Abstract
Data from the Framingham Heart Study suggest that women may be more sensitive to the deleterious cardiovascular remodeling effects of aldosterone. Previous studies from our laboratory have shown that chronic treatment with spironolactone, a mineralocorticoid receptor (MR) antagonist, decreases ischemic cerebral infarct size and prevents remodeling of the middle cerebral artery (MCA) in male spontaneously hypertensive stroke-prone rats (SHRSP). Therefore, we hypothesized that MR antagonism would reduce ischemic infarct size and prevent MCA remodeling in female SHRSP. Six-week-old female SHRSP were treated for 6 wk with spironolactone (25 or 50 mg·kg−1 ·day−1) or eplerenone (100 mg·kg−1 ·day−1) and compared with untreated controls. At 12 wk, cerebral ischemia was induced for 18 h using the intraluminal suture occlusion technique, or the MCA was isolated for analysis of passive structure using a pressurized arteriograph. MR antagonism had no effect on infarct size or passive MCA structure in female SHRSP. To study the potential effects of estrogen, the above experiments were repeated in bilaterally ovariectomized (OVX) female SHRSP treated with spironolactone (25 mg·kg−1·day−1). Infarct size and vessel structure in OVX SHRSP were not different from control SHRSP. Spironolactone had no effect on infarct size in OVX SHRSP. However, MCA lumen and outer diameters were increased in spironolactone-treated OVX SHRSP, suggesting an effect of estrogen. Cerebral artery MR expression, assessed by Western blotting, was increased in female, compared with male, SHRSP. These studies highlight an apparent sexual dimorphism of MR expression and activity in the cerebral vasculature from hypertensive rats.
Keywords: sex differences, cerebral ischemia, vascular remodeling, spironolactone, eplerenone, spontaneously hypertensive stroke-prone rats, middle cerebral artery
Stroke is the third leading cause of death and is a leading cause of long-term disability in the United States (53). Ischemic strokes, caused by a blockage in a cerebral blood vessel, account for 88% of all stroke occurrences (53). Hypertension is a primary risk factor for stroke (59). Spontaneously hypertensive stroke-prone rats (SHRSP) are a powerful model for studying hypertension-related cerebrovascular disease and stroke, due to their pathological similarity to the human disease (32, 58). After induction of ischemia using middle cerebral artery (MCA) occlusion (MCAO) (26), male SHRSP have significantly larger cerebral infarcts compared with normotensive Wistar Kyoto (WKY) rats (15). The relaxed luminal diameters of cerebral collateral vessels in SHRSP are significantly smaller compared with WKY rats (12). The difference in cerebral infarct size between the two strains has been linked to this, rather than collateral vessel density (12–14). This suggests that the damage caused by an ischemic stroke is directly related to the structure of the cerebral vasculature.
Aldosterone, the primary mineralocorticoid secreted by the adrenal gland, is implicated in the pathogenesis of cardiovascular disease (9, 45), mainly via activation of the mineralocorticoid receptor (MR) (23). Patients with primary aldosteronism have increased rates of cardiovascular events, including stroke, compared with patients with essential hypertension, independent of blood pressure (30). Studies from our laboratory revealed that activation of the MR, using deoxycorticosterone acetate, in intact male Wistar rats on a normal salt diet leads to increased damage after ischemic stroke and causes hypertrophic remodeling of the MCA (20). Conversely, MR blockade, using spironolactone or eplerenone, is effective in reducing mortality when added to standard therapies in heart failure patients (34, 35). Studies using MR antagonists in animal models have also shown a beneficial effect of these drugs on the cardiovascular system (44, 49). Chronic spironolactone treatment in male SHRSP significantly reduces damage caused by cerebral ischemia (18), and this may be due to the prevention of eutrophic remodeling in the MCA, as demonstrated previously by our laboratory (42).
Interestingly, more women than men have and die from strokes each year (53). Although stroke risk is increased in postmenopausal women, it is still unknown whether aging or hormonal status is to blame. In general, postmenopausal hormone replacement therapy, as illustrated by multiple clinical trials, has not been successful in reducing stroke risk in women (3, 38), which contradicts the numerous animal studies demonstrating neuroprotective effects of estrogen (29). The absence of a clear effect of estrogen for stroke protection in women warrants further study of alternatives, which could invoke protection from stroke. The impressive cardiovascular benefits of MR antagonism in men makes this therapy an exciting candidate for use in women. Although multiple cardiovascular benefits have been attributed to the use of MR antagonists, few studies have been performed in women. Recent analysis of data from the Framingham Heart Study found that serum aldosterone levels are positively correlated with cardiac wall thickness in women, but not in men (55). These data suggest that women may be more sensitive to the deleterious remodeling effects of aldosterone (21). Therefore, the aim of the present study was to evaluate the effect of MR antagonism on the outcome of cerebral ischemia and MCA remodeling in female SHRSP. The experiments presented here will assess whether the cerebral vasculature of female SHRSP is as responsive to MR antagonism as has been previously shown in male SHRSP.
MATERIALS AND METHODS
Animals and Treatments
All experiments were conducted in 12-wk-old rats. Virgin female and male SHRSP were obtained from the laboratory’s breeding colony at the Medical College of Georgia. Age-matched virgin female inbred WKY rats were purchased from Harlan (Indianapolis, IN) to serve as normotensive controls for SHRSP and to indicate the severity of the damage from ischemic stroke and the extent of cerebral vessel remodeling. Animals were maintained in a temperature-controlled environment on a 12:12-h light-dark cycle and were provided regular rat chow, with normal sodium content (0.25%; Harlan Teklad, diet no. 8656), and tap water ad libitum. The phytoestrogen content of the diet was not controlled, but all rat groups received the same chow. Six-week-old female SHRSP were treated with spironolactone (25 or 50 mg·kg−1·day−1) or eplerenone (100 mg·kg−1 ·day−1) in the food for 6 wk. An additional group of 6-wk-old female SHRSP were bilaterally ovariectomized (OVX) and treated with spironolactone (25 mg·kg−1·day−1) in the food for 6 wk; untreated OVX SHRSP were used as controls. Briefly, bilateral ovariectomy was performed under ketamine/xylazine (67/6.6 mg/kg im) anesthesia. Each ovary was exposed via a flank incision and excised using an electocautery. All experimental protocols were approved by the Medical College of Georgia Institutional Animal Care and Use Committee, following the guidelines of the American Physiological Society.
Blood Pressure Measurement
Blood pressure was continuously monitored using telemetry in separate groups of control and spironolactone-treated (25 and 50 mg/kg) SHRSP and control WKY rats for 6 wk, starting at 6 wk of age. Telemetry transmitters (Data Sciences, St. Paul, MN) were implanted in the abdominal aorta, as previously described (16), before spironolactone treatment; averages of 12-h day and night periods for the last week of treatment were reported. In the eplerenone-treated and OVX SHRSP groups, mean arterial pressure was measured during the last week of treatment using tail-cuff plethysmography with a CODA 6 VPR sensor rat-tail blood pressure system (Kent Scientific, Torrington, CT), as described previously (42). Previous studies indicate a very high level of similarity between the measurements obtained using the Kent Scientific tail-cuff system and telemetry (42). Studies comparing the two methods in rodents found that both methods give similar results (22, 25, 57). A study performed in rats comparing the Kent Scientific VPR tail-cuff system and telemetry found 99% correlation in systolic measurements between the two methods (personal communication with Irina Heald, Kent Scientific). For statistical analysis, all intact SHRSP groups, excluding the eplerenone-treated SHRSP, were compared, and the OVX SHRSP groups were compared.
