Abstract
Extracellular heat shock protein 72 (Hsp72; inducible form of the 70-kDa heat shock protein) plays a critical role in innate and adaptive immune responses and has shown promise as an ideal adjuvant for the optimization of antigen-specific anti-tumor vaccines. Recent studies suggest that to correctly elucidate the mechanisms by which Hsp72 exerts its beneficial effects in vitro, great care must be taken to ensure that endotoxin by-products do not invalidate the findings. In this study, we have taken advantage of the baculovirus expression vector system for production of endotoxin-free recombinant Hsp72. The coding sequence of human hsp72 was recombined into the baculovirus immediately downstream of the strong polyhedron gene promoter. Ninety-six h post-infection of Sf9 insect cells with recombinant baculovirus, maximal levels of Hsp72 protein were detected. The recombinant human Hsp72 was purified by affinity chromatography from insect cells, and purity was confirmed by SDS-PAGE and mass spectrometry. The purified human recombinant Hsp72bv (Hsp72 produced using the BEVS) was demonstrated to have no endotoxin contamination and was shown to have stimulated potent calcium flux in the human monocytic cell line. Furthermore, recombinant Hsp72bv enhanced the tolerance of neuroblastoma cells to heat stress-induced cell death and displayed classical chaperokine functions including augmentation of inflammatory cytokine productions in mouse splenocytes. The production of functional, endotoxin-free recombinant human Hsp72bv in insect cells is inexpensive and convenient and eliminates the need of special procedures for endotoxin depletion. Endotoxin-free recombinant human Hsp72bv can now be used to unlock the important role Hsp72 plays in modulating immune function.
Introduction
Heat shock proteins (HSP)2 act as a class of molecular chaperones involved in numerous processes, such as protein folding, assembly, and intracellular transportation (1, 2). HSP used to be considered to function exclusively inside the cells; however, several family members of HSP, including Hsp60 and Hsp72, have been reported to exist in the extracellular compartment following necrotic release or mild secretion in response to cellular stress (3–5). Extracellular HSP, especially Hsp72, is thought to play an important role in augmenting the immune system and to break the tolerance to recognize “dangerous signal” of an infection or a disease (6, 7). Two unique features of Hsp72 adorn it with its ability to stimulate immune responses. The first feature is the peptide-binding activity of Hsp72; the peptides bound by Hsp72 act as the fingerprint of the diseased cells of origin, which the immune system recognizes (8). The chaperoned peptides are then transferred to APC to induce priming CD8+ T lymphocytes targeting the specific peptide and consequently elicits antigen-specific immunity (9, 10). Second, Hsp72 has the ability to induce nonspecific stimulation of proinflammatory cytokine (11–13) and chemokine production (14). Therefore, extracellular Hsp72 plays a critical role in both innate and adaptive immune activation (15, 16).
However, it is now accepted that recombinant Hsp72 exerts immune-stimulating effects (17, 18). Studies that initially cast doubt on these findings (19–23) were helpful in cautioning the investigators to take special care to ensure that recombinant Hsp72 proteins prepared from endotoxin sources exhibited low endotoxin levels. To revisit the question and confirm that “clean” Hsp72 has the ability to stimulate host chaperokine activity, we expressed the recombinant Hsp72 in the baculovirus expression vector system (BEVS). The BEVS has been well established and extensively used to express a large variety of proteins in insect cells (24). The BEVS can generate large quantities of proteins, and the proteins are more likely to have biological activities of the original proteins under the natural condition than proteins expressed in bacterial systems. More importantly, using insect cells can help exclude the obvious endotoxin contamination in the recombinant Hsp72 protein. Our results demonstrate that recombinant Hsp72bv generated from BEVS is free of endotoxin and retains the ability to stimulate potent calcium flux, augment cytokine secretion, and increase the relative number of CD4+ T lymphocytes and CD11c+ monocytes.
EXPERIMENTAL PROCEDURES
Materials, Cells, and Virus
Spodoptera frugiperda Sf9 insect cells, the baculovirus transfer vector pBACgus-2cp, the BacVector-1000 triple cut Autographa californica multiple nuclear polyhedrosis virus DNA, insect GeneJuice transfection reagent, insect PopCulture reagent, Ni-NTA His·Bind resin, Ni-NTA buffer kit, 5-bromo-4-chloro-3-indolyl-b-d-glucuronide solution, the serum-free BacVector insect cell medium, and endotoxin-free water were all from Novagen. Protease inhibitor mixture tablets were from Roche Diagnostics. pOTB7 vector containing the MGC full-length hsp72 gene was obtained from Invitrogen. Pluronic F68 Prill was obtained from BASF Chemical Company. Fetal bovine serum (FBS) was purchased from Hyclone. T25, T75 flasks, and LUX 60 × 15 mm culture dishes were ordered from Nunclon. Erlenmeyer flasks were from VWR. Low melting temperature Seaplaque agarose was from Cambrex Corp., and 2× Grace's insect medium was from Invitrogen. The Advantage 2 PCR enzyme system was purchased from Clontech. T4 DNA ligase and restriction endonucleases HindIII and XhoI were obtained from New England Biolabs. The mouse monoclonal anti-Hsp72 antibody was purchased from Stressgen. Cytometric bead assay flex sets, anti-mouse CD4 (L3T4), CD8 (Ly2), and anti-mouse CD11c-phycoerythrin conjugated antibody were ordered from BD Biosciences. Fluorescein isothiocyanate-conjugated anti-mouse IgG was from Sigma. The Centricon Ultracel YM-50 column was purchased from Millipore. All other chemicals were reagent grade. BALB/c mice were purchased from Charles River Laboratories.
