Abstract
Taenia crassiceps, like other helminths, can exert regulatory effects on the immune system of its host. This study investigates the effect of chronic T. crassiceps infection on the outcome of Multiple Low Dose Streptozotocin-Induced Diabetes (MLDS). Healthy or previously T. crassiceps-infected mice received MLDS and type 1 diabetes (T1D) symptoms were evaluated for 6 weeks following the induction of MLDS. T. crassiceps-infected mice displayed lower blood glucose levels throughout the study. A significantly lower percentage of T. crassiceps-infected mice (40%) developed T1D compared to the uninfected group (100%). Insulitis was remarkably absent in T. crassiceps-infected mice, which had normal pancreatic insulin content, whereas uninfected mice showed a dramatic reduction in pancreatic insulin. Infected mice that received MLDS did not show an increase in their regulatory T cell population, however, they had a greater number of alternatively activated macrophages, higher levels of the cytokine IL-4, and lower levels of TNF-α. Therefore, infection with T. crassiceps causes an immunomodulation that modifies the incidence and development of MLDS-induced autoimmune diabetes.
1. Introduction
Parasitic helminths are a highly diverse group of organisms that display different morphologies, accessory structures, sexual and feeding behaviors and life cycle stages. Helminths distribute themselves across a variety of niches inside their hosts where they can cause a multiplicity of diseases. Helminth parasites also appear to follow varied and complicated oral and cutaneous routes of infection within host tissues. Surprisingly, despite these differences in features and behavior, helminths share a unique ability to exert profound regulatory effects on the immune systems of their hosts. One of the first observations made concerning helminth infection was the elicitation of a strong Th2-biased immune response [1, 2]. In the last few years, new regulatory has been identified to play a role in helminth infection [2, 3]. One of these mechanisms includes the induction of regulatory T cells (Tregs), which are now known to be involved in pathogen susceptibility and the control of inflammation in helminth infections caused by Litomosoides sigmodontis [4], Trichuris muris [5], Brugia malayi [6], Trichinella spiralis [7], and Heligmosomoides polygyrus [8]. Dendritic cells (DCs) have also been reported to be affected by helminth-derived products [3, 9] and, more recently, a new population of macrophages called alternatively activated macrophages (AAMsϕ) has been consistently observed in several worm infection models [10, 11].
There are a series of epidemiological and experimental studies supporting the idea that helminth infections can induce a protective effect against the development of both autoimmune and allergic diseases [12]. The “hygiene hypothesis” was the first to suggest that the increase in the prevalence of allergies and asthma in developed countries might be linked to the reduction in infections with parasitic and bacterial pathogens. Thus, parasitic infection might somehow “educate” the immune system to avoid exacerbated inflammatory responses [13, 14]. Type 1 diabetes (T1D) is an autoimmune disease that has increased in prevalence over the last several years in developed countries and is caused by the selective destruction of insulin-producing β cells located in pancreatic Langerhans' islets by autoantigen-reactive inflammatory T cells. When the majority of β cells are destroyed, the pancreas' ability to secrete insulin in response to blood glucose levels is impaired, resulting in a disruption of glucose homeostasis. Previous studies have shown that the MLDS-induced diabetes model is a useful tool for understanding the basic mechanisms associated with the origin and modulation of induced T1D. T1D has been correlated predominantly with genetic background as well as proinflammatory cytokine profiles for TNF-α, IL-12 and IFN-γ [15, 16]. For example, C57BL/6 mice are more susceptible to developing MLDS-induced T1D than mice lacking STAT-4, a transcription factor that is essential for IL-12 signaling. STAT-4 deficient mice have a delayed onset of MLDS-induced T1D and show a milder form of the disease [17].
Taenia crassiceps is a cestode parasite that is useful in infection model systems for cysticercosis [18]. Infection of inbred mice with T. crassiceps induces a strong Th2-like immune response that is similar to the response elicited by infection with other helminths [19]. In addition to this Th2-like response, T. crassiceps infection is associated with a series of immunomodulatory events including the induction of AAMϕ [20] and the inhibition of T cell proliferative responses to bystander and polyclonal stimuli [2]. We and others have found that infection with T. crassiceps alters the immune response to and susceptibility to concomitant pathogens such as Trypanosoma cruzi [21], vaccinia virus [22], or Leishmania [18], and also reduces the efficacy of vaccination [23]. Thus, it is clear that T. crassiceps infection is able to modify immune responses to concomitant pathogens.
The purpose of this study is to determine whether the immune modulation that is induced by T. crassiceps infection might affect the outcome of Multiple Low Dose Streptozotocin-Induced Diabetes (MLDS). To address this question, we compared the course of MLDS development in both healthy and T. crassiceps-infected mice. Our data suggest that T. crassiceps infection might modify the incidence and development of MLDS-induced autoimmune diabetes.
2. Materials and Methods
2.1. Mice
Six- to eight-week-old male BALB/cAnN mice and C57BL/6NHsd mice were purchased from Harlan Laboratories (México) and were maintained in a pathogen-free environment at the FES-Iztacala, U.N.A.M. animal facility in accordance with institutional and national guidelines.
2.2. Parasites and Infection Protocols
Metacestodes of Taenia crassiceps (ORF) were harvested in sterile conditions from the peritoneal cavity of female BALB/c mice after 2–4 months of infection. The cysticerci were washed four times in phosphate-buffered saline (PBS; 0.15 M NaCl, 0.01 M sodium phosphate buffer, pH 7.2) and used for mouse infection. Male BALB/c and C57BL/6 mice were infected with an intraperitoneal (i.p.) injection of 20 small, nonbudding cysticerci of T. crassiceps resuspended in 0.3 mL of PBS.
