Abstract
Bacterial flagella contain a rotor-mounted protein complex termed the switch complex that functions in flagellar assembly, rotation, and clockwise/counterclockwise direction control. In Escherichia coli and Salmonella, the switch complex contains the proteins FliG, FliM, and FliN and corresponds structurally with the C-ring in the flagellar basal body. Certain features of subunit organization in the switch complex have been deduced previously, but details of subunit organization in the lower part of the C-ring and the molecular movements responsible for motor switching remain unclear. In this study, we use cross-linking, binding, and mutational experiments to examine subunit organization in the bottom of the C-ring and to probe movements that occur upon switching. The results show that FliN tetramers alternate with FliM C-terminal domains to form the bottom of the C-ring in an arrangement that closely reproduces the major features observed in electron microscopic reconstructions. When motors were switched to clockwise rotation by a repellent stimulus, cross-link yields were altered in a pattern indicating relative movement of FliN and FliMC. These results are discussed in the framework of a structurally grounded hypothesis for the switching mechanism.
Introduction
Many species of motile bacteria can direct their swimming toward favorable stimuli by regulating reversals between clockwise and counterclockwise rotation of the flagella. In Escherichia coli or Salmonella enterica, for example, counterclockwise rotation causes the helical flagellar filaments to join in a bundle that propels the cell smoothly, whereas clockwise rotation of one or more filaments disrupts the bundle and leads to a rapid somersaulting motion termed a tumble (1). In the absence of an external stimulus, cells execute a random walk consisting of smooth runs of ∼1 s punctuated by short (∼0.1-s) tumbles that reorient the cell. By suppressing clockwise rotation in response to increasing attractant or decreasing repellent stimuli, cells prolong the runs that happen to be in a favorable direction, biasing their motion toward nutrients, temperatures, or other conditions favorable for growth (2, 3).
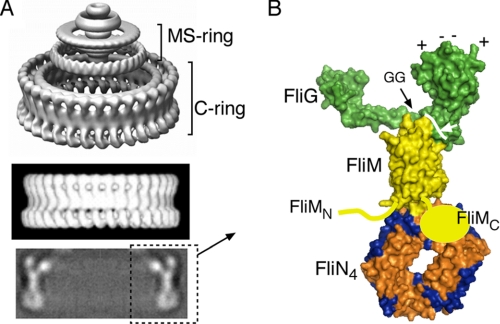
The direction of flagellar rotation is regulated by a structure at the bottom of the basal body called the switch complex, constructed from the proteins FliG, FliM, and FliN. The switch complex contains several copies of each protein; in Salmonella, the subunit numbers are ∼26 FliG, 35 FliM, and 140 FliN, for a total mass of ∼4000 kDa. Morphologically, the switch complex corresponds to a drum-shaped, ∼45-nm diameter feature at the bottom of the basal body termed the C-ring. The C-ring has been imaged most clearly in single-particle reconstructions in Salmonella (4). Salient features include well resolved lobes of density on the upper (membrane-proximal) edge, a relatively smooth, thin side wall, and an array of ring-shaped densities (“donuts”) at the bottom (see Fig. 1A).
FIGURE 1.
A, images of the flagellar basal body from EM reconstructions. The upper panel shows the C-ring, MS-ring, and proximal rod. The lower, thicker part of the MS-ring is situated in the membrane; the C-ring is in the cytoplasm. The middle panel is a side view of only the C-ring, and the lower panel shows a cross-section through the C-ring. The figure has been taken from Ref. 4 with permission. B, molecular model for major portions of the C-ring based on crystal structures of major portions of the proteins and biochemical and mutational studies of subunit relationships. FliGC is on the right and contains a set of conserved charged residues (top) that interact with the stator protein MotA. FliGC is joined to the rest of the protein by a conserved Gly-Gly linker that might allow relative movement of the domains. The structure of FliMC is unknown; this domain is known to interact with FliN, but the details of this relationship are likewise unknown.
The switch complex proteins are multifunctional, performing roles in flagellar assembly and rotation as well as direction switching (5, 6). FliG is the rotor protein most closely involved in rotation and contains conserved charged residues that interact directly with MotA, an integral membrane protein that, together with MotB, forms the stator (7). FliM is extensively involved in direction switching. Numerous FliM mutations have been shown to affect the clockwise/counterclockwise motor bias, and a segment near the N terminus of the protein binds to the clockwise-signaling molecule phospho-CheY (CheY-P).2 A recent NMR study provided evidence suggesting that once captured by the N-terminal segment, CheY-P might also interact with the central domain of FliM (8). FliN is closely involved in flagellar assembly (9, 10) and binds to FliH, a protein that functions in the export process that delivers protein subunits needed to form exterior flagellar structures such as the filament and hook (11–13). FliN is also involved in direction switching (10, 14), and a recent mutational analysis identified a specific region on the protein surface that is essential for clockwise rotation and that might provide an additional, functionally critical binding site for CheY-P (13).
Flagellar motors obtain energy from the membrane gradient of protons (15–17) or, in some alkalophilic and marine species, sodium ions (18, 19). The energizing ions are conducted through complexes formed from the membrane proteins MotA and MotB. The complexes have the subunit composition MotA4-MotB2 and are believed to constitute the stator, secured to the cell wall though a peptidoglycan-binding domain in MotB (20–22). Motors typically contain 10 or more stator complexes (23, 24) arrayed in the membrane around the basal body (25, 26), which can function independently to generate torque (23, 27). An invariant aspartate residue of MotB (Asp32 in the E. coli protein) is critical for rotation and has been proposed to function directly in proton conduction (28). In a current model for the rotation mechanism, proton association/dissociation at Asp32 of MotB drives cyclical conformational changes in the stator complex that apply force at the MotA-FliG interface to drive movement of the rotor (7). Thus, although the direction of rotation is determined by the FliG-FliM-FliN switch, the MotA-MotB complexes appear to be the actual drivers of movement.
