Abstract
The mammalian circadian system, which is composed of a master pacemaker in the suprachiasmatic nuclei (SCN) as well as other oscillators in the brain and peripheral tissues, controls daily rhythms of behavior and physiology. Lesions of the SCN abolish circadian rhythms of locomotor activity and transplants of fetal SCN tissue restore rhythmic behavior with the periodicity of the donor's genotype, suggesting that the SCN determines the period of the circadian behavioral rhythm. According to the model of timekeeping in the SCN, the Period (Per) genes are important elements of the transcriptional/translational feedback loops that generate the endogenous circadian rhythm. Previous studies have investigated the functions of the Per genes by examining locomotor activity in mice lacking functional PERIOD proteins. Variable behavioral phenotypes were observed depending on the line and genetic background of the mice. In the current study we assessed both wheel-running activity and Per1-promoter-driven luciferase expression (Per1-luc) in cultured SCN, pituitary, and lung explants from Per2−/− and Per3−/− mice congenic with the C57BL/6J strain. We found that the Per2−/− phenotype is enhanced in vitro compared to in vivo, such that the period of Per1-luc expression in Per2−/− SCN explants is 1.5 hours shorter than in Per2+/+ SCN, while the free-running period of wheel-running activity is only 11 minutes shorter in Per2−/− compared to Per2+/+ mice. In contrast, circadian rhythms in SCN explants from Per3−/− mice do not differ from Per3+/+ mice. Instead, the period and phase of Per1-luc expression are significantly altered in Per3−/− pituitary and lung explants compared to Per3+/+ mice. Taken together these data suggest that the function of each Per gene may differ between tissues. Per2 appears to be important for period determination in the SCN, while Per3 participates in timekeeping in the pituitary and lung.
Introduction
Circadian rhythms are self-sustained oscillations in physiology and behavior with endogenous periods of approximately 24 hours that can be entrained to environmental cues such as the light/dark cycle and temperature [1]. In mammals, a light-entrainable circadian pacemaker, located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus, plays a critical role in the generation of daily rhythms. Surgical destruction of the SCN abolishes most circadian rhythms, including rhythmic locomotor activity [2], [3], [4]. Transplants of fetal SCN tissue to SCN-lesioned animals restore rhythmic behavior that is consistent with the periodicity of the donor's genotype [5], [6], [7], suggesting that the SCN controls the period of the circadian behavioral rhythm.
The SCN is not the only endogenously rhythmic structure. Ex vivo adrenals, liver, and retinas exhibit circadian rhythmicity [8], [9], [10], [11], [12]. In addition, genes important for circadian timekeeping are expressed not only in the SCN, but also in many peripheral tissues [13], [14], [15], [16], [17]. Following the discovery that immortalized rat embryonic fibroblasts have circadian rhythms of gene expression [18], real-time monitoring of circadian promoter-driven reporters revealed that peripheral tissues and extra-SCN brain regions exhibit circadian oscillations in vitro [19], [20], [21]. Consequently, there is now direct evidence that the mammalian circadian system is composed of multiple circadian oscillators: a light-entrainable central pacemaker in the SCN and many oscillators in other regions of the brain and peripheral tissues.
The molecular mechanism of endogenous rhythm generation in the SCN is modeled as interlocking positive and negative transcriptional and translational feedback loops of circadian gene expression [22], [23], [24]. According to this model, BMAL1/CLOCK or BMAL1/NPAS2 heterodimers activate the transcription of the Period (Per) and Cryptochrome (Cry) genes. As PER and CRY proteins accumulate, they form complexes and directly bind to BMAL1-CLOCK/NPAS2 heterodimers, thereby inhibiting their own transcription. In addition, post-translational modifications regulate the stability and cellular localization of circadian proteins. The mRNAs of three Period homologs (Per1, 2, and 3) are rhythmically expressed in the SCN, yet only Per1 and Per2 have been implicated as important negative elements in the feedback loops that generate the endogenous SCN rhythm [14], [15], [17], [25], [26], [27]. Per1−/− or Per2−/− mice, for example, have more severe behavioral phenotypes than Per3−/− mice. The periods of wheel-running activity of Per1−/− and Per2−/− mice can be 1.5 hrs shorter than wildtype mice (depending on the line and genetic background) and they sometimes become arrhythmic in constant darkness (DD), while only a 0.5 h period difference between Per3−/− and wildtype mice has been observed and Per3−/− mice never become arrhythmic in DD [28], [29], [30], [31], [32]. In addition, Per1/Per2 double mutants, but not Per1/Per3 or Per2/Per3 double mutants, have arrhythmic locomotor activity in DD [28]. While most of these experiments have been performed in mice with mixed genetic backgrounds or congenic with the 129/sv strain, one study found that Per2−/− mice congenic with the C57BL/6J strain have periods of wheel-running activity that are indistinguishable from wildtype mice and they do not become arrhythmic in DD [33].
