Abstract
In animals, thiamine deficiency leads to specific brain lesions, generally attributed to decreased levels of thiamine diphosphate, an essential cofactor in brain energy metabolism. However, another far less abundant derivative, thiamine triphosphate (ThTP), may also have a neuronal function. Here, we show that in the rat brain, ThTP is essentially present and synthesized in mitochondria. In mitochondrial preparations from brain (but not liver), ThTP can be produced from thiamine diphosphate and Pi. This endergonic process is coupled to the oxidation of succinate or NADH through the respiratory chain but cannot be energized by ATP hydrolysis. ThTP synthesis is strongly inhibited by respiratory chain inhibitors, such as myxothiazol and inhibitors of the H+ channel of F0F1-ATPase. It is also impaired by disruption of the mitochondria or by depolarization of the inner membrane (by protonophores or valinomycin), indicating that a proton-motive force (Δp) is required. Collapsing Δp after ThTP synthesis causes its rapid disappearance, suggesting that both synthesis and hydrolysis are catalyzed by a reversible H+-translocating ThTP synthase. The synthesized ThTP can be released from mitochondria in the presence of external Pi. However, ThTP probably does not accumulate in the cytoplasm in vivo, because it is not detected in the cytosolic fraction obtained from a brain homogenate. Our results show for the first time that a high energy triphosphate compound other than ATP can be produced by a chemiosmotic type of mechanism. This might shed a new light on our understanding of the mechanisms of thiamine deficiency-induced brain lesions.
Keywords: Alcohol, Immunophilin, Mass Spectrometry (MS), Receptor regulation, Ubiquitination, Brain, Chemiosmotic Mechanism, Thiamine Triphosphate
Introduction
Thiamine (vitamin B1) is essential for brain function, and its deficiency causes specific brain lesions (1, 2). It is thought that this selective vulnerability results from decreased levels of thiamine diphosphate (ThDP),3 an indispensable cofactor for several key enzymes in cell energy metabolism (especially pyruvate and oxoglutarate dehydrogenases and transketolase).
In the brain, as in most other mammalian tissues, ThDP is the most abundant thiamine derivative, amounting to 80–90% of total thiamine, but several other thiamine derivatives, such as ThTP, might play physiological roles (3, 4). Recently, we discovered the first thiamine adenine nucleotide, adenosine thiamine triphosphate (AThTP), that is accumulated in Escherichia coli during carbon starvation (5). AThTP is also present in mammalian tissues. Furthermore, another adenine thiamine nucleotide, adenosine thiamine diphosphate (AThDP), seems to exist, at least in the liver (6). Although ThTP is found in most organisms, from bacteria to mammals, it exists only in small amounts (0.1–1% of total thiamine) in animal tissues (7), but much higher amounts of ThTP are found in E. coli during amino acid starvation (8).
In eukaryotic organisms, the physiological role(s) of ThTP remain unknown. In view of the particular sensitivity of the nervous system to thiamine deprivation, some authors considered over 30 years ago that nerve cells might contain a specific “neuroactive” form of thiamine, different from ThDP, and that it might be ThTP (9–12).
There was, however, no firm experimental basis for this hypothesis. Indeed, the amounts of ThTP present in nerve and other tissue could be accurately and reliably determined only after the development of HPLC methods in the early 1980s (4, 6). Actually, the compound(s) identified as ThTP in earlier studies may not have been authentic ThTP, and the existence of a “neuroactive” form of thiamine other than ThDP has remained doubtful.
More recently, it was shown that ThTP activates high conductance anion channels in excised patches of neuroblastoma cells, possibly via a phosphorylation-dependent mechanism (13). Later, it was shown that ThTP may indeed phosphorylate proteins (14). Although there is presently no proof that these effects are of physiological significance, it is possible that ThTP may be part of a signaling cascade involved in some kind of cellular regulation.
ThTP can be hydrolyzed by thiamine triphosphatases (ThTPases). Membrane-bound ThTPases are present in many animal tissues, including rat brain (15, 16), but so far, those enzymes could be neither purified nor characterized at the molecular level. However, a soluble 25-kDa cytosolic ThTPase has been purified from bovine brain (17) and characterized at the molecular level (18). This enzyme exhibits nearly absolute specificity for ThTP and a high catalytic efficiency.
Although ThTP hydrolysis has been rather well studied, the mechanism of its synthesis remains unclear. Eckert and Möbus (19) originally proposed that ThTP synthesis is catalyzed by a ThDP kinase (ThDP:ATP phosphoryltransferase), according to the reaction, ThDP + ATP ⇆ ThTP + ADP. In 1984, Chernikevich et al. (20) reported the purification of a ThDP kinase from brewers' yeast, but the specific activity of the enzyme was very low (kcat on the order of 1 min−1). Moreover, the authenticity of ThTP was not checked in these studies. It is possible that the reaction product was AThTP or AThDP rather than ThTP. Indeed, AThTP may be synthesized in vitro by a soluble high molecular mass enzyme complex according to the reaction ThDP + ATP (ADP) ⇆ AThTP + PPi (Pi) (21). On the other hand, Nishino et al. (22) reported the purification of a ThDP kinase from a crude bovine brain mitochondrial fraction, but in this case, the substrate was protein-bound rather than free ThDP. Again, the purified preparation had a very low specific activity, and these results could not be reproduced elsewhere (23).
At the end of the 1980s, Kawasaki and co-workers (24–26) demonstrated that adenylate kinase (AK; EC 2.7.4.3) 1 (AK1; a cytosolic form found mainly in skeletal muscle) was able to synthesize ThTP according to the reaction ThDP + ADP ⇆ ThTP + AMP. They suggested that this reaction, although very slow (kcat ∼ 0.5 min−1 at physiological pH) (25) was of physiological significance. However, the observation that transgenic mice deficient in AK1 have normal ThTP levels was not in agreement with this conclusion (27). Furthermore, we recently showed that when an E. coli strain with a thermo-sensitive AK was grown in minimal medium supplemented with glucose, the ThTP level strongly increased, although the AK activity was completely inactivated (28). All AKs that we tested had the property of synthesizing ThTP at a very slow rate, but this reaction did not appear to be the main mechanism for ThTP synthesis.
Except for AK, we have been unable to measure any ThTP-synthesizing activity involving a soluble enzyme, but we have previously shown that when rat brain homogenates were incubated with ThDP, a significant amount of ThTP was detected in the particulate fraction containing cell debris, synaptosomes, and mitochondria (29, 30).
At that time, we thought that ThTP synthesis took place inside the synaptosomal compartment, but this poses the problem of the transport of ThDP into these structures. Indeed, there is no known ThDP transporter in plasma membranes, but the internal mitochondrial membrane does contain a ThDP transporter (31, 32). It was therefore possible that the synthesis observed took place inside the free mitochondria rather than in the synaptosomes.
The present study demonstrates that ThTP synthesis indeed occurs in the mitochondrial matrix with ThDP as precursor, but we find, unexpectedly, that the phosphate donor is not ATP or ADP but Pi. We further demonstrate that the free energy required for driving the reaction ThDP + Pi ⇆ ThTP + H2O is provided by electron transfer through the respiratory chain and the resulting proton-motive force. Thus, ThTP synthesis in brain mitochondria appears to be carried out through an utterly new type of mechanism reminiscent of ATP synthesis, which ceases to be operative when the organelle is disrupted.
EXPERIMENTAL PROCEDURES
Chemicals and Radiochemicals
DMSO and tetrahydrofuran (HPLC grade) were from Biosolve B.V. Percoll, mannitol, sucrose, EDTA, EGTA, bovine serum albumin (essentially fatty acid-free), sodium pyruvate, succinic acid, l-malic acid, Triton X-100, antimycin A, stigmatellin, myxothiazol, oligomycin, N,N′-dicyclohexylcarbodiimide (DCCD), carbonyl cyanide m-chlorophenylhydrazone (CCCP), 2,4-dinitrophenol, digitonin, ATP, ADP, and ThDP were from Sigma. Valinomycin was from Fluka (Sigma). Protease inhibitor mixture was from Roche Applied Science. All solutions were prepared with Milli-Q water. Radiolabeled phosphoric acid (H332PO4, 314–337 TBq/mmol, 370 MBq/ml) was from PerkinElmer Life Sciences. ThTP was prepared as previously described (33).
Preparation of Cytosolic and Mitochondrial Fractions
All animal experiments were made following the directives of the committee for animal care and use of the University of Liège, in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Rat brain mitochondria were prepared by a modification of the method described by Rosenthal et al. (34). Male Wistar rats (200 g) were killed by decapitation. The brain was rapidly removed, minced, and homogenized on ice with a motor-driven glass-Teflon Potter-Elvehjem homogenizer in 10 ml of homogenizing medium A (225 mm mannitol, 75 mm sucrose, 7.5 mm Hepes, 0.5 mm EDTA, 0.1% (w/v) bovine serum albumin (fatty acid-free), pH adjusted to 7.3 with KOH) in the presence of protease inhibitor mixture and filtered on a double layer of gauze. The homogenate was then centrifuged at 1000 × g for 10 min in a Beckman Avanti 30 centrifuge fixed angle rotor. The supernatant was carefully decanted, and the pellet (P1; Table 1) was precipitated with 2 ml of trichloroacetic acid 12%).
TABLE 1.
