Abstract
Background
The relationship of body mass index (BMI) with heart failure (HF) risk before and after kidney transplant is not well-described.
Methods
We examined United States Renal Data System records for 67,591 kidney transplant candidates (1995–2004) with Medicare insurance and BMI data at listing. HF diagnoses were ascertained from Medicare billing claims. BMI was categorized per World Health Organization criteria. We modeled time-dependent associations (adjusted hazard ratio, aHR) of transplant with HF risk after listing compared to waiting in each BMI group by multivariable, stratified Cox regression. The time-dependent exposure variables partitioned relative risk of HF following transplant versus waiting into early (≤90d) and late (>90d) posttransplant periods.
Results
The BMI distribution of listed candidates was: 3.7% under, 40.4% normal, 32.0% over, 16.2% obese, and 7.7% morbidly-obese weight. The prevalence of HF among patients awaiting transplant reached 57.4% by three years. Deceased donor transplant was associated with increased early HF risk compared to continued waiting – aHRs ranged from 2.23 for normal-BMI to 2.82 for morbidly obese patients. However, transplant reduced the risk of HF in the late posttransplant period, from 54% (aHR 0.46) in normal BMI to 32% (aHR 0.68) for morbidly obese patients. Relative benefits were largest for normal weight candidates who received live-donor transplants (aHR 0.31).
Conclusions
HF risk improves in obese patients in the long-term after kidney transplant, but not as much as for non-obese. There is need for close monitoring and for new strategies to reduce HF risk in obese patients before and after transplant.
Keywords: Body mass index, Heart failure, Kidney transplantation, Medicare, Registry
Introduction
Heart failure (HF) is common among patients with end-stage renal disease (ESRD). Concomitant dysfunction of the heart and kidneys results in part from shared risk factors, as well as from a “vicious cycle” of reciprocal causation 1. Overt HF portends a poor prognosis in dialysis-dependent patients 2, and even asymptomatic cardiac dysfunction in kidney transplant candidates bears an independent association with mortality 3. Kidney transplantation improves a number of factors predisposing to HF including volume overload, uremic toxicity and anemia. Although left ventricular systolic dysfunction was previously considered a contraindication to kidney transplantation, reversals of cardiac dysfunction after transplant have been documented 4–6.
Obesity, a risk factor for HF in the general population 7, predicts increased risks of mortality and perioperative complications in kidney transplant recipients including delayed graft function, elevated transplant costs, and allograft loss 8–10. Single-center and registry-based observational studies have reported higher frequencies of HF after kidney transplant in obese compared to non- obese recipients 11–14. However, the joint effects of BMI and transplantation upon HF after listing are not well-described despite the increasing prevalence of obese patients on the transplant waiting list. Therefore, to advance understanding of the risks of HF before and after kidney transplantation, we performed a retrospective study of a national cohort of recent renal allograft candidates recorded in the United States Renal Data System (USRDS). We aimed to describe the frequency of total and new-onset HF diagnoses after listing, and to estimate the time-dependent relative risk of HF associated with deceased or living donor kidney transplantation, compared to continuing waiting, in early and late post-transplant periods. The joint effects of candidate BMI with transplant-related HF risk were considered across BMI classifications.
Methods
Data Sources
We performed sample selection, outcomes ascertainment, and covariate determinations using registry data collected by the USRDS that incorporates information from the Organ Procurement and Transplantation Network (OPTN) and Medicare billing claims records. Details of the source USRDS data, as well as limitations of Medicare claims data, have been described previously 15
Participant Selection
The primary sample included adult (≥18 years-old) ESRD patients listed for kidney transplantation from January 1995 to December 2004 with Medicare Part A and B benefits and available BMI data at listing. We identified Medicare beneficiaries per the “Payer History” file of the USRDS. In secondary analyses we studied patients with one-year of continuous Medicare coverage before listing, with continuity defined to allow unlimited gaps up to three days (administrative gaps) and/or a single gap of four to sixty days, and no indication of pre-listing HF as defined below.
Definitions of Outcomes and Covariates
Heart failure
HF was defined by identification of qualifying Medicare bills with a HF diagnosis per the claims definition of the USRDS Annual Data Report (ADR) (International Classification of Diseases Code 9th Edition (ICD-9) codes 398.91, 422, 425, 428, 402.×1, 404.×1, 404.×3) 16. We defined claims-based HF diagnosis as ≥1 inpatient Part A institutional claim or ≥2 Part B physician/supplier claims. The date of the earliest claim was taken to define HF onset. We recently demonstrated this algorithm to have 92.5% sensitivity for detection of clinically diagnosed HF events in a validation study among kidney transplant recipients 17. Pre-listing HF diagnosis also included indications of HF on the United Network for Organ Sharing (UNOS) Candidate Registration Form and the Center for Medicare and Medicaid Studies (CMS) Form 2728, which queries select comorbidities at the time of ESRD reporting.
BMI at listing and other baseline patient traits
Candidate demographic and clinical information were obtained from the CMS Form 2728 and the OPTN Transplant Candidate Registration Form, including age, gender, race, Hispanic ethnicity, primary cause of ESRD, and date of first dialysis, and comorbidities (coronary disease or myocardial infarction, cerebrovascular disease, peripheral vascular disease, tobacco use and alcohol dependence). BMI (kg/m2) was computed from height and weight information reported at waitlist entry, and was categorized by World Health Organization criteria into five categories ranging from underweight to morbidly obese weight (Table 1).
Table 1.
Baseline characteristics of the full study cohort at listing for kidney transplantation.