Cell Culture and Development of Recombinant Viruses
Sf9 insect cells were maintained in suspension culture at a density of 0.5 × 106 to 3 × 106 cells/ml in BacVector Insect Cell Medium with 5% FBS and 0.1% Pluronic F68 Prill. The growth temperature was maintained at 27 °C throughout all the experiments. The cell count was made with a hemocytometer after trypan blue staining; an experimental error of ± 10% must be accounted for. To develop the construct required for expression of the human Hsp72 protein, the human hsp72 open reading frame gene was first synthesized by PCR using pOTB7 vector (containing the MGC-full-length hsp72 gene) as a template and then was cloned into the pBACgus-2cp baculovirus transfer vector at the HindIII and XhoI sites. The pBACgus-2cp vector encoding an N-terminal His-tag followed by an S-peptide tag was used for protein purification. The final recombinant transfer vector pBACgus-72 was sequenced to conform the reading frame, and the sequence of the corresponding gene was inserted. To develop recombinant baculovirus, 2.0 × 106 Sf9 insect cells were seeded into a T25 flask and were co-transfected with 500 ng of recombinant transfer vector pBACgus-72 and 100 ng of BacVector-1000 triple cut linearized A. californica multiple nuclear polyhedrosis virus DNA, using insect GeneJuice transfection reagent as directed by the manufacturer. Supernatant was collected 6 days post-transfection, and the recombinant baculovirus was isolated by plaque assay. The recombinant baculovirus was plaque-purified 3× to eliminate contaminated wild-type baculovirus. The presence of the desired human hsp72 gene in the recombinant virus was confirmed by PCR amplification using two primers EcoRV forward (5′-CCATTGTAATGAGACGCAC-3′) and DOWN1629 reverse (5′-CTGTAAATCAACAACGCACAG-3′). The purified recombinant virus was amplified to generate high titer viral stock according to standard techniques and were used for protein expression.
Recombinant Human Hsp72bv, Purification, and Analysis
Sf9 insect cells were plated onto T75 flasks at a density of 2.0 × 106/flask and allowed adhering to the flask for ∼1 h. High titer recombinant virus was then added to obtain the desired multiplicity of infection. Upon infection, the cells were maintained at 27 °C. The infected cells were collected 96 h post-infection, and 0.05 culture volume of insect PopCulture reagent containing 2× protease inhibitor mixture was added, followed by 4 units benzonase nuclease/1 ml of the original culture volume. The mixture was inverted gently and incubated at room temperature for 15 min. The cell debris was removed by centrifugation for 15 min at 15,000 rpm (4 °C). For purification of His-tagged proteins, the supernatants were subjected to metal chelation column chromatography using Ni-NTA His·Bind resin equilibrated with column buffer (300 mm NaCl, 50 mm sodium phosphate buffer, 1 mm imidazole, pH 8.0). The column was washed twice with 10 ml of wash buffer (300 mm NaCl, 50 mm sodium phosphate buffer, pH 8.0) containing 5 mm imidazole. The bound proteins were eluted with elute buffer (300 mm NaCl, 50 mm sodium phosphate buffer, pH 8.0) containing 250 mm imidazole. Fractions containing Hsp72 fusion proteins were further desalted by a Centricon YM-50 column and identified by SDS-PAGE, immunoblotting, and mass spectrometry analysis. SDS-PAGE and immunoblotting were performed under reducing and denaturing conditions. The appropriate amount of proteins were separated on 12% SDS-PAGE gel and either visualized by Coomassie Blue staining or transferred to a polyvinylidene difluoride transfer membrane. The membranes were blocked with 5% nonfat milk for 1 h at room temperature and incubated overnight with mouse monoclonal anti-Hsp72 antibody at a 1:3,000 dilution overnight at 4 °C. After washing 3× with tris-buffered saline (containing 0.01% Tween 20), the membranes were incubated for 1 h at room temperature at a 1:20,000 dilution of horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (Sigma). Immunoblots were developed by chemiluminescence employing ECL Western blotting reagents (Amersham Biosciences International, UK) according to the manufacturer's instructions. For mass spectrometry analysis, desalted purified Hsp72bv protein was dried completely in a SpeedVac and then was dissolved in 100 μl of 200 mm ammonium bicarbonate. 20 μl of freshly made 10 mm dithiothreitol (made with 100 mm ammonium bicarbonate) was added to the protein solution followed by heating at 65 °C for 1 h. Then 20 μl of freshly prepared 55 mm iodoacetamide (made with 100 mm ammonium bicarbonate) was added, and the samples were wrapped in foil and shaken for 1 h. Proteins were precipitated with 1 ml ice-cold acetone and resuspended in 50 μl of 50 mm ammonium bicarbonate. Samples were subjected to trypsin digestion overnight at 37 °C before mass spectrometry analysis. All of the water used is endotoxin-free water. Purified proteins were analyzed for endotoxin content using the Limulus amebocyte lysate assay (Cambrex Co.). Protein concentration was measured by RC DC protein assay (Bio-Rad).
Calcium Influx Measurement
THP-1 cells were incubated at 37 °C for 30 min in cell-loading medium (RPMI 1640, 10% FBS, 30 mm HEPES, 1 mm CaCl2, 1 mm MgCl2) containing 0.04% pluronic, 3 μm fluo-3 and 9 μm Fura Red (protected from light). Then the cells were spun down and washed twice with 6 ml of wash buffer (RPMI 1640, 10% FBS, 30 mm HEPES, 2 mm probenecid). 0.7 ml cell wash buffer was added to make 1 × 107 cells/ml cell suspension. 200 μl of cell suspension was transferred to a 5-ml tube containing 1 ml of cell wash buffer, wrapped in foil paper, and stored at room temperature in the dark. Samples were warmed up at 37 °C for 5∼10 min and were analyzed on a BD FACSAria flow cytometer (BD Biosciences) equipped with a 488 nm argon laser. Base-line values were recorded for 1 min before the addition of modulators. Data of fluo-3 mean fluorescence intensity at 515–535 nm and Fura Red mean fluorescence intensity at 665–685 nm using linear amplification. At least 10,000 events were collected for each sample. Data were then analyzed using FlowJo software to obtain the mean fluorescence intensity of fluo-3/Fura Red ratio versus time.
