Abstract
The islet in type 2 diabetes (T2DM) and the brain in neurodegenerative diseases share progressive cell dysfunction, increased apoptosis, and accumulation of locally expressed amyloidogenic proteins (islet amyloid polypeptide (IAPP) in T2DM). Excessive activation of the Ca2+-sensitive protease calpain-2 has been implicated as a mediator of oligomer-induced cell death and dysfunction in neurodegenerative diseases. To establish if human IAPP toxicity is mediated by a comparable mechanism, we overexpressed human IAPP in rat insulinoma cells and freshly isolated human islets. Pancreas was also obtained at autopsy from humans with T2DM and nondiabetic controls. We report that overexpression of human IAPP leads to the formation of toxic oligomers and increases beta cell apoptosis mediated by increased cytosolic Ca2+ and hyperactivation of calpain-2. Cleavage of α-spectrin, a marker of calpain hyperactivation, is increased in beta cells in T2DM. We conclude that overactivation of Ca2+-calpain pathways contributes to beta cell dysfunction and apoptosis in T2DM.
Introduction
Hyperglycemia in type 2 diabetes mellitus (T2DM)3 is due to impaired insulin secretion in the setting of relative insulin resistance (1). The islets of Langerhans in T2DM are characterized by a deficit in beta cells, increased beta cell apoptosis, and islet amyloid derived from islet amyloid polypeptide (IAPP), a 37-amino acid highly conserved peptide co-expressed and secreted with insulin by pancreatic beta cells (2, 3).
The pathology of the islet in T2DM and brain in neurodegenerative diseases such as Alzheimer disease share several parallels. In both, the loss of functional tissue is associated with deposition of a locally expressed protein with the potential to form amyloid fibrils (Alzheimer beta protein in Alzheimer disease and IAPP in T2DM) (2, 4). In both T2DM and Alzheimer disease, there has been a debate as to whether the amyloid deposits contribute to cell loss (the so-called amyloid hypothesis) or are secondary to the processes leading to cell loss. Evidence against a direct role of amyloid deposits on cell loss is the poor correlation between the extent of amyloid deposits and the severity of disease in both human and animal models (3, 5, 6). Moreover, preformed amyloid fibrils are not cytotoxic when applied to cells (7).
However, several lines of evidence are supportive of a role of cytotoxicity by amyloidogenic proteins. These include genetic predisposition in occasional families with mutations leading to increased amyloidogenicity of the amyloid protein (8) and reproduction of the disease phenotype in rodent models transgenic for the relevant human amyloidogenic protein (9). There is an increasing appreciation that the cytotoxic forms of amyloidogenic proteins are small nonfibrillar oligomers that may form in membranes and cause nonselective membrane permeability (7, 10, 11), the toxic oligomer hypothesis. Moreover, misfolding and aggregation of amyloidogenic proteins into toxic oligomers induce apoptosis through the mechanism of endoplasmic reticulum stress (ER stress) (12, 13).
The proximal molecular events that link formation of toxic oligomers and induction of ER stress are unknown. One plausible explanation is that local membrane instability caused by toxic oligomers permits unregulated Ca2+ surges from the ER or other intracellular Ca2+-enriched compartments. Toxic IAPP oligomers appear to form and act intracellularly (14) within the secretory pathway (15). Moreover, they escape the secretory pathway, apparently by disrupting intracellular membranes (15). Therefore, disturbance of the usually discrete cellular compartmentalization of Ca2+ is a logical candidate to link membrane-permeant toxic oligomers and induction of cellular dysfunction and, under more extreme circumstances, cell death. There is as much as a 10,000-fold concentration difference between cytoplasmic Ca2+ (100 nm) and ER lumen Ca2+ (0.5–1.0 mm) so that even modest disruption of the ER membrane integrity might activate aberrant cytoplasmic Ca2+-activated signaling pathways. Also, because a high ER Ca2+ concentration is required for appropriate ER function, ER membrane disturbance might be expected to exacerbate the ER dysfunction that permitted protein misfolding initially.
In support of the postulate that unregulated Ca2+ release from the ER to the cytoplasm might be a mediator of beta cell dysfunction and apoptosis in T2DM, it is well recognized that cytoplasmic Ca2+ overload is a ubiquitous cause of cell death in neurons, cardiomyocytes, and insulin-producing beta cells (16, 17). Effectors or executors of calcium overload include protease calpains, kinases/phosphatases, calmodulin, and calcineurin (18). Sustained hyperactivation of calpain is provoked in many pathological processes, including ischemia, traumatic injury, and neurodegenerative disorders such as Alzheimer disease (17, 19, 20).
Calcium-dependent protease calpains belong to the cysteine protease family that has previously been implicated in the pathophysiology of several inflammatory disorders, including myocardial reperfusion injury, cerebral ischemia/reperfusion, circulatory shock, and T2DM (17). Insulin-producing beta cells express several calpains, including calpain-10, μ-calpain (or calpain-1), and m-calpain (or calpain-2). Polymorphisms in calpain-10 are associated with the risk of developing T2DM in some ethnic groups (21). The ubiquitous μ-calpains and m-calpains are activated by micromolar and millimolar levels of calcium, respectively. Calpains mediate a variety of physiological functions such as cytoskeleton remodeling, vesicle trafficking. and membrane fusion (22).
This study was designed to test the hypothesis that IAPP-mediated ER stress-induced apoptosis is mediated in part through increased cytosolic Ca2+ and activation of Ca2+-dependent calpain. We report that overexpression of human IAPP in INS 832/13 cells and isolated human islets led to increased cytoplasmic Ca2+, activation of Ca2+-sensitive calpain-2. and beta cell apoptosis. Inhibition of calpain by calpeptin attenuated the toxicity of human IAPP. Also, we detected calpain-cleaved fragments of the cytoskeleton protein α-spectrin, a surrogate indicator of Ca2+-initiated and calpain-mediated cytotoxicity, in beta cells of humans with T2DM.
EXPERIMENTAL PROCEDURES
Human and Rodent Prepro-IAPP Adenovirus
Adenovirus generation and transduction were performed according to the procedure described by Huang et al. (13). Briefly, to generate human and rat prepro-IAPP adenovirus, KpnI and XhoI or EcoRI and EcoRV restriction sites, respectively, were introduced in the front of ATG and after the stop codon. A 290-bp human prepro-IAPP PCR fragment was digested with KpnI and XhoI, and a 300-bp rat prepro-IAPP PCR fragment was digested with EcoRI and EcoRV. The fragments were inserted into pENTR2B and subsequently into pAd/CMV/DEST adenovirus vector (Invitrogen). Recombinant adenoviruses expressing human and rat prepro-IAPP (hIAPP and rIAPP, respectively) were generated and purified according to the manufacturer's instructions (Clontech).
Cell Lines
Rat insulinoma cell line INS 832/13 was kindly provided by Dr. C. Newgard (Durham, NC) (23). Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 10 mm Hepes, 1 mm sodium pyruvate, 100 units/ml penicillin, 100 μg/ml streptomycin (Invitrogen), and 50 μm 2-mercaptoethanol (Sigma) in a 37 °C 5% CO2 tissue culture incubator. Rat insulinoma cells (RIN) that express a low level of endogenous IAPP were used to establish the dose-response relationship between the m.o.i. of adenovirus-expressing hIAPP or rIAPP (Fig. 1, A and B) and protein expression levels. We chose to use 400 m.o.i. for all experiments with INS 832/13 cells. The cells were transduced with adenoviruses expressing human or rodent IAPP and then 48 or 72 h later were washed with PBS and either fixed for immunocytochemistry or lysed by boiling in Laemmli sample buffer for immunoblotting. Protein concentrations were determined using the DC protein assay (Bio-Rad). Calpeptin and BAPTA-AM were from Calbiochem.