Crystal structures have been solved for major portions of the FliG, FliM, and FliN proteins (29–31). The structurally characterized part of FliG includes the C-terminal two-thirds of the protein and consists of two globular domains joined by an α-helix and a short, relatively extended linker. The conserved charged residues that interact with the stator lie together on a ridge at the top of the FliG C-terminal domain (termed FliGC). At the end of the domain opposite the charged ridge, FliGC has a conserved surface hydrophobic patch (29) that was shown to interact with FliM (32). The other domain of FliG present in the crystal structure displays another well conserved surface feature, termed the EHPQR motif for its constituent residues, that was also found to interact with FliM (32). In the case of FliM, the structure was determined for a central domain comprising residues 46–215 of the ∼330-residue protein (31). This middle domain of FliM (termed FliMM) is folded into an ellipsoidal shape with the approximate dimensions 3 × 4 × 5 nm. A motif containing residues GGXG is strongly conserved in FliM and is displayed at one end of this core domain. The GGXG motif was implicated in binding to FliG (33) and is thus predicted to point toward the top (i.e. membrane-proximal part) of the C-ring. The structures of the N- and C-terminal domains of FliM are not known, except for a short segment near the N terminus, for which the structure was obtained in complex with the signaling protein CheY (34). The structure of FliMM shows both the N and C termini at the end opposite the GGXG motif, and so the N- and C-terminal domains not present in the crystal structure should attach near the bottom, relatively distant from FliG and from the membrane. For FliN, the structure is known for the C-terminal two-thirds of the protein, lacking only an N-terminal region that is relatively poorly conserved and largely dispensable for function (35). The FliN subunits are tightly intertwined into dimers, which are further associated, in one crystal form, into donut-shaped tetramers (30). The E. coli FliN protein was shown to form stable tetramers in solution (30), and targeted disulfide cross-linking experiments indicated a donut-like organization for the protein in the cell (36). A conserved hydrophobic patch on FliN, centered on the dimer 2-fold axis, forms a site of interaction with the flagellar export protein FliH (11, 13). The hydrophobic patch and adjoining regions are also important for the switch to clockwise rotation and accordingly were suggested to interact with the signaling protein CheY-P (13).
Recent electron microscopic (EM) reconstructions of the C-ring have resolved features as small as individual protein domains and can thus provide strong constraints on the organization of components (4, 37). Using the overall shape observed in the EM images in combination with crystal structures, cross-linking, protein binding, and mutational analyses, we previously developed a model for the organization of most components in the switch complex, illustrated in Fig. 1B. In this model, FliG is at the top of the C-ring, where it can interact with the stator; FliM is just below FliG and forms the relatively smooth side wall of the ring; and FliN is at the bottom, where the EM images show rings of density of a shape and size that match the donut-shaped FliN tetramers observed in the crystal structure (30). The model can account satisfactorily for the overall shape and dimensions of the C-ring, as well as for results from diverse mutational and biochemical studies (36, 38–41). Certain aspects of subunit organization in the C-ring remain unclear, however. Although FliN is known to bind to the C-terminal domain of FliM (termed FliMC), the location of FliMC and the details of the FliMC-FliN relationship are unknown. A clearer picture of subunit organization in this region will be essential for the development of molecular models of switching, particularly given the likelihood that FliN interacts with the signaling molecule CheY-P.
Here, we report biochemical and mutational studies that address the major questions of subunit organization in the lower part of the switch complex. The results indicate that the FliMC domains are inserted between adjoining FliN tetramers in the C-ring. The bottom part of the ring thus consists of an alternating array of FliM and FliN molecules, accounting for the cooperative action of these proteins in forming the ring (42). A structural model based on the results accurately reproduces the major features of the lower part of the C-ring observed in EM reconstructions. Repellent-induced changes in cross-link yield reveal subunit movements that occur upon switching. The switch between counterclockwise and clockwise rotation is accompanied by a displacement of FliMC along one of its interfaces with FliN. An explicit, structurally grounded model for switching is proposed that accords with present knowledge of switch complex organization and function.
EXPERIMENTAL PROCEDURES
Strains, Plasmids, and Media
The E. coli strains and plasmids used are listed in Table 1. Procedures for transformation and plasmid isolation were described previously (43). TB broth contained 10 g/liter Tryptone and 5 g/liter NaCl, and LB medium contained these plus 5 g of yeast extract. Ampicillin and chloramphenicol were used in liquid media at 125 and 50 μg/ml, respectively, and in soft agar plates at 50 and 12.5 μg/ml, respectively. Isopropyl β-d-thiogalactopyranoside (IPTG) was prepared as a 0.1 m stock in water and used at the concentrations indicated. Leucine was prepared as a 100 mm stock in water. DNA sequencing and oligonucleotide synthesis were carried out by core facilities at the University of Utah.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or property | Ref. or source |
|---|---|---|
| DFB223 | fliN null strain | Ref. 35 |
| DFB228 | fliM null strain | This work |
| DFB232 | fliMN null strain | Ref. 35 |
| RP3098 | ΔflhDC; no expression of chromosomal flagellar genes | J. S. Parkinson |
| pTBM30 | Ptac expression vector; Apr; parent of pHT39 | Ref. 51 |
| pLS4 | fliN in pAlter-1; Apr | Ref. 35 |
| pHT41 | fliM in pAlter-1, vector for site-directed mutagenesis | Ref. 43 |
| pHT39 | fliN under Ptac control; Apr | Ref. 48 |
| pDFB94a | fliM under Ptac control; Cmr | This work |
| pDFB66 | cheY under Para control; Cmr | Ref. 13 |
| pHT100 | GST-only under Ptac control; Kmr | Ref. 44 |
| pHT86 | GST-FliM under Ptac control; Kmr | Ref. 44 |
Site-directed Mutagenesis and Assays of Swarming
Mutagenesis used either the QuikChange method (Qiagen) or the altered sites procedure (Promega). Mutations were confirmed by sequencing, and then mutant fliN and fliM genes were transferred into plasmids pHT39 and pDFB94, respectively, to allow IPTG-regulated expression from the tac promoter. For assays of function, strains with deletions in the relevant genes (see Table 1) were transformed with wild-type or mutant plasmids, and motility in soft agar, swimming in liquid, and flagellation were measured as described (43). Soft agar plates contained Tryptone broth, 0.27% agar, appropriate antibiotic(s), and IPTG at concentrations of 0, 10, and 100 μm to give FliM or FliN expression levels ranging from slightly below to significantly above wild-type levels (35).
