Abstract
Changes in the cerebrovascular system due to age or disease can significantly alter the blood-oxygenation-level-dependent (BOLD) signal and complicate its interpretation. The simultaneous acquisition of arterial spin labeling (ASL) and BOLD data represents a useful technique to more fully characterize the neurovascular underpinnings of functional brain response to cognition. We conducted a functional magnetic resonance imaging (FMRI) study of episodic memory encoding to investigate whether age is related to cerebral blood flow (CBF) and BOLD response in the medial temporal lobe (MTL). Results demonstrated a significant reduction in resting state CBF in older compared to young adults. Conversely, older adults showed significantly increased CBF but not BOLD response in the MTL during picture encoding relative to young adults. Correlations between CBF response and cognition were demonstrated whereas associations with BOLD were not observed. Stroke risk was associated with both CBF and BOLD response. Results suggest that aging effects on CBF and BOLD responses to encoding are dissociable and that cerebrovascular alterations contribute to findings of age-related differences.
Keywords: Aging, Episodic memory, Learning, Picture encoding, Functional MRI, fMRI, ASL, CBF, BOLD, Medial temporal lobe
1. Introduction
Functional neuroimaging has been used to study the neural substrates of age-related cognitive decline. Functional magnetic resonance imaging (FMRI) studies typically measure the blood-oxygen-level-dependent (BOLD) signal, an indirect measurement of neuronal activity that depends on several physiological variables (cerebral blood flow [CBF], cerebral blood volume [CBV], and cerebral oxygen consumption [CMRO2]). With advancing age, there are alterations in the cerebrovascular system that may affect neurovascular coupling (for review see D’Esposito et al., 2003), including altered cerebrovascular ultrastructure, reduced elasticity of vessels, increased atherosclerosis, reduced CBF in the resting state, decreased resting CMRO2, and reduced vascular reactivity to chemical modulators (Bentourkia et al., 2001; Claus et al., 1998; D’Esposito et al., 2003; Kawamura et al., 1993; Markus et al., 2001; Yamaguchi et al., 1986). Even ‘healthy’ older subjects may have undetected, clinically silent vascular pathology (DeCarli et al., 2005; D’Esposito et al., 2003). Thus, the interpretation of findings from FMRI studies is complicated by age-related differences in cerebrovascular dynamics that affect neurovascular coupling. Therefore, it is critical to assess for cerebrovascular function (e.g., CBF, stroke risk) in older adults and to consider this information when interpreting FMRI results.
Despite these considerations, results of studies examining age-related differences in resting state global and regional CBF have not been consistent. Some studies have yielded no significant association between advancing age and global (Waldemar et al., 1991) or regional CBF (Yamaguchi et al., 1986). In contrast, other have shown the coupling of age-related atrophy with decreases in perfusion (Kaszniak et al., 1979; Kety et al., 1956; Meyer et al., 1994), suggesting that atrophy results in reduced cerebral metabolism. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies have shown age-related reductions in both global CBF (Akiyama et al., 1997; Hagstadius and Risberg, 1989; Krausz et al., 1998; Matsuda et al., 2003) as well as regional CBF in the medial temporal lobe (MTL; Krausz et al., 1998), parahippocampus (Martin et al., 1991; Matsuda et al., 2003) and hippocampus (Larsson et al., 2001; Tanaka et al., 2000).
FMRI has also been used to measure brain changes with aging. For example, with regard to learning and memory processes, some studies have demonstrated reduced MTL activation as measured by PET or BOLD FMRI signal in older compared to young adults during encoding of faces (Grady et al., 1995) and complex scenes (Gutchess et al., 2005). Decreased activation in older adults in these studies typically has been proposed to reflect reduced efficiency in cognitive processing (Cabeza et al., 1997; Grady et al., 1995; Madden et al., 1996). This age-related reduction during encoding is often accompanied by increased recruitment of non-MTL regions, such as frontal regions, thought to reflect an age-related strategic mechanism in which older adults engage more regions in order to maintain performance (Daselaar et al., 2006; Gutchess et al., 2005).
In contrast, other groups have reported increased MTL activation with advancing age (Madden et al., 1999). Increased MTL activation in these studies has been proposed to reflect compensatory mechanisms or less efficient processing (Cabeza et al., 1997). Other studies have demonstrated no age-related differences in brain activity between older adults with normal memory performance and young adults during episodic encoding (Daselaar et al., 2003). However, the young adults showed significantly greater activation in the perirhinal/parahippocampal region relative to a group of older adults demonstrating reduced memory performance. It has been proposed that there is a correlation between the degree of MTL activation and the number of associations formed during encoding of stimuli (Henke et al., 1997) so decreased activation in the older adults demonstrating reduced memory performance may reflect the formation of fewer memory associations. Thus, to date, studies of normal aging have shown increases (e.g., Madden et al., 1999), decreases (e.g., Grady et al., 1995), and no age-related differences (e.g., Daselaar et al., 2003) in BOLD response to encoding in the MTL.
Although discrepancies across studies regarding increases versus decreases in the BOLD response may be related to such theoretical distinctions, differences across samples may also relate more simply to the coupling between the hemodynamic response and its underlying neurophysiology and neuroanatomy. Another issue is that differences in resting state may influence the magnitude of the BOLD response (Cohen et al., 2002). If the BOLD signal is not calibrated based on resting state, greater BOLD response may be falsely interpreted as a compensatory response when it may actually be a consequence of the resting state. The incorporation of techniques to measure resting state has the potential to directly address some of the current inconsistencies in the literature.
Most neuroimaging studies have relied on PET, SPECT, and BOLD FMRI technologies, although another MRI technique termed arterial spin labeling (ASL) is an attractive alternative. ASL is a noninvasive MRI procedure in which arterial water is magnetically labeled and used as an endogenous tracer to measure CBF (Detre et al., 1992; Williams et al., 1992). Unlike PET and SPECT techniques, ASL involves the use of an endogenous tracer rather than an intravenously administered contrast agent and relatively brief scan times (typically five to ten minutes). Due to T1 relaxation, the magnetization of the labeled blood water decays within seconds allowing ASL scans to be repeated in short succession (Johnson et al., 2005). In addition, ASL may have advantages over the BOLD signal. The BOLD signal depends on several physiological effects whereas ASL directly measures CBF (typically in physiological units of millimeters of blood per 100 grams of tissue per minute). ASL signal may be more closely related to neuronal activity and may better localize functional activity as it involves the arterial side of the vascular tree whereas BOLD is suggested to mainly involve the venous side (Lee et al., 2001). Functional changes in CBF have demonstrated less inter-subject variability and have been shown to be more robust to baseline vascular changes relative to BOLD (Aguirre et al., 2002; Brown et al., 2003; Stefanovic et al., 2006). Combining both techniques (e.g., FMRI using ASL/BOLD) would provide valuable complements to more fully characterize the neurovascular underpinnings of encoding but would also require the acquisition and joint analysis of quantitative measurement of functional CBF and BOLD responses. Preliminary studies demonstrate the feasibility of using combined ASL/BOLD to measure the functional perfusion and BOLD response to encoding (Restom et al., 2006; Restom et al., 2007).