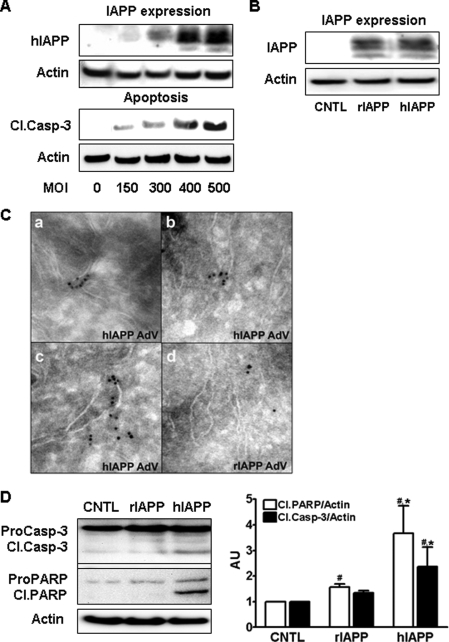
FIGURE 1.
Overexpression of hIAPP induces apoptosis in beta cells. A, there was an m.o.i.-dependent increase in IAPP expression and apoptosis (cleaved caspase-3 (Cl.Casp-3) in rat insulinoma cells (RIN) transduced with hIAPP adenovirus at different m.o.i. for 48 h. B, in RIN cells, IAPP overexpression at 400 m.o.i. was comparable for human and rodent IAPP. Cntl, control. C, toxic IAPP oligomers were detected by cryo-immunogold labeling (A11 antibody) in INS 832/13 cells overexpressing hIAPP (panels a–c) to a greater extent than in cells overexpressing rIAPP (panel d) at the same m.o.i. AdV, adenovirus. D, hIAPP overexpression induces apoptosis in INS 832/13 cells to a much greater extent than rIAPP (400 m.o.i., 48 h; please note comparable protein overexpression shown in B). Data are the mean ± S.E. from four independent experiments; #, p < 0.05 compared with control; *, p < 0.05 compared with rIAPP overexpressing cells. ProCasp-3, pro-caspase; PARP, poly(ADP-ribose) polymerase.
Human Islets
Isolated human islets were obtained from the Islet Cell Resource Consortium. The islet purity was 90–95% as assessed by dithizone staining. The donors, aged 35–55 years, were heart-beating cadaver organ donors, and none had a previous history of diabetes or metabolic disorders. Islet viability was assessed by the live/dead kit (Molecular Probes). Islets were cultured in a 6-well plate or 4-well chamber slide in RPMI 1640 medium (5.5 mm glucose) containing 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (Invitrogen).
To ensure that islets were functional and viable during the course of the experiment, we performed functional evaluation (glucose-stimulated insulin secretion) of isolated islets. After 72 h in culture, 40 islets were preincubated for 1 h in complete RPMI 1640 medium containing 4.0 mm glucose. The medium was replaced with fresh medium containing either 4.0 mm glucose or 16.7 mm glucose for 5 min, and aliquots were collected for insulin measurements. Islets were lysed for insulin content measurement. Insulin concentration was measured by a human insulin enzyme-linked immunosorbent assay kit (Linco Inc., St. Charles, MO).
Western Blot Analysis
Proteins (20–40 μg per lane) were separated on 4–12% BisTris NuPAGE gels and blotted onto a polyvinylidene difluoride membrane (Pall, Ann Arbor, MI). Membranes were probed with rabbit antibodies against calpain-2, cleaved caspase-3, poly(ADP-ribose) polymerase, β-actin (Cell Signaling Technologies, Beverly, MA), and cleaved spectrin (Santa Cruz Biotechnology, Santa Cruz, CA). Horseradish peroxidase-conjugated secondary antibodies were from Zymed Laboratories Inc.. Proteins were visualized using enhanced chemiluminescence (Millipore), and protein expression levels were quantified using Labworks software (UVP, Upland, CA). The membranes were reused after stripping with Pierce stripping buffer for 20 min at room temperature (Pierce).
Cytosolic Ca2+ Measurement Using Reporter System
To measure the elevation of cytosolic Ca2+, we used the NFAT-SEAP reporter system (Clontech) based on the activation of calcineurin by the elevation of cytosolic Ca2+ (24). Calcineurin is a calmodulin-dependent phosphatase (PP2B) that de-phosphorylates and activates the transcriptional factor nuclear factor of activated T-cells (24). INS 832/13 cells were plated in 4-well chamber permanox slides (Nunc, Rochester, NY) at 80,000 cells per well and cultured for 16–24 h. Cells were transfected overnight with the NFAT-SEAP construct (1.0 μg of plasmid DNA per well) using Lipofectamine (Invitrogen) and then transduced with adenoviruses expressing hIAPP versus rIAPP at 400 m.o.i. Medium was collected 6, 12, and 18 h after transduction, and the SEAP activity was measured according to the manufacturer's instructions (Calbiochem). Similar procedure was used for isolated human islets.
Ratiometric Measurement of Cytosolic Ca2+
INS 832/13 cells on coverslips were loaded with the fluorescent Ca2+ indicator Fura-2 by incubation in media containing 5 μm Fura-2/AM (Molecular Probes, Eugene, OR) for 60 min at 37 °C (25). Coverslips were then mounted in an experimental chamber (RC-25F; Warner Instrument Corp.) that was perfused with culture medium at 1.5 ml/min. The perfusion medium was heated using an in line heater (TC-344B; Warner Instrument Corp.), which maintained bath temperature at 37 °C. The chamber was placed on the stage of an inverted microscope (Zeiss TV 100; Carl Zeiss, Inc., Thornwood, NY) with attached digital imaging system (Attofluor; Atto Instruments, Rockville, MD) with electronically controlled excitation filter positions (software RatioVision). Cells were continuously perfused with media for 3–5 min before the start of the acquisition of data. Ratios of images (340 nm excitation/380 nm excitation, emission filter 520 nm) of 30–50 cells per field were obtained at 30-s intervals. A region of interest was defined over each cell, and the average ratio intensity over the region was converted to [Ca2+]i using a calibration curve constructed with a series of calibrated buffered calcium solutions (calcium calibration buffer kit number 2; Molecular Probes). For each cell, [Ca2+]i values and their times of acquisition were stored on computer disk.
Calpain Activity Assay
The fluorometric calpain activity assay is based on the detection of the cleavage of the calpain substrate Ac-LLY-amino-4-trifluoromethylcoumarin (calpain activity assay kit, Biovision, Sunnyvale, CA). Upon cleavage of this substrate in lysates, free amino-4-trifluoromethylcoumarin emits a yellow-green fluorescence (505 nm), which is detected by the CCD camera (UVP, Upland, CA). The cells were plated on a 6-well plate for overnight and transduced with hIAPP, rIAPP, adenoviruses, or none as a control. After 12 h, cells were washed with cold PBS and lysed with 100 μl of lysis buffer on ice for 20 min. Protein content in the lysates was measured by DC-protein assay (Bio-Rad). Equal amount of protein lysates were subjected to calpain assay according to the manufacturer's instruction. Purified calpain (provided in the kit) was used as a positive control, and calpain inhibitor (benzyloxycarbonyl-LLY-fluoromethyl ketone) was included as a negative control (data not shown).