The C57BL/6J strain is ideal for analysis of circadian behavior because the mice have high amplitude (robust), consolidated (not dissociated or fragmented) locomotor activity with a stable free-running period in DD [34], [35]. Since the phenotypes of Per mutant mice may vary depending on the genetic background, we sought to investigate the circadian phenotypes of wheel-running behavior and of SCN and peripheral tissue explants in Per2−/− and Per3−/− mice congenic with the C57BL/6J strain. In our previous study of C57BL/6J Per1−/− mice, we found that the real-time circadian gene promoter activity rhythm was weak or absent in SCN slices in vitro even though the free-running wheel-running activity rhythm was indistinguishable from wildtype mice [36]. Pituitary and lung explants were also arrhythmic in C57BL/6J Per1−/− mice. In the current study, we assessed wheel-running activity and rhythmicity in the SCN and peripheral tissues in Per2−/− and Per3−/− mice congenic with the C57BL/6J strain. Our approach allowed us to compare the behavioral rhythms to the rhythms of explanted tissues of Per2−/− and Per3−/− mice with genetic background held constant.
Results
Circadian Wheel-Running Behavior of C57BL/6J Per2−/− and Per3−/− Mice
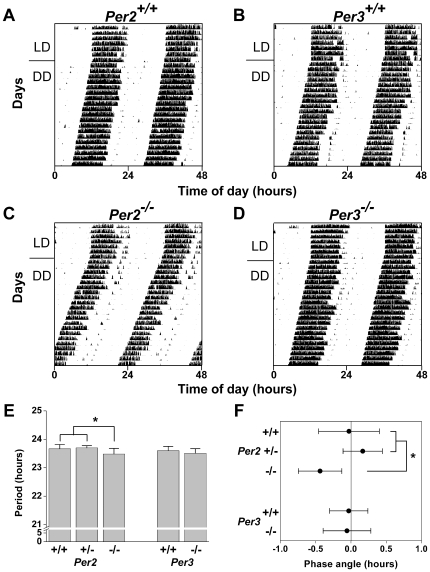
We generated Per2−/− and Per3−/− mice congenic with the C57BL/6J strain by backcrossing mPer2−/− and mPer3−/− mutants on an inbred 129/sv genetic background [28], [32] for 10 to 11 generations with C57BL/6J wildtype mice (The Jackson Laboratory, Bar Harbor, ME). We found that Per2−/− mice had a significantly shorter period of locomotor activity (by ∼11 minutes) compared to Per2+/+ and Per2+/− mice (F2, 28 = 5.61, p<0.01, LSD p<0.05; Figure 1A, C, E). In contrast, the period of wheel-running activity in Per3−/− mice did not differ from Per3+/+ controls (t17 = 1.26, p = 0.22; Figure 1B, D, E). The phase angle of entrainment was significantly advanced by 25 minutes in Per2−/− mice compared to Per2+/+ and Per2+/− mice (F2, 28 = 8.13, p<0.01, LSD p<0.05; Figure 1F). The phase angle of entrainment of Per3−/− mice did not differ from Per3+/+ mice (t17 = 0.18, p = 0.86; Figure 1F). Total activity levels and the amplitudes (Qp) of wheel-running activity in Per2−/− and Per3−/− mice were indistinguishable from wildtype controls (Table 1). The Qp of wheel-running activity in Per2+/− mice was significantly greater than in Per2+/+ and Per2−/− mice (F2, 28 = 3.77, p = 0.04, LSD p<0.05; Table 1). None of the mice in our study became arrhythmic even after 3 weeks in DD.
Figure 1. Characterization of Circadian Behavior in C57BL/6J Per2−/− and Per3−/− Mice.