Co-pelleting Assay for Protein Binding
Glutathione S-transferase (GST) pulldown experiments were carried out essentially as described (33, 44) with minor modifications. The experiments used a ΔflhDC strain that expresses no chromosomal flagellar genes and a “two-cell” protocol in which GST-FliM and FliN (wild-type or mutant) were expressed in separate cells. Control experiments used GST only in place of the GST-FliM fusion, expressed from plasmid pHT100 (44). Cells were cultured overnight at 32 °C in 40 ml of LB medium with appropriate antibiotics and 400 μm IPTG. Cells expressing GST-FliM were mixed with cells expressing FliN or its mutant variants, and then the mixed cells were pelleted and resuspended in 1 ml of phosphate-buffered saline (140 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, 1.8 mm KH2PO4) to which 100 μl of lysozyme solution (5 mg/ml in 50% glycerol) and 10 μl of 4-amidinophenylmethanesulfonyl fluoride (10 mm stock) had been added. After 1 h on ice, cells were disrupted by sonication; unbroken cells were pelleted (16,000 × g, 30 min, 4 °C); and 50 μl of the supernatant was saved for use in estimating the amount of FliM present before addition of affinity beads. The rest (∼1 ml) was transferred to a fresh tube, mixed with 150 μl of a 50% slurry of glutathione-Sepharose 4B (GE Healthcare) prepared according to the manufacturer's directions, and incubated for 3 h at room temperature with gentle rotation to allow binding. The beads were pelleted (14,000 × g, 1 min), washed three times with 1 ml of phosphate-buffered saline, and repelleted. The supernatant was removed, and GST-FliM and associated proteins were released from the beads by addition of 50 μl of elution buffer (50 mm reduced glutathione in 50 mm Tris-HCl, pH 8) for 10 min at room temperature with gentle rotation. Beads were pelleted, and the supernatant was collected for analysis by SDS-PAGE and immunoblotting using anti-FliN antibody as described (36, 44).
Cross-linking
Initial cross-linking experiments used the catalyst Cu(1,10-phenanthroline)3 and were performed as described (36, 45) with minor modifications. Plasmids expressing the Cys-substituted FliM and FliN proteins were cotransformed into the fliMN deletion strain DFB232. The flhDC strain was used as a negative control to verify that the FliM-FliN cross-linking depended upon the assembly of flagella. Cells were cultured at 37 °C for 4–5 h in LB medium containing appropriate antibiotics and then diluted 100-fold into TB broth containing appropriate antibiotics and grown overnight with 50 μm IPTG at 37 °C. Using A600 readings to estimate culture density, equal numbers of cells from each culture were transferred to a centrifuge tube, pelleted (3000 × g, 10 min), and resuspended in 200 μl of lysis buffer (50 mm Tris, pH 8, 0.5 m sucrose, 10 mm EDTA, 0.2 mg/ml lysozyme). After 1 h on ice, cells were rapidly diluted by addition of 1.8 ml of ice-cold water and then divided into two 1-ml fractions. Cross-linking reagent (110 μl in 50% ethanol) was added to one sample, whereas non-cross-linked controls received just 50% ethanol. The cross-linking reagent contained 4 mm CuSO4 and 16 mm 1,10-phenanthroline and was freshly prepared from a 1 m stock of 1,10-phenanthroline in 95% ethanol and a 400 mm stock of CuSO4 in water. Samples were rotated gently for 5 min at room temperature, sonicated for 30 s (Branson Model 450 sonifier, 50% duty cycle, power level 3), and left at room temperature for an additional 5 min. Reactions were quenched by addition of N-ethylmaleimide (22 μl from a 1 m stock in 95% ethanol) and EDTA (126 μl from a 0.5 m stock). A total of 100 μl of each sample was mixed with 100 μl of nonreducing gel loading buffer, boiled, and used for electrophoresis.
For cross-linking experiments using iodine, cells were cultured and collected as described above and then treated directly (without cell lysis) with 0.2 mm I2 added from a 2 mm stock in 95% ethanol, with non-cross-linked controls receiving just ethanol. N-Ethylmaleimide was added after 5 min, and then cells were mixed with nonreducing gel loading buffer, boiled, and used for electrophoresis. In cross-linking experiments examining repellent effects, leucine (30 mm final concentration) or glycerol (10% final concentration) was added to resuspended cells, and following a 2-min incubation at room temperature, cross-linking was induced with iodine as described above.
SDS-PAGE and Immunoblotting
Protein samples were separated on 8.5 or 12% SDS-polyacrylamide minigels (Bio-Rad Mini-PROTEAN system) and transferred to nitrocellulose using a semidry transfer apparatus (Bio-Rad). Rabbit polyclonal antibodies against FliM or FliN were prepared as described previously (43) and used at 2000- and 1500-fold dilution, respectively. Bands were visualized using the SuperSignal West Pico Luminol system (Pierce) and x-ray film.
FliMC Homology Model
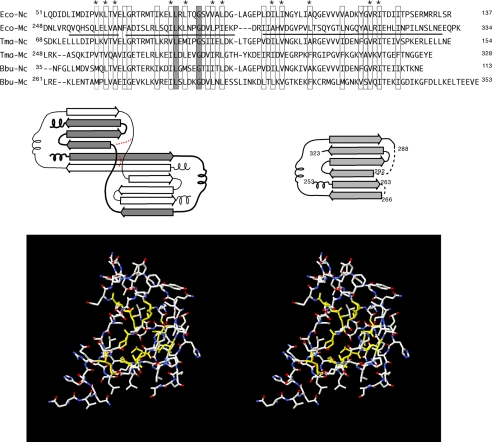
A homology model for the C-terminal domain of FliM was constructed using its sequence similarity to FliN. With the FliN coordinates opened in Swiss- PdbViewer, segments of FliMC showing similarity to FliN were imported and aligned with the corresponding segments of FliN. Side chain torsion angles were adjusted to eliminate clash, and the structure was subjected to one round of energy minimization. A FliM-FliN sequence alignment and further details are provided in Fig. 2.
FIGURE 2.
