Abstract
Background and objective
OSA is associated with increased incidence of cardiovascular diseases. Pathogenic mechanisms of vascular diseases include thickened vascular walls due to the increased number of smooth muscle cells (SMC). Retinoic acid (RA) suppresses the growth of SMC, and reduced retinoid levels are associated with vascular diseases. Oxidant signalling promotes SMC growth, thus antioxidant levels may also influence the development of cardiovascular diseases. The present study tested the hypothesis that plasmas from OSA patients contain altered levels of retinoids, carotenoids and tocopherols.
Methods
Plasma samples were taken before and after sleep from patients with OSA (mostly mild) without known cardiovascular diseases and from control subjects. Levels of retinoids, carotenoids and tocopherols were measured using sensitive gas chromatograph-mass spectrometry and high pressure liquid chromatography methods and total antioxidant capacity was assessed fluorometrically.
Results
Results showed that plasmas from patients with OSA had significantly lower retinyl palmitate and 9-cis RA compared with control subjects, while levels of retinol, all-trans RA and 13-cis RA were indifferent. All-trans β-carotene and 9-cis β-carotene were also lower in OSA patients. Levels of all-trans RA and 13-cis RA in OSA patients were reduced after sleep compared with before sleep. OSA patients showed significantly higher δ-tocopherol compared with controls. Treatment of cultured human vascular SMC with post-sleep OSA patient plasmas promoted cell growth, but not in controls.
Conclusions
Mild OSA exhibits altered levels of specific retinoids, carotenoids and tocopherols, which may be markers and/or mediators for the increased susceptibility of patients to vascular diseases.
Keywords: antioxidant, carotenoid, retinoid, sleep apnoea, tocopherol
INTRODUCTION
OSA has been recognized as a risk for developing cardiovascular diseases.1–3 The Wisconsin Sleep Cohort Study, a prospective study investigating a possible relationship between OSA and high blood pressure, reported that sleep-disordered breathing is associated with increased risk for the development of hypertension.4 The Sleep Heart Health Study revealed a link between OSA and myocardial infarction as well as stroke.5 OSA may also play a role in the development of congestive heart failure and coronary heart disease.6,7 The prevalence of pulmonary hypertension in OSA patients has been reported in some studies to be as high as 15–20%.8,9
Several mechanisms have been proposed for the relationship between OSA and cardiovascular diseases including repeated episodes of hypoxia/reoxygenation (intermittent hypoxia), hypercapnia, inflammation and changes in intrathoracic pressures.1 Abnormal oxygenation and pressures result in compensatory responses including sympathetic nerve activity, elevated urinary and plasma norepinephrine and endothelin, vasoconstriction and haemodynamic modification.1,10,11 Hypertension (pulmonary or systemic) can result from hyperresponsive vasoconstriction, vascular extracellular matrix remodelling, and smooth muscle cell (SMC) growth.12–14 The normal phenotype of vascular SMC is contractile, non-proliferative and non-migratory; however, SMC taken from patients with pulmonary or systemic hypertension have been demonstrated to have increased proliferation compared with normal controls.14,15 The growth of SMC is believed to be regulated by the balance between factors which promote SMC proliferation and inhibit apoptosis and agents which inhibit proliferation and induce apoptosis.14–16
Retinoic acid (RA) can negatively regulate cell growth. All-trans RA (ATRA) has been shown to inhibit growth of cancer cells,17–19 epithelial cells20,21 and vascular SMC.22–26 In rats, ATRA reduces vascular remodelling in the carotid artery balloon injury model23 and in monocrotaline-induced pulmonary hypertension. 27 In our previous study, plasmas taken from patients with idiopathic pulmonary arterial hypertension were found to have reduced levels of ATRA and 13-cis RA.22 Thus, RA may participate in maintaining the normal balance of SMC growth inhibition, and alterations of RA levels may trigger vascular changes. In this study of idiopathic pulmonary arterial hypertension,22 patients were also found to have reduced levels of β-carotene (the primary source of retinoids) and α-tocopherol (antioxidants and indicators of oxidative stress which may influence retinoid metabolism). The present study examined whether plasmas from OSA patients contain altered levels of retinoids, carotenoids, tocopherols and oxidative stress.
METHODS
Patients and control subjects
Patients attending the sleep clinics at Georgetown University Medical Center and George Washington University-Medical Faculty Associates were recruited prospectively from December 2004 through December 2006. Patients suspected of having OSA-hypopnea syndrome, but without existing cardiovascular disease, cancer, liver or kidney disease, underwent a full overnight polysomnography at either sleep clinic. The polysomnography included recording of oronasal flow (using a thermistor), thoracoabdominal movements, electrocardiography, submental and pretibial electromyography, electrooculography, electroencephalography and transcutaneous measurement of oxygen desaturation. Obstructive apnoea was defined by the absence of airflow for more than 10 s, with continuation of effort. Hypopnea was defined as any airflow reduction that lasted for more than 10 s, accompanied by an oxygen desaturation of >4%, with or without an electroencephalography arousal.28–30 The apnoea-hypopnea index (AHI) was defined as the sum of the number of apnoeas and hypopneas per hour of sleep. An AHI < 5 was considered to be OSA-negative. AHI between 5 and 15 signified mild disease, 15–30 was moderate disease, and AHI > 30 was considered to be severe disease.28–30
Control individuals were selected by age/gender matching and no clinical suspicion for OSA including excessive daytime hypersomnolence, snoring choking or gasping for air during sleep, witnessed apneic episodes and neck circumference of greater than 42 cm. Controls also had no known cardiovascular diseases, cancer, liver or kidney diseases, based on clinical histories and most recent physician exams. Some controls underwent a full overnight polysomnography and was found to be OSA-negative.
The Institutional Review Boards at Georgetown University and George Washington University approved the protocols, and all participants gave written, informed consent. All patient information was kept confidential in accordance with The Health Insurance Portability and Accountability Act regulations. All subjects were identified with a unique number. Relevant clinical information for collected plasma from each subject was recorded in research charts and coded only with the unique code number. Research on plasma samples was performed double-blind in coded tubes. Blood samples were taken from peripheral veins of subjects at seated position before (~9 pm) and after sleep (~6 am). Night-time samples were not taken under fasting condition; morning samples were taken right after subjects woke up before eating or drinking. Immediately after sampling, blood samples were centrifuged in heparin coated tubes, and plasma was transferred to a sterile tube. Samples were stored at −80°C under N2 to prevent oxidation until analyses.
