Abstract
In addition to a primary somatosensory cortex (SI), the cerebral cortex of all mammals contains a second somatosensory area (SII); however, the functions of SII are largely unknown. Our aim was to explore the functions of SII by comparing response properties of whisker-related neurons in this area with their counterparts in the SI. We obtained extracellular unit recordings from narcotized rats, in response to whisker deflections evoked by a piezoelectric device, and compared response properties of SI barrel (layer IV) neurons with those of SII (layers II to VI) neurons. Neurons in both cortical areas have similar response latencies and spontaneous activity levels. However, SI and SII neurons differ in several significant properties. The receptive fields of SII neurons are at least five times as large as those of barrel neurons, and they respond equally strongly to several principal whiskers. The response magnitude of SII neurons is significantly smaller than that of neurons in SI, and SII neurons are more selective for the angle of whisker deflection. Furthermore, whereas in SI fast-spiking (inhibitory) and regular-spiking (excitatory) units have different spontaneous and evoked activity levels and differ in their responses to stimulus onset and offset, SII neurons do not show significant differences in these properties. The response properties of SII neurons suggest that they are driven by thalamic inputs that are part of the paralemniscal system. Thus whisker-related inputs are processed in parallel by a lemniscal system involving SI and a paralemniscal system that processes complimentary aspects of somatosensation.
INTRODUCTION
The cerebral cortex of all mammals contains several representations for each sensory modality. For example, there exist upward of 32 morphologically and functionally discrete visual cortical areas in primates (Van Essen et al. 1992). Similarly, in all species, there exist several discrete somatosensory and auditory cortical areas (Disbrow et al. 2003; Harel et al. 2000; Huang and Winer 2000; Welker and Sinha 1972; Woolsey 1967). The roles and relationships of these multiple sensory representations are controversial. In the visual system, there is evidence that different cortical regions process information hierarchically, such that visual information is processed serially from one cortical area to the next (Casagrande and Kaas 1994; Kennedy and Bullier 1985; Stone et al. 1979). However, there is also evidence that visual information is processed in parallel by multiple cortical areas (Herkenham 1980; Weyand and Swadlow 1986). A similar ambiguity characterizes somatosensory cortical regions, with evidence existing for both serial and parallel processing by different cortical areas (Burton 1991; Coleman et al. 1999; Dykes 1983; Pons et al. 1992; Turman et al. 1995; Zhang et al. 1996).
In rodents, stimulation of the mystacial vibrissae (whiskers) evokes responses both in the first somatosensory (“barrel”) cortex (SI) and in the second somatosensory cortex (SII; so named because it was discovered later than SI; White 1987). However, apart from the fact that whisker-related neurons in SII have large receptive fields (Carvell and Simons 1986; Fabri and Burton 1991; Remple et al. 2003), relatively little is known about the functional organization of this cortical area. For example, whereas the response properties of SI neurons may be shaped primarily by inputs from the lemniscal pathway (Keller 1995), it is not known whether SII responses are mediated by inputs from SI or by direct subcortical inputs from the paralemniscal pathway.
Our aim was to take advantage of the extensive information on response properties of neurons in barrel cortex (SI) and the unique anatomical structure of the whisker system to compare response properties of whisker-related neurons in SI and SII. These comparisons provide strong support for the hypothesis that SII is part of the paralemniscal system that processes information in parallel with lemniscal pathways, including SI. Some of these results were reported previously in abstract form (Kwegyir-Afful and Keller 2002).
METHODS
Surgical procedures
Twelve female Sprague-Dawley rats weighing 250 –350 g were used in this study. All procedures strictly adhered to institutional and federal guidelines. Under halothane anesthesia (3%) and infusion of local anesthetics at surgical sites, a craniotomy was performed over the right primary and second somatosensory cortex. The dura was removed, and agarose (1.4% in buffered saline) was poured over the craniotomy to prevent drying. A venous catheter was inserted in the jugular vein for drug delivery, and a second catheter placed in the femoral artery for monitoring blood pressure and heart rate. Following the surgical procedures, administration of halothane was discontinued, and the rats were infused intravenously with fentanyl (10 μg/kg/h) for the rest of the experiment. The rats were then immobilized with pancuronium bromide (1.5 mg/kg/h) and artificially respired with a positive pressure respirator at 90 breaths/min. Blood pressure, heart rate, and electroencephalographs were monitored throughout the experiment to ensure that the animal was in no pain or distress. Body temperature was maintained at 37°C with a servo-controlled heating blanket.
A separate series of experiments was conducted with three additional rats, using approaches identical to the ones described above, except that urethane (1.5 g/kg) was used as an anesthetic for the duration of the experiments.
Recording and stimulation
Extracellular unit recordings were obtained with quartz-insulated platinum electrodes (2–4 MΩ). Electrodes were advanced perpendicular to the cortical surface, using either a stepper motor (Burleigh, Fishers, NY) or a seven-channel manipulator (Thomas Recording). Whiskers on the contralateral face were continually stimulated during electrode penetrations to detect units with low or no spontaneous activity. Waveforms recorded from well-isolated units were digitized through a Plexon data acquisition system (Dallas, TX) at 40 kHz. Units were isolated off-line with Plexon’s Off-line Sorter, and auto-correlograms were generated with Neuroexplorer software (Littleton, MA) to confirm that recordings were obtained from single units.
At the end of the experiment, recording sites were marked with electrolytic lesions (5 μA for 10 s). The animals were deeply anesthetized with sodium pentobarbital (60 mg/kg) and perfused transcardially with buffered saline followed by 4% buffered paraformaldehyde. Recording sites were identified in Nissl-stained coronal sections.
Whisker stimulation
Receptive fields were initially determined by manually deflecting individual whiskers. Whiskers evoking detectable responses were then individually attached—10 mm from their base—to a computer-controlled piezoelectric stimulator that can be deflected in eight different directions. Ramp-and-hold stimuli, 200 ms in duration and having onset/offset velocity of 102 mm/s, were applied at 1 Hz. To reduce mechanical ringing, the trapezoid ramp-and-hold waveforms were filtered with a Bessel filter. The peak onset and offset velocity were measured as the slope of the linear portion of the deflection ramp. The stimulator was calibrated with a photodiode device. Individual whiskers were deflected in one of eight directions (in 45° increments), delivered randomly for a total of 50 stimuli per deflection angle. In experiments involving urethane-anesthetized rats, whiskers were manually deflected with a handheld probe.
Data analysis
Time stamps of well-isolated units and of stimulus triggers were exported to Matlab (MathWorks, Natick, MA) for analyses using custom written software. Peristimulus time histograms (PSTHs, 1-ms bins) were constructed from these time stamps. PSTHs were constructed from responses to stimulation of a whisker at the cell’s preferred angle. Significant stimulus-evoked responses were defined as PSTH bins whose response magnitude significantly exceeded (99% CI) spontaneous activity levels, computed from a 200-ms period preceding the stimuli. Response onset latency was identified as the time of occurrence of two consecutive poststimulus bins displaying significant responses.
Statistical analyses were performed in SPSS and Microsoft Excel. Where appropriate, results are displayed using a boxplot to depict the median and distribution of the data (see Fig. 1B). Between-group statistical comparisons were assessed with the nonparametric Kolmogorov-Smirnov (K-S) test, because it is sensitive to any type of distributional differences (i.e., central tendency, variability, skewness, and kurtosis) and makes no assumptions regarding normality or equivalence of variance. Multiple-group comparisons were assessed with ANOVA with nonparametric posthoc tests. Within-group (individual neuron) comparisons made use of one-tailed Student’s t-tests. Categorized data were analyzed using a χ2 test. To fully describe data distributions, both means ± SD and medians are presented.