Construction of the FliMC homology model. An alignment of FliN and FliM C-terminal domains is shown in the upper panel. Boxes indicate positions where hydrophobic character is conserved, and asterisks indicate the positions forming the core in both FliN and the homology-modeled FliMC. The solid line under the E. coli C-terminal sequence represents the residues that are included in the FliMC homology model. FliN forms intertwined dimers, with topology sketched in the middle panel. Altered connectivity in two places (indicated in red) allows the FliM peptide to fold as a monomer (consistent with the known properties of FliM, which interacts with FliN to form 1:4 complexes). The homology model has conserved hydrophobic residues at core positions (shown in yellow in the lower panel). Ec-Nc and Ec-Mc, E. coli FliN and FliM C-terminal domains, respectively; Tma-Nc and Tma-Mc, T. maritima FliN and FliM C-terminal domains, respectively; Bbu-Nc and Bbu-Mc, B. burgdorferi FliN and FliM C-terminal domains, respectively.
RESULTS
FliM-binding Surfaces on FliN
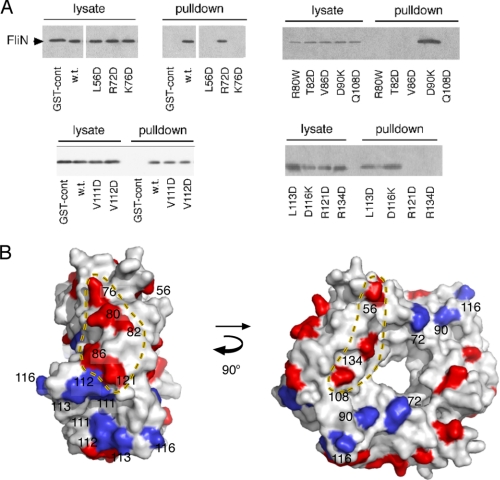
FliN was shown to bind specifically to FliMC in studies that used either GST pulldown assays (44) or blot overlay assays (46). Those studies predate the FliN crystal structure, and the regions on FliN that bind to FliMC were not mapped. To identify the regions on FliN that interact with FliM, we utilized the previously developed GST-FliM pulldown assay with a collection of FliN mutations sampling the protein surface (13). The FliN mutations all involved non-conservative replacements (e.g. reversal of charge, replacement of hydrophobic residues by Asp). Positions were chosen using a homology model for the E. coli FliN protein based on the crystal structure determined for Thermotoga maritima FliN (30). The sequences of E. coli and T. maritima FliN are 55% identical in the region of interest, and the homology model contained hydrophobic residues at all core positions. Pulldown experiments used a two-cell protocol in which the interacting proteins were expressed in different cells. Cells expressing FliN (wild-type or mutant) were mixed with cells expressing GST-FliM; the cells were lysed; and glutathione-Sepharose beads were used to isolate GST-FliM and associated protein(s). Following elution with glutathione, samples were resolved by SDS-PAGE, and FliN was detected by immunoblotting. Representative blots and a summary of all the binding results mapped onto the FliN structure are shown in Fig. 3.
FIGURE 3.
Regions on FliN important for the interaction with FliN. A, representative gels from the GST-FliM/FliN pulldown experiments. B, results of the pulldown experiments mapped onto the structural model of the FliN tetramer. Residues important for the FliM-FliN interaction fall in two widely separated regions. The hydrophobic patch on FliN, discussed under “Discussion,” is centered on the 2-fold axis of the dimer and contains, among others, residues 111–113, which are not important for the FliM interaction. cont, control; w.t., wild-type.
As reported previously (44), FliN was readily co-isolated with GST-FliM in this assay, whereas the negative controls using GST alone were clear. Of 14 FliN surface residue mutations studied, eight prevented binding to FliM in the pulldown assay. The residues important for FliM binding fell in two distinct regions; positions 76, 80, 82, 86, and 121 are grouped on an outward-facing part of the FliN donut, whereas residues 56, 108, and 134 lie along the side of the donut, with positions 108 and 134 lying near the inner edge. The FliM-FliN interaction was not affected by substitutions at residues 111, 112, 113, and 116, which are in and around the hydrophobic patch, or at residues 72 and 90, which are together on the side of the FliN donut roughly opposite the 56/108/134 face (Fig. 3B). In a previous study of FliN mutant phenotypes, mutations in the residue 76 and residue 134 regions were found to alter the level of FliN expression needed for optimal function, and it was proposed that these regions might interact with FliM (13). The present experiments demonstrate the FliM interaction more directly and show, in particular, that two regions are involved.
