Abstract
Molecular medicine can benefit greatly from antibodies that deliver therapeutic and imaging agents to select organs and diseased tissues. Yet the development of complex and defined composite nanostructures remains a challenge that requires both designed stoichiometric assembly and superior in vivo testing ability. Here, we generate nanostructures called nanostreptabodies by controlled sequential assembly of biotin-engineered antibody fragments on a streptavidin scaffold with a defined capacity for additional biotinylated payloads such as other antibodies to create bispecific antibodies as well as organic and non-organic moieties. When injected intravenously, these novel and stable nanostructures exhibit exquisite targeting with tissue-specific imaging and delivery, including rapid transendothelial transport that enhances tissue penetration. This “tinkertoy construction” strategy provides a very flexible and efficient way to link targeting vectors with reporter and/or effector agents, thereby providing virtually endless combinations potentially useful for multipurpose molecular and functional imaging in vivo as well as therapies.
Introduction
In recent years, there has been an increasing demand to build multipurpose nanocomplexes with enhanced tissue-specific targeting in vivo, ultimately to improve molecular and functional imaging as well as therapy. Multispecific nano-immunocomplexes can theoretically facilitate pharmacodelivery for many diseases, including cancer, by combining targeting probes and therapeutic payloads; yet, both their assembly and targeting remain a challenge. Numerous design strategies for engineering multispecific antibodies and protein/antibody fusions have been developed that produce molecules with varied geometry and valence (1–6). Antibody engineering seems to offer a near-endless variety of structures. Single chain Fv (scFv)2 modules can be fused to each other, fused to either the C-terminal or the N-terminal end of an antibody or both (1, 4), or alternatively fused to a multimerization domain to create miniantibodies (5). Similarly, Fab fragments can be manipulated as single chains (2, 3) or chained together as heterodimeric fusions (6). In any case, to be of practical use in animal models and ultimately in patients, these heteromeric assemblies need to be: 1) stable over time with little aggregation, rearrangement, or dissociation of the different moieties, 2) well-defined chemically and structurally without domain swapping, varied or varying degrees of multimerization, and/or heteromerization, and 3) finally, expressed well enough to give high production yields and sizeable amounts of purified material at reasonable costs. Recent approaches have focused on using defined interactions between binding moieties to generate heteromeric complexes. Several strong interactions have been proposed, including the interaction between S-protein and S-tag (7, 8), barnase and bastar (9), or protein kinase A and protein kinase A-anchoring protein (10), and some of them are even further secured with a covalent bond introduced afterward (10–13). Despite their elegance, these methods have so far gained limited success, mostly because of limitations imposed by protein folding, toxicity, or the difficulty of directly incorporating synthetic molecules.
The very high affinity interaction between avidin or streptavidin and biotin (KD of 6 × 10−14 m and 4 × 10−14 m, respectively (14)) seems well-suited to associate targeting moieties with effector molecules. Unfortunately, both the tetravalent character of avidin or streptavidin and the practical challenge in controlling the number and location of biotin residues through chemical biotinylation have prevented the use of the strongest known non-covalent interaction for building well-characterized heteromeric complexes on a routine basis. Here, we introduce biotin moieties by an enzymatic reaction at specific locations in the protein structure and develop methodologies to assemble in a controlled manner multispecific and/or multivalent antibody complexes on a streptavidin scaffold suitable for testing in vivo targeting and delivery. These novel, stable nanostreptabodies, when injected intravenously, achieve rapid, highly tissue-specific targeting and tissue penetration of engineered antibody nanocomplexes. The methodology proposed in this report provides a versatile and simple way to achieve the controlled assembly of varied targeting antibody fragments with reporter and/or effector modules to create novel multifunctional nanocomplexes. Together with current efforts to engineer avidin and streptavidin (15), e.g. by controlling the number of biotin binding sites (16), and the added possibility of integrating avidin and streptavidin fusion molecules (17), this approach offers a nearly endless number of combinations that could be useful and suitable for a variety of applications.
EXPERIMENTAL PROCEDURES
Vector Construction-tSK Vector Series
The Fc-encoding DNA of the human IGHG1 locus was cloned from HMVEC cells (Lonza) and, after removal of the SfiI site located in the second intron by overlapping PCR, transferred into the miniantibody vector mSK1 (33) to replace the equivalent murine Fc part. The resulting vector had the same mammalian/bacterial hybrid leader peptide with a double SfiI cloning site (5′-GGCCCAGCCGGCCATGCTAGTGGCCCGGGAGGCC) followed by the IGHG1 hinge region, the CH2 domain, and the CH3 domain, together with introns. The construct was terminated by a SalI site and a His tag encoding the C-terminal sequence VDH6 in place of the CH3 terminal sequence PGK. The cassette was amplified by PCR with a primer adding a NotI site and BglII site after the stop codon and cloned after EcoRI-BglII restriction into the EcoRI-BamHI fragment of the PTT3 episomal vector generously provided by Dr. Y. Durocher (47) to give the tSK-Fc vector. For the heavy chain vector, the IGHV1.2 leader sequence (Fig. 1) was assembled from 4 overlapping primers with an EcoRI site on the 5′-side and an MfeI site followed by a NotI site on the 3′-side, and transferred into the PTT3 vector as above. The human CH1 domain was amplified from genomic DNA, assembled with the hinge-CH2-CH3 genomic fragment by overlapping PCR, and cloned between the MfeI and the NotI sites to give the tSK-HC vector. The light chain vector was similarly constructed. The IGKV3–20 leader sequence (Fig. 1) was assembled from six overlapping primers with an EcoRI site on the 5′-side and an XbaI site and a NotI site on the 3′-side. After transfer into the PTT3 vector, the CK domain was amplified from genomic DNA and cloned as an XbaI-NotI fragment to give the tSK-LC vector. Initial attempts using polyethylenimine transfection of 293-EBNA cells only yielded poor expression levels.
FIGURE 1.