Phenotypic Analysis and Cytometric Bead Assay
Spleens were removed from BALB/c mice (6–8 weeks old). Splenocytes were isolated and treated with a hypotonic solution to lyse the erythrocytes. Primary splenocytes were brought to a concentration of 10 × 106 cells/ml in enriched RPMI in 6-well plates. The next day, cells were treated with phosphate-buffered saline, 100 μg/ml of bovine serum albumin (BSA) and 100 μg/ml or 200 μg/ml of recombinant Hsp72bv, respectively. Cultures were incubated at 37 °C for 3 days. Then, the cells and supernatant were separated by spinning down at 1,000 rpm for 5 min. The cell pellets were fixed with 1% paraformaldehyde and then analyzed for cell components. Surface expression of molecules was determined by flow cytometry. Cells (0.5 × 106) were stained with specific antibodies (anti-mouse CD4 (L3T4), CD8 (Ly2), or anti-mouse CD11c-phycoerythrin conjugated antibody) for 30 min on ice (protected from light). Fluorescein isothiocyanate-conjugated anti-mouse IgG secondary antibody was used. BD FACAria and Diva software were used for immunofluorescence analyses. The supernatants were stored at −80 °C for cytokine assay. For cytokine assay, the Cytometric bead assay flex sets, including IFN-γ, IL-12p70, tumor necrosis factor-α, and IL-4, were employed following the standard procedure. Briefly, to prepare the mixed capture beads and the mixed detection reagents, each tube requires 50 μl of the diluted beads. Then, 50 μl of the mixed capture beads and 50 μl of sample was added. After a 1-h incubation at room temperature, 50 μl of mixed detection reagent was added to each assay tube. The assay tubes were incubated for 2 h at room temperature. Thereafter, the assay tubes were washed once with 1 ml of wash buffer. Lastly, 300 μl of wash buffer was added to each assay tube, and the data were collected on a BD FACSAria flow cytometer. Raw data were analyzed using FCAP Array software.
RESULTS
Isolation of Recombinant Baculovirus Encoding Human hsp72 Gene
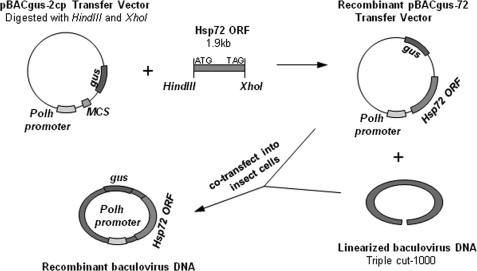
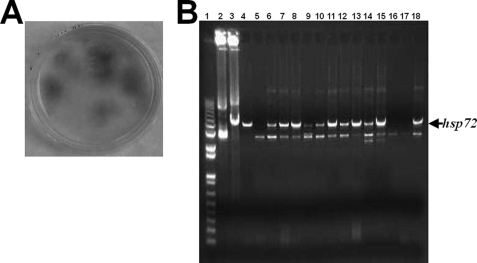
We cloned the entire coding sequence of the human hsp72 gene into the baculovirus transfer plasmid pBACgus-2cp at HindIII and XhoI restriction sites to generate recombinant pBACgus-72 baculovirus transfer vector. The hsp72 gene was located downstream from the baculovirus polyhedron protein promoter (Fig. 1). The recombinant baculovirus transfer vector pBACgus-72 was then co-transfected into Sf9 insect cells with linearized wild-type baculovirus DNA, and the recombinant baculovirus was isolated by plaque staining (Fig. 2A). The chromogenic substrate 5-bromo-4-chloro-3-indolyl-b-d-glucuronide was applied at the last step of plaque staining for the detection of the gus gene. Recombinant pBACgus-containing viruses express β-glucuronidase, which can reduce 5-bromo-4-chloro-3-indolyl-b-d-glucuronide to produce a localized blue color (Fig. 2A). Three rounds of plaque screening were employed to remove any residual wild-type baculovirus. To confirm that the purified isolates contain the human hsp72 gene sequence, the recombinant viral DNAs were isolated and verified by PCR amplification (Fig. 2B) and gene sequencing (data no shown). In a separate experiment, the selected primers were shown to detect Hsp72 in a normal human cell line (data not shown). Only the positive recombinant viral isolates, containing the hsp72 gene, were propagated to generate high titer viral stock for later expression.
FIGURE 1.
Schematic representation of the pBACgus-70 transfer vector construct. The coding sequence of the human hsp72 gene was cloned into baculovirus transfer plasmid pBACgus-2cp between HindIII and XhoI restriction sites to form the pBACgus-70 transfer vector. This pBACgus-70 transfer vector was cotransfected into Sf9 insect cells along with the baculovirus A. californica multiple nuclear polyhedrosis virus linear DNA to form recombinant baculovirus containing the hsp72 gene after the polyhedron (polh) gene promoter in its genome. kb, kilobase; ORF, open reading frame.
FIGURE 2.
Identification of recombinant baculovirus containing target gene. Sf9 insect cells were cotransfected with A. californica multiple nuclear polyhedrosis virus linear DNA and baculovirus transfer vectors pBACgus-70. A, cotransfected Sf9 insect cells were overlaid with SeaPlaque agarose and grown for 5 days. 5-bromo-4-chloro-3-indolyl-b-d-glucuronide (50 μl) was spread on each plate. After 3–24 h, blue plaque identified the cells containing potential recombinant virus. The recombinant virus was purified by three rounds of plaque assay. B, recombinant viral genome was extracted and verified by PCR using EcoRV forward and DOWN1629 reverse primers as described under “Experimental Procedures.” Lane 1, 1-kb DNA ladder; lane 2, pBACgus-2cp transfer vector as negative control; lane 3, pBACgus-70 transfer vector as positive control; lanes 4-18, different viral DNA extracted from independent recombinant viral isolates. The arrow indicated the recombinant baculovirus containing the hsp72 gene within its genome. The results were representative experiments from at least 10 independently performed experiments with similar results.