2.3. Multiple Low-Dose Streptozotocin-Induced Diabetes (MLDS)
Mice infected with T. crassiceps for 6 weeks or uninfected controls received daily intraperitoneal injections of 40 mg/kg streptozotocin (Sigma-Aldrich; dissolved in 0.1 M sodium citrate, pH 4.5) for 5 consecutive days. Blood glucose was measured in animals that were fasted for 6 hours by an Accu-chek Advantage glucometer (Roche Diagnostics) once per week over a 6-week period. Untreated mice were included as controls. Animals were considered diabetic when fasting blood glucose was greater than means ±2 SD on two consecutive tests.
2.4. Intraperitoneal Glucose Tolerance Test
Uninfected and T. crassiceps-infected mice were subjected to an intraperitoneal glucose tolerance test in order to establish the effects of the MLDS-induced diabetes model on glucose metabolism (n = 6–10 for each group). Uninfected and infected MLDS-treated mice were fasted for 6 hours prior to sample collection. A basal blood sample (0 min) was collected by tail-snip and plasma glucose was evaluated using an Accu-chek Advantage glucometer. Mice were then injected i.p. with filtered d-glucose (1.5 mg/kg). Glucose levels were evaluated again at 30-, 60-, and 120-minute time points.
2.5. Histology
Pancreata from C57Bl/6 and BALB/c mice were collected 6 weeks after the induction of diabetes. Tissue was processed and embedded in paraffin, and 5-μm sections were cut for histological analysis. Thin sections were stained with hematoxylin-eosin and evaluated microscopically for the presence of insulitis using the following scoring system: grade 0, normal; grade 1, minor peri-islet cell infiltration; grade 2, moderate infiltration (<50% of islet area); grade 3, severe infiltration (>50% of islet area) with damage to islet architecture.
2.6. Immunohistochemistry
Immunoperoxidase staining was performed on 5-μm paraffin sections using an avidin-biotin complex system, the Insulin Ab-6 (INS04 + INS05) mouse monoclonal antibody, and the commercial kit Dako EnVision + System-HRP (DAB). Sections were counterstained with hematoxylin. Images were captured using AxioVision Rel 4.6An and an AxioCam ICc3 connected to a Zeiss Microscope recorded the images.
2.7. Cytokine ELISAs
Peripheral blood was collected from tail snips once a week over a 6-week period. Serum IL-4 and TNF-α levels were measured by sandwich ELISA using commercial kits purchased from Peprotech (Rocky Hill, NJ, USA).
2.8. Isolation of Peritoneal Macrophages
BALB/c and C57BL/6 were sacrificed 6 weeks after induction diabetes. Peritoneal exudate cells (PECs) from mice STZ, STZ/Tc, and Normal mice were obtained using 5 mL of ice-cold sterile PBS and the red blood cells were lysed by resuspending the cells in Boyle's solution. Following two washes, the viable cells were counted by trypan blue exclusion with a Neubauer hemocytometer. PECs were adjusted to 5 × 106/mL in RPMI medium and then cultured in six-well plates (Costar, Cambridge, Mass). After 2 hours at 37°C and in 5% CO2, nonadherent cells were removed by washing them with warm supplemented RPMI medium. Peritoneal macrophages were aseptically removed and processed for RNA extraction using the TRIzol reagent (Invitrogen, Carlsbad, CA).
2.9. Analysis of Cell Surface Markers in Macrophages
The Fc receptors on peritoneal macrophages were blocked with antimouse CD16/CD32 (Biolegend, CA, USA) and then stained with an APC-conjugated monoclonal antibody against F4/80 (Biolegend, CA, USA), PE-conjugated antibodies against PD-L1 and PD-L2, FITC-conjugated antibodies against CD23, or Alexafluor-conjugated antimannose receptor antibody (all obtained from Biolegend). The stained cells were analyzed on a FACsCalibur flow cytometer using Cell Quest software (Becton Dickinson).
2.10. T-Reg Cells Detection
Limph nodes were macerated individually using frosted glass slides. The staining Treg cells were according to the manufacturer instruction (Mouse Treg Flow Kit, Biolegend).
2.11. Reverse Transcriptase-PCR
Total RNA was extracted from purified peritoneal macrophages obtained from BALB/c and C57BL/6. mRNA transcripts in peritoneal macrophages was determined by reverse transcription (RT)-PCR. The RNA was quantified and 3 mg of RNA were reverse transcribed using the Superscript II First Strand Synthesis Kit (Invitrogen) and an oligo dT primer, as recommended by the manufacturer. Once cDNA was obtained, conventional PCR was performed. The PCR reactions contained (in a 25 mL final volume) 5 × PCR buffer blue, 10 mM dNTP, 40 nM each forward and reverse primer, 1 unit of Taq DNA polymerase (Sacace Biotechnologies, Italy), and 2 mL of the cDNA. The program used for the amplification of each gene was an initial denaturation at 95 8°C for 5 minutes, 35 cycles of 95 8°C for 40 seconds, the indicated melting temperature for 50 seconds and 72 8°C for 40 seconds and a final extension step of 72 8°C for 4 minutes. All reactions were carried out in a thermal cycler (Corbett Research, Australia). Finally, to observe the amplified products, a 1.5% agarose gel was prepared and samples were loaded with blue juice buffer containing SYBR Green (Invitrogen). The gels were visualized using a Fujifilm FLA 5000 scanner (Fuji, Japan) with FLA 5000 image reader V2.1 software to capture the shown images.