Cerebral Ischemia
In separate groups of rats, cerebral ischemia was induced using a modified version of the intraluminal suture MCAO technique, as previously described (19, 26). Rats were anesthetized using an inhaled isoflurane (2–5%)/oxygen gas mixture. A laser Doppler probe (Perimed, N. Royalton, OH) attached to the skull was used to indicate successful occlusion of the MCA. A piece of 3-0 suture (Dermalon, monofilament nylon), with a rounded tip, was advanced through the internal carotid artery to the branch point of the MCA at the circle of Willis and was secured in place. Eighteen hours post-MCAO, brains were removed, sectioned (2 mm), stained in 2% 2,3,5-triphenyltetrazolium, and postfixed in 2% paraformaldehyde for 24 h. Pink areas were considered viable tissue, and white areas were considered infarcted regions. Slices were digitized and analyzed using ImageJ software, as described previously (19). The percent hemisphere infarcted (%HI) was determined using an equation to account for edema (50).
Artery Preparation
Separately treated rats that had not undergone MCAO were anesthetized with pentobarbital sodium (100 mg/kg ip) for blood and tissue collection. Heparanized blood samples, obtained via cardiac puncture, were centrifuged, and plasma was collected for electrolyte analysis. The first branch-free segment of the MCA most proximal to the circle of Willis was dissected from the brain and placed in cold physiological salt solution (PSS) (in mmol/l: 141.9 NaCl, 4.7 KCl, 1.7 MgSO4, 0.5 EDTA, 2.8 CaCl2, 10.0 HEPES, 1.2 KH2PO4, and 5.0 glucose; pH 7.4). The vessel was mounted on two glass micropipettes in a small vessel arteriograph (Living Systems Instrumentation, Burlington, VT). The distal pipette was closed off, creating a zero-flow or blind-sac condition; any vessels possessing leaks were discarded. All experiments in this study were performed without flow. Luminal diameter and left/right wall thickness measurements (in micrometers) were obtained using video microscopy. To maintain vessel integrity, the time between animal death and vessel mounting was kept within 45 min.
Myogenic Tone, Vessel Reactivity, and Passive Structure
Vessels were allowed to equilibrate for 1 h at 75 mmHg in heated, oxygenated PSS. Seventy-five millimeters of mercury were chosen based on previous experiments performed in the rat MCA (8, 42). This pressure lies within the autoregulatory range of the rat MCA and is close to the pressure normally experienced by the MCA (11). Initially, luminal diameter measurements were taken at 75 and 125 mmHg to represent active tone. Similar measurements were taken at the end of each experiment in calcium-free conditions for passive tone. Myogenic tone was calculated using the following formula: 1 − (active diameter/passive diameter) * 100 (8).
After active tone measurements were performed, cumulative dose responses (10−9–10−5 mol/l), first to acetylcholine and then to bradykinin, were performed with the intraluminal pressure maintained at 75 mmHg. Luminal diameter measurements were made after a 5-min equilibration at each dose.
Passive vessel structure was assessed in calcium-free PSS containing 2.0 mmol/l EGTA over a range of pressures from 0 to 180 mmHg in 20-mmHg increments. After a 3-min equilibration at each pressure, measurements of luminal diameter and wall thickness (left and right) were taken.
Calculations
Equations used to assess vessel structure were as follows and as described previously (42):
β-Coefficients were obtained from the equation, y = αeβx, after exponential regression was performed on individual stress-strain curves using KaleidaGraph software (version 4.0).
Western Blotting
All animals were anesthetized with pentobarbital sodium (100 mg/kg ip), and the brain was removed at 12 wk of age. Cerebral arteries, including the middle cerebral, anterior cerebral, ophthalmic, posterior communicating, superior cerebellar, and basilar, were dissected and placed in cold lysis buffer (0.25 M Tris·HCl, 10% SDS, 40% glycerol, 0.25% sodium orthovanadate, 0.25% phenylmethane-sulfonyl fluoride, 0.025% aprotinin, and 0.025% leupeptin; pH = 6.8). Samples were processed, and total protein was quantified using a bicinchoninic acid protein kit (Pierce, Rockford, IL). Samples matched for total protein content were resolved and transferred to polyvinylidene difluoride membranes. The membranes were blocked and then incubated with a mouse monoclonal anti-MR primary antibody (1:200; MRN2 2B7, a generous gift from laboratory of Dr. Gomez-Sanchez, Jackson, MS) (24), followed by a goat anti-mouse fluorescent secondary antibody (1:10,000; Rockland, Gilbertsville, PA). Stripped membranes were reprobed using a mouse monoclonal anti-β-tubulin primary antibody (3:10,000; Upstate, Charlottesville, VA) followed by the same secondary as previously mentioned. Membranes were scanned using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE) to visualize and quantify the bands. The ratio of MR to β-tubulin was calculated for each band; the ratios were averaged for each group and compared.
Statistics
Statistical significance was determined using two-way repeated-measures ANOVA, with the Bonferroni multiple-comparison posttest for all dose and pressure responses. One-way ANOVA was used for physiological measurements, blood pressure, %HI, percent tone, EC50 data, and β-coefficients with the Bonferroni multiple-comparison posttest. A t-test was used to determine statistical significance for the Western blot data. A P value of ≤0.05 was considered significant. All values presented are means ± SE; n represents the number of animals. Statistical comparisons were not made between intact and OVX SHRSP groups; these were treated as separate studies.
RESULTS
Part I: Effects of MR Antagonism in Intact Female SHRSP
Blood pressure
Mean arterial pressure was not different between SHRSP groups (P > 0.05, Table 1). Control WKY rats had significantly lower mean arterial pressure compared with all SHRSP groups (P > 0.05, Table 1). Eplerenone-treated SHRSP were not included in the statistical analysis of blood pressure.
Table 1.
Physiological parameters
| Experimental Groups |
||||||
|---|---|---|---|---|---|---|
| Physiological Parameter | SHRSP Control | SHRSP Spir 25 mg/kg | SHRSP Spir 50 mg/kg | SHRSP Eplerenone | WKY Control | P < 0.05 |
| Mean arterial pressure, mmHg | 141±4 (5) | 134±4 (5) | 137±9 (4) | 134±6 (6) | 98±1* (5) | WKY vs. all other groups*‡ |
| Body weight, g | 172.6±2.7 (8) | 194.5±3.4* (8) | 180.7±3.0 (5) | 177.6±3.1 (5) | 182.1±2.3 (7) | Spir 25 vs. all other groups* |
| Heart weight, g | 0.724±0.02 (8) | 0.808±0.02* (8) | 0.708±0.01 (5) | 0.694±0.02 (5) | 0.594±0.01* (7) | WKY and Spir 25 vs. all other groups* |
| Heart-to-body weight ratio × 100 | 0.419±0.01 (8) | 0.416±0.01 (8) | 0.392±0.005 (5) | 0.391±0.01 (5) | 0.326±0.005* (7) | WKY vs. all other groups* |
| Brain weight, g | 1.665±0.01 (5) | 1.667±0.02 (8) | 1.662±0.03 (5) | 1.604±0.02 (5) | 1.918±0.01* (7) | WKY vs. all other groups* |
| Brain-to-body weight ratio × 100 | 0.951±0.02 (5) | 0.858±0.01† (8) | 0.898±0.01 (5) | 0.904±0.02 (5) | 1.050±0.01* (7) | WKY vs. all other groups* Spir 25 vs. SHRSP control† |
| Plasma potassium, mmol/l | 4.6±0.4 (6) | 4.5±0.1 (6) | 5.0±0.2 (6) | 4.6±0.1 (5) | NS | |
| Plasma sodium, mmol/l | 141.6±2.8 (6) | 141.2±1.9 (6) | 140.1±1.1 (6) | 145.6±0.4 (5) | NS | |
Values are mean ± SE; nos. in parentheses are n values. Treatments were started at 6 wk of age in female spontaneously hypertensive stroke-prone rats (SHRSP). All parameters were measured in 12-wk-old female SHRSP and Wistar Kyoto (WKY) rats. Spir, spironolactone; NS, not significant.
P < 0.05 vs. all other groups;
P < 0.05 vs. SHRSP-control.
Eplerenone-treated SHRSP were not included in the statistical comparison for mean arterial pressure.