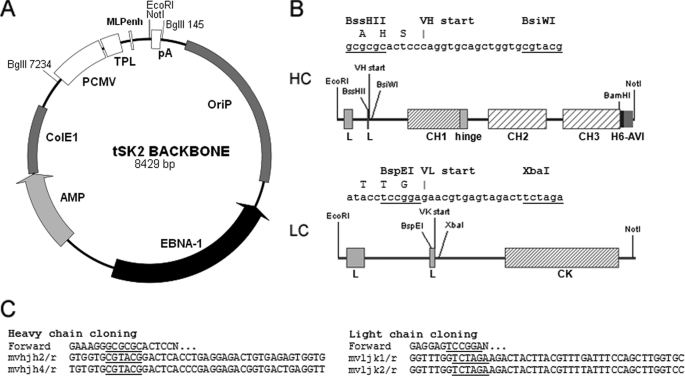
tSK2 antibody expression vector series. A, backbone of the tSK2 episomal vector. Expression cassettes are inserted between the EcoRI site and the NotI site. PCMV, CMV promoter; TPL, adenovirus 5 tripartite leader sequence; MLPenh, adenovirus major late promoter enhancer; pA, polyadenylation site. B, heavy chain and light chain expression cassettes. Sequence of the cloning sites and translation before the start of variable regions are indicated above each diagram. L, leader peptide containing its own intron; H6, hexahistidine-tag; AVI, AviTag sequence. C, primer design. In the forward primers, N … indicates the start of the variable region sequence; sequence of the reverse primers is given for chains terminating by murine JH2, JH4, JK1, or JK2.
tSK2 Vector Series
The PCEP4 vector (Invitrogen, Carlsbad, CA) was used as a backbone and successively cut, filled-in, and religated after digestion by XbaI, then BglII, then NruI and SalI, and finally by EcoRI. The BglII fragment from tSK-HC was transferred in its original orientation to give tSK2-HC. Similarly, the tSK2-LC vector was built after removal of the XbaI site by fill-in reaction and removal of the BspE1 site in the EBNA gene by site-directed mutagenesis. The AviTag was introduced in the heavy chain vector immediately after the His tag by ligating overlapping oligonucleotides between the SalI site and the NotI site (final sequence 5′-GTCGACGGAGGATCCCACCATCACCATCATCATGGCGGTCTGAACGACATCTTCGAGGCTCAGAAAATTGAATGGCACGAATAAGCGGCCGC) to give the tSK2-HCavi vector. Finally the tSK2-FHavi vector encoding the CH1 domain for Fab expression was derived by insertion of a BamHI site after the lysine codon in the hinge region and excision between the 2 BamHI sites.
tSK2-BF Vector
The Escherichia coli BirA gene was amplified by PCR from the pBirAcm plasmid (Avidity, Denver, CO) using the oligonucleotides BspE1BirAFor (5′-ACCTCCGGAGACGTCAAGGATAACACCGTG) and BirARev (5′- CTCACGCGTTTTTTCTGCACTACGCAGGGATATTTC). The IMAGE clone MHS1011 containing the human furin cDNA was purchased from Open Biosystems (Huntsville, Al). The sequence encoding the Golgi localization and transmembrane domains was amplified with primers FurBir3F (5′-CTGCGTAGTGCAGAAAAAACGCGTGAGGCGGGGCAACGGCTG) and FurRevNotHA (5′-GGGCGGCCGCTCAAGCATAATCTGGAACATCATATGGATAGAGGGCGCTCTGGTCTTTGATAAA), which added a hemagglutinin (HA) peptide tag. The final product was assembled by PCR and cloned into the tSK2-LC vector after digestion by BspEI and NotI to give the tSK2-BF vector.
Antibody Expression
Antibodies and Fab fragments were expressed transiently in human embryonic kidney 293F cells according to the manufacturer's recommendations (Invitrogen). Cells were maintained in suspension in 293 FreeStyle serum-free medium (Invitrogen) supplemented with penicillin and streptomycin. Standard transfections were performed in 20-ml culture aliquots at a density 1 × 106 cells/ml. DNA (20 μg) in OptiMEM (750 μl) was mixed with 293fectin (Invitrogen) (25 μl) in OptiMEM (750 μl); after 15 min of incubation at room temperature, the mixture was transferred to the culture, and cells were incubated over a 6-day period at 37 °C, in an 8% CO2, 100% humidity incubator with a constant agitation of 130 rpm on a rotating platform (IKA KS 260). For antibody production, heavy chain and light chain vectors were mixed at a 1:2 ratio; for biotinylation experiments, the tSK2-BF was also mixed (in a typical experiment, ratios were heavy chain vector 6 μg, light chain vector 12 μg, and tSK2-BF 2 μg), and free biotin (50 μm, Research Organic, OH) was added to the culture medium. Antibody concentrations were measured in the culture supernatants by sandwich ELISA.
Biotinylated mCherry Expression
mCherry was amplified by PCR using the primers 5′-CACTCCACCTGCCTCGGATCCACCTCCCTTGTACAGCTCGTCCATGCC and 5′-GAGTGCACCTGCGAGGCGCGCACTCCGTGAGCAAGGGCGAGGAGGATAAC and cloned into tSK2-HCdelta(BssH2/BamHI) after AarI digestion. Expression in the above conditions yielded biotinylated mCherry 36 mg/liter.
Protein Analysis and Purification
Recombinant proteins were purified on an AKTApurifier UPC 10 (GE Healthcare). For IMAC, HisTrap FF, 1-ml columns were loaded with cleared culture supernatants, washed with 10 ml of sodium phosphate buffer 20 mm, NaCl 0.5 m, pH 7.4 containing imidazole 20 mm, and eluted in the same buffer at an imidazole concentration of 200 mm over a 2-min gradient. SEC purifications and analyses were conducted on a Superdex 200 column in sodium phosphate buffer, 50 mm, NaCl 0.15 m, pH 7.0. SEC-HPLC analyses were conducted on a BioSep-SEC-S 3000 column (Phenomenex) mounted on an Agilent 1100 system (Agilent) in sodium phosphate buffer, 50 mm, pH 6.8. Protein concentrations were determined by UV spectrophotometry on a Nanodrop ND-1000 (Thermo Fisher Scientific) with extension coefficients estimated by the ProtParam tool available on the ExPASy website.
Biotin Quantification
2-(4-Hydoxyphenylazo)-benzoic acid (HABA) displacements were measured with an EZ Biotin Quantitation kit from Pierce; fluorescent detection was made with the FluoReporter Biotin Quantitation assay kit from Molecular Probes (Invitrogen). Dynabeads Biotin Binder beads from Dynal (Invitrogen) were used for the immunoprecipitation experiments. MALDI-TOF-MS analyses were performed on a Bruker AutoFlex II mass spectrometer. Briefly, samples were mixed in 1:1 ratio with an aqueous matrix solution containing sinapic acid 2% w/v in acetonitrile 50% v/v and trifluoroacetic acid 0.1% v/v. MS spectra were collected from random points per sample, where each spectrum was obtained with 200 laser shots per point. Operating conditions were as follows: ion source 20.00 kV, ion source 2D18.50 kV, lens voltage 8.00 kV, linear detector gain voltage 1400 V, optimized pulsed ion extraction time 350 ns, matrix suppression 3000 Da, and positive linear mode. Samples and controls were clustered around calibrant protein mix (Bruker) spots for enhanced mass accuracy.