Caspase-3 Activity Assay
We employed a functional assay to detect the activation of procaspase-3 in human islets (Caspase-Glo 3/7 Assay, Promega, Madison, WI). Briefly, 100 μl of reagents were used to measure the activity of about 100 islets at the end of the experiments. The values were normalized by the protein content. A specific inhibitor of caspase-3 (catalog no. 218832, Calbiochem) was used to verify the specificity of the assay. Calcium ATPase inhibitor, 0.1 μm thapsigargin, was used as a positive control.
Human Pancreatic Tissue
Institutional Review Board approval was obtained from both the Mayo Clinic (Institutional Review Board number 1516-03) and UCLA (number 06-04-021-01). We obtained human pancreatic tissue at autopsy from seven obese humans with T2DM and seven age- and BMI-matched nondiabetic controls (Table 1). In addition, aliquots of nine surgically removed human pancreatic tissue specimens (pancreatectomy for tumor but non-tumor-affected pancreas used for study) from T2DM (n = 3) and control subjects (n = 6) were studied with UCLA Institutional Review Board approval (Fig. 6C). The detection of IAPP toxic oligomers in these pancreas samples was reported previously (15).
TABLE 1.
Clinical data of human cases used in spectrin immunohistochemistry study and spectrin cleavage analysis
Note: two pancreatic tissue sections per case were used for immunohistological study. A total of 3581 beta cells were evaluated in cases with T2DM and 2695 beta cells in obese non-diabetic controls. F, female; M, male.
| Group | Age | Gender | BMI | Cleaved spectrin-positive beta cells |
|---|---|---|---|---|
| % | ||||
| Controls | ||||
| 1 | 75 | F | 35.5 | 0.0 |
| 2 | 63 | F | 32.2 | 0.0 |
| 3 | 43 | F | 44.2 | 0.5 |
| 4 | 84 | F | 33.4 | 1.1 |
| 5 | 82 | M | 30.3 | 0.9 |
| 6 | 50 | F | 37.8 | 1.7 |
| 7 | 77 | F | 34.9 | 0.0 |
| Mean | 68 | 36.0 | 0.6 | |
| S.E. | 6.1 | 1.7 | 0.2 | |
| Type 2 diabetes | ||||
| 1 | 49 | F | 47.0 | 0.8 |
| 2 | 68 | F | 37.8 | 79.3 |
| 3 | 65 | M | 34.6 | 7.8 |
| 4 | 63 | M | 33.4 | 53.0 |
| 5 | 76 | F | 30.7 | 2.0 |
| 6 | 64 | M | 33.3 | 60.9 |
| 7 | 68 | M | 33.2 | 26.3 |
| Mean | 65 | 36.0 | 33.0 | |
| S.E. | 3.1 | 2.0 | 11.3 | |
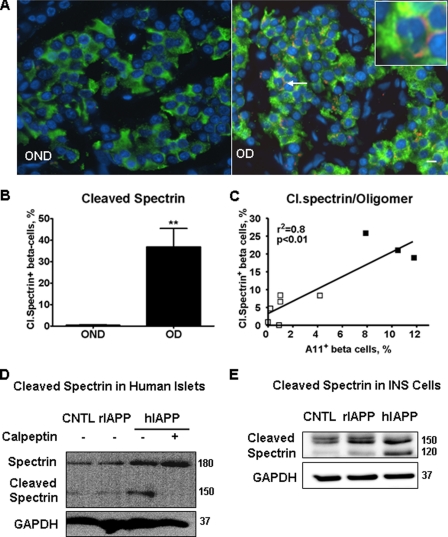
FIGURE 6.
Cleavage of calpain substrate spectrin was detected in beta cells of paraffin-embedded pancreatic tissues from humans with T2DM. A, immunofluorescent images of pancreatic sections stained with anti-cleaved spectrin (red) and anti-insulin antibodies (green). Nuclei were visualized by DAPI (blue). Beta cell with cleaved (Cl.) spectrin was indicated by an arrow (inset shows beta cell with cleaved spectrin in the periphery). B, quantification of beta cells positive for cleaved spectrin in tissue sections from age- and BMI-matched obese nondiabetic (OND, n = 7) and from obese diabetic subjects (OD, n = 7). C, there was a positive correlation between frequency of beta cells with cleaved spectrin and frequency of beta cells positive for toxic oligomers detected by immunofluorescent labeling with oligomer-specific antibody A11 in surgically obtained human pancreas. These tissue samples were previously used to report the increased frequency of toxic IAPP oligomers in beta cells of individuals with T2DM compared with nondiabetic controls (15). □, nondiabetic; ■, T2DM. D, overexpression of hIAPP in human islets induced spectrin cleavage that was blocked by calpain inhibitor calpeptin. Islets were transduced with hIAPP or rIAPP as control at m.o.i. 400 for 72 h. CNTL, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. E, cleavage of spectrin was induced by overexpression of hIAPP in INS 832/13 cells. Cells were transduced with hIAPP or rIAPP as control at 400 m.o.i. for 48 h. Data are the mean ± S.E.; **, p < 0.01.
Immunocytochemistry
For immunocytochemical analysis, cells were plated in 8-well chamber Permanox slides (Nunc, Rochester, NY) at 80,000 cells per well and cultured for 48 h. Cells were then transduced with adenoviruses expressing human or rodent IAPP at 400 m.o.i. Forty eight hours later, cells were gently washed with PBS and fixed with 4% paraformaldehyde (Sigma) for 20 min at room temperature. After washing, cells were permeabilized with 0.4% Triton X-100/Tris-buffered saline for 20 min at room temperature, blocked with 3% bovine serum albumin, 0.2% Triton X-100/Tris-buffered saline for 1 h at room temperature, and incubated overnight at 4 °C with anti-calpain antibody (catalog no. 208755, Calbiochem) diluted 1:100 with 0.2% Tween 20, 3% bovine serum albumin/Tris-buffered saline, followed by Cy3-anti-rabbit IgG (1:200, 1 h at room temperature; Jackson ImmunoResearch Laboratories, West Grove, PA).
To assess cell death, 48 or 72 h after transduction, culture medium was replaced with medium containing 50 μg/ml propidium iodide (PI) (Sigma). Cells were cultured at 37 °C for 30 min, then washed once with PBS, and fixed with 4% paraformaldehyde for 20 min at room temperature. Slides were mounted with Vectashield with DAPI (Vector Laboratories, Burlingame, CA). Random areas (six per chamber) were imaged first in blue (DAPI) and then in red (PI or Cy3) channel.
Immunofluorescent staining of human pancreatic sections was performed as described (13). A specific antibody against the calpain-cleaved α-spectrin (Santa Cruz Biotechnology) (26, 27) was used as a surrogate for calpain hyperactivation. Sections were double-stained with cleaved spectrin and insulin antibodies.