We combined ASL and BOLD FMRI to examine age-related differences in MTL at rest and during episodic memory encoding. The use of both CBF and BOLD data may help disentangle the complex functions these techniques involve by eliminating some of the assumptions on which FMRI techniques rely (Hoge et al., 2005). We also examined the effects of age and cerebrovascular function (e.g., stroke risk) on CBF and cognition. With changes in aging and cerebrovascular function, we expected MTL hypoperfusion in older relative to young adults at rest. However, we expected a greater increase in CBF and BOLD response in older compared to young adults during novel picture encoding, which may reflect the operation of compensatory mechanisms (Bondi et al., 2005; Han et al., 2007; Wierenga et al., under editorial review). Similar increases in BOLD and CBF response would provide support for the integrity of neurovascular system. On the contrary, disparities between simultaneously acquired CBF and BOLD response to identical stimuli would suggest cerebrovascular alterations. Thus, combined ASL/BOLD imaging will address the confounds of using BOLD measures alone to characterize age changes in brain function and may shed light on heretofore inconsistencies across studies.
2. Methods
2.1 Participants
Fifteen healthy and independently living older adults and 15 young adults were recruited with institutional review board-approved procedures. Older participants were recruited from a larger pool of individuals participating in the UCSD Alzheimer’s Disease Research Center as well as from another longitudinal study of normal aging. Young adults were recruited from the local university community. Participants were selected without regard to race, ethnicity, or socioeconomic status. Older participants were non-demented based on extensive medical, neurological, and neuropsychological examinations. Individuals with a reported history of substance abuse, neurological illness, or psychiatric disorders were excluded from the study. All subjects with the exception of one older adult were right-handed.
2.2 Neuropsychological assessment
All participants were administered measures of learning and memory (California Verbal Learning Test-II; [Delis et al., 2000]) and executive function (Trail Making, Verbal Fluency, and Design Fluency subtests of the Delis-Kaplan Executive Function System; [Delis et al., 2001]). As part of their participation in the ongoing longitudinal studies, older adults were also administered measures of global cognition (Mattis Dementia Rating Scale; [Mattis, 1988]) and memory (WAIS-R Digit Span; [Wechsler, 1981]; WMS-R Logical Memory; [Wecshler, 1987]). Demographically-corrected standard scores from each of the test’s normative reference groups were used in all analyses.
2.3 Stroke risk assessment
Validated health risk appraisal functions were used for the basis of the evaluation of stroke risk (Framingham Stroke Risk Profile; FSRP; [D’Agostino et al., 1994]). The following stroke risk factors were included: age, systolic blood pressure, diabetes mellitus, cigarette smoking, prior cardiovascular disease, atrial fibrillation, left ventricular hypertrophy by electrocardiogram, and the use of antihypertensive medication. Using methods previously described by D’Agostino and colleagues (1994), a total stroke risk score summing the assigned number of points related to each of the individuals stroke risk factors was calculated for each older participant. Prior to scanning, systolic blood pressure was twice measured using a sphygmomanometer on the left and right arms of seated participants on the day of study participation. The mean of the two measurements was used in all analyses. Other stroke risk information was collected via participant interviews.
2.4 FMRI behavioral task
Functional scanning involved a picture-encoding task consisting of the presentation of novel and familiar landscape images (Stern et al., 1996). Prior to the functional scanning, participants viewed four landscape images (two with horizontal and two with vertical aspect ratio) for approximately 10 minutes and these images then served as the familiar images during scanning. During the functional scanning session, images were displayed in a blocked design with each block consisting of either 10 familiar (i.e., repeated) or 10 novel images. Each image was displayed for 2 seconds with a 0.5 second interval between images. Five blocks of novel and five blocks of familiar scenes were presented per run (250 seconds) in an alternating fashion with three runs per subject. The order of images within each block was pseudorandom. The stimuli were generated using the Psychophysics Toolbox for Matlab (Mathworks, Natick, MA, USA). Stimuli were presented via an LCD projector that was back-projected onto a screen at the participant’s feet. To ensure that participants maintained adequate attention, they were instructed to determine whether each image had a horizontal or vertical aspect ratio and make an appropriate response using a two-button response box. Responses were monitored by the scanner operators to ensure compliance and accuracy.
2.5 Imaging acquisition and analyses
2.5.1 Structural and functional imaging acquisition
Participants were scanned on a 3.0 Tesla General Electric Medical Systems EXCITE whole body imager with an 8-channel receive-only head coil (General Electric Medical Systems, Milwaukee, WI, USA). Functional BOLD and ASL data were simultaneously acquired using a quantitative pulsed ASL sequence (PICORE QUIPSS II; [Wong et al., 1998]) with a dual-echo single-shot spiral acquisition (TE1 = 2.4 and TE2 = 24 ms). Five contiguous oblique slices, each 6 mm thick, were acquired at the level of the hippocampus. A tagging slab of 200 mm thickness was placed 10 mm below the most inferior slice. Tag images were interleaved with control images. Other parameters include TI1 = 700 ms, TI2 = 1400 ms, matrix size 64×64, FOV 240 mm, flip angle = 90 degrees, TR = 3000 ms, and 84 repetitions. The total scan time for each ASL run was approximately 4 minutes and 20 seconds. One resting-state and three functional scans were acquired. In addition, a cerebral spinal fluid (CSF) reference scan was acquired for use in CBF quantification. The CSF scan consisted of a single-echo, single repetition scan acquired at full relaxation and echo time equal to 2.4 ms. This scan used the same in-plane parameters as the resting ASL scan, but the number of slices was increased to cover the lateral ventricles. A high-resolution structural scan was acquired with a fast spoiled gradient echo pulse sequence (172 contiguous sagittal slices; 1 mm thickness; FOV = 25 cm; TR = 8 ms; TE = 3.1 ms; flip angle = 12 degrees; T1 = 450; 256 × 192 matrix; Bandwidth = 31.25 kHZ; frequency direction = S-I; NEX =1; scan time = approximately 7 minutes 30 seconds). During all 4 ASL runs, cardiac oximetry and respiratory effort signals were recorded using a pulse oximeter (INVIVO Magnitude 3150M patient monitor, Orlando, FL, USA) and a respiratory effort transducer (TSD201, BioPac Systems, Inc., Goleta, CA, USA) in order to reduce physiological noise and improve the signal-to-noise ratios in our ASL data (Restom et al., 2006). Contrasts between the familiar and novel conditions were performed.
