Abstract
Atherosclerosis and its associated complications represent major causes of morbidity and mortality in the industrialized or Western countries. Monocyte chemoattractant protein-1 (MCP-1) is critical for the initiating and developing of atherosclerotic lesions. Interleukin-8 (IL-8), a CXC chemokine, stimulates neutrophil chemotaxis. Ticlopidine is one of the antiplatelet drugs used to prevent thrombus formation relevant to the pathophysiology of atherothrombosis. In this study, we found that ticlopidine dose-dependently decreased the mRNA and protein levels of TNF-α-stimulated MCP-1, IL-8, and vascular cell adhesion molecule-1 (VCAM-1) in human umbilical vein endothelial cells (HUVECs). Ticlopidine declined U937 cells adhesion and chemotaxis as compared to TNF-α stimulated alone. Furthermore, the inhibitory effects were neither due to decreased HUVEC viability, nor through NF-kB inhibition. These results suggest that ticlopidine decreased TNF-α induced MCP-1, IL-8, and VCAM-1 levels in HUVECs, and monocyte adhesion. Therefore, the data provide additional therapeutic machinery of ticlopidine in treatment and prevention of atherosclerosis.
1. Introduction
Atherosclerosis is a chronic inflammatory disease. The recruitment of inflammatory cells is an essential step in the development and progression of the disease. It is well documented that monocyte adhesion, migration, and infiltration into the atheroma are crucial to the pathogenesis of atherosclerosis. Chemokines play an important role in recruiting monocytes and lymphocytes into the atherosclerotic lesions [1–4].
We have monocyte chemoattractants protein-1 (MCP-1) is a 14-kDa glycoprotein of the CC chemokine family and a potent chemotactant for monocyte, T cell, and NK cell recruitment [5–7]. MCP-1 is expressed by monocytes, smooth muscle cells, and endothelial cells, including human vascular endothelial cells (HUVECs) in response to several different stimuli such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α , and angiotensin II [8–10]. MCP-1 has been found as one of the key factors in the initiation of the inflammatory process of atherogenesis [1, 5, 11]. MCP-1 has been detected in macrophage-rich areas of atherosclerotic lesions [12, 13], and MCP-1 mRNA expression increases in endothelial cells, macrophages, and vascular smooth muscle cells in the atherosclerotic arteries from the patients receiving bypass revascularization [14]. Therefore, MCP-1 is critical to the initiation and development of atherosclerotic lesions.
IL-8, another important chemokine, belongs to a CXC chemokine family. It has been found to act mainly on neutrophils [15–17] but it also was found to recruit monocytes in some studies [2, 18]. IL-8 was found in human atheroma [19], and is implicated in atherosclerosis development [4]. Mice lacking IL-8 receptors are less susceptible to form atherosclerosis and have fewer monocytes accumulated in vascular lesions [16]. In addition to chemokines, vascular cell adhesion molecule-1 (VCAM-1) mediates adhesion to and rolling of monocytes along endothelial cells [20, 21]. Overexpression of VCAM-1 was found in atherosclerotic lesion [22, 23], and deficiency of VCAM-1 Ig domain 4 reduces monocyte migration and inhibits atheroselerotic lesion formation in mice [24]. Therefore, chemokines together with adhesion molecules play a key role in development of atherosclerosis.
Ticlopidine is widely used in the prevention of thrombosis during and after coronary stent placement and has been found to be at least equivalent to aspirin in the prevention of events in patients with cerebrovascular disease [25]. In several studies, ticlopidine even appeared to be slightly more effective than aspirin in preventing serious vascular occlusive events in patients with atherosclerotic disease [26]. The ticlopidine-aspirin stroke study (TASS) demonstrated that ticlopidine was a more effective agent than aspirin for the prevention of recurrent transient ischemic attacks [27].
In this study, in addition to the antiplatelet function, we examined the effects of ticlopidine on the expressions of MCP-1, IL-8, and VCAM-1 in an in vitro atherosclerosis model which consists of TNF-α-stimulated HUVECs to evaluate the possible role of ticlopidine for its potential antiatherosclerotic therapy.
2. Materials and Methods
2.1. Materials
Ticlopidine was purchased from Sigma (St. Louis, MO), and U937 cells from Culture Collection and Research Center CCRC No. 68002 (Hsinchu, Taiwan). All reagents were of analytical grade.
2.2. Cell Culture
We purchased HUVECs and endothelial cell growth medium (EGM-2, CC3156) from Clonetics (San Diego, CA), which contained 10% fetal bovine serum, hydrocortisone, hFGF-B, vEGF, R3-IGF-I, ascorbic acid, hEGF, GA-1000, and heparin. HUVECs which were used between passages 1 and 6, were maintained in EGM-2 medium in a humidified chamber containing 5% CO2 at 37°C. Cells were cultured in 6-well or 24-well plates until confluent and were washed twice and incubated with serum-free medium for 12 hours before different concentrations of ticlopidine (0.1, 2, 10, 30 μg/mL) were added. After being incubated for 12 hours, cells were stimulated with TNF-α (10 ng/mL) (R&D Systems; Minneapolis, MN) for 24 hours for MCP-1 and IL-8 expression. After incubation, the supernatants were collected for ELISA analysis, and the cells were used for RNA isolation.
