Abstract
Interstitial flow in and around bone tissue is oscillatory in nature and affects the mechanical microenvironment for bone cell growth and formation. We investigated the role of oscillatory shear stress (OSS) in modulating the proliferation of human osteoblast-like MG63 cells and its underlying mechanisms. Application of OSS (0.5 ± 4 dynes/cm2) to MG63 cells induced sustained activation of phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR/p70S6K (p70S6 kinase) signaling cascades and hence cell proliferation, which was accompanied by increased expression of cyclins A and D1, cyclin-dependent protein kinases-2, -4, and -6, and bone formation-related genes (c-fos, Egr-1, and Cox-2) and decreased expression of p21CIP1 and p27KIP1. OSS-induced activation of PI3K/Akt/mTOR/p70S6K and cell proliferation were inhibited by specific antibodies or small interference RNAs of αvβ3 and β1 integrins and by dominant-negative mutants of Shc (Shc-SH2) and focal adhesion kinase (FAK) (FAK(F397Y)). Co-immunoprecipitation assay showed that OSS induces sustained increases in association of Shc and FAK with αvβ3 and β1 integrins and PI3K subunit p85, which were abolished by transfecting the cells with FAK(F397Y) or Shc-SH2. OSS also induced sustained activation of ERK, which was inhibited by the specific PI3K inhibitor LY294002 and was required for OSS-induced activation of mTOR/p70S6K and proliferation in MG63 cells. Our findings provide insights into the mechanisms by which OSS induces osteoblast-like cell proliferation through activation of αvβ3 and β1 integrins and synergistic interactions of FAK and Shc with PI3K, leading to the modulation of downstream ERK and Akt/mTOR/p70S6K pathways.
Keywords: Mechanotransduction, Integrins, Oscillatory Flow, Osteoblast, Proliferation
Introduction
Mechanical loading is critical for the formation of new bone (1–3). During dynamic and periodic loading of intact bone, the reciprocating flow of interstitial fluid through the canaliculi generates oscillatory shear stress (OSS),3 which is detected by osteocytes in the canaliculi and osteoblasts lining the endosteal and periosteal surfaces of bone (4, 5). Stimulation of osteocytes by fluid shear stress induces their release of osteoblastic factors, which are transferred via gap junctions of the osteocyte-interconnecting network to induce osteoblast recruitment and hence bone growth (4, 6). There is increasing evidence that fluid shear stress regulates signaling, gene expression, and differentiation in osteocytes and osteoblasts (4–9). Recent studies using flow channels have demonstrated that application of steady fluid shear stress to osteoblasts induces cell proliferation (10, 11) and the expression of many genes, including c-fos (8, 12), Egr-1 (early growth response-1) (8, 13), and Cox-2 (cyclooxygenase-2) (8, 12), all of which have been shown to play a role in bone formation in vivo (14–17).
The signaling molecules that have been shown to regulate mechanically induced proliferation in osteoblasts include NO (10, 18–20), prostaglandin E2, prostacyclin (10, 18–20), and ERK (10, 11, 18). Kapur et al. (11) demonstrated that ERK1/2 are required for mitogenic response of human osteoblasts to steady fluid shear stress. There is evidence that the mTOR/p70S6K (p70S6 kinase) pathway, which is downstream from phosphatidylinositol 3-kinase (PI3K)/Akt (21), is required for osteoblast proliferation and differentiation (22). However, whether the PI3K/Akt/mTOR/p70S6K pathway is involved in mechanotransduction in osteoblasts and the consequent modulation of their function in response to fluid shear stress remains unclear. Integrins, as the main molecules that connect the cytoskeleton with the extracellular matrix, have been shown to play important roles in transmitting mechanical stimuli into chemical signals in a wide variety of cells seeded on the extracellular matrix (23). In several systems including endothelial cells, integrin activation leads to increases in association with focal adhesion kinase (FAK), which is a nonreceptor protein-tyrosine kinase containing a tyrosine 397 residue (YpAEI motif), and Shc, which is an adaptor protein containing a C-terminal Src homology 2 (SH2) domain, and subsequently the activation of several intracellular signaling cascades, including ERK (24). In osteoblasts, FAK has been shown to play important roles in OSS-induced ERK activation, leading to up-regulation of the bone formation-related genes c-fos, Cox-2, and osteopontin (9). Although FAK and Shc have been shown to be critical for integrin-mediated signaling activation, whether they play synergistic roles in modulating the integrin activation of downstream signaling cascades remains unclear. In addition, whether integrins modulate the activation of PI3K/Akt/mTOR/p70S6K through FAK and Shc in osteoblasts in response to shear stress also remains to be determined.
The aim of the present study was to investigate the role and its underlying molecular mechanisms of OSS in modulating the proliferation of human osteoblast-like MG63 cells, which are originally derived from an osteogenic sarcoma of a 14-year-old male and exhibit many osteoblast traits characterized for bone-forming cells (25). Our findings have provided a molecular basis for the mechanisms by which OSS induces proliferation of osteoblast-like cells through integrin-mediated synergistic association of FAK and Shc with PI3K and their modulation of the downstream ERK and Akt/mTOR/p70S6K pathways.
EXPERIMENTAL PROCEDURES
Materials
Mouse monoclonal antibodies against human αvβ3 and β1 integrins (MAB1976 and MAB2253, respectively) were purchased from Chemicon (Temecula, CA). Mouse monoclonal antibody against FAK and phospho-FAK were purchased from BD Biosciences (Bedford, MA). Wortmannin, LY294002, rapamycin, mouse monoclonal antibodies against cyclins A and D1, cyclin-dependent protein kinases (Cdk)-4 and -6, and p21CIP1, and rabbit polyclonal antibodies against phospho-Akt (Ser-473), Akt, phospho-mTOR (Ser-2448), mTOR, phospho-p70S6K (Thr-389), p70S6K, and p27KIP1 were purchased from Cell Signaling Technology (Beverly, MA). The monoclonal antibodies against β3 and β1 integrins, Cdk-2, ERK2 (sc-1647), and phospho-ERK1/2 (sc-7383) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies against Shc and p85, a PI3K regulatory subunit, were obtained from Upstate Biotechnology Inc. (Lake Placid, NY). The control small interfering RNA (siRNA) and specific siRNAs of β1 and β3 integrins were purchased from Invitrogen. The dominant-negative mutants of FAK (i.e. FAK(F397Y)) and Shc (i.e. Shc-SH2) and the wild type FAK (FAK-wt) were previously described (26, 27). The wild type Shc (Shc-wt) was a gift from Dr. Yasuo Fukami (Research Center for Environmental Genomics, Kobe University, Japan). Bromodeoxyuridine (BrdUrd) was purchased from BD Pharmingen (La Jolla, CA). PD98059 was purchased from Calbiochem (La Jolla, CA). All other chemicals of reagent grade were obtained from Sigma, unless otherwise noted.
Cell Culture
The human osteoblast-like MG63 cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in a medium consisting of Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen). After reaching confluence (1–2 × 105 cells/cm2), the cells were trypsinized and seeded onto glass slides (75 × 38 mm; Corning, Corning, NY) that had been precoated with fibronectin (30 μg/ml) for 24 h prior to the flow experiment.
Oscillatory Flow Apparatus
The cultured MG63 cells were subjected to oscillatory fluid flow in a parallel plate flow chamber, as previously described (28, 29). In brief, the flow channel in the chamber was created by a silicon gasket with dimensions of 2.5 cm in width (w), 5.0 cm in length, and 0.025 cm in height (h). The cell-seeded glass slide and the gasket were fastened between a polycarbonate base plate and a stainless plate. The chamber was connected to a perfusion loop system, kept in a constantly temperature-controlled enclosure, and maintained at pH 7.4 by continuous gassing with a humidified mixture of 5% CO2 in air. The osmolality of the perfusate was adjusted to 285–295 mOsm/kg H2O during the perfusion. The flow of the perfusate in the flow channel is laminar with a parabolic velocity profile. The fluid shear stress (τ) generated on the cells seeded on the glass slide can be estimated as τ = 6 Qμ/wh2, where Q is the flow rate, and μ is the dynamic viscosity of the perfusate. The oscillatory flow is composed of a low level of mean flow (shear stress = 0.5 dynes/cm2) supplied by a hydrostatic flow system to provide the basal nutrient and oxygen delivery and the superimposition of a sinusoidal oscillation using a piston pump with a frequency of 1 Hz and a peak-to-peak amplitude of ±4 dynes/cm2. These parameters of the oscillatory flow were chosen on the basis of the measurement of fluid flow through the bone canaliculi (30). In some experiments, MG63 cells were incubated with a specific inhibitor for signaling molecules for 1 h before and during exposure to flow. The inhibitors used include wortmannin (100 nm) and LY294002 (10 μm) for PI3K, rapamycin (10 nm) for mTOR, and PD98059 (30 μm) for MEK1 (which is an upstream signaling molecule of ERK). For integrin blocking assays, MG63 cells were pretreated with an antibody (10 μg/ml) against αvβ3 or β1 integrin before seeding onto the glass slides and during OSS application.
