Abstract
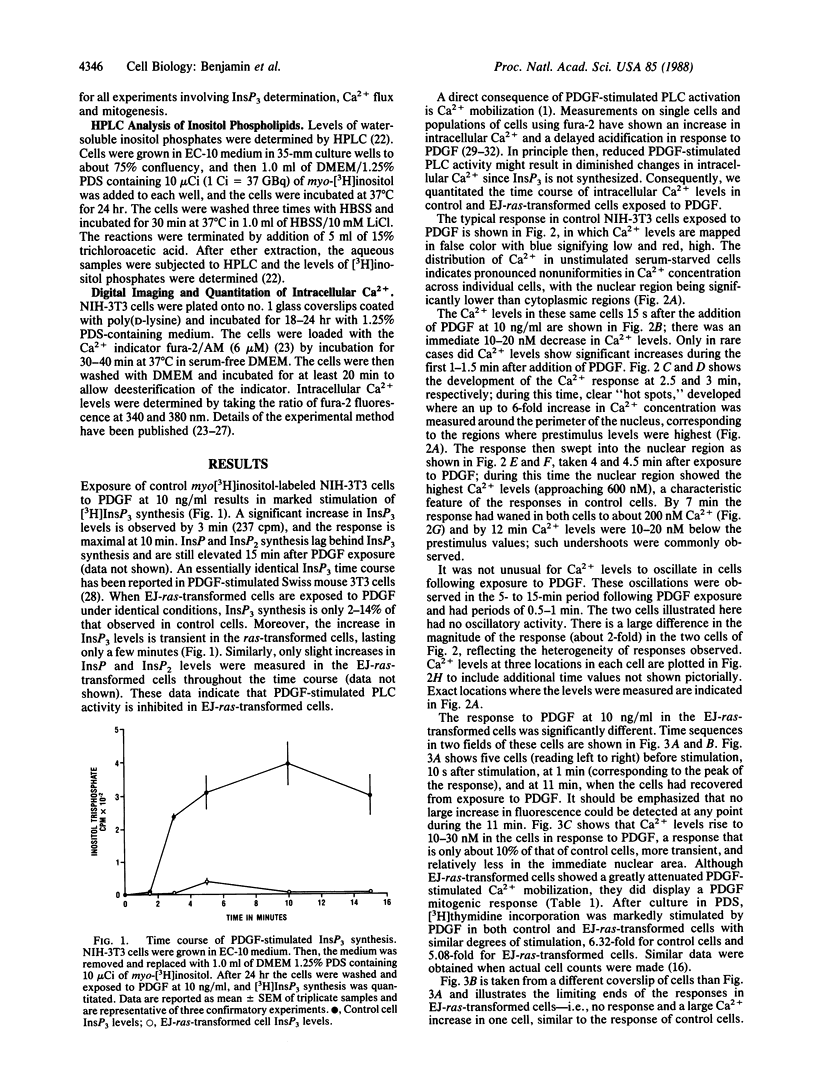
NIH-3T3 cells transformed by the EJ-ras oncogene synthesize only 10-15% as much inositol 1,4,5-trisphosphate (InsP3) as control cells after stimulation with platelet-derived growth factor (PDGF). This is despite the fact that the basal (unstimulated) levels of InsP3 synthesized in control and EJ-ras-transformed cells are not significantly different. Using the fluorescent indicator fura-2 and digital-imaging techniques, we have visualized and quantified changes in intracellular Ca2+ concentrations in control and EJ-ras-transformed NIH-3T3 cells in response to PDGF. Within 3 min after exposure of control cells to PDGF, intracellular Ca2+ levels are increased 3- to 9-fold, paralleling the increase in InsP3. In contrast, the majority (greater than 90%) of the EJ-ras-transformed cells show no increase in Ca2+ levels after PDGF exposure and the few that did respond exhibited only a small transient increase. Pronounced differences in the intracellular localization of Ca2+ increases in control and the responding EJ-ras-transformed cells were also observed. Despite the inhibition of InsP3 synthesis and subsequent Ca2+ mobilization, the EJ-ras-transformed cells respond mitogenically to PDGF. These data do not support the hypothesis that the EJ-ras gene product (p21) stimulates a phosphatidylinositol 4,5-bisphosphate-specific phospholipase C in NIH-3T3 cells; instead they suggest that the EJ-ras p21 may uncouple the PDGF receptor from phospholipase C resulting in inhibition of PDGF-stimulated activity of phospholipase C, InsP3 synthesis, and Ca2+ mobilization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Benjamin C. W., Tarpley W. G., Gorman R. R. Loss of platelet-derived growth factor-stimulated phospholipase activity in NIH-3T3 cells expressing the EJ-ras oncogene. Proc Natl Acad Sci U S A. 1987 Jan;84(2):546–550. doi: 10.1073/pnas.84.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin C. W., Tarpley W. G., Gorman R. R. The lack of PDGE-stimulated PGE2 release from ras-transformed NIH-3T3 cells results from reduced phospholipase C but not phospholipase A2 activity. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1254–1259. doi: 10.1016/0006-291x(87)91572-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Cornwall M. C., Williams G. H. Spatially resolved cytosolic calcium response to angiotensin II and potassium in rat glomerulosa cells measured by digital imaging techniques. J Biol Chem. 1987 Feb 25;262(6):2919–2927. [PubMed] [Google Scholar]
- Connor J. A. Digital imaging of free calcium changes and of spatial gradients in growing processes in single, mammalian central nervous system cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6179–6183. doi: 10.1073/pnas.83.16.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Tseng H. Y., Hockberger P. E. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Proffitt R. T., Baenziger J. U., Chang D., Kennedy B. B. Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem. 1981 Sep 10;256(17):8896–8899. [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., Ross R. Relation of cholesterol and mevalonic acid to the cell cycle in smooth muscle and swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1980 Jun 10;255(11):5134–5140. [PubMed] [Google Scholar]