2.3. MTT Assay for Cell Viability
Mitochondrial dehydrogenase activity, which reduces 3-(4,5-dimethyl-thiazol-2-yl)-2,5- diphenyl tetrazolium bromide (MTT, Sigma, St. Louis, USA) in active mitochondria to purple formazan, was used to determine cell survival in a colorimetric assay. Cell viability was calculated accordingly
| (1) |
2.4. RNA Isolation, Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Real-Time PCR Analysis
Total RNA was extracted from 1 × 106 cells according to manufacturer's instructions (Life Technologies, Grand Island, NY). The concentrations of the RNA samples were measured with a spectrophotometer (GeneQuant II, Pharmacia Biotech) to determine the OD 260 and OD260/280 values. In total, 5 μg of RNA samples was reverse transcribed with an oligo-dT primer to synthesize first-strand cDNA according to a previous report [28]. cDNA was amplified by PCR with specific primers for MCP-1 and IL-8; primers for MCP-1 were 5′-GCTCATAGCAGCCACCTTCATTC-3′ (sense) and 5′-TGCAGATTCTTGGGTTGTGGAG-3′ (antisense). Primers for IL-8 were 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ (sense) and TCTCAGCCCTCTTCATCAAAAACTTCTC-3′ (anti-sense). The PCR conditions used for MCP-1 and IL-8 were based on the previous reports [29, 30]. Primers for human β-actin were 5′-ATGGATGATGATATCGCCGCG-3′ (sense) and 5′-CATGAAGCATTTGCGGTGGACGATGGAGGGGCC-3′ which used as an internal control. PCR products were visualized by agarose gel electrophoresis. Photos were scanned by Scan Maker II sp and analyzed by Image-ProR Plus software.
Real-time PCR amplification employed reagents supplied in a DyNAmo Flash SYBR Green qPCR Master Mix Kit (Finnzymes, Finland), and each reaction contained 20 ng of cDNA, 10 pmol each of both primers, and 5 μL of SYBR Green master mix.
The following gene-specific primer sets were used: MCP-1 5′-CCCCAGTCACCTGCTGTTAT-3′ (sense) and 5′-AGATCTCCTTGGCCACAATG-3′ (antisense) [31]; IL-8, 5′-GTGCAGTTTTGCCAAGGAGT-3′ (sense) and 5′-TAATTTCTGTGTTGGCGCAG-3′ (antisense) [32]; VCAM-1, 5′-TCTCATTGACTTGCAGCACC-3′ (sense) and 5′-TTCTTGCAGCTTTGTGGATG-3′. (antisense) [33]; β-actin, 5′-CCCTTTTTGTCCCCCAAC-3′ (sense) and 5′-CTGGTCTCAAGTCAGTGTACAGGT-3′ (antisense) [34]. Specific mRNA levels were quantified by real-time RT-PCR (MJ Research DNA Ebgine OPTICON 2) using β-actin levels for normalization. The amplification conditions were 10 minutes at 95°C, 40 cycles of 10 seconds at 95°C, and 1 minute at 60°C. The relative quantity of MCP-1, IL-8, and VCAM-1 transcripts was calculated using the 2−△Ct method. Data were first normalized to the corresponding β-actin levels, and then expressed as fold increase with relative to untreated controls. All samples were assayed in triplicates. Data represents mean ± SEM of three independent experiments.
2.5. MCP-1, IL-8, and VCAM-1 Analysis by Enzyme-Linked Immunosorbent Assay (ELISA)
Concentrations of MCP-1, IL-8, and VCAM-1 were quantified using commercially available DuoSet ELISA development system (R&D Systems; Minneapolis, MN) according to the manufacturer's instructions. The sensitivity for MCP-1, IL-8, and VCAM-1 was 6.25–1000 pg/mL, 31.25–2000 pg/mL and 15.625–1000 pg/mL, respectively. All the samples were tested in the duplicated wells.
2.6. U937 Adhesion Assay
The adhesion assay was modified as described in [35]. In brief, U937 cells were labeled with 2′, 7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acethoxymethyl ester (BCECF/AM, 10 μg/mL; Sigma, MO) for 30 minutes at 37°C. RPMI-1640 containing 2% FCS was added to stop the reaction, and cells were suspended in M199 medium. HUVECs were cultured in 24-well plates until confluent, at which time different concentrations of ticlopidine were added and then incubated for an additional 12 hours. After incubation, TNF-α (10 ng/mL) was added to the wells and incubated for another 24 hours. Then HUVECs were coincubated with 106BCECF/AM-labelled U937 cells/well for 30 minutes at 37°C. Nonadhering U937 cells were removed, and the 24-well plates were washed twice with M199 medium. The plates were inverted and centrifuged for 2200 rpm 5 minutes to remove M199 medium. Cells were lysed in 0.1% Triton X-100 in 0.1 mol/L Tris buffer. Fluorescence was measured with an F-4500 Fluorescence Spectrophotometer (HITACHI) (using excitation at 510 nm and emission at 531 ± 25 nm). The areas under emission wavelength were calculated for the fluorescence intensity of adherent cells using F-4500 software. Media from untreated cells were used to determine the basal adhesion.
2.7. Chemotaxic Assay
HUVECs were seeded in the upper chamber of a transwell tissue culture insert (8 μm pore size) (Costar Cambridge, MA) until they reached confluent density. Different concentrations of ticlopidine were added to the upper wells and incubated for 30 minutes at 37°C, 5% CO2 incubator. Media from the upper chamber were aspirated and U937 cells (106 cell/100 μl/well) were added to the upper chambers. Recombinant IL-8 (rIL-8) (50 ng/mL) and rMCP-1 (50 ng/mL) were added into the lower wells. The plates were then incubated in a 37°C, 5% CO2 incubator for 1.5 hours. M199 media containing 0.5 mM EDTA were used for washing the U937 cells from the lower part of the membrane to lower wells. Cells in the lower wells were collected by centrifugation at 3000 g for 5 minute and direct cell counting was conducted under microscope.