Western Blot Analysis
MG63 cells were lysed with a buffer containing 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and a protease inhibitor mixture (phenylmethylsulfonyl fluoride, aprotinin, and sodium orthovanadate). The total cell lysate (100 μg of protein) was separated by SDS-PAGE (12% running, 4% stacking) and analyzed by using the designated antibodies and the Western-Light chemiluminescent detection system (Applied Biosystems, Foster City, CA).
Immunoprecipitation
MG63 cells were lysed with a buffer containing 25 mm HEPES, pH 7.4, 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 0.125 m NaCl, 5 mm EDTA, 50 mm NaF, 1 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, 10 mg/ml leupeptin, and 2 mm β-glycerophosphate. The same amount of protein from each sample was incubated with an antibody against αvβ3 or β1 integrin or p85 for 2 h at 4 °C, followed by incubation with protein A/G plus agarose for 1 h. The agarose-bound immunoprecipitates were collected by centrifugation and incubated with a boiling sample buffer containing 62 mm Tris-HCl, pH 6.7, 1.25% (w/v) SDS, 10% (v/v) glycerol, 3.75% (v/v) mercaptoethanol, and 0.05% (w/v) bromphenol blue and then subjected to SDS-PAGE and Western blotting.
DNA Plasmids, siRNA, and Transfection
DNA plasmids (4 μg/ml) were transfected into MG63 cells at 60% confluence by using Lipofectamine (Invitrogen). The pSV-β-galactosidase plasmid was co-transfected to normalize the transfection efficiency. The cells were kept as static controls or subjected to oscillatory flow 48 h after transfection. For siRNA transfection, MG63 cells at 70–80% confluence were transfected with the designated siRNA at various concentrations (10–40 nm) for 48 h using the RNAiMAX transfection kit (Invitrogen) before shear stress experiments.
BrdUrd Incorporation Assay
BrdUrd, an analog of the DNA precursor thymidine, can be incorporated into newly synthesized DNA by cells entering and progressing through the synthetic phase (S phase; DNA synthesis) of the cell cycle (31). Thus, BrdUrd incorporation assay can be used to assess cells undergoing active DNA synthesis during cell proliferation. In brief, MG63 cells were treated with BrdUrd (10 μm) 1 h before the end of flow. The cells were rinsed and fixed for 30 min at room temperature with acetic alcohol (90% ethanol, 5% acetic acid, 5% distilled H2O) and then washed in phosphate-buffered saline and incubated in 1.5 n HCl at 37 °C for 15 min, followed by extensive washing in phosphate-buffered saline. The samples were stained with mouse anti-BrdUrd antibody (Sigma), fluorescein-conjugated anti-mouse antibody (Molecular Probes) as a secondary antibody, goat serum (Sigma) as a blocking medium, and propidium iodide stain as a counterstain for the nucleus. The stained samples were examined using an inverted microscope (Axiovert 200M; Zeiss) with a 20× objective (NA = 0.4, Plan-Neofluar). The cells were rinsed, mounted over glycerol/phosphate-buffered saline (1:1), and photographed. Six separate regions of each sample were counted for a total of ∼100 cells. Proliferation is assessed from the percentage of nuclei that show BrdUrd incorporation.
RNA Isolation and Reverse Transcription-PCR
The total RNA was isolated by the guanidium isothiocyanate/phenochloroform method and converted to cDNA as described (8). The cDNA was diluted 1:20 before the performance of PCR using 1 μl of cDNA in 20 mm Tris-HCl, pH 8.4, 3 mm MgCl2, 0.2 mm dNTP mix, 0.5 μm sense and antisense primers of c-fos, Egr-1, or Cox-2 (8), and Taq polymerase (2 units/μl; Takara Shuzo, Shiga, Japan). Amplification of the cDNA was performed in parallel samples using human glyceraldehyde-3-phosphate dehydrogenase primers. The samples were amplified using primers with denaturation at 94 °C for 1 min, annealing at 60 °C for 1 min, and extension at 72 °C for 2 min, using a GeneAmp System 9700 (PE Biosystems, Foster City, CA). Amplification was linear with respect to the cDNA concentration by optimizing the primer concentration, amplification cycles, and MgCl2 concentration for each PCR. The amplified cDNAs were analyzed by 1% agarose gel electrophoresis and ethidium bromide staining. The band intensities were quantified directly from the stained agarose gels using video imaging and a densitometry software system (GDS-8000 ImagingWorkstation; UVP, Upland, CA).
Statistical Analysis
The results are expressed as the means ± S.E. Statistical analysis was performed by using an independent Student t test for two groups of data and analysis of variance followed by Scheffe's test for multiple comparisons. A p value less than 0.05 was considered significant.
RESULTS
OSS Induces MG63 Cell Proliferation via the Activation of PI3K/Akt/mTOR/p70S6K Pathway
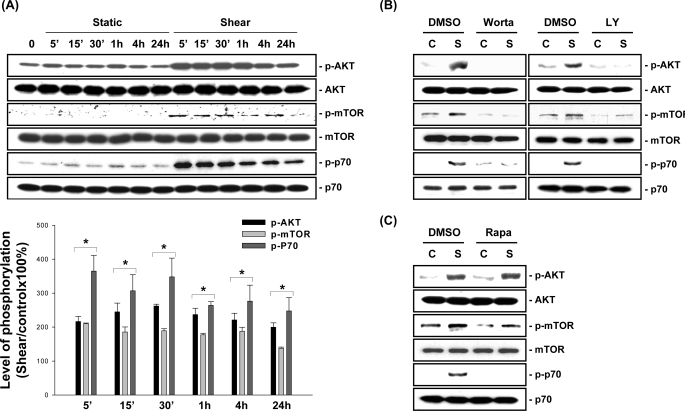
MG63 cells were kept as controls or subjected to OSS (0.5 ± 4 dynes/cm2) for 5 min, 15 min, 30 min, 1 h, 4 h, and 24 h, and their phosphorylation of Akt, mTOR, and p70S6K was examined by using Western blot analysis. MG63 cells incubated under static conditions did not alter their basal levels of Akt, mTOR, and p70S6K phosphorylation for the 24-h period tested (Fig. 1A). OSS applied to MG63 cells induced rapid increases (significant within 5 min) in the phosphorylation of these molecules, which were sustained over the 24-h period tested. Pretreating MG63 cells with the specific PI3K inhibitors wortmannin (100 nm) and LY294002 (10 μm) abolished the OSS-induced Akt, mTOR, and p70S6K phosphorylation (Fig. 1B). However, pretreating MG63 cells with the specific mTOR inhibitor rapamycin (10 nm) only inhibited OSS-induced phosphorylation of mTOR and p70S6K, but not Akt, which is upstream from mTOR (Fig. 1C).
FIGURE 1.
OSS induces activation of the PI3K/AKT/mTOR/p70S6K pathway in MG63 cells. A, MG63 cells were kept under static conditions as controls or subjected to OSS for 5 min (5′), 15 min (15′), 30 min (30′), 1 h, 4 h, and 24 h. The phosphorylation of AKT, mTOR, and p70S6K was determined by Western blot analysis. The data are presented as the amounts (band densities normalized to the total protein levels) of phosphorylated Akt, mTOR, and p70S6K proteins relative to those in static control cells and are shown as the means ± S.E. from three independent experiments. *, p < 0.05 versus static control cells at corresponding time points. B and C, MG63 cells were pretreated with vehicle control DMSO or a specific inhibitor for PI3K (wortmannin, 100 nm; and LY294002, 10 μm) (B) or mTOR (rapamycin, 10 nm) (C) for 1 h before and during exposure to OSS. The cells were kept under static conditions (lanes C) or subjected to OSS for 1 h (lanes S), and the phosphorylation of AKT, mTOR, and p70S6K was determined by Western blot analysis. The results are representative of three independent experiments with similar results.
