Abstract
The AMP-activated protein kinase (AMPK) is an αβγ heterotrimer that regulates appetite and fuel metabolism. We have generated AMPK β1−/− mice on a C57Bl/6 background that are viable, fertile, survived greater than 2 years, and display no visible brain developmental defects. These mice have a 90% reduction in hepatic AMPK activity due to loss of the catalytic α subunits, with modest reductions of activity detected in the hypothalamus and white adipose tissue and no change in skeletal muscle or heart. On a low fat or an obesity-inducing high fat diet, β1−/− mice had reduced food intake, reduced adiposity, and reduced total body mass. Metabolic rate, physical activity, adipose tissue lipolysis, and lipogenesis were similar to wild type littermates. The reduced appetite and body mass of β1−/− mice were associated with protection from high fat diet-induced hyperinsulinemia, hepatic steatosis, and insulin resistance. We demonstrate that the loss of β1 reduces food intake and protects against the deleterious effects of an obesity-inducing diet.
Keywords: Cytokines, Diseases/Diabetes, Diseases/Metabolic, Diseases/Neurodegeneration, Diseases/Obesity, Gene/Knockout, Metabolism/Energy, Signal Transduction/Protein Kinases/Serine/Threonine
Introduction
The AMP-activated protein kinase (AMPK)6 is an evolutionarily conserved serine/threonine protein kinase that functions as a metabolic regulatory enzyme at both the cellular and whole body level (1). AMPK is activated in response to physiological processes that raise intracellular levels of AMP, such as exercise and hypoxia. It restores cellular energy balance by switching off ATP-consuming anabolic pathways and switching on ATP-generating catabolic pathways by direct phosphorylation of downstream targets. Modulation of AMPK activity by hormones adds an additional layer of control, allowing cellular energy supply and demand to be balanced with the energy requirements of the whole organism (2).
AMPK functions as an αβγ heterotrimer. Different isoforms for each of the subunits exist (α1, α2, β1, β2, γ1, γ2, and γ3) as well as some splice variants, allowing more than 12 heterotrimeric combinations to be generated that may mediate unique tissue-specific functions (3, 4). The 63-kDa AMPK α subunits, designated α1 and α2, contain a serine/threonine protein kinase catalytic domain that is activated by phosphorylation of Thr-172 in the activation loop (5, 6). We, and others have shown that the C terminus of the β subunits are essential for AMPK heterotrimer assembly by anchoring the α and γ subunits (7, 8). The β1 and β2 subunits show 82% identity from residue 73 to 270, but only 43% identity for the N-terminal residues 1–72 (9). The β1 subunit is N-terminally myristoylated and is phosphorylated on multiple serines (10); however, the physiological importance of these phosphorylation sites is poorly understood. Northern blot analysis of human tissues revealed that AMPK β1 expression is highest in the liver and brain and low in kidney and skeletal muscle, whereas β2 is most highly expressed in skeletal muscle with lower expression in kidney, liver, and lung (11).
Hepatic AMPK is thought to play important roles in regulating lipid metabolism, glucose homeostasis, and insulin sensitivity (1). Here, activation of AMPK suppresses fatty acid synthesis and increases oxidation through direct phosphorylation of acetyl-CoA carboxylase 1 and 2, respectively (12). AMPK signaling also inhibits hepatic glucose output by transcriptional control. This is mediated by phosphorylation of CRTC2 (CREB-regulated transcription coactivator 2), (13), a co-activator of CREB, leading to down-regulation of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) transcription (13, 14). There is also evidence for AMPK signaling via GSK3β phosphorylation of CREB to reduce hepatic PEPCK transcription (15). Recently, three genetically modified animal models have provided important tools for dissecting the role AMPK plays in regulating hepatic glucose homeostasis. Liver-specific deletion of LKB1, one of three AMPK upstream kinases, leads to inhibition of AMPK signaling and up-regulation of gluconeogenic enzyme expression, resulting in elevated fasting blood glucose in mice (14). In a second model, transgenic overexpression of a constitutively active AMPKα2 allele in the liver was reported to cause hypoglycemia (14, 16). Finally, AMPK α2 null mice display fasting hyperglycemia, glucose intolerance, and increased hepatic glucose output due to elevated adrenergic signaling (17). Interestingly, AMPK α1 null mice were reported as having no detectable metabolic phenotype (17). In summary, these studies support the view that hepatic AMPK lowers systemic glucose levels by inhibiting hepatic glucose output.
Hypothalamic AMPK regulates appetite in response to circulating nutrients, such as glucose and fatty acids, as well as hormonal signals derived from the pancreas, adipose tissue, and gut (1). The activation of hypothalamic AMPK is associated with increased expression of neuropeptide Y and agouti-related peptide and leads to increased appetite and food intake (1). Inhibition of AMPK has the reciprocal effect. Recent studies suggest that many of the effects of AMPK on appetite may be mediated indirectly through modulation of hypothalamic ACC activity, resulting in altered malonyl-CoA and fatty acid levels (18). Surprisingly, neither whole-body AMPKα1 nor AMPKα2 null mice have altered appetite or body mass (19). However, in mice lacking whole-body CAMKK2 or lacking both AMPKα1 and AMPKα2 in agouti-related peptide containing neurons, there are modest reductions in appetite and body mass (20, 21).
The current study examined the effect of whole-body AMPK β1 deletion (β1−/−) on energy homeostasis and metabolism. AMPK β1−/− mice displayed tissue-specific defects in phosphorylation of AMPK on Thr-172 and AMPK activity, with the most dramatic effect observed in the liver and more modest effects in the hypothalamus and adipose tissue. Consistent with the known role of AMPK as a regulator of appetite, we found that AMPKβ1 null mice consumed significantly less food and were protected from developing high fat diet-induced obesity. Surprisingly, AMPK β1 null mice also had markedly reduced fasting gluconeogenic enzyme expression and enhanced hepatic insulin sensitivity. In contrast, isolated hepatocytes from the β1 null mice had increased gluconeogenic enzyme expression, increased rates of fatty acid synthesis, and reduced rates of fatty acid oxidation. These data demonstrate that the deletion of AMPKβ1 results in reduced appetite and protection from diet-induced obesity and hepatic insulin resistance.