Representative double-plotted actograms of wheel-running activity of Per2+/+ (A; n = 10), Per3+/+ (B; n = 10), Per2−/− (C; n = 12), and Per3−/− (D; n = 10) mice maintained in 12L∶12D LD (lights on at 0 h and lights off at 12 h) for 7 days and then released into constant darkness (DD). The free-running period was determined by using χ2 periodogram for days 1-15 in DD (E). The phase angle of entrainment (F) was determined by drawing a regression line to activity onset for days 1-5 in DD and then extending the regression line to the last day in LD. A negative phase angle was obtained when activity started before the time of lights off and a positive phase angle was obtained when activity started after the time of lights off. Data are the mean±SD; *p<0.05.
Table 1. Circadian Behavior of C57BL/6J Per2−/− and Per3−/− Mice.
| Genotype | Mean (revs/day) | SD | n | p | |
| Activity/day | Per2+/+ | 3726.37 | 1618.62 | 9 | F 2, 28 = 2.12, p = 0.14 |
| Per2+/− | 4713.71 | 756.35 | 8 | ||
| Per2−/− | 3410.36 | 1558.60 | 12 | ||
| Per3+/+ | 4797.62 | 931.28 | 9 | t 17 = 0.96, p = 0.35 | |
| Per3−/− | 4257.75 | 1428.48 | 10 | ||
| Genotype | Mean | SD | n | p | |
| Qp | Per2+/+ | 1793.54 | 658.27 | 9 | F 2, 28 = 3.77, p = 0.04 |
| Per2+/− | 2466.54 | 184.55 | 8 | aLSD p<0.05 | |
| Per2−/− | 1805.20 | 685.741 | 12 | ||
| Per3+/+ | 2304.96 | 423.78 | 9 | t 17 = 1.08, p = 0.30 | |
| Per3−/− | 2063.37 | 538.76 | 10 |
Per2+/− is significantly greater than Per2+/+ and Per2−/−.
The same mice were used to determine period and phase angle of entrainment (Figure 1E, F).
Lack of Functional PER2 Affects the Circadian Rhythm in C57BL/6J SCN Explants
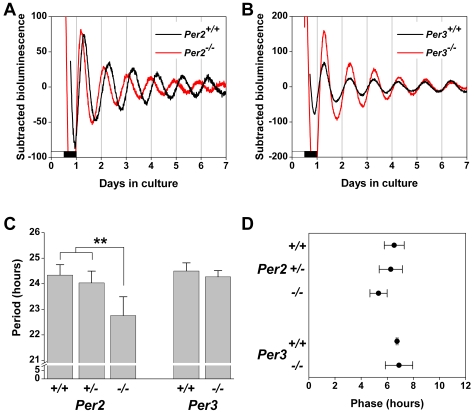
We next assessed Per1-luc expression in cultured SCN explanted from Period mutant mice (Figure 2). Per2−/− SCN explants exhibited robust rhythmicity, but the period of Per1-luc expression was significantly shorter than in SCN from Per2+/+ and Per2+/− mice (F2, 14 = 11.61, p<0.01, LSD p<0.01; Figure 2A, C). There was also a trend for the phase of the Per1-luc expression rhythm in Per2−/− SCN explants to be advanced compared to Per2+/+ and Per2+/− SCN (F2, 14 = 3.83, p = 0.05; Figure 2D).
Figure 2. Per1-luc Expression in SCN Explants from C57BL/6J Per2−/− and Per3−/− Mice.
Representative baseline-subtracted bioluminescence rhythms in SCN explants from Per2+/+ (black trace; A; n = 5) and Per2−/− (red trace; A; n = 6) mice and from Per3+/+ (black trace; B; n = 4) and Per3−/− (red trace; B; n = 5) mice. The lighting condition of the previous light/dark cycle is indicated for the first day; open bars are light and black bars are dark. (C) The period was determined by fitting a regression line to the acrophase of the Per1-luc rhythm. (D) The phase was designated as the first peak of Per1-luc expression in vitro and is plotted relative to the light-dark cycle before culture, where 0 h is the time of lights on and 12 h is the time of lights off (only the subjective day is shown). All data are presented as the mean±SD; **p<0.01.
Per1-luc expression in Per3−/− SCN explants was indistinguishable from Per3+/+ SCN. The period (t6 = 1.17, p = 0.29; Figure 2B, C) and phase (t7 = −0.28, p = 0.79; Figure 2D) of Per1-luc expression in Per3−/− SCN did not differ from Per3+/+ SCN.