| Baseline Characteristics | Full Sample of Candidates (N=67,591) | Underweight (BMI <18.5) (N=2,473) | Normal weight (BMI 18.5 to<25) (N=27,274) | Overweight (BMI 25 to <30) (N=21,678) | Obese (BMI 30 to <35) (N=10,970) | Morbidly Obese (BMI ≥35) (N=5,196) |
|---|---|---|---|---|---|---|
| n (%) | ||||||
| Age at listing (years) | § | |||||
| 18–30 | 8,549 (12.6) | 728 (29.4) | 4,500 (16.5) | 1,877 (8.7) | 903 (8.2) | 541 (10.4) |
| 31–45 | 19,845 (29.4) | 840 (34.0) | 8,818 (32.3) | 5,646 (26.0) | 2,864 (26.1) | 1,677 (32.3) |
| 46–60 | 25,187 (37.3) | 637 (25.8) | 9,038 (33.1) | 8,589 (39.6) | 4,708 (42.9) | 2,215 (42.6) |
| > 60 | 14,010 (20.7) | 268 (10.8) | 4,918 (18.0) | 5,566 (25.7) | 2,495 (22.7) | 763 (14.7) |
| Female gender | 27,543 (40.8) § | 1,506 (60.9) | 11,047 (40.5) | 7,454 (34.4) | 4,793 (43.7) | 2,743 (52.8) |
| Race | § | |||||
| Black | 23,076 (34.1) | 653 (26.4) | 8,497 (31.2) | 7,508 (34.6) | 4,177 (38.1) | 2,241 (43.1) |
| White | 39,416 (58.3) | 1,483 (60.0) | 16,237 (59.5) | 12,787 (59.0) | 6,183 (56.4) | 2,726 (52.5) |
| Other | 5,099 (7.5) | 337 (13.6) | 2,540 (9.3) | 1,383 (6.4) | 610 (5.6) | 229 (4.4) |
| Hispanic ethnicity | 11,427 (16.9) § | 362 (14.6) | 4,511 (16.5) | 4,047 (18.7) | 1,790 (16.3) | 717 (13.8) |
| Cause of ESRD | § | |||||
| Diabetes | 19,983 (29.6) | 328 (13.3) | 6,476 (23.7) | 7,037 (32.5) | 4,177 (38.1) | 1,965 (37.8) |
| Hypertension | 16,773 (24.8) | 469 (19.0) | 6,663 (24.4) | 5,717 (26.4) | 2,674 (24.4) | 1,250 (24.1) |
| Glomerulonephritis | 15,561 (23.0) | 737 (29.8) | 6,885 (25.2) | 4,589 (21.2) | 2,229 (20.3) | 1,121 (21.6) |
| Other | 15,274 (22.6) | 939 (38.0) | 7,250 (26.6) | 4,335 (20.0) | 1,890 (17.2) | 860 (16.6) |
| Duration of dialysis prior to listing | § | |||||
| Not on dialysis | 24,604 (34.6) | 756 (30.6) | 8,979 (32.9) | 8,042 (37.1) | 4,405 (40.2) | 2,422 (46.6) |
| 1–12 months | 15,004 (22.2) | 519 (21.0) | 6,197 (22.7) | 4,957 (22.9) | 2,372 (21.6) | 959 (18.5) |
| 13–24 months | 8,678 (12.8) | 235 (9.5) | 3,448 (12.6) | 2,902 (13.4) | 1,519 (13.9) | 574 (11.1) |
| ≥25 months | 18,892 (27.9) | 939 (38.0) | 8,451 (31.0) | 5,658 (26.1) | 2,626 (23.9) | 1,218 (23.4) |
| Unreported | 413 (0.6) | 24 (1.0) | 199 (0.7) | 119 (0.6) | 48 (0.4) | 23 (0.4) |
| Dialysis modality at listing | § | |||||
| Peritoneal | 5,548 (8.2) | 197 (8.0) | 2269 (8.3) | 1850 (8.5) | 908 (8.3) | 324 (6.2) |
| Hemodialysis, none or unreported | 62,043 (91.7) | 2,276 (92.0) | 25,005 (91.7) | , (91.5) | 10,062 (91.7) | 4,872 (93.8) |
| Comorbidities | ||||||
| Coronary disease or myocardial infarction | 3117 (4.6) § | 43 (1.7) | 983 (3.6) | 1,228 (5.7) | 673 (6.1) | 190 (3.7) |
| Cerebrovascular disease | 1,768 (2.6) ‡ | 55 (2.2) | 674 (2.5) | 637 (2.9) | 290 (2.6) | 112 (2.2) |
| Peripheral vascular disease | 2,770 (4.1) § | 62 (2.5) | 1029 (3.8) | 968 (4.5) | 509 (4.6) | 202 (3.9) |
| Tobacco use | 1,459 (2.2) § | 65 (2.6) | 701 (2.6) | 439 (2.0) | 180 (1.6) | 74 (1.4) |
| Alcohol dependence | 311 (0.5) ‡ | 11 (0.4) | 141 (0.5) | 111 (0.5) | 36 (0.3) | 12 (0.2) |
| Listing year | § | |||||
| 1995–1998 | 24,604 (36.4) | 1,057 (42.7) | 10,957 (40.2) | 7,660 (35.3) | 3,377 (30.8) | 1,553 (29.9) |
| 1999–2001 | 20,224 (29.9) | 767 (31.0) | 7,925 (29.1) | 6,522 (30.1) | 3,372 (30.7) | 1,638 (31.5) |
| 2002–2004 | 22,763 (33.7) | 649 (26.2) | 8,392 (30.8) | 7,496 (35.6) | 4,221 (38.5) | 2,005 (38.6) |
BMI, body mass index; ESRD, end-stage renal disease
Percentages reflect proportions within the full sample or BMI category with the indicated demographic or clinical traits (“column percentages”).
P-value for Chi-square test of differences in distributions of clinical characteristics according to BMI category:
0.002–0.01;
<0.001
Statistical Analysis
Baseline demographic and clinical traits of the study patients are described as counts and proportions. Continuous variables were categorized into clinically relevant strata. Missing categorical covariate data were grouped with the absence of a characteristic when such categories were relevant, or into a category distinct from the reference group.
At-risk time for all the time-to-event analyses was censored at end of Medicare enrollment, death not concurrent with HF diagnosis, or end of study (December 31, 2004). Time to first post-listing HF diagnosis in the full study sample was examined to describe the burden of clinically recognized HF in the target population (cumulative prevalence). Time to first post-listing HF diagnosis in a sample selected as free of indicated HF before listing in the registry was also examined as a representation of cumulative incidence. We estimated the observed, cumulative frequencies and 95% confidence intervals (CI) of HF after listing and after transplant by the product-limit (Kaplan-Meier) method. At-risk time for the Kaplan-Meier estimates while waiting was also censored at transplantation to constrain these frequency estimates to the period between listing and transplant. Waitlist entry was considered according the principle of “intention to treat”, and thus risk-time was not censored at waitlist removal for reasons other than transplant or death.
We employed multivariable, stratified Cox regression to estimate the time-dependent relative risk (adjusted hazard ratio, aHR) of HF diagnosis (total and new-onset) associated with deceased or living donor kidney transplantation compared to continuing waiting without transplant for each BMI group. Living donor renal allografts tend to function more promptly and have superior long-term graft survival compared to deceased donor transplants for reasons such as less pre-transplant ischemia. As an underlying hypothesis of this analysis was that restoration of kidney function may improve HF risk in ESRD patients in the long-term, living and deceased donor transplantations were considered as distinct exposure events. Kidney transplant time-dependent exposure variables partitioned relative risk associated with transplant versus waiting into early (≤90 days) and late (>90 days) post-transplant periods based on an a priori hypothesis that HF risk differs peri-operatively compared to late after transplant. Stratification by BMI group at the time of listing established patients within a given BMI category who remained without transplant as references, while allowing estimation of all time-dependent variables within a single model and thus facilitating direct comparison of effect estimates. Models were adjusted for baseline patient demographic and clinical traits at listing including age, gender, race, ethnicity, cause of ESRD, duration of dialysis prior to listing, dialysis modality, reported comorbidities and listing year. To assess consistency of effects across demographic groups, subgroup analyses limited by gender or race were performed.
Authorship and Funding
This work was approved by the Institutional Review Board of Saint Louis University. Dr. Lentine received support from a grant from the National Institute of Diabetes Digestive and Kidney Diseases (NIDDK), K08DK073036. Drs. Brennan and Schnitzler received support from a grant from the NIDDK, P30DK079333. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
BMI distribution and baseline characteristics according to BMI
There were 180,233 unique adults listed for kidney transplantation per USRDS records in the study period, of whom 89,297 had Medicare primary insurance at the time of listing. Of these Medicare-insured transplant candidates, 67,591 (75.7%) also had BMI data at listing and were selected for analysis. The BMI distribution of this sample of waitlisted subjects was: 3.7% underweight, 40.4% normal, 32.0% overweight, 16.2% obese, and 7.7% morbidly obese weight. Forty-one percent of the sampled patients were women and 34% were black race. The distribution of patient characteristics according to BMI at listing is shown in Table 1. Trait distributions varied significantly according to listing BMI, with overweight, obese and morbidly groups having higher prevalence of black race, ESRD due to diabetes, listing before dialysis initiation and listing in the most recent years of the study compared to normal weight candidates. Overweight and obese candidates were more likely to be aged >45 years and to have known coronary artery disease and peripheral vascular disease at listing. Morbidly obese and underweight candidates were more commonly female. Of 38,997 candidates with continuous Medicare coverage for at least one year before transplant, 21,120 (54%) did not have identified HF prior to listing. The BMI distribution of this sub-sample was similar to that of the full cohort (Table 2).