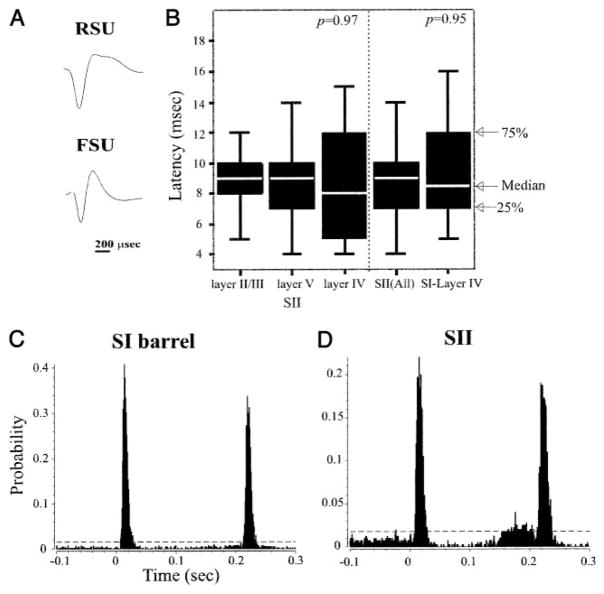
FIG. 1.
A: representative waveforms recorded from a regular spike unit (RSU) and a fast spike unit (FSU). RSUs have longer duration waveforms. B: distribution of response onset latency of second somatosensory cortex (SII) and primary somatosensory cortex (SI) neurons. SI and SII neurons and neurons in different layers of SII have similar onset latencies. P values computed from Kolmogorov-Smirnov (K-S) tests. Depicted are the median and 1st and 3rd quartiles of the data distribution. Whiskers represent data within 1.5 times the range from the 1st to the 3rd quartile. C and D: representative peristimulus time histograms (PSTHs) constructed from responses of an SI barrel (layer IV) neuron (C) and an SII neuron (D) to a 200-ms ramp and hold whisker deflection at time t = 0. In these and subsequent PSTHs, horizontal broken lines depict response magnitude levels exceeding (99% CI) spontaneous activity levels (computed from a 200-ms period preceding stimulus onset; note different ordinate scales).
RESULTS
Recording sites and cell classification
Included in the following analyses are data from 140 well-isolated units, of which 124 units were recorded from 35 electrode penetrations in the SII. We compared these data with previously published findings on neurons in SI, recorded under identical conditions (Table 1). In addition, we include data from 16 units we recorded in layer IV of SI. This was done to ensure that, under our recording conditions, response properties of SI neurons are comparable to previously reported data. All recording sites were identified posthoc by histologically examining electrode tracks and lesion sites (see METHODS). SI units were all in layer IV of the granular posteromedial barrel subfield (Woolsey and Van der Loos 1970), the cortical area containing representations of the large whiskers (Welker and Woolsey 1974). We specifically targeted recording to neurons in barrel hollows by identifying neuronal clusters exhibiting a strong preference to stimulation of a single, principal whisker. All SII units were in the dysgranular cortical region immediately lateral to SI (Fabri and Burton 1991; Remple et al. 2003) and medial to primary auditory cortex (Fabri and Burton 1991; Remple et al. 2003). Laminar location was determined from microdrive readings and histological analyses. SII units in layers II/III (n = 44) were recorded at depths of 400–900 μm from the pial surface; units in layer V (n = 57) were at depths of 900–1,400 μm; units in layer VI (n = 23) were at depths of 1,400–1,900 μm. As previously reported (Carvell and Simons 1986; Fabri and Burton 1991), we did not observe a well-defined layer IV in SII.
TABLE 1.
Response properties of SI and SII neurons
| SII-FSU (n = 9) | SII-RSU (n = 115) | SI-RSU (n = 16) | SII layer II/III (n = 44) | SII layer V (n = 57) | SII layer VI (n = 23) | |
|---|---|---|---|---|---|---|
| Latency, ms | 8.60 ± 2.41 | 9.13 ± 3.66 | 9.33 ± 3.05 | 9.11 ± 2.84 | 8.98 ± 3.02 | 9.32 ± 5.76 |
| Spontaneous activity, Hz | 7.95 ± 8.74 | 5.20 ± 5.70 | 3.27 ± 3.99 | 5.72 ± 6.48 | 5.74 ± 6.20 | 3.74 ± 3.01 |
| ON response magnitude, spikes/stimulus | ||||||
| At cells’ preferred direction | 1.11 ± 0.42 | 1.07 ± 0.83 | 1.62 ± 0.97 | 1.27 ± 0.97 | 0.93 ± 0.65 | 0.99 ± 0.64 |
| Average of all directions | 0.91 ± 0.45 | 0.88 ± 0.74 | 1.60 ± 1.03 | 1.04 ± 0.83 | 0.76 ± 0.60 | 0.83 ± 0.64 |
| OFF/ON response magnitude | ||||||
| At cells’ preferred direction | 0.48 ± 0.32 | 0.49 ± 0.33 | 0.66 ± 0.34 | 0.48 ± 0.24 | 0.49 ± 0.40 | 0.53 ± 0.30 |
| Average of all directions | 1.00 ± 0.34 | 0.71 ± 0.31 | 0.72 ± 0.34 | 0.79 ± 0.33 | 0.70 ± 0.31 | 0.72 ± 0.32 |
| Receptive field size, no. of whiskers | 7.8 ± 3.1 | 6.7 ± 2.6 | 7.7 ± 3.1 | 6.0 ± 2.3 | 6.1 ± 1.8 | |
| Principal whisker size, no. of whiskers | 1.6 ± 1.0 | 2.3 ± 2.0 | 2.5 ± 1.96 | 2.6 ± 2.0 | 1.5 ± 0.78 | |
| Angular tuning category | ||||||
| Range | 2–6 | 0–7 | 0–7 | 0–6 | 0–7 | 3–7 |
| Median | 6.0 | 3.0 | 1.5 | 3.0 | 3.0 | 5.5 |
With the exception of n values, all values reported here are means ± SD. FSU, fast spike units; RSU, regular spike units.
Previous studies reported that cortical neurons could be grouped into two classes based on the duration of their extracellularly recorded waveforms (Mountcastle et al. 1969; Simons 1978). Fast spiking units (FSUs)—which correspond to GABAergic, parvalbumin-expressing inhibitory interneurons (Connors and Gutnick 1990)— have significantly shorter waveforms compared with regular spiking units (RSUs), most of which are excitatory neurons. We applied the criteria developed by Bruno and Simons (2002) to distinguish between these populations. RSUs were defined as units whose waveforms had an initial negativity (N1) lasting >180 μs, followed by a positivity (P1) lasting >400 μs (Fig. 1A). FSUs had an N1 component ≤175 μs and a P1 component ≤350 μs (Fig. 1A). According to these criteria, 92% of SII neurons were classified as RSUs and 8% as FSUs. This ratio of RSUs and FSUs is similar to the one reported in studies of SI barrel cortex (Simons and Carvell 1989). Our sample includes RSUs recorded in layers II to VI of SII (40 units in layer II/III; 53 in layer V, and 22 in layer VI) and FSUs recorded in the same layers (4 units in layer II/III, 4 in layer V, and 1 in layer VI). All SI units in our study were RSUs recorded from layer IV barrels of SI.