We performed a BrdUrd incorporation assay to investigate the effect of OSS on the modulation of MG63 cell proliferation. Exposure of MG63 cells to OSS for 24 h resulted in an increase in the percentage of cells in the S phase compared with the static control cells (Table 1). For static control cells, pretreating MG63 cells with LY294002 (10 μm) and rapamycin (10 nm) did not alter the percentage of cells in the S phase compared with the untreated cells. For cells exposed to OSS for 24 h, pretreatment with LY294002 or rapamycin (compared with untreated cells) resulted in a significantly lower percentage of cells in the S phase (Table 1; the remaining data in Table 1 will be discussed below). Taken together, these results indicate that OSS induces MG63 cell proliferation through the activation of the PI3K/Akt/mTOR/p70S6K pathway.
TABLE 1.
Oscillatory shear stress induces MG63 cell proliferation via the PI3K/Akt/ERK/mTOR/p70S6K pathway
MG63 cells were pretreated with vehicle control DMSO or specific inhibitor for PI3K (LY294002, 10 μm), mTOR (rapamycin, 10 nm), or MEK1 (an upstream signaling molecule of ERK) (PD98059, 30 μm) for 1 h and then were kept under static conditions or exposed to oscillatory flow for 24 h in the presence of reagent. The cells were fixed and stained with an anti-BrdUrd antibody with propidium iodide stain as a counterstain for the nucleus, as described under “Experimental Procedures.” The data are the means ± S.E. from three independent experiments.
| Percentage of BrdUrd-positive cells |
||
|---|---|---|
| Static | Oscillatory shear stress | |
| DMSO | 32.5 ± 1.4 | 52.2 ± 4.4b |
| LY294002 | 35.2 ± 2.9 | 37.8 ± 1.9a |
| DMSO | 33.9 ± 1.8 | 60.6 ± 0.6b |
| Rapamycin | 37.1 ± 1.2 | 39.3 ± 2.6a |
| DMSO | 30.5 ± 1.4 | 57.2 ± 2.6b |
| PD98059 | 24.4 ± 1.1a | 31.4 ± 1.5a |
ap < 0.05 vs. cells treated with vehicle control.
b p < 0.05 vs. static control cells.
OSS Induces Sustained Increases in the Association of αvβ3 and β1 Integrins with Shc and FAK and Its Phosphorylation in MG63 Cells
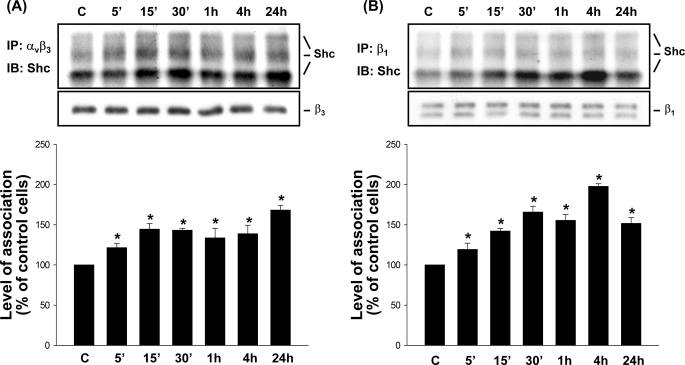
The integrin/Shc association has been used as a readout of integrin activation (32, 33). We investigated whether OSS can induce activation of αvβ3 and β1 integrins, in terms of their increased association with Shc. MG63 cells were kept as controls or exposed to OSS for 5 min, 15 min, 30 min, 1 h, 4 h, and 24 h, and their extracts were immunoprecipitated with an antibody against αvβ3 or β1 integrin, followed by Western blot analysis with an antibody against Shc. Application of OSS to MG63 cells induced a rapid increase (significant within 5 min) in the association of αvβ3 (Fig. 2A) and β1 (Fig. 2B) integrins with Shc, as demonstrated by their co-immunoprecipitation, in comparison with the static controls. These increases in association of αvβ3 and β1 integrins with Shc were sustained over the 24-h period tested. Incubation of MG63 cells under static conditions over the 24-h period tested did not alter the basal levels of αvβ3-Shc and β1-Shc association in these cells (supplemental Fig. S1, A and B).
FIGURE 2.
OSS induces sustained increases in the association of αvβ3 and β1 integrins with Shc in MG63 cells. MG63 cells were kept under static conditions as controls (lanes C) or subjected to OSS for 5 min (5′), 15 min (15′), 30 min (30′), 1 h, 4 h, and 24 h. The association of αvβ3 (A) and β1 (B) integrins with Shc was determined by using co-immunoprecipitation assay. Three bands of integrin/Shc association result from three isoforms of Shc, i.e. p46, p52, and p66. The amounts of integrin·Shc complexes in sheared cells are presented as band densities (normalized to β3 (A) or β1 (B) integrin) relative to those in control cells. The results are the means ± S.E. from three independent experiments. *, p < 0.05 versus static control cells at corresponding time points. IP, immunoprecipitation; IB, immunoblot.
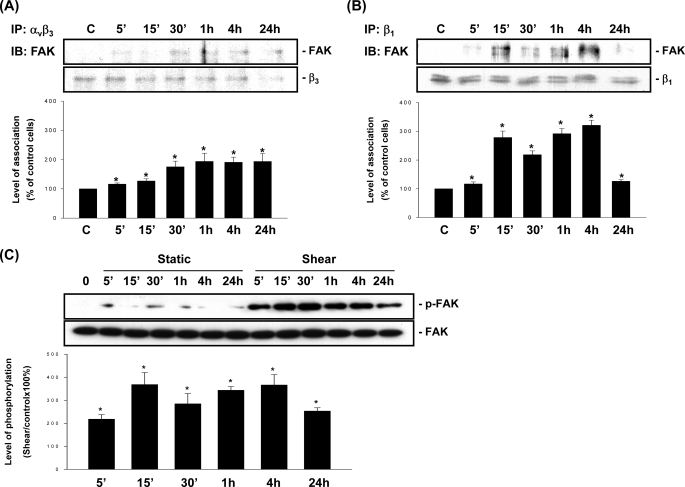
Previous studies have shown that activation of integrins induces the accumulation of FAK in focal adhesion sites and its activation and increased association with integrins (34). We investigated whether OSS induces increases in association of αvβ3 and β1 integrins with FAK and its activation. The results of co-immunoprecipitation assay showed that OSS induces increases in the association of FAK with αvβ3 (Fig. 3A) and β1 (Fig. 3B) integrins in MG63 cells as early as 5 min after exposure to flow, as compared with the static controls. These increases in association of FAK with αvβ3 and β1 integrins were sustained over the 24-h period tested, although the FAK/β1 association declined after 24 h of flow, but it was still significantly higher than the static control level (Fig. 3B). Exposure of MG63 cells to OSS also induced rapid and sustained increases (significant within 5 min and maintained at 24 h) in FAK phosphorylation (Fig. 3C). Incubation of MG63 cells under static conditions over the 24-h period tested did not alter the basal levels of association of αvβ3 and β1 integrins with FAK (supplemental Fig. S1, A and B) and its phosphorylation (Fig. 3C) in these cells.
FIGURE 3.
OSS induces sustained increases in the association of FAK with αvβ3 and β1 integrins and its phosphorylation in MG63 cells. MG63 cells were kept under static conditions as controls (lanes C) or subjected to OSS for the indicated times. The association of FAK with αvβ3 (A) and β1 (B) integrins and the phosphorylation of FAK (C) was determined by using co-immunoprecipitation assays (A and B) and Western blot analysis (C). The amounts of integrin·FAK complexes (A and B) and phosphorylated FAK protein (C) in sheared cells are presented as band densities (normalized to β3 (A) or β1 (B) integrin, or the total protein levels of FAK (C)) relative to those in static control cells at corresponding time points. The results are the means ± S.E. from three independent experiments. *, p < 0.05 versus static control cells. IP, immunoprecipitation; IB, immunoblot.