EXPERIMENTAL PROCEDURES
Generation of β1−/− Mice
We generated AMPK β1 subunit null mice on a pure C57Bl/6 background using standard homologous recombination techniques. We recently used hepatocytes from the β−/− mice to study the effects of thioenpyridine drugs on AMPK (22). Our targeting strategy was designed to delete exons 2, 3, and 4 of the prkab1 gene. Because the β1 subunit binds the α and γ subunits via its C terminus (23), it was necessary to verify that the germ line deletion of exons 2–4 resulted in a transcript with the expected frame shift mutation and premature translation termination in exon 6. RNA from wild type and null mouse heart tissue was reverse transcribed and PCR-amplified with primers designed to the 3′- and 5′-ends of the mouse β1 open reading frame. Amplification of β1 cDNA resulted in two products: the expected 440-bp species and an unexpected 320-bp product (supplemental Fig. 1). DNA sequencing revealed the longer product resulted from mRNA splicing of exon 1 to exon 5 with the expected frameshift, whereas the 320-bp product resulted from an alternative splicing event whereby exon 1 spliced in frame to exon 6. BLAST (24) searches performed using the predicted translated protein products from both of these AMPK β null transcripts revealed no matches in the data base. Because the unexpected splice variant occurred only in the null mouse sample, it is most likely the result of the gene targeting/deletion event and therefore not naturally occurring. To ascertain if either gene product was being translated, an antibody to the N-terminal residues 2–24 of AMPK β1 (within exon 1 residues 1–53) was used for immunoprecipitation studies. The N-terminal AMPK β1 antibody successfully immunoprecipitated native AMPK β1 from wild type mouse liver; however, no AMPK β1 protein products could be immunoprecipitated from AMPK β1−/− mouse liver (supplemental Fig. 2). Had the putative truncated AMPK β1 exon 1–6 splice variant been translated, it would not have contained the CBM, but it would have had the capacity to bind the AMPK γ but not α subunit.
Animal Experiments
For all experiments, male homozygous β1−/− mice were compared with wild type littermate controls and were generated from heterozygous intercross matings. Mice were housed in SPF microisolators and maintained on a 12-h light/dark cycle with lights on at 0700 h. Mice were fed a control low fat chow diet (diet 2014, Harlan Teklad) for 6 weeks following weaning and were then maintained on this diet or switched to a diet containing 45% kcal fat (diet SF-01-028, Specialty Feeds (Glen Forrest, Australia)). The St. Vincent's Health animal ethics committee approved all procedures.
For food intake studies, mice were housed individually, and food was weighed daily over 7 days in mice 12–16 weeks of age. Metabolic rate and activity levels were measured using a Columbus Instruments laboratory animal-monitoring system over 48 h and following a 6-h acclimatization period. Glucose (1 g/kg d-glucose) and insulin (0.5 unit/kg) tolerance tests were performed 6 h after the removal of food as described previously (25). Hyperinsulinemic-euglycemic clamps were performed in conscious, 22–29-week-old mice as described previously (26). Briefly, 3 days prior to the clamp, two catheters were inserted into the right jugular vein. The clamp was conducted after a 6-h fast, which commenced at the start of the light cycle. At −60 min, tracer (d-[3-3H]glucose) was infused at a constant rate (7.5 μCi/h, 0.12 ml/h) for 1 h for determination of basal glucose turnover. At 0 min, an infusion of 10 milliunits/kg/min of insulin diluted in saline containing d-[3-3H]glucose (7.5 μCi/h, 0.12 ml/h) was begun. A 50% dextrose solution was infused at a variable rate to maintain euglycemia. Once steady state was achieved, glucose-specific activity was measured in whole blood after deproteinization with BaOH and ZnSO4. Hepatic glucose production and glucose disposal rate for the basal and clamp period were calculated using Steele's equation for steady state conditions. Clamp insulin was measured by an enzyme-linked immunosorbent assay (Mercodia, Diagenics Ltd.). For serum cytokines and insulin measurements, fasting and fed blood samples (∼300 μl) were collected retroorbitally using a non-heparinized capillary tube, allowed to clot, and centrifuged (3000 rpm, 5 min). Serum was removed and stored at −80 °C until analysis as described previously (25). Hypothalamic dissections were performed in fasting and fed mice as described previously (25).
Isolated Hepatocytes
Hepatocytes were prepared by the collagenase perfusion method with minor modifications as recently described (22). The following day, experiments were performed. For measurement of the mRNA expression of gluconeogenic enzymes, hepatocytes were incubated with either vehicle or insulin (at the concentrations indicated) for 4 h before lysis in TRIzol. For lipogenesis and fatty acid oxidation experiments, cells were washed with PBS and incubated in serum-free Medium 199 (Invitrogen) for 2 h. Lipogenesis was assessed by incubating cells with serum-free Medium 199 containing [1-14C]acetate (0.5 μCi/ml) (Amersham Biosciences) and 0.5 mm unlabeled sodium acetate. After 4 h of incubation, cells were washed twice with PBS and harvested by scraping in methanol with lipids extracted and quantitated as described previously (27, 28). For fatty acid oxidation, serum-free Medium 199 containing [1-14C]palmitic acid (0.5 μCi/ml) (Amersham Biosciences) and 0.5 mm unlabeled palmitate was used with fatty acid oxidation determined by measuring labeled CO2 and acid-soluble metabolites as described previously (29).