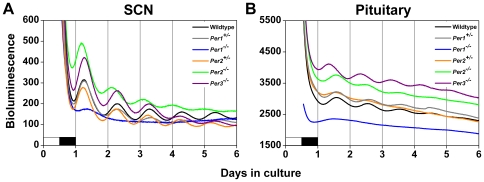
Baseline Per1 promoter activity in the SCN was also altered by loss of Per function. In SCN explants, Per1 promoter activity was elevated in Per2−/− and Per3−/− mice compared to wildtypes and heterozygotes (Figure 3A), suggesting that PER2 and PER3 may inhibit the activation of the Per1 promoter in wildtype SCN.
Figure 3. Per1 Promoter Activity Is Altered in PER-deficient Tissues.
Average Per1-luc bioluminescence (raw data) from SCN explants (A) and whole pituitary glands (B) from wildtype, heterozygous, or PER-deficient mice. Bioluminescence data from Per1−/− mice were taken from [36]. The lighting condition of the previous light/dark cycle is indicated for the first day; open bars are light and black bars are dark.
Circadian Rhythms in Pituitary and Lung Explants Are Altered in Per3−/− Mice
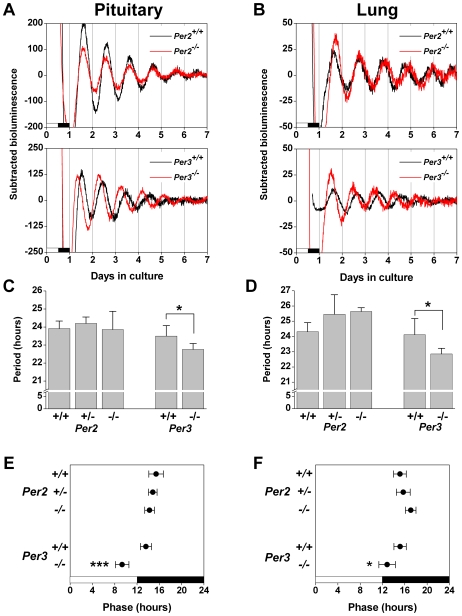
To determine if peripheral tissues have altered circadian rhythms in C57BL/6J Per2−/− and Per3−/− mice, we assessed Per1-luc expression in ex vivo pituitary glands and lungs (Figure 4). The period (H = 0.76, d.f. = 2, p = 0.68; Figure 4A, C) and phase (F2, 16 = 1.98, p = 0.18; Figure 4A, E) of Per1-luc expression in Per2−/− pituitary explants did not differ from Per2+/+ and Per2+/− mice.
Figure 4. Per1-luc Expression in Peripheral Tissues Explanted from C57BL/6J Per2−/− and Per3−/− Mice.
Representative baseline-subtracted bioluminescence rhythms in pituitary (A) and lung (B) explants from Per2+/+ (black trace; top panel; n = 6) and Per2−/− (red trace; top panel; n = 4-6) mice and from Per3+/+ (black trace; bottom panel; n = 4) and Per3−/− (red trace; bottom panel; n = 5) mice. The lighting condition of the previous light/dark cycle is indicated for the first day; open bars are light and black bars are dark. The period of pituitary (C) and lung (D) explants was determined by fitting a regression line to the acrophase of the Per1-luc rhythm. The phase of pituitary (E) and lung (F) explants was designated as the first peak of Per1-luc expression in vitro and is plotted relative to the light-dark cycle before culture, where 0 h is the time of lights on and 12 h is the time of lights off (open bars are light and black bars are dark). All data are presented as the mean±SD; *p<0.05, ***p<0.001.
The period of Per1-luc expression in Per3−/− pituitary explants was significantly shorter than in Per3+/+ pituitary (t7 = 2.43, p<0.05; Figure 4A, C). In addition, the phase of Per1-luc expression was significantly advanced in Per3−/− pituitary compared to Per3+/+ mice (t7 = 5.69, p<0.001; Figure 4A, E).
In pituitary explants, baseline Per1 promoter activity was elevated in Per2−/− and Per3−/− mice compared to wildtypes and heterozygotes (Figure 3B), suggesting that PER2 and PER3 may inhibit the activation of the Per1 promoter in wildtype pituitary.