Confocal Microscopy
Cells were allowed to attach for 30 min at 37 °C on poly-d-lysine-coated coverslips (MatTek Corporation, Ashland, MA) and then fixed with methanol at −20 °C for 10 min. The fixed cells were incubated with a Giantin antibody (Abcam, Cambridge, MA) followed by Alexa Fluor-conjugated reporter antibody (Invitrogen) and then fluorescein isothiocyanate-conjugated HA tag antibodies (Sigma-Aldrich Co.). Following immunostaining, cells were examined using a Nikon TE2000 microscope with a spinning disk confocal system (UltraView ERS5; Perkin Elmer, Waltham, MA) equipped with a CCD camera (ORCA-ER, Hamamatsu, Japan). A plan Apo 60× NA 1.4 oil immersion objective lens was controlled by a piezoelectric Z stepper. Z-sections through the cells were acquired every 0.5 μm, and exposures were kept under 300 ms. Images in the figures are composites of all Z-sections. Biotinylated mCherry-containing complex was visualized in rat lung live sections (250 μm) prepared as described in Ref. 48. Images of rat lung live sections (Fig. 6E) correspond to the projection of 5 consecutive confocal Z-sections at 2-μm Z-step. The supplemental movie was created with Volocity v.3 (Improvision) after three-dimensional resolution of a Z-stack composed of twenty images at the 2-μm step.
FIGURE 6.
Targeting of 833 nanostreptabodies. A and B, in vivo CT-SPECT imaging of rats injected with 30 μCi of 833cb Sab obtained by reacting 125I-SA with 833cb Fab at a 4:1 molar ratio 10 min before injection (top panel) or 125I-SA alone (bottom panel). C, targeting of the 833cb Sab prepared with an Alexa Fluor 488 SA conjugate in rat lung tissue grown in a dorsal skinfold chamber in nude mice following tail vein injection (30 μg). D, time-space analysis of signal intensity on a vessel cross-section illustrating the transendothelial transport of 833cb Sab (37). E and F, lung targeting of the monoSa/833cb/mCherry complex analyzed by confocal microscopy in a rat lung, 250 μm, live section 1 h following tail vein injection of 400 μg (E) of the complex or 500 μg (F) mCherry alone; a few nonspecific large dots are visible on both images (yellow arrows) (bar, 50 μm, Z-stack, 10 μm).
Radiolabeling and SPECT/CT Imaging
Streptavidin (New England Biolabs) was radiolabeled with 125-iodine (Perkin Elmer) using iodobeads according to the manufacturer protocol (Pierce). Sprague-Dawley rats (Harlan) females 150–175 g were injected in the tail vein with 30 μCi of either 125I-SA or 833cb Sab assembled from radiolabeled SA (at molar ratio 4:1). CT and SPECT scans were acquired 30 min after injection with an X-SPECT (Gamma Medica, Inc.) second generation MicroSPECT® imaging system. SPECT-images data were collected in a 360° orbit with 30 s sampling every 6° with high-resolution parallel hole colimators and pulse-height analyzer window set over the 30 keV photopeak of 125-iodine. Tomographic reconstructions were done using a standard filtered back projection. Three-dimensional data sets after CT-SPECT fusion were processed on the AMIRA System (TGS, Inc.).
Imaging via Intravital Microscopy
In vivo imaging experiments were done using intravital fluorescent video microscopy as previously described (37). Briefly, dorsal skinfold chambers were implanted with rat lung tissue in nude mice. 10–14 days later, when the lung tissue is fully vascularized, mice were injected in the tail vein with 30 μg of 833cb Sab (Alexa Fluor 488, Invitrogen). Fluorescence microscopy was performed using a Mikron Instrument Microscope (Mikron Instrument) equipped with epi-illuminator and video-triggered stroboscopic illumination from a xenon arc (X-400, PerkinElmer Optoelectronics) (37).
ELISA and Dot-Blots
GST-CAV1 fusion and GST alone were either dried on the plate or deposited on nitrocellulose. All incubations were done in TBS containing Tween 20 0.1% and nonfat milk 5% w/v; all antibodies were used at 2 μg/ml; bispecific antibodies were auto-assembled prior to dilution in TBS. Recombinant soluble APP (33) was conjugated to HRP using EZ-Link kit from Pierce and used at a 1:500 dilution.
RESULTS
Antibody Production by Transient Gene Expression
Transient gene expression offers a short development time and scales up easily, and therefore, was chosen for this project (18, 19). First, antibody cassettes were designed (20). Briefly, we made genomic constructs and chose leader peptides whose encoding sequences accommodate a restriction site in the second exon near the peptide cleavage site (Fig. 1B). For the heavy chain, we chose the leader sequence from the human locus IGHV1–2 and modified the second exon to introduce a BSSHII site (20), which together with a BsiWI site, created the cloning site for the VH element. For the light chain, we chose the leader sequence from the human locus IGKV3–20, which accounts for almost 30% of all human light chains (21). We introduced a BspE1 site in the leader sequence, which together with an XbaI site, created the cloning site for the VL element (Fig. 1B). The BspE1 site created a T to S mutation in the leader peptide that was apparently inconsequential. Both leaders and cloning sites were assembled with constant regions and cloned into the tSK2 episomal vector containing the EBNA1 gene (see Fig. 1A and “Experimental Procedures” for construction details); a polyhistidine tag and an AviTag were also added during construction on the C-terminal side of the CH3 domain of the heavy chain (see Fig. 1, B and C). Using a GFP derivative and 293fectin, we reached transfection levels above 90% with 293F cells adapted to suspension culture and serum-free conditions. Table 1 summarizes the results of antibody expression obtained by co-transfection of both vectors (tSK2-LC and tSK2-HC for light chain and heavy chain, respectively). Expression yields ranged from 13 to 91 mg/liter for full-length antibodies with batch volumes varying between 20 and 100 ml; expression was dependent on the antibody used with 833c giving the highest yields. We obtained better expression levels using a 2-fold excess of light chain vector as previously reported (22, 23). All purifications were done by immobilized metal affinity chromatography (IMAC), which produced well-purified recombinant antibodies (Fig. 2A). During the building of our vector series, we also constructed the tSK2-Fc vector for expression of miniantibodies and C-terminal Fc-fusion proteins (see expression of H6 and 17.31 scFv-Fc fusion in Table 1).
TABLE 1.