OSS Activation of Akt/mTOR/p70S6K Pathway Is Mediated by αvβ3 and β1 Integrins through FAK and Shc
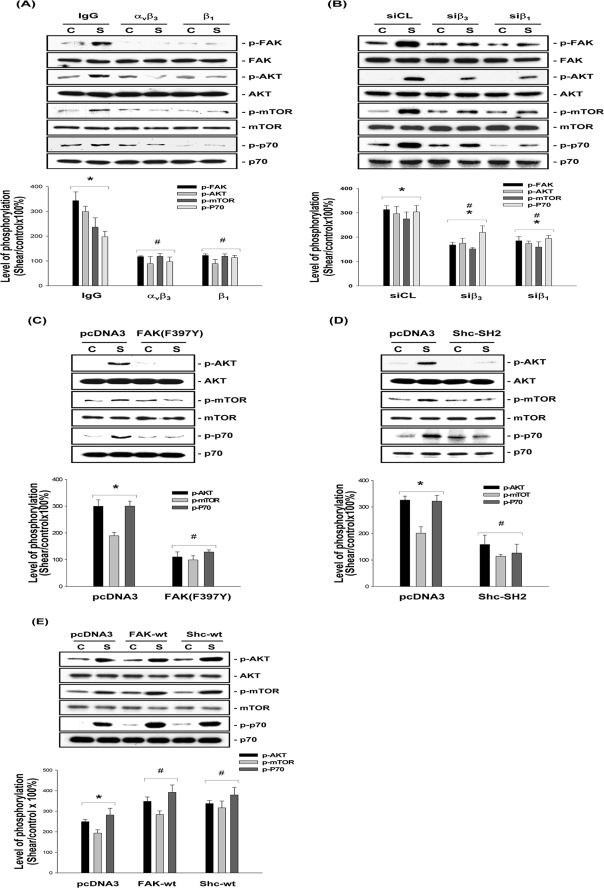
MG63 cells were preincubated with an antibody against αvβ3 or β1 integrin for 1 h and then exposed to OSS for 1 h. Preincubation with either anti-αvβ3 or anti-β1 antibody totally abolished the OSS-induced phosphorylation of FAK, Akt, mTOR, and p70S6K in MG63 cells, as compared with the cells preincubated with control IgG (Fig. 4A). The involvement of αvβ3 and β1 integrins in OSS activation of FAK/Akt/mTOR/p70S6K signaling cascades was further confirmed by the attenuations of shear-induced phosphorylation of these signaling molecules by transfecting MG63 cells with β3- and β1-specific siRNAs (40 nm for each) (Fig. 4B), which caused reductions in β3 and β1 protein expression by ∼60 and 90%, respectively (compared with control siRNA) (35).
FIGURE 4.
OSS-induced activation of the Akt/mTOR/p70S6K pathway is mediated by αvβ3 and β1 integrins through FAK and Shc. MG63 cells were pretreated with specific antibodies (10 μg/ml) against αvβ3 (anti-αvβ3) or β1 (anti-β1) for 1 h (A) or transfected with specific siRNA (40 nm for each) of β3 (siβ3) or β1 (siβ1) integrin (B), dominant-negative mutant FAK(F397Y) (C), Shc-SH2 (D), or FAK-wt or Shc-wt (E) (4 μg for each) for 48 h. As controls, the cells were pretreated with nonspecific control IgG (A) or transfected with control siRNA (siCL) (B) or empty vector pcDNA3 (C–E). The cells were kept under static conditions (lanes C) or subjected to OSS (lanes S) for 1 h, and the phosphorylation of FAK, AKT, mTOR, and p70S6K was determined by Western blot analysis. The data are presented as the amounts (band densities normalized to the total protein levels) of phosphorylated FAK, Akt, mTOR, or p70S6K proteins relative to those in static control cells pretreated with control IgG (A) or transfected with siCL (B) or pcDNA3 empty vector (C–E) and are shown as the means ± S.E. from three independent experiments. *, p < 0.05 versus static control cells. #, p < 0.05 versus sheared cells pretreated with control IgG (A) or transfected with siCL (B) or pcDNA3 (C–E).
We further investigated whether FAK and Shc are upstream of the shear-induced Akt/mTOR/p70S6K signaling events. MG63 cells were transfected with a dominant-negative mutant of FAK (i.e. FAK(F397Y)) or Shc (i.e. Shc-SH2), or a wild type FAK (FAK-wt) or Shc (Shc-wt) and then were either kept as static controls or subjected to OSS for 1 h. Transfections with FAK(F397Y) (Fig. 4C), Shc-SH2 (Fig. 4D), and FAK-wt or Shc-wt (Fig. 4E) (compared with control pcDNA3) slightly increased the basal levels of phosphorylation of mTOR and p70S6K in static control cells. Following the exposure to OSS, the cells transfected with FAK(F397Y) and Shc-SH2 (compared with control pcDNA3) showed lower levels of Akt, mTOR, and p70S6K phosphorylation in comparison with the static control cells (Fig. 4, C and D). In contrast, MG63 cells transfected with FAK-wt or Shc-wt had higher levels of Akt, mTOR, and p70S6K phosphorylation in response to OSS when compared with control pcDNA3 (Fig. 4E) and also with FAK(F397Y) and Shc-SH2 transfections (Fig. 4, C and D).
FAK Is Required for OSS Activation of αvβ3 and β1 Integrins in MG63 Cells
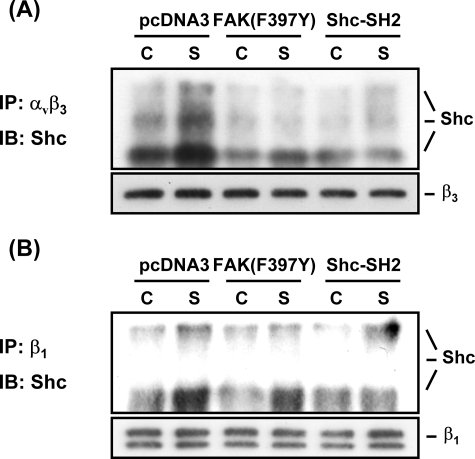
MG63 cells were transfected with FAK(F397Y) and then exposed to OSS for 1 h, and the association of αvβ3 and β1 integrins with Shc were examined by immunoprecipitating the cell extracts with an antibody against αvβ3 or β1 integrin, followed by Western blot analysis with an antibody against Shc. Transfecting MG63 cells with FAK(F397Y) (compared with control pcDNA3) resulted in inhibitions of the OSS-induced activation of αvβ3 (Fig. 5A) and β1 (Fig. 5B) integrins, as indicated by the decreased levels of shear-induced association of Shc with these integrins. Transfection of MG63 cells with Shc-SH2 (compared with control pcDNA3) totally abolished the OSS-induced increases in αvβ3/Shc and β1/Shc association. These results suggest that FAK and Shc are required for the OSS-induced activation of αvβ3 and β1 integrins in MG63 cells.
FIGURE 5.
FAK is required for OSS-induced activation of αvβ3 and β1 integrins in MG63 cells. MG63 cells were transfected with FAK(F397Y), Shc-SH2, or pcDNA3 (4 μg for each) for 48 h and then kept under static conditions (lanes C) or subjected to OSS (lanes S) for 1 h. The extracts of these cells were immunoprecipitated with an antibody against αvβ3 (A) or β1 (B) integrin, followed by Western blot analysis with an antibody against Shc. The results are representative of duplicate experiments with similar results. IP, immunoprecipitation; IB, immunoblot.
FAK and Shc Play Synergistic Roles to Form Heteromeric Complexes with PI3K in MG63 Cells in Response to OSS
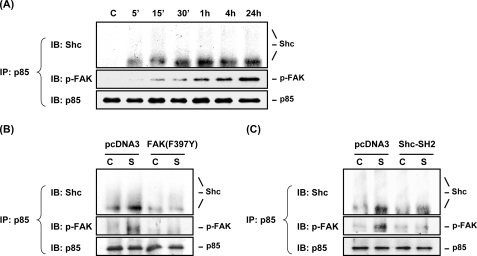
PI3K is a heterodimer phospholipid kinase consisting of a 110-kDa catalytic subunit (p110) and a 85-kDa regulatory subunit (p85), which has been shown to associate with activated tyrosine kinases, including integrin-associated FAK and Shc (36, 37). We investigated whether FAK and Shc play synergistic roles in cooperation with PI3K to form FAK·Shc·PI3K complexes in MG63 cells in response to OSS. The results of co-immunoprecipitation assays showed that the association of p85 with Shc and phospho-FAK in MG63 cells increased rapidly within 5 min after exposure to flow (Fig. 6A). These increases in association of p85 with phosphorylated FAK and Shc were sustained 24 h after the onset of flow. Only the low molecular weight isoform of Shc (i.e. p46), but not the other two isoforms (i.e. p52 and p66), could associate with p85 in MG63 cells in response to OSS. Incubation of MG63 cell under static conditions did not alter the basal levels of these molecule association over the 24-h period tested (supplemental Fig. S1C). Transfection of MG63 cells with the dominant-negative mutant of either FAK (FAK(F397Y)) (Fig. 6B) or Shc (Shc-SH2) (Fig. 6C) (compared with control pcDNA3) abolished the OSS-induced association of p85 with both phosphorylated FAK and Shc. These results suggest that FAK and Shc play synergistic roles in the OSS-induced formation of complexes in MG63 cells. Thus, FAK and Shc may be cooperative in forming heteromeric complexes with PI3K in mediating the PI3K-eliciting signaling cascades in MG63 cells in response to OSS.