2.8. Western Blot Analysis of NF-Kb p65 Subunit Translocation
HUVECs were incubated with different concentrations of ticlopidine and 100 μg/mL of aspirin, which was added as the control. Cells were stimulated with or without TNF-α (10 ng/mL) for 30 minutes and nuclear proteins were prepared. Briefly, the cells were washed with ice-cold PBS, lyzed in a hypotonic lysis buffer containing protease inhibitors (20 mM Hepes, 10 mM KCl, 1 mM MgCl2, 0.5% NP-40, 0.5 mM DTT, 10 mM NaF, 1 mM PMSF, 10 μg/mL Aprotinin, 10 μg/mL Leupeptin, pH7.4), and incubated on ice for 5 minutes. After incubation, the lysate was centrifuged at 10,000 xg for 5 minutes to separate nucleus pellet from supernatant (cytosolic part). The supernatant was saved. The resultant pellet was, subsequently, washed with the hypotonic lysis buffer twice, suspended in a hypertonic buffer (20 mM HEPES (pH 7.4), 10 mM KCl, 1 mM MgCl2, 0.4 M NaCl, 0.5 mM DTT, 10 mM NaF, 1 mM PMSF, 10 mg/mL Aprotinin, 10 mg/mL Leupeptin), and vigorously vortex for 30 minutes. After incubation on ice for 15 minutes, the nuclear extract was collected by centrifuging at 25,000 xg, 4°C for 30 minutes and transferred to new microcentrifuge tubes. The concentration of the nuclear and cytosolic extracts was determined by protein assay (Bio-Rad). Thirty μg/mL of protein were resolved on 10% SDS-PAGE and were transferred to nitrocellulose membrane. The blots were immediately blocked with 5% nonfat milk-containing Tris-buffered saline and probed with anti-NF-kB p65 Ab (1 : 1000; Santa Cruz Biotechnology, Inc.) for 1.5 hours at room temperature. The resultant immunocomplex was visualized in chemoluminescence enhanced system (Amersham). The same blot was restriped and probed with anti-B23 polyclonal antibody for nuclear protein loading control (1 : 1000 dilution; Santa Cruz Biotechnology, Inc).
2.9. Statistical Analysis
We used Student's t-test to analyze the differences of continuous variables which were presented as the mean ± SEM. The differences were considered significant if the P-value was smaller than 0.05.
3. Results
3.1. Ticlopidine Decreased TNF-α-Induced MCP-1 mRNA and Protein Levels in HUVECs
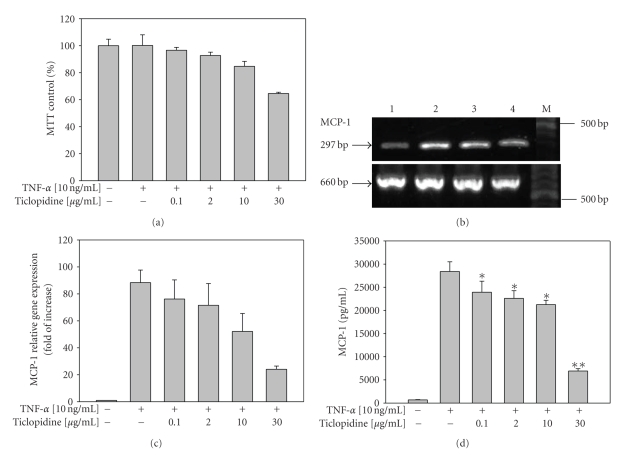
The concentrations of ticlopidine used in this study were physiological concentrations according to the Klein-Soyer et al. [36]. The daily recommended dose of ticlopidine is 250–500 mg, demonstrated plasma concentrations of the drug in low microgram/mL range [37], and concentrations of 10–30 μg/mL are pharmacologically irrelevant. The MTT assay was performed to investigate whether ticlopidine affect the growth of HUVECs. As shown in Figure 1(a), more than 85% of the cells remained viable after treatment of TNF-α combined with different concentrations ofticlopidine 0.1, 2, and 10 μg/mL respectively, indicating that there were no significant toxic effects detected as compared to the controls (100%). When HUVECs were treated with 30 μg/mL of ticlopidine, the cell viability was above 65% as compared to the controls.
Figure 1.
Reduction of MCP-1 mRNA and protein levels by ticlopidine in HUVECs. HUVECs were incubated with serum-free medium for 12 hours before different concentrations of ticlopidine were added, after 12 hours incubation cells were stimulated with/without TNF-α (10 ng/mL) for another 24 hours. (a) Cell growth was analyzed by MTT. Results are mean ± SEM (n = 3). (b) Total RNA was extracted and analyzed by RT-PCR. Lane 1, without TNF-α; lane 2, TNF-α (10 ng/mL) alone; lane 3, TNF-α + ticlopidine (0.1 μg/mL); lane 4 TNF-α + ticlopidine (30 μg/mL); M denotes molecular size marker. The lower panel is β-actin as internal control. Results are representative of one of three independent experiments. (c) Relative amount of MCP-1 mRNA level was determined by quantitative real-time RT-PCR. (d) Culture supernatants were analyzed by ELISA. Data are expressed as the mean ± SEM of duplicate wells and are representative of five individual experiments. Significantly different versus TNF-treated alone *P < .05, **P < .001.
We tested whether ticlopidine was able to regulate MCP-1 level elicited by TNF-α on HUVECs. By using specific MCP-1 primers, RT-PCR analysis detected a single band with the expected size of 297 base pairs (bp). As shown in Figure 1(b), MCP-1 mRNA level decreased significantly when treated with 30 μg/mL of ticlopidine (lane 4) as compared to those with 0.1 μg/mL (lane 3) and without treatment (lane 2). In addition, we analyzed the level of MCP-1 mRNA using quantitative real-time PCR. We found that MCP-1 mRNA was up-regulated in TNF-α treated HUVECs, and downregulated by ticlopidine at examined concentrations (Figure 1(c)). These results indicated that ticlopidine declined the MCP-1 mRNA level induced by TNF-α on HUVECs. The negative controls for PCR, either without cDNA or without reverse transcriptase, were performed simultaneously.
Furthermore, our ELISA data indicated that TNF-α significantly upregulated the level of MCP-1 (28,410 ± 2,088 pg/mL) secretion in HUVECs as compared to that in samples without TNF-α treatment (619.2 ± 54.02 pg/mL, n = 6) (P < .0001). TNF-α-stimulated MCP-1 secretion was reduced when cells were pretreated with ticlopidine in a dose dependent manner (Figure 1(d)). Doses of ticlopidine at as low as 0.1 μg/mL showed obvious reduction in MCP-1 secretion by 15.8% (23,920 ± 2,387 pg/mL) (P = .0416) (Figure 1(d)).