Production and Purification of Recombinant Human Hsp72bv from Insect Cells
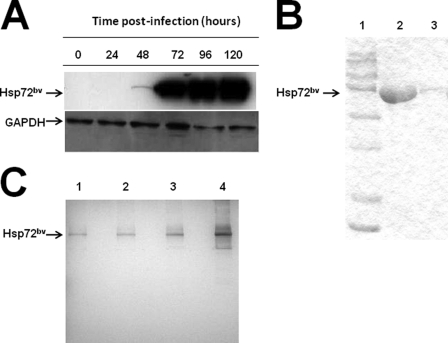
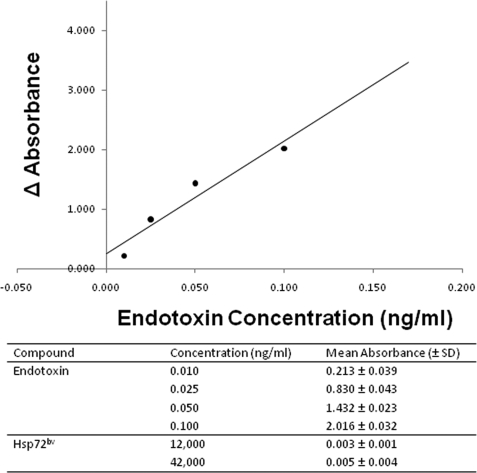
The recombinant human Hsp72bv was expressed in Sf9 insect cells by infection of the purified recombinant baculovirus at a multiplicity of infection of 5. Virus-infected cells were collected at different post-infection time points, and cell lysates were probed with anti-Hsp72 antibody to determine the amount of expression (Fig. 3A). Hsp72bv protein was detectable 48 h post-infection and reached the maximal level at 96 h (Fig. 3A). Recombinant Hsp72bv protein has an N-terminal polyhistidine tag (His-tag) to facilitate protein purification by affinity chromatography. Large amounts of recombinant Hsp72 protein were produced in virus-infected Sf9 insect cells and purified by a Ni-NTA His·Bind column under native conditions. Purified recombinant protein was further desalted and analyzed by SDS-PAGE (Fig. 3B). The Coomassie Blue-stained image demonstrates that the single protein band migrated at ∼72 kDa, and no nonspecific proteins were detectable even when 30 μg of sample was loaded onto the gel. To further confirm the purity of recombinant Hsp72bv, silver staining after SDS-PAGE was performed. Silver staining is a very sensitive tool for protein visualization with a detection level down to the 0.3–10 ng level (25). It is 30–50-fold more sensitive than Coomassie Blue staining. We demonstrate that 50-, 100-, and even 200-ng samples of recombinant Hsp72bv show no other band. When 500 ng of recombinant Hsp72bv was loaded onto the gel, there was only one very faint nonspecific protein band (Fig. 3C). These results confirm that the recombinant Hsp72bv is of very high purity. The purified recombinant Hsp72bv protein was then digested and further identified by mass spectrometry. Bioinformatics confirmed that the purified recombinant protein preparation was indeed Hsp72 and exhibited 71% amino acid coverage with human Hsp72 in the data base (Table 1). Purified recombinant Hsp72bv was further analyzed for the presence of endotoxin by Limulus amebocyte lysate assay. The Limulus amebocyte lysate test is a quantitative test for bacterial endotoxin. The correlation between the absorbance and the endotoxin concentration is linear in the 0.01–0.1 ng/ml range. This in vitro end product endotoxin test can detect as low as 0.01 ng/ml endotoxin in the samples. In our hands, the standard curve of the positive endotoxin control exhibited a regression curve of 0.952 in the range of 0.01–0.1 ng/ml (Fig. 4B). The endotoxin content of 40 μg recombinant Hsp72bv protein sample was below detectable levels (Fig. 4A). These data demonstrate that the recombinant Hsp72bv protein expressed by BEVS in Sf9 cells is free of endotoxin. Taken together, these results confirm that we have successfully produced and purified bioactive endotoxin-free Hsp72bv from insect cells.
FIGURE 3.
Expression of recombinant human Hsp72bv in Sf9 insect cells. A, Sf9 insect cells (2 × 106 cells/ml) were infected with recombinant baculovirus virus containing the hsp72 gene. Samples were collected every 24 h post-infection and examined for the expression of Hsp72 by Western blot analysis. Briefly, membranes were probed with mouse anti-Hsp72 monoclonal antibody (top panel) or anti-GAPDH (loading control; bottom panel) followed by incubation with peroxidase-conjugated goat anti-mouse IgG secondary antibody. Lane 1, 0 h; lane 2, 24 h; lane 3, 48 h, lane 4, 72 h; lane 5, 96 h; and lane 6, 120 h. In a separate experiment 96 h post-infection, cells were collected, and clear cell lysate was applied to a Ni-NTA His·Bind resin column. Purified protein Hsp72bv was collected and desalted using Centricon Ultracel YM-50. Hsp72bv protein was analyzed using SDS-PAGE followed by either Coomassie blue staining (B); lane 1, protein maker; lane 2, 30 μg Hsp72bv; lane 3, 1.5 μg Hsp72bv or Silver staining (C); lane 1, 50 ng Hsp72bv; lane 2, 100 ng Hsp72bv; lane 3, 200 ng Hsp72bv; lane 4, 500 ng Hsp72bv. The data is a representative experiment from three independently performed experiments with similar results.
TABLE 1.
Measurement of recombinant Hsp70bv protein purity
| Hsp72bv (No. of spectra) | Protein molecular mass | Protein pI | Species | Amino acid coverage | Distinct peptides | Protein name |
|---|---|---|---|---|---|---|
| Da | % | n | ||||
| 46 | 70052.6 | 5.48 | Human | 71 | 53 | Hsp70.1 (Hsp70-1/Hsp70-2) |
| 39 | 70185.6 | 5.60 | Human | 57 | 43 | Hsp70-1A/1B (Hsp70-1/2) (Hsp70.1/2) |
| 16 | 71109.6 | 5.77 | Human | 27 | 17 | Hsp70-6 (hsp70-B′) |
| 15 | 71028.5 | 5.81 | Human | 24 | 15 | Hsp70-6 (Hsp70-B′) |
| 6 | 70021.3 | 5.56 | Human | 17 | 10 | Hsp70-2 (Hsp70-2) |
| 0 | 137911.4 | 5.58 | Human | 4 | 3 | Receptor tyrosine-protein kinase ErbB2 precursor (ECs7.10.1; p185ErbB2; C-ErbB2; NEU proto-oncogene; tyrosine kinase-type cell surface receptor Her2; MLN19) |
FIGURE 4.