Measurements of retinoids, carotenoids and tocopherols
Retinol, carotenoids and tocopherols were analysed by high pressure liquid chromatography (HPLC), and RA levels were determined by combining HPLC and gas chromatograph-mass spectrometry (GC/MS) as previously described.22 In brief, 200 µL of plasma samples with13C4-retinoic acid as an internal standard were extracted with chloroform/methanol (2 : 1 volume). The extract was dried under N2, and 200 µL of chloroform was added. Samples were loaded onto an amino column (Phenomenex, Torrance, CA, USA) and the RA fraction was eluted with diethyl ether/3% acetic acid. The residue was evaporated under N2, re-suspended in ethanol, and then injected onto HPLC system. The RA peak was eluted at 7 min of the HPLC chromatogram, evaporated under N2 and derivatized for GC/MS analysis.
Measurement of total antioxidant performance
Plasma total antioxidant performance (TAP) was determined fluorometrically with a 1420-multilabel counter (Wallac Victor 2; Perkin-Elmer Life Sciences, Boston, MA, USA) as described previously31 with minor modifications. This method measures 1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (BODIPY581/591;Molecular Probes, Eugene, OR, USA), a lipid-soluble fluorescent probe, and uses the lipid-soluble radical initiator 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitrile) (MeO-AMVN; Wako Chemicals, Richmond, VA, USA). Oxidation was determined by monitoring the appearance of green fluorescence of the oxidation product of BODIPY (λex = 500 nm, λem = 520 nm). The results are expressed as TAP values (% protection), which represents the percentage of inhibition of BODIPY oxidation in human plasma in respect to that occurring in a control sample, phosphatidylcholine liposomes (2.5 mg/mL) in PBS (100 mmol/L, pH 7.4) using the equation: [(ValueControl − ValuePlasma)/ValueControl] × 100.31
Culture of human vascular SMC
Human pulmonary artery SMC (Cell Applications, San Diego, CA, USA) were used for experiments after three to six passages in cell culture at 5%CO2 and 37°C in medium supplied by the manufacturer. To prepare lysates, the cells were washed in PBS and solubilized with 50 mmol/L Hepes solution (pH 7.4) containing 1%(v/v) Triton X-100, 4 mmol/L thylenediaminetetra-acetic acid, 1 mmol/L sodium fluoride, 0.1 mmol/L sodium orthovanadate, 1 mmol/L tetrasodium pyrophosphate, 2 mmol/L phenylmethylsulphonyl fluoride, 10 µg/mL leupeptin and 10 µg/mL aprotinin. Total protein concentrations were determined spectrophotometrically using Bio-Rad Protein Assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Neutral comet assays to measure double-stranded DNA breaks, as indication of apoptosis, were performed as previously described.32 Briefly, cells were washed in PBS and embedded in 1% agarose before being placed in lysis solution (2.5 mol/L NaCl, 1% Na-lauryl sarcosinate, 100 mmol/L EDTA, 10 mmol/L Tris base, 0.01% Triton X-100) for 30 min. Nuclei were electrophoresed for 20 min, and then stained using SYBR Green. Comet tails were visualized at 478-nm excitation and 507-nm emission wavelengths.
Statistical analysis
Statistical comparisons using the Student’s t-test were performed for OSA patients versus control subjects. Pair Student’s t-test was performed to compare before and after sleep using SPSS software 15.0 (Chicago, IL, USA). Statistical significance was determined at P < 0.05.
RESULTS
Patients
Sixteen patients who underwent sleep studies without known cardiovascular diseases were recruited to the study. Nine subjects were found to be positive for OSA, while seven subjects were negative. Plasma samples were taken, and sensitive and reliable methods were used to assess the levels of retinoids, carotenoids, tocopherols and total antioxidant capacity. At the end of the study, HPLC and GC/MS results on the plasma levels of retinoids, carotenoids and tocopherols were successfully measured in nine OSA patients and in age/gender matched control subjects. All subjects were healthy with apparent normal dietary intake of vitamin A and vitamin E, according to the analyses of plasma retinol and total tocopherol levels. The median age of OSA patients was 43.7 ± 2.9 (range 31–55 years), while the median age for controls was 44.3 ± 3.5 (range 27–60 years). The body-mass indices of the OSA patients were not significantly different from controls (26 ± 3 vs 29 ± 6). The profiles of OSA patients include mean weight of 88.6 kg and the AHI of 18.0 ± 5.7 events/h with mostly mild OSA (Table 1).
Table 1.
Profiles of OSA patients
| Age | Gender | Height (cm) |
Weight (kg) |
RDI (events/h) |
OSA | Min. % O2 saturation |
Epworth sleepiness score |
|
|---|---|---|---|---|---|---|---|---|
| OSA-A | 55 | M | 173 | 82 | 9 | Mild | 89% | 17 |
| OSA-B | 48 | M | 188 | 100 | 60 | Severe | 77% | 6 |
| OSA-C | 31 | M | 168 | 73 | 9 | Mild | 89% | 14 |
| OSA-D | 46 | M | 183 | 71 | 7 | Mild | 93% | 6 |
| OSA-E | 48 | F | 158 | 77 | 6 | Mild | 93% | 6 |
| OSA-F | 54 | M | 178 | 80 | 14 | Mild | 80% | 8 |
| OSA-G | 32 | M | 196 | 91 | 26 | Moderate | 92% | 3 |
| OSA-H | 42 | M | 183 | 96 | 10 | Mild | 85% | 3 |
| OSA-I | 37 | M | 173 | 127 | 21 | Moderate | 61% | 13 |
RDI, Respiratory Disturbance Index.
Retinoid levels
Retinoids were measured in plasma samples of OSA patients and control subjects before sleep using the sensitive and reliable HPLC and GC/MS techniques (Table 2). In contrast to the previous study of idiopathic pulmonary arterial hypertension patients,22 plasma levels of ATRA and 13-cis RA in OSA patients were not significantly different from those of control subjects. Interestingly, however, the mean 9-cis RA level in OSA patients was about 40% lower than that of control subjects (P < 0.05). Notably, while the levels of the major vitamin A, retinol were not different, the level of retinyl palmitate in OSA patients was only 1/3 of the control (P < 0.01).