Summary of antibody expression levels.
| Name | Protein | Antigen | Yieldsa |
|---|---|---|---|
| 833cb | mAb | Rat APP | 65.5 ± 11.9 |
| 833cbb | mAb | Rat APP | 50.0 ± 4.2 |
| 833cb Fabb | Fab | Rat APP | 91 |
| P27cc | mAb | Caveolin 1 | 21.5 |
| P27cbb | mAb | Caveolin 1 | 34.9 ± 16.7 |
| P27cb Fabb | Fab | Caveolin 1 | 15.8 |
| P34cc | mAb | Caveolin 1 | 12.6 |
| P35cc | mAb | Unknown | 32 |
| P7cc | mAb | Rat PV-1 | 13 |
| H6 | mAb | Unknown | 18 ± 2.8 |
| E6 | scFv-Fc | Unknown | 6.7 |
| 17.31 | scFv-Fc | Unknown | 38 |
| ratAPP-D1.Fc | Fc-fusion | 4.8 |
a In mg/liter, S.D. indicated when replicate experiments are available.
b c indicates human/mouse chimeric antibody and b indicates biotinylation.
c See Ref. 33.
FIGURE 2.
Antibody biotinylation. A, SEC-HPLC elugram of IMAC purified full-length bisbiotinylated chimeric antibody 833cb. B, Western analysis by SA-HRP conjugate of 833cb versus polyclonal human IgG; only the heavy chain is biotinylated as seen in reducing (R) conditions (NR, non-reducing conditions). C, expression of BirA in 293 cells (in green, left panel) follows a perinuclear membranous network that extends deep in the cytoplasm and substantially colocalizes with the Golgi network (in red, all panels); most of antibody-overexpressing 293 cells have an enlarged Golgi network staining that colocalizes with expression of 833cb (in green, right panel).
Enzymatic Antibody Biotinylation
Post-translational biotinylation carried out by biotin protein ligases is highly sequence-specific and depends on the presence of key biotin acceptor sites (24). Recently, a minimal 15 amino acid substrate for the E. coli biotin ligase (BirA) has been found which exhibits reactivity similar to the natural biotin acceptor, biotin carboxyl carrier protein (BCCP) (25, 26). The short size of this tag (AviTag) is ideal to confine biotin moieties to precise locations in proteins with minimal foreign insertion. When fused to antibody fragments, this tag undergoes efficient biotinylation in a variety of systems, including bacteria, yeast, and mammalian cells (27–30). However, effective in vivo biotinylation of secreted antibody fragments may require localization of BirA to the secretory pathway in both yeast (29) and mammalian cells (30). Thus, we cloned the BirA enzyme as a fusion protein with an N-terminal kappa leader peptide and the C-terminal Golgi localization domain of furin terminated by an HA tag. As shown in Fig. 2, a biotinylation occurs that is exclusively located on the heavy chain when the resulting episomal vector tSK2-BF is cotransfected with the antibody vectors (tSK2-LC/tSK2-HC/tSK2BF ratio at 12/6/2 w/w/w) in cells supplemented with 50 μm exogenous biotin (31). Examination by confocal microscopy using an HA antibody 24 h post-transfection revealed a strong expression of BirA in the Golgi network sometimes extending into a perinuclear network (Fig. 2C). Many transfected cells presented an enlarged Golgi network that correlated with higher antibody expression levels, witnessing the intense work of the protein secretory machinery (Fig. 2C).
Attempts to directly measure the number of biotin residues using either HABA biotin displacement or a fluorometric assay (32) gave non-reproducible results. Immunoprecipitation using streptavidin-covered magnetic beads preincubated with or without free biotin followed by Western blot analysis showed a nearly complete biotin-dependent binding (data not shown), indicating that the average number of biotin residues per acceptor site was between 0.5 and 1.
We confirmed that the main protein species was indeed a bisbiotinylated antibody by MALDI-TOF analysis, which found a mass shift between the biotinylated antibody and the non-biotinylated control of 480 Da, quite near the expected mass of two biotin residues of 452 Da (2 × 244 Da-2H2O). This slight mass discrepancy between the observed and expected values is likely due to the presence of the sodium ion adduct (M+ H+ Na)+ of the protein. Moreover, the uncertainty in mass calibration at 150 kDa (size of biotinylated antibody) may add a slight mass discrepancy, but most likely the former is the case.
We also analyzed the influence of the dose of tSK2-BF plasmid in the transfection preparation to determine optimal conditions for expression and biotinylation (Fig. 3). Increasing amounts of tSK2-BF resulted in higher levels of BirA expression (whole extract, Fig. 3C). Above 0.1 μg of tSK2-BF per ml of culture (10% total DNA per transfection) the production of antibody decreased very rapidly (Fig. 3A) whereas below 0.02 μg/ml (2%) the degree of biotinylation declined (Fig. 3B), leaving a window between 2 and 10% to achieve both high degrees of biotinylation and high levels of antibody production.
FIGURE 3.
Influence of BirA concentration. The amount of BirA-expressing plasmid tSK2-BF was tested between 0 and 25% of the total plasmid content in the transfection mixture: A, concentration of 833cb antibody in the culture supernatant (ELISA), B, degree of antibody biotinylation by Western analysis using an SA-HRP conjugate (upper panel) and a human IgG1 reagent (bottom panel), and C, expression of BirA in 293 cells using an HA-antibody (upper panel) and an actin antibody (bottom panel).
Fab Expression and Building of Streptabodies
We removed the hinge and the Fc-encoding part from the heavy chain vector tSK2-HC. Coexpression with the light chain vector and tSK2-BF resulted in the secretion of large amounts of biotinylated Fab fragments at levels comparable to those of the parent antibodies (Table 1). Western blot analyses revealed the presence of a biotinylated band at 50 kDa under non-reducing conditions and half that size after reduction. There were also additional bands only recognized by anti-human kappa antibodies, which were not biotinylated and did not bind to nickel-containing resin. Purification by IMAC isolated a highly pure Fab-containing fraction (Fig. 4A). Measurement of the biotin content by immunoprecipitation found a degree of biotinylation of 95% for 833cb; 833c is a chimeric rat membrane-bound aminopeptidase P antibody (see Table 1). This result was confirmed by MALDI-TOF analysis, which showed a single peak at 50092.081 Da for the biotinylated 833cb Fab preparation and a mass difference with the non-biotinylated Fab preparation of 226 Da, precisely the mass of a single biotin residue minus the mass of a molecule of H2O.
FIGURE 4.