There was a trend toward an elongated period of Per1-luc expression in lung explants from Per2−/− and Per2+/− mice compared to Per2+/+ mice (F2, 14 = 3.84, p = 0.05; Figure 4B, D). There was also a trend for Per2−/− lung explants to have a delayed phase of Per1-luc expression compared to Per2+/+ and Per2+/− lung (F2, 14 = 3.60, p = 0.06; Figure 4B, F).
In Per3−/− lung explants, the period was shorter (t7 = 2.53, p<0.05; Figure 4B, D) and the phase of Per1-luc expression was advanced (t7 = 2.61, p<0.05; Figure 4B, F) compared to Per3+/+ lung.
Discussion
Previous studies have demonstrated that the SCN controls the period of the circadian locomotor activity rhythm in mammals [7], [37], [38]. Therefore, it is reasonable to predict that if a molecular alteration of the timekeeping mechanism changes the period of the circadian rhythm in the SCN, then the period of locomotor activity should also change (in a similar direction and magnitude). This hypothesis is supported by previous studies in tau mutant hamsters and Clock (Δ19) mutant mice [39], [40], [41]. In contrast, we found that clock gene promoter-driven luciferase activity was arrhythmic or had low amplitude, irregular rhythms in SCN explants from C57BL/6J Per1−/− mice even though their locomotor behavior was indistinguishable from wildtype mice [36]. In the current study we assessed locomotor activity and rhythmicity in the SCN of Per2−/− and Per3−/− mice to determine if the in vitro phenotypes of the mutant SCN were congruent with behavior.
We found that the period of wheel-running activity was 11 minutes shorter and the phase angle of entrainment was advanced by 25 minutes in C57BL/6J Per2−/− mice compared to Per2+/+ mice. Since we observed only small differences in behavior between Per2−/− and Per2+/+ mice, we expected to observe a mild phenotype of Per1-luc expression in the Per2−/− SCN. Surprisingly, though, we found that the period of Per1-luc expression in the Per2−/− SCN was 1.5 hours shorter than in the Per2+/+ SCN. The period was shorter in both the SCN and behavior, but the Per2 mutant phenotype was enhanced in SCN explants. Since the phenotype was more pronounced in vitro compared to in vivo, we hypothesize that in vivo factors such as temperature fluctuations, activity feedback, or coupling to extra-SCN oscillators may compensate for the mutant phenotype.
To our knowledge, our study is the first to analyze real-time circadian rhythms of gene expression in SCN explants from Per2−/− mice. A previous study found that bioluminescence was arrhythmic or had low amplitude, irregular rhythms in immortalized fibroblasts derived from Per2−/− mice and infected with a lentiviral construct in which the Per2 promoter drives the expression of luciferase (mPer2-dluc) [42]. SCN explants (or dissociated SCN neurons) were not investigated using this lentivirally-mediated method [42]. In contrast to the dissociated Per2−/− fibroblasts analyzed by Liu et al. [42], we found that SCN, pituitary, and lung explants from C57BL/6J Per2−/− mice had robust, rhythmic expression of Per1-luc. While the period of Per1-luc expression in Per2−/− SCN explants was significantly shorter than in Per2+/+ SCN, circadian rhythms in ex vivo pituitary and lung from Per2−/− mice were largely unaffected compared to Per2+/+ mice.
In contrast to Per2−/− mice, wheel-running behavior and rhythmicity in the SCN of C57BL/6J Per3−/− mice were indistinguishable from Per3+/+ mice. Interestingly, though, we found that the period and phase of Per1-luc expression in the pituitary and lung were altered when PER3 was not functional. Our findings in Per3−/− SCN and lung explants are in agreement with a previous study that assessed the expression of the PERIOD2::LUCIFERASE fusion protein in ex vivo Per3−/− tissues [42]. Since we also found that circadian rhythms were altered in pituitary explants from Per3−/− mice compared to Per3+/+ mice, it is possible that Per3 is important for timekeeping in peripheral tissues. Future studies could assess the physiological outputs of peripheral tissues to determine if the in vitro phenotypes of Per3−/− tissues are present in vivo.
Taken together, our data suggest that the function of each Per gene may differ between tissues. Per2 appears to be important for period determination in the SCN, while Per3 participates in timekeeping in the pituitary and lung.