Image Analysis
To assess cell death, propidium iodide-positive cells were counted in each image and related to the total numbers of cells in the image (DAPI-labeled nuclei). To quantify the expression of calpain 2, the area labeled with anti-calpain 2-Cy3 was measured using Image-Pro Plus software (Media Cybernetics, Inc., Silver Spring, MD) and related to the total number of cells in the image (DAPI-labeled nuclei). For cleaved spectrin analysis, 10–15 islets from each human pancreas section were imaged. Total beta cells and beta cells stained for cleaved spectrin were counted (Table 1).
Cryo-immunogold Labeling and Electron Microscopy
Sample Preparation
INS 832/13 cells were transduced with hIAPP or rIAPP for 30 h and then fixed with 4% paraformaldehyde + 0.1% glutaraldehyde for 6 h, scraped, spun, and embedded and sectioned as described previously (28).
Staining Procedure
Sections were incubated with antibody against toxic oligomers (A11 (29)) diluted in 20 mm Tris, 150 mm NaCl, 1% bovine serum albumin at 4 °C overnight, washed three times with the same buffer, and then incubated with secondary antibody for 45 min at room temperature. After washing, the sections were fixed with 0.8% glutaraldehyde, treated with 1% uranyl acetate in 1.3% methylcellulose, and air-dried. Samples were analyzed within 1–3 days after staining using a JEM 1200-EX transmission microscope (JEOL, Japan) equipped with a BioScan 600W digital camera (Gatan, Inc., Pleasanton, CA).
Confocal Time Lapse Microscopy
Time-lapse confocal microscopy was used as described previously (30). Briefly, INS 832/13 cells were transfected with plasmid DNA expressing NFAT-GFP for 12 h, and then the same cells were transduced with Ad-hIAPP at 400 m.o.i. Confocal time-lapse microscopy was conducted to follow individual cells in the presence with 500 ng/ml of PI for 2 h.
Statistical Analysis
All values were presented as the mean ± S.E. Student's t test was performed to compare the differences between the rIAPP versus hIAPP adenovirus expressing cells or islets. Values of p < 0.05 were considered to be statistically significant.
RESULTS
Overexpression of Human IAPP in INS 832/13 Cells Reduces Cell Viability and Increases Cell Apoptosis
We previously reported that the overexpression of hIAPP with a green fluorescent protein tag induced ER stress in INS 832/13 cells (13). Because the presence of the green fluorescent protein tag may interfere or distort the toxic effects of hIAPP, we created an adenovirus expressing hIAPP without any tag. The nonamyloidogenic rIAPP was used as a control because it contains three proline residues in the 20–29-amino acid region of IAPP rendering it soluble. The sequence of IAPP in the mouse and rat is identical, hence the term rodent. To establish a dose response for m.o.i. of adenoviral expression and toxicity, and to ensure comparable expression of experimental hIAPP and control rIAPP, RIN cells were transduced with an m.o.i. of 150–500 with a resulting progressive increase in apoptosis quantified by cleavage of caspase-3 48 h after transduction (Fig. 1A). For subsequent experiments, we used an m.o.i. of 400 for hIAPP or rIAPP as a control for a comparable burden of protein expression (Fig. 1B). hIAPP expression caused increased apoptosis compared with control or rIAPP (Fig. 1D). Furthermore, toxic IAPP oligomers were detected in hIAPP-transduced INS 832/13 cells but rarely in cells transduced with rIAPP (Fig. 1C).
Cytosolic Calcium Is Increased in INS 832/13 Cells Overexpressing Human IAPP
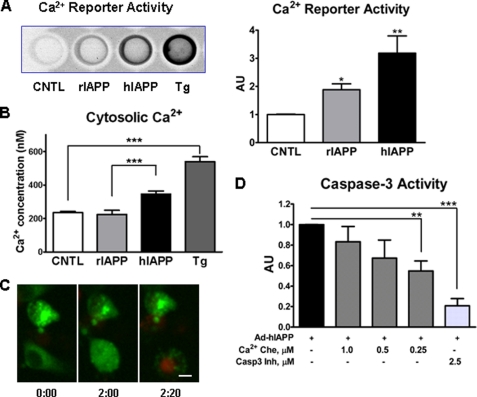
Having established that the new hIAPP adenovirus without a tag induced apoptosis in INS 832/13 cells, we sought to determine what caused apoptosis in INS 832/13 cells overexpressing hIAPP. Because hIAPP oligomers may compromise cellular membrane integrity (7, 31, 32), we designed several experiments to establish if overexpression of hIAPP induces an increase in cytosolic Ca2+. First, we established that hIAPP protein expression was detected 4–6 h after viral infection (data not shown), and the cytosolic Ca2+ was elevated 12 h (Fig. 2A, reporter assay) and 6 h (Fig. 2B, ratiometric assay) after adenoviral transduction. We observed an increased level of cytosolic Ca2+ in cells overexpressing hIAPP and a more modest increase in cells overexpressing rIAPP compared with nontransduced control cells (Fig. 2A). The minor effect of rIAPP was not detected by the ratiometric assay, which measured the cytosolic Ca2+ in less than a second, but was detectable by the reporter assay, which measured the accumulative effects of cytosolic Ca2+ elevation over 12 h. The increased Ca2+ levels in rIAPP-expressing cells may come from two sources as follows: adenoviral transfection itself can increase Ca2+ levels (33) or the increased number of peptides entering the ER can permit some passive leakage of Ca2+ through the ER translocon pore (34). We employed confocal time-lapse video micrography to show that hIAPP-induced abnormal elevation of cytosolic Ca2+ ions is followed by cell death (Fig. 2C, right panel). In Fig. 2C, left panel, one cell in the lower portion of the image is positive for cytosolic NFAT-GFP at the beginning of imaging (8 h post-transduction). Two hours later, in the same cell, we observed an abnormally high level of dephosphorylated NFAT-GFP (Fig. 2C, green color) due to Ca2+-activated calcineurin. This dephosphorylated NFAT-GFP translocated to the nucleus (Fig. 2C, middle panel). Twenty minutes later, PI was incorporated to the nucleus of this cell indicating cell death (Fig. 2C, right panel). As further evidence of the role of elevated Ca2+ in mediating hIAPP, induced apoptosis was obtained using Ca2+ chelator. When the intracellular calcium chelator BAPTA-AM was added 4 h after viral transduction, apoptosis (caspase-3 activation) was reduced at a dose of 0.25 μm (Fig. 2D). We do not see significant protective effect at higher concentrations of BAPTA-AM. We believe that at higher concentrations BAPTA-AM penetrates the ER lumen and depletes ER Ca2+ leading to loss of function of ER chaperones, compromising protein folding and thus compromising its protective effects (35).
FIGURE 2.