Table 2.
Baseline characteristics of the sample without heart failure before listing for kidney transplantation*.
| Baseline Characteristics | Candidates Without HF before Listing (N=21,120) | Underweight (BMI <18.5) (N=811) | Normal weight (BMI 18.5 to<25) (N=8,596) | Overweight (BMI 25 to <30) (N=6,714) | Obese (BMI 30 to <35) (N=3,393) | Morbidly Obese (BMI ≥35) (N=1,606) |
|---|---|---|---|---|---|---|
| n (%) | ||||||
| Age at listing (years) | § | |||||
| 18–30 | 2,973 (14.1) | 265 (32.7) | 1,526 (17.8) | 644 (9.6) | 315 (9.3) | 223 (13.9) |
| 31–45 | 6,884 (32.6) | 283 (34.9) | 3,031 (35.3) | 1,991 (29.7) | 1,033 (30.5) | 546 (34.0) |
| 46–60 | 7,744 (36.7) | 198 (24.4) | 2,804 (32.6) | 2,688 (40.0) | 1,381 (40.7) | 673 (41.9) |
| > 60 | 3,519 (16.7) | 65 (8.0) | 1,235 (14.4) | 1,391 (20.7) | 664 (19.6) | 164 (10.2) |
| Female gender | 8,827 (41.8) § | 512 (63.1) | 3,474 (40.4) | 2,378 (35.4) | 1,568 (46.2) | 895 (55.7) |
| Race | § | |||||
| Black | 7,648 (36.2) | 209 (25.8) | 2,792 (32.5) | 2,497 (37.2) | 1,411 (41.6) | 739 (46.0) |
| White | 11,873 (56.2) | 490 (60.4) | 5,006 (58.2) | 3,801 (56.6) | 1,780 (52.5) | 796 (49.6) |
| Other | 1,599 (7.6) | 112 (13.8) | 798 (9.3) | 416 (6.2) | 202 (6.0) | 71 (4.4) |
| Hispanic ethnicity | 3,776 (17.9) § | 130 (16.0) | 1,537 (17.9) | 1,336 (19.9) | 562 (16.6) | 211 (13.1) |
| Cause of ESRD | § | |||||
| Diabetes | 4,971 (23.5) | 99 (12.2) | 1,639 (19.1) | 1,751 (26.1) | 1,009 (29.7) | 474 (29.5) |
| Hypertension | 5,528 (26.2) | 150 (18.5) | 2,175 (25.3) | 1,858 (27.7) | 925 (27.3) | 420 (26.2) |
| Glomerulonephritis | 5,399 (25.6) | 257 (31.7) | 2,351 (27.4) | 1,630 (24.3) | 766 (22.6) | 395 (24.6) |
| Other | 5,221 (24.7) | 305 (37.6) | 2,431 (28.3) | 1,475 (22.0) | 693 (20.4) | 317 (19.7) |
| Duration of dialysis prior to listing | § | |||||
| Not on dialysis | 7,665 (36.3) | 249 (30.7) | 2,800 (32.6) | 2,495 (37.2) | 1,376 (40.6) | 745 (46.4) |
| 1–12 months | 590 (2.8) | 18 (2.2) | 265 (3.1) | 192 (2.9) | 89 (2.6) | 26 (1.6) |
| 13–24 months | 4,457 (21.1) | 119 (14.7) | 1,764 (20.5) | 1,503 (22.4) | 784 (23.1) | 287 (17.9) |
| ≥25 months | 8,238 (39.0) | 412 (50.8) | 3,682 (42.8) | 2,478 (36.9) | 1,130 (33.3) | 536 (33.4) |
| Unreported | 170 (0.8) | 13 (1.6) | 85 (1.0) | 46 (0.7) | 14 (0.4) | 12 (0.8) |
| Dialysis modality at listing | ||||||
| Peritoneal | 1,772 (8.4) | 77 (9.5) | 712 (8.3) | 586 (8.7) | 290 (8.6) | 107 (6.7) |
| Hemodialysis, none or unreported | 19,348 (91.2) | 734 (90.5) | 7,884 (91.7) | 6,128 (91.3) | 3103 (91.5) | 1,499 (93.3) |
| Comorbidities | ||||||
| Coronary disease or myocardial infarction | 425 (2.0) † | 4 (0.5) | 120 (1.4) | 177 (2.6) | 90 (2.7) | 34 (2.1) |
| Cerebrovascular disease | 467 (2.2) | 19 (2.3) | 197 (2.3) | 161 (2.4) | 62 (1.8) | 28 (1.7) |
| Peripheral vascular disease | 616 (2.9) ‡ | 12 (1.5) | 241 (2.8) | 238 (3.5) | 83 (2.5) | 42 (2.6) |
| Tobacco use | 391 (1.8) ‡ | 15 (1.9) | 187 (2.2) | 122 (1.8) | 53 (1.6) | 14 (0.9) |
| Alcohol dependence | 86 (0.4) ‡ | 1 (0.1) | 46 (0.5) | 31 (0.5) | 7 (0.2) | 1 (0.1) |
| Listing year | § | |||||
| 1995–1998 | 8,154 (38.6) | 389 (48.0) | 3,638 (42.3) | 2,489 (37.1) | 1,112 (32.8) | 526 (32.8) |
| 1999–2001 | 6,217 (29.4) | 226 (27.9) | 2,451 (28.5) | 2,053 (30.6) | 1,005 (29.6) | 482 (30.0) |
| 2002–2004 | 6,749 (32.0) | 196 (24.2) | 2,507 (29.2) | 2,172 (32.4) | 1,276 (37.6) | 598 (37.2) |
BMI, body mass index; ESRD, end-stage renal disease
Percentages reflect proportions within the full sample or BMI category with the indicated demographic or clinical traits (“column percentages”).
Restricted to patients with one-year of continuous Medicare coverage before listing and no indication of pre-listing HF.
P-value for Chi-square test of differences in distributions of clinical characteristics according to BMI category:
0.02–0.05;
0.002–0.01;
<0.001
Fifty four percent (36,433) of the full cohort were transplanted during the observation period, after a median waiting period of 411 days in those who received an allograft. BMI at transplant could be calculated for 78.4% (28,550) of these subjects. Among transplant recipients with complete BMI data at transplant and listing, the median change in BMI from listing to transplant was 0 kg/m2 (interquartile range: −1.26 to 0.45 kg/m2). Because not all candidates were transplanted and because HF risk was considered from the candidate’s perspective, BMI at listing was used to classify patients in the analytic models.