3.2. Ticlopidine Decreased TNF-α-Induced IL-8 mRNA and Protein Levels in HUVECs
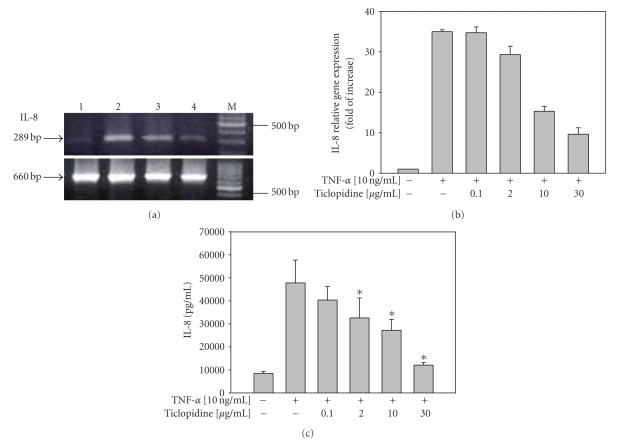
The effect of ticlopidine on the IL-8 production of TNF-α stimulated HUVECs was also examined simultaneously. By using specific IL-8 primers, RT-PCR analysis detected a single band with the expected size of 289 bp. As shown in Figure 2(a), ticlopidine at 30 μg/mL strongly decreased the induction of IL-8 mRNA level in HUVECs treated with TNF-α. The β-actin gene amplification was included as the internal control to normalize the gene expression. Moreover, real-time PCR experiment showed the increased level of IL-8 mRNA expression upon TNF-α treatment, and decreased at different ticlopidine concentrations tested (Figure 2(b)). Similarly, TNF-α up-regulated the level of IL-8 (47,830 ± 9,882 pg/mL) secretion in HUVECs as compared to that in samples without TNF-α treatment (8,494 ± 801 pg/mL, n = 6) (P = .0078). TNF-α-timulated IL-8 secretion was reduced (Figure 2(c)) when cells were pretreated with ticlopidine in a dose dependent way. Doses of ticlopidine at as low as 2 μg/mL significantly (P = .0135) decreased IL-8 secretion by 31.9% (32,580 ± 8,711 pg/mL) presumably due to the reduction in the steady-state level of IL-8 mRNA (Figure 2(b)). Taken together, our data suggested ticlopidine declined IL-8 mRNA and protein levels in TNF-α stimulated HUVECs.
Figure 2.
Reduction of IL-8 mRNA and protein levels by ticlopidine in HUVECs. HUVECs were incubated with serum-free medium for 12 hours before different concentrations of ticlopidine were added, after 12 hours incubation cells were stimulated with/without TNF-α (10 ng/mL) for another 24 hours. (a) Total RNA was extracted and analyzed by RT-PCR. Lane 1, without TNF-α; lane 2, TNF-α (10 ng/mL) alone; lane 3, TNF-α + ticlopidine (0.1 μg/mL); lane 4 TNF-α + ticlopidine (30 μg/mL); M denotes molecular size marker. The lower panel is β-actin as the internal control. Results are representative one of three independent experiments. (b) Relative amount of IL-8 mRNA level was determined by quantitative real-time RT-PCR. (c) Culture supernatants were analyzed by ELISA. Data are expressed as the mean ± SEM of duplicate wells and are representative of six individual experiments. Significantly different versus TNF-treated alone*P < .05, **P < .001.
3.3. Reduction of VCAM-1 mRNA and Protein Levels on TNF-α Treated HUVECs by Ticlopidine
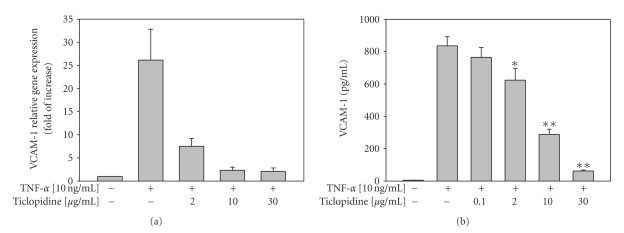
To examine whether ticlopidine could affect VCAM-1 level, we assayed the VCAM-1 level by real-time PCR and ELISA. VCAM-1 mRNA level was downregulated at different ticlopidine concentrations treated (Figure 3(a)). As shown in Figure 3(b), ticlopidine declined VCAM-1 protein levels in a dose-dependent manner. Ticlopidine at 2, 10, and 30 μg/mL significantly decreased TNF-α induced VCAM-1 levels to 624.3 ± 71.34 pg/mL (P = .04), 288.3 ± 32.45 pg/mL (P < .001), and 62.0 ± 5.86 pg/mL (P < .001), respectively, as compared to TNF-α treated alone (836.0 ± 56.45 pg/mL). The VCAM-1 levels analyzed by flow cytometry (data not shown) were consistent with those measured using the ELISA method.
Figure 3.
Reduction of VCAM-1 mRNA and protein levels by ticlopidine in HUVECs. HUVECs were incubated with serum-free medium for 12 hours before different concentrations of ticlopidine were added, after 12 hours incubation cells were stimulated with/without TNF-α (10 ng/mL) for another 24 hours. (a) Relative amount of VCAM-1 mRNA level was determined by quantitative real-time RT-PCR. (b) Culture supernatants were analyzed by ELISA. Data are expressed as the mean ± SEM of duplicate wells and are representative of three individual experiments. Significantly different versus TNF-treated alone *P < .05, **P < .001.