Recombinant human Hsp72bv expressed in Sf9 insect cells is endotoxin-free. Recombinant human Hsp72bv protein was expressed by BEVS in Sf9 cells. Purified protein was examined for endotoxin contamination using a Limulus amebocyte lysate QCL-1000 kit (Cambrex). Briefly, the determination includes a blank for endotoxin standards in quadruplicate. Blank wells contained 50 μl of Limulus amebocyte lysate reagent water instead of the sample. Absorbance was measured at 405–410 nm. Data are the standard curve using the formula: y = 18.923x + 0.2472 (top panel). The mean absorbance (405–410 nm) and corresponding concentrations of the four standards (endotoxin) ranging from 0.01 to 0.1 ng/ml and recombinant human Hsp72bv protein (bottom panel).
Recombinant Human Hsp72bv Enhanced the Tolerance of neuroblastma Cells to Heat Shock-induced Cell Death
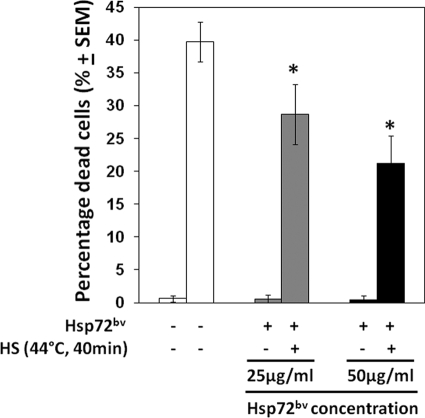
Exogenous Hsp70 renders neuroblastoma cells resistant to heat shock-induced cell death and the apoptotic effects of staurosprine (26). We therefore examined if recombinant Hsp72bv purified from insect cells functions in a similar fashion when added to the human neuroblastoma cell line, SH-SY5Y. SH-SY5Y cells were incubated in FBS-free medium containing different concentration of Hsp72bv (25 μg/ml or 50 μg/ml), respectively; and then subjected to heat shock (44 °C, 40 min). We demonstrated that pretreatment of SH-SY5Y cells with Hsp72bv protein significantly reduced cell death-induced heat shock (44 °C, 40 min), in a dose-dependent fashion but did not significantly affect the viability of cells maintained at control temperatures (37 °C, 40 min) (Fig. 5).
FIGURE 5.
Exogenously added recombinant human Hsp72bv protects neuroblastoma cells against lethal heat stress. Neuroblastoma SH-SY5Y cells (106/ml) were incubated in the presence of recombinant human Hsp72bv protein (25 μg/ml; gray bars) or recombinant human Hsp72bv protein 50 μg/ml; filled bars) or 50 μg/ml BSA (control protein; open bars) for 3 h at 37 °C. Cells were then exposed to heat shock (HS; 44 °C for 40 min), and incubated for a further 24 h at 37 °C, and cell death was measured by trypan exclusion assay. Data represent the percentage of dead cells ± S.E. and are the sum of three independently performed experiments. *, p < 0.05 versus control (BSA).
Recombinant Human Hsp72bv Induces a Rapid Intracellular Calcium Flux
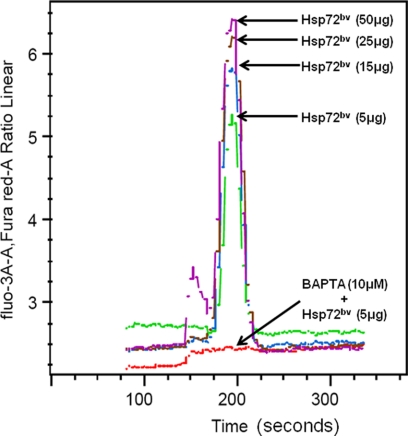
Previous experiments demonstrated that endotoxin does not induce intracellular calcium flux in cells, however, Hsp72 does (12, 27). To determine whether the purified recombinant protein Hsp72bv stimulates intracellular calcium flux, THP-1 cells were treated with recombinant Hsp72bv protein, and intracellular calcium flux was measured by flow cytometry. We demonstrated that the recombinant Hsp72bv elicits a rapid dose-dependent intracellular calcium flux (Fig. 6). Pretreatment of THP-1 monocytes with the intracellular calcium chelator BAPTA-AM completely abrogated recombinant Hsp72bv-induced intracellular calcium flux (Fig. 6).
FIGURE 6.
Recombinant human Hsp72bv induces rapid intracellular calcium flux. THP-1 cells were treated with 3 μm fluo-3 and 9 μm Fura Red cell loading medium as described in detail under “Experimental Procedures.” Briefly, after incubating at 37 °C for 30 min, cells were spun down and washed with 6 ml wash buffer to make 1 × 107cells/ml cell suspension. Samples were warmed up at 37 °C for 5 min and loaded to a BD FACSAria flow cytometer for analysis. Baseline values were initially recorded for 1 min before the addition of 5 μg Hsp72bv (green line), or 15 μg Hsp72bv (blue line), or 25 μg Hsp72bv (brown line), or 50 μg Hsp72bv (purple line), or 10 μm BAPTA + 5 μg Hsp72bv (red line). Data were plotted as fluo-3 fluorescence versus time and Fura Red fluorescence versus time. FlowJo software was used to analyze the fluo-3/Fura Red ratio versus time. The result is a representative experiment from three independently performed experiments with similar results.