Cumulative frequencies of HF on the waitlist and after transplant
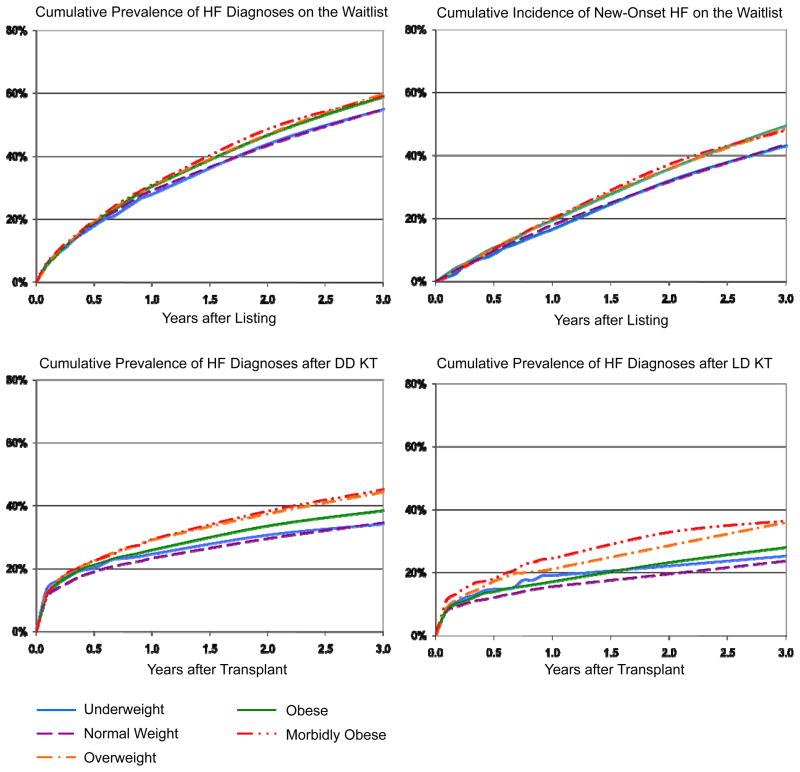
HF was common after waitlist entry. By three years, the cumulative prevalence of HF diagnoses on the waitlist was 57.4% (95% CI 56.8–57.9%). Among candidates without a diagnosis of HF prior to listing, the incidence of new-onset HF was 46.1% (95% CI 45.2–47.0%) at three years. The crude frequencies of total and new-onset HF diagnoses varied significantly by baseline BMI strata (P<0.0001 by Log-Rank test) (Figure 1). Specifically, among obese or morbidly obese candidates the cumulative prevalence of HF at three years after listing was 59.6% (95% CI 58.5–60.6%) compared to 55.0% (95% CI 54.2–55.8%) in normal weight candidates. In obese or morbidly obese candidates the cumulative incidence of HF at three years after listing was 48.4% (95% CI 46.5–50.3%) compared to 43.2% (95% CI 41.8–44.7%) in normal weight candidates.
Figure 1. Frequency of heart failure diagnoses after kidney transplant listing and after transplantation, according to listing BMI category.
DD, deceased donor; HF, heart failure; LD, living donor; KT, kidney transplant
Among patients transplanted during observation, 36.1% (95% CI 35.5–36.6%) acquired HF diagnoses by three years after transplant. The cumulative frequency of HF rose most sharply early after transplant and then achieved a more gradual slope (Figure 1). The prevalence of HF at three years after transplant was lower among recipients of living compared to deceased donor allografts, 28.2% (95% CI 27.0–29.5%) vs. 37.8% (95% CI 37.2–38.4%). Post-transplant HF was more common among transplant recipients with higher BMI at listing (Figure 1). Estimates of HF prevalence according to BMI at transplant in the subgroup with these data available were very similar (point estimates at three years within 0 to 1.5%).
Relative risk of HF within 90 days of transplant compared to waiting
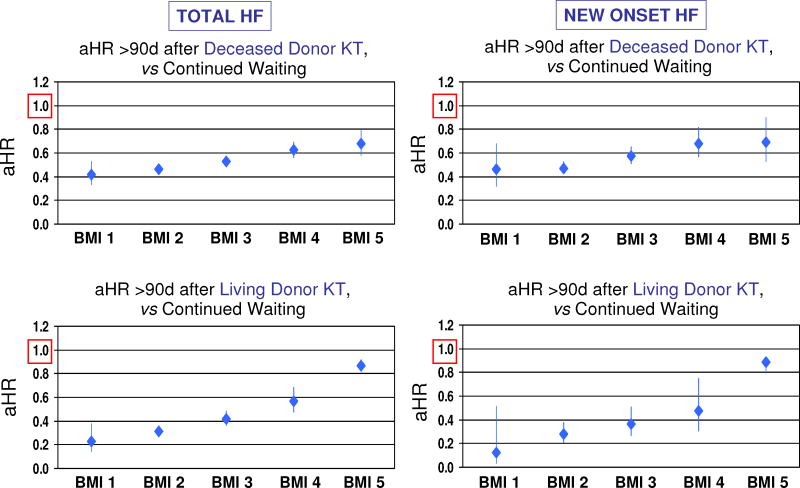
Associations of kidney transplantation with relative risk of HF compared to waiting were examined by time-dependent regression. Figure 2 displays the time-dependent relative risks of HF associated with transplant within the first 90-day period post-transplant, compared to continued experience without transplant, including the joint effect of candidate BMI. Deceased-donor kidney transplant was associated with increased early (≤90 days) HF risk compared to waiting without transplant: aHRs ranged from approximately 2.23 (95% CI 2.08–2.39) for normal weight candidates (BMI group 2) to an aHR of 2.82 (95% CI 2.41–3.30) for morbidly obese patients (BMI group 5). Living-donor kidney transplant was also associated with increased early HF risk. Notably, normal weight candidates who received living-donor allografts faced a modest 20% relative risk increase (aHR 1.21, 95% CI 1.02–1.44) in the early post- transplant period, but early relative risk was more than two times that of waiting (aHR 2.36 95% CI 1.74–3.21) for morbidly obese patients receiving a living donor transplant. Patterns were similar for the relative risk of new-onset HF early after kidney transplant, with both deceased and living donor transplantation predicting increased risk, but suggestion of lowest relative risk in normal weight patients transplanted with living-donor grafts.
Figure 2. Early increases in heart failure risk after kidney transplant comparing to waiting, by candidate BMI and donor type. *.
* Transplant modeled as a time-dependent predictor of HF after listing in stratified multivariable regression. Models were adjusted for baseline patient traits and comorbidities as listed in Tables 1 and 2. Reference groups are patients in a given BMI category who remain without transplant. BMI group by WHO: 1-Underweight (<18.5); 2-Normal weight (18.5 to <25); 3-Overweight (25 to <30); 4-Obese (30 to <35); 5-Morbidly obese (≥35).
Relative risk of HF late after transplant compared to waiting
In contrast, there was significant reduction in HF risk late (>90 days) after transplant compared to waiting (Figure 3). For deceased donor transplant, late risk reduction varied from 54% (aHR 0.46, 95% CI 0.43–0.96) in normal weight candidates to 32% (aHR 0.68, 95% CI 0.58–0.79) in morbidly obese patients. Relative benefits were largest for normal weight candidates receiving living donor transplants, who achieved up to 69% relative risk reduction (aHR 0.31, 95% CI 0.27–0.36). Patterns were similar for the relative risk of new-onset HF late after transplant, with both deceased and living donor transplants predicting benefit across candidate BMI strata, but appearance of a graded decline the long-term protective effect of transplantation in patients at higher BMI levels.