Cross-linking of FliN to FliMC
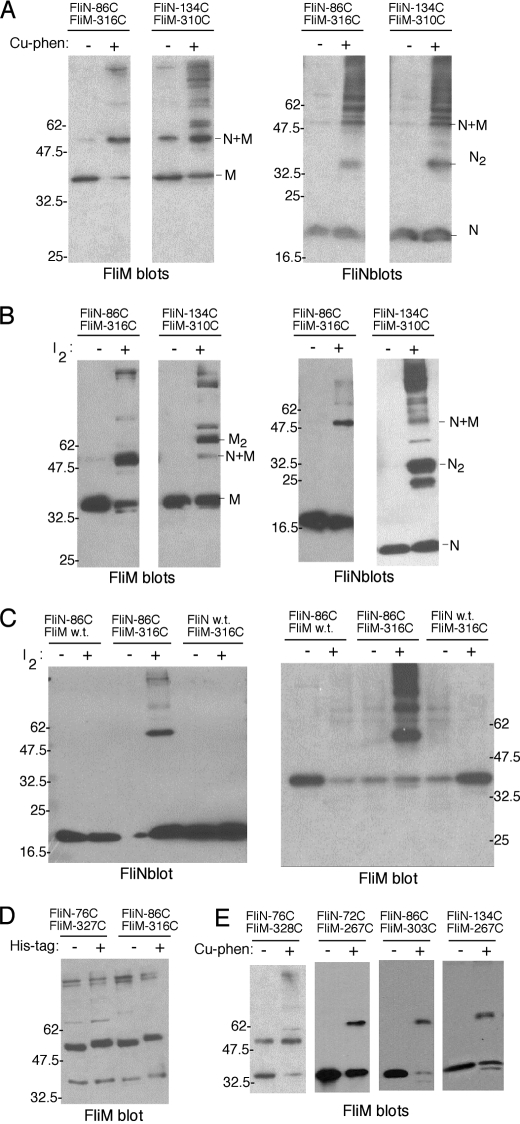
Next, to define the FliMC-FliN relationship more fully, we conducted targeted disulfide cross-linking experiments. Cysteine replacements were made at 12 positions sampling the surface of FliN and at 16 positions sampling the surface of FliMC. The structure of FliMC is not known, but this domain exhibits sequence similarity to FliN (33) that allowed us to construct a homology model for use in choosing positions of the Cys replacements (Fig. 2, center panel). Amino acids forming the core of the FliMC homology model all exhibit conserved hydrophobic character (Fig. 2, lower panel). For the cross-linking experiments, the FliN and FliMC Cys mutant proteins were expressed from two compatible plasmids in a ΔfliMN strain, in all pairwise combinations including the single-Cys controls (13 × 19, or 247, strains in all). Initial experiments used the oxidation catalyst copper-phenanthroline, which has been shown to be relatively permissive in the sense of allowing cross-linking at a somewhat extended range, presumably by generating relatively long-lived reaction intermediates (47). Copper-phenanthroline does not readily cross the membrane, and so these experiments used lysed cells. For the Cys pairs that showed cross-linking with copper-phenanthroline, the experiments were repeated using iodine, which requires closer proximity of the Cys residues and provides tighter constraints (45). Iodine crosses the membrane, and so these experiments could be done using intact cells. Products of cross-linking were characterized on immunoblots using polyclonal antibodies against FliM and FliN. A band was assigned as the FliM-FliN heterodimer if it was detected on both the FliM and FliN immunoblots, required Cys replacements in both of the proteins, was eliminated by treatment with 2-mercaptoethanol, and migrated with an apparent mass of ∼50 kDa. Representative blots are shown in Fig. 4, and results are summarized on the FliN and FliMC structures in Fig. 5.
FIGURE 4.
Targeted disulfide cross-linking between FliN and FliMC. A, cross-linking by the relatively high-yield Cys pairs N86/M316 and N134/M310 catalyzed by copper-phenanthroline (Cu-phen). B, cross-linking of the N86/M316 and N134/M310 pairs by oxidation with iodine. C, single-Cys controls demonstrating the requirement for Cys residues in both proteins. D, shift in mobility of the FliM-FliN heterodimer when a His tag is introduced in FliN. The cross-linking used copper-phenanthroline. E, cross-linking involving additional Cys pairs. Results with copper-phenanthroline are shown; with iodine, FliM-FliN cross-linking was observed reproducibly, but yields were lower (not shown). w.t., wild-type.
FIGURE 5.
Positions of the Cys replacements used in the cross-linking experiments and summary of the positions that showed cross-linking. Arrow thicknesses represent the relative strength of cross-links seen with iodine. A, Cys positions in FliMC. B, Cys positions in FliN.
Wild-type FliN has no Cys residues, and FliM has just a single Cys residue that is fully buried in the folded protein; as expected, neither of the native proteins showed significant cross-linking upon treatment with copper-phenanthroline. The majority of the double-Cys mutants also did not show any clear reproducible cross-linking. Of the 216 double-Cys mutants studied, two (N86/M316 and N134/M310, where “N” is FliN and “M” is FliM) reproducibly gave a moderate yield of cross-linked FliM-FliN heterodimer. Seven other Cys pairs (N72/M260, N72/M267, N76/M327, N76/M328, N86/M303, N134/M265, and N134/M267) also gave reproducible cross-linking, but in lower yield (Fig. 5).
The yield of FliM-FliN heterodimer appeared lower on FliN blots than on FliM blots, even though the two antibodies showed similar sensitivity in detecting the native proteins. As a further test of the heterodimer band assignment, a His tag was fused to the N terminus of FliN, and the N86/M316 cross-linking experiment was repeated. On a FliM immunoblot, the FliM-FliN heterodimer showed the expected mobility shift when the His tag was attached to FliN, confirming the presence of FliN. The cause of the different apparent intensities in FliM versus FliN blots is uncertain but might have to do with the masking of some FliN epitopes in the cross-linked product.
To verify that the cross-linking occurred between FliM and FliN proteins in the flagellum rather than by collision of the diffusing proteins, the cross-linking experiments were repeated in a mutant strain that does not assemble flagella (RP3098, ΔflhDC). No FliM-FliN cross-linking was observed in the non-flagellate strain (supplemental Fig. 1).
In FliN, the positions that cross-linked to FliM fell in the same regions implicated in the binding experiments and, in both FliN and FliMC, the cross-linking residues occurred in widely separated locations that cannot be accounted for in terms of a single FliM-FliN interface. Both the cross-linking and binding data are most easily accounted for in a model in which FliMC is positioned between two adjacent FliN tetramers. (A model with two FliMC domains flanking a single FliN tetramer would be equivalent in the sense of having the same interfaces, and the actual C-ring structure will presumably contain many copies of both proteins.) A model for the FliN4-FliMC-FliN4 assembly was constructed by positioning the cross-linking Cys pairs in proximity while orienting the protein surfaces to avoid steric clash. The resulting spacing between FliN tetramers, as determined from just the cross-linking results and the protein dimensions, was 4 nm, close to what has been measured in EM reconstructions. Because of the shape of the subunit interfaces, the FliN tetramers are forced into a tilted orientation that also resembles the features seen in micrographs (Fig. 6; compare with EM reconstruction in Fig. 8).
FIGURE 6.
Structural model for the FliN4-FliMC-FliN4 unit. The Cys pairs that showed reproducible cross-linking using iodine are indicated. Numbers in parentheses indicate the approximate distance between sulfur atoms (in Å). Two views are shown; FliMC is shown in green, and the FliN tetramers are shown in blue and light blue.