2.12. Statistical Analysis
One-tail Student t-test (glycemia, cytokine, and glucose tolerance test) was applied. P < .05 was considered statistically significant.
3. Results
3.1. Taenia crassiceps Infection Modulates Hyperglycemia and Diabetes Incidence by Multiple Low Dose Streptozotocin-Induced Diabetes
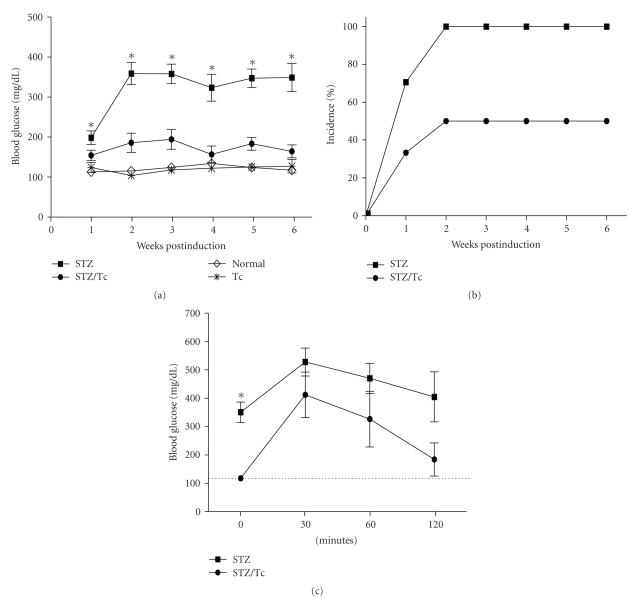
This study investigates whether T. crassiceps infection modifies the onset and development of MLDS-induced diabetes. Six weeks after infection with T. crassiceps, male BALB/c and C57BL/6 mice received MLDS (40 mg/kg) for 5 consecutive days. Blood glucose levels were analyzed each week for six weeks following the treatment with MLDS. Uninfected BALB/c mice became hyperglycemic one week post-MLDS injection (Figure 1(a)). By the second week following the induction of diabetes 100% of the uninfected mice were diabetic and remained diabetic until the end of the experimental period. In contrast, T. crassiceps-infected BALB/c mice displayed significantly lower blood glucose levels throughout the six-week period following MLDS injection compared to uninfected mice (Figure 1(a)). Interestingly, the onset of diabetes (as determined by glucose levels) was also different between uninfected and T. crassiceps-infected mice. The onset of diabetes in T. crassiceps-infected BALB/c mice occurred 2 weeks after MLDS induction; whereas the onset of diabetes occurred within the first week following MLDS-induction in uninfected mice. Additionally, only 40% of T. crassiceps-infected mice developed hyperglycemia compared to 100% in uninfected mice (Figure 1(b); P < .05). Interestingly, T. crassiceps-infected mice had normal glucose tolerance test values (Figure 1(c)). In contrast, uninfected mice could not down-modulate the hyperglycemia until 2 hours after the glucose tolerance test was administered.
Figure 1.
Hyperglycemia and diabetes incidence is modified by T. crassiceps infection in BALB/c mice. (a) Blood glucose levels throughout the MLDS protocol for uninfected BALB/c mice and BALB/c mice infected with T. crassiceps for 6 weeks. (b) Percent incidence of diabetes between uninfected and T. crassiceps-infected BALB/c mice. (c) Intraperitoneal glucose tolerance test for MLDS-treated uninfected and T. crassiceps-infected mice. Dotted line indicates normal glucose values.*P < .05, n = 12.
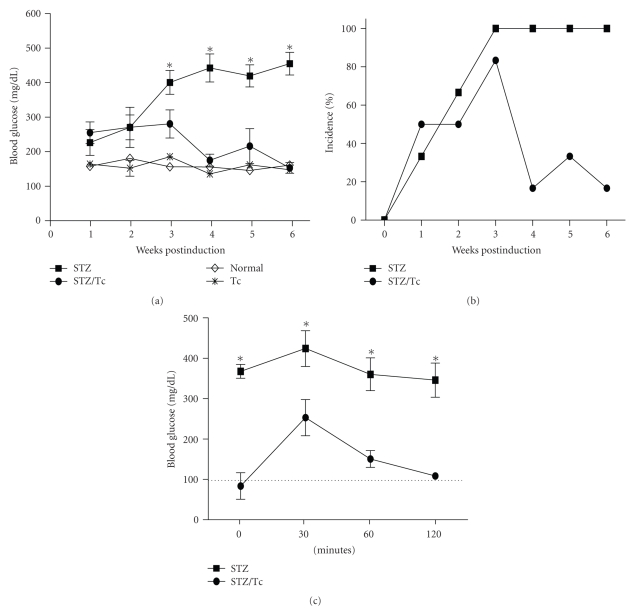
A different trend was observed in C57BL/6 mice. C57BL/6 mice are known to be more susceptible to MLDS-induced diabetes [24] and are more resistant to T. crassiceps infection [25, 26]. C57BL/6 mice developed higher levels of hyperglycemia than BALB/c mice, but these high glucose levels were controlled after 4 weeks by T. crassiceps infection (Figure 2(a)). Shortly after MLDS-induction, 80% of T. crassiceps-infected C57BL/6 mice were diabetic (Figure 2(b)). However, by 4 weeks following MLDS-induction infected C57BL/6 mice began to recover from the hyperglycemia. Only 20% of T. crassiceps-infected C57BL/6 mice were diabetic at the end of this experiment. MLDS treatment caused significant elevations in glucose levels during an intraperitoneal glucose tolerance test in uninfected mice, but mice carrying T. crassiceps displayed normal glucose tolerance test values (Figure 2(c)).