Effect of Recombinant Hsp72bv on Cytokine Production and Leukocyte Phenotype
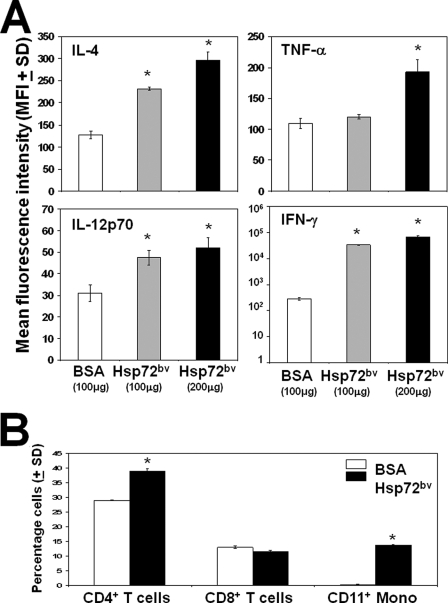
To determine whether the recombinant Hsp72bv protein can enhance proinflammatory cytokine production, primary mouse splenocytes isolated from BALB/c mice were treated for 3 days with 100 μg or 200 μg of Hsp72bv or 100 μg BSA control. We demonstrated that treatment of mouse splenocytes with exogenous recombinant Hsp72bv protein significantly increased the expressions of proinflammatory cytokines tumor necrosis factor-α (1.8-fold), IL-12p70 (1.7-fold), IFN-γ (2,000-fold), and the anti-inflammatory cytokine, IL-4 (2.3-fold) as compared with BSA controls (Fig. 7A). To determine the effect of recombinant Hsp72bv on leukocyte phenotype, splenocytes were treated in a similar fashion, and phenotypic changes were analyzed by flow cytometry. We demonstrated that recombinant Hsp72bv significantly increases the relative number of CD4+ T lymphocyte (1.3-fold) and CD11c+ monocyte (55.2-fold), but not CD8+ T lymphocytes, as compared with BSA controls (Fig. 7B). Taken together, these data suggest that recombinant Hsp72bv protein retains its chaperokine activity.
FIGURE 7.
Effect of recombinant human Hsp72bv on splenocyte functions. Mouse splenocytes (106 cells) were treated with BSA (100 μg) or recombinant Hsp72bv (100 or 200 μg) for 96 h in a 37 °C incubator. A, supernatant was recovered, and IL-4, tumor necrosis factor-α, IL-12p70, or IFN-γ was measured using cytometric bead assay according to the manufacturer's instructions (BD Biosciences) on a BD FACSAria flow cytometer. Raw data were analyzed using FCAP array software. Data are mean fluorescence intensity ± S.D. and are the sum of three independently performed experiments. *, p < 0.05 versus control (BSA). B, splenocytes were collected and stained with anti-mouse CD11-phycoerythrin, CD4-fluorescein isothiocyanate, and CD8-phycoerythrin and analyzed using a BD FACSAria flow cytometer. Individual cells were gated on the basis of forward and orthogonal scatter. The photomultiplier for fluorescein isothiocyanate (FL1-height) or PE (FL2-height) was set on a logarithmic scale. Cell debris was excluded by raising the forward scatter-height photomultiplier tube threshold. The flow rate was adjusted to <200 cells/second, and at least 30,000 cells were analyzed for each sample. Data are the sum of four independently performed experiments. *, p < 0.05 versus control (BSA).
DISCUSSION
In addition to its classical intracellular chaperone function, Hsp72 has been found to also have extracellular functions. Extracellular Hsp72 released through the passive or active pathway is thought to exert a number of immunological properties, including enhancing productions of cytokines and chemokines, promoting cell activation and maturation (5, 28), assisting antigen cross-presentation, and eliciting antigen-specific immunity (29, 30). A number of studies have demonstrated that extracellular Hsp72-peptide complexes can lead to the transport of peptide antigens into APC cells and delivery to MHC class I molecules and, therefore, induce the production of peptide-specific CD8+ cytotoxic T lymphocytes (9, 15, 31, 32). Recently, intense studies have been initiated into the use of extracellular Hsp72 as an agent for tumor immunotherapy (33). The Hsp72-mediated effects on APC have been reported to be receptor-mediated processors occurring via TLR2/4 (11, 34–39). However, it was reported that the two commonly used standards, heat inactivation and polymyxin B inhibition, which rule out the possibility that the observed immunological effects were due to the contamination of endotoxin, were suggested to be inadequate (40). These authors report that endotoxin is heat-sensitive, particularly at low concentrations, and the endotoxin inhibitor, polymyxin B, is not able to completely abolish the function of minute amounts of endotoxin (40). To avoid endotoxin contamination and to circumvent the endotoxin-depletion procedure, in this study, we expressed the recombinant Hsp72 using the BEVS. The most attractive feature offered by the BEVS is its ability to produce significant amounts of the desired protein in a cellular environment that are more likely to have biological activities of the original proteins under the natural condition than proteins expressed in bacterial systems (41). More importantly, using insect cells excludes the possibility of endotoxin contamination. Our data demonstrate that immunologically active recombinant human Hsp72 can be produced in recombinant baculovirus-infected insect cells. There was no detectable endotoxin in the purified recombinant proteins as judged by SDS-PAGE, immunoblotting (Figs. 3 and 4), and mass spectrometry (Table 1).
Previous studies have evaluated the role of Hsp72 in various signal transduction steps and demonstrated that Hsp72 binds with high affinity to the plasma membrane of APC to elicit a rapid intracellular Ca2+ flux within 10 s (12, 27, 42). This is an important distinction between LPS- and Hsp72-mediated events because the treatment of APC with LPS does not result in an intracellular calcium flux (43). Our data demonstrate that recombinant Hsp72bv protein from insect cells elicits a rapid, dose-dependent intracellular calcium flux in human monocytes, which is completely abrogated by pretreatment with the intracellular calcium chelator, BAPTA-AM (Fig. 6). Taken together, these data suggest that the recombinant Hsp72bv is active and has normal chaperokine function attributed to the Hsp72 protein, not endotoxin contamination.