Table 2.
Levels of retinoids
| Subject | Total RA, (ng/mL) |
ATRA, (ng/mL) |
13-cis-RA, (ng/mL) |
9-cis-RA, (ng/mL) |
Retinol, (µg/dL) |
Retinyl palmitate, (µg/dL) |
|---|---|---|---|---|---|---|
| OSA-A | 4.13 | 1.34 | 1.69 | 0.36 | 55.31 | 0.42 |
| OSA-B | 4.00 | 1.47 | 1.14 | 0.43 | 79.89 | 0.28 |
| OSA-C | 2.74 | 1.09 | 0.86 | 0.43 | 63.31 | 0.60 |
| OSA-D | 2.58 | 1.18 | 0.81 | 0.23 | 52.67 | 0.86 |
| OSA-E | 3.89 | 1.19 | 1.20 | 0.43 | 60.49 | 0.50 |
| OSA-F | 5.68 | 1.22 | 2.03 | 0.55 | 45.06 | 0.28 |
| OSA-G | 4.85 | 1.41 | 1.65 | 0.62 | 60.01 | 0.83 |
| OSA-H | 3.01 | 1.24 | 0.95 | 0.19 | 41.29 | 0.45 |
| OSA-I | 3.54 | 1.31 | 0.72 | 0.23 | 45.65 | 0.23 |
| Mean | 3.82 | 1.27 | 1.23 | 0.39* | 56.0 | 0.49** |
| SEM | 0.34 | 0.04 | 0.15 | 0.05 | 3.9 | 0.08 |
| Cont-A | 3.34 | 0.73 | 1.21 | 0.53 | 38.65 | 3.07 |
| Cont-B | 2.69 | 0.97 | 0.91 | 0.30 | 43.78 | 1.36 |
| Cont-C | 3.31 | 1.30 | 0.78 | 0.53 | 92.33 | 0.44 |
| Cont-D | 5.28 | 1.37 | 1.96 | 0.58 | 70.82 | 0.92 |
| Cont-E | — | — | — | — | 82.08 | 1.56 |
| Cont-F | 6.36 | 1.53 | 2.11 | 0.86 | 61.21 | 2.37 |
| Cont-G | 3.99 | 1.34 | 1.11 | 0.82 | 90.96 | 2.08 |
| Cont-H | — | — | — | — | 73.77 | 1.20 |
| Cont-I | 5.19 | 1.44 | 1.43 | 0.87 | 56.38 | 0.56 |
| Mean | 4.31 | 1.24 | 1.36 | 0.64 | 67.8 | 1.51 |
| SEM | 0.50 | 0.11 | 0.19 | 0.08 | 6.4 | 0.29 |
denotes significant difference from control, P < 0.05;
denotes significant difference from control, P < 0.01.
A dashed line indicates that no data were obtained. Samples were obtained before sleep.
9-cis-RA, 9-cis retinoic acid; 13-cis-RA, 13-cis retinoic acid; ATRA, all-trans retinoic acid; Cont, control; RA, retinoic acid.
Examination of before and after sleep levels of retinoids in OSA patients revealed that plasma ATRA and 13-cis RA, but not 9-cis RA, were significantly reduced after sleep (P < 0.05) (Table 3). Neither retinol nor retinyl palmitate levels were altered by sleep in OSA patients (Table 3). No significant changes in any of retinoids were noted in plasmas from OSA-negative individuals before and after sleep (data not shown). These results demonstrate that otherwise healthy OSA patients have altered plasma retinoid levels. Specifically, pre-sleep levels of retinyl palmitate and 9-cis RA are lower in OSA patients compared with control subjects; and post-sleep levels of ATRA and 13-cis RA are lower compared with pre-sleep levels in OSA patients.
Table 3.
Before and after sleep levels of retinoids
| OSA group | Total RA, (ng/mL) |
ATRA, (ng/mL) |
13-cis-RA, (ng/mL) |
9-cis-RA, (ng/mL) |
Retinol, (µg/dL) |
Retinyl palmitate, (µg/dL) |
|---|---|---|---|---|---|---|
| Before mean | 4.22† | 1.29† | 1.43† | 0.47† | 59.5‡ | 0.54‡ |
| SEM | 0.40 | 0.06 | 0.18 | 0.04 | 4.1 | 0.09 |
| After mean | 3.91 | 1.10* | 1.15* | 0.55 | 63.1 | 0.55 |
| SEM | 0.17 | 0.08 | 0.12 | 0.04 | 5.1 | 0.07 |
denotes significant difference from before sleep level, P < 0.05.
Because of a limitation of sample volumes, not all patient samples could be used for all of the paired t-test analyses:
comparison made using a subset of six patients;
comparison made using a subset of seven patients.
9-cis-RA, 9-cis retinoic acid; 13-cis-RA, 13-cis retinoic acid; ATRA, all-trans retinoic acid; RA, retinoic acid.
Carotenoid levels
Retinoids, the active forms of vitamin A, cannot be synthesized by mammalian cells and must be obtained from the diet as provitamin A carotenoids or as retinyl esters.33 Although isomerases exist for retinoids, the primary source of a specific RA stereoisomer is the corresponding stereoisomer of β-carotene.34 Therefore, we hypothesized that the reduction of 9-cis RA in the plasma of OSA patients may be reflected by a reduction 9-cis β-carotene in these patients.
Pre-sleep levels of carotenoids are shown in Table 4. Plasma all-trans β-carotene was ~50% lower in OSA patients compared with controls (P < 0.05); and 9-cis β-carotene was ~35% lower (P < 0.05). No significant differences were noted for other carotenoids. Further, none of carotenoids were different before and after sleep in OSA patients (Table 5) or in OSA-negative subjects (data not shown).
Table 4.