Overexpression of hIAPP in INS 832/13 cells leads to elevation of cytosolic Ca2+, NFAT-GFP nuclear translocation, and cell death. INS 832/13 cells were transduced with rIAPP or hIAPP adenovirus at 400 m.o.i. A, cytosolic Ca2+ was measured using NFAT-SEAP gene reporter system 12 h post-transduction with adenovirus (see “Experimental Procedures” for details). CNTL, control. B, Ca2+ concentrations were measured ratiometrically 6 h post-transduction by monitoring more than 100 individual cells loading with 5 μm Fura-2/AM. Positive control was 1 μm of Ca2+-ATPase inhibitor thapsigargin (Tg). C, INS 832/13 cells were transfected with plasmid DNA expressing NFAT-GFP for 12 h and then transduced with Ad-hIAPP at 400 m.o.i. for 8 h. Then laser confocal microscopy was performed to follow individual cells with elevated cytosolic Ca2+ for 2 h in the presence of 500 ng/ml PI. The cell at the bottom showed cytosolic NFAT-GFP distribution at the beginning of observation. Two hours later, NFAT-GFP in this cell translocated to the nucleus, and then 20 min later the nucleus incorporated propidium iodide indicating cell death. The scale bar is 5 μm. D, INS 832/13 cells were transduced with hIAPP adenovirus (Ad) for 6 h, and then three doses of Ca2+ chelator (Ca2+ Che) BAPTA-AM were applied for 48 h. Caspase-3 (Casp-3) inhibitor was used to show the assay specificity and as a positive control. Data are the mean ± S.E. from four (A) and five experiments (D); *, p < 0.05; **, p < 0.001; ***, p < 0.0001. AU, arbitrary unit.
Calcium-dependent Calpain Activity Is Increased in INS 832–13 Cells Overexpressing Human IAPP
We further asked what was the consequence of increased cytosolic Ca2+ induced by hIAPP. Among many cellular events initiated by increased cytosolic Ca2+, calpain, a neutral Ca2+-dependent protease, was a prime suspect. Previous study has shown that overexpression of Alzheimer amyloid precursor protein in cultured neurons induced activation of calpain (19). The expression of hIAPP in INS 823/13 cells induced increased calpain protease activity (Fig. 3A). This functional assay of calpain protease activity does not specify which calpain is activated, so a calpain-2-specific antibody was then used to address this question.
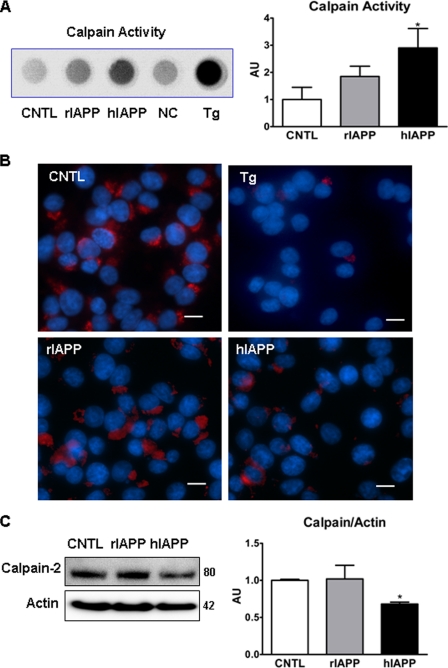
FIGURE 3.
Overexpression of hIAPP in INS 832/13 cells reduces amount of inactive calpain 2. A, calpain proteolytic activity was measured by a kit 12 h post-transduction. Tg, cells treated with 1.0 μm thapsigargin for 4 h (positive control); NC, negative control, cell lysate without the assay substrate; CNTL, control. B, immunofluorescent images of INS 832/13 cells transduced with hIAPP and rIAPP for 48 h or treated with thapsigargin (Tg, positive control) and stained with anti-calpain-2 antibody (red) and DAPI (blue). Scale bar is 10 μm. C, representative Western blot and quantification of calpain-2 protein expression relative to actin. Data are the mean ± S.E. from four (A) and three experiments (B and C); *, p < 0.05. AU, arbitrary unit.
The antibody used to detect calpain-2 recognizes both the 80-kDa inactive and 58-kDa active forms of calpain-2 (catalog no. 208755, Calbiochem). Inactive calpain-2 is associated with the ER membrane at the cytosolic side (36), but following activation, calpain-2 is released from this site and rapidly degraded. Rapid degradation of activated calpain-2 is presumably to prevent this potent protease from degrading proteins distant from the intended local site of activation (37). Consistent with this, treatment with thapsigargin (ER Ca2+-ATPase inhibitor) induced an almost complete loss of calpain-2 immunoreactivity on the ER surface (Fig. 3B, top right panel). Overexpression of hIAPP also caused a loss of calpain-2 immunoreactivity (Fig. 3B, lower right panel) compared with cells overexpressing rIAPP (Fig. 3B, lower left panel). To ensure the localization of calpain-2 on the ER of beta cells, we performed ER fractionation and performed immunoblots using the same calpain-2-specific antibody. The result of enriched ER protein immunoblotting confirmed the observation from immunocytochemistry that treatment with thapsigargin led to the loss of inactive calpain in the ER-enriched fraction (data not shown). Immunoblotting showed that overexpression of hIAPP decreased total calpain content (Fig. 3C), presumably as a consequence of rapid degradation following Ca2+ activation (see above, Fig. 3B) (37). Furthermore, the observed hIAPP-induced elevated cytosolic Ca2+, increased calpain activity, and the decrease in total calpain protein content by immunoblot are consistent with Etoposide-induced cytosolic Ca2+ elevation, increased calpain activity, and decreased total calpain protein content (38).
Inhibition of Calpain Activity by Calpeptin Reduces Toxicity of hIAPP in INS 832/13 Cells
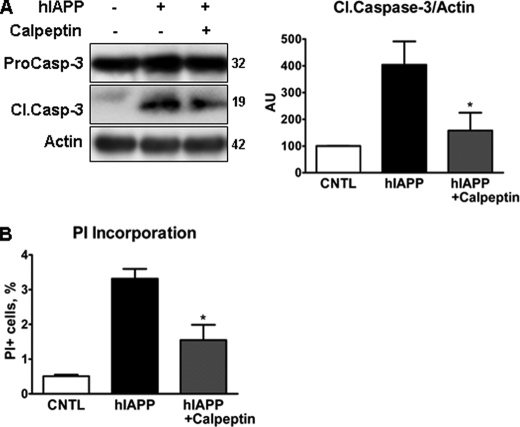
If calpain mediates hIAPP-induced apoptosis, then inhibition of calpain would be expected to reduce apoptosis. We chose the specific calpain inhibitor calpeptin, a fragment of the endogenous calpain inhibitor calpastatin, to test this hypothesis. A dose-response study was undertaken in INS 832/13 to identify a nontoxic dose (data not shown). Based on those findings, we chose to use calpeptin at 1.25 μm for 66 h. At that dose, caspase-3 cleavage and PI incorporation were decreased by 30 and 50%, respectively, in calpeptin-treated hIAPP-expressing cells compared with hIAPP-expressing cells without treatment (Fig. 4, A and B).
FIGURE 4.
Calpain inhibitor, calpeptin, protects beta cells from hIAPP-induced death. INS 832/13 cells were transduced with rIAPP or hIAPP adenovirus at 400 m.o.i. for 72 h (A) and 48 h (B). AU, arbitrary unit. Calpeptin (1.25 μm) was added 6 h post-transduction. Cell viability was assessed by cleaved caspase-3 immunoblotting (A) and PI incorporation (B). Data are the mean ± S.E. from three independent experiments; *, p < 0.05, hIAPP + calpeptin compared with hIAPP only. Casp, caspase; Cl.Casp-3, cleaved caspase-3; CNTL, control.