Figure 3. Late reductions in heart failure risk after kidney transplant comparing to waiting, by candidate BMI and donor type.
* Transplant modeled as a time-dependent predictor of HF after listing in stratified multivariable regression. Models were adjusted for baseline patient traits and comorbidities as listed in Tables 1 and 2. Reference groups are patients in a given BMI category who remain without transplant. BMI group by WHO: 1-Underweight (<18.5); 2-Normal weight (18.5 to <25); 3-Overweight (25 to <30); 4-Obese (30 to <35); 5-Morbidly obese (≥35).
Subgroup analyses
Associations of transplant with early and late HF risk compared to waiting were similar in men compared to women, and in persons of black, white and other races, albeit with some loss of statistical precision (wider confidence intervals) due to smaller sample sizes (Table 3).
Table 3.
Associations of kidney transplantation with heart failure risk compared to waiting within gender and race-stratified sub-groups.*
| Heart failure after listing in the full cohort according to candidate gender | |||||
|---|---|---|---|---|---|
| aHR ≤90d after KT vs Waiting | aHR >90d after KT vs Waiting | ||||
| Men (N=40,048) | Women (N=27,543) | Men (N=40,048) | Women (N=27,543) | ||
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | ||
| Deceased Donor KT | |||||
| Underweight | 3.56 (2.62–4.84)§ | 2.35 (1.74–3.16)§ | 0.56 (0.41–0.75)‡ | 0.40 (0.30–0.52)§ | |
| Normal weight | 2.27 (2.08–2.47)§ | 2.27 (2.04–2.52)§ | 0.43 (0.39–0.46)§ | 0.51 (0.46–0.56)§ | |
| Overweight | 2.41 (2.21–2.63)§ | 2.63 (2.34–2.97)§ | 0.50 (0.46–0.55)§ | 0.60 (0.54–0.67)§ | |
| Obese | 2.58 (2.27–2.94)§ | 2.74 (2.37–3.17)§ | 0.61 (0.54–0.69)§ | 0.61 (0.54–0.70)§ | |
| Morbidly Obese | 3.09 (2.50–3.81)§ | 2.67 (2.15–3.31)§ | 0.69 (0.57–0.84)‡ | 0.71 (0.59–0.85)‡ | |
| Living Donor KT | |||||
| Underweight | 0.96(0.31–2.97) | 2.78 (1.75–4.41)§ | 0.23 (0.09–0.54)‡ | 0.25 (0.14–0.44)§ | |
| Normal weight | 1.05 (0.82–1.34) | 1.49 (1.17–1.89)‡ | 0.28 (0.23–0.34)§ | 0.36 (0.29–0.44)§ | |
| Overweight | 1.33 (1.05–1.67)† | 1.63 (1.22–2.16)‡ | 0.36 (0.30–0.44)§ | 0.52 (0.42–0.65)§ | |
| Obese | 1.52 (1.10–2.10)† | 2.08 (1.48–2.91)§ | 0.59 (0.47–0.74)§ | 0.54 (0.40–0.73)§ | |
| Morbidly Obese | 3.00 (1.93–4.65)§ | 1.99 (1.31–3.02)‡ | 0.86 (0.78–0.96)‡ | 0.88 (0.81–0.94)‡ | |
| Total HF after listing according to candidate gender | ||||
|---|---|---|---|---|
| aHR ≤90d after KT vs Waiting | aHR >90d after KT vs Waiting | |||
| Men (N=12,293) | Women Men (N=8,827) | (N=12,293) | Women (N=8,827) | |
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Deceased Donor KT | ||||
| Underweight | 3.50 (1.88–6.53)§ | 2.81 (1.69–4.67)§ | 0.57 (0.35–0.93)† | 0.44 (0.29–0.69)‡ |
| Normal weight | 2.48 (2.11–2.90)§ | 2.74 (2.26–3.32)§ | 0.41 (0.35–0.47)§ | 0.57 (0.48–0.67)§ |
| Overweight | 2.76 (2.34–3.26)§ | 3.23 (2.59–4.02)§ | 0.56 (0.49–0.65)§ | 0.64 (0.53–0.77)§ |
| Obese | 3.24 (2.56–4.12)§ | 3.74 (2.91–4.79)§ | 0.66 (0.54–0.81)§ | 0.65 (0.52–0.82)‡ |
| Morbidly Obese | 2.71 (1.70–4.31)§ | 2.75 (1.82–4.16)§ | 0.61 (0.42–0.89)‡ | 0.76 (0.56–1.03) |
| Living Donor KT | ||||
| Underweight | 2.76 (0.39–19.60) | 5.48 (2.60–11.56)§ | --- | 0.18 (0.04–0.71)† |
| Normal weight | 1.09 (0.63–1.89) | 1.97 (1.25–3.11)‡ | 0.26 (0.18–0.40)§ | 0.29 (0.19–0.44)§ |
| Overweight | 1.52 (0.88–2.63) | 1.67 (0.87–3.22) | 0.28 (0.18–0.45)§ | 0.54 (0.34–0.83)‡ |
| Obese | 1.32 (0.55–3.17) | 2.15 (0.96–4.79) | 0.68 (0.42–1.10) | 0.16 (0.05–0.50)‡ |
| Morbidly Obese | 0.80 (0.11–5.66) | 3.10 (1.29–7.47)† | 0.90 (0.76–1.08) | 0.90 (0.78–1.05) |
| Total HF after listing according to candidate race | ||||||
|---|---|---|---|---|---|---|
| aHR ≤90d after KT vs Waiting | aHR >90d after KT vs Waiting | |||||
| White (N=39,416) | Black (N=23,076) | Other (N=5,099) | White (N=39,416) | Black (N=23,076) | Other (N=5,099) | |
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Deceased Donor KT | ||||||
| Underweight | 2.89 (2.24–3.72)§ | 2.82 (1.79–4.42)§ | 1.95 (0.81–4.71) | 0.39 (0.30–0.50)§ | 0.74 (0.52–1.05) | 0.33 (0.14–0.80)† |
| Normal weight | 2.22 (2.04–2.41)§ | 2.28 (2.01–2.58)§ | 2.71 (2.12–3.47)§ | 0.44 (0.40–0.47)§ | 0.50 (0.45–0.56)§ | 0.47 (0.36–0.61)§ |
| Overweight | 2.33 (2.13–2.55)§ | 2.77 (2.45–3.13)§ | 3.00 (2.17–4.16)§ | 0.50 (0.46–0.55)§ | 0.61 (0.54–0.68)§ | 0.55 (0.39–0.77)‡ |
| Obese | 2.58 (2.28–2.91)§ | 2.79 (2.37–3.29)§ | 2.72 (1.60–4.64)‡ | 0.55 (0.49–0.62)§ | 0.75 (0.65–0.87)‡ | 0.45 (0.25–0.79)‡ |
| Morbidly Obese | 2.89 (2.39–3.49)§ | 2.93 (2.28–3.76)§ | 1.50 (0.37–6.02) | 0.70 (0.59–0.83)§ | 0.75 (0.60–0.93)‡ | --- |
| Living Donor KT | ||||||
| Underweight | 2.37 (1.45–3.87)‡ | 2.33 (0.87–6.21) | 0.94 (0.13–6.67) | 0.19 (0.10–0.37)§ | 0.49 (0.22–1.09) | 0.13 (0.02–0.94)† |
| Normal weight | 1.24 (1.02–1.52)† | 1.24 (0.84–1.82) | 1.26 (0.63–2.53) | 0.31 (0.27–0.37)§ | 0.37 (0.27–0.49)§ | 0.11 (0.04–0.29)§ |
| Overweight | 1.31 (1.05–1.63)† | 1.81 (1.28–2.56)‡ | 1.62 (0.72–3.62) | 0.38 (0.32–0.45)§ | 0.53 (0.40–0.69)§ | 0.47 (0.25–0.88)† |
| Obese | 1.74 (1.32–2.30)§ | 1.73 (1.07–2.78)† | 1.94 (0.62–6.06) | 0.57 (0.46–0.71)§ | 0.55 (0.38–0.80)‡ | 0.64 (0.24–1.72) |
| Morbidly Obese | 2.47 (1.73–3.51)§ | 2.38 (1.32–4.31)‡ | --- | 0.88 (0.82–0.94)‡ | 0.87 (0.78–0.97)† | --- |
