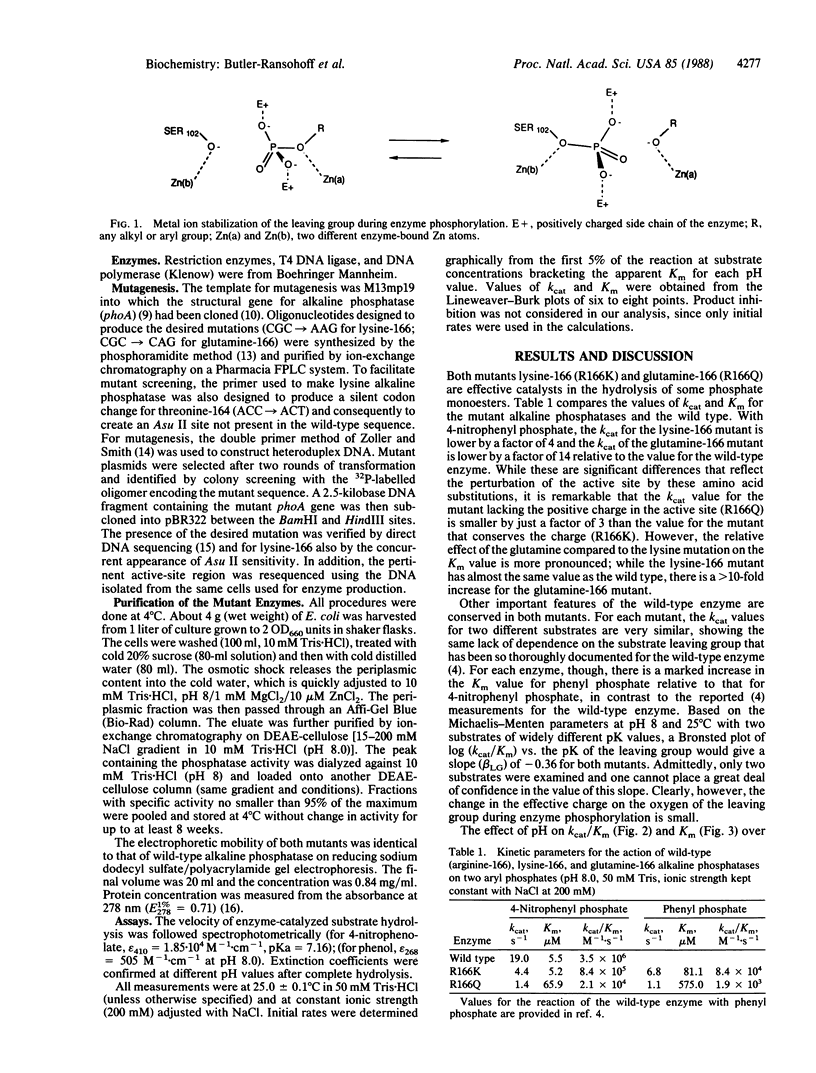
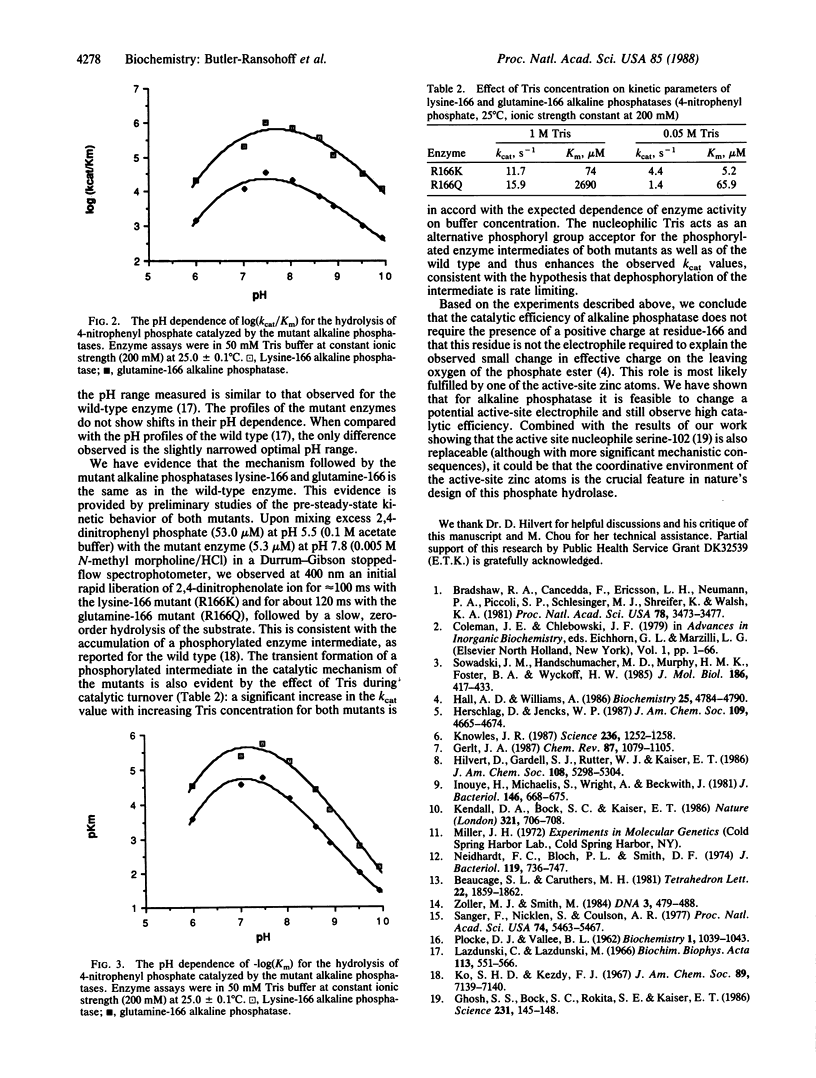
Abstract
The guanidinium group of arginine-166 has been postulated to act as an electrophilic species during phosphorylation of alkaline phosphatase. Its role could be either to stabilize the developing negative charge on the oxygen of the leaving group or the pentacoordinate transition state or to help bind the -PO2-3 group. We have produced via site-directed mutagenesis two Escherichia coli alkaline phosphatase mutants (lysine-166 and glutamine-166) to test whether the guanidinium group plays a critical role in catalysis. Comparative kinetic characterization of the lysine-166 and glutamine-166 mutants indicates that the charge at residue 166 is not required for the hydrolysis of phosphate monoesters. Small decreases in kcat are observed for both the lysine and glutamine mutants, relative to the wild-type enzyme, but the value for the uncharged glutamine mutant is only one-third that of lysine. Thus, the stabilizing effect of the positively charged guanidinium group does not appear to play a major role in the rate-limiting step for substrate hydrolysis. A significant effect on the Km value is seen only for the glutamine mutant.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradshaw R. A., Cancedda F., Ericsson L. H., Neumann P. A., Piccoli S. P., Schlesinger M. J., Shriefer K., Walsh K. A. Amino acid sequence of Escherichia coli alkaline phosphatase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3473–3477. doi: 10.1073/pnas.78.6.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. S., Bock S. C., Rokita S. E., Kaiser E. T. Modification of the active site of alkaline phosphatase by site-directed mutagenesis. Science. 1986 Jan 10;231(4734):145–148. doi: 10.1126/science.3510454. [DOI] [PubMed] [Google Scholar]
- Ghosh S. S., Bock S. C., Rokita S. E., Kaiser E. T. Modification of the active site of alkaline phosphatase by site-directed mutagenesis. Science. 1986 Jan 10;231(4734):145–148. doi: 10.1126/science.3510454. [DOI] [PubMed] [Google Scholar]