Calcium Elevation and Calpain Activation Are Observed in Human Islets Overexpressing Human IAPP
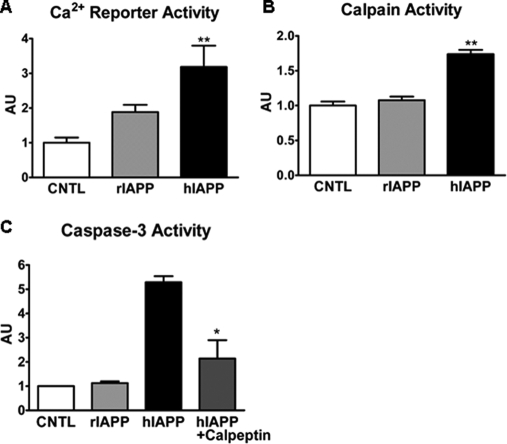
After establishing that overexpression of hIAPP induced increased cytosolic Ca2+, activation of calpain, and apoptosis in INS 832/13 cells, we extended these findings to isolated human islets. Expression of hIAPP increased cytosolic Ca2+ levels, monitored by the same Ca2+ reporter system (Fig. 5A). Activation of calpain by hIAPP was also confirmed in human islets assayed by calpain activity (Fig. 5B). Inhibition of calpain by calpeptin in human islets again led to attenuation of caspase-3 activation (Fig. 5C).
FIGURE 5.
Overexpression of hIAPP in human islets leads to an increase in cytosolic Ca2+ and calpain activation, and inhibition of calpain activity attenuates caspase-3 activity. Human islets were transduced with rIAPP or hIAPP adenovirus at 400 m.o.i. for 24 h (A and B) or 72 h (C). A, intracellular Ca2+ elevation was measured using NFAT-SEAP gene reporter system (see “Experimental Procedures” for details). B, calpain proteolytic activity was detected by calpain activity assay. C, caspase-3 activity was measured in islet lysates using caspase-3 activity kit. AU, arbitrary unit. Data are the mean ± S.E. from four experiments; *, p < 0.05; **, p < 0.01. CNTL, control.
Cleaved Spectrin Was Detected in Beta Cells in Humans with T2DM
Having demonstrated that high expression levels of human IAPP induce increased cytosolic Ca2+ and hyperactivation of calpain, we posed the following question. Is there evidence of chronically increased cytosolic Ca2+ and hyperactivation of calpain in beta cells of humans with T2DM? Living beta cells from humans with T2DM are rarely available. Therefore, to address this, we analyzed the consequences of sustained elevated cytosolic Ca2+ and hyperactivation of calpain in sections of fixed paraffin-embedded human tissue available from T2DM.
The cytoskeletal protein α-spectrin (also called α-fodrin), when subjected to calpain cleavage, generates a specific α-spectrin fragment that can be detected as a measurement of calpain activity (39, 40). Using a specific antibody against the calpain-specific (26), cleaved fragment of α-spectrin, we detected increased cleaved fragments (Fig. 6, A and B) in beta cells from humans with T2DM (n = 7), compared with age- and BMI-matched nondiabetic subjects (n = 7). Exocrine cells also express α-spectrin, but the cleaved spectrin in T2DM was rarely detected in exocrine cells, ensuring that the detected calpain hyperactivation in beta cells was not an artifact of tissue collection or preservation. To substantiate this observation, we examined the relationship between the frequency of cleaved spectrin and the frequency of intracellular toxic IAPP oligomers detected with a specific anti-toxic oligomer antibody (A11) in pancreatic beta cells of nine human pancreas samples obtained at surgery as reported previously (15). We observed a positive correlation (r2 = 0.8, p < 0.01) between the frequency of beta cells with cleaved spectrin and the oligomer-specific immunoreactivity (Fig. 6C). Using immunoblotting, we also detected cleaved spectrin in isolated human islets (Fig. 6D) and in INS 832/13 cells overexpressing hIAPP (Fig. 6E). In the same preparation, inhibition of calpain activation by calpeptin reduced the cleavage of spectrin induced by overexpressing hIAPP (Fig. 6D).
DISCUSSION
We tested the hypothesis that human IAPP-induced apoptosis is mediated through disturbance of intracellular Ca2+ homeostasis. We report the following: 1) that overexpression of hIAPP induced the formation of toxic IAPP oligomers and increased cytosolic Ca2+ concentration and hyperactivation of calpain-2 protease in insulin-secreting beta cells and/or isolated human islets; 2) that inhibition of calpain attenuated the toxicity of human IAPP in INS 832/13 cells and isolated human islets; and 3) that calpain-cleaved products of α-spectrin were present in isolated human islets and INS 832/13 cells overexpressing hIAPP and were identified in pancreatic beta cells from humans with T2DM. These studies imply that chronically and aberrantly activated Ca2+/calpain pathways contribute to the cytotoxicity of aggregated human IAPP. Moreover, these data suggest that dysfunction and increased beta cell apoptosis in T2DM are mediated, at least partially, through chronic overactive Ca2+/calpain pathways. These findings support and extend the IAPP toxic oligomer hypothesis in T2DM.
Aberrantly increased cytoplasmic Ca2+ has been shown to mediate cellular dysfunction and cell death in neurodegenerative diseases, also characterized by abnormal intracellular aggregates of amyloidogenic proteins (17, 18, 41). We report that the overexpression of hIAPP in insulin-producing cells or islets leads to an early increase in cytoplasmic Ca2+. This finding was obtained by using a gene reporter system, in which the Ca2+-sensitive transcriptional factor nuclear factor of activated T-cells was linked to the secreted alkaline phosphatase to measure the accumulated effects of Ca2+ elevation. This finding was substantiated by the ratiometric measurement of intracellular Ca2+ using fura-2-AM (Fig. 2B), a standard protocol in the field (25).
The mechanism by which hIAPP aggregates cause inappropriately increased cytoplasmic Ca2+ is unknown but is most likely mediated by the property of hIAPP oligomers to induce nonselective membrane leakage (7). In support of this, it has been shown that application of β-amyloid aggregates to cells caused increased cytoplasmic Ca2+ concentrations and subsequently reduced ER Ca2+ stores (42). It has been reported that exogenous hIAPP can activate transient receptor potential channels (43). Cytosolic Ca2+ elevation induced by hIAPP overexpression may arise as a result of hIAPP induced leakage into the cytosol from the secretory pathway or mitochondria because membrane-permeant IAPP toxic oligomers are identified at each of these sites (14, 15). Also, oligomeric β-amyloid-induced Ca2+ release can be blocked by the inhibition of the ER calcium release channels (42). Furthermore, increased cytoplasmic Ca2+ can be associated with the malfunction of both ER ryanodine and inositol 1,4.5-triphosphate receptors, which are substrates of activated calpains (44, 45). Because toxic hIAPP oligomers have been detected in the secretory pathway (15), they might also be secreted and act on the plasma membrane, a site that also leads to cytotoxicity (7, 10, 32, 46, 47) and elevated cytoplasmic calcium levels (18, 42).
An increase in cytosolic Ca2+ leads to activation of calpain, which is implicated in a number of pathological disorders, including brain ischemia, injury, and neurodegeneration (17). Inappropriately high calpain activation has been reported in the affected hippocampus in Alzheimer disease (16). Our data indicate that increased cytosolic Ca2+ concentrations preceded the hyperactivation of calpain. Furthermore, we showed that inhibition of calpain attenuated hIAPP-induced cytotoxicity, which is consistent with a recent report showing that the inhibition of calpain improves memory and synaptic transmission in a mouse model of Alzheimer disease with overexpression of human β-amyloid protein (48).