Fab expression and streptabody formation. A, SEC-HPLC elugram of 833cb streptabody (Sab, red trace) prepared by incubating SA with a 5x excess of monobiotinylated 833cb Fab; remaining unbound Fab comigrates with the Fab alone (blue trace). B, titration of SA by increasing amounts of 833cb Fab; Fab/SA indicates the Fab to SA molar ratio and I, II, and III complexes between SA and one, two, and three Fabs, respectively.
In the presence of escalating amounts of Fab, streptavidin/Fab complexes of higher molecular weights appeared at shorter elution times on the size-exclusion high-performance liquid chromatography (SEC-HPLC) elugram. The peaks corresponding to streptavidin SA in complex with one, two, or three Fabs were clearly separated; a fourth transition appeared above 3 molar excesses of Fab and was seen by a net increase in peak intensity (Fig. 4B). Above 4 excesses of Fab per SA molecule, a single final product was made and unreacted Fab started to accumulate (Fig. 4A and 4B). As expected (28), the 833cb streptabody (Sab) showed better binding to the antigen than the parent antibody by ELISA and appeared quite stable with storage over a week at 4 °C and multiple freeze-thaw cycles neither modifying binding nor the SEC-HPLC elution profile and thus, did not show evidence of aggregation or breakdown (data not shown).
Reactivity of Streptavidin with Full Antibodies
The reaction of P27cb, a rat caveolin 1 antibody (33), with SA at a one-to-one molar ratio (50 pmol P27cb in 100 μl of PBS, 10 min at room temperature) gave two main reaction products (Fig. 5A). Between 1 and 4 molar excesses of SA, the amount of product I slightly increased with a maximum around 2 excesses. At 0.5 molar excess, product I almost disappeared and product II became the major product of the reaction. 833cb antibody gave a similar reaction profile with both SA and avidin. Reactions at a 10-fold lower concentration did not favor the formation of one product versus the other for both antibodies. We also created a variant of 833cb antibody with an extra 15-amino acid long linker [GGGGS]3 inserted N-terminally to the His tag (833cb-link), which reacted similarly with SA.
FIGURE 5.
Reactivity of bisbiotinylated antibodies. A, SEC-HPLC elugram of the reaction mixture of P27cb and SA at a 1:1 molar ratio. B, SEC-HPLC elugram of the reaction mixture of 833cb-link and SA at 1:1 molar ratio; Alexa Fluor 488 biocytin was added at the end of the reaction and fluorescence measurement on a 100-μl fraction collection is shown superimposed in arbitrary units. C, SEC-HPLC elugram of the reaction mixture of P27cb compound I with (red) or without (blue) 833cb Fab at a 1:2 molar ratio. D, Coomassie staining after SDS-PAGE in non-reducing conditions of: P27cb (lane 1), purified P27cb compound I (lane 2), purified P27cb compound III (lane 3), 833cb Fab (lane 4), and SA (lane 5). E, ELISA with GST-CAV1 (gray) or GST alone (white) coating and rat APP-HRP conjugate with the following primaries: nothing, P27cb Fab, 833cb Fab, P27cb, 833cb, monoSA/P27cb, monoSA/833cb, monoSA/P27cb-833cb Fab, monoSA/833cb-P27cb Fab, respectively from 1 to 9. F, same as E by dot-blots.
To confirm the structure of these two products, we added Alexa Fluor 488 biocytin to the reaction mixture prior to chromatography and measured the fluorescence of the elution. As seen in Fig. 5B, product II was not labeled, indicating that it does not contain any available biotin binding sites. We prepared compound I from P27cb by incubating 500 μg of antibody in 66 ml of PBS in the presence of 1.5 molar equivalents of SA. We separated the reaction mixture on a Superdex 200 column and produced 218 μg of compound I (33% yield from initial antibody), pure at 95% by SEC-HPLC analysis. Similarly, we reacted 1 mg of 833cb-link antibody with 530 μg of SA (1.5 molar equivalent) in 33 ml of PBS and got 468 μg of compound I (47% yield from initial antibody), pure at 97%. Analysis of the reaction of each compound I with 1 molar equivalent of the respective parent antibody showed their transformation into compound II by SEC-HPLC. The association of two bisbiotinylated antibodies on one molecule of SA is the simplest complex that does not have any free biotin binding sites available. Removal of one full antibody from this structure gives a monoSA antibody complex, which is the most likely structure of compound I. Accordingly, SDS-PAGE analysis of purified compounds revealed a heteromeric complex made of SA and antibody (Fig. 5D, lane 2); a mass of 216 kDa was calculated from the elution time of compound I during SEC-HPLC, consistent with the proposed structure. We analyzed the stability of the monoSA/833cb complex I and did not detect any significant degradation or aggregation by SEC-HPLC analysis after 10 days in PBS at 4 °C.
Assembly of Bispecific Antibody Complexes
As expected, the reaction of monoSA/P27cb complex I with monobiotinylated 833cb Fab gave a bispecific complex III (Fig. 5C). Two equivalents of Fab were needed to complete the transformation. Western-blot analysis after purification by SEC-HPLC of complex III showed predictably the presence of the full antibody, the Fab, and SA (Fig. 5D). Reaction of monoSA/833cb complex I with P27cb Fab (>97% biotinylated) gave a similar bispecific complex (data not shown). To establish the bispecific character of both complexes III, we analyzed concomitant binding to caveolin I (as a fusion to GST (33)) and APP (as a conjugate between the ectodomain of rat APP (33) and horseradish peroxidase). Only the bispecific complexes gave a signal well above background by ELISA (Fig. 5E). Analysis by dot-blot produced an identical result (Fig. 5F). In these experiments, we mixed the monostreptavidin/bisbiotin-antibody complex I with the biotinylated Fab of different specificity in stoichiometric amounts (monoSA/mAb and Fab, 2 μg and 1 μg, respectively, in PBS) and verified, prior to dilution in the blocking buffer, that only the bispecific complex III was present in the reaction mixture. SEC-HPLC analysis of this complex showed no detectable degradation or aggregation over 5 days of storage at 4 °C.