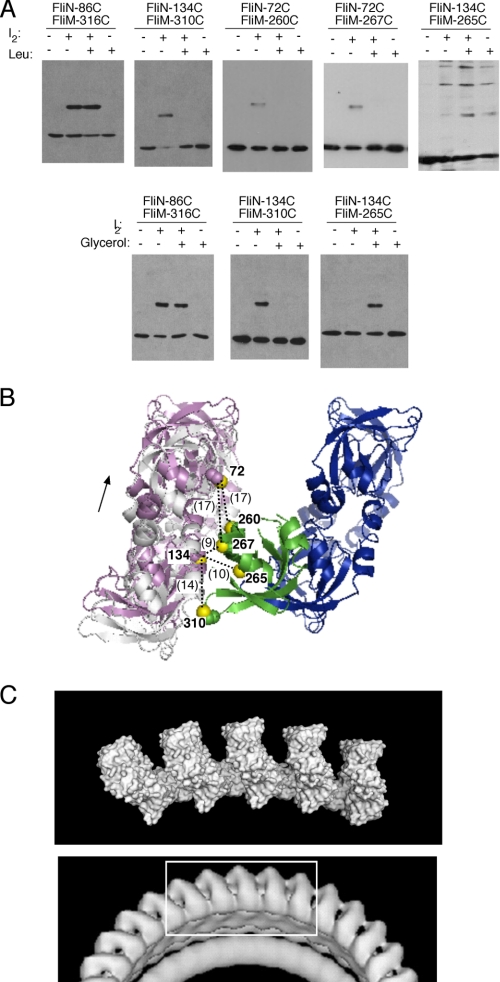
FIGURE 8.
Movements associated with switching to clockwise rotation. A, effects of repellent stimuli on cross-link yields. The experiments used iodine to induce cross-linking in intact cells. Upper panels, effects of leucine (30 mm). Lower panels, effects of glycerol (10%). B, Cys pairs that showed altered cross-link yields upon treatment with repellent mapped onto the FliN4-FliMC-FliN4 structural model. The affected cross-links are on the left-hand interface, and the changes in cross-link yield can be accounted for by a displacement along the interface in the direction indicated. The magnitude of the displacement cannot yet be estimated precisely; inter-sulfur distances that would result from a 7-Ådisplacement are indicated. C, comparison of the FliMC-FliN structural model (upper panel, five FliN tetramers and four FliMC domains are shown in the arrangement corresponding to the clockwise state) with an image of the bottom of the basal body from EM reconstructions on a clockwise-locked mutant (4).
Phenotypic Data in the Framework of the Model
Available mutational data were used to provide additional tests of the structural model of the FliN4-FliMC-FliN4 unit. It was noted above that the cross-linking positions occur in roughly the same regions where mutations disrupted the FliM-FliN interaction, and as expected, this correspondence was confirmed when the binding data were mapped onto the model of the FliN4-FliMC-FliN4 unit. Positions where mutations disrupted the FliM-FliN interaction lie within or adjacent to the FliM-FliN interfaces, whereas positions insensitive to mutation are more distant from the interfaces (Fig. 7A). Next, the function of each of the FliM and FliN Cys mutant proteins was measured using soft agar motility assays. Measurements were made over a range of inducer concentrations to allow function to be assayed over a range of FliM and FliN expression levels. Results of all the assays are given in supplemental Fig. 2; key findings are as follows. None of the single-Cys replacements in FliM disrupted function very strongly. The four FliM replacements that caused the strongest defects (motility less than half of that of the wild type) were at positions 309, 316, 321, and 328, all of which lie along the FliMC-FliN interface in the model (Fig. 7B). In FliN, most single-Cys replacements had only minor effects, but fairly strong defects occurred at positions 126 and 134, both of which are predicted to lie at the interface with FliMC (Fig. 7B). Thus, the Cys mutant phenotypes are in agreement with the model.
FIGURE 7.
Mutational data mapped onto the model of the FliN4-FliMC-FliN4 unit. A, FliMC-FliN binding data mapped onto a stereoview of the FliN4-FliMC-FliN4 model. Positions where mutations disrupted binding are colored red, and positions where binding was unaffected are blue. B, positions of Cys replacements that had the largest effect on function. Mutations at the four positions indicated in FliMC and position 126 in FliN reduced swarming to about half of that of the wild type; the mutation at position 134 in FliN reduced function to ∼10% of that of the wild type. (Functional data on all Cys mutants are provided in the supplemental data.) C, additional mutations. Four mutations in FliN that gave a moderate assembly defect and severe motility defect, isolated in an extensive study of spontaneous mutants (13), are shown in orange. These cluster on a surface of FliN that is in contact with FliMC (the left-hand interface in the figure). Two additional mutations that were made as tests of the model are indicated in red-orange (FliM position 292) and purple (FliN position 87). D, effects of the site-directed mutations I292E and V87D. Both mutations prevented swarming in the soft agar assay (upper panels) and FliM-FliN binding in the pulldown assay (lower panels). cont, control; wt, wild-type.
In an extensive study of spontaneous fliN mutations, Macnab and co-workers (10) identified a small number of alleles that affected motility more strongly than flagellar synthesis. These mot mutants were later found to be partially rescuable by overexpression of the mutant proteins, and it was proposed that they affect regions important for the incorporation of FliN into the C-ring (48, 49). Three of these mutations occur at residues that are buried at the interface between FliN dimers, where they are expected to interfere with assembly of the tetramer. The remaining mot alleles (four) are clustered on the FliN surface near the inner face of the donut, in a region that contacts FliMC in the model (Fig. 7C).
Finally, as a test of the predictive power of the model, additional mutations were made at residues predicted to lie in the center of the larger FliMC-FliN interface (position 292 in FliM and position 87 in FliN). Each of these mutations eliminated motility in the swarming assay and FliM-FliN binding in the pulldown assay (Fig. 7D). Mutations giving such strong defects are very rare in FliM and FliN and in fact have not previously been seen except in core residues that are likely to affect protein folding (33, 50); thus, the present results provide strong support for the importance of the interface predicted by the model.
