Abstract
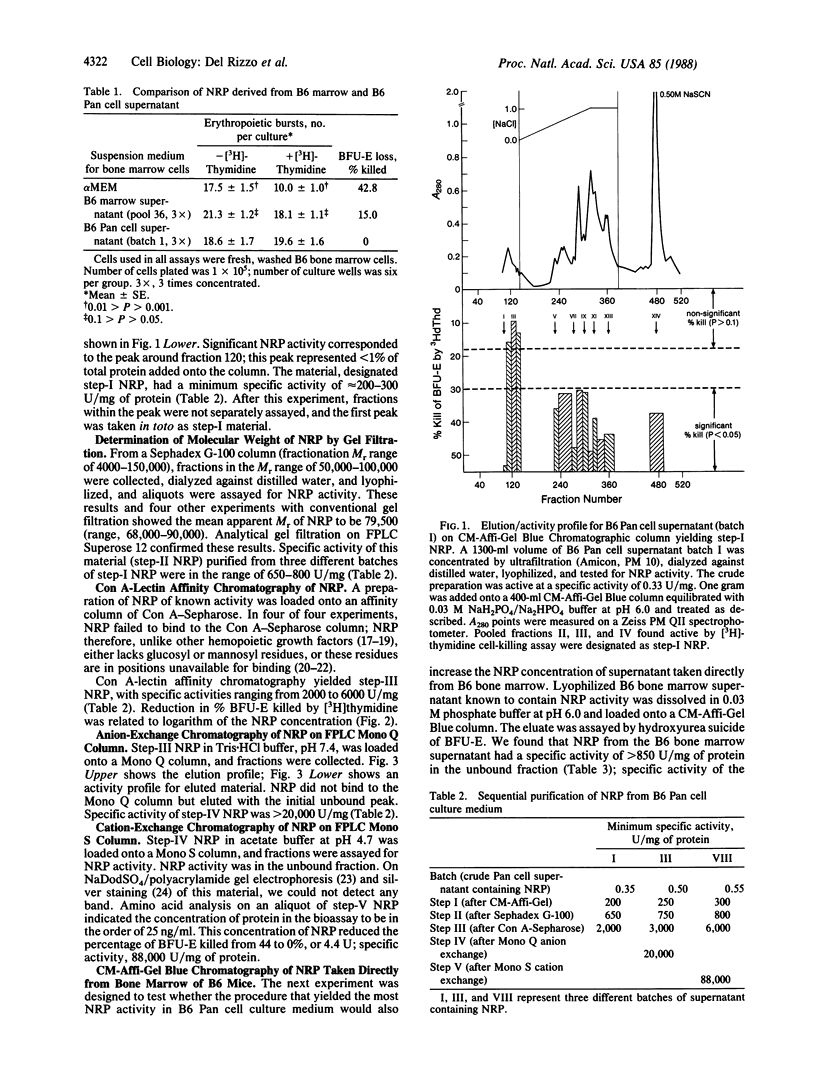
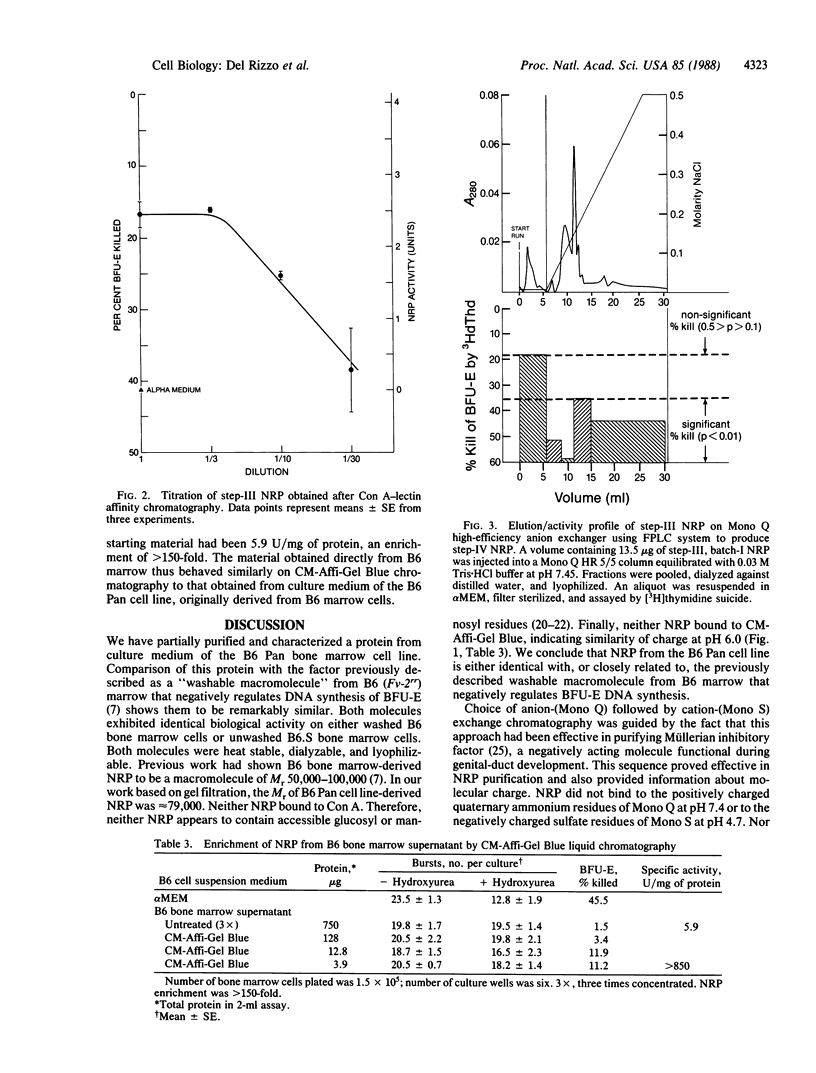
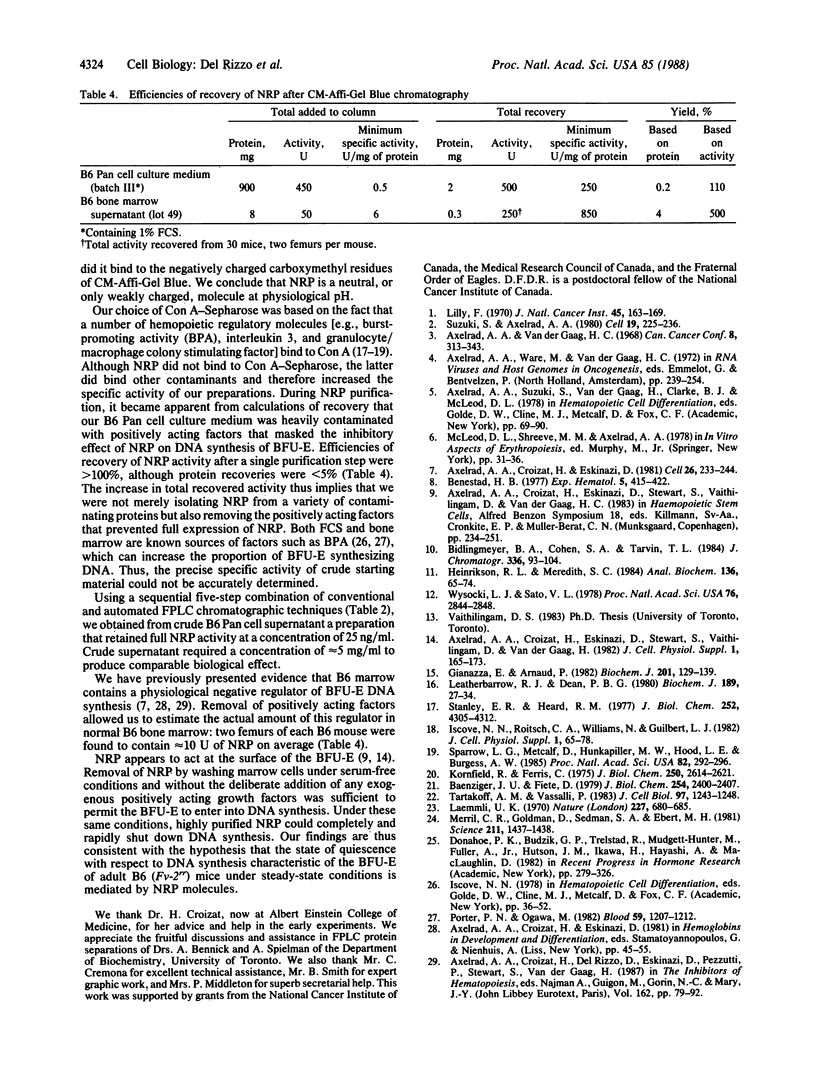
During studies on the influence of Fv-2 on the cycle state of the erythroid burst-forming unit (BFU-E), an activity was found in bone marrow supernatants from C57BL/6 (B6) mice that shut down DNA synthesis of the BFU-E in vitro. It acted within minutes, its action was completely reversed by a single wash, and it appeared specific to the BFU-E. The activity-causing substance, being macromolecular, heat stable, and trypsin-sensitive, was evidently a protein and was named negative regulatory protein. We purified a negative regulatory protein from a bone marrow-derived "B6 Pan" cell line with properties thus far indistinguishable from those of the negative regulatory protein obtained directly from B6 marrow. By gel filtration the protein has a Mr of approximately equal to 79,000, by cation- and anion-exchange chromatography it appears to be a neutral molecule at physiological pH, and the molecule does not bind to Con A. After five sequential chromatographic steps, we obtained a preparation active at a concentration of 25 ng/ml. Our findings are compatible with the hypothesis that quiescence of BFU-E with respect to DNA synthesis in vivo in B6 mice is mediated by negative regulatory protein molecules.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad A. A., Croizat H., Eskinazi D. A washable macromolecule from Fv2rr marrow negatively regulates DNA synthesis in erythropoietic progenitor cells BFU-E. Cell. 1981 Oct;26(2 Pt 2):233–244. doi: 10.1016/0092-8674(81)90306-8. [DOI] [PubMed] [Google Scholar]