FIGURE 6.
FAK and Shc are cooperative in forming heteromeric complexes with PI3K in MG63 cells in response to OSS. A, MG63 cells were kept under static conditions as controls (lane C) or subjected to OSS for the indicated times. B and C, MG63 cells were transfected with FAK(F397Y) (B) or Shc-SH2 (C), or pcDNA3 empty vector (4 μg for each) for 48 h and then were kept as controls (lanes C) or subjected to OSS (lanes S) for 1 h. The association of p85 with Shc and phosphorylated FAK was determined by using co-immunoprecipitation assay. The results showed that only the low molecular weight isoform of Shc (i.e. p46), but not the other two isoforms (i.e. p52 and p66), can associate with p85 in MG63 cells in response to OSS. The blots are representative of three independent experiments with similar results. IP, immunoprecipitation; IB, immunoblot.
OSS-induced MG63 Cell Proliferation Is Mediated by αvβ3 and β1 Integrins through FAK and Shc
BrdUrd incorporation assays showed that for the cells transfected with control siRNA or pcDNA3 empty vector, OSS induced increases in the percentage of cells in the S phase as compared with static controls (Table 2). For the cells exposed to OSS for 24 h, transfections with specific siRNA of either β1 or β3 integrin or dominant-negative mutant of FAK or Shc caused decreases in the percentage of cells in the S phase in comparison with transfections with control siRNA or pcDNA3, respectively (Table 2). These results are in concert with the findings above that OSS-induced MG63 cell proliferation is mediated by αvβ3 and β1 integrins through FAK and Shc.
TABLE 2.
Oscillatory shear-induced MG63 cell proliferation is mediated by integrins through FAK and Shc
MG63 cells were transfected with control siRNA (siCL) or specific siRNA of β1 (siβ1) or β3 (siβ3) integrin for 48 h and then were kept under static conditions or exposed to oscillatory shear stress for 24 h. In other experiments, the cells were transfected with pcDNA3 or a dominant-negative mutant of FAK (FAK[F397Y]) or Shc (Shc-SH2). The cells were fixed and stained with an anti-BrdUrd antibody, with propidium iodide stain as a counterstain for the nucleus, as described under “Experimental Procedures.” The data are the means ± S.E. from three independent experiments.
| Percentage of BrdUrd-positive cells |
||
|---|---|---|
| Static | Oscillatory shear stress | |
| siCL | 19.4 ± 1.4 | 33.5 ± 2.1a |
| siβ 1 | 17.5 ± 1.1 | 20.6 ± 0.8b |
| siβ 3 | 16.4 ± 0.8 | 21.2 ± 2.0b |
| pcDNA3 | 13.4 ± 1.0 | 22.2 ± 2.0a |
| FAK(F397Y) | 11.7 ± 2.0 | 12.9 ± 1.5b |
| Shc-SH2 | 13.7 ± 1.1 | 11.8 ± 0.4b |
a p < 0.05 vs. static control cells receiving the same treatment.
b p < 0.05 vs. sheared cells transfected with control siRNA or pcDNA3.
OSS Induces Changes in Cell Cycle Regulatory Protein Expression and c-fos, Egr-1, and Cox-2 Gene Expression in MG63 Cells through the mTOR/p70S6K Pathway
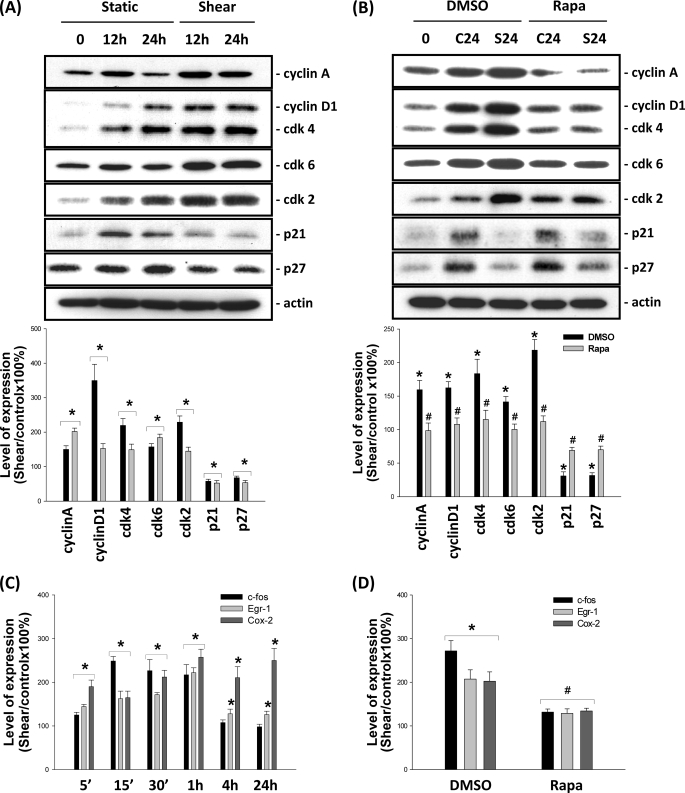
We further investigated whether OSS-induced MG63 cell proliferation is accompanied by corresponding changes in cell cycle regulatory protein and bone formation-related gene expression. As shown in Fig. 7A, incubation of MG63 cells under static conditions for 12 or 24 h resulted in slight increases in the expression of cyclins A and D1, Cdk-2, -4, and -6, p21CIP1, and p27KIP1 in these cells. Exposure of MG63 cells to OSS resulted in increased expression of cyclins A and D1 and Cdk-2, -4, and -6, and decreased expression of p21CIP1 and p27KIP1 as compared with the static control cells at the corresponding time points. These OSS-induced changes in cell cycle regulatory protein expression were inhibited by pretreating MG63 cells with rapamycin (10 nm) (Fig. 7B), indicating that the mTOR/p70S6K pathway is required for OSS modulation of cell cycle regulatory protein expression.
FIGURE 7.
OSS induced changes in cell cycle regulatory protein expression and c-fos, Egr-1, and Cox-2 gene expression through the mTOR/p70S6K pathway. A, MG63 cells were kept under static conditions as controls or subjected to OSS for 12 h and 24 h. The expression of cell cycle regulatory proteins was determined by Western blot analysis. B, MG63 cells were pretreated with vehicle control DMSO or rapamycin (Rapa, 10 nm) for 1 h and then kept under static conditions as controls (lanes C) or subjected to OSS (lanes S) for 24 h. The expression of cell cycle regulatory proteins was determined by Western blot analysis. C, MG63 cells were kept as controls or subjected to OSS for the indicated times, and their mRNA expression of c-fos, Egr-1, and Cox-2 was examined by using reverse transcription-PCR analysis. D, MG63 cells were pretreated with vehicle control (DMSO) or rapamycin (10 nm) for 1 h before and during exposure to OSS for 15 min, and the mRNA expression of c-fos, Egr-1, and Cox-2 was examined by using reverse transcription-PCR analysis. The data are shown as the means ± S.E. from three independent experiments. *, p < 0.05 versus static control cells at corresponding time points. #, p < 0.05 versus sheared cells pretreated with vehicle control DMSO.
As shown in Fig. 7C, OSS caused rapid induction (significant within 5 min) of expression of c-fos, Egr-1, and Cox-2 mRNAs. At 24 h of flow application, these increased levels of mRNAs were sustained for Egr-1 and Cox-2 but declined for c-fos to the basal level. Pretreatments of MG63 cells with rapamycin (10 nm) inhibited these OSS inductions of c-fos, Egr-1, and Cox-2, suggesting the importance of mTOR/p70S6K in modulating bone formation-related gene expression in osteoblast-like cells in response to OSS (Fig. 7D).