Physiological parameters
Body and heart weight were increased in spironolactone-treated (25 mg/kg) SHRSP compared with all other groups, but the heart-to-body weight ratio was not different compared with all other groups (Table 1). The brain-to-body weight ratio was increased in spironolac-tone-treated (25 mg/kg) SHRSP compared with control SHRSP (Table 1). Spironolactone (50 mg/kg) and eplerenone had no effect on body, heart, brain, heart-to-body weight ratio, and brain-to-body weight ratio compared with control SHRSP (P < 0.05, Table 1). Heart weight and heart-to-body weight ratios were lower in control WKY rats compared with all SHRSP groups (P < 0.05, Table 1). Brain weight and brain-to-body weight ratios were significantly larger in control WKY rats compared with all SHRSP groups (P < 0.05, Table 1). Plasma sodium and potassium levels were not significantly different between groups (P > 0.05, Table 1).
Effect of MR antagonists on ischemic cerebral infarct size
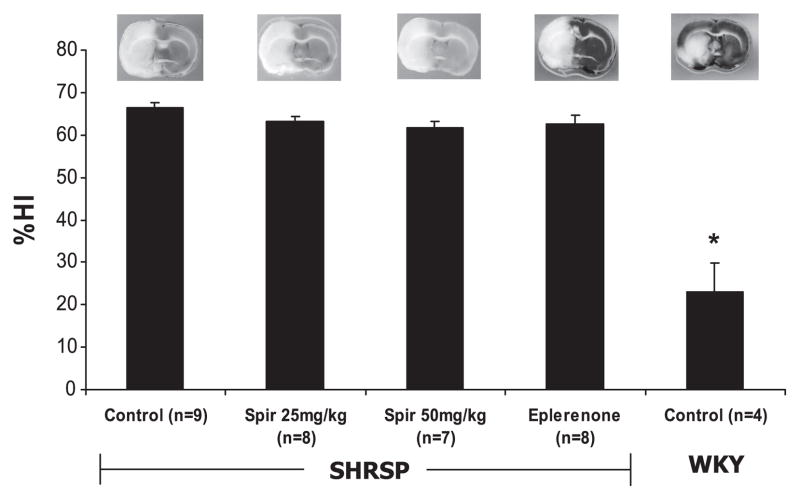
Spironolactone (25 and 50 mg/kg) and eplerenone had no effect on ischemic infarct size, as expressed as the %HI compared with control SHRSP (P > 0.05, Fig. 1). %HI was significantly lower in control WKY rats compared with all SHRSP groups, indicating less damage from MCAO (P < 0.05, Fig. 1).
Fig. 1.
Effect of mineralocorticoid receptor (MR) antagonists on cerebral infarct size after 18 h of permanent ischemia in female spontaneously hypertensive stroke-prone rats (SHRSP) and Wistar Kyoto (WKY) rats. Treatments were started at 6 wk of age in female SHRSP. Permanent cerebral ischemia was induced using middle cerebral artery occlusion (MCAO) at 12 wk of age. Brains were sliced in 2-mm-thick sections and stained to visualize the infarcted regions, which are indicated by the white areas on the brain slice. Representative brain slices are shown for each group. The percent hemisphere infarcted (%HI) was calculated using the Swanson equation after digital analysis of 3 brain slices from each animal. Spironolactone (Spir; 25 and 50 mg/kg) and eplerenone treatment had no effect on ischemic infarct size in female SHRSP. Infarct sizes were significantly smaller in control WKY rats compared with all SHRSP groups (*P <0.05, ANOVA).
Effect of MR antagonists on MCA mechanics and reactivity
MCA STRUCTURE
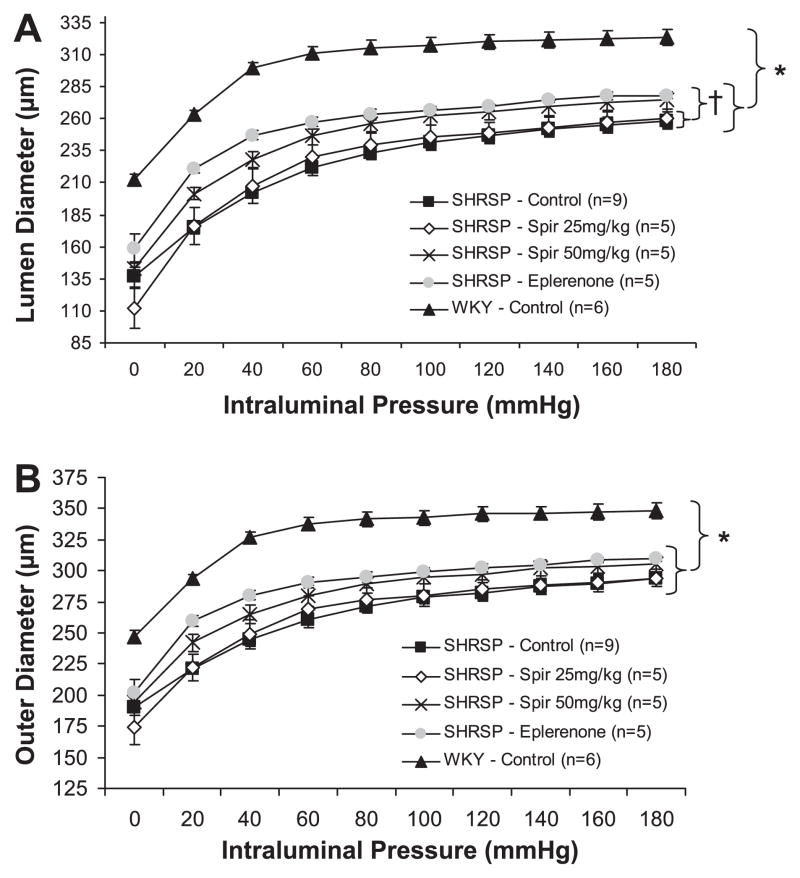
MCAs from female SHRSP underwent inward-eutrophic remodeling, as indicated by the smaller luminal and outer diameters, with no change in wall area compared with control WKY rats (P < 0.05, Fig. 2 and Table 2). Control WKY rats had significantly larger luminal and outer diameters compared with all SHRSP groups (P < 0.05, Fig. 2). Wall thickness and wall-to-lumen ratio were significantly smaller in control WKY rats compared with all SHRSP groups (P < 0.05, Table 2). Wall area was not different between groups (P < 0.05, Table 2). Eplerenone treatment increased luminal diameter and decreased wall thickness and wall-to-lumen ratio compared with control and spironolactone-treated (25 mg/kg) SHRSP (P > 0.05), but did not affect outer diameter (P < 0.05) (Fig. 2 and Table 2). Wall-to-lumen ratio was significantly decreased in spironolactone (50 mg/kg) compared with spironolactone-treated (25 mg/kg) SHRSP (P > 0.05, Table 2). Only the measurements made at 80 mmHg are displayed in Table 2, but are representative of the data taken over the range of pressures. The P values indicated in Table 2 are of the ANOVAs from the entire curve for each structural parameter.
Fig. 2.
Effect of MR antagonists on middle cerebral artery (MCA) remodeling in female SHRSP. Treatments were started at 6 wk of age in female SHRSP. MCAs from 12-wk-old female SHRSP and WKY rats were mounted in a pressurized arteriograph, and measurements of passive lumen (A) and outer (B) diameters were acquired over a range of pressures using video microscopy. MCA luminal diameters were larger in control WKY rats compared with all SHRSP groups (*P < 0.05, ANOVA). In eplerenone-treated SHRSP, MCA luminal diameters were increased compared with control and spironolactone-treated (25 mg/kg) SHRSP (†P < 0.05, ANOVA). MR antagonists had no effect on MCA outer diameters in SHRSP. MCA outer diameters were larger in control WKY rats compared with all SHRSP groups (*P < 0.05, ANOVA).
Table 2.