Distribution of ThTP in subcellular fractions from rat brain
Subcellular fractions were isolated on a Percoll gradient, and ThTP was determined in each fraction by HPLC. The specific activities of two marker enzymes (fumarase and LDH) were measured in the synaptosomal and mitochondrial fractions. ThTP synthesis was determined in synaptosomes and free mitochondria. To estimate the rate of synthesis, the ThTP content of mitochondria was measured after a 10-min incubation (37 °C) in the presence of ThDP (1 mm), succinate (5 mm), and Pi (5 mm). The incubation medium contained KCl (140 mm), MgCl2 (2 mm), EGTA (0.5 mm), K-MOPS (10 mm, pH adjusted to 7.3 with KOH). Means ± S.D. for four experiments.
| ThTP content | Fumarase | LDH | ThTP synthesis | |
|---|---|---|---|---|
| pmol mg−1 | nmol mg−1min−1 | nmol mg−1min−1 | pmol mg−110 min−1 | |
| Homogenate | 0.59 ± 0.24 | |||
| P1 | 0.99 ± 0.36 | |||
| Myelin | 0.40 ± 0.08 | |||
| Synaptosomes | 0.70 ± 0.21 | 0.38 ± 0.06 | 1.4 ± 0.2 | 4.6 ± 0.7 |
| Mitochondria | 3.5 ± 0.6a | 1.04 ± 0.07b | 0.19 ± 0.01a | 60 ± 4c |
ap < 0.01, analysis of variance followed by Dunnett's test for comparison with the synaptosomes.
b p < 0.05.
c p < 0.001.
The supernatant was centrifuged at 11,000 × g for 10 min, and the pellet, containing free mitochondria and synaptosomes, was resuspended in 10 volumes of medium A containing digitonin 0.015% to disrupt synaptosomal plasma membranes and release the mitochondria trapped within (34). After 30 s at 4 °C, the suspension was centrifuged at 11,000 × g for 10 min, the pellet was resuspended in medium B (225 mm mannitol, 75 mm sucrose, 7.5 mm Hepes, 0.5 mm EDTA, pH adjusted to 7.4 with KOH). The suspension, called digitonin-treated fraction (DTMF), was split in several aliquots that were kept on ice until use, generally within 1 h.
The protocol for the isolation of rat liver mitochondria was a modification of previously published methods (35, 36); the liver was removed, minced, and homogenized on ice in 40 ml of solution 1 (220 mm mannitol, 70 mm sucrose, 5 mm Hepes, 0.5 mm EDTA, 0.3% bovine serum albumin (fatty acid-free), pH adjusted to pH 7.4 with KOH). After homogenization, a centrifugation (600 × g, 10 min) was performed, the supernatant was decanted, and the previous step was repeated in half of the original volume. The supernatants were pooled and centrifuged at 7000 × g for 10 min. The resulting pellet contained the mitochondria, which were resupended in 40 ml of solution 2 (220 mm mannitol, 70 mm sucrose, 5 mm Hepes, and 0.1 mm EGTA, pH adjusted to 7.4 with KOH) and centrifuged (7000 × g, 10 min). The final pellet was kept on ice and used within 1 h. Mouse and quail brain mitochondrial fractions were prepared as described for the rat brain.
Cytosolic fractions of various rat tissues and quail brain were prepared after homogenization in medium A using a glass- Teflon Potter-Elvehjem homogenizer and centrifugation (100,000 × g, 1 h). Proteins were precipitated by the addition of 40 μl of trichloroacetic acid (72%) to 200 μl of supernatant (cytosolic fraction) and 2 ml of trichloroacetic acid (12%) to the pellet. Protein concentration was determined by the method of Peterson (37).
Isolation and Purification of Mitochondria from Rat Brain Using a Discontinuous Percoll Gradient
The protocol was adapted from previously published methods (38, 39). The rat brains were homogenized as described above. After centrifugation (1000 × g, 10 min), the supernatant was carefully decanted, and the pellet was resuspended in half of the original volume. The previous step was repeated, and the supernatants were pooled (S1). The discontinuous density gradient was prepared in 15 × 97-mm centrifuge tubes by layering 4 ml of S1 on three preformed layers consisting of 3 ml of 10% Percoll, 3 ml of 20% Percoll, and 3 ml of 40% Percoll. The gradients were centrifuged for 13 min at 30,000 × g in a Beckman Avanti 30 centrifuge fixed angle rotor. The synaptosomal fraction was near the interface between the 20 and 10% Percoll layers, whereas the fraction at the interface between the 40 and 20% Percoll layers corresponded to free mitochondria. The myelin fraction was in the 10% layer. The fractions were collected using a Pasteur pipette and diluted with medium B. The suspensions were centrifuged at 15,000 × g for 20 min. The pellets were washed once again to eliminate the residual Percoll and resuspended in a medium suitable for assay of ThTP synthesis (see below) in Eppendorf tubes on ice. Cross-contamination between synaptosomal and mitochondrial fractions was tested by the enzyme markers fumarase (40) and lactate dehydrogenase (41).
Electron Microscopy
Different fractions were fixed in 2.5% glutaraldehyde in 0.1 m phosphate (Sorensen) buffer (pH 7.4, 30 min, 4 °C) and postfixed in 0.1 m phosphate buffer containing 1% OsO4 (aqueous osmium tetroxide) for 30 min at 4 °C. Then the preparations were dehydrated in a graded series of ethanol and embedded in Epon. Ultrathin sections (50–80 nm) were contrasted with uranyl acetate and lead citrate before examination with a Jeol (Tokyo, Japan) CX100 electron microscope at 60 kV.
Estimation of ThTP Synthesis in Mitochondria
The mitochondrial preparations were incubated at 30 or 37 °C, under different conditions. Unless otherwise stated, the incubation medium was a saline containing 140 mm KCl, 2 mm MgCl2, 0.5 mm EGTA, 10 mm K-MOPS, pH adjusted to 7.3 with KOH. A small volume of mitochondrial preparation was added so that the final protein concentration was about 0.7 mg/ml. The final volume of incubation medium was 1 ml. The duration of incubation was usually 10 or 15 min. When the incubation medium contained added ThDP, the mitochondria were washed twice by centrifugation (11,000 × g, 10 min) in medium B in order to remove excess ThDP that would interfere with ThTP estimation. The final pellet was resuspended in 200 μl of 12% trichloroacetic acid and centrifuged at 5000 × g for 3 min. The supernatant was extracted with diethyl ether, and thiamine derivatives were determined by HPLC (42).
In order to check whether ThTP synthesized in mitochondria is free or bound to matrix proteins, the mitochondrial pellet was suspended in 500 μl of hypotonic phosphate buffer (10 mm, pH 7.5) for 10 min and freeze-thawed (−80 °C) twice. After centrifugation (20,000 × g, 10 min), the pellet was suspended in 200 μl of 12% trichloroacetic acid, and 80 μl of 72% trichloroacetic acid were added to the supernatant (400 μl). After centrifugation and trichloroacetic acid extraction, ThTP was determined in both fractions.
The authenticity of ThTP synthesized was checked by enzymatic hydrolysis using a specific recombinant ThTPase, as previously described (7). To 50 μl of mitochondrial trichloroacetic acid extract treated with diethyl ether we added 10 μl of Bistris propane buffer (final concentration 156 mm, pH 8.0), 2 μl of MgCl2 (final concentration 3.125 mm), and 2 μl of (9.6 mg/ml) purified human recombinant ThTPase (43). The mixture was incubated at 37 °C for 30 min. The samples were then analyzed by HPLC.
Functional Characterization of Isolated Mitochondria
Oxygen consumption was measured polarographically using a Clark-type electrode (Hansatech, King's Lynn, Norfolk, UK) in a 2-ml cell at 25 °C in the same saline as for ThTP synthesis (140 mm KCl, 2 mm MgCl2, 0.5 mm EGTA, and 10 mm K-MOPS, pH adjusted to 7.3 with KOH) in the presence of Pi (5 mm), succinate (5 mm), and ADP (0.2 mm). Typically, the respiratory control ratio (RCR) is calculated from the ratio of O2 consumption at state 3 over state 4, but because our preparation contained a significant ATPase activity, we instead estimated RCR from state 3 over state 2 respiration. The uncoupled respiratory control (URC) was calculated from the ratio of O2 consumption in the presence of CCCP (1 μm) over the O2 consumption in the presence of oligomycin (1 μg/ml). ATP synthesis was measured in the presence of Pi (5 mm), ADP (0.2 mm), succinate (5 mm), or other substrates and various metabolic inhibitors at the concentrations indicated.
Incorporation of Labeled Pi into ThDP
An aliquot (1 ml) of the mitochondrial suspension was incubated for 10 min at 37 °C in the presence of 1 mm ThDP, 5 mm pyruvate, 5 mm Pi and 10 μl of H332PO4 (3.7 MBq final). The mitochondria were then washed twice, treated with 12% trichloroacetic acid, and centrifuged for 3 min at 5000 × g. After extraction with diethyl ether, the supernatant was used for the assay of thiamine derivatives by HPLC, and the pellet was used for the determination of protein concentration by the method of Peterson (37). The HPLC effluent was collected (0.5-ml samples), and the radioactivity was determined by liquid scintillation.