2.5.2 Structural MRI processing
2.5.2.1 Tissue segmentation
Whole brain images were skull-stripped and segmented into gray matter, white matter, and cerebrospinal fluid (CSF) compartments. Following N3 bias correction of field inhomogeneities (Sled et al., 1998), each scan was processed with Brain Surface Extractor (Version 3.3; [Shattuck et al., 2001]), an automated method to most fully remove all non-brain material. This approach has been shown to be very effective when working with the images of older adults (Fennema-Notestine et al., 2006). Scans were also manually edited when necessary to remove any residual non-brain material. Anatomical images were down-sampled to the resolution of the ASL image using Analysis of Functional NeuroImages (AFNI) software. Tissue segmentation was performed using FSL’s FAST software (FMRIB’s Automated Segmentation Tool; [Zhang et al., 2001]). Whole brain volume was also derived and used in normalizing hippocampal, total gray matter, white matter, and CSF volumes (Bigler and Tate, 2001). Specifically, volumes were normalized by dividing the structure volumes by the whole brain volume and multiplying by 100 to correct for intersubject differences in overall brain size.
2.5.2.2 Automated medial temporal lobe masks created in standard Talaiarch space
For the functional neuroimaging data analysis, regions of interest (ROIs) were delineated consisting of the bilateral hippocampus and parahippocampal gyrus. An automatic subcortical segmentation program (Freesurfer ASEG; Fischl et al., 2002) was applied to the anatomical image to delineate an ROI containing the hippocampus. A region growing algorithm was next used to derive an ROI containing the parahippocampal gyrus. Both ROIs were then visually inspected and manually edited in the coronal plane to increase precision. The hippocampal and parahippocampal ROIs were combined to form one MTL ROI to be used in the CBF and BOLD signal data analysis.
2.5.2.3 Manually outlined hippocampal and parahippocampal masks created in native space
To improve precision for volumetric analyses through native space renderings, hippocampal and parahippocampal volumes were also manually outlined in the coronal plane. Volumes were delineated using AFNI software and completed by an experienced operator (KJB), who was blind to participant identity and group. High levels of inter-rater reliability for the procedure were established on a separate set of images not among those studied presently (intraclass correlation coefficients [ICC] > .90). Hippocampal volumes were delineated using a stereotactic approach adapted from methods published previously (Han et al., 2007; Jak et al., 2007; Nagel et al., 2004). The anterior boundary of the hippocampus was chosen as the coronal slice through the fullest portion of the mammillary bodies. At this level, the posterior boundary was traced on the last coronal slice on which the superior colliculi could be fully visualized. The temporal horn and alveus demarcated the dorsal and lateral boundaries. Ventrally, the hippocampus was bound by the parahippocampal white matter. Medially, the ambient cistern defined the hippocampal boundary.
Parahippocampal gyrus volumes were similarly estimated using a stereotactic approach adapted from methods published previously (Raz et al., 1997). The anterior and posterior bounds of the parahippocampal gyrus were identical to those used to delineate the hippocampus. Dorsally, a horizontal line drawn from the most medial portion of the parahippocampal gyrus white matter to the most lateral point of the collateral sulcus demarcated the parahippocamal gyrus. Ventrally and laterally, the boundary was a line drawn from the most inferior part of the collateral sulcus to the white matter lateral to the collateral sulcus. Medially, the parahippocampal gyrus was bounded by CSF.
2.5.3 Functional MRI processing
Using AFNI software, a three-dimensional (3D) brick (or brik) was created from the structural scan slices. A 3D brick of each image file was generated for each TR of the time course of the PICORE QUIPSS II scanning sequence. Motion in the time series data was corrected by using the AFNI 3D volume registration program to register each acquisition to a selected reference volume with an iterated least squares algorithm to estimate three rotational and three displacement parameters for each participant. The anatomical volume was aligned with the functional volume with a MATLAB program that uses the scanner coordinates of each volume. Functional CBF images were generated from the running subtraction of the control and tag images (Liu and Wong, 2005). Functional BOLD images were computed from the running average (average of each image with the mean of its two nearest neighbors) of the second echo (Liu and Wong, 2005). For each subject, a mean ASL image was formed from the average difference of the control and tag images from the resting state scan data. This mean ASL image was then converted to absolute units of cerebral blood flow (mL/100 g tissue/minute) with use of the CSF image (Chalela et al., 2000).
For the functional scans, ASL and BOLD runs were concatenated to form one time series per voxel for each type of scan and analyzed with a general linear model (GLM) framework as described in Restom and colleagues (Restom et al., 2006). Pre-whitening with the assumption of an autoregressive AR(1) model (Burock & Dale, 2000; Woolrich et al., 2001) was performed to account for temporal autocorrelations. Different physiological noise and low frequency nuisance term regressors were used for each run (Restom et al., 2006). An overall threshold of p < 0.05 (with correction for multiple comparisons) was used to define voxels with significant activation. The AFNI program AlphaSim (Cox, 1996) was used to account for multiple comparisons.
2.5.4 Partial volume correction
To correct the CBF measures for partial volume effects, we used the method previously reported by Johnson and colleagues (Johnson et al., 2005). These calculations assume that CSF has zero CBF and that CBF in gray matter is 2.5 times greater than that to white matter. The following formula was used to compute partial volume corrected CBF signal intensities: CBFcorr = CBFuncorr/(GM + 0.4 * WM). CBFcorr and CBFuncorr are corrected and uncorrected CBF values, respectively. GM and WM are gray matter and white matter partial volume fractions, respectively. Information from the high resolution structural image and the FSL Automated Segmentation Tool (FAST) was used to determine the tissue content of each perfusion voxel (Smith et al., 2004).
2.6 Statistical analyses
Independent samples t-tests compared the means of the young and older adults on demographic, cognitive, and structural and functional neuroimaging variables. All group mean comparisons were inspected for homogeneity of variance assumptions via Levene’s test. When Levene’s test was significant (i.e., group variances were significantly different), the p-value associated with unequal variances was reported. Hierarchical multiple regression was used to determine the relationship of age to percent change in CBF and BOLD response after accounting for MTL volume and resting-state CBF, respectively. Pearson’s product-moment correlations examined the association among age, cognitive performance, stroke risk, and FMRI (CBF and BOLD) response during picture encoding in the entire sample. Additional correlations limited to the older adult group were also computed. All analyses were run in SPSS (version 14.0).
3. Results
3.1 Demographic variables
The mean age was 77.00 years (S.D. = 7.32) for the older adult group and 25.57 (S.D. = 3.19) for the young participants. The mean years of education was 16.53 years (S.D. = 2.20) for the older adult group and 17.07 (S.D. = 1.79) for the younger group. The two age groups did not significantly differ on education (t = .73, p = .47) or on gender distribution (χ2 = .00, p > .99). Both the older adult and young participant groups consisted of 8 women and 7 men.
3.2 Neuropsychological performance
The two age groups were compared on a number of neuropsychological measures of memory and executive functions. There were no significant differences in learning and memory performance between young and older adults as measured by the CVLT-II (see Table 1), with the exception of one qualitative measure: greater repetitions (t = 2.77, p = .01) by the older adults. This group difference on CVLT-II performance was statistically significant when comparing standard scores but not when comparing raw scores. The mean CVLT-II summary learning measure for both groups was in the above average range (T-score > 55) and long-delay free recall was in the average range (z-score ≥ 0.4), suggesting that neither group had difficulties in learning or memory abilities. Regarding the executive function measures, there were no significant differences in performance between the two groups on a number of the D-KEFS subtests with the exception of one score. Older adults demonstrated poorer performance on switching on the D-KEFS Design Fluency subtest relative to young adults (t = 2.15, p = .04; see Table 1). Again, both groups performed well within the normal range (mean scaled scores ≥10).