OSS Activates ERK1/2, Which Are Involved in OSS-induced Activation of mTOR/p70S6K and Proliferation in MG63 Cells
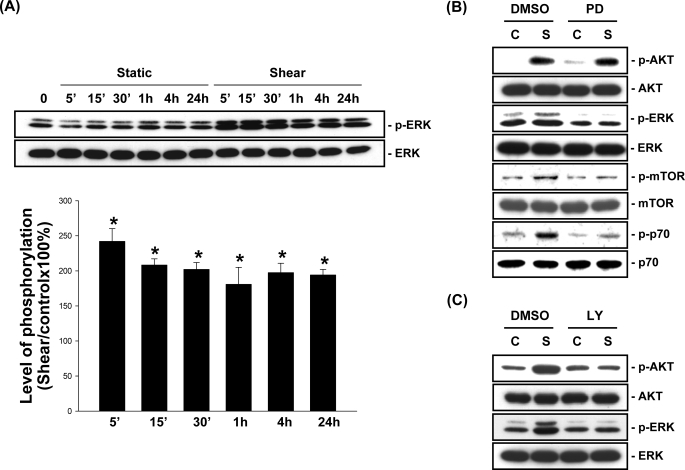
Application of OSS to MG63 cells induced sustained increases in ERK1/2 phosphorylation in these cells (Fig. 8A). Pretreating MG63 cells with PD98059 (30 μm), a specific inhibitor of MEK1 that is upstream of ERK1/2, inhibited the OSS-induced phosphorylation of ERK1/2, mTOR, and p70S6K, but not Akt (Fig. 8B). In contrast, pretreating MG63 cells with the specific PI3K inhibitor LY294002 (10 μm) inhibited the OSS-induced Akt and ERK1/2 phosphorylation (Fig. 8C). These results suggest that PI3K is required for the activation of both Akt and ERK in MG63 cells in response to OSS. BrdUrd incorporation assays showed that the OSS-induced increase in the percentage of cells in the S phase was inhibited by pretreating the cells with PD98059, as compared with the untreated cells. For static control cells, pretreatment with PD98059 also caused a decrease in the percentage of cells in the S phase compared with the untreated cells, suggesting that ERK is involved in the modulation of MG63 cell proliferation under static conditions as well as in response to OSS.
FIGURE 8.
ERK is required for OSS activation of mTOR/p70S6K in MG63 cells. A, MG63 cells were kept under static conditions as controls or subjected to OSS for the indicated times, and their phosphorylation of ERK1/2 was determined by Western blot analysis. The data are presented as the amounts (band densities normalized to the total protein levels) of phosphorylated ERK1/2 proteins relative to those in static control cells and are shown as the means ± S.E. from three independent experiments. *, p < 0.05 versus static control cells at corresponding time points. B and C, MG63 cells were pretreated with vehicle control DMSO or a specific inhibitor for MEK (PD98059, 30 μm) (B) or PI3K (LY294002, 10 μm) (C) for 1 h before and during exposure to OSS. The cells were kept under static conditions as controls (lanes C) or subjected to OSS (lanes S) for 1 h, and the phosphorylation of Akt, ERK1/2, mTOR, or p70S6K was determined by Western blot analysis. The results are representative of three independent experiments with similar results.
DISCUSSION
In the present study, we have characterized a novel mechanism (summarized in Fig. 9) by which OSS induces osteoblast-like MG63 cell proliferation through the integrin-mediated synergistic association of FAK and Shc with PI3K and the activation of downstream ERK and Akt/mTOR/p70S6K pathways. Several lines of evidence support this conclusion. First, OSS induced sustained phosphorylation of Akt, mTOR, and p70S6K, as well as ERK1/2, in MG63 cells; the OSS-induced phosphorylation of all these signaling molecules was inhibited by the specific PI3K inhibitor LY294002, and the phosphorylation of mTOR and p70S6K was inhibited by the specific mTOR inhibitor rapamycin and the MEK1 inhibitor PD98059. Second, OSS induced sustained increases in the association of αvβ3 and β1 integrins with Shc and FAK and the phosphorylation of FAK; blockage of these integrins with their specific antibodies or their siRNAs inhibited the OSS-induced phosphorylation of FAK, Akt, mTOR, and p70S6K. Third, OSS induced increases in association of FAK and Shc with p85, which were inhibited by transfecting MG63 cells with a dominant-negative mutant of either FAK or Shc, indicating that FAK and Shc play synergistic roles in the formation of FAK·Shc·p85 complexes in response to OSS. Finally, OSS induced MG63 cell proliferation, which was accompanied by the increased expression of cyclins A and D1, Cdk-2, -4, and -6, and bone formation-related genes Egr-1, c-fos, and Cox-2 and the decreased expression of p21CIP1 and p27KIP1. These OSS-induced responses of signaling molecules and genes were inhibited by pretreating the cells with rapamycin. In addition, the OSS-induced MG63 cell proliferation was inhibited by pretreatments with LY294002 or rapamycin and transfections with specific siRNAs of β3 or β1 integrin, as well as dominant-negative mutants of FAK or Shc.
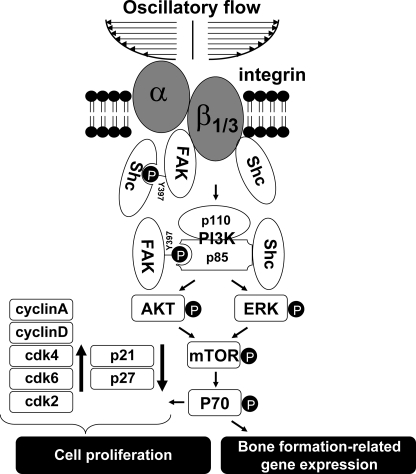
FIGURE 9.
Schematic representation of the signaling pathways regulating osteoblast-like cells proliferation in response to OSS. OSS induces proliferation in osteoblast-like cells through activation of the PI3K/Akt/mTOR/p70S6K pathway, which is mediated by αvβ3 and β1 integrins through their association with FAK and Shc, which play synergistic roles to cooperate with PI3K subunit p85 to form FAK·Shc·p85 heteromeric complexes. Shc may interact with αvβ3 and β1 integrins in response to OSS by direct association with these integrins using the SH2 domain or via the association with FAK through the tyrosine 397 residue of FAK. Thus, FAK is required for maximal activation of αvβ3 and β1 integrins (using integrin/Shc association as readouts) in response to OSS. In addition to Akt, ERK is also downstream from PI3K and required for the OSS activation of mTOR/p70S6K, which in turn are required for the OSS modulation of cell cycle regulatory protein expression and induction of bone formation-related genes.
The PI3K/Akt/mTOR/p70S6K pathway has been shown to play important roles in the modulation of osteoblast functions in response to chemical stimuli, including bone morphogenic protein-7 and insulin-like growth factor-1 (21, 22, 39–41). However, the role of this signaling pathway in modulating osteoblast signaling, gene expression, and functions in response to mechanical stimuli remains unclear. Our present study demonstrated for the first time that OSS induces sustained phosphorylation of Akt, mTOR, and p70S6K in osteoblast-like MG63 cells, with Akt being upstream from mTOR/p70S6K. Pretreatment of MG63 cells with the specific PI3K inhibitor LY294002 and the mTOR inhibitor rapamycin inhibited the shear-induced increase in the percentage of cells in the S phase, indicating the importance of the Akt/mTOR/p70S6K pathway in modulating the proliferation of osteoblast-like cells in response to OSS.
Integrins have been implicated as mechanosensors in many types of cells, including osteocytes and osteoblasts, seeded on extracellular matrix (5, 6, 8, 42, 43). Mechanical stretch prevents osteocytes against apoptosis, which involves integrins, cytoskeletal molecules, and catalytic kinases such as Src (43). Recent studies demonstrated that αvβ3 and β1 integrins are required for osteoblast survival and proliferation (44, 45), and these integrins have been shown to be activated by steady fluid shear stress in different cell types, including osteoblast-like MC3T3-E1 and MG63 cells (8, 12). Because integrin receptors do not possess catalytic activity, the signaling induced by integrin activation must be transmitted into cells through the activation of integrin-associated proteins such as FAK and Shc (24, 46, 47). The tyrosine 397 residue in FAK has been shown to serve as a binding site (YpAEI motif) for the SH2 domain of several signaling and adaptor molecules such as Shc, whose association with integrins has been recognized as a readout of integrin activation (24, 46, 47). Our results using co-immunoprecipitation assays demonstrated that OSS induces sustained activation of αvβ3 and β1 integrins in osteoblast-like MG63 cells, as indicated by their sustained association with FAK and Shc. An interesting finding of the current study is that transfection of MG63 cells with a dominant-negative mutant FAK(F397Y) reduced the OSS-induced activation of αvβ3 and β1 integrins, using their association with Shc as readouts (Fig. 5). In addition, transfection with Shc-SH2 can totally abolish the OSS-induced increases in αvβ3/Shc and β1/Shc association. Thus, it is probable that Shc may interact with αvβ3 and β1 integrins in response to OSS with two possible routes; one is via the direct association of its SH2 domain with the integrins, and the other is via an indirect association through the tyrosine 397 residue of FAK (Fig. 9). The tyrosine 397 residue of FAK and the SH2 domain of Shc are critical for the integrin-mediated signaling in MG63 cells in response to OSS, inasmuch as dominant-negative mutants FAK(F397Y) and Shc-SH2 inhibited the activation of Akt/mTOR/p70S6K and the increase in cell proliferation induced by OSS. Our results suggest that FAK and Shc play critical roles in transducing integrin-mediated signaling in osteoblasts and their functional modulation in response to mechanical forces.