Adipose Tissue Lipogenesis and Lipolysis
Epididymal adipose tissue explants were incubated in Krebs-Henseleit buffer (pH 7.4, supplemented with 8 mm glucose and 4% bovine serum albumin) for 2 h at 37 °C in a shaking water bath. Lipolysis was measured in the presence or absence of the β-adrenergic agonist isoproterenol (10 μm) (Sigma). At the conclusion of the 2-h incubation, the incubation medium was removed and assayed for glycerol using a free glycerol determination kit (Sigma). For the measurement of lipogenesis, the Kreb's buffer was supplemented with 1 mCi/ml d-[3-3H]glucose (Amersham) in the presence or absence of insulin (1 nm). After 2 h, adipose tissue explants were washed twice with phosphate-buffered saline, weighed, and then homogenized in 1 ml of phosphate-buffered saline. The lipid phase was extracted from the homogenate using a 2:1 chloroform/methanol extraction and resolved using thin layer chromatography as described previously (29).
Analytical Methods
Immunoblotting and AMPK activity assays were performed as previously described (22). For AMPK β1 and β2 immunoblots, a rabbit monoclonal antibody to the conserved C-terminal sequence in AMPK β (Epitomics, Burlingame, CA) was used. Akt Ser(P)-473 and total Akt antibodies were purchased from Cell Signaling Technology (Danvers, MA). RNA was prepared and reverse transcribed, and relative gene expression was calculated using the comparative Ct (2−ΔΔCt) method as described (27). Gluconeogenic genes and the housekeeping gene 18 S were determined using assay-on-demand gene expression kits (Applied Biosystems, Foster City, CA). Tissue lipids and glycogen levels were determined as described previously (27, 28).
Statistical Analysis
All data are reported as means ± S.E. Results were analyzed using Student's t test or analysis of variance procedures where appropriate using GraphPad Prism software. Tukey's post hoc test was used to test for significant differences revealed by the analysis of variance. Data were transformed logarithmically when necessary to obtain similar variances among groups. Significance was accepted at p ≤ 0.05. Correlations were performed using GraphPad Prism software.
RESULTS
AMPK β1−/− mice had no detectable β1 protein expression (Fig. 1A). β1−/− mice displayed no overt behavioral or externally visible phenotype and were fertile, and heterozygous intercrosses generated WT, β1−/−, and heterozygous progeny at the expected Mendelian frequency (1:1:2). In contrast to previous reports, β1−/− mice survived for in excess of 2 years. Deletion of β1 did not result in defects in the CNS (supplemental Fig. 3).
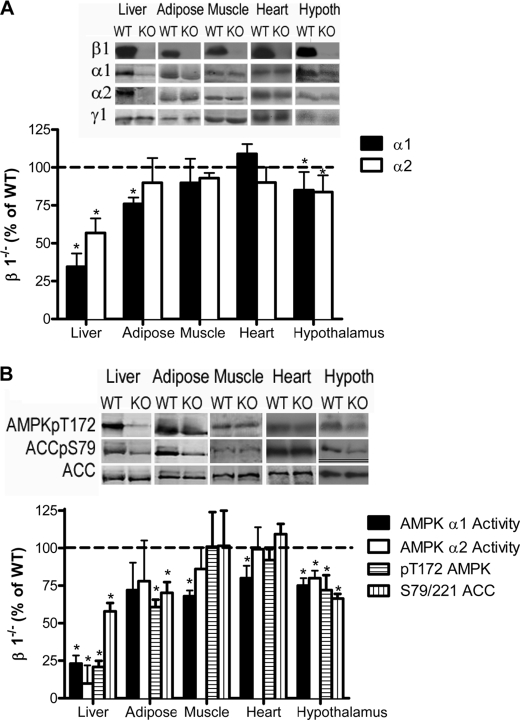
FIGURE 1.
Tissue-specific alterations in AMPK activity and subunit expression in AMPK β1−/− mice. A, representative immunoblots (top) and densitometry (bottom) for AMPK α1, AMPK α2, AMPK β1, AMPK β2, and AMPK γ1 from liver, adipose, muscle, heart, and hypothalamus (Hypoth) of wild type and β1−/− mice. B, AMPK α1 and AMPK α2 activities and AMPK Thr-172 and ACC S79/221/ACC in liver, adipose, muscle, heart, and hypothalamus. Values are mean ± S.E.; n = 5–9; *, p < 0.5 compared with wild type.
The effect of β1 deletion on AMPK subunit protein levels was examined in liver, heart, white adipose tissue, skeletal muscle (mixed vastus), and hypothalamus (predominantly arcuate-containing neurons). β1 deletion resulted in a significant loss of both α1 and α2 catalytic subunits in the liver (Fig. 1A). α1 protein was also modestly reduced in white adipose tissue and hypothalamus (p < 0.05), whereas α1 and α2 protein levels were unaltered in heart and skeletal muscle (Fig. 1A). In contrast, protein levels of the γ1 subunit were unaltered between wild type (WT) and β1−/− mice in all tissues examined (Fig. 1A).
We examined AMPK activity, AMPK α Thr-172 phosphorylation, and phosphorylation of the AMPK downstream target ACC. In the liver of β1−/− mice, the activities of both α1 and α2 and the phosphorylation of AMPK Thr-172 and ACC Ser-79/Ser-221 were substantially reduced (p < 0.001) (Fig. 1B). In adipose tissue, α1 activity tended to be reduced (−28%, p = 0.22), but the reduction in ACC phosphorylation was more dramatic (−30%, p < 0.05) (Fig. 1B). Hypothalamic AMPK activity was reduced in both fed (p = 0.034) (Fig. 1B) and fasting chow-fed β1−/− mice (data not shown) and was associated with significantly reduced ACC Ser-79 phosphorylation (−44%) (Fig. 1B). These findings demonstrate that deletion of AMPK β1 results in prominent changes in AMPK α subunit expression in the liver and more modest effects in the hypothalamus and white adipose tissue, resulting in reduced AMPK activity in these tissues. These tissue-specific effects on AMPK α expression and activity coincide with the more prominent expression of the AMPK β1 isoform (relative to AMPK β2) in these tissues (11).