3.4. Reduction of U937 Cell Adhesion to TNF-Treated HUVECs by Ticlopidine
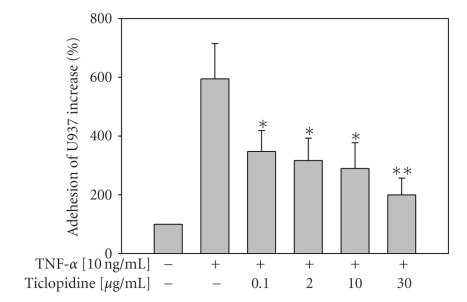
The human premonocytic cell line U937 [38] has been extensively used to investigate leucocyte-endothelial cell interaction [39]. Studies demonstrated IL-8 and MCP-1 receptors; CXCR2 and CCR2B mRNAs were detected in U937 cells [40]. Adhesion of human premonocytic U937 cells to HUVECs, in our study, showed that TNF-α significantly (P < .001) enhanced U937 adhesion (594.6 ± 120.0%) as compared to medium alone (100%). Ticlopidine induced a dose-dependent reduction of TNF-induced adhesion (Figure 4). It at as low as 0.1 μg/mL decreased TNF-α-induced U937 cells adhesion by 347.7 ± 70.88% (P = .032).
Figure 4.
Reduction of monocyte (U937) adhesion by ticlopidine. HUVECs were incubated with serum-free medium for 12 hours before different concentrations of ticlopidine were added, after 12 hours incubation cells were stimulated with/without TNF-α (10 ng/mL) for another 24 hours. Adhesion of fluorescence-labeled U937 cells was determined by adhesion assay. Data are expressed as the mean ± SEM of five individual experiments. Significantly different versus TNF-treated alone *P < .05, **P < .001.
3.5. Reduction of U937 Cell Chemotaxis by Ticlopidine
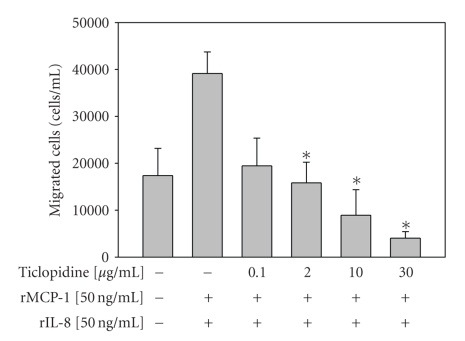
To investigate whether ticlopidine could mediate chemoattractant in response to chemokines, we investigated whether ticlopidine might modulate chemotaxis under TNF-α stimulation. As shown in Figure 5, recombinant MCP-1 and IL-8 (50 μg/mL) increased U937 cells chemotaxis to the lower wells as compared to medium alone. Ticlopidine at 2, 10, and 30 μg/mL concentrations significantly (P = .0497, .0352, and .0352, resp.) inhibited the U937 cell chemotaxis to the lower wells (Figure 5).
Figure 5.
Reduction of U937 cells chemotaxis by ticlopidine. HUVECs were incubated at the upper wells of the transwell plates until confluency, then different concentrations of ticlopidine were added. After 30 minutes of incubation, cells were stimulated with/without TNF-α (10 ng/mL). U937 cells were added to the upper chamber wells and incubated for 1.5 hours. The cells chemotastic to the lower wells were counted. Data are expressed as the mean ± SEM of three individual experiments. Significantly different versus TNF-treated alone *P < .05, **P < .001.
3.6. Translocation of NF-kB p65 Was Not Affected by Ticlopidine
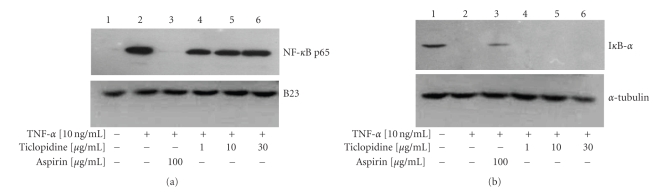
To test whether ticlopidine could inhibit NF-kB translocation and subsequently reduce IL-8 and MCP-1 levels, a Western blot was performed. Western analysis of nuclear protein NF-kB p65 subunit translocation had shown that ticlopidine at all concentrations tested had no effect on p65 subunit translocation (Figure 6(a)). In contrast, aspirin (100 μg/mL) is known to inhibit NF-kB p65 subunit translocation [35, 41]. B2 3 , a major nucleolar protein, was included as the loading control. In the cytoplasm, TNF-α treatment resulted in complete degradation of IkB-α in HUVECs as compared to control (Figure 6(b), lane 2). Aspirin has been shown to inhibit activation of NF-kB by preventing alteration of IkB-α (Figures 6(a) and 6(b), lane 3) [41]. We found that IkB-α was undetectable under different concentrations of ticlopidine treatment. α-tubulin was included as the loading control in cytoplasm. Therefore, our findings suggest that the reduction of IL-8 and MCP-1 by ticlopidine may not occur through the inhibition of NF-kB p65 subunit translocation.
Figure 6.
Ticlopidine has no effect on NF-kB activation. HUVECs were incubated with different concentrations of ticlopidine, and 100 μg/mL of aspirin was added as the control. Cells were stimulated with/without TNF-α (10 ng/mL) for 30 minutes, nuclear and cytosolic proteins were collected. Briefly, the nuclear protein extracts were collected, and western blots were performed using anti-NFkB p65 and anti-B23 Ab (a). Cytosolic protein extracts were collected, and western blots were performed using anti-Ik-B-α and α-tubulin Ab (b). Lane 1, without TNF-α; lane 2, TNF-α (10 ng/mL) alone; lane 3, TNF-α + aspirin (100 μg/mL); lane 4, TNF-α + ticlopidine (1 μg/mL); lane 5, TNF-α + ticlopidine (10 μg/mL); lane 6, TNF-α + ticlopidine (30 μg/mL). Results are representative of one of three independent experiments.
4. Discussion
Atherosclerosis and its associated complications continue to represent major causes of morbidity and mortality in the industrialized or Western countries. Inflammation and leukocyte recruitment play an important role in the pathogenesis of atherosclerosis [42]. Because there is lack of effective drugs for atherosclerosis regression, the current standard therapy for atherosclerosis-related diseases mainly relies on antiplatelet agents, including ticlopidine. Platelet interaction with leukocytes may be crucial for the initiation of inflammation [43–45]. Platelets are involved in various phases of atherothrombosis, including the initial steps of atherogenesis, the progression of atherosclerotic lesions, and the ensuing thrombotic complications. Studies have demonstrated that platelet-endothelial interaction also triggers a local inflammatory reaction that contributes to microcirculatory impairment and an acceleration of atherosclerotic lesion progression in the macrovasculature [46].