Levels of carotenoids
| Subject | All-trans β-carotene, (µg/dL) |
13-cis β-carotene, (µg/dL) |
9-cis β-carotene, (µg/dL) |
Lutein (µg/dL) |
Zeaxanthin, (µg/dL) |
Cryptoxanthin, (µg/dL) |
Lycopene, (µg/dL) |
|---|---|---|---|---|---|---|---|
| OSA-A | 29.66 | 0.95 | 0.37 | 18.83 | 2.51 | 7.35 | 83.46 |
| OSA-B | 13.98 | 0.98 | 0.49 | 7.54 | 2.29 | 4.56 | 43.77 |
| OSA-C | 14.83 | 0.95 | 0.36 | 20.35 | 4.03 | 7.68 | 86.44 |
| OSA-D | 29.52 | 0.93 | 0.27 | 28.21 | 4.24 | 9.08 | 68.01 |
| OSA-E | 21.40 | 0.48 | 0.30 | 7.66 | 1.45 | 1.58 | 28.60 |
| OSA-F | 30.71 | 1.55 | 0.25 | 17.66 | 5.26 | 13.62 | 57.91 |
| OSA-G | 24.73 | 1.44 | 0.15 | 17.03 | 3.99 | 7.63 | 75.61 |
| OSA-H | 4.61 | 0.84 | 0.18 | 10.10 | 3.24 | 5.68 | 80.95 |
| OSA-I | 6.42 | 1.06 | 0.10 | 6.33 | 1.64 | 2.96 | 79.42 |
| Mean | *19.5 | 1.0 | *0.27 | 14.9 | 3.2 | 6.7 | 67.1 |
| SEM | 3.3 | 0.1 | 0.04 | 2.5 | 0.4 | 1.2 | 6.6 |
| Cont-A | 37.15 | 1.47 | 0.39 | 15.44 | 1.65 | 6.92 | 107.53 |
| Cont-B | 47.72 | 0.96 | 0.56 | 15.69 | 1.41 | 3.85 | 48.03 |
| Cont-C | 9.14 | 0.36 | 0.42 | 8.09 | 1.01 | 1.75 | 69.01 |
| Cont-D | 55.88 | 2.12 | 0.50 | 20.97 | 3.28 | 17.38 | 68.71 |
| Cont-E | 25.53 | 3.26 | 0.43 | 29.67 | 5.16 | 12.99 | 110.45 |
| Cont-F | 101.99 | 0.97 | 0.51 | 27.51 | 3.71 | 7.31 | 58.59 |
| Cont-G | 31.37 | 5.13 | 0.45 | 21.03 | 4.19 | 29.10 | 146.33 |
| Cont-H | 30.86 | 2.07 | 0.16 | 23.55 | 5.92 | 13.00 | 63.54 |
| Cont-I | 22.83 | 0.70 | 0.26 | 26.00 | 3.78 | 8.77 | 57.44 |
| Mean | 40.3 | 1.9 | 0.41 | 20.9 | 3.3 | 11.2 | 81.1 |
| SEM | 9.0 | 0.5 | 0.04 | 2.3 | 0.6 | 2.8 | 10.9 |
denotes significant difference from control, P < 0.05.
Samples were obtained before sleep.
Cont, control.
Table 5.
Before and after sleep levels of carotenoids
| OSA group | All-trans β-carotene, (µg/dL) |
13-cis β-carotene, (µg/dL) |
9-cis β-carotene, (µg/dL) |
Lutein (µg/dL) |
Zeaxanthin, (µg/dL) |
Cryptoxanthin (µg/dL) |
Lycopene, (µg/dL) |
|---|---|---|---|---|---|---|---|
| Before mean | 23.6 | 1.0 | 0.31 | 16.8 | 3.4 | 7.4 | 63.4 |
| SEM | 2.7 | 0.1 | 0.04 | 2.7 | 0.5 | 1.4 | 8.0 |
| After mean | 24.9 | 1.2 | 0.32 | 16.7 | 3.6 | 7.6 | 68.4 |
| SEM | 2.8 | 0.2 | 0.05 | 2.3 | 0.6 | 1.4 | 7.7 |
Paired t-test was performed on seven patients.
Antioxidant levels
Oxidative stress has been shown to occur in OSA patients and may alter regulatory mechanisms for retinoid and carotenoid metabolism.35 Tocopherols in human plasma have important cellular functions as antioxidants.36,37 However, we found no significant differences between OSA and control levels of major vitamin E, α-and γ-tocopherols (Table 6). Interestingly, OSA patients had a significantly higher (about twofold) level of a minor vitamin E constituent, δ-tocopherol (P < 0.05). Tocopherol levels were further examined in OSA patients in samples obtained before and after sleep. The data revealed a significant reduction in the level of δ-tocopherol after sleep (P < 0.01), while other major vitamin E isoforms were not altered by sleep in these patients (Table 7). Tocopherol levels were unaltered by sleep in OSA-negative individuals (data not shown).
Table 6.
Levels of tocopherols
| Subject | α-Tocopherol, (µg/dL) |
γ-Tocopherol, (µg/dL) |
δ-Tocopherol, (µg/dL) |
|---|---|---|---|
| OSA-A | 929.2 | 127.4 | 3.3 |
| OSA-B | 1371.2 | 466.6 | 32.7 |
| OSA-C | 1087.6 | 343.6 | 14.2 |
| OSA-D | 864.2 | 186.0 | 6.7 |
| OSA-E | 835.4 | 108.6 | 7.9 |
| OSA-F | 2465.1 | 122.6 | 13.8 |
| OSA-G | 1298.7 | 145.2 | 15.9 |
| OSA-H | 1301.7 | 287.3 | 8.7 |
| OSA-I | 1112.3 | 542.1 | 26.2 |
| Mean | 1252 | 259 | 14.4 * |
| SEM | 165 | 54 | 3.1 |
| Cont-A | 1473.5 | 199.0 | 6.3 |
| Cont-B | 1227.2 | 73.6 | 2.7 |
| Cont-C | 779.4 | 423.3 | 12.5 |
| Cont-D | 1691.3 | 84.8 | 3.4 |
| Cont-E | 1173.0 | 173.8 | 4.5 |
| Cont-F | 1330.3 | 122.7 | 11.4 |
| Cont-G | 1739.2 | 172.8 | 2.6 |
| Cont-H | 1204.2 | 292.8 | 9.4 |
| Cont-I | 1906.7 | 150.4 | 4.7 |
| Mean | 1392 | 118 | 6.4 |
| SEM | 116 | 36 | 1.3 |
denotes significant difference from control, P < 0.05.
