or reduced responsiveness to BAFF [27,39], or to a defect in the FDC populations of BdKO21 mice.
Do different PI3K isoforms merely exist for compensatory reasons or do they have distinct functions? One interpretation is that regulatory isoforms are redundant and that distinct knockout phenotypes reflect differences in expression levels. However, p85β has unique functions in T cells, which like B cells have low expression of p85β relative to p85α. p85βKO but not p85αKO T cells have increased survival and proliferation in vitro [19,23]. There is also a greater reduction in basal motility in vivo of p85βKO T cells compared to p85αKO [40]. Our current results indicate that both p85α and p85β have unique functions in BCR-mediated signaling and function. p85α is required for maximal BCR-stimulated Ca2+ mobilization, whereas p85β appears dispensable for this response even in the absence of p85α.
A similar pattern was observed for NFκB activation, known to be downstream of the BCR/Ca2+ signalosome. Unexpectedly, we find that p85α and p85β have opposing roles in BCR-mediated proliferation. The response is eliminated in BsKO21 B cells, and slightly but reproducibly increased in p85βKO relative to WT B cells. A negative regulatory role for p85β in BCR responses is further supported by the partial restoration of proliferation in BdKO21 vs. BsKO21 B cells, in cultures treated with anti-IgM.
The increased response of p85β-deficient cells did not result from greater NFκB activation but was associated with enhanced ERK phosphorylation. Studies of p85αKO B cells have shown a similar enhancement of BCR-induced ERK phosphorylation [21,22]; BsKO21 cells showed a similar trend here but with greater variability. It has been shown that pan-PI3K inhibitors or p110δ-specific inhibitors partially decrease ERK phosphorylation in B cells [4,22,41]. The opposing actions of PI3K catalytic and regulatory subunits on ERK phosphorylation add to the accumulating evidence that the regulatory subunits of PI3K have functions that are independent of their ability to bind the catalytic subunits [9,42,43]. Further experiments are necessary to uncover the mechanism of enhanced ERK phosphorylation.
The increased proliferation of p85βKO lymph node (FO) B cells in vitro was quantitatively modest, and not detected by thymidine incorporation using unfractionated splenic B cells [23]. However, the small increase in FO B cell responsiveness might have important biological consequences. When we analyzed older p85βKO mice, we observed many with mild lymphocyte infiltrates in the salivary gland, as well as anti-nuclear antibodies in ~ 15% of mice between 3 and 12 months of age [14]. We also detected ~ 30% higher basal serum IgM levels in young adult p85βKO mice (p < 0.05). In Pik3r2-null mice that also have T cell-specific deletion of Pik3r1, a severe autoimmune exocrinopathy develops with lacrimal gland destruction and high titers of anti-nuclear antibodies [14]. It is likely that loss of p85β in a non-T cell is required for disease development, as only subclinical infiltrates are observed when Pik3r2 is intact and Pik3r1 is deleted in T cells (J.S.O., unpublished observations). Hence, mild hyperreactivity of p85βKO B cells might be an important contributing factor to this complex autoimmune syndrome. Human autoimmune diseases are usually associated with complex combinations of genetic risk factors, so it is possible that altered p85β expression or function contributes to antibody-mediated autoimmune pathology.
It is intriguing that p85β could partially compensate for p85α in BCR-triggered AKT activation but not in Ca2+ mobilization. This suggests the existence of distinct pools of 3′-phosphorylated inositol lipids that recruit AKT or PLCγ downstream of different signaling complexes. The complex that promotes AKT recruitment and activation can function with either p85α or p85β, whereas the Ca2+ signalosome is selective for p85α. We speculate that redundant recruitment occurs when assembly is based mainly on the highly conserved SH2 domains, whereas unique recruitment occurs when assembly requires SH3 or RhoGAP-homology domains that are less similar in p85α and p85β.
In summary, our results demonstrate that p85α and p85β have overlapping roles in B cell development and peripheral survival, and that p85β acts in a pathway that dampens ERK activation and proliferation following BCR crosslinking. Considering that mice lacking either p85α or p85β are viable [19,23,44], it might be possible to design therapies that modify expression or function of these regulatory isoforms to treat immune diseases without causing severe toxicity.