Spontaneous activity
RSUs in SII had spontaneous firing rates ranging from 0.05 to 25.15 Hz (median, 3.55 Hz; 5.20 ± 5.70 Hz), whereas spontaneous firing of SI RSUs ranged from 0.15 to 12.52 Hz (median, 1.63 Hz; 3.27 ± 3.99 Hz) similar to values previously reported for SI (<1–12 Hz) (Simons and Carvell 1989). This difference was not statistically significant (K-S test, P = 0.14). FSUs in SII tended to have higher spontaneous firing rates than RSUS, but this difference did not reach statistical significance (K-S test, P = 0.38), with FSUs ranging from 0.87 to 29.8 Hz (median, 5.3 Hz; 7.95 ± 8.74 Hz). This finding is in contrast to that reported for rat SI (Simons and Carvell 1989) and in SII of the rabbit (Swadlow 1991), where FSUs have significantly higher spontaneous activity than RSUs.
Response latency
We reasoned that if the responses of SII neurons are shaped by direct thalamic inputs, the onset latencies of SII neurons would be indistinguishable from those of SI cells. To test this, we compared the onset latency of SI and SII neurons to whisker deflections (at each cell’s preferred direction). Figure 1, C and D, depicts PSTHs computed for representative units recorded from SI and SII, using 1-ms bins (identical results were obtained using 100-μs bins). The response onset latencies of these neurons were nearly identical (7 and 8 ms). The median response latency of all SII neurons was 9.0 ms (4–30 ms; 9.1 ± 3.6 ms), and for SI neurons, the median was 8.5 ms (5–16 ms; 9.3 ± 3.0 ms; K-S test, P = 0.95; Fig. 1B). Although our sample size of SI neurons is smaller than that of SII cells, the onset latencies of SI neurons we recorded is similar to that reported in previous studies (e.g., Brumberg et al. 1999). Thus SII neurons respond to whisker deflections with latencies that are similar to those of their counterparts in SI.
In SI barrel cortex, neurons in layer IV and some neurons in the deeper layers respond to whisker deflections earlier than cells in superficial layers (Brumberg et al. 1999). To determine if a similar difference in response latency characterizes SII neurons, we compared the onset latency of cells in different layers. We found no statistically significant differences in onset latency for units recorded from layer II/III (median, 9.0 ms; n = 44), layer V (median, 9.0 ms; n = 58), or layer VI (median, 8.0 ms; n = 23; ANOVA P = 0.97; Fig. 1B). Similarly, the onset latencies of RSUs and FSUs were indistinguishable (P = 0.65).
These results suggest that, unlike in SI, neurons in all layers of SII receive suprathreshold inputs from thalamic afferents and that the response properties of neurons in all layers of SII are shaped by these inputs. Therefore in the following analyses, we compared the response properties of SII neurons in all layers with those of neurons in layer IV of SI.
Response kinetics
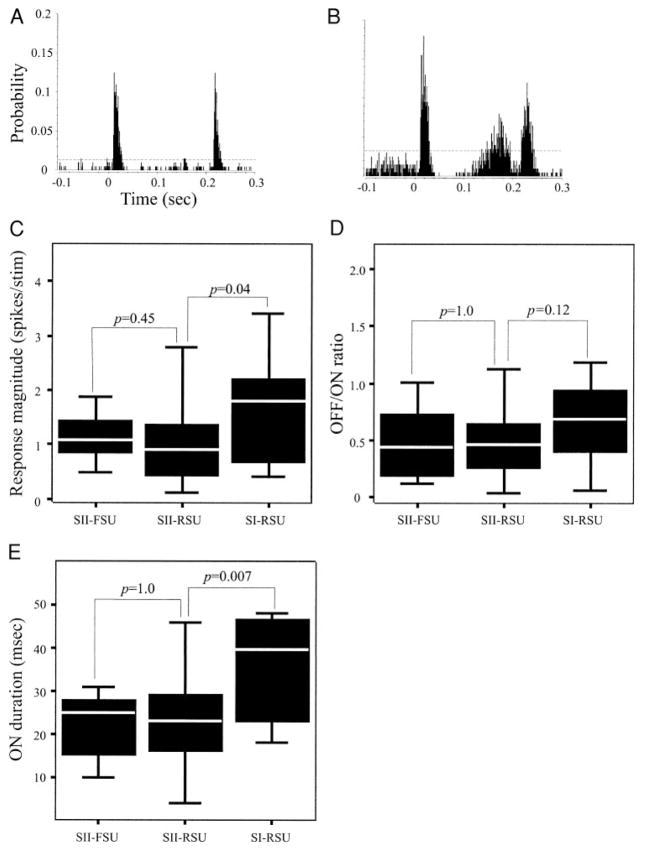
Ramp-and-hold whisker deflections evoked phasic responses in most (90.3%) SII neurons, with cells responding vigorously to stimulus onset (ON) and offset (OFF), while evoking no significant activity during the hold phase of the stimulus (Fig. 2A). We identified the beginning of ON and OFF responses as two consecutive, statistically significant PSTH bins (see METHODS) and the end of each ON and OFF response as three consecutive bins with no significant activity. The duration of the entire ON response ranged from 7 to 54 ms (median, 24 ms; 24.98 ± 10.61 ms). ON response durations of SI neurons were more variable, ranging from 18 to 99 ms. Using criteria introduced by Simons and Carvell (1989), we classified units whose responses (at their preferred direction) were 50 ms or longer as slowly adapting, and the remaining neurons as rapidly adapting. Of SI cells, 18.8% were slowly adapting, whereas all but four neurons (3%) recorded in SII were rapidly adapting. The duration of ON responses in rapidly adapting SI neurons ranged from 18 to 48 ms (median, 25 ms; 31.46 ± 11.21 ms), and in slowly adapting SI neurons, ranged from 84 to 99 ms (median, 91 ms; 91.33 ± 7.51 ms). There was no significant difference between ON response duration of SI rapidly adapting neurons and SII neurons (K-S test, P = 0.09). Additionally, there was no significant difference (K-S test, P = 0.93) in ON response duration between RSUs (median, 24 ms; range, 7–54 ms) and FSUs (median, 25 ms; range, 10–54 ms; Fig. 2E) in SII.
FIG. 2.
Response kinetics of SII neurons. A: PSTH showing the characteristic response pattern recorded from 90.3% of SII neurons. These neurons had a biphasic response, responding to stimulus onset (ON) and stimulus offset (OFF). B: in addition to the ON and OFF responses, in 9.7% of SII neurons, there was a secondary response beginning 146.33 ± 42.07 ms following the ON response and lasting 35.0 ± 27.9 ms. This category of cells included both RSUs and FSUs. C: box-plots of the distribution of response magnitudes of the different classes of neurons. The response magnitude of SII cells was significantly smaller than SI barrel cells, but SII-RSUs and SII-FSUs had similar response magnitudes. D: OFF-ON: response magnitude ratios of SII-FSUs, SII-RSUs, and SI-RSUs were statistically indistinguishable. E: response duration of SI neurons was longer than SII cells due to the fact that a larger proportion of SI barrel neurons were slowly adapting. All statistical comparisons made with K-S tests.