| New-onset heart failure after listing according to candidate race (limited to candidates without HF before listing) | ||||||
|---|---|---|---|---|---|---|
| aHR ≤90d after KT vs Waiting | aHR >90d after KT vs Waiting | |||||
| White (N=11,873) | Black (N=7,648) | Other (N=1,599) | White (N=11,873) | Black (N=7,648) | Other (N=1,599) | |
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Deceased Donor | ||||||
| KT | ||||||
| Underweight | 3.10 (1.95–4.93)§ | 2.81 (1.17–6.78)† | 3.02 (0.74–12.28) | 0.40 (0.26–0.61)§ | 0.88 (0.50–1.56) | 0.37 (0.09–1.52) |
| Normal weight | 2.59 (2.22–3.02)§ | 2.43 (1.93–3.05)§ | 3.04 (1.96–4.72)§ | 0.45 (0.39–0.51)§ | 0.50 (0.41–0.60)§ | 0.49 (0.32–0.76)‡ |
| Overweight | 2.58 (2.16–3.08)§ | 3.49 (2.82–4.31)§ | 3.61 (2.00–6.54)§ | 0.55 (0.48–0.64)§ | 0.67 (0.55–0.81)§ | 0.47 (0.24–0.91)† |
| Obese | 3.95 (3.19–4.89)§ | 2.78 (2.04–3.78)§ | 3.13 (1.27–7.69) | † 0.56 (0.45–0.69)§ | 0.86 (0.68–1.08) | 0.38 (0.14–1.05) |
| Morbidly Obese | 2.72 (1.82–4.08)§ | 2.77 (1.69–4.54)§ | 3.04 (0.42–22.23) | 0.69 (0.51–0.93)† | 0.74 (0.51–1.05) | --- |
| Living Donor KT | ||||||
| Underweight | 6.43 (3.20–12.90)§ | --- | --- | 0.18 (0.05–0.74)† | --- | --- |
| Normal weight | 1.32 (0.86–2.03) | 1.91 (0.95–3.83) | 2.08 (0.66–6.53) | 0.25 (0.18–0.36)§ | 0.37 (0.21–0.66) ‡ | 0.23 (0.06–0.95)† |
| Overweight | 1.21 (0.67–2.18) | 2.80 (1.54–5.07)‡ | --- | 0.31 (0.20–0.47)§ | 0.64 (0.40–1.04) | --- |
| Obese | 1.84 (0.92–3.70) | 1.58 (0.51–4.90) | --- | 0.46 (0.27–0.79)‡ | 0.47 (0.21–1.04) | 0.45 (0.06–3.20) |
| Morbidly Obese | 1.80 (0.67–4.79) | 3.11 (0.78–12.45) | --- | 0.92 (0.82–1.04) | 0.78 (0.53–1.15) | --- |
Transplant modeled as a time-dependent predictor of HF after listing in multivariable regression within indicated gender and racial groups, adjusted for baseline patient traits and comorbidities as listed in Tables 1 and 2. Gender was excluded as a covariate in the gender-stratified analyses; race was excluded as a covariate in the race-stratified analyses. Reference groups for the aHR are patients in a given BMI category who remain without transplant. BMI group by WHO: 1-Underweight (<18.5); 2-Normal weight (18.5 to <25); 3-Overweight (25 to <30); 4-Obese (30 to <35); 5-Morbidly obese (≥35).
P-value for statistical significance compared to reference: 0.02–0.05;
0.002–0.01;
<0.001
“--- ” Not evaluable due to insufficient events within this category.
Discussion
Heart failure is a common but serious complication in patients with renal failure. We examined the joint effects of kidney transplantation and patient BMI on acquisition of HF diagnoses among a large national sample of transplant candidates, and observed several main findings. 1) HF is common among ESRD patients awaiting transplantation, with incidence and prevalence rising progressively with waiting time. 2) Kidney transplantation is associated with a brief early rise in HF risk compared to experience without transplant. 3) Both deceased and living donor kidney transplants are associated with significant long-term reductions in HF risk. This benefit appears more pronounced for recipients (especially normal weight) of living donor allografts. 4) There is variation in the apparent benefit of kidney transplantation for HF risk according to candidate BMI –while obese patients experience benefit in long-term HF risk with transplant compared to waiting, their benefit is less than for non-obese patients.
Our finding that HF is a progressively frequent diagnosis among ESRD patients awaiting kidney transplantation is sobering but not unexpected. Prior studies have demonstrated that chronic kidney disease is an independent risk factor for HF and that a significant proportion of patients with ESRD have left ventriculuar hypertrophy (LVH) and/or reduced left ventricular (LV) systolic function 18–20. Even among chronic kidney disease patients with normal LV systolic function, there is a high prevalence of impaired myocardial performance as seen using modern tissue Doppler techniques 21. Indeed, authors have recognized the pathobiologic interplay of cardiac and kidney dysfunction in the increasingly used term “cardiorenal syndrome” 1.
Given the baseline high degree of cardiac structural abnormalities in patients with ESRD, the increase in HF in the early posttransplantation period (<90 days) seen in our study may be explained by several mediating factors. These include perioperative stresses, fluid overload and delayed or impaired graft function. The predictors of early HF following kidney transplant have not been well studied. In a recent study of 132 kidney transplant recipients, early HF was common and was associated with post-transplant anemia, allograft dysfunction, duration of dialysis prior to transplant, and degree of pretransplant LVH 22. Of note, in our study sample the relative rise in HF risk early after transplant was smaller with living compared to deceased donor transplantation across BMI strata, possibly reflecting superior early graft function in this cohort. It is also possible that the increase in early HF is affected by ascertainment bias, in that patients receive close follow-up early after transplant, compared to more intermittent clinical assessments of patients on the waitlist.