Materials and methods
Mice
The generation of Pik3r1-flox and Pik3r2-null mice is described elsewhere [25,44]. To generate the various conditional knockouts described here, Pik3r1-flox and Pik3r2-null mice were intercrossed and then bred to CD19-Cre or CD21-Cre transgenic mice. Mice were maintained in a C57BL/6 × 129SvEv mixed background and used at 6 to 16 weeks of age. Animals were housed under sterile conditions with irradiated food and autoclaved microisolator cages and water. All procedures were approved by the institutional animal care and use committee of UC Irvine.
FACS analysis
Single cell suspensions of RBC-depleted spleens, lymph nodes, and peritoneal lavages were stained with combinations of the following antibodies: anti-CD5-FITC, anti-CD21-FITC, anti-CD24-PE, anti-CD23-biotin, anti-AA4.1-PE, anti-Thy1.2 PE (eBiosciences, San Diego, CA), anti-CD43-PE, anti-IgM-PerCP, anti-B220-APC (BD Biosciences, San Diego, CA), anti-BrdU-FITC, and Annexin V- PE (Caltag, Burlingame, CA). Samples were acquired on a FACScalibur (BD Biosciences) and data analyzed using FlowJo (Tree Star, Inc., San Carlos, CA) software.
B cell purification and culture
B cells were purified via negative selection using anti-CD43 microbeads on MACS columns (Miltenyi, Auburn, CA), as described [33]. For removal of immature B cells, cells were also incubated with anti-AA4.1-biotin and anti-biotin microbeads. Purity was verified to be 85–95% by FACS analysis. Cells were cultured in RPMI-1640 supplemented with 10% heat-inactivated FCS, 5 mM HEPES, 2 mM L-glutamine, penicillin (100 units/ml), streptomycin (100 μg/ml), and 50 μM β-mercaptoethanol for all assays shown.
Immunoblotting
Purified lymph node B cells (2 or 3 × 106) were lysed in a detergent buffer containing 1% TritonX-100, 50mMTris, pH 7.4, 10% glycerol, 150 mM NaCl and a protease inhibitor cocktail (Sigma, St. Louis, MO). Proteins were resolved via 7.5% or 12.5% SDS-PAGE (Biorad, Hercules, CA) and transferred to nitrocellulose membranes. Membranes were blocked with 5% milk in TBST for 1 hr, washed with TBST, and probed with primary antibodies for 1 hr at room temperature (RT). Antibodies against p110α, p110β, p110δ (Santa Cruz, Santa Cruz, CA), and a rabbit antiserum that recognizes all class IA PI3K regulatory isoforms (anti-pan-p85, kind gift from Lewis Cantley) were used to probe for p85α and p85β expression. mAbs specific for β-Actin (Sigma, St. Louis, MO) were used as loading controls.
RNA expression analysis
Total cellular RNA was extracted by using TRIzol reagent (Invitrogen Life Technologies) and DNase-treated. cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). The p85β primers were designed to detect exons deleted by the gene targeting strategy. Primer sequences were as follows. p85β forward: TTCCAGTACAGGGCTGTGTACCCATT; reverse: TAGCATTCACTGTCCAGCTCTGCT; GAPDH forward: AATTCAACGGCACAGTCA; reverse: GCGGCACGTCAGATCCACGACGGAC. PCR was done for 30 cycles as follows: 94°C for 1 min, 58°C for 1 min, 72°C for 1 min, with a 7-min final extension at 72°C.
B cell proliferation
B cells from the spleen or lymph nodes were purified as above. Cells were stimulated with 10 μg/mL F(ab′)2 fragment of anti-IgM (Jackson ImmunoResearch, West Grove, PA), 10 μg/mL LPS (Sigma), or 1 μg/mL anti-CD40 (BD Biosciences) and 2 ng/mL IL-4 (R&D) for 72 hrs. Cell division and death were tracked following CFSE dilution and Annexin-V staining, respectively.
BrdU incorporation
Mice were injected intraperitoneally with 1200 μL of 3 mg/mL BrdU in PBS every 12 h for the times described. Splenocytes were then harvested, stained with anti-CD23-PE, anti-IgM PerCP, and anti-AA4.1-biotin/SA-APC, and stained for BrdU incorporation using the BrdU Flow Kit (BD Biosciences).