Table 1.
Demographic variables, cognitive characteristics, and brain volume by age group
| Variable | Young Adults (n=15) mean (SD) | Older Adults (n=15) mean (SD) | t | P | Cohen’s d |
|---|---|---|---|---|---|
| Age | 25.57 (3.19) | 77.00 (7.32) | . | . | . |
| Years of Education | 17.07 (1.79) | 16.53 (2.20) | .73 | .47 | .28 |
| Gender (women/men) | 8/7 | 8/7 | χ2 =.00 | .64 | . |
| CVLT-II List 1–5 Total Recall Standard Score | 60.54 (8.00) | 59.38 (12.32) | .28 | .78 | .12 |
| CVLT-II Short Delay Free Recall Standard Score | .77 (.95) | .58 (1.16) | .44 | .67 | .19 |
| CVLT-II Long Delay Free Recall Standard Score | .50 (.68) | .46 (1.20) | .11 | .92 | .04 |
| CVLT-II Repetitions Standard Score | −.42 (.67) | .46 (.86) | 2.77 | .01 | 1.18 |
| CVLT-II Intrusions Standard Score | .00 (.80) | −.42 (1.10) | 1.06 | .30 | .45 |
| D-KEFS Trail Making Motor Sequencing Scaled Score | 13.54 (2.70) | 12.82 (1.08) | .83 | .42 | .36 |
| D-KEFS Letter Fluency Scaled Score | 13.62 (2.22) | 13.36 (3.88) | .19 | .85 | .09 |
| D-KEFS Category Fluency Scaled Score | 13.31 (3.33) | 13.27 (2.53) | .03 | .98 | .01 |
| D-KEFS Category Switching Correct Responses Scaled Score | 12.92 (2.25) | 11.36 (3.61) | 1.29 | .21 | .54 |
| D-KEFS Design Fluency Percent Accuracy Scaled Score | 10.00 (1.71) | 10.80 (1.40) | 1.19 | .25 | .53 |
| D-KEFS Design Fluency Switching Scaled Score | 15.00 (3.14) | 12.64 (2.01) | 2.15 | .04 | .93 |
| Total Brain Volume (mm3) | 1377804.33 (112556.79) | 1302708.60 (95923.72) | 1.97 | .06 | .74 |
| Normalized Total Gray Matter Volume | .53 (.01) | .43 (.02) | 11.54 | <.001 | 6.55 |
| Normalized MTL Volume | .60 (.08) | .56 (.09) | 1.71 | .25 | .49 |
CVLT-II – California Verbal Learning Test-second edition
D-KEFS – The Delis-Kaplan Executive Function System
3.3 Resting state CBF
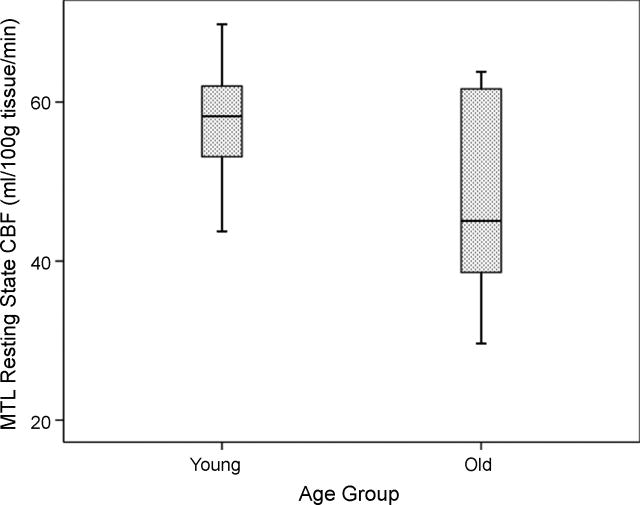
During rest, older adults demonstrated significantly reduced MTL perfusion relative to their young counterparts (t = 2.50, p = .02; see Table 2 and Figure 1). When each hemisphere was analyzed separately, older adults displayed decreased resting CBF in both right (t = 2.07, p = .05) and left MTL (t = 2.58, p = .02).
Table 2.
Cerebral blood flow (CBF) at rest and percent change in CBF and blood oxygenation level dependent (BOLD) response to novel picture encoding by age
| Variable | Young Adults mean (SD) | Older Adults mean (SD) | t | p | Cohen’s d |
|---|---|---|---|---|---|
| Baseline CBF (ml/100g/min) | 57.09 (7.06) | 47.94 (12.30) | 2.50 | .02 | .94 |
| Percent Change CBF | 42.90% (18.53) | 100.12% (55.69) | 3.72 | .002 | 1.43 |
| Percent Change BOLD | 0.45% (0.13) | 0.56% (0.20) | 1.81 | .08 | .68 |
Figure 1.
Boxplot of medial temporal lobe (MTL) resting state cerebral blood flow (CBF) by age group.
3.4 Functional CBF and BOLD response during picture encoding
3.4.1 Age group comparisons
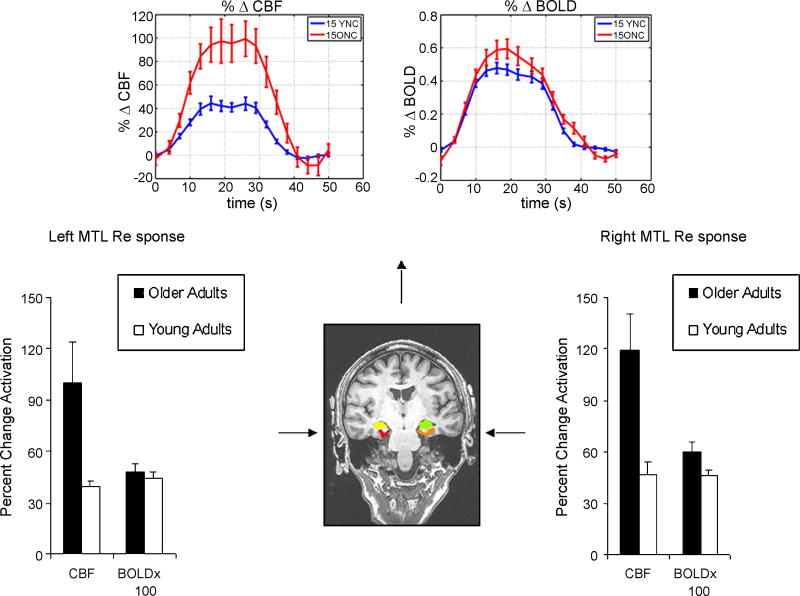
During novel picture encoding, older adults displayed a significantly larger percent change in CBF response compared to the young adults (t = 3.72, p = .002; see Table 2 and Figure 2), although there was no significant between-group difference in percent change BOLD signal during novel picture encoding (t = 1.81 p = .08). When each hemisphere was analyzed separately, older adults demonstrated a significantly larger percent change in right (t = 3.29, p = .004) and left hemisphere CBF response (t = 2.46, p = .02). Also, as expected there were no differences in right or left hemisphere BOLD response (both p-values > .05).