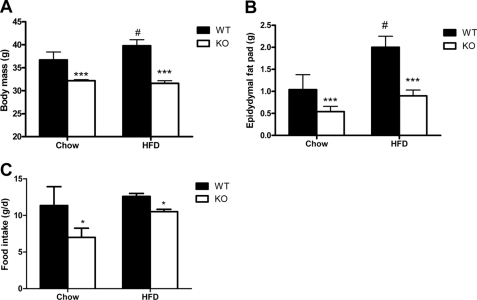
AMPK is an important regulator of whole body energy metabolism; therefore, we fed β1−/− mice a control chow or high fat diet (HFD) over 25 weeks. On both chow and HFD, β1−/− mice weighed less than their wild type littermates (Fig. 2A) and had reduced epidydymal adipose tissue mass (Fig. 2B). Reduced body and fat mass were also observed in β1−/− female mice (data not shown). Reduced adipose tissue mass in β1−/− mice was not due to reduced adipose tissue lipogenesis, which was increased in adipocytes from β1−/− mice (supplemental Fig. 4A) or elevated adipose tissue lipolysis (supplemental Fig. 4B). Markers of adipose tissue differentiation (PPARγ and AP2) were not different between WT and β1−/− mice fed either chow or a HFD (data not shown). A significant correlation between body weight and adiposity was observed for all mice, irrespective of diet (R2 = 0. 74, p < 0.0001). Consistent with the reduced adiposity of β1−/− mice, serum concentrations of the adipokines leptin, resistin, and IL-6 were lower in β1−/− mice (Table 1).
FIGURE 2.
AMPK β1−/− mice have reduced body mass and food intake. A, body mass of wild type and AMPK β1−/− mice fed a control chow or HFD at 22 and 29 weeks of age, respectively. B, reduced epidydymal white adipose tissue in AMPK β1−/− mice. C, average daily food intake in β1−/− mice fed a control chow or HFD. *, p < 0.05, and ***, p < 0.001 compared with wild type; #, p < 0.05 relative to chow.
TABLE 1.
Serum measurements in chow- and HFD-fed wild type and AMPK β1−/− mice
Blood was collected by retro-orbital bleed using non-heparanized capillary tubes. Values are mean ± S.E., n = 6–8.
| Chow |
HFD |
|||
|---|---|---|---|---|
| WT | β1 KO | WT | β1 KO | |
| Glucose (mm) | 3.9 ± 0.7 | 4.2 ± 0.3 | 6.22 ± 0.18a | 6.05 ± 0.12a |
| Non-esterified fatty acid (mm) | 1.48 ± 0.19 | 1.58 ± 0.18 | 1.25 ± 0.16 | 1.65 ± 0.11 |
| Triglyceride (mm) | 1.21 ± 0.3 | 0.94 ± 0.2 | 1.91 ± 0.2 | 2.23 ± 0.2 |
| Glycerol (mm) | 0.44 ± 0.07 | 0.41 ± 0.04 | NDb | ND |
| Leptin (pg/ml) | 1107 ± 288 | 1182 ± 267 | 8520 ± 2144a | 2714 ± 338a,c |
| Tumor necrosis factor α (pg/ml) | 1.85 ± 0.32 | 1.69 ± 0.28 | 4.97 ± 0.68a | 4.45 ± 0.27a |
| IL-6 (pg/ml) | 16.4 ± 4.1 | 2.7 ± 1.1c | 15.85 ± 4.5 | 4.55 ± 2.01c |
| Resistin (pg/ml) | 1312 ± 57 | 1167 ± 83c | 3807 ± 514a | 2414 ± 374a,c |
| PAI-1 (pg/ml) | 2128 ± 418 | 1576 ± 191 | 2660 ± 457 | 2102 ± 253 |
| Adiponectin (μg/ml) | 15.84 ± 1.51 | 13.77 ± 0.92 | 16.52 ± 1.36 | 13.91 ± 2.47 |
a p < 0.05 compared with chow for same dietary condition.
b ND, not determined.
c p < 0.05 compared with wild type for same dietary condition.
In contracting skeletal muscle and adipocytes, there is a strong correlation between the production of IL-6 and the activation of AMPK (30–32). We examined IL-6 mRNA levels in white adipose and liver and found that IL-6 expression was similar between WT and β1−/− mice (data not shown). These data suggest that the reduced serum levels of IL-6 were lower because of reduced adiposity and not an inherent effect of AMPK on IL-6 transcription.
We measured energy expenditure and food intake in chow- and HFD-fed mice. WT and β1−/− mice had similar oxygen consumption, respiratory exchange ratios, and activity levels (Table 2), but β1−/− mice on both chow and HFD had significantly reduced food intake (Fig. 2C). Food intake was also reduced in HFD fed β1−/− female mice (data not shown). These findings are consistent with the role of hypothalamic AMPK as an important regulator of appetite (20, 21, 33, 34).
TABLE 2.
VO2, respiratory exchange ratio (VCO2/VO2), and activity levels in WT and β1−/− during the light and dark cycles
All values are means measured over 72 h ± S.E., n = 6.