Samples were obtained before sleep.
Cont, control.
Table 7.
Before and after sleep levels of tocopherols
| OSA group | α-Tocopherol, (µg/dL) |
γ-Tocopherol, (µg/dL) |
δ-Tocopherol, (µg/dL) |
|---|---|---|---|
| Before mean | 1264 | 214 | 14 |
| SEM | 214 | 52 | 3 |
| After mean | 1335 | 202 | 8** |
| SEM | 230 | 56 | 2 |
denotes significant difference from before sleep level, P < 0.01.
Paired t-test was performed on seven patients.
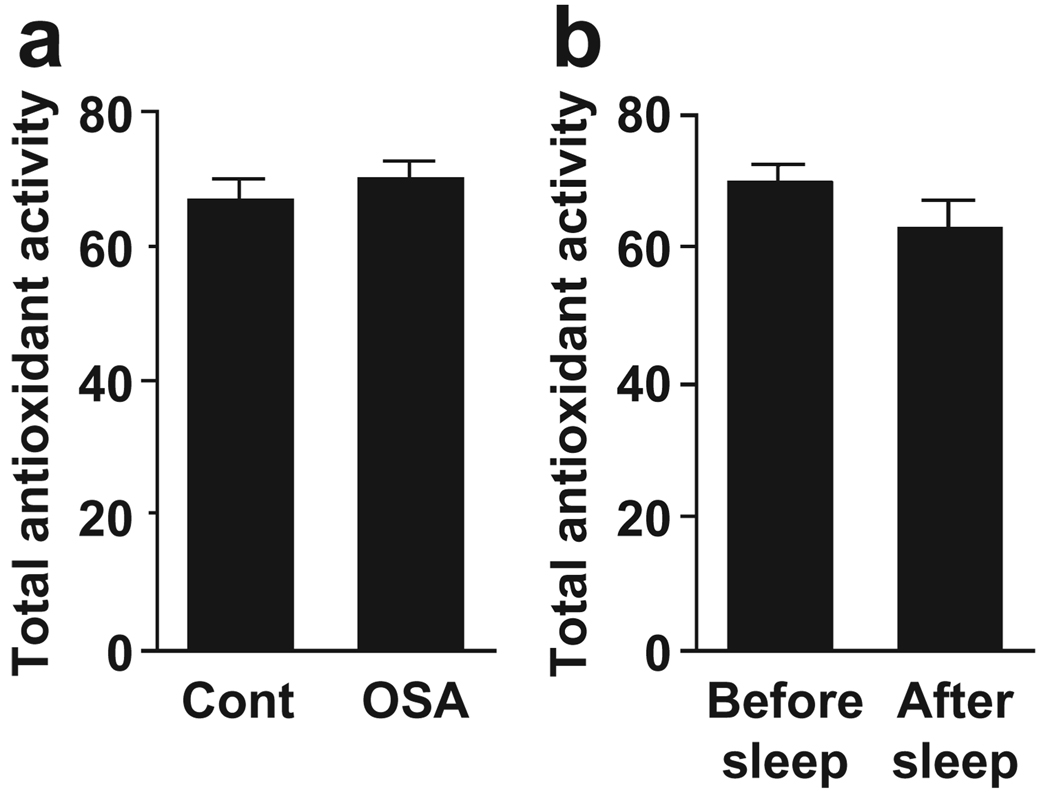
Total antioxidant performance measurements, which provide a more global antioxidant capacity, did not revealed significantly different oxidative stress levels between OSA patients and control subjects (Fig. 1a) or between before and after sleep in OSA patients (Fig. 1b).
Figure 1.
Antioxidant capacity. (a) Total antioxidant activity was measured in plasma samples from OSA patients and control subjects fluorometrically as described in Methods. Values represent means ± SEM (n = 8). (b) Total antioxidant activity was measured in plasma samples from OSA patients before and after sleep. Values represent means ± SEM (n = 6–8).
Effects of plasma from OSA patients on vascular smooth muscle cell growth
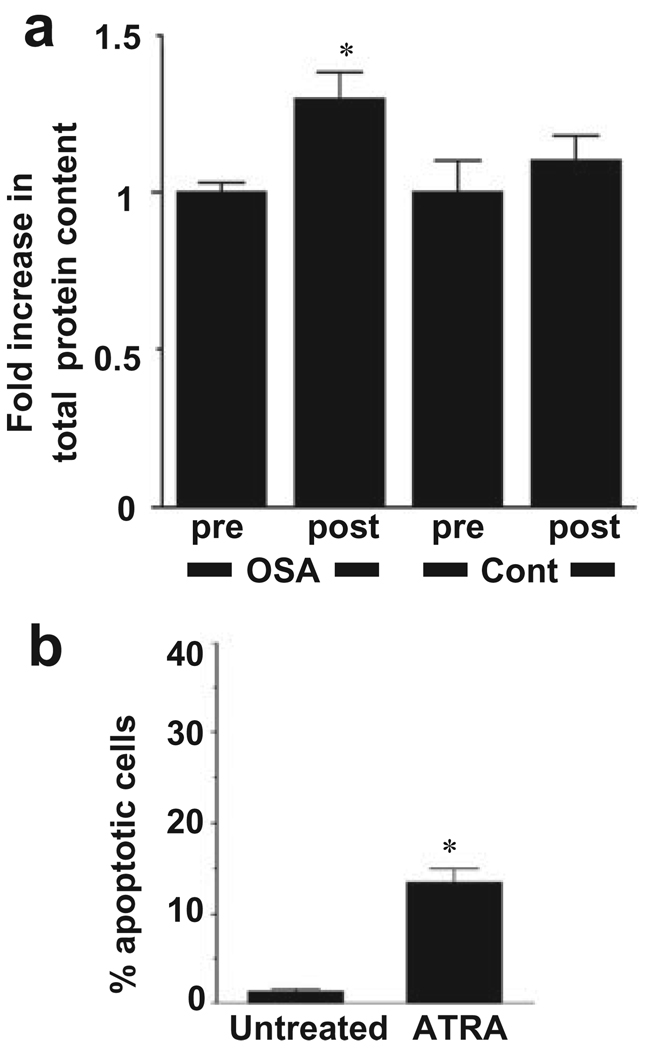
In the previous study, we found that ATRA has ability to suppress cell growth in human pulmonary artery SMC.22 Thus, we hypothesized that plasma of OSA patients after sleep may have the capacity to promote SMC growth. To test this hypothesis, equal numbers of human pulmonary artery SMC were plated in wells of six-well plates and plasma samples were added at 5% final concentration. After 3 day, cells were harvested and total protein content was determined to estimate cell growth. As shown in Figure 2a, we found that the total protein content of cells plated in wells which contained post-sleep OSA patients was significantly higher than that from pre-sleep OSA patients (P < 0.05). In contrast, no significant differences were noted between the effects of plasma samples from post- and pre-sleep subjects without OSA. The growth of SMC is depending on events which regulate cell hyperplasia, cell hypertrophy and cell survival. Our experiments using neutral comet assay revealed that ATRA has capacity to induce apoptosis of human pulmonary artery SMC (Fig. 2b).