Figure 2.
Hyperglycemia and diabetes incidence is modified by T. crassiceps infection in C57BL/6 mice. (a) Blood glucose levels throughout the MLDS protocol for uninfected C57BL/6 mice and mice infected with T. crassiceps for 6-weeks. (b) Percent incidence of diabetes between uninfected and T. crassiceps-infected C57BL/6 mice. (c) Intraperitoneal glucose tolerance test for MLDS-treated uninfected and T. crassiceps-infected mice. *P < .05, n = 8.
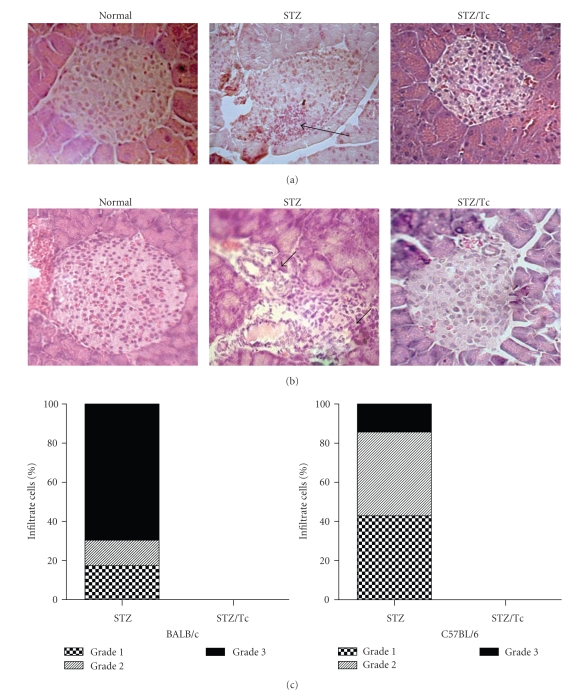
3.2. Lack of Insulitis in T. crassiceps Infected Mice Treated with MLDS
It is known that MLDS stimulates the recruitment of leukocytes to pancreatic islets, resulting in insulitis [15]. Pancreata were harvested at the end of the 6-week experimental period for histopathological analysis. Figure 3 illustrates islet histopathology and the development of insulitis in uninfected and T. crassiceps-infected BALB/c and C57BL/6 mice. A significant number of infiltrating leukocytes and the presence of insulitis was observed in the pancreata from uninfected mice (Figures 3(a) and 3(b)), which was associated with loss of islet architecture in some cases. Conversely, the islet histopathology from T. crassiceps-infected mice was devoid of both cellular infiltrates and insulitis (Figures 3(a) and 3(b)). Similar results were observed in the pancreata from BALB/c and C57BL/6 mice.
Figure 3.
MLDS-mediated insulitis in BALB/c mice. All mice received MLDS for 5 consecutive days and were sacrificed 6 weeks later to harvest tissues for histopathology. (a) H&E stained BALB/c mouse pancreas sections: normal islet, STZ (uninfected and MLDS-treated), STZ/Tc (T. crassiceps-infected and MLDS-treated). (b) H&E stained C57BL/6 mouse pancreas sections. (c) Score of cell infiltrates in BALB/c and C57BL/6 islets. Note the lack of leukocyte infiltrates in T. crassiceps-infected mice. Arrows indicate infiltration. Magnification ×400.
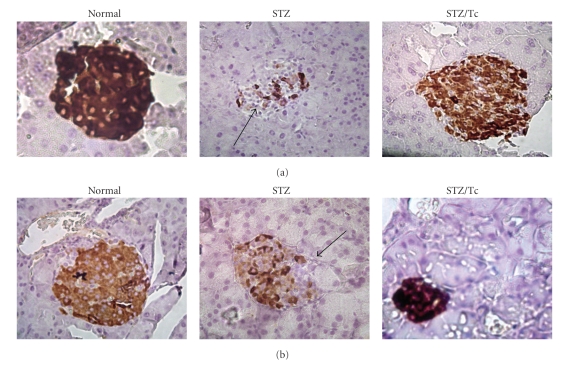
3.3. Immunohistochemistry of Insulin in MLDS-Treated Mice
To determine whether islet cells from either uninfected or T. crassiceps-infected mice are able to produce insulin, we performed specific immunostaining of pancreatic tissue using an anti-insulin antibody. MLDS-treated T. crassiceps infected mice demonstrated strong insulin staining in their pancreatic islets as compared with normal (untreated and uninfected) mice (Figure 4(a)). In contrast, MLDS-treated uninfected mice had weak insulin staining, suggesting a substantial loss of insulin granules in islet β-cells. A similar result was obtained from C57BL/6 mouse tissues (Figure 4(b)).
Figure 4.