On the other hand, MCP-1 and IL-8 levels were elevated in lesions with monocyte infiltration [13, 19]. Both increased MCP-1 and IL-8 levels have been observed in patients with acute coronary syndromes. The elevation of those and other possible chemokines can be used to predict overall atherosclerotic burden [47]. In Figures 1 and 2 we showed that ticlopidine significantly decreased TNF-α-induced MCP-1 and IL-8 levels in HUVECs and monocyte adhesion onto endothelial cells. According to previous studies [8, 48], cytokine–activated endothelial cells may secrete monocyte-specific chemoattractant molecules, to amass monocytes at sites of vascular injury and/or inflammation such as atherosclerosis.
MCP-1-deficient mice deposit fewer lipids and have fewer macrophages within the aortic walls after LDL receptor-deficient mice have been fed with a high cholesterol diet [49]. Mice lacking one of the MCP-1 receptors, CCR-2 showed an overall decrease in atherosclerotic lesion size [50, 51]. Further, macrophages and monocytes were less significantly present in the aorta of CCR-2-deficient mice although the overall plasma cholesterol levels were unaffected by the CCR-2 genotype. Blocking CCR-2 and CXCR-3 has differential effects during atherogenesis [52]. These data suggest that MCP-1 plays a crucial role in initiating atherosclerosis by recruiting macrophages and monocytes to the vessel wall, and that the process of monocyte recruitment is a major determinant of lesion size.
IL-8 belongs to ELR+ CXC chemokines family which plays a role in neutrophil chemotaxis and regulates CXC chemokine-induced angiogenesis [53, 54]. A previous study showed that the CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-mediated angiogenesis [55]. IL-8, shown to be an angiogenic factor in vivo and a chemotactic and mitogenic factor for vascular smooth muscle and endothelial cells in vitro, is remarkably elevated in atherectomized specimens and may be a primary signal for angiogenesis in artherosclerotic tissue [56]. IL-8, which is found colocalized with vascular endothelial cells, supports a role for chemokines in atherogenesis, and, in turn, may contribute to the development and progression of coronary atherosclerotic disease in humans [56].
Ticlopidine, an antiplatelet drug, was clinically used for the secondary prevention of transient ischemic attacks (TIA), ischemic stroke, and coronary heart diseases, which are closely associated with atherosclerosis [19, 20]. Several clinical studies of ticlopidine in the prevention of stroke have shown it to be an effective prophylactic agent not only in patients with previous transient cerebral ischemia but also in patients with a previous complete stroke [26, 57]. Recently, studies revealed ticlopidine attenuates progression of atherosclerosis in apolipoprotein E and low density lipoprotein receptor double knockout (apoE/LDLR-/-) mice and improve the endothelial function in those mice [58]. The size of the atherosclerotic plaques and the number of macrophages were significantly reduced by ticlopidine treated mice as compared to their nontreated counterparts. Thus, ticlopidine treatment limited the vascular inflammatory response of atherosclerosis in addition to the antiplatelet effects.
In examining ticlopidine's effects on TNF-α-induced levels of MCP-1 and IL-8 chemokines and VCAM-1 in HUVECs, we found that ticlopidine lowered both the mRNA and protein levels of MCP-1, IL-8, and VCAM-1. Studies suggested that aspirin also suppresses monocyte adhesion and chemotaxis to endothelial cells. Aspirin is widely used as an antiinflammatory, antithrombotic, and even an anti-malignant agent. It was found to inactivate the transcription factor, NF-kB, which contains a DNA-binding domain for consensus sequences of various cytokines and chemokines including MCP-1 and IL-8 [59–62]. Studies showed inhibition of NF-kB attenuated atherosclerosis in apoE/LDLR double knockout mice [63]. Therefore, aspirin may inhibit NF-kB activities and then decrease chemokine expression (Figure 5). However, we found ticlopidine failed to inhibit the translocation of NF-kB into the nucleus. Therefore, the reduction of MCP-1 and IL-8 by ticlopidine may occur through an NF-kB-independent pathway.
Taking all the findings together, we suggest that ticlopidine may decline atherosclerotic process by blocking the crucial step of monocyte adhesion and migration through reducing MCP-1, IL-8, and VCAM-1 level in cytokine-stimulated endothelial cells, and this effect does not occur through NF-kB inhibition.
Acknowledgments
This study was supported by a Grant from the National Science Council of Taiwan, Grants NSC 96-2314-B-038-022 and NSC 97-2314-038-004-MY3 (Dr. S-J. Leu). The authors thank Dr. Fang Liao from Academic Sinica, Taipei, Taiwan for his assistance in migration assay.