Determination of Thiamine Derivatives and ATP
Thiamine and its phosphate esters were determined by HPLC after oxidation to fluorescent thiochromes (42). ATP was determined by HPLC using either UV detection (44) or fluorescence detection after ethenylation with chloroacetaldehyde by a slight modification of previously published methods (45, 46). After precipitation, the trichloroacetic acid (12%) was extracted from the supernatants by diethyl ether. To 150 μl of sample were added 25 μl of sodium acetate (1 m, pH 4.0) and 25 μl of chloroacetaldehyde. After incubation (30 min, 72 °C), the samples were put on ice, and 50 μl of NH4HCO3 (0.5 m) were added. Control solutions containing ATP, ADP, or AMP were treated the same way and used as standards. Ethenylated adenine nucleotides were separated by HPLC and detected fluorimetrically (300 nm for excitation and 420 nm for emission) using a 5-μm Apollo C18 column (150 × 4.6 mm, Varian B.V. Middelburg, The Netherlands) at a flow rate of 1 ml/min using a gradient elution. Solvent A was composed of sodium phosphate buffer (100 mm, pH 6.0). Solvent B contained 70% solvent A and 30% methanol. A two-step gradient was applied: from 0 to 20% B in 9 min; from 20 to 100% B from 9 to 24 min; from 100 to 0% B from 24 to 30 min, followed by 1 min at 0% B before the next injection.
Separation of Free and Bound ThDP Using a TSK Molecular Sieve
The DTMF was suspended in a hypotonic medium (Tris-HCl 20 mm, pH 7.8), freeze-thawed twice, and centrifuged at 11,000 × g for 5 min. The supernatant (100 μl) was injected on a TSK column (G3000SW, 30 × 0.75 cm, 10 mm (Tosoh Bioscience GmbH, Stuttgart, Germany)) equilibrated in sodium acetate buffer (25 mm, pH 7.2) at a flow rate of 0.5 ml/min. Fractions of 1 ml were collected, and thiamine derivatives were determined after treatment with trichloroacetic acid as described above.
Preparation of Rat Brain Slices
Slices were prepared as described (47, 48) and incubated in artificial cerebro-spinal fluid (36 °C; 95% O2, 5% CO2) for 40 min. Coronal slices (500 μm thick) (D.S.K. microslicer DTK-1000, Dosaka EM Co. Ltd., Kyoto-shi, Japan) from one hemisphere were used as controls, whereas the slices from the other hemisphere were incubated in the presence of the inhibitors. After incubation, slices were homogenized in 500 μl of 12% trichloroacetic acid and centrifuged at 5000 × g for 5 min. The supernatants were extracted with diethyl ether for ThTP and ATP determination, and the pellets were dissolved in 0.8 n NaOH for the protein assay.
Statistical Analyses
Statistical analyses were performed by one-way or two-way analysis of variance, followed by a post hoc test (Dunnett's multiple comparisons test).
RESULTS
In Rat Brain, ThTP Is Mainly Localized in Mitochondria
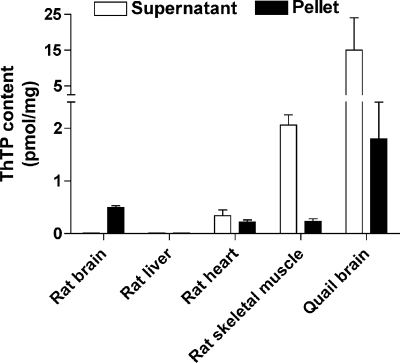
In order to study the subcellular localization of ThTP, we submitted rat brain homogenates to subcellular fractioning on Percoll gradients, and we determined ThTP levels in each fraction (Table 1). The mitochondrial fraction was highly enriched in ThTP compared with other fractions. We checked the cross-contamination of the fractions by measuring the activities of fumarase and l-lactate dehydrogenase. As expected, the specific activity of l-lactate dehydrogenase was much higher in the synaptosomal fraction than in free mitochondria. For fumarase, both fractions possessed a significant enzyme activity, but specific activity was highest in the mitochondrial fraction. The presence of fumarase in synaptosomes is not surprising because this structure is rich in mitochondria (38). These data suggest that free mitochondria are well separated from synaptosomes. The purity of the mitochondrial fraction was also checked by electron microscopy (Fig. 1). The mitochondrial fraction was essentially composed of mitochondria with very few contaminations from synaptosomes (Fig. 1A). The synaptosomal fraction contained some axons and mitochondria, mainly in nerve terminals (Fig. 1B). When the rat brain homogenate was directly centrifuged at high speed (100,000 × g, 1 h), no significant amount of ThTP was detected in the supernatant (Fig. 2), suggesting that there is little cytosolic ThTP in rat brain and that it is mainly localized in mitochondria. In rat heart, ThTP was equally distributed between the two fractions, whereas in skeletal muscle, it was found mainly in the cytosolic fraction. This relatively high content may be due, at least in part, to the high activity of the soluble cytosolic AK1 in skeletal muscle. In chicken skeletal muscle, where cytosolic ThTP is even more abundant, Kawasaki and co-workers (25, 26) have shown that its accumulation was linked to AK1, catalyzing the slow reaction ThDP + ADP ⇆ ThTP + AMP. No significant amount of ThTP was found in rat liver. It is remarkable that, in the quail brain, ThTP content is over 10 times higher than in the rat brain, in agreement with our previous results (7), and most of it is found in the supernatant. This might be due to the absence of cytosolic 25-kDa ThTPase in birds. Until now, we were unable to demonstrate such an activity in quail or chicken brain (7), and we have also been unable to find any sequence with homology to 25-kDa ThTPase in the chicken genome, making birds the only major vertebrate class where this enzyme would be absent.
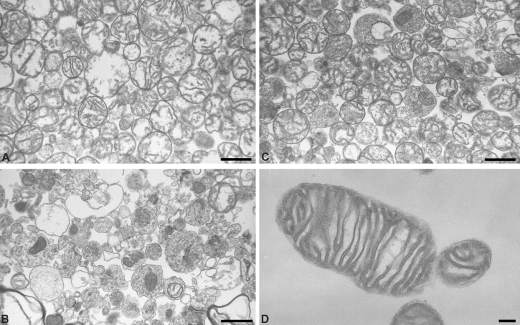
FIGURE 1.
Electron micrographic examination of subcellular fractions from rat brain. Shown are mitochondrial (A) and synaptosomal (B) fractions after Percoll gradient. C, DTMF (magnification ×9000). D, higher magnification of DTMF (×24,500), showing the intact double membrane and the cristae of a mitochondrion in a DTMF. Scale bar, 1 μm in A–C and 2 μm in D.
FIGURE 2.
Distribution of ThTP between cytosolic and particulate fractions in various tissues. The organs were homogenized in medium A and centrifuged at 100,000 × g for 1 h. ThTP was determined in the supernatant (cytosolic fraction) and the particulate fractions (mean ± S.D., n = 4–6).
ThTP Synthesis Occurs in Isolated Brain Mitochondria
We have previously shown (29) that, when rat brain homogenates were incubated with glucose and ThDP (1 mm), significant amounts of ThTP appeared in the particulate fraction (containing cell debris, synaptosomes, and mitochondria) obtained after centrifugation of the homogenate. We thought at that time that ThTP synthesis took place in the cytoplasm of cell bodies and nerve terminals, from ThDP and ATP. However, we were never able to demonstrate the occurrence of such a reaction in a soluble fraction. Actually, the present results indicate that ThTP is mostly present in mitochondria and virtually undetectable in cytosolic fractions from rodent brain.
In order to check whether ThTP synthesis may indeed occur in mitochondria, synaptosomal and mitochondrial fractions were isolated from rat brain as above and incubated with ThDP (1 mm), Pi (5 mm), and succinate (5 mm) for 10 min at 37 °C, and the ThTP content was determined by HPLC (Table 1). ThTP synthesis mainly occurred in the mitochondrial fraction (Table 1), whereas only little ThTP was produced in other fractions. The experimental conditions used were those found optimal for ThTP synthesis (see below).
The authenticity of synthesized ThTP was checked by hydrolysis with the highly specific purified recombinant human ThTPase (18), as previously described (7). After incubation with ThTPase, the ThTP peak completely disappeared, confirming its authenticity.
ThTP Synthesis in Rat Brain Mitochondria Requires Pi and a Respiratory Substrate
Because the mitochondrial yield after Percoll gradient is low, we routinely used mitochondria obtained by differential centrifugation (DTMF; see “Experimental Procedures”). This fraction is supposed to contain mitochondria originating from glial and neuronal cells. As shown in Fig. 1C, this DTMF mainly consists of intact mitochondria. A single mitochondrion is shown at higher magnification (Fig. 1D) to illustrate its morphological integrity (double membrane and intact cristae).