- Axelrad A., Croizat H., Eskinazi D., Stewart S., Vaithilingam D., van der Gaag H. Gene controlled negative regulation of DNA synthesis in erythropoietic progenitor cells. J Cell Physiol Suppl. 1982;1:165–173. doi: 10.1002/jcp.1041130424. [DOI] [PubMed] [Google Scholar]
- Axelrad A. Genetic and cellular basis of susceptibility or resistance to Friend leukemia virus infection in mice. Proc Can Cancer Conf. 1969;8:313–343. [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979 Apr 10;254(7):2400–2407. [PubMed] [Google Scholar]
- Benestad H. B. Hydroxyurea (HU) in experimental hematology. II. Similarities and dissimilarities between HU and 3H-thymidine killing. Exp Hematol. 1977 Sep;5(5):415–422. [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Donahoe P. K., Budzik G. P., Trelstad R., Mudgett-Hunter M., Fuller A., Jr, Hutson J. M., Ikawa H., Hayashi A., MacLaughlin D. Müllerian-inhibiting substance: an update. Recent Prog Horm Res. 1982;38:279–330. doi: 10.1016/b978-0-12-571138-8.50013-5. [DOI] [PubMed] [Google Scholar]
- Gianazza E., Arnaud P. A general method for fractionation of plasma proteins. Dye-ligand affinity chromatography on immobilized Cibacron blue F3-GA. Biochem J. 1982 Jan 1;201(1):129–136. doi: 10.1042/bj2010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Roitsch C. A., Williams N., Guilbert L. J. Molecules stimulating early red cell, granulocyte, macrophage, and megakaryocyte precursors in culture: similarity in size, hydrophobicity, and charge. J Cell Physiol Suppl. 