Although most SII neurons responded only to stimulus onset and offset (Fig. 2A), a minority (n = 12/124; 9.7%) of these units had a secondary response during the hold phase of the stimulus (Fig. 2B). In most (8 of 12) neurons, this secondary component occurred in response to stimulation in the cell’s nonpreferred direction. We therefore analyzed the kinetics of the secondary responses from angle-averaged data. Of these 12 cells, 16.7% were FSUs and 83.3% were RSUs. The onset latency of this secondary response ranged from 41 to 182 ms (median, 158 ms), with a duration lasting between 2 and 105 ms (median, 27.5 ms). The response magnitude of the secondary response ranged from 0.01 to 1.05 spikes/stimulus (median, 0.06 spikes/stimulus). We did not observe this secondary response in our SI recording, and to our knowledge, it has not been reported in other studies of SI neurons. Thus while most SII neurons are rapidly adapting and their response duration is similar to that of SI rapidly adapting neurons, slowly adapting neurons with long ON responses, and tonically firing neurons, are rarely found in SII.
Units recorded from SI and SII differ in their ON response magnitude (computed for the entire duration of the ON response; K-S test, P = 0.04; Fig. 2C). SI RSU responses ranged from 0.40 to 3.42 spikes/stimulus (median, 1.80 spikes/stimulus; 1.62 ± 0.97 spikes/stimulus). SII units response magnitudes were significantly smaller, ranging from 0.11 to 4.52 spikes/stimulus (median, 0.93 spikes/stimulus; 1.07 ± 0.8 spikes/stimulus). In contrast, SII RSUs and FSUs did not differ in response magnitudes (K-S test, P = 0.45; Fig. 2C).
In SI neurons, the magnitude of ON responses is usually larger than that of OFF responses (Kyriazi et al. 1994). We found a similar preference for ON responses in both SI and SII neurons, with OFF-ON response magnitude ratios of 0.66 ± 0.34 for SI cells and 0.49 ± 0.33 for SII neurons. In SII, OFF-ON ratios of RSUs (0.04–2.14; median, 0.46; 0.49 ± 0.33) were similar to those of FSUs (0.12–1.01; median, 0.43; 0.48 ± 0.32; K-S test, P = 1.0; Fig. 2D). Similarly, we found no significant differences (K-S test, P = 0.12) in OFF-ON ratios between SI and SII RSUs: RSUs in SI had ratios ranging from 0.07 to 1.19 (median, 0.68; 0.66 ± 0.34; similar to ratios reported by Kyriazi et al. 1994). Thus OFF-ON ratios of RSUs are similar in SI and SII neurons. However, whereas in SII, RSUs and FSUs have similar OFF-ON ratios, in SI, RSUs have a significantly smaller OFF-ON ratio compared with FSUs (Kyriazi et al. 1994).
Receptive field size
We defined receptive field size as the number of whiskers whose stimulation evokes a significant response, averaged across all eight angles, in SII neurons. In initial experiments (see METHODS), we recorded responses to manual deflection of individual whiskers in urethan-anesthetized rats. Under these conditions, SII neurons responded to stimulation of 5–13 whiskers (median, 9 whiskers; n = 30 neurons). Whiskers whose stimulation evoked responses in a particular neuron were always located contiguously along the whisker pad. In other experiments— under fentanyl analgesia—we recorded responses of individual neurons to piezoelectric stimulation of individual whiskers. Although this approach is advantageous in that stimulation parameters are better controlled, the time needed to fully characterize the responses of a neuron limits the number of whiskers that can be tested. As a result, values obtained with this approach underestimate the size of SII receptive fields. We included in this analysis only cases in which we recorded responses to stimulation of ≥4 adjacent whiskers (n = 58 units). These cells responded to stimulation of ≥4 whiskers and as many as 12 whiskers (median, 6 whiskers). For 18 units, we were able to test responses to stimulation of eight or more individual whiskers. These units responded to stimulation of 5–12 whiskers (median, 10 whiskers). Thus the receptive field size of SII neurons does not appear to be affected significantly by the choice of anesthetic. Under both fentanyl analgesia and urethane anesthesia, the median receptive field size was 10 whiskers. This contrasts sharply with the receptive field size of SI neurons, the majority of which respond to stimulation of a single whisker (e.g., Bruno and Simons 2002).
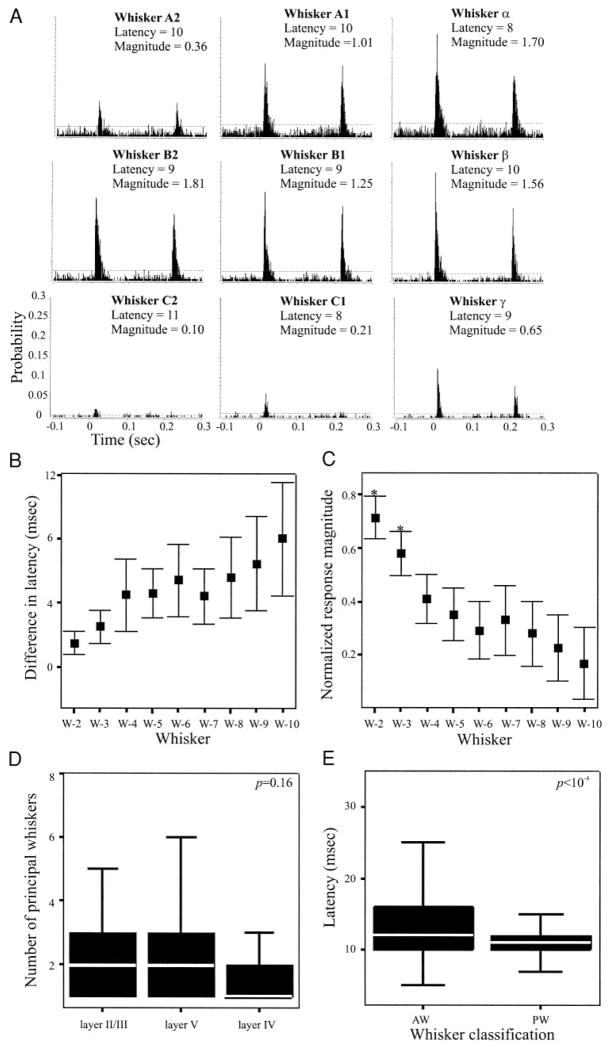
Neurons in the SI barrel field that respond to stimulation of more than one whisker respond preferentially to stimulation of the whisker associated with their anatomically defined barrel. That whisker is termed the principal whisker (PW; Welker 1971). Stimulation of the PW evokes responses having larger magnitude and shorter onset latency compared with responses to adjacent whiskers (AW; Armstrong-James 1995; Simons 1995). We sought to determine whether a similar distinction between PW and AW responses characterized SII neurons. Figure 3A shows PSTHs constructed from responses of a representative layer V SII neuron to deflections of different whiskers. Robust responses were evoked in this cell by stimulation of each of nine different whiskers. Note that stimulation of different whiskers evoked responses having different magnitudes but similar onset latencies. We obtained similar results from each of the 58 neurons in which we tested responses to four or more whiskers. To quantitatively assess whether different whiskers evoke responses having significantly different onset latencies, we identified, for each neuron, the whisker evoking the shortest latency response (termed “W-1”). The onset latency of responses to the remaining whiskers, rank ordered by response latency (W-2, W-3…), were then normalized to the onset latency of W-1. Figure 3B shows the means (±99% CIs) of these normalized latencies, computed from responses recorded from the 58 neurons described above. Note that, although the mean difference in latency is somewhat variable, there are no significant differences in onset latency in response to stimulation of different whiskers. Thus individual SII neurons respond to stimulation of different whiskers with similar onset latency, and as a result, onset latency cannot be used to distinguish between PW and AW responses.