| WT |
β1−/− |
|||
|---|---|---|---|---|
| Light | Dark | Light | Dark | |
| VO2 (ml/kg/h) | 3431 ± 206 | 3989 ± 221 | 3567 ± 194 | 4136 ± 191 |
| Respiratory exchange ratio | 0.84 ± 0.02 | 0.90 ± 0.02 | 0.86 ± 0.02 | 0.89 ± 0.02 |
| Xtotal (beam breaks/12 h) | 12,317 ± 2457 | 52,465 ± 12,402 | 15,970 ± 2117 | 62,060 ± 6838 |
| Xamb (beam breaks/12 h) | 4457 ± 1203 | 25,899 ± 7273 | 5900 ± 1029 | 29,659 ± 4294 |
| Ztotal (beam breaks/12 h) | 3676 ± 1783 | 26,921 ± 10421 | 4104 ± 1410 | 28,021 ± 6105 |
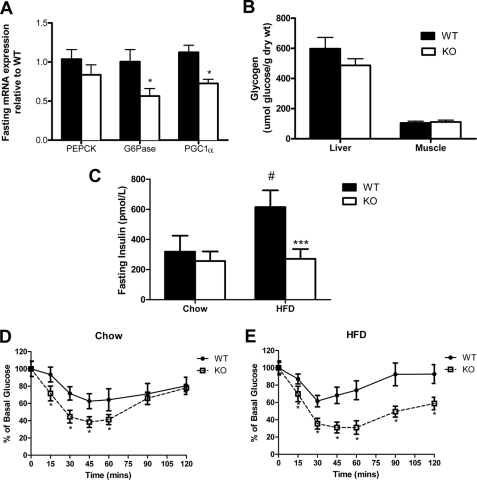
Previous studies in whole-body α2−/− mice (17), liver-specific α2−/− mice (35), or liver-specific LKB1−/− mice (14) showed that reduced hepatic AMPK activity results in increased PEPCK and G6Pase expression and hyperglycemia, effects that may be mediated through phosphorylation and nuclear exclusion of CRTC2 (13). In contrast to the anticipated role of AMPK in the liver, after an overnight fast, AMPK β1−/− mice had reduced expression of G6Pase, PGC1α, and PEPCK (p = 0.07) (Fig. 3A). There was no difference in liver or muscle glycogen contents between WT and β1−/− mice (Fig. 3B). On a control chow diet, β1−/− mice were normoglycemic; however, surprisingly when fed a HFD, β1−/− mice were protected against the development of hyperinsulinemia (Fig. 3C). Moreover, insulin tolerance tests showed that β1−/− mice exhibited improved whole-body insulin sensitivity on either chow or HFDs (Fig. 3, D and E).
FIGURE 3.
Reduced fasting gluconeogenic gene expression and protection against obesity-induced insulin resistance. A, G6Pase, PEPCK, and PGC1α mRNA expression in livers from overnight fasted wild type and β1−/− mice. B, liver and muscle glycogen levels from wild type and β1−/− mice. C, serum insulin in fasted wild type and β1−/− mice. Shown are insulin tolerance tests in wild type and β1−/− mice fed chow (D) or HFD (E). All values are mean ± S.E.; n = 6–8; *, p < 0.05, and ***, p < 0.001 compared with wild type; #, p < 0.05 relative to chow.
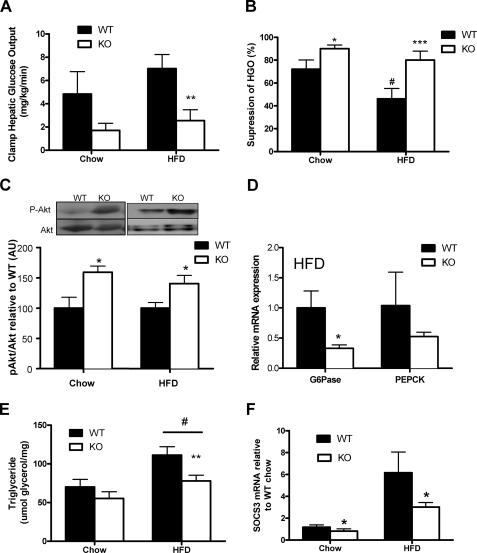
To assess whether increased insulin sensitivity in β1−/− mice was due to improvements in peripheral or hepatic insulin sensitivity, we conducted hyperinsulinemic-euglycemic clamps. Blood glucose, serum insulin, non-esterified fatty acid, and basal glucose turnover were similar between β1−/− and WT mice during the clamp (data not shown). In chow-fed mice, the clamp glucose infusion rate tended to be higher in β1−/− mice relative to WT mice (+10%, p = 0.3), an effect that was significant in HFD-fed β1−/− mice (+42%, p < 0.05). Insulin-stimulated glucose disposal rate was similar between chow-fed β1−/− and WT mice (data not shown); however, when fed an HFD, β1−/− mice had an increased glucose infusion rate, suggesting improved skeletal muscle insulin sensitivity (+24%, p < 0.05). β1−/− mice had reduced hepatic glucose output (Fig. 4A) and greater insulin-induced suppression of hepatic glucose production when fed either diet, indicating marked improvements in hepatic insulin sensitivity (Fig. 4B). Consistent with this, we found that insulin-stimulated Akt phosphorylation following the clamp was higher in livers of both chow and HFD β1−/− compared with WT mice (Fig. 4C) and was associated with a greater suppression of G6Pase mRNA following the clamp (Fig. 4D). A similar trend was also observed for PEPCK (Fig. 4D). Because liver lipids impair insulin sensitivity (36), we measured triglyceride (Fig. 4E), diglyceride, and ceramide levels (data not shown) and found that they were reduced in β1−/− mice. Liver lipids were positively associated with body mass (triglyceride, R2 = 0.35, p = 0.001), suggesting that improvements in hepatic insulin sensitivity may have been secondary to reductions in appetite.
FIGURE 4.
Improved liver insulin sensitivity in AMPK β1−/− mice and protection from HFD-induced insulin resistance. Hepatic glucose production (A) and percentage suppression (B) of hepatic glucose production measured during hyperinsulinemic-euglycemic clamps. C, representative immunoblots and densitometric quantification of Akt Ser-473 phosphorylation and total Akt in liver following hyperinsulinemic-euglycemic clamps. D, mRNA expression of G6Pase and PEPCK in liver of wild type and β1−/− mice fed a HFD following a hyperinsulinemic euglycemic clamp. Shown is liver triglyceride (E) and SOCS3 mRNA expression (F) from wild type and β1−/− mice fed chow or a HFD. All values are mean ± S.E.; n = 6–8; *, p < 0.05, **, p < 0.01, and ***, p < 0.001 compared with wild type; #, p < 0.05 relative to chow. AU, arbitrary units.