Importantly, however, transplant was associated with significantly decreased risk for late HF as compared to ongoing waiting in our analysis. There are several likely explanations for this observation. First, it has been demonstrated that dialysis imparts specific hemodynamic stressors that may predispose patients to adverse cardiac remodeling and subsequent HF. In a recent study utilizing implantable hemodynamic monitoring, patients undergoing hemodialysis three times weekly were demonstrated to have significant increases in right ventricular and pulmonary artery pressures between dialysis sessions 23. Similarly, LV mass, LV myocardial interstitial edema and LV intraventricular synchrony have been shown to adversely fluctuate between hemodialysis sessions, all potentially leading to negative cardiac remodeling and subsequent heart failure 24, 25. The degree of overt fluid retention and weight gain between hemodialysis sessions is an independent risk factor for all-cause cardiovascular death 26. A recent study demonstrated that dialysis patients have significant alterations in systolic and diastolic function by tissue Doppler even if LV ejection fraction is normal 27. It is therefore plausible that kidney transplantation may significantly lower HF risk by restoring renal function, interrupting the ongoing stressors listed above related to dialysis, as well as possibly improving other mediators of HF such as anemia and inflammatory markers.
Our findings of HF risk reduction after transplant resonate with serial echocardiographic observations of average increases in ejection fraction and regression of left ventricular hypertrophy following kidney transplantation 5, 6, as well as retrospective data demonstrating reduced HF incidence in diabetic patients following transplant 28. Pilot studies have suggested that regression of ventricular hypertrophy requires adequate allograft function 4, which is congruent with our finding of larger long-term, relative HF risk reductions with living compared to deceased donor grafts. Others have demonstrated that LVH regression is also significantly dependent on degree of posttransplant blood pressure control 29–31.
Another key observation of the current study was variation in the apparent benefit of kidney transplant for HF risk according to candidate BMI. We found that obese patients, who composed nearly 25% of our study population, had higher risk of HF after listing than normal weight candidates. While obese patients did acquire benefit in long-term HF risk with transplant compared to waiting, their benefit was less than for non-obese patients. In the Framingham Heart Study, obesity was shown to be a significant independent risk factor for the development of HF as well as overall cardiovascular mortality 7, 32. Similarly, increased BMI and abdominal adiposity were recently shown to be strong independent risk factors for HF hospitalizations and HF mortality in a middle-aged to older adult population 33. However, the implications of obesity in ESRD are complex and controversial. High BMI is associated with a mortality benefit in dialysis patients 34–37 and a large, registry-based study found no apparent survival benefit with weight loss among transplant candidates 37. These relationship are confounded by underlying comorbidities and malnutrition that both reduce BMI and increase death risk 38.
Several prior studies including clinical record and registry-based data have shown higher risk of HF in obese compared to non-obese kidney transplant candidates. Additionally, retrospective studies have suggested that BMI at time of transplant and posttransplant weight gains are associated with acquisition of cardiovascular risk factors and decreased patient and graft survival 9, 39. Recently, in a retrospective study of 1102 patients who underwent kidney transplant between 1991 and 2004 a one center, we found that elevated BMI at transplant predicted significantly increased risk of posttransplant HF and atrial fibrillation 11. The current study expands on this knowledge by examining a large, national cohort of ESRD patients with analyses performed to compare HF risk before and following transplant. Our findings indicate that obesity at listing modifies the impact of transplantation on HF by increasing the early risk to some degree and, most notably, attenuating the long-term risk reduction associated with transplantation.
Limitations of this study include the retrospective design. We were unable to objectively confirm or grade severity of HF diagnoses based on the use of Medicare claims as binary outcome measures, and the ICD9 coding scheme does not distinguish uremia as a cause of HF. Notably, the claims-based algorithm employed was recently shown to have very good sensitivity for detection of clinically diagnosed HF events in a validation study among a similar population 17, but misclassification of other conditions such as primary fluid retention with HF diagnosis codes cannot be excluded. Claims data for beneficiaries of insurance systems other than Medicare are not captured in the USRDS and our results may not generalize to privately insured patients. However, as Medicare is the single largest payer for end-stage renal disease services in the United States due to disease-specific entitlement, sampling of Medicare beneficiaries captures a large and relevant portion of the target population. The USRDS registry lacks quantitative information on clinical parameters such as blood pressure, cardiac ejection fractions, laboratory values such as hemoglobin, and the use of cardiovascular medications. Because follow-up in the registry is not protocol based, there is potential for ascertainment bias. In particular, early monitoring in the peri-transplant period may account for some of the increase in HF diagnoses early after transplant compared to waiting.
In conclusion, in this large retrospective study of Medicare-insured kidney transplant candidates, we found that the incidence and prevalence of HF increases during the waiting period for an allograft. Kidney transplantation is associated with a brief rise in HF risk early following transplant compared to risk in patients who remained on the waiting list. However, both deceased and living donor transplants are associated with significant long-term reductions in HF risk. There is variation in the benefit of transplantation on HF risk according to candidate BMI – while HF risk is reduced in obese patients in the long-term after kidney transplant, the benefit is not as much as for non-obese patients. There is need for close monitoring and for new strategies to reduce HF risk in obese ESRD patients before and after transplant.
Acknowledgments
The data reported here have been supplied by the United States Renal Data System. Dr. Lentine received support from a grant from the National Institute of Diabetes Digestive and Kidney Diseases (NIDDK), K08DK073036. Dr. Brennan received support from a grant from the NIDDK, P30DK079333. Dr. Lentine received a “Top Ten Abstract Award” for presentation of portions of this work at the 9th Annual State of the Art Winter Symposium of the American Society of Transplant Surgeons; January 17, 2009, Marco Island, FL. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government, the NIDDK or the National Institutes of Health.
Funding Sources: Dr. Lentine received support from a grant from the National Institute of Diabetes Digestive and Kidney Diseases (NIDDK), K08DK073036. Drs. Brennan and Schnitzler received support from a grant from the NIDDK, P30DK079333.
Footnotes
Institution at which work was performed: Saint Louis University Center for Outcomes Research, St. Louis, MO
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527–39. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47(3):884–90. doi: 10.1038/ki.1995.132. [DOI] [PubMed] [Google Scholar]
- 3.de Mattos AM, Siedlecki A, Gaston RS, Perry GJ, Julian BA, Kew CE, 2nd, et al. Systolic dysfunction portends increased mortality among those waiting for renal transplant. J Am Soc Nephrol. 2008;19(6):1191–6. doi: 10.1681/ASN.2007040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira SR, Moises VA, Tavares A, Pacheco-Silva A. Cardiovascular effects of successful renal transplantation: a 1-year sequential study of left ventricular morphology and function, and 24-hour blood pressure profile. Transplantation. 2002;74(11):1580–7. doi: 10.1097/00007890-200212150-00016. [DOI] [PubMed] [Google Scholar]
- 5.Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. [see comment] Journal of the American College of Cardiology. 2005;45(7):1051–60. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 6.Stokkel M, Duchateau CS, Jukema W, de Fijter HW. Noninvasive assessment of left ventricular function prior to and 6 months after renal transplantation. Transplant Proc. 2007;39(10):3159–62. doi: 10.1016/j.transproceed.2007.06.083. [DOI] [PubMed] [Google Scholar]
- 7.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 8.Johnson DW, Isbel NM, Brown AM, Kay TD, Franzen K, Hawley CM, et al. The effect of obesity on renal transplant outcomes. Transplantation. 2002;74(5):675–81. doi: 10.1097/00007890-200209150-00015. [DOI] [PubMed] [Google Scholar]
- 9.Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. [see comment] Transplantation. 2002;73(1):70–4. doi: 10.1097/00007890-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Gore JL, Pham PT, Danovitch GM, Wilkinson AH, Rosenthal JT, Lipshutz GS, et al. Obesity and outcome following renal transplantation. Am J Transplant. 2006;6(2):357–63. doi: 10.1111/j.1600-6143.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- 11.Lentine KL, Rocca-Rey LA, Bacchi G, Wasi N, Schmitz L, Salvalaggio PR, et al. Obesity and cardiac risk after kidney transplantation: experience at one center and comprehensive literature review. Transplantation. 2008;86(2):303–12. doi: 10.1097/TP.0b013e31817ef0f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott KC, Hypolite IO, Hshieh P, Cruess D, Taylor AJ, Agodoa LY. Hospitalized congestive heart failure after renal transplantation in the United States. Annals of Epidemiology. 2002;12(2):115–22. doi: 10.1016/s1047-2797(01)00272-1. [DOI] [PubMed] [Google Scholar]
- 13.Abbott KC, Yuan CM, Taylor AJ, Cruess DF, Agodoa LY. Early renal insufficiency and hospitalized heart disease after renal transplantation in the era of modern immunosuppression. Journal of the American Society of Nephrology. 2003;14(9):2358–65. doi: 10.1097/01.asn.0000083008.25305.67. [DOI] [PubMed] [Google Scholar]
- 14.Lentine KL, Schnitzler MA, Abbott KC, Li L, Burroughs TE, Irish W, et al. De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. American Journal of Kidney Diseases. 2005;46(4):720–33. doi: 10.1053/j.ajkd.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Researcher’s Guide to the United States Renal Data System Database. 2007 http://www.usrds.org/research.htm.