Figure 2.
Effects of plasma from OSA patients on human pulmonary artery smooth muscle cell growth. (a) Human pulmonary artery smooth muscle cells were treated with 5% plasma from OSA patients and individuals who were found to be negative for OSA (cont) before (pre) or after (post) sleep for 3 days. Cell lysates were prepared and total protein content was monitored by Bradford assay. The values represent means ± SEM of fold increase in protein content relative to pre-sleep controls (n = 5–7). * denotes significant difference from the pre-sleep control value at P < 0.05. (b) Human pulmonary artery smooth muscle cells were treated with all-trans retinoic acid (ATRA) (2 µmol/L) for 24 h. Incidence of apoptosis was determined by the neutral comet assay. The values represent means ± SEM of percentage apoptotic cells (n = 4). * denotes significant difference from the untreated control value at P < 0.05.
DISCUSSION
The major findings of the present study are that plasma levels of specific retinoids, carotenoids and tocopherols, some of which have been shown to regulate growth of vascular SMC, are altered in otherwise healthy patients with mostly mild OSA; and that post-sleep plasmas from these patients exhibit higher capacity to promote SMC growth. Pre-sleep retinyl palmitate and 9-cis RA levels were significantly lower in OSA patients compared with control subjects. Levels of ATRA and 13-cis RA, which were previously found to be lower in idiopathic pulmonary arterial hypertension patients,22 were significantly reduced after sleep in OSA patients. Reduced 9-cis RA may be related to lower 9-cis β-carotene, but carotenoid levels appear not to be responsible for reduced ATRA and 13-cis RA levels after sleep. While the levels of major vitamin E constituents and the total antioxidant capacity are similar between OSA patients and control subjects, OSA patients were found to have significantly higher plasma δ-tocopherol. Although the relationship between retinoids, carotenoids and tocopherol levels and vascular biology is not completely understood, these results showing the altered levels of specific retinoids, carotenoids and tocopherols in otherwise healthy patients with mostly mild OSA may have clinical importance in the mechanism of increased risk for cardiovascular diseases in OSA patients.
Retinoid regulation of cell growth is mediated by two families of nuclear receptors as well as cytoplasmic binding protein. SMC contain these as well as enzymes for the metabolism and storage of retinoids. Interestingly, relative expressions of some of these enzymes are altered between the SMC synthetic and contractile phenotypes.25,26 9-cis RA and ATRA are the isoforms that bind to and activate the nuclear receptors and are important for gene regulation.38 Therefore, our findings of the reduction of retinoids, which negatively regulate SMC growth, may have implications for the predisposition of OSA patients to cardiovascular diseases. Consistent with this hypothesis, in post-sleep OSA patient plasma, in which ATRA and 13-cis RA levels are reduced compared with before sleep, the ability to promote SMC growth was increased.
It appears that there are cellular mechanisms by which OSA patients have reduced levels of retinoids. Plasma levels of retinyl palmitate may increase after a meal containing a large amount of provitamin A39 and fasting levels of retinyl ester are significantly increased with the intake of vitamin A supplement.40 However, since vitamin A (retinol) levels in plasma of both OSA patients and control subjects are similar, differences from controls are not likely due to differences in dietary intake and the specific isomers may be homeostatically controlled.
Some studies have suggested that intestinal absorption of vitamins and antioxidants is inversely associated with the systemic inflammatory response, independent of underlying diseases such as cancer.41 Increased oxidative stress markers, such as exhaled 8-isoprostane, are also detected in OSA following a night of apneic events.35,42 Thus, increased oxidative stress and inflammation in OSA could potentially contribute to reduced absorption of vitamin A precursors and analogues. Oxidative stress and inflammatory diseases also down-regulate levels and activities of retinoid and carotenoid binding proteins and receptors.43 This would potentially exert compounding effects of reduced retinoids/carotenoids and down-regulated activities of responsive proteins. The activities of retinoid isomerases, esterases and retinyl ester synthases may also be affected by oxidative stress. In these patients with mostly mild OSA, however, we did not detect significant alterations of oxidative stress parameter such as total vitamin E levels and total radical quenching activities. Lloret, et al.44 recently reported their studies of collecting plasma from OSA patients during sleep using a catheter in the antecubital vein, allowing concurrent measurement of oxygen desaturation, increases in lipid peroxidation and oxidized blood glutathione levels with the first apneic events. Their studies show time-specific detection of oxidative stress, indicating that timing of sample collections may be critical for detecting oxidative stress in OSA patients. In this regard, measurements of oxidative stress response genes might give more stable assessments of oxidative stress as recently reported by Somers and co-workers.45
Interestingly, we found that one minor vitamin E constituent, δ-tocopherol, was found to be twofold higher in OSA patients than in controls. The antioxidant capacities of tocopherols have been extensively studied, and the relative antioxidant capacities have been rated as α ≫ γ > δ.36 As reactive oxygen species may mediate cell signal transduction,46 cell growth inhibitory properties of tocopherols may be associated with their antioxidant activities. Thus, the significance of the specific increase in δ-tocopherol in OSA is not known, but it is intriguing to speculate that this minor vitamin E constituent may play specific roles in cell growth signalling.