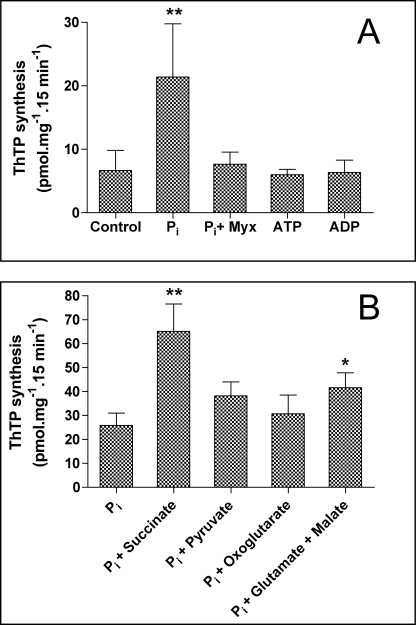
We incubated the DTMF at 37 °C in an isotonic medium containing KCl, MgCl2, and ThDP (1 mm). After 15 min, the mitochondria were washed to eliminate excess ThDP (that would interfere with ThDP estimation), and we measured the mitochondrial ThTP content. In the absence of thiamine compounds other than ThDP, little ThTP was formed. We then tested the effects of compounds expected to be phosphate donors for the phosphorylation of ThDP (Fig. 3A). No significant activation of ThTP synthesis was observed in the presence of added ATP or ADP, suggesting that neither a ThDP kinase nor AK is involved in the synthesis. Quite unexpectedly, Pi was able to significantly stimulate ThTP production by brain mitochondria. This was reminiscent of the mechanism of ATP synthesis by oxidative phosphorylation from ADP and Pi. Because it is well known that this reaction is energized by the oxidation of respiratory substrates, we tested the effects of such substrates on ThTP synthesis in the presence of Pi (5 mm). As shown in Fig. 3B, the addition of respiratory substrates further activated ThTP synthesis, with succinate being the most effective.
FIGURE 3.
ThTP synthesis by a DTMF. A, effect of Pi (5 mm), myxothiazol (1 μm; Myx), ATP (1 mm), and ADP (1 mm) on ThTP synthesis. The control was incubated without exogenous Pi. B, effect of various substrates (5 mm each except for malate (2.5 mm)) in the presence of Pi (5 mm). The control was incubated with Pi but without added substrate. The incubations were carried out for 15 min at 30 °C in a saline medium containing 140 mm KCl, 2 mm MgCl2, 0.5 mm EGTA, 10 mm K-MOPS, pH adjusted to 7.3 with KOH, and ThDP (1 mm). Then the mitochondria were washed twice by centrifugation (11,000 × g, 5 min), and the pellet was suspended in 200 μl of trichloroacetic acid (12%) for ThTP determination (means ± S.D.; n = 5–15; **, p < 0.01; *, p < 0.05 for comparison with the control group, Dunnett's post-test).
We also found (Fig. 3A) that the synthesis observed in the presence of Pi without added respiratory substrates was completely blocked by low concentrations of myxothiazol, a specific inhibitor of complex III of the respiratory chain. Thus, the appreciable ThTP synthesis measured with Pi alone is probably due to the presence of endogenous substrates (NADH and/or succinate) in the mitochondria.
We consistently observed that succinate was a better substrate for ThTP synthesis than pyruvate, oxoglutarate, or a combination of glutamate and malate, although this difference was not observed for ATP synthesis (Table 2). In any event, the data indicate that ThTP is synthesized from ThDP and Pi and that this endergonic reaction is coupled to electron flow trough the respiratory chain, as is the case for ATP synthesis by oxidative phosphorylation.
TABLE 2.
Synthesis of ATP and ThTP in a rat brain mitochondrial fraction (DTMF) under different conditions
The mitochondria were incubated (10 min, 37 °C) in saline (cf. Table I) containing 0.2 mm ADP for ATP synthesis and 1 mm ThDP for ThTP synthesis. After centrifugation (11,000 × g, 5 min), the ThTP and ATP contents were determined in the pellet. The results are expressed as mean ± S.D., and the number of experiments is indicated in parentheses.
| [ATP] | [ThTP] | |
|---|---|---|
| nmol/mg of protein | pmol/mg of protein | |
| Control | 6.3 ± 2.4 (8) | 2.1 ± 1.5 (4) |
| Pi (5 mm) | 7.6 + 3.1 (9) | 17 ± 3 (20) |
| Pi + pyruvate (5 mm) | 140 ± 20 (4) | 42 ± 6 (52) |
| Pi + pyruvate + CCCP (0.2 μm) | 5.5 ± 2.7 (4) | 10 ± 2 (4) |
| Pi + succinate (5 mm) | 130 ± 50 (8) | 69 ± 12 (21) |
| Pi + succinate + CCCP (0.2 mm) | 2.4 ± 2.9 (4) | 7.5 ± 0.6 (3) |
| Pi + succinate + valinomycin (10 nm) | 2.2 ± 3.0 (4) | 2.2 ± 1.9 (6) |
| Pi + succinate + valinomycin (1 nm) | 9.0 ± 4.0 (3) | 19 ± 2 (6) |
It is nonetheless important to exclude the possibility that ThTP is synthesized outside the mitochondria and subsequently transported into the organelle. Therefore, we incubated (10 min at 37 °C) a DTMF mitochondrial preparation in a KCl-saline containing 10 μm ThTP, and we checked that ThTP was not taken up by the mitochondria. In a control experiment, ThTP was added at the end of the incubation to correct for nonspecific adsorption to membranes. After incubation, the suspension was centrifuged (10 min, 11,000 × g), and its ThTP content was measured. It was not significantly higher than in the control, indicating that ThTP does not readily cross the inner mitochondrial membrane. However, it should be stressed that ThDP, which is the likely precursor of ThTP, is probably able to easily enter mitochondria and accumulate in the matrix. Indeed, a specific mitochondrial ThDP transporter was recently characterized in mammalian cells (32), and earlier studies (49) have shown a rapid uptake of labeled ThDP by rat liver mitochondria. When the mitochondria were lysed by incubation in hypotonic phosphate buffer followed by a freeze-thaw cycle and centrifugation, over 80% of the ThTP was in the supernatant, showing that it is not tightly bound to membrane structures.
Taken together, those results strongly suggest that a net synthesis of ThTP occurs in mitochondria (but not in the cytosol) and that functional mitochondria are required for optimal ThTP synthesis. We checked that our mitochondria were well coupled by measuring respiratory chain parameters using oximetry. The URC was 5.0 ± 0.5 (n = 4), and the RCR was 3.7 ± 0.7 (n = 6) with succinate as substrate. These results are in agreement with the literature; synaptic mitochondria routinely have a RCR of ∼5.0 with glutamate and malate as substrates (50). Antimycin A (2 μg/ml) and oligomycin (1 μg/ml) inhibited the state 3 respiration by 95 and 81%, respectively (not shown).
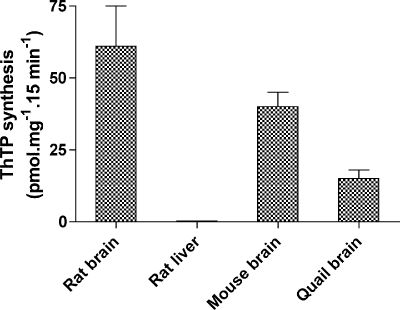
We tested whether ThTP synthesis may occur in mitochondria isolated from tissues other than rat brain (Fig. 4). An important synthesis was observed in mouse and quail brains, but no synthesis occurred in liver mitochondria. Therefore, using oximetry, we checked whether our liver mitochondria were functional. We obtained the following RCR and URC values: RCRsuccinate = 2.7 ± 0.1 (n = 8) and URCsuccinate = 2.8 ± 1.1 (n = 5); RCRglutamate + malate = 4.2 ± 0.4 (n = 4) and URCglutamate + malate = 4.7 ± 0.6 (n = 2). Thus, our liver mitochondria, like brain mitochondria, appear to be well coupled but unable to synthesize ThTP, in agreement with the absence of this compound in rat liver (Fig. 2).
FIGURE 4.
ThTP synthesis in mitochondria isolated from various tissues. Mitochondria were incubated 15 min at 30 °C in saline (cf. Fig. 3) containing Pi (5 mm), succinate (5 mm), and ThDP (1 mm).
Kinetics and Temperature Dependence of ThTP Synthesis
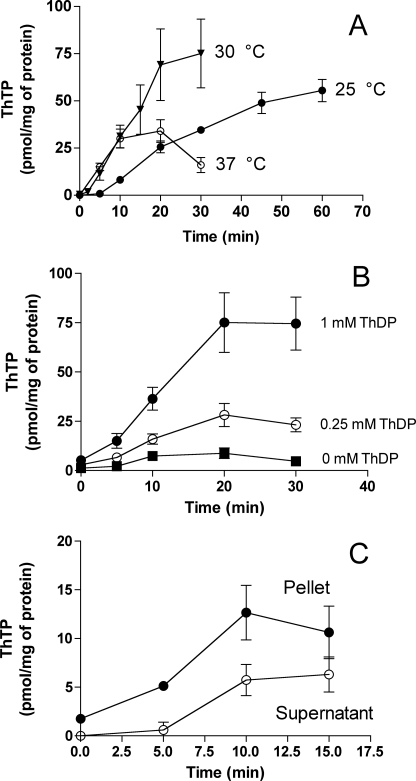
At 25 °C, the synthesis, after an initial lag, proceeded nearly linearly for at least 40 min (Fig. 5A). At 37 °C, the initial rate was higher than at 25 °C, but the reaction stopped after 10 min, and thereafter ThTP levels decreased, suggesting either a hydrolysis or a release of synthesized ThTP. The optimum temperature was 30 °C, and the reaction proceeded linearly for 20 min.
FIGURE 5.
Kinetics of ThTP synthesis in a rat brain DTMF. All incubations were carried out in saline (same composition as in Fig. 3) containing succinate (5 mm) and Pi (5 mm). A, the synthesis was measured in the presence of ThDP (1 mm) either at 25 °C (●), 30 °C (▾), or 37 °C (○) for the time indicated. B, the incubation was carried out at 30 °C in the absence (■) or the presence of 0.25 (○) or 1 mm (●) ThDP. C, the mitochondrial fraction was incubated (30 °C) in the presence of succinate (5 mm) and Pi (5 mm) for various periods of time in the absence of added ThDP. At the time indicated, the fraction was centrifuged (11,000 × g), and ThTP was determined in the supernatant (○) and in the pellet (●). The results are expressed as mean ± S.D. (n = 3).