Vessel dynamics
| Experimental Groups |
||||||
|---|---|---|---|---|---|---|
| Vessel Dynamics | SHRSP Control (9) | SHRSP Spir 25 mg/kg (5) | SHRSP Spir 50 mg/kg (5) | SHRSP Eplerenone (5) | WKY Control (6) | P < 0.05 |
| Wall thickness at 80 mmHg, μm | 38.0±1.4 | 37.6±1.7 | 33.6±1.1 | 31.6±1.4† | 26.0±0.7* | WKY vs. all groups* Epl vs. SHRSP control and Spir 25† |
| Wall-to-lumen ratio at 80 mmHg | 0.16±0.01 | 0.16±0.01 | 0.13±0.01† | 0.12±0.01† | 0.08±0.002* | WKY vs. all groups* Epl vs. SHRSP control and Spir 25† Spir 50 vs. Spir 25‡ |
| Wall area at 80 mmHg, μm2 | 15,045.6±528.2 | 15,203.4±780.6 | 14,359.6±491.2 | 13,842.2±620.6 | 13,423.5±478.4 | NS |
| Remodeling index at 80 mmHg, % | 94.6 | 93.7 | 96.1 | 98.0 | ||
| Growth index at 80 mmHg, % | 12.1 | 13.3 | 7.0 | 3.1 | ||
| Bradykin log EC50 | −8.99±0.4 | −8.32±0.1 | −8.20±0.2 | −4.47±3.1 | −9.63±1.3 | NS |
| Myogenic tone at 75 mmHg, % | 13.0±2.7 | 15.1±2.3 | 11.1±3.6 | 21.2±5.4 | 10.2±2.4 | NS |
| β-Coefficient | 4.76±0.4§ | 3.96±0.6§ | 5.25±0.2§ | 7.23±1.0† | 8.84±0.2 | Epl vs. SHRSP control and Spir 25† vs. WKY§ |
Values are mean ± SE; nos. in parentheses are n values. Treatments were started at 6 wk of age in female SHRSP. All experiments were conducted in 12-wk-old female SHRSP and WKY rats. Only the measurements made at 80 mmHg for the structural parameters are displayed in the table, but are representative of the data taken over the range of pressures (0 to 180 mmHg). Epl, eplerenone. The P values indicated stand for the ANOVAs from the entire curve.
P < 0.05 vs. all other groups.
P < 0.05 vs. SHRSP-control and SHRSP-Spir 25 mg/kg.
P < 0.05 vs. SHRSP-Spir 25 mg/kg.
P < 0.05 vs. WKY control.
MCA COMPLIANCE AND STIFFNESS
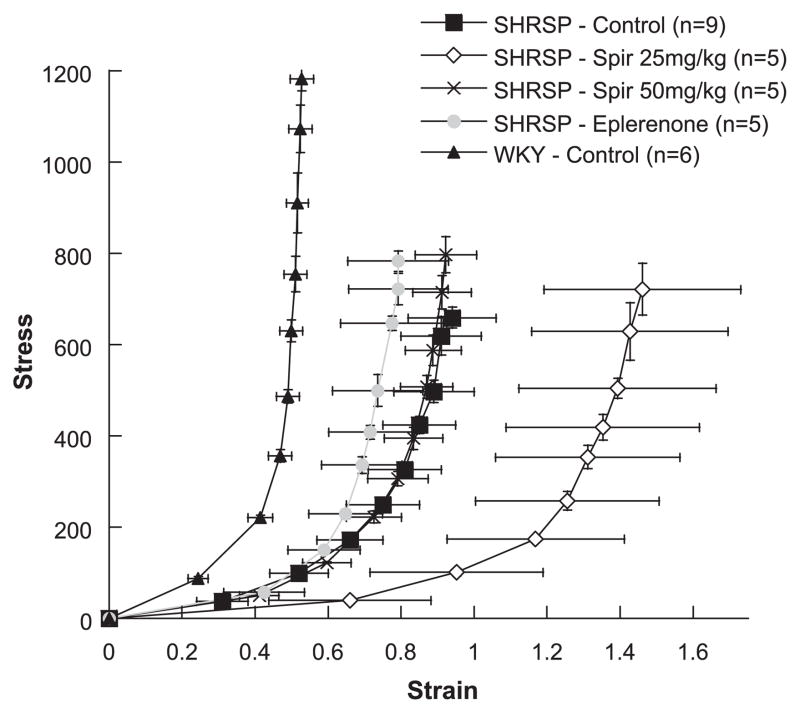
There was a rightward shift in the stress-strain curve from spironolactone-treated (25 mg/kg) SHRSP compared with control SHRSP (Fig. 3), indicating an increase in vessel compliance. Spironolactone (50 mg/kg) and eplerenone had no effect on compliance in SHRSP (Fig. 3). The paradoxical leftward shift in control WKY rats compared with control SHRSP has been demonstrated previously for males (2). The β-coefficient, calculated from exponential regression on individual stress-strain curves, is used as a measure of vessel stiffness (11). Stiffness was significantly less in the control and both spironolactone (25 and 50 mg/kg) SHRSP groups compared with control WKY rats, as indicated by smaller β-coefficients (P < 0.05, Table 2). Eplerenone treatment increased β-coefficients compared with control and spironolactone-treated (25 mg/kg) SHRSP (P < 0.05, Table 2), indicating increased stiffness in eplerenone-treated SHRSP.
Fig. 3.
Effect of MR antagonists on MCA compliance in female SHRSP. Circumferential wall stress and strain were calculated from passive MCA luminal diameter and wall thickness measurements obtained over a range of pressures using a pressurized arteriograph system. A rightward shift in the stress-strain curve indicates increased compliance, and a leftward shift indicates decreased compliance. There was a rightward shift in the MCA stress-strain curve from spironolactone-treated (25 mg/kg) SHRSP.
MCA REACTIVITY TO ACETYLCHOLINE AND BRADYKININ
MR antagonists had no effect on the percent increase in luminal diameter in response to acetylcholine or bradykinin over the range of doses (data not shown). The log EC50 values were also unchanged, suggesting there was no difference in sensitivity to acetylcholine (data not shown) or bradykinin (P > 0.05, Table 2) in the MCA. There was no difference in responses to acetylcholine or bradykinin and log EC50 values between control WKY rats and all SHRSP groups.
MCA MYOGENIC TONE
MR antagonists had no effect on myogenic tone at 75 mmHg (P > 0.05, Table 2) or at 125 mmHg (data not shown). There was also no difference in myogenic tone at 75 or 125 mmHg between control WKY rats and all SHRSP groups.
Effects of Spironolactone in OVX SHRSP
Due to the lack of a difference of MR antagonists on cerebral infarct size or MCA structure in intact SHRSP, similar studies were carried out using spironolactone (25 mg/kg) treatment in OVX SHRSP to elucidate if the absence of an effect of spironolactone was due to the presence of estrogen.
Physiological parameters
Spironolactone (25 mg/kg) had no effect on mean arterial blood pressure, body weight, heart weight, heart-to-body weight ratio, or brain weight in OVX SHRSP (P > 0.05, Table 3). Mean arterial pressure was not different between intact and OVX SHRSP (P > 0.05, ANOVA). However, the brain-to-body weight ratio was lower in spironolactone-treated (25 mg/kg) OVX SHRSP (P < 0.05, Table 3). There was no difference in plasma sodium levels between groups (P > 0.05), but spironolactone (25 mg/kg) treatment significantly increased plasma potassium levels in OVX SHRSP (P < 0.05, Table 3).
Table 3.
Physiological parameters for OVX SHRSP
| Experimental Group |
|||
|---|---|---|---|
| Physiological Parameter | SHRSP OVX | SHRSP OVX + Spir 25 mg/kg | P |
| Mean arterial pressure, mmHg | 126±8 (4) | 139±3 (5) | NS |
| Body weight, g | 190.4±1.6 (7) | 197.4±6.4 (8) | NS |
| Heart weight, g | 0.697±0.01 (7) | 0.699±0.03 (8) | NS |
| Heart/body weight × 100 | 0.366±0.004 (7) | 0.354±0.004 (8) | NS |
| Brain weight, g | 1.723±0.01 (3) | 1.662±0.03 (8) | NS |
| Brain/body weight × 100 | 0.922±0.01 (3) | 0.846±0.02 (8) | P < 0.05 |
| Plasma potassium, mmol/l | 4.6±0.2 (6) | 5.2±0.1 (6) | P < 0.05 |
| Plasma sodium, mmol/l | 140.0±0.6 (6) | 135.9±4.1 (6) | NS |
Values are mean ± SE; nos. in parentheses are n values. Bilateral ovariectomy (OVX) was performed and spironolactone treatment was started at 6 wk of age in female SHRSP. All parameters were measured in 12-wk-old OVX SHRSP.