References
- 1.Terkeltaub R, Boisvert WA, Curtiss LK. Chemokines and atherosclerosis. Current Opinion in Lipidology. 1998;9(5):397–405. doi: 10.1097/00041433-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Reape TJ, Groot PHE. Chemokines and atherosclerosis. Atherosclerosis. 1999;147(2):213–225. doi: 10.1016/s0021-9150(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 3.Dosquet C, Weill D, Wautier JL. Cytokines and thrombosis. Journal of Cardiovascular Pharmacology. 1995;25(supplement 2):S13–S19. doi: 10.1097/00005344-199500252-00004. [DOI] [PubMed] [Google Scholar]
- 4.Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thrombosis and Haemostasis. 2007;97(5):714–721. [PubMed] [Google Scholar]
- 5.Navab M, Imes SS, Hama SY, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. Journal of Clinical Investigation. 1991;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams DH, Lloyd AR. Chemokines: leucocyte recruitment and activation cytokines. The Lancet. 1997;349(9050):490–495. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- 7.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annual Review of Immunology. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 8.Sica A, Wang JM, Colotta F, et al. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. Journal of Immunology. 1990;144(8):3034–3038. [PubMed] [Google Scholar]
- 9.Rollins BJ, Yoshimura T, Leonard EJ, Pober JS. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. American Journal of Pathology. 1990;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- 10.Satriano JA, Shuldiner M, Hora K, Xing Y, Shan Z. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-α and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase. Journal of Clinical Investigation. 1993;92(3):1564–1571. doi: 10.1172/JCI116737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yla-Herttuala S, Lipton BA, Rosenfeld ME, et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(12):5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. Journal of Clinical Investigation. 1991;88(4):1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeya M, Yoshimura T, Leonard EJ, Takahashi K. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Human Pathology. 1993;24(5):534–539. doi: 10.1016/0046-8177(93)90166-e. [DOI] [PubMed] [Google Scholar]
- 14.Seino Y, Ikeda U, Takahashi M, et al. Expression of monocyte chemoattractant protein-1 in vascular tissue. Cytokine. 1995;7(6):575–579. doi: 10.1006/cyto.1995.0078. [DOI] [PubMed] [Google Scholar]
- 15.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Advances in Immunology. 1994;55:97–179. [PubMed] [Google Scholar]
- 16.Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. Journal of Clinical Investigation. 1998;101(2):353–363. doi: 10.1172/JCI1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boisvert WA, Curtiss LK, Terkeltaub RA. Interleukin-8 and its receptor CXCR2 in atherosclerosis. Immunologic Research. 2000;21(2-3):129–137. doi: 10.1385/ir:21:2-3:129. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez ER, Kannewurf BS. Atherosclerosis: a unifying disorder with diverse manifestations. American Journal of Health-System Pharmacy. 1998;55(19, supplement 1):S4–S7. doi: 10.1093/ajhp/55.suppl_1.S4. [DOI] [PubMed] [Google Scholar]
- 19.Koch AE, Kunkel SL, Pearce WH, et al. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. American Journal of Pathology. 1993;142(5):1423–1431. [PMC free article] [PubMed] [Google Scholar]
- 20.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 21.Tian J, Pei H, James JC, et al. Circulating adhesion molecules in apoE-deficient mouse strains with different atherosclerosis susceptibility. Biochemical and Biophysical Research Communications. 2005;329(3):1102–1107. doi: 10.1016/j.bbrc.2005.02.090. [DOI] [PubMed] [Google Scholar]
- 22.Ramos CL, Huo Y, Jung U, et al. Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circulation Research. 1999;84(11):1237–1244. doi: 10.1161/01.res.84.11.1237. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien KD, Allen MD, McDonald TO, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques: implications for the mode of progression of advanced coronary atherosclerosis. Journal of Clinical Investigation. 1993;92(2):945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cybulsky MI, Iiyama K, Li H, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. Journal of Clinical Investigation. 2001;107(10):1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn MJ, Fitzgerald DJ. Ticlopidine and clopidogrel. Circulation. 1999;100(15):1667–1672. doi: 10.1161/01.cir.100.15.1667. [DOI] [PubMed] [Google Scholar]
- 26.Gent M, Blakely JA, Easton JD, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. The Lancet. 1989;1(8649):1215–1220. doi: 10.1016/s0140-6736(89)92327-1. [DOI] [PubMed] [Google Scholar]
- 27.Bellavance A. Efficacy of ticlopidine and aspirin for prevention of reversible cerebrovascular ischemic events. The Ticlopidine Aspirin Stroke Study. Stroke. 1993;24(10):1452–1457. doi: 10.1161/01.str.24.10.1452. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y-Y, Hu C-J, Chang S-M, Tai T-Y, Leu S-J. Aspirin inhibits monocyte chemoattractant protein-1 and interleukin-8 expression in TNF-α stimulated human umbilical vein endothelial cells. Atherosclerosis. 2004;174(2):207–213. doi: 10.1016/j.atherosclerosis.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Hayes IM, Jordan NJ, Towers S, et al. Human vascular smooth muscle cells express receptors for CC chemokines. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18(3):397–403. doi: 10.1161/01.atv.18.3.397. [DOI] [PubMed] [Google Scholar]
- 30.Lagoo-Deenadayalan S, Lagoo AS, Barber WH, Hardy KJ. A standardized approach to PCR-based semiquantitation of multiple cytokine gene transcripts from small cell samples. Lymphokine and Cytokine Research. 1993;12(2):59–67. [PubMed] [Google Scholar]
- 31.Stern JL, Slobedman B. Human cytomegalovirus latent infection of myeloid cells directs monocyte migration by up-regulating monocyte chemotactic protein-1. Journal of Immunology. 2008;180(10):6577–6585. doi: 10.4049/jimmunol.180.10.6577. [DOI] [PubMed] [Google Scholar]
- 32.Huber TB, Reinhardt HC, Exner M, et al. Expression of functional CCR and CXCR chemokine receptors in podocytes. Journal of Immunology. 2002;168(12):6244–6252. doi: 10.4049/jimmunol.168.12.6244. [DOI] [PubMed] [Google Scholar]