The rate of ThTP synthesis was greatly increased by exogenous ThDP (Fig. 5B), but a significant synthesis was already observed in the absence of added ThDP, suggesting the presence of endogenous unbound ThDP. In order to check for the presence of free ThDP, the mitochondrial fraction was lysed by a freeze/thaw cycle and centrifuged, and the supernatant was filtered on a gel permeation column to separate free and bound ThDP. About 35% of ThDP was eluted in the void volume, suggesting that a significant portion of ThDP is free inside the mitochondria. We can estimate the free intramitochondrial ThDP concentration at ∼100 μm.
Finally, we checked whether some of the ThTP could be released from the mitochondrial fraction. After synthesis, the fraction was centrifuged (11,000 × g), and ThTP was determined in the pellets and the supernatants (Fig. 5C). This experiment was done only without added ThDP (which would interfere with the determination of ThTP). As can be seen, there is an increase in ThTP content in the supernatant with time, indicating that it is indeed released.
ThTP Synthesis Is Tightly Coupled to Electron Flow through the Respiratory Chain
Data shown in Figs. 6 and 7 give further support to the hypothesis that electron flow through the respiratory chain is absolutely required for ThTP synthesis.
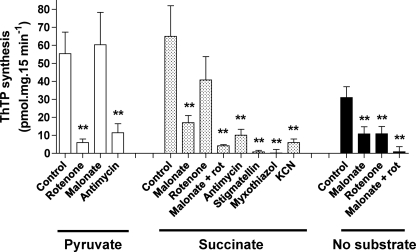
FIGURE 6.
Effect of respiratory chain inhibitors on ThTP synthesis in a DTMF. Mitochondria were incubated for 10 min at 37 °C in the presence of various substrates and inhibitors. All of the tests were done in the presence of Pi (5 mm) and ThDP (1 mm), and the concentrations of substrates and inhibitors were as follows: pyruvate, 5 mm; succinate, 5 mm; rotenone, 2 μm; malonate, 5 mm; antimycin A, 2 μg/ml; stigmatellin, 1 μm; myxothiazol, 1 μm; KCN, 1 mm (mean ± S.D.; n = 3–31; **, p < 0.01 for comparison with respective control).
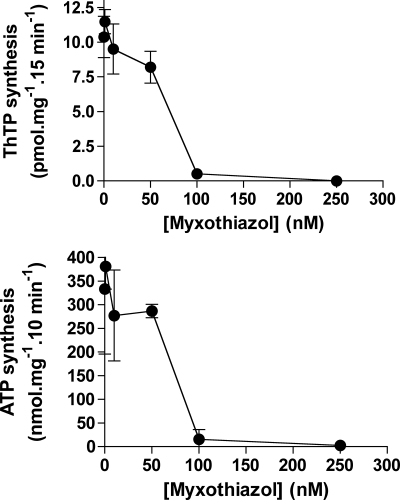
FIGURE 7.
ThTP (top) and ATP synthesis (bottom) by brain mitochondria are blocked by the same concentrations of the complex III inhibitor myxothiazol. The DTMF was incubated for 15 min in the presence of Pi (5 mm), succinate (5 mm), and various concentrations of myxothiazol. No ThDP was present. For ATP synthesis, ADP (0.2 mm) was present, and the incubation time was 10 min (means ± S.D., n = 2–8).
First, in the absence of any added respiratory substrate, we found an inhibition of the synthesis by rotenone (inhibitor of complex I) or malonate (inhibitor of complex II). Combining both inhibitors led to a complete inhibition of synthesis (Fig. 6). These results suggest that a ThTP synthesis without added substrates may occur because of available endogenous substrate, but no synthesis occurs when electron flow is blocked.
In the presence of pyruvate, ThTP synthesis was significantly inhibited by rotenone and antimycin (inhibitor of complex III) but not by malonate (Fig. 6). In the presence of succinate, malonate was a good inhibitor, but rotenone was without effect. The combination of both rotenone and malonate completely inhibited ThTP synthesis. The additional effect of rotenone was probably due to the fact that it prevents the oxidation of endogenous substrates via complex I. Rotenone was very effective in the presence of pyruvate (which produces NADH, a substrate for complex I). By contrast, when succinate (which reduces complex II) was used as substrate, rotenone had no significant effect, but malonate (a competitive inhibitor of succinate dehydrogenase) suppressed ThTP synthesis. ThTP synthesis was also inhibited by antimycin A, stigmatellin, and myxothiazol, three compounds inhibiting complex III by different mechanisms (51). Myxothiazol was particularly effective, and dose-dependent inhibition of ThTP synthesis paralleled the inhibition of ATP synthesis (Fig. 7). Here (in contrast to Fig. 6), ThTP synthesis was measured in the absence of added ThDP, in order to be sure that the effect of myxothiazol is on ThTP synthesis and not ThDP transport. A very similar dose-response curve was obtained for the effect of myxothiazol on complex III respiration in synaptic mitochondria (50). ThTP synthesis was also impaired by cyanide (Fig. 6) as well as anoxia (not shown), confirming that oxygen reduction through complex IV is required to energize the reaction. Because no ADP was added to the preparation under normal assay conditions, the mitochondrial ATP content was always very low (<1 nmol/mg), confirming that ATP is not required for ThTP synthesis.
In any event, the addition of ATP never induced any net synthesis of ThTP, whatever the experimental conditions. These data strongly suggest that no active ThDP kinase exists in the rat brain, either in cytoplasm or mitochondria.
Incorporation of Labeled Pi into ThTP
The mitochondrial fraction was incubated in the presence of [32P]PO43− and pyruvate, and then thiamine derivatives were separated by HPLC, the ThTP peak was collected, and the specific radioactivity was determined as described previously (29). Based on the final specific radioactivity of 32Pi (9.99 GBq/mmol), the specific radioactivity of ThTP was 11.1 ± 3.3 GBq/mmol (n = 4). The fact that the specific activities of 32Pi and [32P]ThTP were the same suggests that 32Pi is directly incorporated into ThTP without the formation of an intermediate (ATP for instance), which would have resulted in a dilution of the specific radioactivity.
ThTP Synthesis Requires Intact and Well Coupled Mitochondria and Is Linked to the Proton-motive Force
When the mitochondria were disrupted by sonication (100 kHz, three times for 15 s at 60-s intervals) or by adding the neutral detergent Triton X-100 (final concentration of 0.5%), no ThTP synthesis was observed after the addition of ThDP, Pi, and succinate.
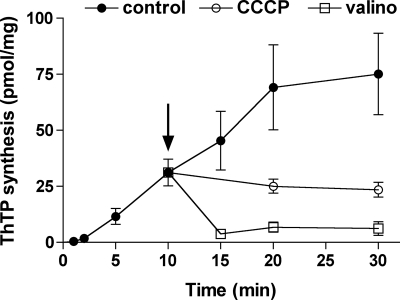
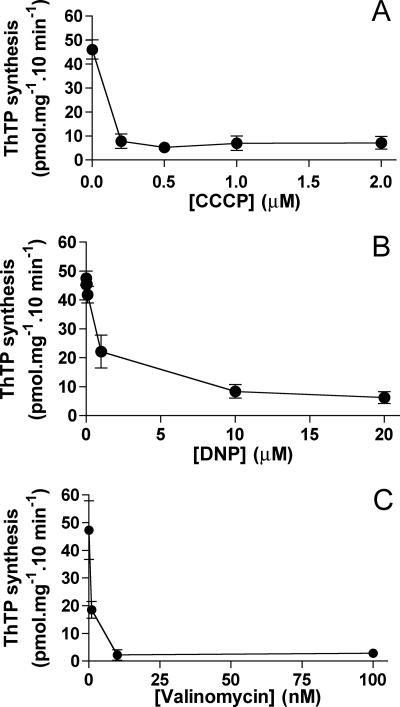
The uncoupler CCCP, which dissipates the electrochemical proton gradient, abolishes both ATP and ThTP synthesis (Table 2). When ThTP was synthesized for 10 min and then either the uncoupler CCCP or the K+ ionophore valinomycin was added to the preparation, the synthesis was immediately stopped, and with valinomycin ThTP was even hydrolyzed (Fig. 8). The maximum effect of CCCP was already observed at 0.2 μm (Fig. 9A). 2,4-Dinitrophenol, another protonophore, was also highly efficient with an IC50 of ∼1 μm (Fig. 9B). The K+ ionophore valinomycin was even more efficient and inhibited ThTP synthesis at 10 nm (Fig. 9C). Strangely, with both protonophores, maximum inhibition was about 80–90% but never as complete as with valinomycin.
FIGURE 8.
The addition of CCCP or valinomycin blocks ThTP synthesis. The DTMF was incubated for various time intervals at 30 °C in the presence of Pi (5 mm), succinate (5 mm), and ThDP (1 mm). After 10 min (arrow), either CCCP (0.2 μm) or valinomycin (10 nm) was added.
FIGURE 9.