Effects of Direction Switching
We suggested previously that CheY-P might bind to FliN (13) and that switching might involve changes in the part of the C-ring containing the FliN donuts (36). Changes in the conformation of FliN or its relationship to FliM might be expected to change the FliMC-FliN interface(s) and alter the pattern of cross-linking. To test this, we examined the effects of a repellent (leucine) stimulus on yields of FliM-FliN cross-linking. The experiments used both Cys pairs that had previously been found to cross-link and some additional pairs that were situated relatively close to the FliMC-FliN interface (and might come into cross-linking range as a result of conformational change). Key findings are shown in Fig. 8 and are summarized as follows. Cross-link yield in the N86/M316 mutant was unaffected by leucine, indicating that this FliMC-FliN interface is not greatly affected by switching. In contrast, several positions on the other FliMC-FliN interface showed strong responses to repellent; cross-linking was eliminated in the N134/M310, N72/M260, and N72/M267 Cys pairs, whereas the N134/M265 Cys pair, which cross-linked very little in the absence of a stimulus, gave substantial cross-linking in the presence of leucine. To confirm that these effects were caused by motor switching rather than any other effects of leucine, the experiments were repeated using glycerol (an alternative repellent stimulus). The results were similar and, in some cases, more pronounced than those seen with leucine (Fig. 8A). All of the observed changes in cross-linking are accounted for in a model in which one of the FliMC-FliN interfaces remains unchanged while the other undergoes a substantial (of order 5 Å) displacement (Fig. 8B).
DISCUSSION
Although the bacterial chemotaxis system is among the most intensively studied signaling systems in biology, the mechanism of the motor-switching event that culminates chemotactic signaling has remained poorly understood. The development of molecular hypotheses for switching has been hampered by a shortage of detailed structural information on the switch complex as well as insufficient information on the molecular movements occurring upon motor reversal. The present results cast significant new light on the switching process by clarifying aspects of subunit organization in the lower portion of the switch complex, a part that includes binding sites for the signaling molecule CheY-P, and by providing a glimpse of subunit movements that occur within the complex upon switching.
The present cross-linking, binding, and functional data, as well as results of previous mutational studies, are well accounted for in a structural model in which the lower portion of the C-ring is formed from an alternating array of FliN tetramers and FliM C-terminal domains (illustrated in Fig. 6). This model accounts naturally for the observation that FliM and FliN function cooperatively in forming the C-ring (i.e. the structure fails to form in the absence of either protein) (42) as well as for the resiliency of the proteins to mutation. Because FliM and FliN each form two distinct interfaces with each other, most single-site mutations have only relatively minor effects on flagellar assembly and function (13, 33), and the only surface residue mutations in FliN or FliM known to prevent motility completely are the N87 and M292 replacements made in this study as tests of the model. Evidently, the FliM-FliN interfaces can tolerate some disruption (enough to eliminate binding in the pulldown assay) without preventing assembly of the C-ring structure as a whole. Finally, the present structural model provides a close match to the major features seen in EM reconstructions of the basal body, most notably the spacing and tilt of the FliN tetramers (Fig. 8C).
Previous mutational studies highlighted the importance of a conserved hydrophobic patch on FliN in both flagellar assembly and direction switching. The hydrophobic patch is centered on the symmetry axis of the FliN dimer, and so the FliN tetramer has two patches, displayed on opposite faces of the donut. The hydrophobic patch was shown to bind to the flagellar export protein FliH, which presumably accounts for its importance in assembly (11, 13). Several counterclockwise-biased mutations in and around the patch pointed to a role in switching. These mutations could be suppressed in most cases by overexpression of CheY, and we proposed that the patch might be the site of action of CheY-P (13). (This proposal has been corroborated in further study of the FliN-CheY interaction.3) In the present structural model for the lower part of the C-ring, the hydrophobic patches on the FliN tetramer do not interact with the adjacent FliMC domains but remain exposed to the solvent, as required for interaction with FliH and CheY-P.
Changes in cross-link yield in the presence of repellents (Fig. 8) show that switching is associated with a movement of FliMC relative to FliN. Although the present constraints are not sufficient to define the movement precisely, it clearly involves a substantial displacement of FliMC along one of its interfaces with FliN, with less movement at the other interface. In the context of the C-ring, such a movement would be associated with rotation of the FliMC domains relative to the ring as a whole (supplemental Fig. 3). We note further that this movement will alter the accessibility of the hydrophobic patch and thus its ability to interact with CheY-P. The model thus provides a straightforward mechanism for linking CheY-P binding to the conformational change. Previously, we noted that the FliN donut is puckered and suggested that switching might be associated with a reversal in this puckering (36). Although such puckering motions might occur in concert with the displacement observed here, there are presently no results bearing on it directly, and so it is not included as a feature of the present model.
Direction reversal must presumably involve changes at the rotor-stator interface, formed between the charge-bearing ridge on FliGC and the cytoplasmic domain of MotA (7). Movements taking place in the lower part of the C-ring must therefore be transmitted “up” through FliMM to alter the orientation or position of FliGC. This would imply some movement in the FliMM domains. Preliminary cross-linking experiments indicate that the FliMM domains do undergo relative movement (as evidenced by altered yields of FliM-FliM cross-linking in the presence of repellent).4 Experiments are under way to define the movement more fully. We note that the motions occurring in FliMM and FliMC might be different, as these are distinct domains joined by a linker that is relatively non-conserved, variable in length, and possibly flexible.
Molecular hypotheses for flagellar direction reversal have been difficult to formulate, largely because of the lack of information on subunit organization and movements occurring in the switch. The present results provide a fuller structural framework for the development of switching models, reveal movements occurring in the lower part of the switch, and demonstrate the feasibility of applying cross-linking and related approaches to define the switching event in molecular detail.
Acknowledgments
We gratefully acknowledge Brian Lowder, Moises Terrazas, and Eun A Kim for helpful discussions and technical assistance with the experiments.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM64664 from NIGMS and Grant GM64116.
This article was selected as a Paper of the Week.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
M. K. Sarkar, K. Paul, and D. F. Blair, manuscript in preparation.
K. Paul, M. K. Sarkar, D. Brunstetter, and D. F. Blair, unpublished data.
- CheY-P
- phospho-CheY
- EM
- electron microscopic
- IPTG
- isopropyl β-d-thiogalactopyranoside
- GST
- glutathione S-transferase.