Insulin immunostaining of pancreatic islet cell from MLDS-treated mice. (a) Immunostaining of insulin in islets from BALB/c mice, insulin immunostaining in pancreatic islets of different uninfected (normal), uninfected and MLDS-treated mice (STZ), or T. crassiceps-infected MLDS-treated mice (STZ/Tc). Mice (n = 4) were sacrificed 6 weeks after the initial injection of MLDS and pancreata were processed for immunohistochemistry to specifically detect insulin. (b) Same as (a), just the sections belong to C57BL/6 mice. Note the lack of insulin staining in uninfected MLDS-treated mice. Magnification ×400.
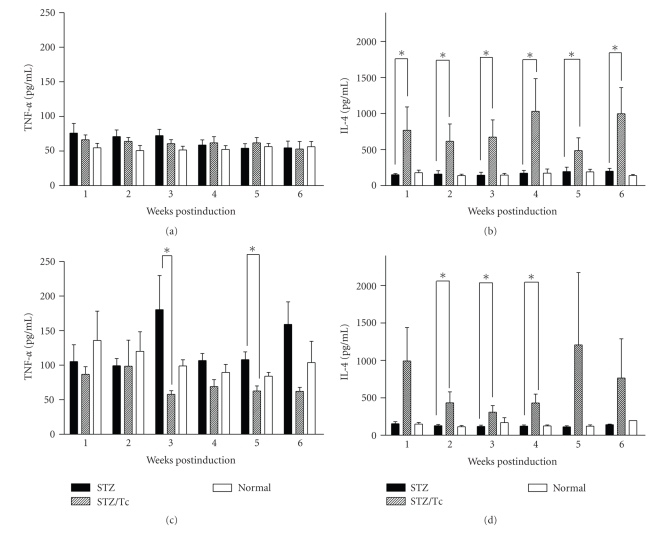
3.4. T. crassiceps Infection Increases IL-4 Levels in MLDS-Treated Mice
To analyze whether the inhibition of diabetes in helminth-infected MLDS-treated mice was due to an alteration in the Th1/Th2 balance, cytokine levels from the sera of uninfected and infected mice were measured. The Th1-associated cytokines IFN-γ and TNF-α have been reported to accompany the development of diabetes in both NOD and MLDS-induced mouse models [14, 27]. While BALB/c mice did not show significant changes in TNF-α levels in response to MLDS treatment (Figure 5(a)), uninfected C57BL/6J mice had high levels of TNF-α and IFN-γ in their sera (Figure 5(c), and data not shown). Interestingly, C57BL/6 mice that were previously infected with T. crassiceps and then treated with MLDS displayed lower sera TNF-α levels but maintained elevated levels of IFN-γ , as compared to uninfected mice (data not shown). We also evaluated the effect of T. crassiceps infection on the presence of the Th2-associated cytokine IL-4. At some experimental time points, IL-4 levels were significantly enhanced in both T. crassiceps-infected BALB/c and C57BL/6 mice that received MLDS whereas uninfected mice with T1D had lower levels of serum IL-4 (Figures 5(c) and 5(d)).
Figure 5.
Cytokine profiles from the sera of mice treated with MLDS. BALB/c mice were bled at indicated time points and (a) TNF-α and (b) IL-4 were detected by ELISA. (c) TNF-α and (d) IL-4 for C57BL/6 mice. * P < .05, n = 10.
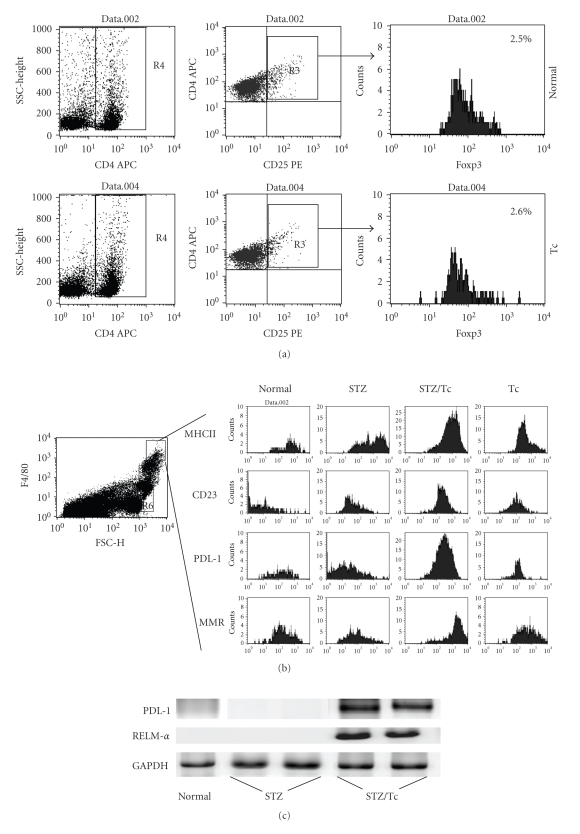
3.5. Presence of AAMϕ, but Not Tregs, in MLDS-Treated T. crassiceps-Infected Mice
In the last few years, new classes of regulatory cells that are induced by helminth infections have been identified. Tregs and AAMϕ have been implicated in the control of immune pathology associated with helminth infection, but both cell types have also been associated with suppression of the immune response [28, 29]. In order to determine whether these cells play a role in T. crassiceps-mediated diabetes prevention, we performed FACS analysis of peritoneal cells and T cells from the mesenteric lymph node. Following MLDS treatment, neither uninfected nor T. crassiceps-infected mice displayed an increase in the population of Treg in the mesenteric lymph node (Figure 6(a)). Interestingly, T. crassiceps-infected mice displayed an increase in the percentage of AAMϕ in the peritoneum, as determined by the expression of the mannose receptor, CD23, PDL1, and MHCII (Figure 6(b)). To confirm the presence of AAMϕ, RT-PCR was performed to detect mRNA transcripts for Fizz-1 and PDL-1. These transcripts were present in peritoneal cells from T. crassiceps-infected mice treated with MLDS, but not in uninfected, MLDS-treated mice (Figure 6(c)).