Dose-dependent effects of uncouplers and valinomycin on ThTP synthesis. The DTMF was incubated (10 min, 37 °C) in the presence of Pi (5 mm), succinate (5 mm), and ThDP (1 mm) with various concentrations of CCCP (A), 2,4-dinitrophenol (DNP) (B), or valinomycin (C) (mean ± S.D., n = 2–8).
In order to test whether mitochondrial ThTP synthesis can have a physiological significance, rat brain slices were incubated for 40 min in artificial cerebro-spinal fluid at 37 °C in the absence or the presence of 10 μm CCCP, and ThTP was determined. After treatment with CCCP, ThTP levels were decreased to less than 30%. This suggests that, in vivo, ThTP is synthesized through the same mechanism as in isolated mitochondria.
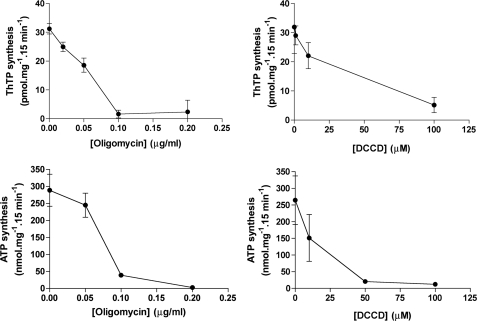
ThTP Synthesis Is Inhibited by Oligomycin and DCCD
We compared the inhibition of ThTP and ATP synthesis by oligomycin and DCCD (Fig. 10). Oligomycin inhibited both ThTP and ATP synthesis with an IC50 close to 0.05 μg/ml. With DCCD, half-maximal inhibition was close to 25 μm. Because it is well known that both oligomycin and DCCD inhibit ATP synthase by blocking proton flux in F0, these results suggest that at least the F0 component of the mitochondrial F0F1-ATP synthase is involved in ThTP synthesis.
FIGURE 10.
Oligomycin and DCCD block ThTP synthesis. The DTMF was incubated (15 min, 30 °C) in the presence of Pi (5 mm), succinate (5 mm), and either ThDP (1 mm) (top) for ThTP synthesis or ADP (0.2 mm) (bottom) for ATP synthesis with various concentrations of oligomycin (left) or DCCD (right). Then the samples were centrifuged (11,000 × g, 5 min), and the pellets were suspended in 200 μl of trichloroacetic acid (TCA) (12%) for ThTP and ATP determination (mean ± S.D., n = 3).
Fate of Synthesized ThTP
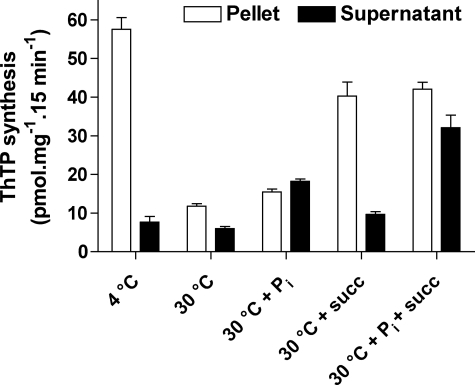
In the following experiments (Fig. 11 and 12), we first incubated a DTMF fraction for 15 min at 30 °C in the presence of 1 mm ThDP and succinate in order to obtain a maximal accumulation of ThTP. Then the mitochondria were washed to remove substrates in excess, and a second incubation was carried out under various conditions but without added ThDP. As shown in Fig. 11, when the second incubation was carried out at 30 °C followed by centrifugation of the mitochondria, little ThTP was found in the pellet, whereas a small amount appeared in the supernatant. This global disappearance of ThTP suggests that it was hydrolyzed by the reversible ThTP synthase because Δp may be dissipated, since no respiratory substrate was present during the second incubation. It is noteworthy that, when the second incubation was carried out at 4 °C, no disappearance of ThTP was observed, in agreement with the idea that it is due to enzymatic hydrolysis. The disappearance of ThTP was also slowed down when succinate was present during the second incubation, presumably because Δp is maintained in the presence of this substrate.
FIGURE 11.
Effect of Pi on the release of synthesized ThTP. The DTMF was incubated (15 min, 30 °C) in the presence of Pi (5 mm), succinate (5 mm), and ThDP (1 mm). Then the mitochondria were washed twice by centrifugation (11,000 × g, 5 min, 4 °C) in medium B. The pellets were resuspended in saline (140 mm KCl, 2 mm MgCl2, 0.5 mm EGTA, and 10 mm K-MOPS, pH adjusted to 7.3 with KOH) and incubated for 15 min at 30 °C under various conditions as indicated. The second incubation was followed by a centrifugation (11,000 × g, 5 min), and ThTP was determined in the supernatants and the pellets.
FIGURE 12.
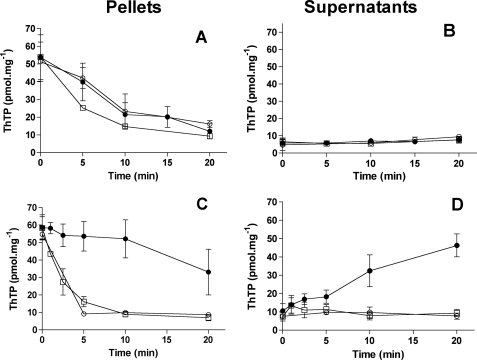
Effect of substrates (succinate and Pi) on the hydrolysis and release of synthesized ThTP. The mitochondrial fraction was preincubated (15 min, 30 °C) in the presence of Pi (5 mm), succinate (5 mm), and ThDP (1 mm). Then the samples were washed twice by centrifugation (11,000 × g, 5 min), and the mitochondria were resuspended in saline and incubated for the time indicated either in the absence (A and B) or the presence (C and D) of Pi (5 mm) and succinate (10 mm). After centrifugation (11,000 × g, 5 min), ThTP was determined in the pellets (A and C) and the supernatants (B and D) (mean ± S.D., n = 2–8). ●, control; ○, 0.2 μm CCCP; □, 10 nm valinomycin.
A significant amount of ThTP was apparently released from the mitochondria during the second incubation and was recovered in the last supernatant. Interestingly, this release was increased in the presence of 5 mm Pi and was maximal when both Pi and succinate were present in the incubation medium. A possible explanation for the activating effect of Pi is that ThTP extrusion occurs through an exchange mechanism Pi/ThTP, the putative exchanger probably being associated with the inner mitochondrial membrane. We have so far no explanation for the fact that succinate is also required for maximal release of ThTP.
Data from Fig. 12 present kinetic data confirming the above conclusions. Fig. 12A shows the decrease in ThTP level in the absence of an energy substrate. Because no ThTP appeared in the supernatant (Fig. 12B), this decrease was probably the result of a hydrolysis. When the same experiment was carried out in the presence of substrates (5 mm Pi and 5 mm succinate), ThTP was released from the mitochondria in the absence of uncouplers (Fig. 12, C and D). However, in the presence of valinomycin or CCCP, a rapid hydrolysis occurred. These results suggest that in energized mitochondria, ThTP synthesized is released, and this release probably depends on the presence of both external Pi and an energy substrate.
DISCUSSION
Synthesis, Accumulation, and Hydrolysis of ThTP in Different Tissues and Subcellular Compartments
In animal tissues, ThTP levels can be highly variable. In mouse tissues, ThTP levels are extremely low, representing less than 0.1% of total thiamine (0.15 pmol/mg protein in mouse skeletal muscle) (6). At the other end of the spectrum, we find adult pig skeletal muscle (18 nmol/g wet weight or 45 pmol/mg protein (52)), chicken white muscle (7 pmol/mg protein (25)), and Electrophorus electricus electric organ (3.9 nmol/g wet weight or 30 pmol/mg protein (53)), with ThTP representing 69, 80, and 87%, respectively, of total thiamine. In all three cases, ThTP is mainly localized in the cytosolic fraction. Furthermore, there is no significant soluble 25-kDa ThTPase activity in E. electricus electric organ (53). The enzyme is also absent in quail tissues (birds seem to lack the ThTPase orthologous gene) (4), and pig tissues express a catalytically inactive protein (54). It is therefore likely that, in those tissues with a high AK1 activity, ThTP is synthesized by this enzyme, as proposed by Kawasaki and co-workers (25), and accumulates in the cytosol because of the absence of cytosolic ThTPase activity. In rodent muscle, a high ThTPase activity prevents ThTP accumulation, and the small amounts formed through the action of AK are immediately hydrolyzed. Thus, cytosolic ThTP levels can be highly variable according to the species and tissue analyzed, which raises doubts about the physiological significance of the cytosolic pool.
Concerning other tissues, the situation is further complicated by our recent discovery of adenosine thiamine phosphate compounds eluting close to ThTP. This might explain that some earlier studies reported higher amounts of ThTP in rat liver and heart than in brain (55–57), whereas we find that ThTP is practically undetectable in rat liver. In contrast, there are significant levels of AThDP (in liver) and AThTP (liver and heart) (5, 6). On the other hand, ThTP is consistently found in the brain (human (58); baboon (59); pig, quail, and trout (7); and rodents (5, 6)). In mammalian brains, ThTP levels are kept rather low, presumably because of the 25-kDa ThTPase activity. In the rat brain, for instance, its intracellular concentration would be around 10−7 m if it were homogeneously distributed. However, because our results suggest that it is essentially localized in mitochondria, its concentration in the matrix would be ∼5–10 μm, far from negligible.