Survival assays
Freshly isolated splenocytes were TUNEL stained using the In Situ Cell Death Detection Kit, fluorescein (Roche, Indianapolis, IN). Purified mature B cells were incubated in medium alone or stimulated with 20 μg/mL anti-IgM F(ab′)2 (Jackson Immunoresearch). Cells were stained with 2 mg/ml propidium iodide (Invitrogen, Carlsbad, CA) and incubated for 5 min at RT prior to analysis.
Calcium flux assay
Splenocytes were stained with CD1d-biotin/SA APC and CD24-PE prior to loading with the calcium indicator dyes Fluo-3 and Fura Red (Invitrogen), as described previously [30]. The baseline level of Fluo-3/Fura Red was collected for 1 min before cells were stimulated with 10 μg/mL anti-IgM for an additional 10 min.
NFκB translocation assay
B cells were initially stimulated for 5 min in a 37°C water bath then allowed to settle onto poly-lysine coated coverslips for an additional 55 min in a 37°C incubator. Cells were fixed with 1 mL 4% paraformaldehyde for 10 min, washed with PBS, then stained with anti-p65 (Santa Cruz) in 0.1% Triton-X/PBS for 1 hr at RT, washed, then stained with anti-rabbit IgG Alexa 488 (Invitrogen) for 15 min at 4°C. The coverslips were mounted using Vectashield with DAPI (Vector Laboratories, Burlingame, CA), sealed, then visualized with a Zeiss Axiovert microscope equipped with an Axiocam CCD camera. Digital images were scored for percent translocation by an observer who was blinded to the sample description.
Phosflow assays for pAKT, pERK, pS6
Lymph node B cells incubated for 20 min at 37°C in media alone or in the presence of 50 nM wortmannin, 10 ng/mL rapamycin, or 10 mM U0126 (EMD, San Diego, CA) prior to stimulation. Cells were stimulated with 10 μg/mL F(ab′)2 fragment of anti-IgM for 5 min (pAKT, pERK) or 15 min (pS6) and stained intracellularly as described [28]. Briefly, cells were fixed in 2% MeOH-free paraformaldehyde (Polysciences Inc., Warrington, PA), pelleted, and permeabilized with 90% MeOH for 30 min on ice. Cells were then washed, blocked with 0.5% BSA in PBS, then stained with anti-pAKT (S473), anti-pERK (T202/Y204), or anti-pS6 (S235/S236) (Cell Signaling Techonology, Danvers, MA) for 1 hr. pAKT and pERK samples were additionally stained with donkey anti-rabbit biotin (Jackson ImmunoResearch) for 30 min. Cells were then stained with B220-FITC, Thy1.2-PE, and anti-rabbit IgG Alexa 647 (pS6) or SA-APC (pAKT, pERK).
Statistical analyses
To test for significant differences, data were analyzed using either Student’s t-tests or one-way ANOVA and Tukey post-test, as noted in the figure legends.
Supplementary Material
Acknowledgments
We thank Tim Bender, Craig Walsh, Aimee Edinger and Jack Xu for helpful comments, Robert Rickert, Klaus Rajewsky and Lewis Cantley for providing mouse strains, and Melissa Chavez for scoring immunofluorescence images. Supported by NIH grants RO1-AI50831 (D.A.F.) and T32-AI60573 (J.S.O.). J.S.O. was an Achievement Rewards for College Scientists (A.R.C.S.) Scholar.