Effect of spironolactone on ischemic cerebral infarct size in OVX SHRSP
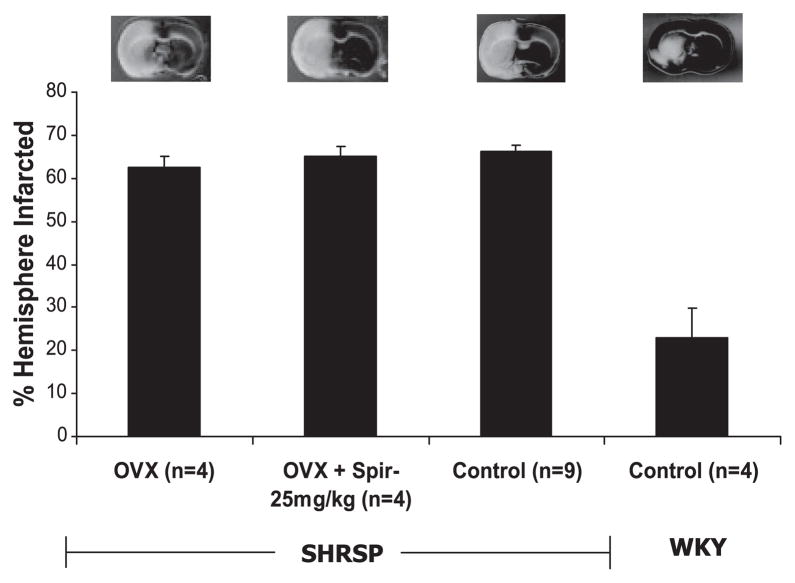
Spironolactone (25 mg/kg) treatment had no effect on ischemic infarct size, as indicated by the lack of a difference in %HI between groups (P > 0.05, Fig. 4). %HI is similar between control and OVX SHRSP.
Fig. 4.
Effect of spironolactone (25 mg/kg) treatment on cerebral infarct size after 18 h of permanent ischemia in ovariectomized (OVX) SHRSP. Bilateral ovariectomy was performed, and spironolactone treatment was started, at 6 wk of age in female SHRSP. Permanent cerebral ischemia was induced using MCAO at 12 wk of age. Brains were sliced in 2-mm-thick sections and stained to visualize the infarcted regions, which are indicated by the white areas on the brain slice. Representative brain slices are shown for each group. The %HI was calculated using the Swanson equation after digital analysis of 3 brain slices from each animal. Spironolactone (25 mg/kg) treatment had no effect on ischemic infarct size in OVX SHRSP. Infarct sizes from control female WKY rats were included on the graph to highlight the severity of cerebral damage in SHRSP.
Effect of spironolactone on MCA mechanics and reactivity in OVX SHRSP
MCA STRUCTURE
Spironolactone (25 mg/kg) treatment decreased lumen and outer diameters in OVX SHRSP (P < 0.05, Fig. 5). Lumen and outer diameters for control SHRSP and WKY rats were included as a reference to indicate the severity of eutrophic remodeling in OVX SHRSP. Wall thickness and wall-to-lumen ratio were also decreased in spironolactone-treated (25 mg/kg) OVX SHRSP (P < 0.05, Table 4). Wall area was not altered by spironolactone (25 mg/kg) treatment in OVX SHRSP (P > 0.05, Table 4). Only the measurements made at 80 mmHg are displayed in Table 4, but are representative of the data taken over the range of pressures. The P values indicated in Table 4 are of the ANOVAs from the entire curve for each structural parameter.
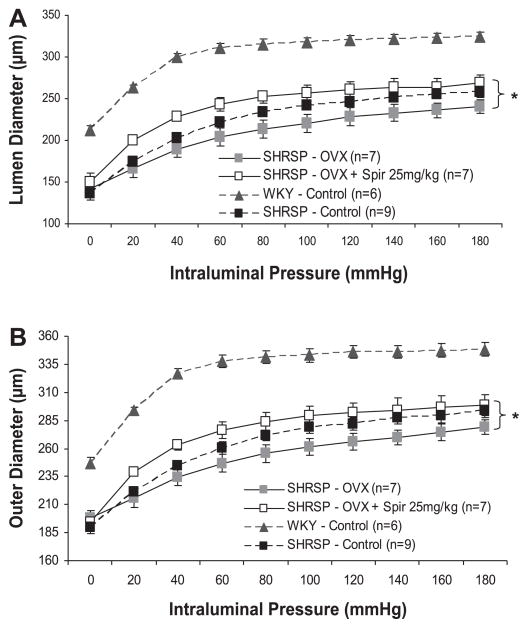
Fig. 5.
Effect of spironolactone (25 mg/kg) treatment on MCA remodeling in OVX SHRSP. Bilateral ovariectomy was performed, and spironolactone treatment was started, at 6 wk of age in female SHRSP. MCAs from 12-wk-old OVX SHRSP were mounted in a pressurized arteriograph, and measurements of passive luminal (A) and outer (B) diameters were acquired over a range of pressures using video microscopy. Spironolactone (25 mg/kg) treatment increased MCA luminal and outer diameters in OVX SHRSP (*P <0.05, ANOVA). MCA luminal and outer diameters from control WKY rats were included to show remodeling of MCAs from SHRSP.
Table 4.
Vessel dynamics for OVX SHRSP
| Experimental Group |
|||
|---|---|---|---|
| Vessel Dynamics | SHRSP OVX | SHRSP OVX + Spir 25 mg/kg | P |
| Wall thickness at 80 mmHg, μm | 42.0±3.5 (7) | 31.9±1.1 (7) | P < 0.05 |
| Wall-to-lumen ratio at 80 mmHg | 0.21±0.03 (7) | 0.13±0.01 (7) | P < 0.05 |
| Wall area at 80 mmHg, μm2 | 15,186.9±690.7 (7) | 13,403.5±557.1 (7) | NS |
| Bradykinin log EC50 | −8.66±0.3 (7) | −9.36±0.3 (4) | NS |
| Myogenic tone at 75 mmHg, % | 7.28±2.6 (7) | 24.0±7.9 (4) | P < 0.05 |
| β-Coefficient | 5.54±0.6 (7) | 6.26±1.0 (7) | NS |
Values are mean ± SE; nos. in parentheses are n values. Bilateral ovariectomy was performed and spironolactone treatment was started at 6 wk of age in female SHRSP. All experiments were conducted in 12-wk-old OVX SHRSP. Only the measurements made at 80 mmHg for the structural parameters are displayed in the table, but are representative of the data taken over the range of pressures (0–180 mmHg). The P values indicated stand for the ANOVAs from the entire curve.
MCA COMPLIANCE AND STIFFNESS
Spironolactone (25 mg/kg) treatment had no effect on compliance in OVX SHRSP, as there was not a shift in the stress-strain curve (Fig. 6). Vessel stiffness was also not altered by spironolactone (25 mg/kg) treatment in OVX SHRSP, as indicated by the lack of a change in the β-coefficient (P > 0.05, Table 4).