Hsp72 has the unique ability to chaperone antigenic peptides for presentation to the APC and stimulate signaling pathways that subsequently result in the production of inflammatory mediators (11–13, 38, 44, 45). In a similar fashion, the treatment of splenocytes with recombinant Hsp72bv for 3–5 days significantly augmented the expression of proinflammatory cytokines tumor necrosis factor-α, IL-12p70, and IFN-γ (Fig. 7A). Because our working hypothesis is that Hsp72 nonspecifically augments proinflammatory cytokine production, we were initially surprised that recombinant Hsp72bv also increased the expression of the anti-inflammatory cytokine IL-4 (Fig. 7A). This is because IL-4 and IFN-γ reciprocally antagonize each other's actions on B cells, in particular at the level of IgE synthesis (46), and each inhibits the differentiation of naïve T cells into secretors of the other cytokines (47). IL-4 exerts an inhibitory effect on Th1-like responses, and IFN-γ does the same to Th2-like responses. IL-4 also antagonizes the macrophage-activating effects of IFN-γ and inhibits cell-mediated immune reactions. Closer examination of the expression levels suggests that our hypothesis still holds because recombinant Hsp72bv increased IFN-γ expression levels by 2,000-fold, and IL-4 expression by only 2.3-fold (Fig. 7A). Therefore, the regulatory effect of these two cytokines will favor the immune responses of Th1-like proinflammatory immune responses.
CD4+ T lymphocytes play an important role in establishing and maximizing the capabilities of the immune system. They are essential in determining B cell antibody class switching, in the activation and growth of cytotoxic T lymphocytes and in maximizing bactericidal activity of professional phagocytes including monocytes and macrophages. Our data demonstrate that recombinant Hsp72bv treatment increases the relative numbers of CD4+ T lymphocytes and CD11c+ monocytes (Fig. 7B). The observation that recombinant Hsp72bv increased the relative number of CD11c+ mononcytes by 55.3-fold (Fig. 7B), can be explained by the fact that in response to inflammation signals, monocytes move quickly to sites of inflammatory foci and divide/differentiate into macrophage/dendritic cells in preparation for further responses and to elicit an immune response. Recombinant Hsp72bv treatment did not significantly alter the relative number of CD8+ T lymphocytes (Fig. 7B). Because CD8+ T lymphocytes target infected somatic cells and neoplastically transformed tumor cells and because their activation requires MHC class I-restricted antigen, Hsp72bv treatment did not significantly alter the relative number of CD8+ T lymphocytes.
Recombinant proteins have been used extensively to elucidate signaling pathways and their biological significance. Such information has been harnessed to design effective therapy against numerous diseases and disorders. Therefore, the lessons learned in this study are easily applicable to wider biomedical and therapeutic issues. We have demonstrated that the infection of recombinant baculovirus in Sf9 insect cells results in functional Hsp72 protein. The recombinant protein is endotoxin-free and retains its chaperokine activity. This now provides an easy and inexpensive technique for production of specific endotoxin-free Hsp72 proteins. Studies are now underway in our laboratory to use this protein to elucidate the mechanism by which the HSP-based vaccination enhances anti-tumor immunity and inhibits tumor growth.3
Acknowledgments
We thank the Scott & White Proteomics Core Facility for expert proteomics assistance.
This work was supported in part by National Institutes of Health Grant RO1CA91889. This work was also supported by institutional support from Scott & White Memorial Hospital and Clinic, TX A&M Health Science Center College of Medicine, the Central Texas Veterans Health Administration, and an endowment from the Cain Foundation (to A. A.).
Zheng, H., Nagaraja, G. M., Kaur, P., Asea, E. E., and Asea, A., manuscript in preparation.
- HSP
- heat shock proteins
- APC
- antigen-presenting cells
- BEVS
- baculovirus expression vector system
- BSA
- bovine serum albumin
- IFN
- interferon
- Ni-NTA
- nickel-nitrilotriacetic acid
- FBS
- fetal bovine serum
- IL
- interleukin
- BAPTA-AM
- 1,2-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, tetraacetoxymethyl ester.
REFERENCES
- 1.Hartl F. U. (1996) Nature 381, 571–579 [DOI] [PubMed] [Google Scholar]
- 2.Lindquist S., Craig E. A. (1988) Annu. Rev. Genet. 22, 631–677 [DOI] [PubMed] [Google Scholar]
- 3.Asea A. (2008) Novartis. Found. Symp. 291, 173–179; discussion 291, 179–183 [DOI] [PubMed] [Google Scholar]
- 4.Asea A. (2006) Curr. Immunol. Rev. 2, 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asea A. (2007) in Heat Shock Proteins: Potent Mediators of Inflammation and Immunity (Asea A., De Maio A. eds) Vol. 1, pp. 3–20, Springer Publishers, Dordrecht, The Netherlands [Google Scholar]
- 6.Asea A. (2005) Exerc. Immunol. Rev. 11, 34–45 [PMC free article] [PubMed] [Google Scholar]
- 7.Calderwood S. K., Theriault J. R., Gong J. (2005) Eur. J. Immunol. 35, 2518–2527 [DOI] [PubMed] [Google Scholar]