Abbreviations
- PI3K
phosphoinositide 3-kinase
- PtdIns
phosphatidylinositol
- mTOR
mammalian target of rapamycin
- WT
wild-type
- KO
knockout
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Donahue AC, Fruman DA. PI3K signaling controls cell fate at many points in B lymphocyte development and activation. Semin Cell Dev Biol. 2004;5:183–197. doi: 10.1016/j.semcdb.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Fruman DA, Satterthwaite AB, Witte ON. Xid-like phenotypes: A B cell signalosome takes shape. Immunity. 2000;13:1–3. doi: 10.1016/s1074-7613(00)00002-9. [DOI] [PubMed] [Google Scholar]
- 3.Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, Cantley LC. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 4.Bilancio A, Okkenhaug K, Camps M, Emery JL, Ruckle T, Rommel C, Vanhaesebroeck B. Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: Comparative analysis of genetic and pharmacologic interference with p110delta function in B cells. Blood. 2006;107:642–650. doi: 10.1182/blood-2005-07-3041. [DOI] [PubMed] [Google Scholar]
- 5.Shi J, Cinek T, Truitt KE, Imboden JB. Wortmannin, a phosphatidylinositol 3-kinase inhibitor, blocks antigen-mediated, but not CD3 monoclonal antibody-induced, activation of murine CD4 + T cells. J Immunol. 1997;158:4688–4695. [PubMed] [Google Scholar]
- 6.Deane JA, Fruman DA. Phosphoinositide 3-kinase: Diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 7.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J. Binding of Ras to phosphoinositide 3-kinase p110alpha is required for Ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi CM, Aleman JO, Ueki K, Luo J, Asano T, Kaneto H, Stephanopoulos G, Cantley LC, Kahn CR. The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance. Mol Cell Biol. 2007;27:2830–2840. doi: 10.1128/MCB.00079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: Insights from gene-targeted mice. Trends Biochem Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 12.Patteon DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, Rowan WC, Sancho S, Walker LS, Vanhaesebroeck B, Okkenhaug K. Cutting edge: The phosphoinositide 3-kinase p110 delta is critical for the function of CD4 + CD25 + Foxp3 + regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 13.Deane JA, Kharas MG, Oak JS, Stiles LN, Luo J, Moore TI, Ji H, Rommel C, Cantley LC, Lane TE, Fruman DA. T-cell function is partially maintained in the absence of class IA phosphoinositide 3-kinase signaling. Blood. 2007;109:2894–2902. doi: 10.1182/blood-2006-07-038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oak JS, Deane JA, Kharas MG, Luo J, Lane TE, Cantley LC, Fruman DA. Sjogren’s syndrome-like disease in mice with T cells lacking class 1A phosphoinositide-3-kinase. Proc Natl Acad Sci USA. 2006;103:16882–16887. doi: 10.1073/pnas.0607984103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deane JA, Fruman DA. Phosphoinositide 3-kinase: Diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 16.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJH, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 17.Glassford J, Soeiro I, Skarell SM, Banerji L, Holman M, Klaus GG, Kadowaki T, Koyasu S, Lam EW. BCR targets cyclin D2 via Btk and the p85alpha subunit of PI3-K to induce cell cycle progression in primary mouse B cells. Oncogene. 2003;22:2248–2259. doi: 10.1038/sj.onc.1206425. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Matsuda S, Terauchi Y, Fujiwara M, Ohteki T, Asano T, Behrens TW, Kouro T, Takatsu K, Kadowaki T, Koyasu S. PI3K and Btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat Immunol. 2003;4:280–286. doi: 10.1038/ni890. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, Koyasu S. Xid-like immunodeficiency in mice with disruption of the p85 subunit of phosphoinositide 3-kinase. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 20.Donahue AC, Hess KL, Ng KL, Fruman DA. Altered splenic B cell subset development in mice lacking phosphoinositide 3-kinase p85alpha. Int Immunol. 2004;16:1789–1798. doi: 10.1093/intimm/dxh180. [DOI] [PubMed] [Google Scholar]
- 21.Glassford J, Vigorito E, Soeiro I, Madureira PA, Zoumpoulidou G, Brosens JJ, Turner M, Lam EW. Phosphatidylinositol 3-kinase is required for the transcriptional activation of cyclin D2 in BCR activated primary mouse B lymphocytes. Eur J Immunol. 2005;35:2748–2761. doi: 10.1002/eji.200425812. [DOI] [PubMed] [Google Scholar]