FIG. 3.
Receptive field size of SII neurons, determined from 58 cells for which ≥5 whiskers were stimulated. A: PSTHs depicting responses of a layer V SII neuron to stimulation of different whiskers. Indicated above each PSTH are the whisker stimulated, the response onset latency (ms), and the response magnitude (spikes/stimulus). Stimulation of different whiskers results in responses with similar latencies, but different magnitude. B: relative differences in onset latency to stimulation of different whiskers, computed for all 58 neurons. For each neuron, latencies were normalized to responses to the whisker evoking the shortest latency response (W-1; see text for additional details). Error bars depict ±99% CIs. As a population, SII neurons responded with statistically indistinguishable onset latencies to stimulation of 9 different whiskers. C: similar analysis applied to response magnitudes, normalized to responses of the whisker evoking the largest response (W-1). As a population, SII neurons responded to 3 whiskers with similar response magnitudes [defined as the principal whiskers (PWs)] and with significantly smaller magnitude responses to the adjacent whiskers (AWs). D: neurons in all layers of SII had a similar number of PWs. E: onset latencies of responses to PWs were significantly shorter that those to AWs. All statistical comparisons made with K-S test.
The responses evoked by each whisker for the representative neuron (Fig. 3A) suggests that differences in response magnitude may be significant. To determine, for each neuron, whether the response magnitude evoked by individual whiskers were significantly different from each other, the whisker evoking the largest response was identified and designated W-1. Responses evoked by the remaining whiskers were rank ordered, as above and normalized to the response magnitude evoked by W-1. Data obtained from 58 neurons were used to construct the plot in Fig. 3C, which shows the means (±99% CIs) of the normalized response magnitude. Responses evoked by the first three whiskers were significantly different from responses evoked by the remaining whiskers. Thus using response magnitude as a metric, it is possible to distinguish between PWs and AWs for each neuron. Layer II/III neurons had between 1 and 10 PWs (2.5 ± 1.96), whereas the number of PWs for layer V cells ranged from 1 to 7 (2.6 ± 2.0) and layer VI cells from 1 to 3 whiskers (1.5 ± 0.78). These differences are not statistically significant (ANOVA, P = 0.16; Fig. 3D).
Interestingly, although response latency proved an inadequate metric to define PWs, PWs— defined as the group of whiskers evoking significantly stronger responses— have onset latencies that were significantly shorter than responses to AWs (K-S test, P < 10−4; Fig. 3E).
Angular selectivity
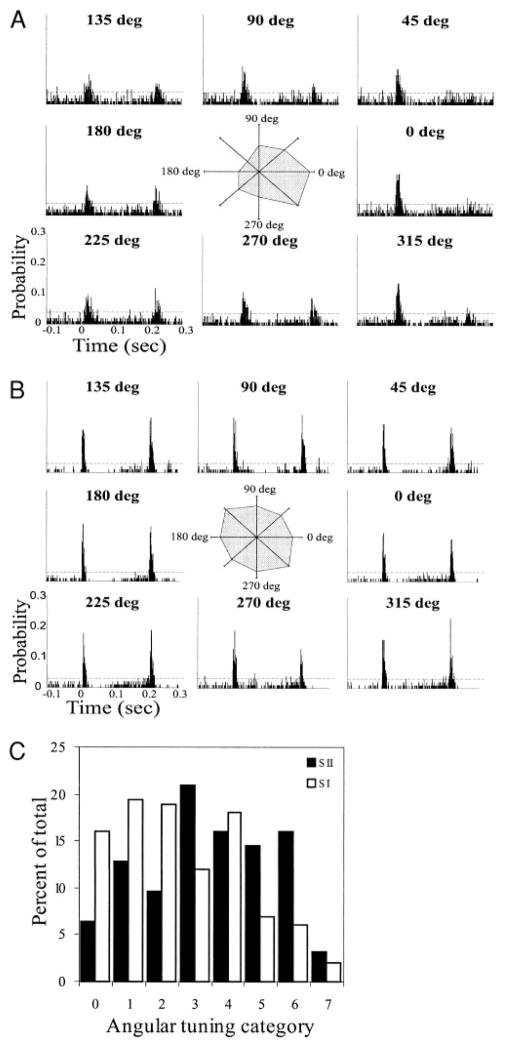
Neurons throughout the lemniscal pathway of the rat whisker system are selective for the angle of whisker deflection (Hartings et al. 2000; Lee and Simons 2004; Minnery and Simons 2003; Simons 1978; Simons and Carvell 1989), eliciting larger magnitude responses to preferred angle deflections. To determine if this property also characterizes SII neurons, we recorded responses to whisker deflections in eight different angles. Figure 4A presents results from an SII neuron with strong angular preference, and Fig. 4B depicts results from a poorly tuned neuron. PSTHs were constructed from responses to deflections in the angles indicated, with 0° representing deflection in the caudal direction and 90° in the dorsal direction. ON response magnitudes to the different deflections were normalized to the maximum ON response and used to construct polar plots (Fig. 4, A and B, insets) showing the normalized response magnitudes against the direction of deflection.
FIG. 4.
Angular selectivity of SII neurons. PSTHs of responses of a well-tuned (category 6, A) and a poorly tuned (category 0, B) SII neuron to deflection of a single whisker in 8 different directions. Polar plots were constructed by plotting the normalized response magnitude against the direction of whisker deflection. 0° represents deflection in the caudal direction and 90° is deflection in the dorsal direction. C: histogram comparing the distribution of angular tuning of SII neurons with those of SI barrels cells (the latter reproduced, with permission, from Simons and Carvell 1989). Angular tuning was defined as the number of deflection angles evoking responses that are statistically smaller than that to the maximally activating angle. Category 0 represents the least-tuned cells (cells that respond equally to all deflection angles) and category 7 represents the best-tuned neurons. A larger proportion of SII neurons have high angular tuning.
To quantify angular preferences, we determined, for each neuron, the number of deflection angles evoking an ON response magnitude that was statistically different from responses to the remaining deflection angles (compared using Student’s t-test, P < 0.05). We then categorized cells into eight groups (0–7; Simons and Carvell 1989) representing the number of angles with responses that are statistically smaller than responses to the maximally activating angle. Category 0 represents the least-tuned cells (cells that respond equally to all deflection angles), and category 7 represents the best-tuned cells (cells that responds preferentially to one deflection angle). The polar plot in Fig. 4A depicts a category 6 neuron, whereas that in Fig. 4B depicts a category 0 neuron. Figure 4C shows the distribution of angular tuning for SII and SI neurons (the latter reproduced from Simons and Carvell 1989). Well-tuned cells (categories 5–7) constituted 33.9% of SII neurons, but only 15.7% of SI cells. Thus SII contains a larger proportion of neurons with strong angular selectivity.
We investigated the possibility that there is a laminar segregation of angular tuning of SII neurons. Neurons in layer II/III had angular tuning ranging from category 0 to 6 (median, 3.0), layer V neurons ranged from category 0 to 7 (median, 3.0), and layer VI neurons ranged from category 3 to 7 (median, 5.5). Although layer VI neurons tended to have a higher angular selectivity, this difference is not statistically significant (χ2 = 16.52; P > 0.05; df = 14).