Figure 2.
Sample of the manually drawn hippocampal and parahippocampal gyrus regions of interest (displayed in radiological orientation) and the young and older adult group mean percent change CBF and BOLD response in these regions during picture encoding by hemisphere. Please note that the BOLD signal data in the bar graphs have been multiplied by 100. Also displayed is the average hemodynamic curve of the CBF and BOLD response for young and older adults (shown in blue and red, respectively) during picture encoding.
3.4.2 Regression models
Results of hierarchical multiple regression analysis demonstrated that advancing age (β=.58, ΔR2 = .25, p = .003) significantly predicted a larger percent change in the functional CBF response to encoding above and beyond that accounted for by MTL volume (β= −.22, ΔR2 = .05, p = .24) and resting-state CBF (β= −.33, ΔR2 = .11, p = .08), and the resulting overall regression model was statistically significant (Overall Model R2 = .41, F = 5.90, p = .003). However, the model was not significant for predicting percent change in functional BOLD response to encoding (Overall Model R2 = .19, F = 2.05, p = .13; MTL volume [β= −.20, ΔR2 = .04, p = .28]; resting-state CBF [β= −.07, ΔR2 < .001, p = .71]; age [β=.44, ΔR2 = .15, p = .04]).
3.5 Structural volumetry
The older and young adults did not differ on normalized volumes of the right hippocampus (t = 0.29, p = .78), left hippocampus (t = 0.77, p = .45), right parahippocampal gyrus (t = 1.92, p = .07), left parahippocampal gyrus (t = 1.40, p = .17), or whole brain white matter (t = 0.71, p = .49). However, normalized whole brain gray matter volume was significantly smaller (t = 11.54, p < .001) and normalized whole brain CSF volume (t = 11.99, p < .001) was significantly larger in older relative to young participants.
3.6 Correlational analyses
3.6.1 Older and young participants
Bivariate correlations were performed to examine the associations between age, cognition, and CBF and BOLD response for all participants. Across all participants, increasing age was significantly associated with decreased bilateral (r = −.42, p = .02), right (r = −.38, p = .04), and left (r = −.40, p = .03) MTL resting state CBF. Age was also significantly associated with percent change in bilateral (r = .63, p < .001), right (r = .59, p = .001), and left (r = .47, p = .009) CBF response to encoding. Memory abilities as measured by the CVLT-II Trials 1–5 Total Recall T-score were significantly associated with left MTL percent change in CBF response (r = .48, p = .01) during novel picture encoding. Percent change in BOLD response to encoding was significantly associated with age (r = .42, p = .02) but not with CBF measures (all p-values > .05) or any measures of cognition (all p-values > .05).
3.6.2 Older adults
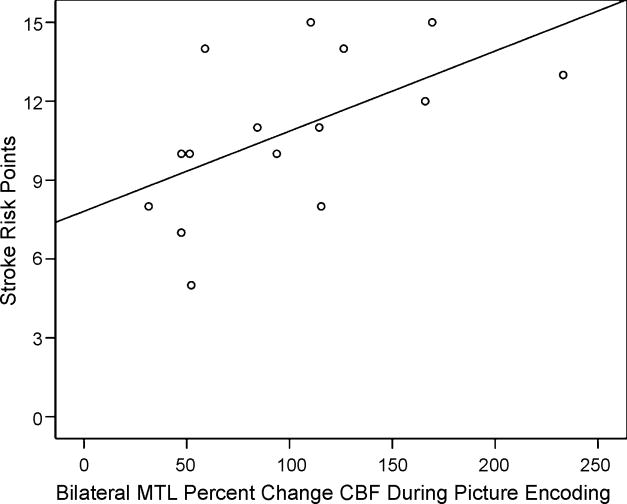
In the older participant group, stroke risk was positively correlated with bilateral percent change in CBF response (r = .57, p = .03; see Figure 3) and BOLD response (r = .63, p = .01), both of which appeared to be driven more by significant right-sided MTL associations (right CBF response: r = .65, p = .009; right BOLD response: r = .59, p = .02) than left-sided MTL associations (left CBF response: r = .28, p = .31; left BOLD response: r = .27, p = .33). Also, consistent with several previous reports, individuals with higher blood pressure demonstrated poorer performance on cognitive measures (Kilander et al., 1998; Knopman et al., 2001; Swan et al., 1998b; Tzourio et al., 1999). Specifically, there was a significant association between systolic blood pressure and memory (CVLT-II recognition memory accuracy: r = −.56, p = .05) measures. Finally, corroborating previous findings (Seshadri et al., 2004), there was an inverse association between stroke risk and brain volume: Normalized whole brain gray matter volume was inversely associated with stroke risk (r = −.65, p = .04).
Figure 3.
Scatterplot of the correlation between stroke risk and bilateral medical temporal lobe (MTL) percent change cerebral blood flow (CBF) during picture encoding for the older adult participants.
4. Discussion
Results of the present study revealed a number of important considerations for FMRI studies of aging. First, a significant reduction in resting state perfusion in older adults relative to their younger counterparts was observed. This finding is consistent with several ASL, PET, and SPECT studies demonstrating age-related reductions in resting state CBF and cerebral metabolic rate of glucose (e.g., Hagstadius & Risberg, 1989; Parkes et al., 2004; Petit-Taboue et al., 1998), although rates of perfusion decreases have varied. One particularly relevant study of 34 healthy adults (aged 20–67 years) using continuous arterial spin labeling (CASL) reported gray-matter perfusion reduction of 0.45% each year (Parkes et al. 2004). The current study corroborates this and other previous findings of age-related differences in CBF and demonstrates consistency of the ASL method with the broader literature for the quantitative study of perfusion in aging.
Past findings regarding the impact of baseline CBF on BOLD response during functional tasks have been somewhat more equivocal (Stefanovic et al., 2006). For instance, increased baseline CBF produced by carbon dioxide (CO2) inhalation, acetazolamide administration, hyperventilation, or caffeine use has been associated with both decreased (Bandettini and Wong, 1997; Brown et al., 2003; Cohen et al., 2002) and increased BOLD response (Cohen et al., 2002; Mulderink et al., 2002). In addition, vasoconstrictive indomethacin administration has been accompanied by a decreased BOLD response (Bruhn et al., 2001). Despite these inconsistencies in the literature, studies demonstrate that baseline physiology impacts the magnitude of BOLD activation. Age-related baseline CBF and cerebrovascular alterations have been reported and the present findings of baseline hemodynamic differences across age may contribute, in part, to findings of age-related differences in BOLD contrast analyses. Therefore, resting state CBF is an important consideration when interpreting functional neuroimaging results of the BOLD response.