Another possibility for the improved liver insulin sensitivity in β1−/− mice may involve the large reduction in circulating IL-6. IL-6 induces hepatic insulin resistance through up-regulation of SOCS3 (suppressor of cytokine-3) (37–39). Consistent with reductions in IL-6 levels, liver SOCS3 expression was reduced in chow- and HFD-fed β1 null mice (Fig. 4F).
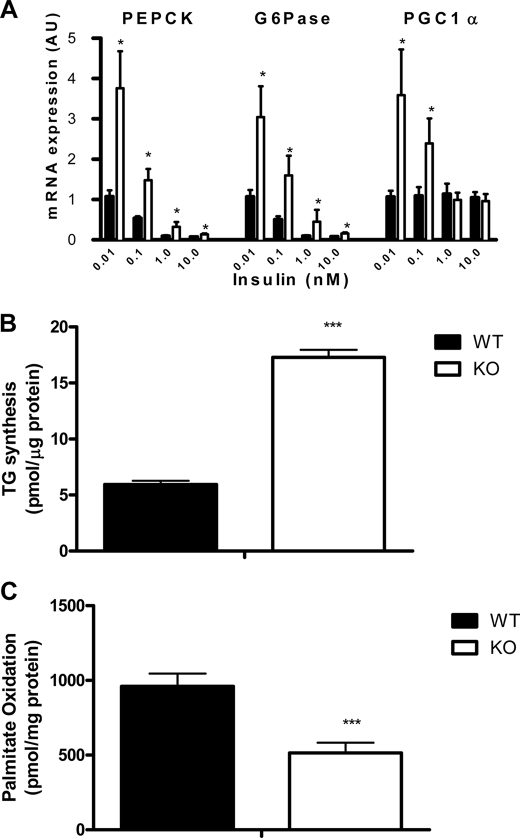
To test whether improved hepatic insulin sensitivity in β1−/− mice was intrinsic to hepatocytes, we isolated hepatocytes from β1−/− and WT mice. In contrast to the findings in vivo, β1−/− hepatocytes had increased basal gluconeogenic enzyme expression of G6Pase, PEPCK, and PGC1α but did not have altered sensitivity to insulin (Fig. 5A). SOCS3 expression was not altered between WT and β1−/− hepatocytes (data not shown). In addition β1−/− hepatocytes had increased fatty acid synthesis (Fig. 5B) and reduced fatty acid oxidation (Fig. 5C), findings consistent with the expected role of AMPK in regulating glucose and lipid metabolism in the liver (1, 2).
FIGURE 5.
Hepatocytes from AMPK β1−/− mice have increased gluconeogenic gene expression and fatty acid synthesis but reduced rates of fatty acid oxidation. A, mRNA expression of enzymes regulating gluconeogenesis (PEPCK, G6Pase, and PGC1α) in isolated hepatocytes from WT and β1−/− mice treated with saline or insulin for 4 h at concentrations as indicated. Shown are fatty acid synthesis into triglyceride (B) and fatty acid oxidation (C) in hepatocytes isolated from WT and β1−/− mice. All values are mean ± S.E.; n = 6–8 of two independent experiments; *, p < 0.05 compared with wild type; ***, p < 0.001 compared with wild type.
DISCUSSION
AMPK exists as an αβγ heterotrimer and shows an absolute dependence on β1 and β2 subunits for complexing the α and γ subunits (7, 8, 23). Despite the importance of the β isoforms for AMPK enzyme function, the physiological role of the β isoforms has received limited attention. This study provides the first in vivo evidence supporting a critical role for the β1 isoform in regulating heterotrimer formation and whole-body glucose metabolism. We demonstrate that germ line deletion of β1 results in reduced expression of the catalytic α1 and α2 proteins, ultimately resulting in a significant reduction of AMPK activity and ACC phosphorylation particularly in the liver where β1 predominates. Despite the reduced AMPK activity, no compensatory increase in the mRNA expression of β2 or the α subunits was seen in the liver (data not shown), highlighting the critical importance of the β1 isoform in this tissue.
AMPK β1−/− mice have a 90% reduction in liver AMPK activity; therefore, our findings of unaltered fasting and fed plasma glucose levels, normal basal glucose turnover during the clamp, and reduced expression of G6Pase in fasted livers were surprising. In addition, β1−/− mice also have enhanced suppression of hepatic glucose output by insulin, a finding consistent with increased Akt phosphorylation and lower mRNA expression of G6Pase during hyperinsulinemic-euglycemic clamps. In contrast to the reduced gluconeogenic enzyme expression of β1−/− mice in vivo, we observed a marked increased in gluconeogenic enzyme expression in isolated hepatocytes from these mice, elevated rates of fatty acid synthesis, and reduced fatty acid oxidation, demonstrating that factors independent of liver β1 are important in vivo.
We believe that there are two potential explanations for our findings. Previous studies by Petersen et al. (36) have shown that weight loss of less than 10% reduces liver fat by 81% and increases hepatic insulin sensitivity by over 200%. Therefore, the modest weight loss induced by a reduction in feeding in β1−/− may have accounted for the significantly reduced levels of liver TG and improved insulin sensitivity. A second mechanism may involve the significantly reduced serum levels of IL-6 in β1−/− mice. IL-6 levels correlate strongly with the development of hepatic insulin resistance (39, 41), which is due to increased expression of SOCS3 (39, 42). Therefore, we believe that the reduced food intake and adiposity are the primary cause for the reduced liver lipid and enhanced hepatic insulin sensitivity seen in vivo in β1−/− mice.