DISCUSSION
We found several similarities between the response properties of SI and SII neurons. Neurons in both cortical areas share similar response onset latencies, and RSUs in the two areas have indistinguishable OFF-ON response magnitude ratios and similar spontaneous activity levels. However, SI and SII neurons differ in several significant properties. The receptive fields of SII neurons are at least five times as large as those of barrel RSUs, and they respond equally to several principal whiskers. Furthermore, the response magnitude of SII neurons is significantly smaller than that of neurons in SI. Also different in the two areas are OFF-ON ratios of FSUs versus RSUs, response magnitude of RSUs versus FSUs, angular tuning, and the proportion of slowly adapting and rapidly adapting neurons.
Response latency
Although neurons in layers II to VI of SI may receive direct thalamic inputs (White and Keller 1989), layer IV (barrel) neurons respond to whisker stimuli at latencies that are significantly shorter than those of neurons in supragranular layers, and most cells in infragranular layers (Armstrong-James et al. 1992; Wilent and Contreras 2004). Laminar comparisons of other response properties also suggest that direct thalamic inputs have a larger influence on the receptive fields of barrel neurons compared with those of cells in other layers of SI (Brumberg et al. 1999; Pinto et al. 2003). In contrast, neurons in all layers of SII respond to whisker stimuli at similar short latencies and have similar response properties. This suggests that, unlike SI, the receptive field properties of neurons in all layers of SII may be shaped, to a significant extent, by direct thalamic inputs.
Neurons in SII and in layer IV of SI (the latter displaying the shortest response latencies in the barrel cortex; Brumberg et al. 1999) respond to whisker deflections with indistinguishable onset latencies. This finding argues against the possibility that SII responses are dependent on inputs from SI, because the conduction time and synaptic delay of SI to SII inputs would result in longer latency responses in SII. Thus SII neurons are likely to receive direct driving inputs from the thalamus in parallel with their counterparts in SI.
Inactivating SI can more directly test the dependence of SII on SI inputs, but the close proximity of these cortical regions in the rat complicates such a manipulation. However, reversible inactivation of SI has been successfully performed in larger species, including cat (Turman et al. 1992, 1995), marmoset (Zhang et al. 1996), and possum (Coleman et al. 1999). In all species, this manipulation failed to abolish SII responses, although it did produce some reductions in both spontaneous and evoked activity of some SII neurons. These findings support the conclusion that SII receives driving inputs from thalamic afferents and suggests that SI inputs are modulatory.
In contrast, SI lesions in Rhesus monkeys have been reported to abolish activity in homotypical SII regions (Burton et al. 1990; Pons et al. 1992). These authors postulate an evolutionary shift from nonprimates and lower primates—in which tactile information is processed in parallel in SI and SII—to a new organization in higher primates in which the processing of tactile information proceeds serially from SI to SII. However, there is also evidence from human and other primates that SII receives direct ascending inputs and operates in parallel with SI (for review, see Burton 1991). It has also been shown that SII of higher primates contains two subdivisions: one that may be driven preferentially by thalamic inputs and the other whose responses depend on inputs from SI (Burton et al. 1995).
The origin of driving inputs to rat SII is currently unknown. Anatomical studies indicate that the ventrolateral portion of the ventral posterior medial thalamus (VPMvl) and the posterior nucleus of the thalamus (POm) project densely to SII (Pierret et al. 2000). These thalamic afferents ramify extensively and diverge in most layers of SII. This laminar divergence is consistent with our finding that the response latency of neurons throughout layers II to VI of SII is indistinguishable (Fig. 1B).
Receptive field size
The receptive field size of SII whisker-related neurons is substantially larger than that of SI cells (Fig. 3). This is in agreement with previous findings in rat and other mammals (Carvell and Simons 1986; Jones and Peters 1984; Swadlow 1991). The large receptive field of SII neurons is not likely the result of converging inputs from SI, for reasons discussed above. Furthermore, anatomical findings show limited convergence of inputs from different SI whisker representations onto SII neurons (Alloway and Burton 1985a; Fabri and Burton 1991). Alternatively, these large receptive fields may be due to convergence of inputs from multi-whisker thalamic neurons in VPM barreloids (Simons and Carvell 1989) or to inputs from thalamic neurons having large receptive fields in VPMvl or POm (Friedberg et al. 1999; Pierret et al. 2000). We favor the latter hypothesis, since VPMvl and POm neurons receive inputs from neurons in the spinal trigeminal nucleus interpolaris (SPVi), and these neurons have some of the largest receptive fields in the whisker-trigeminal system (Jacquin et al. 1986; Timofeeva et al. 2004; Veinante et al. 2000).
Neurons throughout the whisker-to-barrel lemniscal pathway respond preferentially to a well-defined PW. In contrast, most SII neurons respond to several whiskers with similar latency and response magnitude and thus have several PWs (Fig. 3). The difference between the number of PWs in SII and in neurons in the lemniscal system lends further support to the conclusion that SII neurons may be driven by inputs from the paralemniscal system. The large receptive fields and multiple PWs of SII neurons suggest that they are not optimized for single-whisker discrimination. Carvell and Simons (1987) suggested that the large receptive fields in SII imply that this region may have a more contextual role in stimulus detection.
Response kinetics
Response magnitudes of SII neurons to single whisker deflection are significantly lower than those in SI neurons (Fig. 2). This may reflect differences in the efficacies or number of thalamic inputs to SI versus SII neurons. It is possible that SII neurons respond preferentially to simultaneous activation of multiple whiskers, a possibility we are currently exploring. It has been proposed that the paralemniscal system may be essential in the operation of mobile sensory organs (e.g., whiskers; Friedberg et al. 1999; Veinante et al. 2000) and that paralemniscal neurons are preferentially active during voluntary movements. Thus if SII receives driving inputs from the paralemniscal pathway, it may be preferentially active during active whisking.
RSUs and FSUs in SI barrels respond differently to whisker-derived inputs. FSUs have significantly larger response magnitudes (Bruno and Simons 2002), reflecting the higher convergence and efficacy of thalamic inputs to these cells (Gibson et al. 1999; Keller 2001; Porter et al. 2001). As a result of this and other network properties, the barrel circuitry is described as a damping circuit in which inhibition is finely tuned to constrain the temporal responses and receptive field size of RSUs (see Pinto et al. 2003). In contrast, in SII, FSUs and RSUs display similar response magnitudes (Fig. 2), suggesting that there is less net intracortical inhibition in SII compared with SI due to the relatively lower firing rates of FSUs. The reduced efficacy of feed-forward inhibition in SII may contribute to the larger receptive fields of neurons in this region.
RSUs and FSUs in SI differ also in their OFF-ON response ratios, with the former displaying significantly smaller ratios (Kyriazi and Simons 1993). Experimental and modeling studies indicate that the smaller OFF-ON ratios of SI RSUs are also a consequence of the dominance of feed-forward inhibition in the layer IV damping circuit (Kyriazi et al. 1994; Pinto et al. 2003). Thus the similarity in OFF-ON ratios of SII RSUs and FSUs may also reflect reduced feed-forward inhibition in this cortical area.