In addition, older adults demonstrated a significantly larger percent change in CBF response while viewing novel pictures as compared to the young participants, although a concomitant increase in the BOLD response was not observed. These differences in the CBF response were seen in the absence of discrepant performance on primary measures of learning and memory. This finding suggests that perfusion imaging has the potential to detect age-related alterations in MTL response to learning that go undetected when examined with the BOLD signal. Our findings further suggest that CBF and BOLD data yield different information and, therefore, the combination of both techniques may better elucidate brain activation patterns than either technique in isolation. However, it should be noted that greater percent change CBF during novel relative to familiar image presentation could reflect a lower level of absolute CBF response during the familiar image condition. As our recent work (Restom et al., 2007) suggests, the greater percent change CBF response during novel picture encoding in the older participants may reflect a lower level of CBF response during the familiar image condition, which would be consistent with the observed age-related reduction in resting-state CBF.
Regarding cognitive performance, across both age groups, better performance on word-list learning was associated with a larger percent change in left MTL CBF response. These associations between CBF response and episodic memory suggest that participants with better performance on certain memory measures may have been invoking compensatory upsurges in CBF response to support successful memory operations. In contrast, percent change in BOLD signal during novel picture encoding was not significantly associated with any measures of cognition. Thus, in the present study it appears that CBF rather than BOLD response was better related to memory performance in ostensibly healthy and nondemented older adults.
In the older participant group, consistent with several previous reports, we also found higher blood pressure to be associated with poorer performance on cognitive measures (e.g., Elias et al., 1993; Kilander et al., 1998; Knopman et al., 2001; Swan et al., 1998b; Tzourio et al., 1999) and smaller brain volumes (e.g., Akiyama et al., 1997; Swan et al., 1998a). Systolic blood pressure but not stroke risk showed a significant association with cognition. One distinction to note is that the FSRP is comprised of a combination of current and cumulative risk factors, whereas systolic blood pressure is a current index of the cerebrovascular system taken on the day of scanning and, as such, may represent a better index of cerebrovascular integrity than those factors assessing lifetime risk. As Knopman and colleagues (2001) stated, the associations among individual risk factors is very complex. It is possible that the effects of hypertension vary from other risk factors (e.g., diabetes) included in our stroke risk assessment in terms of the locus or mechanisms of brain injury.
In recent years, interest has increased in examining the relative contributions of vascular risk factors (e.g. hypertension, diabetes, coronary artery disease) to the pathogenesis or exacerbation of age-related degenerative diseases like Alzheimer’s disease (AD), and recent studies have shown white matter (WM) lesions to be important predictors of subsequent cognitive decline in mild AD (Honig et al., 2004). Adak et al. (2004) also reported that greater abnormal WM volumes were associated with shorter times to convert either from normal to ‘questionable dementia’ or from ‘questionable’ to mild dementia. Knopman et al. (2005) demonstrated a link between diabetes and cerebral atrophy in their sample of normally-aging older adults and suggested that reduced cerebral perfusion and subtle microinfarction may be linked to the observed atrophy and concomitant cognitive decline. Even older adults in relatively good health experience ischemic brain changes before the development of frank, clinically diagnosed cerebrovascular disease (McPherson and Cummings, 1997; Raz et al., 1998). Although speculative, some of the determinants of abnormal WM likely include those captured by the stroke risk assessment used in the present study. Of course, such risk factors may also interact with one another or with still other risk factors such as advancing age. Thus, interest in the determinants of WM lesions in aging and their impact on cognition and risk for conversion to diseases such as AD has risen, especially because many of these risk factors may prove to be modifiable by medications, diet, or other lifestyle variables.
For functional neuroimaging studies with older adults, if stroke risk is not assessed, cognitive decline may be attributed to aging when it may be at least partially due to cerebrovascular alterations. In the present study, all older adults were healthy and thus there was little variability in our stroke risk profiles (i.e., the stroke risk score only ranged from 5 to 15 points out of a possible 48 points), which resulted in a truncated range for our correlative analyses. Despite these limitations, we nonetheless observed some important relationships between measures of stroke risk and imaging and cognition. A larger sample size and increased variability in stroke risk profile may reveal additional relationships between CBF and BOLD responses and cognitive variables.
Despite the CBF and BOLD changes noted, neither provides a direct quantitative measure of the neural activity within this region. Rather, they provide a qualitative estimation at best. Nevertheless, our data demonstrate that the two measures are not redundant and provide complementary information. Ultimately, combination of ASL with BOLD is a method that, when combined with a vasodilatory stimulus like carbon dioxide inhalation, will allow for a direct quantitative estimate of metabolic change (e.g., CMRO2; [Davis et al., 1998; Hoge et al., 2005]). The estimation of CMRO2 is a well-defined physiological quantity that is tightly linked to neural activity, and the opportunity to non-invasively estimate a quantitative measure linked to neural activity will be particularly valuable for clinical studies where factors such as disease, medication, and age can greatly impact the cerebrovascular system and cause changes in CBF and BOLD measures that are unrelated to changes in neural activity.
Our preliminary work in this area (for reviews, see Brown et al., 2007; Wierenga and Bondi, 2007) suggests that aging results in differences in baseline cerebral blood volume or differences in baseline 02 extraction fraction. According to the deoxyhemoglobin dilution model (see Brown et al., 2007, for discussion), BOLD contrast can give misleading information about the underlying metabolic activity of neurons when differences in baseline CBV or CMRO2 are present. There is an inherent ambiguity of interpreting BOLD response in isolation, especially in the presence of influencing factors such as age, disease, or disease risk. Combined ASL/BOLD imaging thus offers a considerably richer set of indices by which to characterize the hemodynamic response to cognition.
As an applied MRI technique, ASL has only recently begun to be used with clinical populations such as Mild Cognitive Impairment and Alzheimer’s disease (Johnson et al., 2005). Perfusion serves as an index to assess brain function and integrity in certain susceptible regions (e.g., MTL, posterior cingulate). Thus, ASL has important implications for diagnosis, treatment monitoring, and outcome prediction in dementia, stroke, and other neurological diseases (Warach et al., 1996). Initial studies assessing both BOLD and CBF in the MTL during encoding have demonstrated the feasibility of this technique in older adults (Restom et al., 2007). The main challenge is the relatively low contrast-to-noise ratio of the CBF measures. Technological advances (e.g., higher field strength) will further improve the temporal and spatial resolution of ASL and facilitate the use of combined BOLD and ASL data (Fernandez-Seara et al., 2007). The combination of CBF data with BOLD responses to yield CMRO2 information may be particularly useful in interpretation of FMRI study results and has the potential to help reconcile many discrepancies that currently exist from BOLD studies.
Despite our intriguing findings with ASL/BOLD imaging, the present study has several caveats. In imaging-based comparative aging studies, one must account for between-group differences in degree of atrophy by age. In an effort to reduce these potential differences, we used a partial volume correction on each data set and native space ROI tracings to avoid errors that may occur when normalizing brains with varying degrees of atrophy to the same standard space template. Such steps, though helpful, may not completely eliminate atrophic differences between our groups. In correcting for partial voluming, we assumed that the CBF in gray matter is 2.5 times larger than that in white matter. It is important to acknowledge that this ratio may change with age. However, Restom and colleagues (2007) performed 3 sets of CBF estimates: one estimate in which they assumed that gray matter CBF is 2.5 times larger than white matter CBF, a second in which they assumed that CBF reflected gray matter only, and a third estimate in which they made no partial volume correction. Restom and colleagues (2007) reported that significant between-group differences remained the same regardless of which estimate was used to perform the partial volume correction. Corroborating that finding, Johnson and colleagues (2005) also reported no significant differences in their results when they used the partial volume correction compared to when they did not.