We also report that beta cells from humans with T2DM have cleaved α-spectrin, which is an indicator of a compromised cytoskeleton and cellular membrane. Scaffolding protein spectrin (αII) is an actin-binding protein and is expressed in most cells, including pancreatic endocrine and exocrine cells. Spectrin functions as a membrane stabilizer by forming trimers, tetramers, and higher polymers (49). αII spectrin has a μ-calpain cleavage site based on the secondary and tertiary conformational features surrounding the cleavage site, rather than the linear sequence (49). Activated calpain cleaves spectrin and generates 145- and 150-kDa fragments, recognized by a specific antibody (50). It has been reported that calpain-cleaved fragments of α-spectrin are a hallmark of hyperactivation of the calcium-calpain system (51), and injection of 6-hydroxydopamine into rodent brains resulted in calpain activation and the cleavage of α-spectrin. When treated with calpastatin, the cleavage of α-spectrin was greatly reduced (52).
Therefore, the presence of cleaved spectrin in beta cells from humans with T2DM, but rarely in age- and BMI-matched nondiabetic subjects, is consistent with chronic overactivation of Ca2+-calpain pathways. Moreover, we report a positive correlation between the frequency of beta cells with cleaved spectrin (Fig. 6C) and the frequency of toxic oligomer-containing beta cells (15), consistent with the hypothesis that formation of intracellular toxic IAPP oligomers disrupt intracellular membrane fidelity with the adverse consequences of inappropriate activation of Ca2+-sensitive pathways. These observations were further substantiated by the demonstration of the presence of cleaved spectrin in isolated human islets and INS 832/13 cells overexpressing hIAPP and reduction of spectrin cleavage by concurrent use of a calpain inhibitor (Fig. 6, D and E).
There are other lines of evidence to support Ca2+/calpain activation findings. Overexpression of calmodulin in mice results in insulin secretion defects, loss of beta cells, and diabetes (53, 54). Also, abnormal elevation of cytosolic Ca2+ induces ER stress and beta cell apoptosis by palmitate (55). Finally, ER stress is associated with Ca2+ efflux from the ER (56), and the ER stress marker, nuclear CHOP, was detected in beta cells from humans with T2DM but only rarely in beta cells from obese nondiabetic subjects (13).
In conclusion, we report that the overexpression of human islet amyloid protein hIAPP leads to the formation of toxic oligomers, an elevation of cytosolic Ca2+, and hyperactivation of the Ca2+-dependent protease calpain. Furthermore, inhibition of calpain activity attenuates the beta cell apoptosis associated with chronic hyperactivation of Ca2+/calpain. Identification of calpain-cleaved fragments of α-spectrin in beta cells from humans with T2DM but not in control subjects indicates that the Ca2+-dependent protease calpain may play a key role in the pathophysiology of T2DM. These findings suggest that sustained hyperactivity of the Ca2+/calpain signaling pathway may be the molecular basis for oligomeric protein conformational disorders such as T2DM and Alzheimer disease (16, 48).
Acknowledgments
We thank Erica Manesso for statistical advice and Jennifer Jang and Kathleen Linzmeier for technical help. We gratefully acknowledge Dr. Steven H. Young for assistance with the Ca2+ imaging studies.
This work was supported, in whole or in part, by National Institutes of Health Grant DK059579. This work was also supported by The Larry L. Hillblom Foundation Grant 2007-D-003-NET.
- T2DM
- type 2 diabetes
- IAPP
- islet amyloid polypeptide
- ER
- endoplasmic reticulum
- hIAPP
- human prepro-IAPP
- rIAPP
- rat prepro-IAPP
- PBS
- phosphate-buffered saline
- RIN
- rat insulinoma cell
- m.o.i.
- multiplicity of infection
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- DAPI
- 4′,6-diamidino-2-phenylindole
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1, 3-diol
- PI
- propidium iodide
- BMI
- body mass index.
REFERENCES
- 1.Weyer C., Bogardus C., Mott D. M., Pratley R. E. (1999) J. Clin. Invest. 104, 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haataja L., Gurlo T., Huang C. J., Butler P. C. (2008) Endocr. Rev. 29, 303–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Diabetes 52, 102–110 [DOI] [PubMed] [Google Scholar]
- 4.Hardy J., Selkoe D. J. (2002) Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 5.Lue L. F., Kuo Y. M., Roher A. E., Brachova L., Shen Y., Sue L., Beach T., Kurth J. H., Rydel R. E., Rogers J. (1999) Am. J. Pathol. 155, 853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janson J., Soeller W. C., Roche P. C., Nelson R. T., Torchia A. J., Kreutter D. K., Butler P. C. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 7283–7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janson J., Ashley R. H., Harrison D., McIntyre S., Butler P. C. (1999) Diabetes 48, 491–498 [DOI] [PubMed] [Google Scholar]
- 8.Chartier-Harlin M. C., Crawford F., Houlden H., Warren A., Hughes D., Fidani L., Goate A., Rossor M., Roques P., Hardy J., et al. (1991) Nature 353, 844–846 [DOI] [PubMed] [Google Scholar]
- 9.Matveyenko A. V., Butler P. C. (2006) Ilar J. 47, 225–233 [DOI] [PubMed] [Google Scholar]
- 10.Sparr E., Engel M. F., Sakharov D. V., Sprong M., Jacobs J., de Kruijff B., Höppener J. W., Killian J. A. (2004) FEBS Lett. 577, 117–120 [DOI] [PubMed] [Google Scholar]
- 11.Jayasinghe S. A., Langen R. (2007) Biochim. Biophys. Acta 1768, 2002–2009 [DOI] [PubMed] [Google Scholar]
- 12.Huang C. J., Haataja L., Gurlo T., Butler A. E., Wu X., Soeller W. C., Butler P. C. (2007) Am. J. Physiol. Endocrinol. Metab. 293, E1656–E1662 [DOI] [PubMed] [Google Scholar]
- 13.Huang C. J., Lin C. Y., Haataja L., Gurlo T., Butler A. E., Rizza R. A., Butler P. C. (2007) Diabetes 56, 2016–2027 [DOI] [PubMed] [Google Scholar]
- 14.Lin C. Y., Gurlo T., Kayed R., Butler A. E., Haataja L., Glabe C. G., Butler P. C. (2007) Diabetes 56, 1324–1332 [DOI] [PubMed] [Google Scholar]