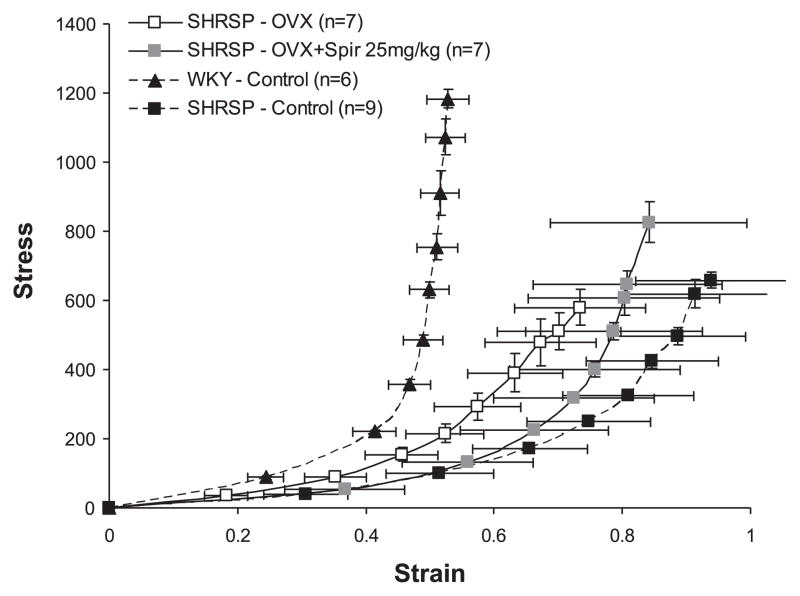
Fig. 6.
Effect of spironolactone (25 mg/kg) treatment on MCA compliance in OVX SHRSP. Circumferential wall stress and strain were calculated from passive MCA luminal diameter and wall thickness measurements obtained over a range of pressures using a pressurized arteriograph system. A rightward shift in the stress-strain curve indicates increased compliance, and a leftward shift indicates decreased compliance. Spironolactone treatment did not cause a shift in the MCA stress-strain curve from OVX SHRSP.
MCA REACTIVITY TO ACETYLCHOLINE AND BRADYKININ
Spironolactone (25 mg/kg) treatment had no effect on the percent increase in luminal diameter in response to acetylcholine or bradykinin over the range of doses in OVX SHRSP (data not shown). The log EC50 values were also unchanged, suggesting there was no difference in sensitivity to acetylcholine (data not shown) or bradykinin (P > 0.05, Table 4) in the MCA of OVX SHRSP.
MCA MYOGENIC TONE
Spironolactone (25 mg/kg) treatment significantly increased myogenic tone at 75 mmHg in the OVX SHRSP compared with nontreated OVX SHRSP (P < 0.05, Table 4). Myogenic tone was also increased at 125 mmHg in the spironolactone-treated (25 mg/kg) compared with non-treated OVX SHRSP (P < 0.05, 28.9 ± 6.8 vs. 11.0 ± 3.4%).
Sexual Dimorphism of MR Expression in Cerebral Arteries from SHRSP
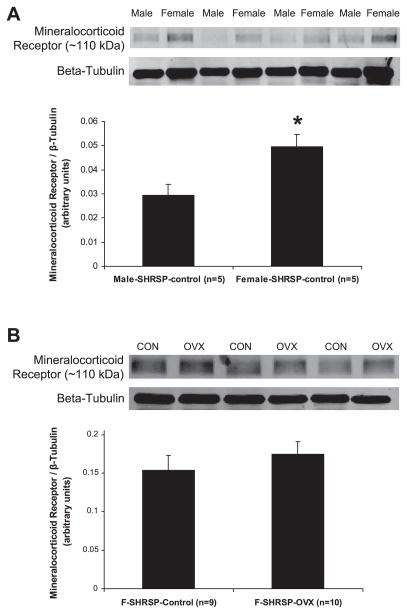
For the first study (Fig. 7A), 12-wk-old male and female SHRSP (n = 5 per sex) were used. Protein expression of the MR assessed by Western blotting was significantly increased in cerebral arteries from female SHRSP compared with male SHRSP, expressed as a ratio of MR to β-tubulin (P < 0.05, Fig. 7A). For the second study (Fig. 7B), 6-wk-old female SHRSP (n = 10) were OVX, as described in the MATERIALS AND METHODS section. Twelve-week-old OVX and intact, female SHRSP (n = 9) were used. Protein expression of the MR assessed by Western blotting was not different in cerebral arteries from intact compared with OVX SHRSP, expressed as a ratio of MR to β-tubulin (P >0.05, Fig. 7B).
Fig. 7.
MR expression in cerebral blood vessels. Cerebral arteries were collected from nontreated 12-wk-old male and female SHRSP and OVX and intact female SHRSP. Western blotting was performed on protein extracts (25 μg for male/female and 30 μg for intact/OVX) using an antibody specific for the MR. To control for equal loading, blots were stripped and reprobed using an antibody specific for β-tubulin. Representative Western blots are shown. A: MR expression was increased in cerebral arteries collected from female SHRSP compared with male SHRSP (*P < 0.05, t-test). B: MR expression was not different in cerebral arteries collected from OVX compared with intact, female SHRSP (P > 0.05, t-test).
DISCUSSION
Previously, we have shown a 50% decrease in cerebral infarct size and prevention of inward eutrophic remodeling of the cerebral vasculature in male SHRSP treated with spironolactone (18, 42), while, in the present study, we did not observe a similar effect of spironolactone in female SHRSP. One difference between these studies and the present study was the route of administration of the spironolactone. However, we have shown that the same dose and route of administration of spironolactone, as used in the present study, caused a reversal of vessel remodeling in older male SHRSP (41). Although eplerenone treatment in female SHRSP elicited some effects on MCA structure, the magnitude of these changes was not as great as the changes observed in male SHRSP treated with eplerenone (100 mg·kg−1·day−1, in food), where the MCA luminal diameter of the treated rats was larger than that of a WKY rat, independent of blood pressure (40). Remarkably, there was also no effect of MR antagonism on cerebral infarct size in intact or OVX SHRSP. The lack of a robust effect of MR antagonism in female SHRSP is even more perplexing, considering they have increased cerebral artery MR expression compared with male SHRSP.
In humans, MR antagonism lowers blood pressure (7, 33). However, mean arterial pressure, measured using telemetry or tail-cuff plethysmography, was not different between control and spironolactone-treated SHRSP groups. This is in agreement with studies showing that MR antagonism has no effect on blood pressure in male SHRSP (18, 41–43, 45) and suggests that MR activation is also not involved in blood pressure maintenance in female SHRSP (32). Previous studies suggest that OVX does not affect blood pressure in spontaneously hypertensive rats and Sprague-Dawley rats (39, 54), but OVX does increase blood pressure in SHRSP (48). It should be noted that the SHRSP were salt-loaded and fed a Stroke-Prone Rodent diet, whereas our studies were performed in nonsalt-loaded rats fed normal chow.
The prevalence of cardiovascular disease is increased in men compared with women before age 55 yr, but by age 65 yr, this is reversed, presenting the possibility that estrogen loss at menopause may be a contributing factor (53). Sex differences in the regulation of blood pressure and the renin-angiotensin-aldosterone system found in humans have also been shown in animal models (27, 37). Studies in female rats indicate that levels of angiotensin-converting enzyme, expression of angiotensin type 1 receptor, and aldosterone secretion are decreased in the presence of estrogen (31, 46, 52). In aged SHRSP, plasma aldosterone is lower in females than in males (28), which could explain the increased cerebral artery MR expression in female SHRSP in the present study.
MR antagonism was effective in reducing mortality in heart failure patients enrolled in two clinical trials, RALES and EPHESUS (34, 35). In these trials, the percentage of female patients included was between 27 and 30%, but the results were not specifically evaluated for sex differences. Animal studies evaluating sex differences of MR antagonism or the effects of MR antagonism in females are limited. One study involving intracerebroventricular administration of a MR antagonist, RU28318, into Wistar rats found that females were less sensitive to central MR antagonism than males (36). Here, neither MR antagonist used could elicit similar dramatic effects in intact or OVX SHRSP, as were seen in male SHRSP in our laboratory’s previous studies (18, 40–42). The lack of an effect of MR antagonism in female SHRSP, even in the presence of increased MR expression, could indicate a sex difference in the metabolism of the antagonists. Pharmacokinetic studies using eplerenone indicate that male rats metabolize the drug more than female rats, due to increased expression of cytochrome P-450 enzymes (10). Since the activity of spironolactone is due to its metabolites, mainly canrenone (17), MR antagonism may be more effective in males because of increased metabolism, as is the case in the present study. It is feasible that both doses of spironolactone used may not have been sufficient to block the MR in female SHRSP; however, a higher dose would have interfered with the estrous cycle (17), causing extraneous effects.
