Abstract
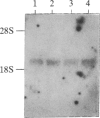
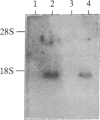
Cells of the monocyte-macrophage series must carry out discrete accessory-cell functions during the process of antigen-specific T-cell activation. One of these functions is the cell-surface expression of major histocompatibility complex (MHC) class II gene products, which are involved in the presentation of foreign antigen to T cells. Previously, we reported that murine peritoneal macrophages infected with the obligate intracellular protozoan Leishmania donovani had suppressed responses to gamma interferon (IFN-gamma) for the induction of MHC class II antigen expression. To determine the molecular basis for this suppression, we examined in the present series of experiments the interaction of this organism with cells of the murine macrophage tumor cell line P388D1. When infected with Leishmania, these cells were also markedly unresponsive to IFN-gamma for the induction of MHC class II antigen expression. This finding was not the result of a defect at the level of the IFN-gamma receptor. Thus, when 125I-labeled IFN-gamma was used, infected macrophages were found to express normal numbers of high-affinity IFN-gamma receptors, and ligand-receptor binding resulted in rapid internalization of labeled IFN-gamma. Despite normal ligand-receptor interactions, the induction in infected cells of mRNA encoding MHC (H-2) class II I-A alpha and beta chains in response to IFN-gamma was markedly suppressed. However, infected cells had normal levels of mRNA encoding the cytoskeletal protein actin. These findings indicate that Leishmania interferes with IFN-gamma induction of macrophage MHC class II antigen expression by down-regulating lymphokine induction of MHC class II mRNA. Suppression of class II expression by this intracellular parasite may prevent subsequent T-cell recognition of infected macrophages and thus favor parasite survival.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyer R. A., Serrano L. E., Jones P. P. Interferon-gamma binds to high and low affinity receptor components on murine macrophages. J Immunol. 1986 May 1;136(9):3329–3334. [PubMed] [Google Scholar]
- Anderson P., Yip Y. K., Vilcek J. Human interferon-gamma is internalized and degraded by cultured fibroblasts. J Biol Chem. 1983 May 25;258(10):6497–6502. [PubMed] [Google Scholar]
- Benoist C. O., Mathis D. J., Kanter M. R., Williams V. E., 2nd, McDevitt H. O. Regions of allelic hypervariability in the murine A alpha immune response gene. Cell. 1983 Aug;34(1):169–177. doi: 10.1016/0092-8674(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada A., Schreiber R. D. Internalization and degradation of receptor-bound interferon-gamma by murine macrophages. Demonstration of receptor recycling. J Immunol. 1987 Jul 1;139(1):147–153. [PubMed] [Google Scholar]
- Celada A., Schreiber R. D. Role of protein kinase C and intracellular calcium mobilization in the induction of macrophage tumoricidal activity by interferon-gamma. J Immunol. 1986 Oct 1;137(7):2373–2379. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Kurt-Jones E. A., Virgin H. W., 4th, Unanue E. R. Relationship of macrophage Ia and membrane IL 1 expression to antigen presentation. J Immunol. 1985 Dec;135(6):3652–3654. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., MacDonald H. R. Binding and internalization of interleukin 1 by T cells. Direct evidence for high- and low-affinity classes of interleukin 1 receptor. J Exp Med. 1986 Oct 1;164(4):1060–1074. doi: 10.1084/jem.164.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas J. M., King D. P., Jones P. P. Biosynthesis and expression of Ia and H-2 antigens on a macrophage cell line are stimulated by products of activated spleen cells. J Immunol. 1983 Jan;130(1):449–456. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Paulnock-King D., Sizer K. C., Freund Y. R., Jones P. P., Parnes J. R. Coordinate induction of Ia alpha, beta, and Ii mRNA in a macrophage cell line. J Immunol. 1985 Jul;135(1):632–636. [PubMed] [Google Scholar]
- Pearson R. D., Wheeler D. A., Harrison L. H., Kay H. D. The immunobiology of leishmaniasis. Rev Infect Dis. 1983 Sep-Oct;5(5):907–927. doi: 10.1093/clinids/5.5.907. [DOI] [PubMed] [Google Scholar]
- Reiner N. E. Host-parasite relationship in murine leishmaniasis: pathophysiological and immunological changes. Infect Immun. 1982 Dec;38(3):1223–1230. doi: 10.1128/iai.38.3.1223-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner N. E., Ng W., McMaster W. R. Parasite-accessory cell interactions in murine leishmaniasis. II. Leishmania donovani suppresses macrophage expression of class I and class II major histocompatibility complex gene products. J Immunol. 1987 Mar 15;138(6):1926–1932. [PubMed] [Google Scholar]
- Reiner N. E. Parasite accessory cell interactions in murine leishmaniasis. I. Evasion and stimulus-dependent suppression of the macrophage interleukin 1 response by Leishmania donovani. J Immunol. 1987 Mar 15;138(6):1919–1925. [PubMed] [Google Scholar]
- Robinson R. R., Germain R. N., McKean D. J., Mescher M., Seidman J. G. Extensive polymorphism surrounding the murine Ia A beta chain gene. J Immunol. 1983 Oct;131(4):2025–2031. [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]