The p85 adaptor protein of PI3K has been shown to employ the inter-SH2 region to bind the p110 catalytic subunit and also using the SH3 and SH2 domains, phosphotyrosine residues, and proline-rich motifs to interact with activated tyrosine kinases (e.g. BCR/Abl and NPM/ALK) and adaptor proteins (e.g. Shc, c-Cbl, Crk, and Gab2); the adaptor proteins enhance the association of PI3K with activated tyrosine kinases (36, 48–50). Wymann and Pirola (50) showed that in BCR/Abl transformed cells, the SH3 domain of p85 can associate with the N-terminal proline-rich region of Shc, which supports the association of p85 with tyrosine-phosphorylated Shc via the C-terminal SH2 domain of p85. Akagi et al. (37) showed that the N-terminal SH2 domain of p85 can associate with tyrosine 397-phosphorylated FAK in Crk-transformed fibroblasts. These results suggest that PI3K can interact with activated FAK and Shc via different regulatory domains. However, whether FAK and Shc play synergistic roles in the interaction with PI3K had not been established. In the present study, we found that transfection of cells with a dominant-negative mutant of either FAK (i.e. FAK(F397Y)) or Shc (i.e. Shc-SH2) totally abolished the association of p85 with both FAK and Shc, suggesting that FAK and Shc may play synergistic roles in cooperation with PI3K to form the FAK·Shc·PI3K heteromeric complexes in response to OSS. These results also indicate that the tyrosine 397 residue of FAK and the SH2 domain of Shc are critical for this OSS-induced formation of FAK·Shc·PI3K heteromeric complexes in osteoblast-like cells.
ERK has been shown to be required for the steady fluid flow-induced mitogenic response in osteoblastic cells (10, 11). Our results on the OSS activation of ERK1/2 and the PD98059 inhibition of OSS-induced increases in the percentage of MG63 cells in the S phase suggest that ERK is involved in OSS-induced proliferation in osteoblast-like cells. In the present study, pretreatment of MG63 cells with the specific PI3K inhibitor LY294002 inhibits OSS-induced Akt and ERK1/2 activation, whereas the specific MEK1 inhibitor PD98059 did not exert an inhibitory effect on OSS-induced Akt activation. These results suggest that ERK and Akt may represent parallel signaling pathways downstream from PI3K. This is consistent with the result by Gayer et al. (51), who showed that mechanical strain induces PI3K activation, which results in independent activation of ERK and Akt, thereby exerting mitogenic effects on intestinal epithelial cells. Our results showed that pretreatment of MG63 cells with PD98059 and LY294002 results in inhibitions in OSS-induced mTOR and p70S6K activation, suggesting that the Akt and ERK signaling pathways may converge to the mTOR/p70S6K pathway in MG63 cells in response to OSS.
The mechanisms underlying MG63 cell proliferation under static conditions and in response to OSS may be different. In the present study, we found that treatment of MG63 cells with PD98058 under static conditions results in significant inhibitions in the percentage of cells in the S phase compared with the untreated cells. In contrast, LY294002 did not show this inhibitory effect under static conditions. Thus, it is probable that ERK, rather than Akt, plays a major role in the modulation of MG63 cell proliferation under static conditions.
The proliferation of eukaryotic cells depends on their progression through the cell cycle, which is controlled by its regulatory proteins, including Cdks and their regulatory subunits cyclins, as well as inhibitors such as p21CIP1 and p27KIP1 (52–55). In cell cycle progression, increasing accumulations of cyclin D·Cdk4/6 and cyclin A·Cdk2 complexes regulate the transition through the G1 and S phases. In the present study, we found that application of OSS to MG63 cells induces increased expression of cyclins A and D1 and Cdk-2, -4, and -6 and decreased expression of p21CIP1 and p27KIP1, confirming that OSS may serve as a positive regulator for cell cycle progression and proliferation in osteoblast-like cells. These OSS-induced changes in cell cycle regulatory protein expression in MG63 cells were inhibited by pretreating the cells with rapamycin, confirming the importance of mTOR/p70S6K in modulating OSS-induced cell cycle progression and proliferation in osteoblast-like cells.
Although most of the previous studies on flow-induced mechanotransduction in bone cells used steady fluid flow as an experimental model (8, 10–12), our present work studied oscillatory flow, which can mimic more closely the physical microenvironment of bone cells in vivo. Both the steady flow used in our previous study (8) and the oscillatory flow used in the present study caused the induction of bone formation-related genes c-fos, Egr-1, and Cox-2 in MG63 cells, but there were significant differences in the magnitudes and durations in the induction of these genes by the two flow patterns. For example, steady shear induced a sustained increase (24 h) in c-fos expression in MG63 cells (8), whereas oscillatory shear only induced a transient induction (1 h) of this gene (Fig. 7C). Because it has been shown that c-fos plays important roles in mitogenic responses of osteoblastic cells (38, 56, 57) and is involved in bone formation or remodeling induced by mechanical loading (15), our new finding of the differential responses to oscillatory versus steady shearing may have significant implications in mechanotransduction in osteoblast-like cells.
In summary, the present study demonstrated for the first time that OSS induces proliferation in osteoblast-like cells through the PI3K/Akt/mTOR/p70S6K pathway. These OSS-induced signaling and cell proliferation are mediated by αvβ3 and β1 integrins through their association with FAK and Shc, which play synergistic roles to cooperate with PI3K subunit p85 to form FAK·Shc·p85 complexes. The identification of this mechanotransduction mechanism in osteoblast-like cells in response to OSS not only provides new insights into the mechanism of mechanical loading-induced bone formation but also aids in future development of bone tissue engineering and therapeutic strategies to increase bone formation.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL080518 and HL085159 (to S. C.). This work was also supported by National Health Research Institutes (Taiwan) Grant ME-097-PP-06 and National Science Council (Taiwan) Grants 97-3112-B-400-006 and 96-2628-B-400-002-MY3 (to J.-J. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- OSS
- oscillatory shear stress
- FAK
- focal adhesion kinase
- PI3K
- phosphatidylinositol 3-kinase
- Cdk
- cyclin-dependent protein kinase
- ERK
- extracellular signal-regulated kinase
- SH
- Src homology
- siRNA
- small interfering RNA
- wt
- wild type
- BrdUrd
- bromodeoxyuridine
- MEK
- mitogen-activated protein kinase/ERK kinase
- DMSO
- dimethyl sulfoxide.