Limitations of the present study are that, while statistically significant differences were obtained in some of the measurements as described in this report, the sample size of the study is small and further investigations with a larger sample size are needed to validate the importance of the findings in this initial study. This study, however, provided evidence for promising novel biological events associated with OSA, which may help elucidating pathogenic mechanisms of OSA-associated complications as well as obtaining clinically useful biomarkers. The future studies should also include examining the effects of CPAP therapy on biological changes observed in this study.
Our previous studies established an inverse association between pulmonary arterial hypertension and the levels of specific retinoids (ATRA and 13-cis RA), carotenoids (β-carotene) and tocopherols (α-tocopherol),22 and reduced levels of these retinoids, carotenoids and tocopherols may play roles in the development of vascular disease. In the present study, results from state-of-the-art measurements in human plasma in conjunction with experiments using cultured human pulmonary artery SMC revealed that otherwise healthy individuals with mild OSA could have higher capacity to develop vascular remodelling and cardiovascular diseases. These events may be regulated by complex mechanisms involving various forms of retinoids, carotenoids and tocopherols. Notably, the present study revealed the possible importance of putative novel cell regulators, retinyl palmitate and δ-tocopherol, whose biological properties are not currently well understood.
SUMMARY AT A GLANCE.
The present study tested the hypothesis that plasmas from OSA patients contain altered levels of retinoids, carotenoids and tocopherols. Results showed that mild OSA exhibits altered levels of specific retinoids, carotenoids and tocopherols, which may be markers and/or mediators for increased susceptibility of patients to vascular diseases.
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health grants HL73929 (to RMD), HL67340 and HL72844 (to YJS), and DK60021 and USDA ARS 51000-065 (to GT). The opinions expressed here are those of the authors and do not reflect the views of the Uniformed Services University of the Health Sciences, the Department of Defense, the Department of Agriculture or the U.S. Federal Government.
REFERENCES
- 1.Quan SF, Gersh BJ. Cardiovascular consequences of sleep-disordered breathing: past, present and future: report of a workshop from the national center on sleep disorders research and the national heart, lung, and blood institute. Circulation. 2004;109:951–957. doi: 10.1161/01.CIR.0000118216.84358.22. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, et al. Clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Morrell MJ, Finn L, Kim H, Peppard PE, Badr MS, et al. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am. J. Respir. Crit. Care Med. 2000;162:2091–2096. doi: 10.1164/ajrccm.162.6.9904008. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am. J. Respir. Crit. Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.Wolk R, Kara T, Somers VK. Sleep-disordered breathing and cardiovascular disease. Circulation. 2003;108:9–12. doi: 10.1161/01.CIR.0000072346.56728.E4. [DOI] [PubMed] [Google Scholar]
- 7.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atwood C, McCrory D, Garcia J, Abman S, Ahearn G. Pulmonary artery hypertension and sleep-disordered breathing. Chest. 2004;126:72S–77S. doi: 10.1378/chest.126.1_suppl.72S. [DOI] [PubMed] [Google Scholar]
- 9.Kessler R, Chaouat A, Weitzenblum E, Oswald M, Ehrhart M, et al. Pulmonary hypertension in the obstructive sleep apnea syndrome: prevalence, causes and therapeutic consequences. Eur. Respir. J. 1996;9:787–794. doi: 10.1183/09031936.96.09040787. [DOI] [PubMed] [Google Scholar]
- 10.Ozturk L, Mansour B, Yuksel M, Yalcin AS, Celikoglu F, et al. Lipid peroxidation and osmotic fragility of red blood cells in sleep-apnea patients. Clin. Chim. Acta. 2003;332:83–88. doi: 10.1016/s0009-8981(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 11.Lavie L. Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Med. Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 12.Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J. Vasc. Surg. 2007;45 Suppl. A:A25–A32. doi: 10.1016/j.jvs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc. Hematol. Disord. Drug Targets. 2006;6:1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 14.Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, et al. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc. Res. 2004;68:75–103. doi: 10.1016/j.mvr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Benisty JI, McLaughlin VV, Landzberg MJ, Rich JD, Newburger JW, et al. Elevated basic fibroblast growth factor levels in patients with pulmonary arterial hypertension. Chest. 2004;126:1255–1261. doi: 10.1378/chest.126.4.1255. [DOI] [PubMed] [Google Scholar]
- 16.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J. Clin. Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillard AC, Lane MA. Retinol decreases beta-catenin protein levels in retinoic acid-resistant colon cancer cell lines. Mol. Carcinog. 2007;46:315–329. doi: 10.1002/mc.20280. [DOI] [PubMed] [Google Scholar]
- 18.Park EY, Dillard A, Williams EA, Wilder ET, Pepper MR, et al. Retinol inhibits the growth of all-trans-retinoic acid-sensitive and all-trans-retinoic acid-resistant colon cancer cells through a retinoic acid receptor-independent mechanism. Cancer Res. 2005;65:9923–9933. doi: 10.1158/0008-5472.CAN-05-1604. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Chongviriyaphan N, Liu C, Russell RM, Wang XD. Combined antioxidant (beta-carotene, alpha-tocopherol and ascorbic acid) supplementation increases the levels of lung retinoic acid and inhibits the activation of mitogen-activated protein kinase in the ferret lung cancer model. Carcinogenesis. 2006;27:1410–1419. doi: 10.1093/carcin/bgi340. [DOI] [PubMed] [Google Scholar]