- 16.U.S. Renal Data System: USRDS. 2008 Annual Data Report. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Access Date March 1, 2009]. p. 33. http://www.usrds.org/2008/rg/A_intro_sec_1_9.pdf. [Google Scholar]
- 17.Lentine KL, Schnitzler MA, Abbott KC, Bramesfeld K, Buchanan PM, Brennan DC. Sensitivity of billing claims for cardiovascular disease events among kidney transplant recipients. Clin J Am Soc Nephrol. 2009;4(7):1213–21. doi: 10.2215/CJN.00670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47(1):186–92. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 19.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 20.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996;27(3):347–54. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 21.Edwards NC, Hirth A, Ferro CJ, Townend JN, Steeds RP. Subclinical abnormalities of left ventricular myocardial deformation in early-stage chronic kidney disease: the precursor of uremic cardiomyopathy? J Am Soc Echocardiogr. 2008;21(12):1293–8. doi: 10.1016/j.echo.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Borrows R, Loucaidou M, Chusney G, Borrows S, Tromp JV, Cairns T, et al. Anaemia and congestive heart failure early post-renal transplantation. Nephrol Dial Transplant. 2008;23(5):1728–34. doi: 10.1093/ndt/gfm815. [DOI] [PubMed] [Google Scholar]
- 23.Kjellstrom B, Braunschweig F, Lofberg E, Fux T, Grandjean PA, Linde C. Changes in right ventricular pressures between hemodialysis sessions recorded by an implantable hemodynamic monitor. Am J Cardiol. 2009;103(1):119–23. doi: 10.1016/j.amjcard.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 24.Fatema K, Hirono O, Takeishi Y, Nitobe J, Kaneko K, Ito M, et al. Hemodialysis improves myocardial interstitial edema and left ventricular diastolic function in patients with end-stage renal disease: noninvasive assessment by ultrasonic tissue characterization. Heart Vessels. 2002;16(6):227–31. doi: 10.1007/s003800200029. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi SY, Seeberger A, Lind B, Nowak J, do Nascimento MM, Lindholm B, et al. A single session of haemodialysis improves left ventricular synchronicity in patients with end-stage renal disease: a pilot tissue synchronization imaging study. Nephrol Dial Transplant. 2008;23(11):3622–8. doi: 10.1093/ndt/gfn311. [DOI] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119(5):671–9. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulel O, Soylu K, Yuksel S, Karaoglanoglu M, Cengiz K, Dilek M, et al. Evidence of left ventricular systolic and diastolic dysfunction by color tissue Doppler imaging despite normal ejection fraction in patients on chronic hemodialysis program. Echocardiography. 2008;25(6):569–74. doi: 10.1111/j.1540-8175.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 28.Abbott KC, Hypolite IO, Hshieh P, Cruess D, Agodoa LY, Welch PG, et al. The impact of renal transplantation on the incidence of congestive heart failure in patients with end-stage renal disease due to diabetes. J Nephrol. 2001;14(5):369–76. [PubMed] [Google Scholar]
- 29.Patel RK, Mark PB, Johnston N, McGregor E, Dargie HJ, Jardine AG. Renal transplantation is not associated with regression of left ventricular hypertrophy: a magnetic resonance study. Clin J Am Soc Nephrol. 2008;3(6):1807–11. doi: 10.2215/CJN.01400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peteiro J, Alvarez N, Calvino R, Penas M, Ribera F, Castro Beiras A. Changes in left ventricular mass and filling after renal transplantation are related to changes in blood pressure: an echocardiographic and pulsed Doppler study. Cardiology. 1994;85(5):273–83. doi: 10.1159/000176695. [DOI] [PubMed] [Google Scholar]
- 31.Becker-Cohen R, Nir A, Ben-Shalom E, Rinat C, Feinstein S, Farber B, et al. Improved left ventricular mass index in children after renal transplantation. Pediatr Nephrol. 2008;23(9):1545–50. doi: 10.1007/s00467-008-0855-5. [DOI] [PubMed] [Google Scholar]
- 32.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 33.Levitan EB, Yang AZ, Wolk A, Mittleman MA. Adiposity and Incidence of Heart Failure Hospitalization and Mortality: A Population-based Prospective Study. Circ Heart Fail. 2009 doi: 10.1161/CIRCHEARTFAILURE.108.794099. Published online before print April 7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63(3):793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 35.Glanton CW, Hypolite IO, Hshieh PB, Agodoa LY, Yuan CM, Abbott KC. Factors associated with improved short term survival in obese end stage renal disease patients. Ann Epidemiol. 2003;13(2):136–43. doi: 10.1016/s1047-2797(02)00251-x. [DOI] [PubMed] [Google Scholar]
- 36.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80(2):324–32. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 37.Schold JD, Srinivas TR, Guerra G, Reed AI, Johnson RJ, Weiner ID, et al. A “weight-listing” paradox for candidates of renal transplantation? [see comment] American Journal of Transplantation. 2007;7(3):550–9. doi: 10.1111/j.1600-6143.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 38.Schnitzler MA, Salvalaggio PR, Axelrod DA, Lentine KL, Takemoto SK. Lack of interventional studies in renal transplant candidates with elevated cardiovascular risk. Am J Transplant. 2007;7(3):493–4. doi: 10.1111/j.1600-6143.2006.01683.x. [DOI] [PubMed] [Google Scholar]
- 39.el-Agroudy AE, Wafa EW, Gheith OE, Shehab el-Dein AB, Ghoneim MA. Weight gain after renal transplantation is a risk factor for patient and graft outcome. Transplantation. 2004;77(9):1381–5. doi: 10.1097/01.tp.0000120949.86038.62. [DOI] [PubMed] [Google Scholar]