Another consideration is that older adult groups may include individuals with preclinical disease. In order to ensure that our older participants were free from cognitive, neurological, or other disease, they were carefully assessed for the presence of cognitive impairment, significant stroke risk factors, and medical and psychiatric conditions known to affect cognition. Our older group was found to be free of medical or psychiatric disease, cognitive impairment, or significant stroke risk. Moreover, 4 of our 15 older adult participants (or 26%) were carriers of the APOE ε4 allele—a genetic susceptibility factor for Alzheimer’s disease—a percentage that roughly corresponds to its 20–25% frequency of occurrence in the population (Corder et al., 1993; Ghebranious et al., 2005). Thus, our sample was not disproportionately at increased risk for preclinical disease. Future efforts targeting at-risk groups (e.g., mild cognitive impairment, genetic risk, etc.) using functional ASL/BOLD imaging will help clarify the complex interactions underlying neurovascular coupling and ultimately provide a better understanding of neural substrates leading to cognitive dysfunction.
In summary, we have demonstrated differences between CBF and BOLD response to learning between ostensibly healthy young and older adults that are suggestive of age-related compensatory mechanisms. However, whereas the lack of difference in the BOLD responses could be interpreted as evidence for no change in neural activity with age, the significant difference in the percent CBF responses could be interpreted as evidence for an age-related compensatory increase in task-related neural activity. However, a definitive interpretation is lacking in the absence of a direct quantitative estimate of neural activity such as from measurement of CMRO2 response during learning. Future studies may be able to obtain such an estimate through simple breath-hold or CO2 inhalation challenges (see Davis et al. 1998).
Acknowledgments
This work was supported by grants from the National Institutes of Health (K24 AG026431, R01 AG012674, P50 AG05131, and RO1 NS051661), and by a grant from the Dana Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. The authors gratefully acknowledge the assistance of staff, patients, and volunteers of the UCSD Alzheimer’s Disease Research Center, and the UCSD Laboratory of Cognitive Imaging.
Footnotes
Disclosure Statement
There were no actual or potential conflicts of interest for the authors that could have inappropriately influenced the present work. Participants were recruited in accordance with Institutional Review Board (IRB) approved policies and procedures. Standard professional and ethical guidelines were upheld during the research study and manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adak S, Illouz D, Gorman W, Tandon R, Zimmerman EA, Guariglia R, Moore MM, Kaye JA. Predicting the rate of cognitive decline in aging and early Alzheimer disease. Neurology. 2004;63(1):108–114. doi: 10.1212/01.wnl.0000132520.69612.ab. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage. 2002;15(3):488–500. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Meyer JS, Mortel KF, Terayama Y, Thornby JI, Konno S. Normal human aging: actors contributing to cerebral atrophy. J Neurol Sci. 1997;152:1–39. doi: 10.1016/s0022-510x(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC. A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. NMR Biomed. 1997;10(4–5):197–203. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<197::aid-nbm466>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bentourkia M. A flexible image segmentation prior to parametric estimation. Comput Med Imaging Graph. 2001;25:6–501. doi: 10.1016/s0895-6111(01)00016-7. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Invest Radiol. 2001;36:9–539. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. FMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer’s disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, Eyler-Zorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB. BOLD and perfusion to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003;23:7–829. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- Brown GG, Perthen J, Liu TT, Buxton R. A primer on functional magnetic resonance imaging. Neuropsychol Rev. 2007 doi: 10.1007/s11065-007-9028-8. [DOI] [PubMed] [Google Scholar]
- Bruhn H, Fransson P, Frahm J. Modulation of cerebral blood oxygenation by indomethacin: MRI at rest and functional brain activation. J Magn Reson Imaging. 2001;13(3):325–334. doi: 10.1002/jmri.1047. [DOI] [PubMed] [Google Scholar]
- Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11:4–249. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FIM. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17:1–391. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31(3):680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- Claus JJ, Breteler MM, Hasan D, Krenning EP, Bots ML, Grobbee DE, Van Swieten JC, Van Harskamp F, Hofman A. Regional cerebral blood flow and cerebrostroke risk factors in the elderly population. Neurobiol Aging. 1998;19(1):57–64. doi: 10.1016/s0197-4580(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI repsonse. J Cereb Blood Flow Metab. 2002;22:9–1042. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene does of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:3–162. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25(1):40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:11–863. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16(12):1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, Jonder C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:4–1834. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System: Examiner’s Manual. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. The Psychological Corporation; San Antonio: 2000. [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:1–37. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993;138:6–353. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Ozyurt IB, Clark CP, Morris S, Bischoff-Grethe A, Bondi MW, Jernigan TL, Fischl B, Segonne F, Shattuck DW, Leahy RM, Rex DE, Toga AW, Zou KH, Brown GG. Quantitative evaluation of automated skull-stripping methods applied to contemporary and legacy images: effects of diagnosis, bias correction, and slice location. Hum Brain Mapp. 2006;27:2–99. doi: 10.1002/hbm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Seara MA, Wang J, Wang Z, Korczykowski M, Guenther M, Feinberg DA, Detre JA. Imaging mesial temporal lobe activation during scene encoding: comparison of fMRI using BOLD and arterial spin labeling. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20366. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Roseb B, Dale AM. Whole Brain Segementation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Ghebranious N, Ivacic L, Mallum J, Dokken C. Detection of ApoE E2, E3 and E4 aleles sing MALDI-TOF mass spectrometry and the homogeneous mass-extended technology. Nucleci Acids Res. 2005;33(17):e149. doi: 10.1093/nar/gni155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapior MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269(5221):218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:1–84. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hagstadius S, Risberg J. Regional cerebral blood flow characteristics and variations with age in resting normal subjects. Brain Cogn. 1989;10:1–28. doi: 10.1016/0278-2626(89)90073-0. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, Brown GG, Corey-Bloom J, Salmon DP, Thal LJ, Bondi MW. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging. 2007;28(2):238–247. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG. Human hippocampus established associations in memory. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Franceschini MA, Covolan RJM, Huppert T, Mandeville JB, Boas DA. Simultaneous recording of task-induced changes in blood oxygenation, volume, and flow using diffuse optical imaging and arterial spin-labeling MRI. Neuroimage. 2005;25(3):701–707. doi: 10.1016/j.neuroimage.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Honig LS, Tang MX, Albert S, Costa R, Luchsinger J, Manly J, Stern Y, Mayeux R. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:12–1707. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Corey-Bloom J, Nagel BJ, Bondi MW. Differential cross-sectional and longitudinal impact of APOR genotype on hippocampal volume in nondemented older adults. Dement Geriatr Cogn Disord. 2007;23:6–282. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Geon-Ho J, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tampini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and Mild Cognitive Impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234(3):851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaszniak AW, Garron DC, Fox JH, Bergen D, Huckman M. Cerebral atrophy, EEG slowing, age, education, and cognitive functioning in suspected dementia. Neurology. 1979;29(Pt 1):1273–1279. doi: 10.1212/wnl.29.9_part_1.1273. [DOI] [PubMed] [Google Scholar]