Figure 6.
Flow cytometry analysis for the detection of regulatory T cells and alternatively activated macrophages. (a) Mesenteric lymph nodes from BALB/c mice were processed and cells were staining for Treg cell detection with anti-CD4, CD25, and Foxp3 (Tregs kit, Biolegend). (b) Peritoneal exudates cells stained with conjugated anti-F480, CD23, MR, PDL1, and MHCII and analyzed by flow cytometry. (c) RT-PCR analysis of PDL-1, RELM-α, and GAPDH in macrophages from uninfected and T.crassiceps-infected BALB/c mice.
4. Discussion
T. crassiceps infection and its associated antigens can induce Th2-type responses in vivo [20] and can modulate the immune response to bystander antigens or live infections [2]. T. crassiceps, like other helminth parasites, has the ability to manipulate and down-modulate the immune responses of its hosts [10, 18]. It is widely accepted that the initiation and development of T1D is mainly caused by an autoimmune cell-mediated destruction of β cells in the pancreas [15]. Genetic, immunological, and environmental factors can also influence the onset and development of T1D [30]. In this study, we found that T. crassiceps infection could alter the development of MLDS-induced diabetes in mice. This study demonstrates that T. crassiceps-infected mice develop a mild, and sometimes transient, form of T1D after MLDS treatment. In contrast, uninfected mice exhibited an accelerated, more severe form of T1D.
There are likely several factors that contribute to the protection by T. crassiceps infection against MLDS-induced diabetes. First, the prominent Th2 environment that is induced by T. crassiceps infection [19, 31] might counteract the proinflammatory responses that are necessary to generate complete MLDS-induced diabetes. Second, T. crassiceps infection might alter T cell recruitment to the pancreas. Third, T. crassiceps infection might induce a regulatory cell response that dampens inflammatory processes during MLDS-induced diabetes.
The findings of this study demonstrate that infection with T. crassiceps maintains high levels of IL-4 and that there is a slight reduction in serum TNF-α levels after infected mice are treated with MLDS. TNF-α has been implicated as a critical player in leukocyte-mediated islet damage [32]. Thus, this type of immune regulation might be responsible for the decrease in pathology and lower incidence of T1D observed in T. crassiceps-infected mice. Consistent with a putative protective role for Th2 type cytokines (IL-4), NOD mice that express IL-4 in their pancreatic β cells are protected from insulitis and autoimmune diabetes [33]. Other experimental models have demonstrated that helminth exposure (Schistosoma mansoni, Trichinella spiralis, Hymenolepis diminuta or Heligmosomoides polygyrus) to mice might drastically reduce the symptoms of autoimmune diseases such as Inflammatory Bowel Disease (IBD), Encephalomyelitis Autoimmune Experimental, and T1D, mainly by reducing IL-12 and IFN-γ production and enhancing IL-4 and IL-10 levels [34–45]. Female NOD mice that spontaneously develop diabetes due to Th1-mediated destruction of pancreatic β-cells are protected from developing diabetes when they are exposed to S. mansoni [34–36], and IL-4 has been shown to play an important role in this protection [46, 47]. It will be necessary to test our system in mice that either lack IL-4 or have been treated with a specific IL-4 inhibitor to fully understand the role that IL-4 plays in T. crassiceps protection against MLDS-induced diabetes.
We found that T. crassiceps infection resulted in an increase in the population of F4/80+ macrophages that express CD23, PDL1, MR, Arg1, Ym1, but not iNOS, an expression profile that is now used to identify AAMϕ. AAMϕ might function as bystander suppressors of the immune response. Alternatively, they might inhibit cell infiltration in the pancreas, given their established suppressive activity, which is mediated through cell-contact, where PD-L1 and PD-L2 play a preponderant role, or by releasing soluble factors [20]. In contrast, Treg cells were not detected in T. crassiceps-infected mice. Mice that were infected with T. crassiceps were protected from both hyperglycemia and lymphocyte infiltration into the pancreatic islet despite the absence of regulatory T cells. This is interesting given the recent findings that demonstrate the importance of Foxp3+ regulatory T cell expansion in mice infected with helminths [4–8]. Tregs induced by S. mansoni and its associated antigens have been implicated in diabetes prevention in NOD mice [36]. This discrepancy might be explained by differences in the classes of helminths used in these infection models, in the route of infection used, or by different suppressive mechanisms that are turned on by T. crassiceps versus S. mansoni [11, 19]. Only one published report demonstrates that macrophages are involved in preventing the pathology associated with IBD model [48]. Therefore, it is possible that AAMϕ might actively participate in dampening the pathology associated with MLDS-induced diabetes, perhaps by the known ability of AAMϕ to strongly suppress T cell responses.