Recently, Dasgupta and Milbrandt (43) reported that genetic deletion of the AMPK β1 isoform using gene-trapping technology resulted in a brain phenotype with atrophy and severe loss of neurons, oligodendrytes, and myelination throughout the central nervous system, resulting in death by postnatal day 21. However, their mice expressed a β1 subunit N-terminal fragment (β1-(2–224)) fused with β-galactacidase that cannot bind either α or γ subunits but retains the midmolecule CBM. Laforin shares an N-terminal CBM (family 20) related to the AMPK β1 CBM (family 48). Disruption of laforin gives rise to a polyglucosan storage disease and neurodegeneration (reviewed in Ref. 40). Since the β1-(20–224)-Gal-expressing mouse phenotype is in marked contrast to the phenotype reported for AMPK α1 null and AMPK α2 null (for a review, see Ref. 2) and the AMPK β1 null mice described herein, we propose that the abnormal central nervous system development of these mice is due to expression of the native AMPK β1-(2–224)-Gal protein and not the loss of the AMPK β1 subunit.
In summary, our data suggest that despite the more pronounced reduction in AMPK activity in the liver of β1−/− mice, the more modest reductions of AMPK activity in the hypothalamus dominate the phenotype, causing reduced food intake. This reduction in feeding protects β1−/− mice from developing diet-induced obesity and hepatic steatosis, thereby overcoming the deleterious liver-specific effects of β1 deletion on hepatic glucose production and lipid deposition.
Supplementary Material
Acknowledgment
We are grateful to Tina Cardamone (Australian Phenomics Network Histopathology and Organ Pathology Service, University of Melbourne).
This study was supported by grants from the Australian Research Council (to B. E. K.), the National Health and Medical Research Council (to M. J. W., D. J. C., B. E. K., and G. R. S.), the Diabetes Australia Research Trust (to G. R. S.), and the National Heart Foundation of Australia (to B. E. K. and D. J. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- AMPK
- AMP-activated protein kinase
- CREB
- cAMP-response element-binding protein
- PEPCK
- phosphenolpyruvate carboxykinase
- G6Pase
- glucose-6-phosphatase
- HFD
- high fat diet
- IL
- interleukin
- ACC
- acetyl-CoA carboxylase.
REFERENCES
- 1.Kahn B. B., Alquier T., Carling D., Hardie D. G. (2005) Cell Metab. 1, 15–25 [DOI] [PubMed] [Google Scholar]
- 2.Steinberg G. R., Kemp B. E. (2009) Physiol. Rev. 89, 1025–1078 [DOI] [PubMed] [Google Scholar]
- 3.Mitchelhill K. I., Stapleton D., Gao G., House C., Michell B., Katsis F., Witters L. A., Kemp B. E. (1994) J. Biol. Chem. 269, 2361–2364 [PubMed] [Google Scholar]
- 4.Carling D., Aguan K., Woods A., Verhoeven A. J., Beri R. K., Brennan C. H., Sidebottom C., Davison M. D., Scott J. (1994) J. Biol. Chem. 269, 11442–11448 [PubMed] [Google Scholar]
- 5.Stapleton D., Mitchelhill K. I., Gao G., Widmer J., Michell B. J., Teh T., House C. M., Fernandez C. S., Cox T., Witters L. A., Kemp B. E. (1996) J. Biol. Chem. 271, 611–614 [DOI] [PubMed] [Google Scholar]
- 6.Hawley S. A., Davison M., Woods A., Davies S. P., Beri R. K., Carling D., Hardie D. G. (1996) J. Biol. Chem. 271, 27879–27887 [DOI] [PubMed] [Google Scholar]
- 7.Townley R., Shapiro L. (2007) Science 315, 1726–1729 [DOI] [PubMed] [Google Scholar]
- 8.Iseli T. J., Oakhill J. S., Bailey M. F., Wee S., Walter M., van Denderen B. J., Castelli L. A., Katsis F., Witters L. A., Stapleton D., Macaulay S. L., Michell B. J., Kemp B. E. (2008) J. Biol. Chem. 283, 4799–4807 [DOI] [PubMed] [Google Scholar]
- 9.Chen Z., Heierhorst J., Mann R. J., Mitchelhill K. I., Michell B. J., Witters L. A., Lynch G. S., Kemp B. E., Stapleton D. (1999) FEBS Lett. 460, 343–348 [DOI] [PubMed] [Google Scholar]
- 10.Mitchelhill K. I., Michell B. J., House C. M., Stapleton D., Dyck J., Gamble J., Ullrich C., Witters L. A., Kemp B. E. (1997) J. Biol. Chem. 272, 24475–24479 [DOI] [PubMed] [Google Scholar]
- 11.Thornton C., Snowden M. A., Carling D. (1998) J. Biol. Chem. 273, 12443–12450 [DOI] [PubMed] [Google Scholar]
- 12.Kemp B. E., Stapleton D., Campbell D. J., Chen Z. P., Murthy S., Walter M., Gupta A., Adams J. J., Katsis F., van Denderen B., Jennings I. G., Iseli T., Michell B. J., Witters L. A. (2003) Biochem. Soc. Trans. 31, 162–168 [DOI] [PubMed] [Google Scholar]
- 13.Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., Takemori H., Montminy M. (2005) Nature 437, 1109–1111 [DOI] [PubMed] [Google Scholar]
- 14.Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horike N., Sakoda H., Kushiyama A., Ono H., Fujishiro M., Kamata H., Nishiyama K., Uchijima Y., Kurihara Y., Kurihara H., Asano T. (2008) J. Biol. Chem. 283, 33902–33910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foretz M., Ancellin N., Andreelli F., Saintillan Y., Grondin P., Kahn A., Thorens B., Vaulont S., Viollet B. (2005) Diabetes 54, 1331–1339 [DOI] [PubMed] [Google Scholar]