Here we show that in rat brain, ThTP synthesis takes place inside mitochondria. A mitochondrial synthesis was also observed in mouse and quail brains. No synthesis was observed in liver mitochondria, in agreement with the observation that ThTP is practically undetectable in rodent liver.
We have checked that our mitochondrial preparation from liver was able to synthesize ATP from ADP and Pi and that its failure to catalyze ThTP synthesis was not due to uncoupling. Like ATP, ThTP formation appears to require a proton-motive force (see below), but it is probably not an automatic consequence of the establishment of a high Δp (otherwise, ThTP might be considered as a mere “by-product” of oxidative phosphorylation). Actually, it appears to be controlled by other unknown regulatory factors that are different in different cell types. The possibility that some activating factor(s) might be specific for neuronal cells or brain is interesting, and their identification would be a challenge for future investigation.
A Chemiosmotic Mechanism for ThTP Biosynthesis in Brain Mitochondria
Data presented in this work show that the mechanism of ThTP synthesis by brain mitochondria is strikingly similar to that of ATP synthesis by oxidative phosphorylation. Our results indeed show the following. (i) ThTP is produced from ThDP + Pi, and this endergonic reaction is tightly coupled to electron flow through the respiratory chain (Figs. 3, 6, and 7). (ii) ThTP synthesis is impaired when Δp is dissipated either by protonophores or by valinomycin in the presence of external K+. Disruption of the mitochondria also abolishes ThTP formation. (iii) The collapse of Δp induced a rapid hydrolysis of the synthesized ThTP, suggesting that the reaction ThDP + Pi ⇆ ThTP + H2O is catalyzed by a reversible H+-translocating ThTP synthase analogous (if not identical) to F0F1-ATP synthase.
We have shown (Fig. 5, B and C) that a significant ThTP synthesis already occurs in the absence of added ThDP, provided that Pi and substrates were present. This synthesis was also inhibited by respiratory chain inhibitors (Fig. 7) and uncouplers, proving that the effects were indeed on ThTP synthesis and not on ThDP uptake.
An especially interesting finding is that ThTP synthesis in the presence of ThDP is inhibited by the same low concentrations of oligomycin as ATP synthesis in the presence of ADP. It is well known that oligomycin inhibits ATP synthesis by blocking proton flux through the F0 part of the ATP synthase. We also found that DCCD, another potent F0 inhibitor, inhibits ThTP synthesis. These results therefore strongly suggest that the mechanism of ThTP synthesis involves a proton circuit that includes F0. The possibility that the catalytic site for ThTP synthesis or hydrolysis is located on F1 remains to be investigated, but in any event, our results clearly indicate that ThTP formation is not coupled to the formation of ATP by oxidative phosphorylation. Rather, ThTP synthesis appears as a process that is alternative to ATP formation by oxidative phosphorylation (e.g. Ca2+ accumulation), with Δp being the obligatory intermediate in all cases. It remains to be discovered why, under special conditions, Δp is used to phosphorylate ThDP rather than ADP. In any case, this is the first demonstration that a triphosphate compound (or any “high energy” compound) other than ATP can be produced by a chemiosmotic type of mechanism.
Fate and Possible Cellular Role of the Synthesized ThTP
ThTP synthesized inside mitochondria can be exported, probably via an exchange with Pi. We did not observe any significant amount of cytosolic ThTP in rat brain, probably because of the presence of a highly active ThTPase. Indeed, as discussed above, cytosolic ThTP only accumulates in tissues where the 25-kDa ThTPase is absent and AK1 is abundant (skeletal muscle, electric organ). Also, the fact that cytosolic ThTP concentrations can be highly variable casts doubt on a possible cytosolic role of ThTP. An interesting possibility would be that ThTP acts in the intermembrane space.
The difference between liver and brain mitochondria raises interesting questions. Under similar conditions, rat liver mitochondria were unable to produce any detectable amount of ThTP. Tissue-specific differences between mitochondria have been shown previously, in particular with respect to the generation of reactive oxygen species (60, 61). It is known that Ca2+-induced mitochondrial permeability transition can lead to apoptosis, and hepatocytes are much more easily destroyed by this mechanism than neurons (62).
This difference probably reflects different strategies used by the two tissues to cope with oxidative stress. Long lived neurons with no regenerative capacity tend to minimize reactive oxygen species generation, whereas short lived liver cells with high regenerative capacity have developed strategies to eliminate reactive oxygen species formed by mitochondria (62). Thiamine deficiency induces oxidative stress in the brains of experimental animals (1, 63). An important production of reactive oxygen species may be a reason why the nervous system is more sensitive to thiamine deficiency than the liver, which, in contrast to the brain, shows no irreversible lesions linked to thiamine deprivation.
Thiamine deficiency-induced lesions have generally been attributed to decreased ThDP-dependent enzyme activities and, in particular, that of 2-oxoglutarate dehydrogenase activity, but the region-selective lesions induced by thiamine deficiency have so far not been satisfactorily explained by this hypothesis (2). Here, we present the first evidence that ThTP might have a mitochondrial function possibly related to protection against oxidative stress. Such a mechanism could help to better understand the events involved in the onset of neurodegenerative diseases (64). It is therefore interesting to point out that thiamine deficiency has been shown to exacerbate the pathology of Alzheimer disease (63, 65, 66) and that there are dysfunctions of thiamine metabolism in neurodegenerative diseases (67, 68).
Acknowledgments
We thank Prof. F. E. Sluse (Bioenergetics and Cellular Physiology, University of Liège) for helpful discussions and Prof. J. Balthazart (Behavioral Neuroendocrinology, GIGA-Neurosciences, University of Liège) for providing the quails.
This work was supported by the “Fonds de la Recherche Fondamentale Collective” (to F. R. F. C.) and the Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture (to M. G.).
- ThDP
- thiamine diphosphate
- AK
- adenylate kinase
- AThDP
- adenosine thiamine diphosphate
- AThTP
- adenosine thiamine triphosphate
- CCCP
- carbonyl cyanide m-chlorophenylhydrazone
- DCCD
- N,N′-dicyclohexylcarbodiimide
- DTMF
- digitonin-treated mitochondrial fraction
- RCR
- respiratory control ratio
- ThTP
- thiamine triphosphate
- ThTPase
- thiamine triphosphatase
- URC
- uncoupled respiratory control
- Bistris propane
- 1,3-bis[tris(hydroxymethyl)methylamino]propane
- HPLC
- high pressure liquid chromatography.
REFERENCES
- 1.Desjardins P., Butterworth R. F. (2005) Mol. Neurobiol. 31, 17–25 [DOI] [PubMed] [Google Scholar]
- 2.Hazell A. S., Butterworth R. F. (2009) Alcohol. Alcohol. 44, 141–147 [DOI] [PubMed] [Google Scholar]
- 3.Bettendorff L. (1994) Metab. Brain Dis. 9, 183–209 [DOI] [PubMed] [Google Scholar]
- 4.Bettendorff L., Wins P. (2009) FEBS J. 276, 2917–2925 [DOI] [PubMed] [Google Scholar]
- 5.Bettendorff L., Wirtzfeld B., Makarchikov A. F., Mazzucchelli G., Frédérich M., Gigliobianco T., Gangolf M., De Pauw E., Angenot L., Wins P. (2007) Nat. Chem. Biol. 3, 211–212 [DOI] [PubMed] [Google Scholar]
- 6.Frédérich M., Delvaux D., Gigliobianco T., Gangolf M., Dive G., Mazzucchelli G., Elias B., De Pauw E., Angenot L., Wins P., Bettendorff L. (2009) FEBS J. 276, 3256–3268 [DOI] [PubMed] [Google Scholar]
- 7.Makarchikov A. F., Lakaye B., Gulyai I. E., Czerniecki J., Coumans B., Wins P., Grisar T., Bettendorff L. (2003) Cell. Mol. Life Sci. 60, 1477–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakaye B., Wirtzfeld B., Wins P., Grisar T., Bettendorff L. (2004) J. Biol. Chem. 279, 17142–17147 [DOI] [PubMed] [Google Scholar]