Materials and Methods
Animals
We obtained mPer2ldc−/− and mPer3−/− mice [28], [32] (provided by Dr. David Weaver, University of MA, congenic with the 129/sv genetic background) and backcrossed the mice with wildtype C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) for 10 to 11 generations (C57BL/6J Per2−/− and Per3−/− mice are available from The Jackson Laboratory, stock #10492 and #10493, respectively). Heterozygous mice were then crossed with C57BL/6J Per1-luc transgenic mice (1-8L) [43] to generate mice that were heterozygous for the Period gene and for the Per1-luc transgene. Period heterozygous (without Per1-luc transgene) mice were then crossed with Period heterozygous mice with the Per1-luc transgene to generate wildtype, heterozygous, and homozygous mutant mice that expressed Per1-luc (N10 to N11) that were used for experiments. Genotype was determined by PCR amplification of tail DNA as previously described [28], [32]. The mice were bred and group-housed in the Vanderbilt University animal facility in a 12 h-light/12 h-dark cycle (12L∶12D) and provided food and water ad libitum. Male and female mice were used for assessing behavior and for preparing tissue explants. The ages at the beginning of the experiment (mean±SD days) and sexes for each experimental condition are: Per2+/+ behavior: 37.8±10.0, 5 males/5 females; Per2+/− behavior: 89.3±60.1, 6 males/3 females; Per2−/− behavior: 49.3±17.2, 8 males/4 females; Per3+/+ behavior: 43.6±9.7, 4 males/6 females; Per3−/− behavior: 43.1±10.2, 5 males/5 females; Per2+/+ tissue: 66.3±7.7, 3 males/3 females; Per2+/− tissue: 77.0±9.3, 3 males/2 females; Per2−/− tissue: 64.3±21.1, 3 males/3 females; Per3+/+ tissue: 113.8±39.9, 3 males/1 female; Per3−/− tissue: 81.8±22.3, 4 males/1 female. All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Vanderbilt University.
Analysis of Wheel-Running Activity
For experiments assessing wheel-running activity, mice were singly housed in cages (33×17×14 cm) with unlimited access to a running wheel (diameter: 11 cm), food, and water. The cages were placed in light-tight, ventilated boxes where the light intensity was 350 lux. Wheel running activity was monitored by a micro-switch-activated signal using the ClockLab system (Actimetrics, Wilmette, IL) and was collected by computer every minute. Analysis was performed using ClockLab software. Free-running period was determined by using a χ2 periodogram for 15 days (days 1–15 in DD). The amplitude (Qp) of the wheel-running rhythm was the peak value of the χ2 periodogram. Total activity level was determined by counting the total number of wheel revolutions from days 1–15 in DD and then averaging them to determine daily activity level. The phase angle of entrainment was defined as the time difference between activity onset and the predicted time of dark onset on the first day in DD. This was calculated by drawing a regression line to activity onset for days 1–5 in DD and then extending the regression line to the last day in LD. A negative phase angle was obtained when activity started before lights off and a positive phase angle was obtained when activity started after lights off.
Luminescence Recording
The detailed methods for real-time measurement of luminescence from ex vivo tissues have been described [44]. Coronal slices of the SCN (300 µm) were prepared by trimming away most extra-SCN tissue and bioluminescence was measured using the LumiCycle apparatus. LumiCycle software (Actimetrics Inc., Wilmette, IL) was used to subtract the 24-hour moving average from the raw luminescence data and to smooth the data by 0.5-hour adjacent averaging. To determine period and phase, the baseline-subtracted and smoothed data was exported to ClockLab (Actimetrics Inc., Wilmette, IL). The period was determined by fitting a regression line to the acrophase of at least 3 days of the Per1-luc rhythm and the phase was determined from the first peak of Per1-luc expression in vitro.
Statistical Analysis
Statistical analysis was performed using SigmaStat (Systat Software, Inc., San Jose, CA). One-way ANOVA followed by post-hoc Fisher's least significant difference (LSD) tests were used for comparison of more than two groups and independent t tests (two-tailed) were used to compare two groups except when data was not normally distributed or variances were not homogeneous. The Kolmogorov-Smirnov test (with Lilliefors' correction) was used to test data for normality. For nonparametric analyses, the Kruskal-Wallis One-way ANOVA on Ranks followed by post-hoc Dunn's Method were used. Significance was ascribed at p<0.05.