Tissue-specific Targeting by Nanostreptabodies
We recently showed that the full 833 IgG binds to APP on the endothelial cell surface specifically within caveolae to be transported rapidly across the endothelial cells to the tissue interstitium in vivo (34–37). To assess whether the nanostreptabodies could also target in vivo, we used SPECT/CT imaging to visualize the targeting of 833cb Sab to the lung. In these experiments, the nanostreptabodies were auto-assembled by mixing the Fab′ and SA 10 min before injection. 833cb Sab prepared with 125I-labeled SA showed nearly complete accumulation in the lung just 30 min after injection in the rat tail vein (Fig. 6A) whereas the control 125I-labeled SA alone showed only some kidney uptake (Fig. 6B). Biodistribution analysis in multiple organs 1 h after the injection confirmed as elective and rapid targeting to the lung of 833cb Sab (75.8 ± 6.2%ID/g, n = 6) almost identical to the parent 833 antibody (34–37) but not targeting of the control P27cb Sab (0.8 ± 0.2%ID/g, n = 5) (see supplemental Table S1). Therefore, the targeting property of the parent antibody appears to be effectively transferred to the nanocomplexes, and the presence of SA does not appear to hinder appreciably the ability of the targeting moiety to reach the lung.
We further analyzed this targeting with an 833cb Sab prepared with an Alexa Fluor 488 SA conjugate by using intravital fluorescence videomicroscopy in a rat lung model implanted in dorsal window chambers in nude mice. This technique has proven instrumental in showing clear evidence of caveolae-mediated transendothelial transport for the 833 antibody (36, 37). Both the fluorescence images (Fig. 6C) taken over the first minutes after intravenous injection and the time-space quantitative analysis of signal intensity (Fig. 6D) showed a rapid radial penetration into the tissue surrounding blood vessels (Fig. 6D), attesting to a transendothelial transport of the nanostreptabody similar to the parent antibody (36, 37), despite the larger size, ∼250 kDa, of the complex. As expected, the control Alexa Fluor 488 SA conjugate did not show any detectable binding or transport into the lung tissue and produced a negative black image (data not shown). These results demonstrate clearly that 833 Fab′ moiety can target and penetrate a single organ in the body; thereby establishing this nanostreptabody targeting system effective in vivo.
The tetravalency of SA enables linkage between biotinylated targeting agents and biotinylated effectors and/or reporter imaging probes. As a model for targeted delivery as the scaffold linking the targetor and the reporter, we prepared monobiotinylated fluorescent mCherry protein (38) and incubated it in excess with the monoSA 833cb complex I. We isolated a fluorescent complex as a single peak of higher molecular weight by SEC chromatography with a 25% yield from the starting antibody material. Examination of live rat lung sections by confocal microscopy 1 h after tail vein injection of 400 μg of this fluorescent nanostreptabody confirmed the expected specific targeting of the lung tissue (Fig. 6E and supplemental Movie S1); none of the other organs tested (heart, kidney, liver, spleen) showed any significant mCherry signal (data not shown), consistent with the SPECT/CT imaging shown in Fig. 6A. To establish targeting specificity and to rule out nonspecific signal from lung vessel leakiness to the imaging agent, we also tested as a control the same biotinylated mCherry alone with a 500-μg dose and detected no signal in the lung tissue (Fig. 6F). These cumulative results showed that auto-assembled streptabodies could indeed achieve tissue-specific targeting and even rapid tissue penetration though a streptavidin scaffold linking targetor and reporter.
DISCUSSION
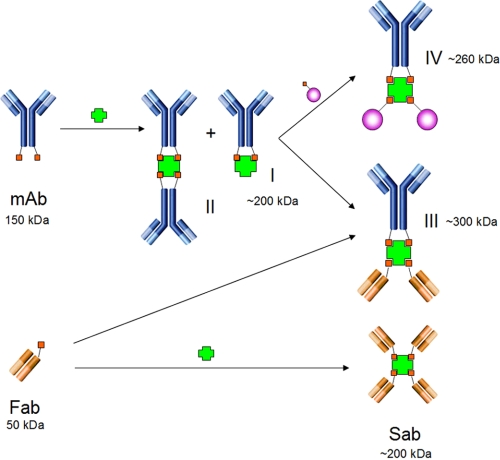
To exploit the potential of antibody targeting in vivo, we needed a rapid method to link targeting antibodies with varied payloads for imaging, diagnostic, or therapeutic benefits. An effective system must be able to rapidly create dependably stable products with defined stoichiometry and a must-have would be the ability to build on-demand complex composite nanostructures capable of including proteins made on different expression platforms, non-polypeptidic biological products such as DNA, and synthetic molecules to cover a large repertoire of targeting molecules for multiple purposes. In this report, we utilize the specific, high affinity interaction between biotin and SA to assemble complex nanostreptabodies and demonstrate for the first time actual in vivo tissue-specific targeting, molecular imaging, and delivery based on these defined heteromeric nanostructures. Although the interaction between biotin and SA is one of the strongest noncovalent association known (14), the classical reaction of chemical biotinylation produces somewhat arbitrary, nonspecifically localized linkages that lead to poorly characterized heterocomplexes. Accordingly, there has been only one report on an attempt to create bispecific antibodies, despite the wide availability of biotinylation reagents (39). We have anticipated that the association between SA and biotinylated proteins would follow precise assembly rules, generating structurally well-defined complexes that would be easy to purify if we were able to incorporate biotin residues at discrete locations on the protein scaffold and control the assembly of the varied biotinylated moieties. Using the biotin acceptor AviTag and a plasmid encoding a fusion between the bacterial biotin ligase BirA enzyme and the furin Golgi localization signal, we have indeed engineered such precise incorporation and are able to generate monobiotinylated and bisbiotinylated antibody fragments to build varied nanostreptabodies as summarized in Fig. 7.
FIGURE 7.
Schematic representation of streptavidin/antibody complex formation. Bisbiotinylated mAbs react with SA (or avidin) to give the monoSA/antibody complex I and the monoSA/bisantibody complex II; monobiotinylated Fabs react with SA to give streptabodies (Sab) or with monoSA/antibodies I to give to bispecific antibodies III. MonoSA/antibody complex I reacts with biotinylated mCherry to give the red tripartite complex IV.
One challenge is obviously to produce antibody fragments that are fully biotinylated. The presence of unreacted, non-biotinylated, or partially biotinylated products will necessitate additional steps of purification. For example, the reaction of an SA acceptor with a mixture of mono- and bis-biotinylated antibodies would undoubtedly generate multiple heterocomplexes, which would be very difficult to separate from each other. The analysis of our monobiotinylated Fab fragments with a single AviTag by both MALDI-TOF and immunoprecipitation shows saturation under our conditions at above 95% of the biotin acceptor sites. This high level of biotinylated is robust enough to allow the preparation of tetraFab nanostreptabodies by direct auto-assembly without downstream purification (Sab complex on Fig. 7). In the case of full-length antibodies, the complete immunoprecipitation by streptavidin beads does not tell us the exact degree of biotinylation but, nevertheless, MALDI-TOF analysis indicates that the biotin acceptor sites are near saturation. Thanks to this very high level of biotinylation, we are able to generate monoSA antibody complexes with 50% yield (complex I on Fig. 7). All our attempts to increase this yield, including the use of diluted mixtures to favor intramolecular reactions, were unsuccessful. We exclude the possibility of a too short linker that would restrict the reaction of the two biotins with a single SA since a variant with a linker 15 amino acids longer that the current 23 amino acids long linker (end of the CH3 domain, after the SLSL motif, to the biocytin residue) exhibits an identical pattern of reactivity. On the other hand, we cannot exclude at this stage that our linker is either too long, interacts with the CH3 domain, or adopts a conformation that would hinder the ability of the biotin residue to access the second biotin binding site, and thus favors the formation of intermolecular complexes.