As in other species (Alloway and Burton 1985b; Coleman et al. 1999; Jones and Peters 1984), the majority of SII neurons are rapidly adapting. Subsequent stations along the lemniscal pathway contain progressively smaller proportions of slowly adapting neurons: 93% in the principal trigeminal nucleus (PrV; Minnery and Simons 2003), 37% in VPM, and 15% in SI (Simons and Carvell 1989). The paralemniscal pathway begins with a smaller proportion of slowly adapting neurons, with 54% of the cells in SPVi described as slowly adapting type I and II neurons (Jacquin et al. 1986). This lends further support for the assumption that SII— having only 3% slowly adapting neurons—is part of the paralemniscal system.
Angular tuning
The relatively strong angular preference of neurons at subcortical levels of the whisker-to-barrel system is thought to reflect the receptor properties and their configuration at the base of the whisker follicle (Lichtenstein et al. 1990; Rice et al. 1986). The proportion of well-tuned cells decreases at subsequent levels of the whisker-to-barrel neuroaxis: 81% of the neurons in trigeminal ganglion are well tuned (Lichtenstein et al. 1990), 46% in PrV (Minnery and Simons 2003), 31.1% in VPm, and 15.7% in SI (Simons and Carvell 1989). This progressive decrease may be due to convergence of inputs from neurons with different and sometimes opposing angular preferences. Surprisingly, 34% of SII neurons are well tuned for whisker deflection angles (Fig. 4). Because the angular tuning properties of neurons in VPMvl and POm—the putative sources of thalamic inputs to SII—are unknown, we cannot speculate as to the mechanisms responsible for the higher angular tuning of SII neurons. Notably, neurons in septa between the layer IV barrels, which are also thought to receive inputs from POm (Lu and Lin 1993; Pierret et al. 2000), have a higher angular preference than their counterparts in the barrel hollows (Lee and Simons 2004).
FSUs in SI of rat and rabbit are reported to exhibit lower angular tuning compared with RSUs (Lee and Simons 2004; Swadlow 1989). Swadlow (1991) reported a similar difference in angular tuning between FSUs and RSUs in rabbit SII. In contrast, we found no significant difference in angular tuning between FSUs and RSUs in SII. Whether these results reflect a species difference, or methodological variability, remains unknown.
The differences between the response properties of SI and SII neurons suggest that these two regions may receive inputs from different subpopulations of thalamic cells. The differences in input transformation in these cortical areas may be a reflection of unique circuit dynamics essential for parallel processing of different, but complementary somatosensory information.
Acknowledgments
We thank Dr. D. J. Simon for insightful discussions, mentorship, and generous gifts of piezoelectric stimulators and other components critical for this study.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-31078.
References
- Alloway KD, Burton H. Homotypical ipsilateral cortical projections between somatosensory areas I and II in the cat. Neuroscience. 1985a;14:15–35. doi: 10.1016/0306-4522(85)90161-7. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Burton H. Submodality and columnar organization of the second somatic sensory area in cats. Exp Brain Res. 1985b;61:128–140. doi: 10.1007/BF00235628. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M. The nature and plasticity of sensory processing within adult rat barrel cortex. In: Jones EG, Diamond IT, editors. Cerebral Cortex: The Barrel Cortex of Rodents. Vol. 11. New York: Plenum Press; 1995. pp. 333–373. [Google Scholar]
- Armstrong-James M, Fox K, Dasgupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Brumberg JC, Pinto DJ, Simons DJ. Cortical columnar processing in the rat whisker-to-barrel system. J Neurophysiol. 1999;82:1808–1817. doi: 10.1152/jn.1999.82.4.1808. [DOI] [PubMed] [Google Scholar]
- Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci. 2002;22:10966–10975. doi: 10.1523/JNEUROSCI.22-24-10966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H. Cerebral cortical regions devoted to the somatosensory system: results from brain imaging studies in humans. In: Nelson RJ, editor. The Somatosensory System: Deciphering the Brain’s Own Body Image. New York: CRC; 1991. pp. 27–72. [Google Scholar]
- Burton H, Fabri M, Alloway K. Cortical areas within the lateral sulcus connected to cutaneous representations in areas 3b and 1: a revised interpretation of the second somatosensory area in macaque monkeys. J Comp Neurol. 1995;355:539–562. doi: 10.1002/cne.903550405. [DOI] [PubMed] [Google Scholar]
- Burton H, Sathian K, Shao DH. Altered responses to cutaneous stimuli in the second somatosensory cortex following lesions of the postcentral gyrus in infant and juvenile macaques. J Comp Neurol. 1990;291:395–414. doi: 10.1002/cne.902910307. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Somatotopic organization of the second somatosensory area (SII) in the cerebral cortex of the mouse. Somatosens Mot Res. 1986;3:213–237. doi: 10.3109/07367228609144585. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Thalamic and corticocortical connections of the second somatic sensory area of the mouse. J Comp Neurol. 1987;265:409–427. doi: 10.1002/cne.902650309. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters A, Rockland KS, editors. Cerebral Cortex. Vol. 10. New York: Plenum Press; 1994. pp. 201–259. [Google Scholar]
- Coleman GT, Zhang HQ, Murray GM, Zachariah MK, Rowe MJ. Organization of somatosensory areas I and II in marsupial cerebral cortex: parallel processing in the possum sensory cortex. J Neurophysiol. 1999;81:2316–2324. doi: 10.1152/jn.1999.81.5.2316. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462:382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- Dykes RW. Parallel processing of somatosensory information: a theory. Brain Res Rev. 1983;6:47–115. doi: 10.1016/0165-0173(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Fabri M, Burton H. Ipsilateral cortical connections of primary somatic sensory cortex in rats. J Comp Neurol. 1991;311:405–424. doi: 10.1002/cne.903110310. [DOI] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Harel N, Mori N, Sawada S, Mount RJ, Harrison RV. Three distinct auditory areas of cortex (AI, AII, and AAF) defined by optical imaging of intrinsic signals. Neuroimage. 2000;11:302–312. doi: 10.1006/nimg.1999.0537. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Temereanca S, Simons DJ. High responsiveness and direction sensitivity of neurons in the rat thalamic reticular nucleus to vibrissa deflections. J Neurophysiol. 2000;83:2791–2801. doi: 10.1152/jn.2000.83.5.2791. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Laminar organization of thalamic projections to the rat neocortex. Science. 1980;207:532–535. doi: 10.1126/science.7352263. [DOI] [PubMed] [Google Scholar]
- Huang CL, Winer JA. Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J Comp Neurol. 2000;427:302–331. doi: 10.1002/1096-9861(20001113)427:2<302::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Mooney RD, Rhoades RW. Morphology, response properties, and collateral projections of trigeminothalamic neurons in brainstem subnucleus interpolaris of rat. Exp Brain Res. 1986;61:457–468. doi: 10.1007/BF00237571. [DOI] [PubMed] [Google Scholar]
- Jones EG, Peters A. Cerebral Cortex: Sensory-Motor Areas and Aspects of Cortical Connectivity. Vol. 5. New York: Plenum Press; 1984. [Google Scholar]