Acknowledgments
We thank David Weaver for mPer2ldc−/− and mPer3−/− mice and Akiko Hida and Hajime Tei for Per1-luc mice.
Footnotes
Competing Interests: Author Shin Yamazaki is an Academic Editor of PLoS ONE.
Funding: This research was supported by a National Institutes of Health grant (NS051278 to S.Y.). R.C.F. was supported by the Vanderbilt Undergraduate Summer Research Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Takahashi JS, Turek FW, Moore RY. New York: Kluwer Academic/Plenum Publishers; 2001. Circadian Clocks. [Google Scholar]
- 2.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 3.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- 5.Sawaki Y, Nihonmatsu I, Kawamura H. Transplantation of the neonatal suprachiasmatic nuclei into rats with complete bilateral suprachiasmatic lesions. Neurosci Res. 1984;1:67–72. doi: 10.1016/0168-0102(84)90031-2. [DOI] [PubMed] [Google Scholar]
- 6.Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, et al. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 8.Andrews RV. Circadian rhythms in adrenal organ cultures. Gegenbaurs Morphol Jahrb. 1971;117:89–98. [PubMed] [Google Scholar]
- 9.Andrews RV, Folk GE., Jr Circadian Metabolic Patterns in Cultured Hamster Adrenal Glands. Comp Biochem Physiol. 1964;11:393–409. doi: 10.1016/0010-406x(64)90006-4. [DOI] [PubMed] [Google Scholar]
- 10.Hardeland R. Circadian rhythmicity in cultured liver cells. I. rhythms in tyrosine aminotransferase activity and inducibility and in [3H]leucine incorporation. Int J Biochem. 1973;4:581–590. [Google Scholar]
- 11.Langner R, Rensing L. Circadian rhythm of oxygen consumption in rat liver suspension culture: changes of pattern. Z Naturforsch [B] 1972;27:1117–1118. doi: 10.1515/znb-1972-0945. [DOI] [PubMed] [Google Scholar]
- 12.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 13.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, et al. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 15.Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, et al. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;90:1003–1011. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- 16.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 17.Takumi T, Matsubara C, Shigeyoshi Y, Taguchi K, Yagita K, et al. A new mammalian period gene predominantly expressed in the suprachiasmatic nucleus. Genes Cells. 1998;3:167–176. doi: 10.1046/j.1365-2443.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- 18.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 20.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, et al. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, et al. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 23.Schibler U. The daily timing of gene expression and physiology in mammals. Dialogues Clin Neurosci. 2007;9:257–272. doi: 10.31887/DCNS.2007.9.3/uschibler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 15 Spec No. 2006;2:R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 25.Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 26.Takumi T, Taguchi K, Miyake S, Sakakida Y, Takashima N, et al. A light-independent oscillatory gene mPer3 in mouse SCN and OVLT. Embo J. 1998;17:4753–4759. doi: 10.1093/emboj/17.16.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 28.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 29.Cermakian N, Monaco L, Pando MP, Dierich A, Sassone-Corsi P. Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. Embo J. 2001;20:3967–3974. doi: 10.1093/emboj/20.15.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 31.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 32.Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol Cell Biol. 2000;20:6269–6275. doi: 10.1128/mcb.20.17.6269-6275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Toh KL, Jones CR, Shin JY, Fu YH, et al. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128:59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, et al. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001;11:959–980. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- 35.Possidente B, Stephan FK. Circadian period in mice: analysis of genetic and maternal contributions to inbred strain differences. Behav Genet. 1988;18:109–117. doi: 10.1007/BF01067080. [DOI] [PubMed] [Google Scholar]
- 36.Pendergast JS, Friday RC, Yamazaki S. Endogenous rhythms in period1 mutant suprachiasmatic nuclei in vitro do not represent circadian behavior. J Neurosci. 2009;29:14681–14686. doi: 10.1523/JNEUROSCI.3261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- 38.Sujino M, Masumoto KH, Yamaguchi S, van der Horst GT, Okamura H, et al. Suprachiasmatic nucleus grafts restore circadian behavioral rhythms of genetically arrhythmic mice. Curr Biol. 2003;13:664–668. doi: 10.1016/s0960-9822(03)00222-7. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- 40.Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- 41.Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, et al. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci U S A. 2005;102:2608–2613. doi: 10.1073/pnas.0409763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 2005;393:288–301. doi: 10.1016/S0076-6879(05)93012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]