Our laboratory has developed a series of antibody probes that specifically target the vascular endothelium in a single tissue and undergo rapid endothelial transcytosis in vivo via caveolae trafficking to actually penetrate deep into the tissue parenchyma (33–37, 40). These vascular and caveolar targeting antibodies may be useful in pharmacodelivery to specific diseased tissues. These antibodies, and others, can be biotinylated as described in this report to enable the straightforward assembly of chemically defined nanostreptabodies with varied payloads to be tested rapidly in vivo for targeting, imaging, and delivery to tissues. For proof of principle, we designed and assembled a nanocomplex containing two monobiotinylated cherry fluorescent protein and one biotinylated antibody specific for aminopeptidase P (complex IV on Fig. 7) and demonstrated exquisitely specific targeting to the lung tissue. We also demonstrate that this approach can be used to build well-defined bispecific antibodies that retain both antigen specificities (complex III on Fig. 7). Together, these methods provide a very flexible and efficient way to assemble antibody-based nanostructures that can incorporate polypeptidic and non- polypeptidic moieties. Biotin can easily be incorporated synthetically and chemically into many imaging and pharmacological agents, including putative chemotherapies, imaging reporters, virus, or nanoparticles. The ease provided in building such nanostructures makes our approach ideal for applications that require high throughput.
This work represents an initial step toward a well-controlled “tinkertoy-based” system that should permit the easy assembly of multiple combination of targeting agents with either imaging reporters for molecular and functional imaging studies in vivo or pharmacological effectors for testing therapeutic impact in animals and ultimately in patients. The idea of using avidin-biotin as a scaffold has clearly been around for many years, as exemplified by the large body of research trying to exploit this high affinity interaction. This includes of course the use of short biotin acceptor tags (25, 26), but until now controlled auto-assembly and in vivo targeting using an avidin scaffold had not been achieved to date. Perhaps the major drawback for the clinical applications of nanostreptabodies is their potential immunogenicity and the appearance of human anti-streptavidin antibodies (HASA), which occur in 60–80% of patients exposed to avidin or streptavidin (41). This limitation has not prevented the ongoing development of the clinical use of streptavidin, in particular for pretargeted radioimmunotherapy (PRIT), in which streptavidin antibody complexes are used to concentrate radioactive biotin derivatives at the tumor site (42, 43). Strategies such as deimmunization (44) or biochemical modifications such as addition of PEG molecules (45, 46) may provide ways to render streptavidin tolerogenic in the long run. In any case, nanostreptabodies provide well-defined nanostructures that could benefit existing clinical uses of the streptavidin/biotin interaction and make excellent preclinical tools allowing progress toward enhancing the combinatorial throughput necessary to analyze targeting and efficacy of multiple targetors and effectors in vivo.
Supplementary Material
Acknowledgments
We thank Dale Winger and Jamie Huberman for technical assistance with the preparation of the dorsal skinfold chambers. We thank Kerri Massey for critical assistance in reviewing the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01CA119378, PO1CA104898, and R24CA95893 from the NCI and Grants R01HL58216 and RO1HL074063 from the NHLBI.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Movie S1.
- scFv
- single chain Fv
- SA
- streptavidin
- HA
- hemagglutinin
- APP
- aminopeptidase P
- BCCP
- biotin carboxyl carrier protein
- ELISA
- enzyme-linked immunosorbent assay
- IMAC
- immobilized metal affinity chromatography
- mAb
- monoclonal antibody
- Sab
- streptabody
- SEC-HPLC
- size-exclusion chromatography by HPLC
- MALDI-TOF
- matrix-assisted laser desorption/ionization-time of flight
- GST
- glutathione S-transferase
- PBS
- phosphate-buffered saline
- HRP
- horseradish peroxidase.
REFERENCES
- 1.Coloma M. J., Morrison S. L. (1997) Nat. Biotechnol. 15, 159–163 [DOI] [PubMed] [Google Scholar]
- 2.Hust M., Jostock T., Menzel C., Voedisch B., Mohr A., Brenneis M., Kirsch M. I., Meier D., Dübel S. (2007) BMC Biotechnol. 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H. S., Shu L., De Pascalis R., Giuliano M., Zhu M., Padlan E. A., Hand P. H., Schlom J., Hong H. J., Kashmiri S. V. (1999) Mol. Immunol. 36, 61–71 [DOI] [PubMed] [Google Scholar]
- 4.Marvin J. S., Zhu Z. (2005) Acta. Pharmacol. Sin. 26, 649–658 [DOI] [PubMed] [Google Scholar]
- 5.Plückthun A., Pack P. (1997) Immunotechnology 3, 83–105 [DOI] [PubMed] [Google Scholar]
- 6.Wu C., Ying H., Grinnell C., Bryant S., Miller R., Clabbers A., Bose S., McCarthy D., Zhu R. R., Santora L., Davis-Taber R., Kunes Y., Fung E., Schwartz A., Sakorafas P., Gu J., Tarcsa E., Murtaza A., Ghayur T. (2007) Nat. Biotechnol. 25, 1290–1297 [DOI] [PubMed] [Google Scholar]
- 7.Gaidamakova E. K., Backer M. V., Backer J. M. (2001) J. Control Release 74, 341–347 [DOI] [PubMed] [Google Scholar]
- 8.Asai T., Wims L. A., Morrison S. L. (2005) J. Immunol. Methods 299, 63–76 [DOI] [PubMed] [Google Scholar]
- 9.Deyev S. M., Waibel R., Lebedenko E. N., Schubiger A. P., Plückthun A. (2003) Nat. Biotechnol. 21, 1486–1492 [DOI] [PubMed] [Google Scholar]