- Keller A. Synaptic organization of the barrel cortex. In: Jones EG, Diamond IT, editors. Cerebral Cortex: The Barrel Cortex of Rodents. Vol. 11. New York: Plenum Press; 1995. pp. 221–262. [Google Scholar]
- Keller A. Intrinsic synaptic interactions in the barrel cortex. Proc 4th Meeting of the German Neuroscience Society. 2001;1:138. [Google Scholar]
- Kennedy H, Bullier J. A double-labeling investigation of the afferent connectivity to cortical areas V1 and V2 of the macaque monkey. J Neurosci. 1985;5:2815–2830. doi: 10.1523/JNEUROSCI.05-10-02815.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwegyir-Afful EE, Keller A. Response properties of whisker related neurons in rat second somatosensory cortex. Soc Neurosci Abstr. 2002;32:450.10. doi: 10.1152/jn.00262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwegyir-Afful EE, Keller A. 2002 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2002. Response properties of whisker related neurons in rat second somatosensory cortex Program no. 450.1. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazi HT, Simons DJ. Thalamocortical response transformations in simulated whisker barrels. J Neurosci. 1993;13:1601–1615. doi: 10.1523/JNEUROSCI.13-04-01601.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazi HT, Carvell GE, Simons DJ. OFF response transformations in the whisker/barrel system. J Neurophysiol. 1994;72:392–401. doi: 10.1152/jn.1994.72.1.392. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simons DJ. Angular tuning and velocity sensitivity in different neuron classes within layer 4 of rat barrel cortex. J Neurophysiol. 2004;91:223–229. doi: 10.1152/jn.00541.2003. [DOI] [PubMed] [Google Scholar]
- Lichtenstein SH, Carvell GE, Simons DJ. Responses of rat trigeminal ganglion neurons to movements of vibrissae in different directions. Somatosens Mot Res. 1990;7:47–65. doi: 10.3109/08990229009144697. [DOI] [PubMed] [Google Scholar]
- Lu S-M, Lin RC-S. Thalamic afferents of the rat barrel cortex: a light and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res. 1993;10:1–16. doi: 10.3109/08990229309028819. [DOI] [PubMed] [Google Scholar]
- Minnery BS, Simons DJ. Response properties of whisker-associated trigeminothalamic neurons in rat nucleus principalis. J Neurophysiol. 2003;89:40–56. doi: 10.1152/jn.00272.2002. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969;32:452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- Pierret T, Lavallee P, Deschênes M. Parallel streams for the relay of vibrissal information through thalamic barreloids. J Neurosci. 2000;20:7455–7462. doi: 10.1523/JNEUROSCI.20-19-07455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto DJ, Hartings JA, Simons DJ. Cortical damping: analysis of thalamocortical response transformations in rodent barrel cortex. Cereb Cortex. 2003;13:33–44. doi: 10.1093/cercor/13.1.33. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Mishkin M. Serial and parallel processing of tactual information in somatosensory cortex of rhesus monkeys. J Neurophysiol. 1992;68:518–527. doi: 10.1152/jn.1992.68.2.518. [DOI] [PubMed] [Google Scholar]
- Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remple MS, Henry EC, Catania KC. Organization of somatosensory cortex in the laboratory rat (Rattus norvegicus): evidence for two lateral areas joined at the representation of the teeth. J Comp Neurol. 2003;467:105–118. doi: 10.1002/cne.10909. [DOI] [PubMed] [Google Scholar]
- Rice FL, Mance A, Munger BL. A comparative light microscopic analysis of the sensory innervation of the mystacial pad. I. Innervation of vibrissal follicle-sinus complexes. J Comp Neurol. 1986;252:154–174. doi: 10.1002/cne.902520203. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Neuronal integration in the somatosensory whisker/barrel cortex. In: Jones EG, Diamond IT, editors. Cerebral Cortex: The Barrel Cortex of Rodents. Vol. 11. New York: Plenum Press; 1995. pp. 263–297. [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in rat vibrissa/barrel system. J Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Stone J, Dreher B, Leventhal A. Hierarchical and parallel mechanisms in the organization of visual cortex. Brain Res Rev. 1979;1:345–394. doi: 10.1016/0165-0173(79)90010-9. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Efferent neurons and suspected interneurons in S-1 vibrissa cortex of the awake rabbit: receptive fields and axonal properties. J Neurophysiol. 1989;62:288–308. doi: 10.1152/jn.1989.62.1.288. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Efferent neurons and suspected interneurons in second somatosensory cortex of the awake rabbit: receptive fields and axonal properties. J Neurophysiol. 1991;66:1392–1409. doi: 10.1152/jn.1991.66.4.1392. [DOI] [PubMed] [Google Scholar]
- Timofeeva E, Lavallee P, Arsenault D, Deschênes M. The synthesis of multi-whisker receptive fields in subcortical stations of the vibrissa system. J Neurophysiol. 2004;91:1510–1515. doi: 10.1152/jn.01109.2003. [DOI] [PubMed] [Google Scholar]
- Turman AB, Ferrington DG, Ghosh S, Morley JW, Rowe MJ. Parallel processing of tactile information in the cerebral cortex of the cat— effect of reversible inactivation of SI on responsiveness of SII neurons. J Neurophysiol. 1992;67:411–429. doi: 10.1152/jn.1992.67.2.411. [DOI] [PubMed] [Google Scholar]
- Turman AB, Morley JW, Zhang HQ, Rowe MJ. Parallel processing of tactile information in cat cerebral cortex: effect of reversible inactivation of SII on SI responses. J Neurophysiol. 1995;73:1063–1075. doi: 10.1152/jn.1995.73.3.1063. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system—an integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- Veinante P, Jacquin MF, Deschênes M. Thalamic projections from the whisker-sensitive regions of the spinal trigeminal complex in the rat. J Comp Neurol. 2000;420:233–243. doi: 10.1002/(sici)1096-9861(20000501)420:2<233::aid-cne6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatopic organization of SmI cerebral neocortex in albino rat. Brain Res. 1971;26:259–275. [PubMed] [Google Scholar]
- Welker C, Sinha MM. Somatotopic organization of SmII neocortex in the albino rat. Brain Res. 1972;37:132–136. doi: 10.1016/0006-8993(72)90354-x. [DOI] [PubMed] [Google Scholar]
- Welker C, Woolsey TA. Structure of layer IV in the somatosensory neocortex of the rat: description and comparison with the mouse. J Comp Neurol. 1974;158:437–454. doi: 10.1002/cne.901580405. [DOI] [PubMed] [Google Scholar]
- Weyand TG, Swadlow HA. Thalamic inputs to visual areas 1 and 2 in the rabbit. J Comp Neurol. 1986;250:521–528. doi: 10.1002/cne.902500410. [DOI] [PubMed] [Google Scholar]
- White EL. Primary areas. In: Adelman G, editor. Encyclopedia of Neuroscience. Boston: Birkhäuser; 1987. p. 213. [Google Scholar]
- White EL, Keller A. An integrative view of cortical circuitry. In: White EL, editor. Cortical Circuits: Synaptic Organization of the Cerebral Cortex—Structure, Function and Theory. Boston: Birkhäuser; 1989. pp. 179–215. [Google Scholar]
- Wilent WB, Contreras D. Synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. J Neurosci. 2004;24:3985–3998. doi: 10.1523/JNEUROSCI.5782-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA. Somatosensory, auditory and visual cortical areas of the mouse. Johns Hopkins Med J. 1967;121:91–112. [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Zhang HQ, Murray GM, Turman AB, Mackie PD, Coleman GT, Rowe MJ. Parallel processing in cerebral cortex of the marmoset monkey: effect of reversible SI inactivation on tactile responses in SII. J Neurophysiol. 1996;76:3633–3655. doi: 10.1152/jn.1996.76.6.3633. [DOI] [PubMed] [Google Scholar]