REFERENCES
- 1.Berg H. C., Anderson R. A. (1973) Nature 245, 380–382 [DOI] [PubMed] [Google Scholar]
- 2.Macnab R. M., Koshland D. E., Jr. (1972) Proc. Natl. Acad. Sci. U.S.A. 69, 2509–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown D. A., Berg H. C. (1974) Proc. Natl. Acad. Sci. U.S.A. 71, 1388–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas D. R., Francis N. R., Xu C., DeRosier D. J. (2006) J. Bacteriol. 188, 7039–7048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi S., Aizawa S., Kihara M., Isomura M., Jones C. J., Macnab R. M. (1986) J. Bacteriol. 168, 1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi S., Fujita H., Ishihara A., Aizawa S., Macnab R. M. (1986) J. Bacteriol. 166, 187–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J., Lloyd S. A., Blair D. F. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6436–6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyer C. M., Vartanian A. S., Zhou H., Dahlquist F. W. (2009) J. Mol. Biol. 388, 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogler A. P., Homma M., Irikura V. M., Macnab R. M. (1991) J. Bacteriol. 173, 3564–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irikura V. M., Kihara M., Yamaguchi S., Sockett H., Macnab R. M. (1993) J. Bacteriol. 175, 802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González-Pedrajo B., Minamino T., Kihara M., Namba K. (2006) Mol. Microbiol. 60, 984–998 [DOI] [PubMed] [Google Scholar]
- 12.McMurry J. L., Murphy J. W., González-Pedrajo B. (2006) Biochemistry 45, 11790–11798 [DOI] [PubMed] [Google Scholar]
- 13.Paul K., Harmon J. G., Blair D. F. (2006) J. Bacteriol. 188, 5240–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sockett H., Yamaguchi S., Kihara M., Irikura V. M., Macnab R. M. (1992) J. Bacteriol. 174, 793–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen S. H., Adler J., Gargus J. J., Hogg R. W. (1974) Proc. Natl. Acad. Sci. U.S.A. 71, 1239–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 3060–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glagolev A. N., Skulachev V. P. (1978) Nature 272, 280–282 [DOI] [PubMed] [Google Scholar]
- 18.Hirota N., Imae Y. (1983) J. Biol. Chem. 258, 10577–10581 [PubMed] [Google Scholar]
- 19.Kawagishi I., Maekawa Y., Atsumi T., Homma M., Imae Y. (1995) J. Bacteriol. 177, 5158–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chun S. Y., Parkinson J. S. (1988) Science 239, 276–278 [DOI] [PubMed] [Google Scholar]
- 21.Blair D. F., Kim D. Y., Berg H. C. (1991) J. Bacteriol. 173, 4049–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Mot R., Vanderleyden J. (1994) Mol. Microbiol. 12, 333–334 [DOI] [PubMed] [Google Scholar]
- 23.Blair D. F., Berg H. C. (1988) Science 242, 1678–1681 [DOI] [PubMed] [Google Scholar]
- 24.Reid S. W., Leake M. C., Chandler J. H., Lo C. J., Armitage J. P., Berry R. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 8066–8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean G. E., Macnab R. M., Stader J., Matsumura P., Burke C. (1984) J. Bacteriol. 143, 991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stader J., Matsumura P., Vacante D., Dean G. E., Macnab R. M. (1986) J. Bacteriol. 166, 244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block S. M., Berg H. C. (1984) Nature 309, 470–472 [DOI] [PubMed] [Google Scholar]
- 28.Zhou J., Sharp L. L., Tang H. L., Lloyd S. A., Billings S., Braun T. F., Blair D. F. (1998) J. Bacteriol. 180, 2729–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown P. N., Hill C. P., Blair D. F. (2002) EMBO J. 21, 3225–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown P. N., Mathews M. A., Joss L. A., Hill C. P., Blair D. F. (2005) J. Bacteriol. 187, 2890–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S. Y., Lowder B., Bilwes A. M., Blair D. F., Crane B. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11886–11891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown P. N., Terrazas M., Paul K., Blair D. F. (2007) J. Bacteriol. 189, 305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathews M. A., Tang H. L., Blair D. F. (1998) J. Bacteriol. 180, 5580–5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S. Y., Cho H. S., Pelton J. G., Yan D., Henderson R. K., King D. S., Huang L., Kustu S., Berry E. A., Wemmer D. E. (2001) Nat. Struct. Biol. 8, 52–56 [DOI] [PubMed] [Google Scholar]
- 35.Tang H., Billings S., Wang X., Sharp L., Blair D. F. (1995) J. Bacteriol. 177, 3496–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul K., Blair D. F. (2006) J. Bacteriol. 188, 2502–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas D., Morgan D. G., DeRosier D. J. (2001) J. Bacteriol. 183, 6404–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan I. H., Reese T. S., Khan S. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 5956–5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oosawa K., Ueno T., Aizawa S. (1994) J. Bacteriol. 176, 3683–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kihara M., Miller G. U., Macnab R. M. (2000) J. Bacteriol. 182, 3022–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowder B. J., Duyvesteyn M. D., Blair D. F. (2005) J. Bacteriol. 187, 5640–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kihara M., Francis N. R., DeRosier D. J., Macnab R. M. (1996) J. Bacteriol. 178, 4582–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang H., Blair D. F. (1995) J. Bacteriol. 177, 3485–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang H., Braun T. F., Blair D. F. (1996) J. Mol. Biol. 261, 209–221 [DOI] [PubMed] [Google Scholar]
- 45.Braun T. F., Blair D. F. (2001) Biochemistry 40, 13051–13059 [DOI] [PubMed] [Google Scholar]
- 46.Toker A. S., Macnab R. M. (1997) J. Mol. Biol. 273, 623–634 [DOI] [PubMed] [Google Scholar]
- 47.Lee G. F., Burrows G. G., Lebert M. R., Dutton D. P. (1994) J. Biol. Chem. 269, 29920–29927 [PubMed] [Google Scholar]
- 48.Lloyd S. A., Tang H., Wang X., Billings S., Blair D. F. (1996) J. Bacteriol. 178, 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao R., Pathak N., Jaffe H., Reese T. S., Khan S. (1996) J. Mol. Biol. 261, 195–208 [DOI] [PubMed] [Google Scholar]
- 50.Zhao R., Schuster S. C., Khan S. (1995) J. Mol. Biol. 251, 400–412 [DOI] [PubMed] [Google Scholar]
- 51.Morrison T. B., Parkinson J. S. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 5485–5489 [DOI] [PMC free article] [PubMed] [Google Scholar]