- 8.Takakura Y., Takemoto S., Nishikawa M. (2007) Curr. Opin. Mol. Ther. 9, 385–391 [PubMed] [Google Scholar]
- 9.Srivastava P. K., Udono H., Blachere N. E., Li Z. (1994) Immunogenetics 39, 93–498 [DOI] [PubMed] [Google Scholar]
- 10.Srivastava P. K. (1994) Experientia. 50, 1054–1060 [DOI] [PubMed] [Google Scholar]
- 11.Asea A., Rehli M., Kabingu E., Boch J. A., Bare O., Auron P. E., Stevenson M. A., Calderwood S. K. (2002) J. Biol. Chem. 277, 15028–15034 [DOI] [PubMed] [Google Scholar]
- 12.Asea A., Kraeft S. K., Kurt-Jones E. A., Stevenson M. A., Chen L. B., Finberg R. W., Koo G. C., Calderwood S. K. (2000) Nat. Med. 6, 435–442 [DOI] [PubMed] [Google Scholar]
- 13.Asea A., Kabingu E., Stevenson M. A., Calderwood S. K. (2000) Cell Stress Chaperones 5, 425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehner T., Bergmeier L. A., Wang Y., Tao L., Sing M., Spallek R., van der Zee R. (2000) Eur. J. Immunol. 30, 594–603 [DOI] [PubMed] [Google Scholar]
- 15.Srivastava P. (2002) Nat. Rev. Immunol. 2, 185–194 [DOI] [PubMed] [Google Scholar]
- 16.Srivastava P. K., Menoret A., Basu S., Binder R. J., McQuade K. L. (1998) Immunity 8, 657–665 [DOI] [PubMed] [Google Scholar]
- 17.Manjili M. H., Wang X. Y., MacDonald I. J., Arnouk H., Yang G. Y., Pritchard M. T., Subjeck J. R. (2004) Expert Opin. Biol. Ther. 4, 363–373 [DOI] [PubMed] [Google Scholar]
- 18.Ménoret A. (2004) Methods 32, 7–12 [DOI] [PubMed] [Google Scholar]
- 19.Gao B., Tsan M. F. (2003) J. Biol. Chem. 278, 22523–22529 [DOI] [PubMed] [Google Scholar]
- 20.Gao B., Tsan M. F. (2003) J. Biol. Chem. 278, 174–179 [DOI] [PubMed] [Google Scholar]
- 21.Gao B., Tsan M. F. (2004) Biochem. Biophys. Res. Commun. 317, 1149–1154 [DOI] [PubMed] [Google Scholar]
- 22.Bausinger H., Lipsker D., Ziylan U., Manié S., Briand J. P., Cazenave J. P., Muller S., Haeuw J. F., Ravanat C., de la Salle H., Hanau D. (2002) Eur. J. Immunol. 32, 3708–3713 [DOI] [PubMed] [Google Scholar]
- 23.Ye Z., Gan Y. H. (2007) J. Biol. Chem. 282, 4479–4484 [DOI] [PubMed] [Google Scholar]
- 24.Summers M. D. (2006) Adv. Virus Res. 68, 3–73 [DOI] [PubMed] [Google Scholar]
- 25.Switzer R. C., 3rd, Merril C. R., Shifrin S. (1979) Anal. Biochem. 98, 231–237 [DOI] [PubMed] [Google Scholar]
- 26.Guzhova I., Kislyakova K., Moskaliova O., Fridlanskaya I., Tytell M., Cheetham M., Margulis B. (2001) Brain Res. 914, 66–73 [DOI] [PubMed] [Google Scholar]
- 27.MacAry P. A., Javid B., Floto R. A., Smith K. G., Oehlmann W., Singh M., Lehner P. J. (2004) Immunity 20, 95–106 [DOI] [PubMed] [Google Scholar]
- 28.Pockley A. G. (2003) Lancet 362, 469–476 [DOI] [PubMed] [Google Scholar]
- 29.Noessner E., Gastpar R., Milani V., Brandl A., Hutzler P. J., Kuppner M. C., Roos M., Kremmer E., Asea A., Calderwood S. K., Issels R. D. (2002) J. Immunol. 169, 5424–5432 [DOI] [PubMed] [Google Scholar]
- 30.Castelli C., Rivoltini L., Rini F., Belli F., Testori A., Maio M., Mazzaferro V., Coppa J., Srivastava P. K., Parmiani G. (2004) Cancer Immunology Immunotherapy 53, 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava P. (2002) Annu. Rev. Immunol. 20, 395–425 [DOI] [PubMed] [Google Scholar]
- 32.Bendz H., Ruhland S. C., Pandya M. J., Hainzl O., Riegelsberger S., Braüchle C., Mayer M. P., Buchner J., Issels R. D., Noessner E. (2007) J. Biol. Chem. 282, 31688–31702 [DOI] [PubMed] [Google Scholar]
- 33.Srivastava P. K., Udono H. (1994) Curr. Opin. Immunol. 6, 728–732 [DOI] [PubMed] [Google Scholar]
- 34.Akira S., Takeda K., Kaisho T. (2001) Nat. Immunol. 2, 675–680 [DOI] [PubMed] [Google Scholar]
- 35.Pulendran B., Palucka K., Banchereau J. (2001) Science 293, 253–256 [DOI] [PubMed] [Google Scholar]
- 36.Kaur P., Asea A. (2009) in Prokaryotic and Eukaryotic Heat Shock Proteins in Infectious Disease (Pockley A. G., Calderwood S. D., Santoro M. G. eds) Vol. 4, pp. 153–167, Springer Publishers, Dordrecht, The Netherlands [Google Scholar]
- 37.Asea A. (2008) Handb. Exp. Pharmacol. 183, 111–127 [DOI] [PubMed] [Google Scholar]
- 38.Vabulas R. M., Ahmad-Nejad P., Ghose S., Kirschning C. J., Issels R. D., Wagner H. (2002) J. Biol. Chem. 277, 15107–15112 [DOI] [PubMed] [Google Scholar]
- 39.Vabulas R. M., Wagner H., Schild H. (2002) Curr. Top Microbiol. Immunol. 270, 169–184 [DOI] [PubMed] [Google Scholar]
- 40.Wallin R. P., Lundqvist A., Moré S. H., von Bonin A., Kiessling R., Ljunggren H. G. (2002) Trends Immunol. 23, 130–135 [DOI] [PubMed] [Google Scholar]
- 41.King L. A. a. P., R. D. (1992) The Baculovirus Expression System: A Laboratory Guide, Chapman & Hall, New York [Google Scholar]
- 42.Johnson J. D., Campisi J., Sharkey C. M., Kennedy S. L., Nickerson M., Fleshner M. (2005) J. Appl. Physiol 99, 1789–1795 [DOI] [PubMed] [Google Scholar]
- 43.McLeish K. R., Dean W. L., Wellhausen S. R., Stelzer G. T. (1989) Inflammation 13, 681–692 [DOI] [PubMed] [Google Scholar]
- 44.Li Z., Menoret A., Srivastava P. (2002) Curr. Opin. Immunol. 14, 45–51 [DOI] [PubMed] [Google Scholar]
- 45.Panjwani N. N., Popova L., Srivastava P. K. (2002) J. Immunol. 168, 2997–3003 [DOI] [PubMed] [Google Scholar]
- 46.Snapper C. M., Paul W. E. (1987) Science 236, 944–947 [DOI] [PubMed] [Google Scholar]
- 47.Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. (1992) J. Immunol. 148, 2142–2147 [PubMed] [Google Scholar]