REFERENCES
- 1.Chow J. W., Wilson A. J., Chambers T. J., Fox S. W. (1998) J. Bone Miner. Res. 13, 1760–1767 [DOI] [PubMed] [Google Scholar]
- 2.Forwood M. R., Owan I., Takano Y., Turner C. H. (1996) Am. J. Physiol. Endocrinol. Metab. 270, E419–E423 [DOI] [PubMed] [Google Scholar]
- 3.Hillam R. A., Skerry T. M. (1995) J. Bone Miner. Res. 10, 683–689 [DOI] [PubMed] [Google Scholar]
- 4.Sikavitsas V. I., Temenoff J. S., Mikos A. G. (2001) Biomaterials 22, 2581–2593 [DOI] [PubMed] [Google Scholar]
- 5.Rubin J., Rubin C., Jacobs C. R. (2006) Gene 367, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner C. H., Pavalko F. M. (1998) J. Orthop. Sci. 3, 346–355 [DOI] [PubMed] [Google Scholar]
- 7.Jiang G. L., White C. R., Stevens H. Y., Frangos J. A. (2002) Am. J. Physiol. Endocrinol. Metab. 283, E383–E389 [DOI] [PubMed] [Google Scholar]
- 8.Lee D. Y., Yeh C. R., Chang S. F., Lee P. L., Chien S., Cheng C. K., Chiu J. J. (2008) J. Bone Miner. Res. 23, 1140–1149 [DOI] [PubMed] [Google Scholar]
- 9.Young S. R., Gerard-O'Riley R., Kim J. B., Pavalko F. M. (2009) J. Bone Miner. Res. 24, 411–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapur S., Baylink D. J., Lau K. H. (2003) Bone 32, 241–251 [DOI] [PubMed] [Google Scholar]
- 11.Kapur S., Chen S. T., Baylink D. J., Lau K. H. (2004) Bone 35, 525–534 [DOI] [PubMed] [Google Scholar]
- 12.Pavalko F. M., Chen N. X., Turner C. H., Burr D. B., Atkinson S., Hsieh Y. F., Qiu J., Duncan R. L. (1998) Am. J. Physiol. Cell Physiol. 275, C1591–C1601 [PubMed] [Google Scholar]
- 13.Ogata T. (1997) J. Cell Physiol. 170, 27–34 [DOI] [PubMed] [Google Scholar]
- 14.McMahon A. P., Champion J. E., McMahon J. A., Sukhatme V. P. (1990) Development 108, 281–287 [DOI] [PubMed] [Google Scholar]
- 15.Turner C. H., Tu Y., Onyia J. E. (1996) Ann. Biomed. Eng. 24, S74 [Google Scholar]
- 16.Forwood M. R. (1996) J. Bone Miner. Res. 11, 1688–1693 [DOI] [PubMed] [Google Scholar]
- 17.Morinobu M., Ishijima M., Rittling S. R., Tsuji K., Yamamoto H., Nifuji A., Denhardt D. T., Noda M. (2003) J. Bone Miner. Res. 18, 1706–1715 [DOI] [PubMed] [Google Scholar]
- 18.McAllister T. N., Frangos J. A. (1999) J. Bone Miner. Res. 14, 930–936 [DOI] [PubMed] [Google Scholar]
- 19.Klein-Nulend J., Semeins C. M., Ajubi N. E., Nijweide P. J., Burger E. H. (1995) Biochem. Biophys. Res. Commun. 217, 640–648 [DOI] [PubMed] [Google Scholar]
- 20.Bakker A. D., Soejima K., Klein-Nulend J., Burger E. H. (2001) J. Biomech. 34, 671–677 [DOI] [PubMed] [Google Scholar]
- 21.Thomas G., Hall M. N. (1997) Curr. Opin. Cell Biol. 9, 782–787 [DOI] [PubMed] [Google Scholar]
- 22.Singha U. K., Jiang Y., Yu S., Luo M., Lu Y., Zhang J., Xiao G. (2008) J. Cell Biochem. 103, 434–446 [DOI] [PubMed] [Google Scholar]
- 23.Giancotti F. G., Ruoslahti E. (1999) Science 285, 1028–1032 [DOI] [PubMed] [Google Scholar]
- 24.Shyy J. Y., Chien S. (2002) Circ. Res. 91, 769–775 [DOI] [PubMed] [Google Scholar]
- 25.Clover J., Gowen M. (1994) Bone 15, 585–591 [DOI] [PubMed] [Google Scholar]
- 26.Li S., Kim M., Hu Y. L., Jalali S., Schlaepfer D. D., Hunter T., Chien S., Shyy J. Y. (1997) J. Biol. Chem. 272, 30455–30462 [DOI] [PubMed] [Google Scholar]
- 27.Chen K. D., Li Y. S., Kim M., Li S., Yuan S., Chien S., Shyy J. Y. (1999) J. Biol. Chem. 274, 18393–18400 [DOI] [PubMed] [Google Scholar]
- 28.Hillsley M. V., Frangos J. A. (1997) Calcif. Tissue Int. 60, 48–53 [DOI] [PubMed] [Google Scholar]
- 29.Wu C. C., Li Y. S., Haga J. H., Wang N., Lian I. Y., Su F. C., Usami S., Chien S. (2006) J. Cell Biochem. 98, 632–641 [DOI] [PubMed] [Google Scholar]
- 30.Weinbaum S., Cowin S. C., Zeng Y. (1994) J. Biomech. 27, 339–360 [DOI] [PubMed] [Google Scholar]
- 31.Miltenburger H. G., Sachse G., Schliermann M. (1987) Dev. Biol. Stand. 66, 91–99 [PubMed] [Google Scholar]
- 32.Wary K. K., Mainiero F., Isakoff S. J., Marcantonio E. E., Giancotti F. G. (1996) Cell 87, 733–743 [DOI] [PubMed] [Google Scholar]
- 33.Jalali S., del Pozo M. A., Chen K., Miao H., Li Y., Schwartz M. A., Shyy J. Y., Chien S. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1042–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyamoto S., Akiyama S. K., Yamada K. M. (1995) Science 267, 883–885 [DOI] [PubMed] [Google Scholar]
- 35.Chang S. F., Chang C. A., Lee D. Y., Lee P. L., Yeh Y. M., Yeh C. R., Cheng C. K., Chien S., Chiu J. J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 3927–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren S. Y., Bolton E., Mohi M. G., Morrione A., Neel B. G., Skorski T. (2005) Mol. Cell Biol. 25, 8001–8008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akagi T., Murata K., Shishido T., Hanafusa H. (2002) Mol. Cell Biol. 22, 7015–7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chattopadhyay N., Yano S., Tfelt-Hansen J., Rooney P., Kanuparthi D., Bandyopadhyay S., Ren X., Terwilliger E., Brown E. M. (2004) Endocrinology 145, 3451–3462 [DOI] [PubMed] [Google Scholar]
- 39.Harris T. E., Lawrence J. C., Jr. (2003) Sci. STKE 2003, re15. [DOI] [PubMed] [Google Scholar]
- 40.Kozawa O., Matsuno H., Uematsu T. (2001) J. Cell Biochem. 31, 430–436 [DOI] [PubMed] [Google Scholar]
- 41.Shoba L. N., Lee J. C. (2003) J. Cell Biochem. 88, 1247–1255 [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., McNamara L. M., Schaffler M. B., Weinbaum S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 15941–15946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plotkin L. I., Mathov I., Aguirre J. I., Parfitt A. M., Manolagas S. C., Bellido T. (2005) Am. J. Physiol. Cell Physiol. 289, C633–C643 [DOI] [PubMed] [Google Scholar]
- 44.Globus R. K., Doty S. B., Lull J. C., Holmuhamedov E., Humphries M. J., Damsky C. H. (1998) J. Cell Sci. 111, 1385–1393 [DOI] [PubMed] [Google Scholar]
- 45.Scarlett A., Parsons M. P., Hanson P. L., Sidhu K. K., Milligan T. P., Burrin J. M. (2008) J. Endocrinol. 196, 509–517 [DOI] [PubMed] [Google Scholar]
- 46.Schlaepfer D. D., Hunter T. (1998) Trends Cell Biol. 8, 151–157 [DOI] [PubMed] [Google Scholar]
- 47.Schlaepfer D. D., Hauck C. R., Sieg D. J. (1999) Prog. Biophys. Mol. Biol. 71, 435–478 [DOI] [PubMed] [Google Scholar]
- 48.Hellyer N. J., Cheng K., Koland J. G. (1998) Biochem. J. 333, 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGlade C. J., Ellis C., Reedijk M., Anderson D., Mbamalu G., Reith A. D., Panayotou G., End P., Bernstein A., Kazlauskas A. (1992) Mol. Cell Biol. 12, 991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wymann M. P., Pirola L. (1998) Biochim. Biophys. Acta 1436, 127–150 [DOI] [PubMed] [Google Scholar]
- 51.Gayer C. P., Chaturvedi L. S., Wang S., Craig D. H., Flanigan T., Basson M. D. (2009) J. Biol. Chem. 284, 2001–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherr C. J. (1993) Cell 73, 1059–1065 [DOI] [PubMed] [Google Scholar]
- 53.Hunter T., Pines J. (1994) Cell 79, 573–582 [DOI] [PubMed] [Google Scholar]
- 54.Sherr C. J., Roberts J. M. (1995) Genes Dev. 9, 1149–1163 [DOI] [PubMed] [Google Scholar]
- 55.Toyoshima H., Hunter T. (1994) Cell 78, 67–74 [DOI] [PubMed] [Google Scholar]
- 56.Glantschnig H., Varga F., Klaushofer K. (1996) Endocrinology 137, 4536–4541 [DOI] [PubMed] [Google Scholar]
- 57.Kousteni S., Han L., Chen J. R., Almeida M., Plotkin L. I., Bellido T., Manolagas S. C. (2003) J. Clin. Invest. 111, 1651–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.