- 20.Chanchevalap S, Nandan MO, Merlin D, Yang VW. All-trans retinoic acid inhibits proliferation of intestinal epithelial cells by inhibiting expression of the gene encoding kruppel-like factor 5. FEBS Lett. 2004;578:99–105. doi: 10.1016/j.febslet.2004.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilhelm J, Smistik Z, Mahelkova G, Vytasek R. Redox regulation of proliferation of lens epithelial cells in culture. Cell Biochem. Funct. 2007;25:317–321. doi: 10.1002/cbf.1390. [DOI] [PubMed] [Google Scholar]
- 22.Preston IR, Tang G, Tilan JU, Hill NS, Suzuki YJ. Retinoids and pulmonary hypertension. Circulation. 2005;111:782–790. doi: 10.1161/01.CIR.0000155254.86840.47. [DOI] [PubMed] [Google Scholar]
- 23.Miano JM, Kelly LA, Artacho CA, Nuckolls TA, Piantedosi R, et al. All-trans-retinoic acid reduces neointimal formation and promotes favorable geometric remodeling of the rat carotid artery after balloon withdrawal injury. Circulation. 1998;98:1219–1227. doi: 10.1161/01.cir.98.12.1219. [DOI] [PubMed] [Google Scholar]
- 24.Haxsen V, Adam-Stitah S, Ritz E, Wagner J. Retinoids inhibit the actions of angiotensin ii on vascular smooth muscle cells. Circ. Res. 2001;88:637–644. doi: 10.1161/01.res.88.6.637. [DOI] [PubMed] [Google Scholar]
- 25.Gidlof AC, Ocaya P, Olofsson PS, Torma H, Sirsjo A. Differences in retinol metabolism and proliferative response between neointimal and medial smooth muscle cells. J. Vasc. Res. 2006;43:392–398. doi: 10.1159/000094415. [DOI] [PubMed] [Google Scholar]
- 26.Wagsater D, Jatta K, Ocaya P, Dimberg J, Sirsjo A. Expression of IL-1beta, IL-1 receptor type I and IL-1 receptor antagonist in human aortic smooth muscle cells: effects of all-trans-retinoic acid. J. Vasc. Res. 2006;43:377–382. doi: 10.1159/000094258. [DOI] [PubMed] [Google Scholar]
- 27.Qin Y, Zhou A, Ben X, Shen J, Liang Y, et al. All-trans retinoic acid in pulmonary vascular structural remodeling in rats with pulmonary hypertension induced by monocrotaline. Chin. Med. J. 2001;114:462–465. [PubMed] [Google Scholar]
- 28.Strollo PJ, Jr, Rogers RM. Obstructive sleep apnea. N. Engl. J. Med. 1996;334:99–104. doi: 10.1056/NEJM199601113340207. [DOI] [PubMed] [Google Scholar]
- 29.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 30.Guilleminault C, Abad VC. Obstructive sleep apnea syndromes. Med. Clin. North Am. 2004;88:611–630. doi: 10.1016/j.mcna.2004.01.002. viii. [DOI] [PubMed] [Google Scholar]
- 31.Beretta G, Aldini G, Facino RM, Russell RM, Krinsky NI, et al. Total antioxidant performance: a validated fluorescence assay for the measurement of plasma oxidizability. Anal. Biochem. 2006;354:290–298. doi: 10.1016/j.ab.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Kitta K, Day RM, Ikeda T, Suzuki YJ. Hepatocyte growth factor protects cardiac myocytes against oxidative stress-induced apoptosis. Free Radic. Biol. Med. 2001;31:902–910. doi: 10.1016/s0891-5849(01)00663-3. [DOI] [PubMed] [Google Scholar]
- 33.Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science. 1990;250:399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- 34.Nagao A, Olson JA. Enzymatic formation of 9-cis, 13-cis, and all-trans retinals from isomers of beta-carotene. FASEB J. 1994;8:968–973. doi: 10.1096/fasebj.8.12.8088462. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki YJ, Jain V, Park AM, Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic. Biol. Med. 2006;40:1683–1692. doi: 10.1016/j.freeradbiomed.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukai K, Tokunaga A, Itoh S, Kanesaki Y, Ohara K, et al. Structure-activity relationship of the free-radical-scavenging reaction by vitamin E (alpha-, beta-, gamma-, delta-tocopherols) and ubiquinol-10: pH dependence of the reaction rates. J. Phys. Chem. B. 2007;111:652–662. doi: 10.1021/jp0650580. [DOI] [PubMed] [Google Scholar]
- 37.Funaba M, Murakami M, Ikeda T, Ogawa K, Tsuchida K, et al. Identification of tocopherol-associated protein as an activin/TGF-beta-inducible gene in mast cells. Biochim. Biophys. Acta. 2006;1763:900–906. doi: 10.1016/j.bbamcr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, et al. International union of pharmacology. Lxiii. Retinoid x receptors. Pharmacol. Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen HM, Dallal GE, Phelan E, Russell RM. Serum concentrations of retinol and retinyl esters in adults in response to mixed vitamin A and carotenoid containing meals. J. Am. Coll. Nutr. 1991;10:460–465. doi: 10.1080/07315724.1991.10718172. [DOI] [PubMed] [Google Scholar]
- 40.Krasinski SD, Russell RM, Otradovec CL, Sadowski JA, Hartz SC, et al. Relationship of vitamin A and vitamin E intake to fasting plasma retinol, retinol-binding protein, retinyl esters, carotene, alpha-tocopherol, and cholesterol among elderly people and young adults: increased plasma retinyl esters among vitamin A-supplement users. Am. J. Clin. Nutr. 1989;49:112–120. doi: 10.1093/ajcn/49.1.112. [DOI] [PubMed] [Google Scholar]
- 41.McMillan DC, Talwar D, Sattar N, Underwood M, O’Reilly DS, et al. The relationship between reduced vitamin antioxidant concentrations and the systemic inflammatory response in patients with common solid tumours. Clin. Nutr. 2002;21:161–164. doi: 10.1054/clnu.2001.0527. [DOI] [PubMed] [Google Scholar]
- 42.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, et al. 8-isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–1392. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 43.Bry K, Lappalainen U. Pathogenesis of bronchopulmonary dysplasia: the role of interleukin 1beta in the regulation of inflammation-mediated pulmonary retinoic acid pathways in transgenic mice. Semin. Perinatol. 2006;30:121–128. doi: 10.1053/j.semperi.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Lloret A, Badia M, Sastre J, Vina J. Obstructive sleep apnea: arterial oxygen desaturation coincides with increases in systemic oxidative stress markers measured with continuous monitoring. Free Radic. Biol. Med. 2007;42:893–894. doi: 10.1016/j.freeradbiomed.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann M, Singh P, Wolk R, Romero-Corral A, Raghavakaimal S, et al. Microarray studies of genomic oxidative stress and cell cycle responses in obstructive sleep apnea. Antioxid. Redox Signal. 2007;9:661–669. doi: 10.1089/ars.2007.1589. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]