Interestingly, MLDS-induced diabetes is associated with Th1-type responses [17] and C57BL/6 mice show a Th1-type response in the MLDS model [24]. We also show that T. crassiceps-infected C57BL/6 mice demonstrated a reversal of MLDS-induced diabetes even though they were more hyperglycemic at earlier time points following MLDS-treatment. Again, the specific regulatory cells and/or cytokines involved in these protective effects still need to be identified, however, according to our data, appears that AAMϕ it may have an important role instead T-regulatory cells that we could not detect in T. crassiceps-infected mice.
5. Conclusion
Our data support the notion that the protection from autoimmunity by helminth infection could be attributed to immunoregulatory mechanisms triggered by these parasites. Our study demonstrates for the first time that T. crassiceps infection protects against Multiple Low Dose Streptozotocin-Induced Diabetes, independently of the genetic background of the host. Furthermore, we present a possible protective role for AAMϕ since we did not detect enhanced Treg cells. The analysis of Taenia-released products and repeating our experiments under conditions of macrophage depletion will be necessary to fully understand the mechanisms involved in this observed protection.
Acknowledgments
This work was supported by Grant IN212909 from PAPIIT-UNAM and Grant no. 60956-M from CONACYT. It is part of the requirement to obtain a PhD degree in the Postgraduate Program in Biomedical Sciences, Facultad de Medicina, UNAM, for the first author who is supported by a fellowship from CONACYT, Mexico.
References
- 1.Maizels RM. Parasite immunomodulation and polymorphisms of the immune system. Journal of Biology. 2009;8, article 62:1–4. doi: 10.1186/jbiol166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites—masters of regulation. Immunological Reviews. 2004;201(1):89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 3.Hewitson JP, Grainger JR, Maizels RM. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Molecular and Biochemical Parasitology. 2009;167(1):1–11. doi: 10.1016/j.molbiopara.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Elia R, Behnke JM, Bradley JE, Else KJ. Regulatory T cells: a role in the control of helminth-driven intestinal pathology and worm survival. Journal of Immunology. 2009;182(4):2340–2348. doi: 10.4049/jimmunol.0802767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor MD, van der Werf N, Harris A, et al. Early recruitment of natural CD4+Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. European Journal of Immunology. 2009;39(1):192–206. doi: 10.1002/eji.200838727. [DOI] [PubMed] [Google Scholar]
- 6.McSorley HJ, Harcus YM, Murray J, Taylor MD, Maizels RM. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. Journal of immunology. 2008;181(9):6456–6466. doi: 10.4049/jimmunol.181.9.6456. [DOI] [PubMed] [Google Scholar]
- 7.Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-β. Journal of Immunology. 2007;178(2):1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- 8.Rausch S, Huehn J, Kirchhoff D, et al. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infection and Immunity. 2008;76(5):1908–1919. doi: 10.1128/IAI.01233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho L, Sun J, Kane C, Marshall F, Krawczyk C, Pearce EJ. Review series on helminths, immune modulation and the hygiene hypothesis: mechanisms underlying helminth modulation of dendritic cell function. Immunology. 2009;126(1):28–34. doi: 10.1111/j.1365-2567.2008.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreider T, Anthony RM, Urban JF, Jr., Gause WC. Alternatively activated macrophages in helminth infections. Current Opinion in Immunology. 2007;19(4):448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes JL, Terrazas LI. The divergent roles of alternatively activated macrophages in helminthic infections. Parasite Immunology. 2007;29(12):609–619. doi: 10.1111/j.1365-3024.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 12.Zaccone P, Burton OT, Cooke A. Interplay of parasite-driven immune responses and autoimmunity. Trends in Parasitology. 2008;24(1):35–42. doi: 10.1016/j.pt.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Vercelli D. Mechanisms of the hygiene hypothesis—molecular and otherwise. Current Opinion in Immunology. 2006;18(6):733–737. doi: 10.1016/j.coi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Cooke A, Zaccone P, Raine T, Phillips JM, Dunne DW. Infection and autoimmunity: are we winning the war, only to lose the peace? Trends in Parasitology. 2004;20(7):316–321. doi: 10.1016/j.pt.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Denis MC, Mahmood U, Benoist C, Mathis D, Weissleder R. Imaging inflammation of the pancreatic islets in type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(34):12634–12639. doi: 10.1073/pnas.0404307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological Research. 2001;50(6):537–546. [PubMed] [Google Scholar]
- 17.Cetkovic-Cvrlje M, Uckun FM. Effect of targeted disruption of signal transducer and activator of transcription (Stat)4 and Stat6 genes on the autoimmune diabetes development induced by multiple low doses of streptozotocin. Clinical Immunology. 2005;114(3):299–306. doi: 10.1016/j.clim.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Sosa M, Rivera-Montoya I, Espinoza A, et al. Acute cysticercosis favours rapid and more severe lesions caused by Leishmania major and Leishmania mexicana infection, a role for alternatively activated macrophages. Cellular Immunology. 2006;242(2):61–71. doi: 10.1016/j.cellimm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 19.McKay DM. The beneficial helminth parasite? Parasitology. 2006;132(1):1–12. doi: 10.1017/S003118200500884X. [DOI] [PubMed] [Google Scholar]