- Hanley M. R., Jackson T. The ras gene. Transformer and transducer. Nature. 1987 Aug 20;328(6132):668–669. doi: 10.1038/328668a0. [DOI] [PubMed] [Google Scholar]
- Hasegawa-Sasaki H. Early changes in inositol lipids and their metabolites induced by platelet-derived growth factor in quiescent Swiss mouse 3T3 cells. Biochem J. 1985 Nov 15;232(1):99–109. doi: 10.1042/bj2320099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives H. E., Daniel T. O. Interrelationship between growth factor-induced pH changes and intracellular Ca2+. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1950–1954. doi: 10.1073/pnas.84.7.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., Moscat J., Aaronson S. A. Novel source of 1,2-diacylglycerol elevated in cells transformed by Ha-ras oncogene. Nature. 1987 Nov 19;330(6145):269–272. doi: 10.1038/330269a0. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., de la Peña P., Moscat J., Garcia-Barreno P., Anderson P. S., Aaronson S. A. Rapid stimulation of diacylglycerol production in Xenopus oocytes by microinjection of H-ras p21. Science. 1987 Oct 23;238(4826):533–536. doi: 10.1126/science.2821623. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Mendoza S. A., Nånberg E., Sinnett-Smith J., Rozengurt E. Ca2+-mobilizing actions of platelet-derived growth factor differ from those of bombesin and vasopressin in Swiss 3T3 mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5768–5772. doi: 10.1073/pnas.84.16.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews W. R., Guido D. M., Huff R. M. Anion-exchange high-performance liquid chromatographic analysis of inositol phosphates. Anal Biochem. 1988 Jan;168(1):63–70. doi: 10.1016/0003-2697(88)90010-3. [DOI] [PubMed] [Google Scholar]
- Michell B. Oncogenes and inositol lipids. 1984 Apr 26-May 2Nature. 308(5962):770–770. doi: 10.1038/308770a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Defize L. H., de Laat S. W. Calcium in the action of growth factors. Ciba Found Symp. 1986;122:212–231. doi: 10.1002/9780470513347.ch13. [DOI] [PubMed] [Google Scholar]
- Pandiella A., Malgaroli A., Meldolesi J., Vicentini L. M. EGF raises cytosolic Ca2+ in A431 and Swiss 3T3 cells by a dual mechanism. Redistribution from intracellular stores and stimulated influx. Exp Cell Res. 1987 May;170(1):175–185. doi: 10.1016/0014-4827(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Parries G., Hoebel R., Racker E. Opposing effects of a ras oncogene on growth factor-stimulated phosphoinositide hydrolysis: desensitization to platelet-derived growth factor and enhanced sensitivity to bradykinin. Proc Natl Acad Sci U S A. 1987 May;84(9):2648–2652. doi: 10.1073/pnas.84.9.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Raines E. W., Ross R. Purification of human platelet-derived growth factor. Methods Enzymol. 1985;109:749–773. doi: 10.1016/0076-6879(85)09128-5. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tarpley W. G., Hopkins N. K., Gorman R. R. Reduced hormone-stimulated adenylate cyclase activity in NIH-3T3 cells expressing the EJ human bladder ras oncogene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3703–3707. doi: 10.1073/pnas.83.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J., Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985 Apr;6(1-2):145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]