- 15.Gurlo T., Ryazantsev S., Huang C. J., Yeh M. W., Reber H. A., Hines O. J., O'Brien T., Glabe C. G., Butler P. C. (2010) Am. J. Pathol., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito K., Elce J. S., Hamos J. E., Nixon R. A. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 2628–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bevers M. B., Neumar R. W. (2008) J. Cereb. Blood Flow Metab. 28, 655–673 [DOI] [PubMed] [Google Scholar]
- 18.Demuro A., Mina E., Kayed R., Milton S. C., Parker I., Glabe C. G. (2005) J. Biol. Chem. 280, 17294–17300 [DOI] [PubMed] [Google Scholar]
- 19.Kuwako K., Nishimura I., Uetsuki T., Saido T. C., Yoshikawa K. (2002) Brain Res. Mol. Brain Res. 107, 166–175 [DOI] [PubMed] [Google Scholar]
- 20.Norberg E., Gogvadze V., Ott M., Horn M., Uhlén P., Orrenius S., Zhivotovsky B. (2008) Cell Death Differ. 15, 1857–1864 [DOI] [PubMed] [Google Scholar]
- 21.Song Y., You N. C., Hsu Y. H., Sul J., Wang L., Tinker L., Eaton C. B., Liu S. (2007) Hum. Mol. Genet 16, 2960–2971 [DOI] [PubMed] [Google Scholar]
- 22.Wu H. Y., Lynch D. R. (2006) Mol. Neurobiol. 33, 215–236 [DOI] [PubMed] [Google Scholar]
- 23.Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 24.Rao A., Luo C., Hogan P. G. (1997) Annu. Rev. Immunol. 15, 707–747 [DOI] [PubMed] [Google Scholar]
- 25.Young S. H., Ennes H. S., McRoberts J. A., Chaban V. V., Dea S. K., Mayer E. A. (1999) Am. J. Physiol. 276, G1204–G1212 [DOI] [PubMed] [Google Scholar]
- 26.Cowan C. M., Fan M. M., Fan J., Shehadeh J., Zhang L. Y., Graham R. K., Hayden M. R., Raymond L. A. (2008) J. Neurosci. 28, 12725–12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutta S., Chiu Y. C., Probert A. W., Wang K. K. (2002) Biol. Chem. 383, 785–791 [DOI] [PubMed] [Google Scholar]
- 28.Ryazantsev S., Yu W. H., Zhao H. Z., Neufeld E. F., Ohmi K. (2007) Mol. Genet. Metab. 90, 393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayed R., Head E., Thompson J. L., McIntire T. M., Milton S. C., Cotman C. W., Glabe C. G. (2003) Science 300, 486–489 [DOI] [PubMed] [Google Scholar]
- 30.Saisho Y., Manesso E., Gurlo T., Huang C. J., Toffolo G. M., Cobelli C., Butler P. C. (2009) Am. J. Physiol. Endocrinol. Metab. 296, E89–E96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khemtémourian L., Killian J. A., Höppener J. W., Engel M. F. (2008) Exp. Diabetes Res. 2008, 421287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engel M. F., Khemtémourian L., Kleijer C. C., Meeldijk H. J., Jacobs J., Verkleij A. J., de Kruijff B., Killian J. A., Höppener J. W. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6033–6038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michelangeli F., Liprandi F., Chemello M. E., Ciarlet M., Ruiz M. C. (1995) J. Virol. 69, 3838–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Coppenolle F., Vanden Abeele F., Slomianny C., Flourakis M., Hesketh J., Dewailly E., Prevarskaya N. (2004) J. Cell Sci. 117, 4135–4142 [DOI] [PubMed] [Google Scholar]
- 35.Paschen W., Hotop S., Aufenberg C. (2003) Cell Calcium 33, 83–89 [DOI] [PubMed] [Google Scholar]
- 36.Hood J. L., Brooks W. H., Roszman T. L. (2004) J. Biol. Chem. 279, 43126–43135 [DOI] [PubMed] [Google Scholar]
- 37.Johnson G. V., Guttmann R. P. (1997) BioEssays 19, 1011–1018 [DOI] [PubMed] [Google Scholar]
- 38.Piwocka K., Vejda S., Cotter T. G., O'Sullivan G. C., McKenna S. L. (2006) Blood 107, 4003–4010 [DOI] [PubMed] [Google Scholar]
- 39.Pettigrew L. C., Holtz M. L., Craddock S. D., Minger S. L., Hall N., Geddes J. W. (1996) J. Cereb. Blood Flow Metab. 16, 1189–1202 [DOI] [PubMed] [Google Scholar]
- 40.Takano J., Tomioka M., Tsubuki S., Higuchi M., Iwata N., Itohara S., Maki M., Saido T. C. (2005) J. Biol. Chem. 280, 16175–16184 [DOI] [PubMed] [Google Scholar]
- 41.Giunti R., Gamberucci A., Fulceri R., Bánhegyi G., Benedetti A. (2007) Arch. Biochem. Biophys. 462, 115–121 [DOI] [PubMed] [Google Scholar]
- 42.Ferreiro E., Oliveira C. R., Pereira C. M. (2008) Neurobiol. Dis. 30, 331–342 [DOI] [PubMed] [Google Scholar]
- 43.Casas S., Novials A., Reimann F., Gomis R., Gribble F. M. (2008) Diabetologia 51, 2252–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rardon D. P., Cefali D. C., Mitchell R. D., Seiler S. M., Hathaway D. R., Jones L. R. (1990) Circ. Res. 67, 84–96 [DOI] [PubMed] [Google Scholar]
- 45.Nagata E., Tanaka K., Gomi S., Mihara B., Shirai T., Nogawa S., Nozaki H., Mikoshiba K., Fukuuchi Y. (1994) Neuroscience 61, 983–990 [DOI] [PubMed] [Google Scholar]
- 46.Mirzabekov T. A., Lin M. C., Kagan B. L. (1996) J. Biol. Chem. 271, 1988–1992 [DOI] [PubMed] [Google Scholar]
- 47.Pereira C., Ferreiro E., Cardoso S. M., de Oliveira C. R. (2004) J. Mol. Neurosci. 23, 97–104 [DOI] [PubMed] [Google Scholar]
- 48.Trinchese F., Fa' M., Liu S., Zhang H., Hidalgo A., Schmidt S. D., Yamaguchi H., Yoshii N., Mathews P. M., Nixon R. A., Arancio O. (2008) J. Clin. Invest. 118, 2796–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stabach P. R., Cianci C. D., Glantz S. B., Zhang Z., Morrow J. S. (1997) Biochemistry 36, 57–65 [DOI] [PubMed] [Google Scholar]
- 50.Rajgopal Y., Vemuri M. C. (2002) Neurosci. Lett. 321, 187–191 [DOI] [PubMed] [Google Scholar]
- 51.Siman R., Zhang C., Roberts V. L., Pitts-Kiefer A., Neumar R. W. (2005) J. Cereb. Blood Flow Metab. 25, 1433–1444 [DOI] [PubMed] [Google Scholar]
- 52.Grant R. J., Sellings L. H., Crocker S. J., Melloni E., Park D. S., Clarke P. B. (2009) Neuroscience 158, 558–569 [DOI] [PubMed] [Google Scholar]
- 53.Epstein P. N., Ribar T. J., Decker G. L., Yaney G., Means A. R. (1992) Endocrinology 130, 1387–1393 [DOI] [PubMed] [Google Scholar]
- 54.Gómez Dumm C. L., Atwater I., Epstein P. N., Gagliardino J. J. (1994) Virchows Arch. 425, 73–77 [DOI] [PubMed] [Google Scholar]
- 55.Gwiazda K. S., Yang T. L., Lin Y., Johnson J. D. (2009) Am. J. Physiol. Endocrinol. Metab. 296, E690–E701 [DOI] [PubMed] [Google Scholar]
- 56.Resende R., Ferreiro E., Pereira C., Oliveira C. R. (2008) J. Neurosci. Res. 86, 2091–2099 [DOI] [PubMed] [Google Scholar]