The classical caveat of using spironolactone is its lack of specificity. While it is an excellent MR antagonist, it also possesses anti-androgenic and weak progestin effects (47). However, the present studies also included eplerenone, a more specific, but less efficacious MR antagonist (47). We have recently shown that eplerenone treatment in male SHRSP elicits similar effects as we have shown with spironolactone treatment (42), i.e., prevention of MCA remodeling (40). This suggests that the possible anti-androgenic effects are not playing a role in the effects we observe with spironolactone in male or female SHRSP. Eplerenone treatment of the female SHRSP did offer some improvements in MCA structure, but the magnitude of these changes is very small compared with the changes observed in male SHRSP treated with MR antagonists (40, 42). Even though there were small improvements in MCA structure, stiffness of MCAs from eplerenone-treated SHRSP was increased compared with control SHRSP, which may partially explain why infarct size was unchanged. MCA compliance was not changed by spironolactone treatment at 50 mg/kg, which may be due to the increased probability of spironolactone to elicit nonspecific effects at higher doses. Also, myogenic tone in spironolactone-treated OVX SHRSP was increased, which is similar to our findings in male SHRSP, where there was an enhancement of overall contractile ability of the MCA (42). Despite this, none of the changes observed in MCA structure were sufficient to reduce the damage caused by cerebral ischemia.
The weak progestin activity of spironolactone can cause disruption of ovulation, but this occurs in rats at a dose that is twofold higher than the highest dose utilized in the present study (17). OVX SHRSP were treated with spironolactone to evaluate whether estrogen may have been modulating the actions of the MR; however, there were only small changes in MCA structure. Interestingly, plasma potassium was increased in these rats. Dietary potassium supplementation and a subsequent increase in plasma potassium has been shown to improve MCA structure (19, 56), presenting the possibility that the small improvements in vascular structure in the spironolactone-treated SHRSP are a result of potassium.
Another limitation of the present study is that the estrous cycle was not monitored. Previous studies show that the cycling status of the female rat affects the damage caused by cerebral ischemia in SHRSP (6). Female SHRSP in the proestrus phase (high circulating estrogen) have ~24% smaller infarcts compared with SHRSP in the metestrus phase (low circulating estrogen) of the cycle. However, it is important to note that SHRSP used in the study were first crossed with WKY rats to alter the sensitivity to experimental stroke (4), and the method of ischemic stroke induction is different. Carswell et al. used a transcranial ischemia technique (5), whereas our study adapted the intraluminal suture occlusion technique (26), although both methods were permanent. Therefore, differences in the techniques employed and the strain of rats used between the two studies could explain the differences between our results. However, in our studies, the variability in infarct size was low within each group of intact SHRSP (SE of the mean of less than ±2.0% HI per group), suggesting either that the female SHRSP were in the same point of the cycle at induction of ischemia, or that the severe damage caused by ischemic stroke in this model cannot be altered by high circulating estrogen levels in the proestrus cycling phase. We would have expected much greater variability in our results, if damage incurred by cerebral ischemia depended on the cycling phase. To highlight this point, OVX SHRSP did not have increased ischemic stroke damage compared with intact control SHRSP. Many studies using normotensive rats have shown that OVX increases damage caused by ischemic stroke (1, 29). A study performed by Alkayed et al. (1) utilized intact and OVX SHRSP and showed that OVX increased infarct size after MCAO, but in that study the investigators used an ischemia (2 h)-reperfusion (22 h) technique, which has an entirely different pathology from the permanent MCAO technique and may explain differences between our findings. However, in a study performed by Carswell et al. (5), exogenous administration of estrogen did not confer protection after transcranial MCAO in OVX SHRSP.
In summary, the present study brings to light an apparent sexual dimorphism in the actions of MR antagonists in SHRSP. The disparity may be partially due to the difference in MR expression in the cerebral arteries. Our data add to the increasing body of literature acknowledging sex differences of the renin-angiotensin-aldosterone system and their involvement in the development and progression of cardiovascular disease and hypertension. Further study is warranted to elucidate the effects that genetics or disease pathology play in creating these sex differences. Data from these studies could lead to more sex-specific therapies for treating patients with cardiovascular disease.
Acknowledgments
We thank Hiram Ocasio for time and surgical expertise in the telemetry studies.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL077385 (A. M. Dorrance) and HL64776 (D. M. Pollock).
References
- 1.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–166. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 2.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–972. doi: 10.1161/01.hyp.13.6.968. [DOI] [PubMed] [Google Scholar]
- 3.Brass LM. Hormone replacement therapy and stroke: clinical trials review. Stroke. 2004;35:2644–2647. doi: 10.1161/01.STR.0000143218.20061.ac. [DOI] [PubMed] [Google Scholar]
- 4.Carswell HV, Anderson NH, Clark JS, Graham D, Jeffs B, Dominiczak AF, Macrae IM. Genetic and gender influences on sensitivity to focal cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension. 1999;33:681–685. doi: 10.1161/01.hyp.33.2.681. [DOI] [PubMed] [Google Scholar]
- 5.Carswell HV, Bingham D, Wallace K, Nilsen M, Graham DI, Dominiczak AF, Macrae IM. Differential effects of 17β-estradiol upon stroke damage in stroke prone and normotensive rats. J Cereb Blood Flow Metab. 2004;24:298–304. doi: 10.1097/01.WCB.0000112322.75217.FD. [DOI] [PubMed] [Google Scholar]
- 6.Carswell HV, Dominiczak AF, Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2000;278:H290–H294. doi: 10.1152/ajpheart.2000.278.1.H290. [DOI] [PubMed] [Google Scholar]
- 7.Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, Poulter NR Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845. doi: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 8.Cipolla MJ, Lessov N, Clark WM, Haley EC., Jr Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–945. doi: 10.1161/01.str.31.4.940. [DOI] [PubMed] [Google Scholar]
- 9.Connell JMC, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186:1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- 10.Cook CS, Zhang L, Ames GB, Fischer J, Zhang J, Levin S. Single- and repeated-dose pharmacokinetics of eplerenone, a selective aldosterone receptor blocker, in rats. Xenobiotica. 2003;33:305–321. doi: 10.1080/0049825021000049277. [DOI] [PubMed] [Google Scholar]
- 11.Coulson RJ, Chesler NC, Vitullo L, Cipolla MJ. Effects of ischemia and myogenic activity on active and passive mechanical properties of rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283:H2268–H2275. doi: 10.1152/ajpheart.00542.2002. [DOI] [PubMed] [Google Scholar]
- 12.Coyle P. Dorsal cerebral collaterals of stroke-prone spontaneously hypertensive rats (SHRSP) and Wistar Kyoto rats (WKY) Anat Rec. 1987;218:40–44. doi: 10.1002/ar.1092180108. [DOI] [PubMed] [Google Scholar]
- 13.Coyle P, Feng X. Risk area and infarct area relations in the hypertensive stroke-prone rat. Stroke. 1993;24:705–710. doi: 10.1161/01.str.24.5.705. [DOI] [PubMed] [Google Scholar]
- 14.Coyle P, Heistad DD. Development of collaterals in the cerebral circulation. Blood Vessels. 1991;28:183–189. doi: 10.1159/000158860. [DOI] [PubMed] [Google Scholar]