- 33.Santos MI, Fuchs S, Gomes ME, Unger RE, Reis RL, Kirkpatrick CJ. Response of micro- and macrovascular endothelial cells to starch-based fiber meshes for bone tissue engineering. Biomaterials. 2007;28(2):240–248. doi: 10.1016/j.biomaterials.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Chen K-L, Liu W-H, Yang Y-Y, Leu S-JC, Shih N-Y. Characterization of novel transforming growth factor-beta type I receptors found in malignant pleural effusion tumor cells. BMC Molecular Biology. 2007;8, article 72 doi: 10.1186/1471-2199-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber C, Erl W, Pietsch A, Weber PC. Aspirin inhibits nuclear factor-κB mobilization and monocyte adhesion in stimulated human endothelial cells. Circulation. 1995;91(7):1914–1917. doi: 10.1161/01.cir.91.7.1914. [DOI] [PubMed] [Google Scholar]
- 36.Klein-Soyer C, Cazenave J-P, Herbert J-M, Maffrand J-P. SR 25989 inhibits healing of a mechanical wound of confluent human saphenous vein endothelial cells which is modulated by standard heparin and growth factors. Journal of Cellular Physiology. 1994;160(2):316–322. doi: 10.1002/jcp.1041600213. [DOI] [PubMed] [Google Scholar]
- 37.Di Perri T, Pasini FL, Frigerio C, et al. Pharmacodynamics of ticlopidine in man in relation to plasma and blood cell concentration. European Journal of Clinical Pharmacology. 1991;41(5):429–434. doi: 10.1007/BF00626364. [DOI] [PubMed] [Google Scholar]
- 38.Harris P, Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. Journal of Leukocyte Biology. 1985;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 39.Carvalho D, Savage COS, Black CM, Pearson JD. IgG antiendothelial cell autoantibodies from scleroderma patients induce leukocyte adhesion to human vascular endothelial cells in vitro: induction of adhesion molecule expression and involvement of endothelium-derived cytokines. Journal of Clinical Investigation. 1996;97(1):111–119. doi: 10.1172/JCI118377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazzarino DA, de Diego M, Hirschman SZ, et al. IL-8 and MCP-1 secretion is enhanced by the peptide-nucleic acid immunomodulator, product R, in U937 cells and primary human monocytes. Cytokine. 2001;14(4):234–239. doi: 10.1006/cyto.2001.0867. [DOI] [PubMed] [Google Scholar]
- 41.Kopp E, Ghosh S. Inhibition of NF-κB by sodium salicylate and aspirin. Science. 1994;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 42.Navab M, Hama SY, Nguyen TB, Fogelman AM. Monocyte adhesion and transmigration in atherosclerosis. Coronary Artery Disease. 1994;5(3):198–204. doi: 10.1097/00019501-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Siegel-Axel DI, Gawaz M. Platelets and endothelial cells. Seminars in Thrombosis and Hemostasis. 2007;33(2):128–135. doi: 10.1055/s-2007-969025. [DOI] [PubMed] [Google Scholar]
- 44.May AE, Langer H, Seizer P, Bigalke B, Lindemann S, Gawaz M. Platelet-leukocyte interactions in inflammation and atherothrombosis. Seminars in Thrombosis and Hemostasis. 2007;33(2):123–127. doi: 10.1055/s-2007-969023. [DOI] [PubMed] [Google Scholar]
- 45.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. Journal of Clinical Investigation. 2005;115(12):3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinhubl SR, Moliterno DJ. The role of the platelet in the pathogenesis of atherothrombosis. American Journal of Cardiovascular Drugs. 2005;5(6):399–408. doi: 10.2165/00129784-200505060-00007. [DOI] [PubMed] [Google Scholar]
- 47.Gerszten RE, Mach F, Sauty A, Rosenzweig A, Luster AD. Chemokines, leukocytes, and atherosclerosis. Journal of Laboratory and Clinical Medicine. 2000;136(2):87–92. doi: 10.1067/mlc.2000.108154. [DOI] [PubMed] [Google Scholar]
- 48.Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Molecular Medicine Today. 1996;2(5):198–204. doi: 10.1016/1357-4310(96)88772-7. [DOI] [PubMed] [Google Scholar]
- 49.Gu L, Okada Y, Clinton SK, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Molecular Cell. 1998;2(2):275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 50.Boring L, Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. Journal of Clinical Investigation. 1997;100(10):2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394(6696):894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 52.Veillard NR, Steffens S, Pelli G, et al. Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo. Circulation. 2005;112(6):870–878. doi: 10.1161/CIRCULATIONAHA.104.520718. [DOI] [PubMed] [Google Scholar]
- 53.Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. Journal of Biological Chemistry. 1995;270(45):27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 54.Belperio JA, Keane MP, Arenberg DA, et al. CXC chemokines in angiogenesis. Journal of Leukocyte Biology. 2000;68(1):1–8. [PubMed] [Google Scholar]
- 55.Addison CL, Daniel TO, Burdick MD, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. Journal of Immunology. 2000;165(9):5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 56.Simonini A, Moscucci M, Muller DWM, et al. IL-8 is an angiogenic factor in human coronary atherectomy tissue. Circulation. 2000;101(13):1519–1526. doi: 10.1161/01.cir.101.13.1519. [DOI] [PubMed] [Google Scholar]
- 57.Hass WK, Easton JD, Adams HP, Jr., et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. New England Journal of Medicine. 1989;321(8):501–507. doi: 10.1056/NEJM198908243210804. [DOI] [PubMed] [Google Scholar]
- 58.Jawien J, Csanyi G, Gajda M, Mateuszuk L, Lomnicka M. Ticlopidine attenuates progression of atherosclerosis in apolipoprotein E and low density lipoprotein receptor double knockout mice. European Journal of Pharmacology. 2007;556(1–3):129–135. doi: 10.1016/j.ejphar.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 59.Kunsch C, Rosen CA. NF-κB subunit-specific regulation of the interleukin-8 promoter. Molecular and Cellular Biology. 1993;13(10):6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasumoto K, Okamoto S-I, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor α and interferon γ synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. Journal of Biological Chemistry. 1992;267(31):22506–22511. [PubMed] [Google Scholar]
- 61.Freter RR, Alberta JA, Hwang GY, Wrentmore AL, Stiles CD. Platelet-derived growth factor induction of the immediate-early gene MCP-1 is mediated by NF-κB and a 90-kDa phosphoprotein coactivator. Journal of Biological Chemistry. 1996;271(29):17417–17424. doi: 10.1074/jbc.271.29.17417. [DOI] [PubMed] [Google Scholar]
- 62.Amberger A, Hala M, Saurwein-Teissl M, et al. Suppressive effects of anti-inflammatory agents on human endothelial cell activation and induction of heat shock proteins. Molecular Medicine. 1999;5(2):117–128. [PMC free article] [PubMed] [Google Scholar]
- 63.Jawien J, Gajda M, Mateuszuk L, et al. Inhibition of nuclear factor-κB attenuates artherosclerosis in apoE/LDLR—double knockout mice. Journal of Physiology and Pharmacology. 2005;56(3):483–489. [PubMed] [Google Scholar]