- Kawamura J, Terayma Y, Takashima S, Obara K, Pavol MA, Meyer JS, Mortel KF, Weathers S. Leuko-araiosis and cerebral perfusion in normal aging. Exp Aging Res. 1993;19:3–225. doi: 10.1080/03610739308253935. [DOI] [PubMed] [Google Scholar]
- Kety SS. Human cerebral blood flow and oxygen consumption as related to aging. J Chronic Dis. 1956;3:5–478. doi: 10.1016/0021-9681(56)90146-1. [DOI] [PubMed] [Google Scholar]
- Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31(3):780–786. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- Knopman D, Boland LL, Moseley T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Mosley TH, Catellier DJ, Sharrett AR Atherosclerosis Risk in Communities (ARIC) Study. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology. 2005;65(6):876–881. doi: 10.1212/01.wnl.0000176074.09733.a8. [DOI] [PubMed] [Google Scholar]
- Krausz Y, Bonne O, Gorfine M, Karger H, Lerer B, Chisin R. Age-related changes in brain perfusion of normal subjects detected by 99mTc-HMPAO SPECT. Neuroradiology. 1998;40(7):428–434. doi: 10.1007/s002340050617. [DOI] [PubMed] [Google Scholar]
- Larsson A, Skoog I, Aevarsson O, Arlig A, Jacobsson L, Larsson L, Ostling S, Wikkelso C. Regional cerebral blood flow in normal individuals aged 40, 75 and 88 years studied by 99Tcm-d,l-HMPAO SPET. Nucl Med Commun. 2001;22:7–741. doi: 10.1097/00006231-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Lee SP, Duong TO, Yang G, Iadecola C, Kim SG. Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow: implication for BOLD fMRI. Magn Reson Med. 2001;45:5–791. doi: 10.1002/mrm.1107. [DOI] [PubMed] [Google Scholar]
- Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. Neuroimage. 2005;24(1):207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Coleman RE, Provenzale JM, DeGrado TR, Hoffman JM. Adult age differences in regional cerebral blood flow during visual work identification: evidence from H215O Pet. Neuroimage. 1996;3(2):127–142. doi: 10.1006/nimg.1996.0015. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999;7:2–115. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. 2001;124(Pt 3):457–467. doi: 10.1093/brain/124.3.457. [DOI] [PubMed] [Google Scholar]
- Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991;11:4–684. doi: 10.1038/jcbfm.1991.121. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Ohnishi T, Asada T, Li ZJ, Kanetaka H, Imabayashi E, Tanaka F, Nakano S. Correction for partial-volume effects on brain perfusion SPECT in healthy men. J Nucl Med. 2003;44:8–1243. [PubMed] [Google Scholar]
- Mattis S. Psychological Assessment Resources. Odessa, FL: 1988. Dementia Rating Scale: Professional Manual. [Google Scholar]
- McPherson SE, Cummings JL. Vascular dementia: clinical assessment, neuropsychological features and treatment. In: Nussbaum PD, editor. Handbook of Neuropsychology and Aging. Plenum Press; New York: 1997. pp. 177–188. [Google Scholar]
- Meyer JS, Takashima S, Terayama Y, Obara K, Muramatsu K, Weathers S. CT changes associated with normal aging of the human brain. J Neurol Sci. 1994;123(1–2):200–208. doi: 10.1016/0022-510x(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Mulderink T, Gitelman D, Mesulam M, Parrish T. On the use of caffeine as a contrast booster for BOLD fMRI studies. Neuroimage. 2002;15(1):37–44. doi: 10.1006/nimg.2001.0973. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Palmer SL, Reddick WE, Glass JO, Helton KJ, Wu S, Xiong X, Kun LE, Gajjar A, Mulhern RK. Abnormal hippocampal development in children with medulloblastoma treated with risk-adapted irradiation. AJNR Am J Neuroradiol. 2004;25(9):1575–1582. [PMC free article] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med. 2004;51:4–736. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Petit-Taboue MC, Landeau B, Desson JF, Desgranges B, Baron JC. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7(3):176–184. doi: 10.1006/nimg.1997.0318. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Aker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Restom K, Behzadi Y, Liu TT. Physiological noise reduction for arterial spin labeling functional MRI. Neuroimage. 2006;31(3):1104–1115. doi: 10.1016/j.neuroimage.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage. 2007;37(2):430–9. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, D’Agostino RD, DeCarli C. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63(9):1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13(5):856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CE, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Rylander KM, Pike GB. The effect of global cerebral vasodilation on focal activation hemodynamics. Neuroimage. 2006;30(3):726–734. doi: 10.1016/j.neuroimage.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Suiguira RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:16–8660. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998a;51(4):986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998b;29(11):2334–2340. doi: 10.1161/01.str.29.11.2334. [DOI] [PubMed] [Google Scholar]
- Tanaka F, Vines D, Tsuchida T, Freedman M, Ichise M. Normal patterns on 99mTc-ECD brain SPECT scan in adults. J Nucl Med. 2000;41:9–1456. [PubMed] [Google Scholar]
- Tzourio C, Doufil C, Ducimetiere P, Alperovitch A. Cognitive decline in individuals with high blood pressure: a longitudinal study in the elderly. EVA Study Group. Epidemiology of Vascular Aging. Neurology. 1999;53(9):1948–1952. doi: 10.1212/wnl.53.9.1948. [DOI] [PubMed] [Google Scholar]
- Waldemar G, Hasselbalch SG, Andersen AR, Delecluse F, Petersen P, Johnsen A, Paulson OB. 99mTc-d,lHMPAO and SPECT of the brain in normal aging. J Cereb Blood Flow Metab. 1991;11:3–508. doi: 10.1038/jcbfm.1991.95. [DOI] [PubMed] [Google Scholar]
- Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab. 1996;16:1–53. doi: 10.1097/00004647-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised. The Psychological Corporation; New York: 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Revised. The Psychological Corporation; New York: 1987. [Google Scholar]
- Wierenga CE, Bondi MW. Use of functional magnetic resonance imaging in the early identification of Alzheimer’s disease. Neuropsychol Rev. 2007 doi: 10.1007/s11065-007-9025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Stricker JL, Houston WS, Eyler LT, Brown GG, Bondi MW. Functional connectivity comparisons of learning by APOE genotype among nondemented older adults. Brain Imaging and Behavior under editorial review. [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA. 1992;89:1–212. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39:5–702. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kanno I, Uemura K, Shishido F, Inugami A, Ogawa T, Murakami M, Suzuki K. Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke. 1986;17(6):1220–1228. doi: 10.1161/01.str.17.6.1220. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]