- 15.Coyle P, Jokelainen PT. Differential outcome to middle cerebral artery occlusion in spontaneously hypertensive stroke-prone rats (SHRSP) and Wistar Kyoto (WKY) rats. Stroke. 1983;14:605–617. doi: 10.1161/01.str.14.4.605. [DOI] [PubMed] [Google Scholar]
- 16.D’Angelo G, Elmarakby AA, Pollock DM, Stepp DW. Fructose feeding increases insulin resistance but not blood pressure in Sprague-Dawley rats. Hypertension. 2005;46:806–811. doi: 10.1161/01.HYP.0000182697.39687.34. [DOI] [PubMed] [Google Scholar]
- 17.de Gasparo M, Joss U, Ramjoue HP, Whitebread SE, Haenni H, Schenkel L, Kraehenbuehl C, Biollaz M, Grob J, Schmidlin J, et al. Three new epoxy-spirolactone derivatives: characterization in vivo and in vitro. J Pharmacol Exp Ther. 1987;240:650–656. [PubMed] [Google Scholar]
- 18.Dorrance AM, Osborn HL, Grekin R, Webb RC. Spironolactone reduces cerebral infarct size and EGF-receptor mRNA in stroke-prone rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R944–R950. doi: 10.1152/ajpregu.2001.281.3.R944. [DOI] [PubMed] [Google Scholar]
- 19.Dorrance AM, Pollock DM, Romanko OP, Stepp DW. A high-potassium diet reduces infarct size and improves vascular structure in hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R415–R422. doi: 10.1152/ajpregu.00438.2005. [DOI] [PubMed] [Google Scholar]
- 20.Dorrance AM, Rupp NC, Nogueira EF. Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension. 2006;47:590–595. doi: 10.1161/01.HYP.0000196945.73586.0d. [DOI] [PubMed] [Google Scholar]
- 21.Duprez DA. Is the female heart more sensitive to aldosterone for early remodeling? Hypertension. 2004;43:936–937. doi: 10.1161/01.HYP.0000124253.98863.86. [DOI] [PubMed] [Google Scholar]
- 22.Fraser TB, Turner SW, Mangos GJ, Ludbrook J, Whitworth JA. Comparison of telemetric and tail-cuff blood pressure monitoring in adrenocorticotrophic hormone-treated rats. Clin Exp Pharmacol Physiol. 2001;28:831–835. doi: 10.1046/j.1440-1681.2001.03531.x. [DOI] [PubMed] [Google Scholar]
- 23.Funder JW. Mineralocorticoid receptors: distribution and activation. Heart Fail Rev. 2005;10:15–22. doi: 10.1007/s10741-005-2344-2. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology. 2006;147:1343–1348. doi: 10.1210/en.2005-0860. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim J, Berk BC, Hughes AD. Comparison of simultaneous measurements of blood pressure by tail-cuff and carotid arterial methods in conscious spontaneously hypertensive and Wistar-Kyoto rats. Clin Exp Hypertens. 2006;28:57–72. doi: 10.1080/10641960500386817. [DOI] [PubMed] [Google Scholar]
- 26.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 27.Maric C. Sex differences in cardiovascular disease and hypertension: involvement of the renin-angiotensin system. Hypertension. 2005;46:475–476. doi: 10.1161/01.HYP.0000178600.88820.b2. [DOI] [PubMed] [Google Scholar]
- 28.Masineni SN, Chander PN, Singh GD, Powers CA, Stier JCT. Male gender and not the severity of hypertension is associated with end-organ damage in aged stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2005;18:878–884. doi: 10.1016/j.amjhyper.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 29.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 30.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JFM, Daemen MJAP, Vetter H, Bohm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto K, Yamori Y, Nagaoka A. Establishment of the stroke-prone spontaneously hypertensive rat (SHR) Circ Res. 1974;34(Suppl I):143–153. [Google Scholar]
- 33.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 34.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 35.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 36.Rahmouni K, Barthelmebs M, Grima M, Imbs JL, De Jong W. Cardiovascular and renal effects of central administration of a mineralo-corticoid receptor antagonist in conscious female rats. Eur J Pharmacol. 1999;385:199–202. doi: 10.1016/s0014-2999(99)00705-0. [DOI] [PubMed] [Google Scholar]
- 37.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 38.Reckelhoff JF. Sex steroids, cardiovascular disease, and hypertension: unanswered questions and some speculations. Hypertension. 2005;45:170–174. doi: 10.1161/01.HYP.0000151825.36598.36. [DOI] [PubMed] [Google Scholar]
- 39.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 40.Rigsby CS, Dorrance AM. Eplerenone prevents cerebral vessel remodeling in male hypertensive rats (Abstract) FASEB J. 2007;21:960. [Google Scholar]
- 41.Rigsby CS, Dorrance AM. Reversal of existing cerebral vessel remodeling with spironolactone in adult spontaneously hypertensive stroke prone rats (SHRSP) Hypertension. 2005;46:813–874. [Google Scholar]
- 42.Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res. 2007;73:198–205. doi: 10.1016/j.mvr.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT., Jr Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension. 1998;31:451–458. doi: 10.1161/01.hyp.31.1.451. [DOI] [PubMed] [Google Scholar]
- 44.Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann NY Acad Sci. 2002;970:89–100. doi: 10.1111/j.1749-6632.2002.tb04415.x. [DOI] [PubMed] [Google Scholar]
- 45.Rocha R, Stier CT., Jr Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab. 2001;12:308–314. doi: 10.1016/s1043-2760(01)00432-5. [DOI] [PubMed] [Google Scholar]
- 46.Roesch DM, Tian Y, Zheng W, Shi M, Verbalis JG, Sandberg K. Estradiol attenuates angiotensin-induced aldosterone secretion in ovariectomized rats. Endocrinology. 2000;141:4629–4636. doi: 10.1210/endo.141.12.7822. [DOI] [PubMed] [Google Scholar]
- 47.Sica DA. Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis. Heart Fail Rev. 2005;10:23–29. doi: 10.1007/s10741-005-2345-1. [DOI] [PubMed] [Google Scholar]
- 48.Stier CT, Jr, Chander PN, Rosenfeld L, Powers CA. Estrogen promotes microvascular pathology in female stroke-prone spontaneously hypertensive rats. Am J Physiol Endocrinol Metab. 2003;285:E232–E239. doi: 10.1152/ajpendo.00029.2003. [DOI] [PubMed] [Google Scholar]
- 49.Stier CT, Jr, Rocha R, Chander PN. Effect of aldosterone and MR blockade on the brain and the kidney. Heart Fail Rev. 2005;10:53–62. doi: 10.1007/s10741-005-2349-x. [DOI] [PubMed] [Google Scholar]
- 50.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 51.Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat. 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka M, Nakaya S, Watanabe M, Kumai T, Tateishi T, Kobayashi S. Effects of ovariectomy and estrogen replacement on aorta angiotensin-converting enzyme activity in rats. Jpn J Pharmacol. 1997;73:361–363. doi: 10.1254/jjp.73.361. [DOI] [PubMed] [Google Scholar]
- 53.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 54.Tsang SY, Yao X, Chan FL, Wong CM, Chen ZY, Laher I, Huang Y. Estrogen and tamoxifen modulate cerebrovascular tone in ovariectomized female rats. Hypertension. 2004;44:78–82. doi: 10.1161/01.HYP.0000131659.27081.19. [DOI] [PubMed] [Google Scholar]
- 55.Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, Murabito JM, Sam F, Colucci WS, Wilson PW. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension. 2004;43:957–962. doi: 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Domenighetti AA, Pedrazzini T, Burnier M. Potassium supplementation reduces cardiac and renal hypertrophy independent of blood pressure in DOCA/salt mice. Hypertension. 2005;46:547–554. doi: 10.1161/01.HYP.0000178572.63064.73. [DOI] [PubMed] [Google Scholar]
- 57.Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol. 2004;286:H2408–H2415. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- 58.Yamori Y, Horie R, Handa H, Sato M, Fukase M. Pathogenetic similarity of strokes in stroke-prone spontaneously hypertensive rats and humans. Stroke. 1976;7:46–53. doi: 10.1161/01.str.7.1.46. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Thijs L, Staessen JA. Blood pressure lowering for primary and secondary prevention of stroke. Hypertension. 2006;48:187–195. doi: 10.1161/01.HYP.0000231939.40959.60. [DOI] [PubMed] [Google Scholar]