- Hall A. D., Williams A. Leaving group dependence in the phosphorylation of Escherichia coli alkaline phosphatase by monophosphate esters. Biochemistry. 1986 Aug 26;25(17):4784–4790. doi: 10.1021/bi00365a010. [DOI] [PubMed] [Google Scholar]
- Inouye H., Michaelis S., Wright A., Beckwith J. Cloning and restriction mapping of the alkaline phosphatase structural gene (phoA) of Escherichia coli and generation of deletion mutants in vitro. J Bacteriol. 1981 May;146(2):668–675. doi: 10.1128/jb.146.2.668-675.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall D. A., Bock S. C., Kaiser E. T. Idealization of the hydrophobic segment of the alkaline phosphatase signal peptide. Nature. 1986 Jun 12;321(6071):706–708. doi: 10.1038/321706a0. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Tinkering with enzymes: what are we learning? Science. 1987 Jun 5;236(4806):1252–1258. doi: 10.1126/science.3296192. [DOI] [PubMed] [Google Scholar]
- Ko S. H., Kézdy F. J. The kinetics of the Escherichia coli alkaline phosphatase catalyzed hydrolysis of 2,4-dinitrophenyl phosphate. J Am Chem Soc. 1967 Dec 20;89(26):7139–7140. doi: 10.1021/ja01002a068. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Lazdunski M. Etude cinétique deu mecanisme d'action catalytique de la phosphatase alcaline d'Escherichia coli. Biochim Biophys Acta. 1966 Mar 7;113(3):551–566. [PubMed] [Google Scholar]
- Lazdunski C., Lazdunski M. Etude cinétique deu mecanisme d'action catalytique de la phosphatase alcaline d'Escherichia coli. Biochim Biophys Acta. 1966 Mar 7;113(3):551–566. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLOCKE D. J., VALLEE B. L. Interaction of alkaline phosphatase of E. coli with metal ions and chelating agents. Biochemistry. 1962 Nov;1:1039–1043. doi: 10.1021/bi00912a014. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowadski J. M., Handschumacher M. D., Murthy H. M., Foster B. A., Wyckoff H. W. Refined structure of alkaline phosphatase from Escherichia coli at 2.8 A resolution. J Mol Biol. 1985 Nov 20;186(2):417–433. doi: 10.1016/0022-2836(85)90115-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]