- 9.Cooper J. R., Itokawa Y., Pincus J. H. (1969) Science 164, 74–75 [DOI] [PubMed] [Google Scholar]
- 10.Itokawa Y., Cooper J. R. (1970) Biochim. Biophys. Acta 196, 274–284 [DOI] [PubMed] [Google Scholar]
- 11.Eliasson S. G., Scarpellini J. D. (1976) Neurochem. Res. 1, 191–200 [DOI] [PubMed] [Google Scholar]
- 12.Eder L., Dunant Y. (1980) J. Neurochem. 35, 1278–1286 [DOI] [PubMed] [Google Scholar]
- 13.Bettendorff L., Kolb H. A., Schoffeniels E. (1993) J. Membr. Biol. 136, 281–288 [DOI] [PubMed] [Google Scholar]
- 14.Nghiêm H. O., Bettendorff L., Changeux J. P. (2000) FASEB J. 14, 543–554 [DOI] [PubMed] [Google Scholar]
- 15.Barchi R. L., Braun P. E. (1972) J. Biol. Chem. 247, 7668–7673 [PubMed] [Google Scholar]
- 16.Bettendorff L., Grandfils C., Wins P., Schoffeniels E. (1989) J. Neurochem. 53, 738–746 [DOI] [PubMed] [Google Scholar]
- 17.Makarchikov A. F., Chernikevich I. P. (1992) Biochim. Biophys. Acta 1117, 326–332 [DOI] [PubMed] [Google Scholar]
- 18.Lakaye B., Makarchikov A. F., Antunes A. F., Zorzi W., Coumans B., De Pauw E., Wins P., Grisar T., Bettendorff L. (2002) J. Biol. Chem. 277, 13771–13777 [DOI] [PubMed] [Google Scholar]
- 19.Eckert T., Möbus W. (1964) Hoppe Seylers Z. Physiol. Chem. 338, 286–288 [DOI] [PubMed] [Google Scholar]
- 20.Chernikevich I. P., Luchko V., Voskoboev A. I., Ostrovsky Y. M. (1984) Biokhimiya 49, 899–907 [Google Scholar]
- 21.Makarchikov A. F., Brans A., Bettendorff L. (2007) BMC Biochem. 8, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishino K., Itokawa Y., Nishino N., Piros K., Cooper J. R. (1983) J. Biol. Chem. 258, 11871–11878 [PubMed] [Google Scholar]
- 23.Voskoboev A. I., Chernikevich I. P. (1985) Biokhimiya 50, 1421–1427 [PubMed] [Google Scholar]
- 24.Shikata H., Koyama S., Egi Y., Yamada K., Kawasaki T. (1989) Biochem. Int. 18, 933–941 [PubMed] [Google Scholar]
- 25.Miyoshi K., Egi Y., Shioda T., Kawasaki T. (1990) J. Biochem. 108, 267–270 [DOI] [PubMed] [Google Scholar]
- 26.Shioda T., Egi Y., Yamada K., Kawasaki T. (1991) Biochim. Biophys. Acta 1115, 30–35 [DOI] [PubMed] [Google Scholar]
- 27.Makarchikov A. F., Wins P., Janssen E., Wieringa B., Grisar T., Bettendorff L. (2002) Biochim. Biophys. Acta 1592, 117–121 [DOI] [PubMed] [Google Scholar]
- 28.Gigliobianco T., Lakaye B., Makarchikov A. F., Wins P., Bettendorff L. (2008) BMC Microbiol. 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bettendorff L., Peeters M., Wins P., Schoffeniels E. (1993) J. Neurochem. 60, 423–434 [DOI] [PubMed] [Google Scholar]
- 30.Bettendorff L., Hennuy B., Wins P., Schoffeniels E. (1993) Neuroscience 52, 1009–1017 [DOI] [PubMed] [Google Scholar]
- 31.Marobbio C. M., Vozza A., Harding M., Bisaccia F., Palmieri F., Walker J. E. (2002) EMBO J. 21, 5653–5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindhurst M. J., Fiermonte G., Song S., Struys E., De Leonardis F., Schwartzberg P. L., Chen A., Castegna A., Verhoeven N., Mathews C. K., Palmieri F., Biesecker L. G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15927–15932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettendorff L., Nghiêm H. O., Wins P., Lakaye B. (2003) Anal. Biochem. 322, 190–197 [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal R. E., Hamud F., Fiskum G., Varghese P. J., Sharpe S. (1987) J. Cereb. Blood Flow Metab. 7, 752–758 [DOI] [PubMed] [Google Scholar]
- 35.Reinhart P. H., Taylor W. M., Bygrave F. L. (1982) Biochem. J. 204, 731–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalmers S., Nicholls D. G. (2003) J. Biol. Chem. 278, 19062–19070 [DOI] [PubMed] [Google Scholar]
- 37.Peterson G. L. (1977) Anal. Biochem. 83, 346–356 [DOI] [PubMed] [Google Scholar]
- 38.Dunkley P. R., Heath J. W., Harrison S. M., Jarvie P. E., Glenfield P. J., Rostas J. A. (1988) Brain Res. 441, 59–71 [DOI] [PubMed] [Google Scholar]
- 39.Sims N. R. (1990) J. Neurochem. 55, 698–707 [DOI] [PubMed] [Google Scholar]
- 40.Hill R. L., Bradshaw R. A. (1969) Methods Enzymol. 13, 91–99 [Google Scholar]
- 41.Bergmeyer H. U., Bernt E. (1974) in Methoden der Enzymatischen Analyse (Bergmeyer H. U. ed) pp. 607–612, Verlag Chemie, Weinheim [Google Scholar]
- 42.Bettendorff L., Peeters M., Jouan C., Wins P., Schoffeniels E. (1991) Anal. Biochem. 198, 52–59 [DOI] [PubMed] [Google Scholar]
- 43.Lakaye B., Makarchikov A. F., Wins P., Margineanu I., Roland S., Lins L., Aichour R., Lebeau L., El Moualij B., Zorzi W., Coumans B., Grisar T., Bettendorff L. (2004) Int. J. Biochem. Cell Biol. 36, 1348–1364 [DOI] [PubMed] [Google Scholar]
- 44.Hill M., Dupaix A., Volfin P., Kurkdian A., Arrio B. (1987) Methods Enzymol. 148, 132–141 [Google Scholar]
- 45.Levitt B., Head R. J., Westfall D. P. (1984) Anal. Biochem. 137, 93–100 [DOI] [PubMed] [Google Scholar]
- 46.Lazarowski E. R., Tarran R., Grubb B. R., van Heusden C. A., Okada S., Boucher R. C. (2004) J. Biol. Chem. 279, 36855–36864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vergara R., Rick C., Hernández-López S., Laville J. A., Guzman J. N., Galarraga E., Surmeier D. J., Bargas J. (2003) J. Physiol. 553, 169–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji H., Hougaard C., Herrik K. F., Strøbaek D., Christophersen P., Shepard P. D. (2009) Eur. J. Neurosci. 29, 1883–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barile M., Passarella S., Quagliariello E. (1990) Arch. Biochem. Biophys. 280, 352–357 [DOI] [PubMed] [Google Scholar]
- 50.Davey G. P., Peuchen S., Clark J. B. (1998) J. Biol. Chem. 273, 12753–12757 [DOI] [PubMed] [Google Scholar]
- 51.Starkov A. A., Fiskum G. (2001) Biochem. Biophys. Res. Commun. 281, 645–650 [DOI] [PubMed] [Google Scholar]
- 52.Egi Y., Koyama S., Shikata H., Yamada K., Kawasaki T. (1986) Biochem. Int. 12, 385–390 [PubMed] [Google Scholar]
- 53.Bettendorff L., Michel-Cahay C., Grandfils C., De Rycker C., Schoffeniels E. (1987) J. Neurochem. 49, 495–502 [DOI] [PubMed] [Google Scholar]
- 54.Szyniarowski P., Lakaye B., Czerniecki J., Makarchikov A. F., Wins P., Margineanu I., Coumans B., Grisar T., Bettendorff L. (2005) Biochim. Biophys. Acta 1725, 93–102 [DOI] [PubMed] [Google Scholar]
- 55.Rindi G., de Giuseppe L. (1961) Biochem. J. 78, 602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishii K., Sarai K., Sanemori H., Kawasaki T. (1979) J. Nutr. Sci. Vitaminol. 25, 517–523 [DOI] [PubMed] [Google Scholar]
- 57.Iwata H., Matsuda T., Tonomura H. (1988) J. Chromatogr. 450, 317–323 [DOI] [PubMed] [Google Scholar]
- 58.Bettendorff L., Mastrogiacomo F., Kish S. J., Grisar T. (1996) J. Neurochem. 66, 250–258 [DOI] [PubMed] [Google Scholar]
- 59.Bettendorff L., Schoffeniels E., Naquet R., Silva-Barrat C., Riche D., Ménini C. (1989) J. Neurochem. 53, 80–87 [DOI] [PubMed] [Google Scholar]
- 60.Johnson D. T., Harris R. A., French S., Blair P. V., You J., Bemis K. G., Wang M., Balaban R. S. (2007) Am. J. Physiol. Cell Physiol. 292, C689–C697 [DOI] [PubMed] [Google Scholar]
- 61.Tahara E. B., Navarete F. D., Kowaltowski A. J. (2009) Free Radic. Biol. Med. 46, 1283–1297 [DOI] [PubMed] [Google Scholar]
- 62.Panov A., Dikalov S., Shalbuyeva N., Hemendinger R., Greenamyre J. T., Rosenfeld J. (2007) Am. J. Physiol. Cell Physiol. 292, C708–C718 [DOI] [PubMed] [Google Scholar]
- 63.Karuppagounder S. S., Xu H., Shi Q., Chen L. H., Pedrini S., Pechman D., Baker H., Beal M. F., Gandy S. E., Gibson G. E. (2009) Neurobiol. Aging 30, 1587–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atamna H., Frey W. H., 2nd (2007) Mitochondrion 7, 297–310 [DOI] [PubMed] [Google Scholar]
- 65.Butterworth R. F. (2003) Nutr. Res. Rev. 16, 277–284 [DOI] [PubMed] [Google Scholar]
- 66.Calingasan N. Y., Ho D. J., Wille E. J., Campagna M. V., Ruan J., Dumont M., Yang L., Shi Q., Gibson G. E., Beal M. F. (2008) Neuroscience 153, 986–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mastrogiacoma F., Bettendorff L., Grisar T., Kish S. J. (1996) Ann. Neurol. 39, 585–591 [DOI] [PubMed] [Google Scholar]
- 68.Bettendorff L., Mastrogiacomo F., Wins P., Kish S. J., Grisar T., Ball M. J. (1997) J. Neurochem. 69, 2005–2010 [DOI] [PubMed] [Google Scholar]