1982;1:65–78. doi: 10.1002/jcp.1041130412. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Ferris C. Interaction of immunoglobulin glycopeptides with concanavalin A. J Biol Chem. 1975 Apr 10;250(7):2614–2619. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Dean P. D. Studies on the mechanism of binding of serum albumins to immobilized cibacron blue F3G A. Biochem J. 1980 Jul 1;189(1):27–34. doi: 10.1042/bj1890027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Porter P. N., Ogawa M. Characterization of human erythroid burst-promoting activity derived from bone marrow conditioned media. Blood. 1982 Jun;59(6):1207–1212. [PubMed] [Google Scholar]
- Sparrow L. G., Metcalf D., Hunkapiller M. W., Hood L. E., Burgess A. W. Purification and partial amino acid sequence of asialo murine granulocyte-macrophage colony stimulating factor. Proc Natl Acad Sci U S A. 1985 Jan;82(2):292–296. doi: 10.1073/pnas.82.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Suzuki S., Axelrad A. A. Fv-2 locus controls the proportion of erythropoietic progenitor cells (BFU-E) synthesizing DNA in normal mice. Cell. 1980 Jan;19(1):225–236. doi: 10.1016/0092-8674(80)90404-3. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Lectin-binding sites as markers of Golgi subcompartments: proximal-to-distal maturation of oligosaccharides. J Cell Biol. 1983 Oct;97(4):1243–1248. doi: 10.1083/jcb.97.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]