- 17.Viollet B., Andreelli F., J⊘rgensen S. B., Perrin C., Geloen A., Flamez D., Mu J., Lenzner C., Baud O., Bennoun M., Gomas E., Nicolas G., Wojtaszewski J. F., Kahn A., Carling D., Schuit F. C., Birnbaum M. J., Richter E. A., Burcelin R., Vaulont S. (2003) J. Clin. Invest. 111, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folmes C. D., Lopaschuk G. D. (2007) Cardiovasc. Res. 73, 278–287 [DOI] [PubMed] [Google Scholar]
- 19.Viollet B., Mounier R., Leclerc J., Yazigi A., Foretz M., Andreelli F. (2007) Diabetes Metab. 33, 395–402 [DOI] [PubMed] [Google Scholar]
- 20.Claret M., Smith M. A., Batterham R. L., Selman C., Choudhury A. I., Fryer L. G., Clements M., Al-Qassab H., Heffron H., Xu A. W., Speakman J. R., Barsh G. S., Viollet B., Vaulont S., Ashford M. L., Carling D., Withers D. J. (2007) J. Clin. Invest. 117, 2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson K. A., Ribar T. J., Lin F., Noeldner P. K., Green M. F., Muehlbauer M. J., Witters L. A., Kemp B. E., Means A. R. (2008) Cell Metab. 7, 377–388 [DOI] [PubMed] [Google Scholar]
- 22.Scott J. W., van Denderen B. J., Jorgensen S. B., Honeyman J. E., Steinberg G. R., Oakhill J. S., Iseli T. J., Koay A., Gooley P. R., Stapleton D., Kemp B. E. (2008) Chem. Biol. 15, 1220–1230 [DOI] [PubMed] [Google Scholar]
- 23.Iseli T. J., Walter M., van Denderen B. J., Katsis F., Witters L. A., Kemp B. E., Michell B. J., Stapleton D. (2005) J. Biol. Chem. 280, 13395–13400 [DOI] [PubMed] [Google Scholar]
- 24.Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990) J. Mol. Biol. 215, 403–410 [DOI] [PubMed] [Google Scholar]
- 25.Steinberg G. R., Watt M. J., Fam B. C., Proietto J., Andrikopoulos S., Allen A. M., Febbraio M. A., Kemp B. E. (2006) Endocrinology 147, 3906–3914 [DOI] [PubMed] [Google Scholar]
- 26.Hevener A. L., He W., Barak Y., Le J., Bandyopadhyay G., Olson P., Wilkes J., Evans R. M., Olefsky J. (2003) Nat. Med. 9, 1491–1497 [DOI] [PubMed] [Google Scholar]
- 27.Watt M. J., Dzamko N., Thomas W. G., Rose-John S., Ernst M., Carling D., Kemp B. E., Febbraio M. A., Steinberg G. R. (2006) Nat. Med. 12, 541–548 [DOI] [PubMed] [Google Scholar]
- 28.Steinberg G. R., Michell B. J., van Denderen B. J., Watt M. J., Carey A. L., Fam B. C., Andrikopoulos S., Proietto J., Görgün C. Z., Carling D., Hotamisligil G. S., Febbraio M. A., Kay T. W., Kemp B. E. (2006) Cell Metab. 4, 465–474 [DOI] [PubMed] [Google Scholar]
- 29.Chen M. B., McAinch A. J., Macaulay S. L., Castelli L. A., O'brien P. E., Dixon J. B., Cameron-Smith D., Kemp B. E., Steinberg G. R. (2005) J. Clin. Endocrinol. Metab. 90, 3665–3672 [DOI] [PubMed] [Google Scholar]
- 30.MacDonald C., Wojtaszewski J. F., Pedersen B. K., Kiens B., Richter E. A. (2003) J. Appl. Physiol. 95, 2273–2277 [DOI] [PubMed] [Google Scholar]
- 31.Glund S., Treebak J. T., Long Y. C., Barres R., Viollet B., Wojtaszewski J. F., Zierath J. R. (2008) Endocrinology 150, 600–606 [DOI] [PubMed] [Google Scholar]
- 32.Sell H., Dietze-Schroeder D., Eckardt K., Eckel J. (2006) Biochem. Biophys. Res. Commun. 343, 700–706 [DOI] [PubMed] [Google Scholar]
- 33.Andersson U., Filipsson K., Abbott C. R., Woods A., Smith K., Bloom S. R., Carling D., Small C. J. (2004) J. Biol. Chem. 279, 12005–12008 [DOI] [PubMed] [Google Scholar]
- 34.Minokoshi Y., Alquier T., Furukawa N., Kim Y. B., Lee A., Xue B., Mu J., Foufelle F., Ferré P., Birnbaum M. J., Stuck B. J., Kahn B. B. (2004) Nature 428, 569–574 [DOI] [PubMed] [Google Scholar]
- 35.Andreelli F., Foretz M., Knauf C., Cani P. D., Perrin C., Iglesias M. A., Pillot B., Bado A., Tronche F., Mithieux G., Vaulont S., Burcelin R., Viollet B. (2006) Endocrinology 147, 2432–2441 [DOI] [PubMed] [Google Scholar]
- 36.Petersen K. F., Dufour S., Befroy D., Lehrke M., Hendler R. E., Shulman G. I. (2005) Diabetes 54, 603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai D., Yuan M., Frantz D. F., Melendez P. A., Hansen L., Lee J., Shoelson S. E. (2005) Nat. Med. 11, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J. H., Kim J. E., Liu H. Y., Cao W., Chen J. (2008) J. Biol. Chem. 283, 708–715 [DOI] [PubMed] [Google Scholar]
- 39.Klover P. J., Zimmers T. A., Koniaris L. G., Mooney R. A. (2003) Diabetes 52, 2784–2789 [DOI] [PubMed] [Google Scholar]
- 40.Gentry M. S., Dowen R. H., 3rd, Worby C. A., Mattoo S., Ecker J. R., Dixon J. E. (2007) J. Cell Biol. 178, 477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazar M. A. (2005) Science 307, 373–375 [DOI] [PubMed] [Google Scholar]
- 42.Ueki K., Kondo T., Kahn C. R. (2004) Mol. Cell. Biol. 24, 5434–5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dasgupta B., Milbrandt J. (2009) Dev. Cell 16, 256–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.