- 10.Chang C. H., Rossi E. A., Goldenberg D. M. (2007) Clin. Cancer Res. 13, 5586s–5591s [DOI] [PubMed] [Google Scholar]
- 11.Backer M. V., Patel V., Jehning B. T., Backer J. M. (2006) Bioconjug. Chem. 17, 912–919 [DOI] [PubMed] [Google Scholar]
- 12.Hodneland C. D., Lee Y. S., Min D. H., Mrksich M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 5048–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo F., Das S., Mueller B. M., Barbas C. F., 3rd, Lerner R. A., Sinha S. C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11009–11014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green N. M. (1990) Methods Enzymol. 184, 51–67 [DOI] [PubMed] [Google Scholar]
- 15.Laitinen O. H., Nordlund H. R., Hytönen V. P., Kulomaa M. S. (2007) Trends in biotechnology 25, 269–277 [DOI] [PubMed] [Google Scholar]
- 16.Howarth M., Chinnapen D. J., Gerrow K., Dorrestein P. C., Grandy M. R., Kelleher N. L., El-Husseini A., Ting A. Y. (2006) Nat. Methods 3, 267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laitinen O. H., Hytönen V. P., Nordlund H. R., Kulomaa M. S. (2006) Cell Mol. Life Sci. 63, 2992–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham P. L., Kamen A., Durocher Y. (2006) Mol. Biotechnol. 34, 225–237 [DOI] [PubMed] [Google Scholar]
- 19.Baldi L., Hacker D. L., Adam M., Wurm F. M. (2007) Biotechnol. Letts. 29, 677–684 [DOI] [PubMed] [Google Scholar]
- 20.Persic L., Roberts A., Wilton J., Cattaneo A., Bradbury A., Hoogenboom H. R. (1997) Gene 187, 9–18 [DOI] [PubMed] [Google Scholar]
- 21.Knappik A., Ge L., Honegger A., Pack P., Fischer M., Wellnhofer G., Hoess A., Wölle J., Plückthun A., Virnekäs B. (2000) J. Mol. Biol. 296, 57–86 [DOI] [PubMed] [Google Scholar]
- 22.Li J., Zhang C., Jostock T., Dübel S. (2007) Protein Eng. Des. Sel. 20, 491–496 [DOI] [PubMed] [Google Scholar]
- 23.Baldi L., Muller N., Picasso S., Jacquet R., Girard P., Thanh H. P., Derow E., Wurm F. M. (2005) Biotechnol. Prog. 21, 148–153 [DOI] [PubMed] [Google Scholar]
- 24.Cronan J. E., Jr. (1990) J. Biol. Chem. 265, 10327–10333 [PubMed] [Google Scholar]
- 25.Cull M. G., Miller J. F., Schatz P. J. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 1865–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckett D., Kovaleva E., Schatz P. J. (1999) Protein. Sci. 8, 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saviranta P., Haavisto T., Rappu P., Karp M., Lövgren T. (1998) Bioconjug Chem 9, 725–735 [DOI] [PubMed] [Google Scholar]
- 28.Cloutier S. M., Couty S., Terskikh A., Marguerat L., Crivelli V., Pugnières M., Mani J. C., Leisinger H. J., Mach J. P., Deperthes D. (2000) Mol. Immunol. 37, 1067–1077 [DOI] [PubMed] [Google Scholar]
- 29.Scholler N., Garvik B., Quarles T., Jiang S., Urban N. (2006) J. Immunol. Methods 317, 132–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barat B., Wu A. M. (2007) Biomol. Eng. 24, 283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulman J. D., Satake M., Harris J. E. (2007) Protein Expr. Purif. 52, 320–328 [DOI] [PubMed] [Google Scholar]
- 32.Batchelor R. H., Sarkez A., Cox W. G., Johnson I. (2007) BioTechniques 43, 503–507 [DOI] [PubMed] [Google Scholar]
- 33.Valadon P., Garnett J. D., Testa J. E., Bauerle M., Oh P., Schnitzer J. E. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntosh D. P., Tan X. Y., Oh P., Schnitzer J. E. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 1996–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carver L. A., Schnitzer J. E. (2003) Nat. Rev. Cancer 3, 571–581 [DOI] [PubMed] [Google Scholar]
- 36.Oh P., Li Y., Yu J., Durr E., Krasinska K. M., Carver L. A., Testa J. E., Schnitzer J. E. (2004) Nature 429, 629–635 [DOI] [PubMed] [Google Scholar]
- 37.Oh P., Borgström P., Witkiewicz H., Li Y., Borgström B. J., Chrastina A., Iwata K., Zinn K. R., Baldwin R., Testa J. E., Schnitzer J. E. (2007) Nat. Biotechnol. 25, 327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2004) Nat. Biotechnol. 22, 1567–1572 [DOI] [PubMed] [Google Scholar]
- 39.Pearce L. A., Oddie G. W., Coia G., Kortt A. A., Hudson P. J., Lilley G. G. (1997) Biochem. Mol. Biol. Int. 42, 1179–1188 [DOI] [PubMed] [Google Scholar]
- 40.Schnitzer J. E. (1998) N. Engl. J. Med. 339, 472–474 [DOI] [PubMed] [Google Scholar]
- 41.Chinol M., Grana C., Gennari R., Cremonesi M., Geraghty J. G., Paganelli G. (2000) in Radioimmunotherapy of Cancer (Abrams P. G., Fritzberg A. R. eds) Informa Healthcare [Google Scholar]
- 42.Goldenberg D. M., Sharkey R. M., Paganelli G., Barbet J., Chatal J. F. (2006) J. Clin. Oncol. 24, 823–834 [DOI] [PubMed] [Google Scholar]
- 43.Weiden P. L., Breitz H. B., Press O., Appelbaum J. W., Bryan J. K., Gaffigan S., Stone D., Axworthy D., Fisher D., Reno J. (2000) Cancer Biother. Radiopharm. 15, 15–29 [DOI] [PubMed] [Google Scholar]
- 44.Jones T. D., Crompton L. J., Carr F. J., Baker M. P. (2009) Methods Mol. Biol. 525, 405–423, xiv [DOI] [PubMed] [Google Scholar]
- 45.Chinol M., Casalini P., Maggiolo M., Canevari S., Omodeo E. S., Caliceti P., Veronese F. M., Cremonesi M., Chiolerio F., Nardone E., Siccardi A. G., Paganelli G. (1998) Br J. Cancer 78, 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall D., Pedley R. B., Boden J. A., Boden R., Melton R. G., Begent R. H. (1996) Br J. Cancer 73, 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durocher Y., Perret S., Kamen A. (2002) Nucleic Acids Res. 30, E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergner A., Sanderson M. J. (2002) J. Gen. Physiol. 119, 187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.