- 22.Hess KL, Donahue AC, Ng KL, Moore TI, Oak J, Fruman DA. Frontline: The p85alpha isoform of phosphoinositide 3-kinase is essential for a subset of B cell receptor-initiated signaling responses. Eur J Immunol. 2004;34:2968–2976. doi: 10.1002/eji.200425326. [DOI] [PubMed] [Google Scholar]
- 23.Deane JA, Trifilo MJ, Yballe CM, Choi S, Lane TE, Fruman DA. Enhanced T cell proliferation in mice lacking the p85{beta} subunit of phosphoinositide 3-kinase. J Immunol. 2004;172:6615–6625. doi: 10.4049/jimmunol.172.11.6615. [DOI] [PubMed] [Google Scholar]
- 24.Brachmann SM, Yballe CM, Innocenti M, Deane JA, Fruman DA, Thomas SM, Cantley LC. Role of phosphoinositide 3-kinase regulatory isoforms in development and actin rearrangement. Mol Cell Biol. 2005;25:2593–2606. doi: 10.1128/MCB.25.7.2593-2606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, Depinho RA, Izumo S, Cantley LC. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Ig[alpha]/[beta] heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Deane JA, Kharas MG, Oak JS, Stiles LN, Luo J, Moore TI, Ji H, Rommel C, Cantley LC, Lane TE, Fruman DA. T-cell function is partially maintained in the absence of class IA phosphoinositide 3-kinase signaling. Blood. 2007;109:2894–2902. doi: 10.1182/blood-2006-07-038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasparakis M, Schmidt-Supprian M, Rajewsky K. I{kappa}B kinase signaling is essential for maintenance of mature B cells. J Exp Med. 2002;196:743–752. doi: 10.1084/jem.20020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donahue AC, Kharas MG, Fruman DA. Measuring phosphorylated Akt and other phosphoinositide 3-kinase-regulated phosphoproteins in primary lymphocytes. Meth Enzymol. 2007;434:131–154. doi: 10.1016/S0076-6879(07)34008-1. [DOI] [PubMed] [Google Scholar]
- 31.Richardson CJ, Schalm SS, Blenis J. PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol. 2004;15:147–159. doi: 10.1016/j.semcdb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Donahue AC, Fruman DA. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur J Immunol. 2007;37:2923–2936. doi: 10.1002/eji.200737281. [DOI] [PubMed] [Google Scholar]
- 34.Okkenhaug K, Ali K, Vanhaesebroeck B. Antigen receptor signalling: A distinctive role for the p110delta isoform of PI3K. Trends Immunol. 2007;28:80–87. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: Partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- 36.Ruckle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: Towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 37.Fukao T, Yamada T, Tanabe M, Terauchi Y, Ota T, Takayama T, Asano T, Takeuchi T, Kadowaki T, Hata Ji J, Koyasu S. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 38.Lu-Kuo JM, Fruman DA, Joyal DM, Cantley LC, Katz HR. Impaired kit- but not Fcepsilon RI-initiated mast cell activation in the absence of phosphoinositide 3-kinase p85alpha gene products. J Biol Chem. 2000;275:6022–6029. doi: 10.1074/jbc.275.8.6022. [DOI] [PubMed] [Google Scholar]
- 39.Woodland RT, Schmidt MR, Thompson CB. BLyS and B cell homeostasis. Semin Immunol. 2006;18:318–326. doi: 10.1016/j.smim.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Matheu MP, Deane JA, Parker I, Fruman DA, Cahalan MD. Class IA phosphoinositide 3-kinase modulates basal lymphocyte motility in the lymph node. J Immunol. 2007;179:2261–2269. doi: 10.4049/jimmunol.179.4.2261. [DOI] [PubMed] [Google Scholar]
- 41.Jacob A, Cooney D, Pradhan M, Coggeshall KM. Convergence of signaling pathways on the activation of ERK in B cells. J Biol Chem. 2002;277:23420–23426. doi: 10.1074/jbc.M202485200. [DOI] [PubMed] [Google Scholar]
- 42.Chamberlain MD, Berry TR, Pastor MC, Anderson DH. The p85alpha subunit of phosphatidylinositol 3′-kinase binds to and stimulates the GTPase activity of Rab proteins. J Biol Chem. 2004;279:48607–48614. doi: 10.1074/jbc.M409769200. [DOI] [PubMed] [Google Scholar]
- 43.Kang H, Schneider H, Rudd CE. Phosphatidylinositol 3-kinase p85 adaptor function in T-cells. Co-stimulation and regulation of cytokine transcription independent of associated p110. J Biol Chem. 2002;277:912–921. doi: 10.1074/jbc.M107648200. [DOI] [PubMed] [Google Scholar]
- 44.Ueki K, Yballe CM, Brachmann SM, Vicent D, Watt JM, Kahn CR, Cantley LC. Increased insulin sensitivity in mice lacking p85beta subunit of phosphoinositide 3-kinase. Proc Natl Acad Sci USA. 2002;99:419–424. doi: 10.1073/pnas.012581799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.