- 20.Terrazas LI, Montero D, Terrazas CA, Reyes JL, Rodríguez-Sosa M. Role of the programmed death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. International Journal for Parasitology. 2005;35(13):1349–1358. doi: 10.1016/j.ijpara.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez M, Terrazas LI, Márquez R, Bojalil R. Susceptibility to Trypanosoma cruzi is modified by a previous non-related infection. Parasite Immunology. 1999;21(4):177–185. doi: 10.1046/j.1365-3024.1999.00218.x. [DOI] [PubMed] [Google Scholar]
- 22.Spolski RJ, Corson J, Thomas PG, Kuhn RE. Parasite-secreted products regulate the host response to larval Taenia crassiceps. Parasite Immunology. 2000;22(6):297–305. doi: 10.1046/j.1365-3024.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- 23.Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infection and Immunity. 2004;72(5):2598–2604. doi: 10.1128/IAI.72.5.2598-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller A, Schott-Ohly P, Dohle C, Gleichmann H. Differential regulation of Th1-type and Th2-type cytokine profiles in pancreatic islets of C57BL/6 and BALB/c mice by multiple low doses of streptozotocin. Immunobiology. 2002;205(1):35–50. doi: 10.1078/0171-2985-00109. [DOI] [PubMed] [Google Scholar]
- 25.Sciutto E, Fragoso G, Diaz ML, et al. Murine Taenia crassiceps cysticercosis: H-2 complex and sex influence on susceptibility. Parasitology Research. 1991;77(3):243–246. doi: 10.1007/BF00930866. [DOI] [PubMed] [Google Scholar]
- 26.Reyes JL, Terrazas CA, Vera-Arias L, Terrazas LI. Differential response of antigen presenting cells from susceptible and resistant strains of mice to Taenia crassiceps infection. Infection, Genetics and Evolution. 2009;9(6):1115–1127. doi: 10.1016/j.meegid.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet β-cell destruction and insulin-dependent diabetes mellitus. Biochemical Pharmacology. 1998;55(8):1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 28.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. Journal of Immunology. 2006;176(10):5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 29.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. Journal of Immunology. 2004;173(2):1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 30.Biros E, Jordan MA, Baxter AG. Genes mediating environment interactions in type 1 diabetes. The Review of Diabetic Studies. 2005;2(4):192–207. doi: 10.1900/RDS.2005.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toenjes SA, Spolski RJ, Mooney KA, Kuhn RE. The systemic immune response of BALB/c mice infected with larval Taenia crassiceps is a mixed Th1/Th2-type response. Parasitology. 1999;118(6):623–633. doi: 10.1017/s0031182099004370. [DOI] [PubMed] [Google Scholar]
- 32.Dahlén E, Dawe K, Ohlsson L, Hedlund G. Dendritic cells and macrophages are the first and major producers of TNF-α in pancreatic islets in the nonobese diabetic mouse. Journal of Immunology. 1998;160(7):3585–3593. [PubMed] [Google Scholar]
- 33.Chen C, Lee W-H, Yun P, Snow P, Liu C-P. Induction of autoantigen-specific Th2 and Tr1 regulatory T cells and modulation of autoimmune diabetes. Journal of Immunology. 2003;171(2):733–744. doi: 10.4049/jimmunol.171.2.733. [DOI] [PubMed] [Google Scholar]
- 34.Cooke A, Tonks P, Jones FM, et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunology. 1999;21(4):169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 35.Zaccone P, Fehérvári Z, Jones FM, et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. European Journal of Immunology. 2003;33(5):1439–1449. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 36.Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. European Journal of Immunology. 2009;39(4):1098–1107. doi: 10.1002/eji.200838871. [DOI] [PubMed] [Google Scholar]
- 37.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infection and Immunity. 2007;75(1):397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Flamme AC, Ruddenklau K, Bäckström BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infection and Immunity. 2003;71(9):4996–5004. doi: 10.1128/IAI.71.9.4996-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sewell D, Qing Z, Reinke E, et al. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. International Immunology. 2003;15(1):59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- 40.Khan WI, Blennerhasset PA, Varghese AK, et al. Intestinal nematode infection ameliorates experimental colitis in mice. Infection and Immunity. 2002;70(11):5931–5937. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elliott DE, Li J, Blum A, et al. Exposure to schistosome eggs protects mice from TNBS-induced colitis. American Journal of Physiology. 2003;284(3):G385–G391. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 42.Smith P, Mangan NE, Walsh CM, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. Journal of Immunology. 2007;178(7):4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- 43.Moreels TG, Nieuwendijk RJ, De Man JG, et al. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut. 2004;53(1):99–107. doi: 10.1136/gut.53.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr., Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. European Journal of Immunology. 2004;34(10):2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 45.Reardon C, Sanchez A, Hogaboam CM, McKay DM. Tapeworm infection reduces epithelial ion transport abnormalities in murine dextran sulfate sodium-induced colitis. Infection and Immunity. 2001;69(7):4417–4423. doi: 10.1128/IAI.69.7.4417-4423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tominaga Y, Nagata M, Yasuda H, et al. Administration of IL-4 prevents autoimmune diabetes but enhances pancreatic insulitis in NOD mice. Clinical Immunology and Immunopathology. 1998;86(2):209–218. doi: 10.1006/clin.1997.4471. [DOI] [PubMed] [Google Scholar]
- 47.Rapoport MJ, Jaramillo A, Zipris D, et al. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. Journal of Experimental Medicine. 1993;178(1):87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grip O, Janciauskiene S, Lindgren S. Macrophages in inflammatory bowel disease. Current Drug Targets—Inflammation & Allergy. 2003;2(2):155–160. doi: 10.2174/1568010033484179. [DOI] [PubMed] [Google Scholar]