Abstract
The 8-residue alanine oligopeptide, Ac-A4KA2Y-NH2 (AKY8), was found to form amyloid-like fibrils upon incubation at room temperature in acidified aqueous solution, at peptide concentrations > 10 mM. The fibril solution exhibits an enhanced VCD couplet in the amide I’ band region, which is nearly 2 orders of magnitude larger than typical polypeptide/protein signals in this region. The UV-CD spectrum of the fibril solution shows circular dichroism in the region associated with the tyrosine side chain absorption. A similar peptide, Ac-A4KA2-NH2 (AK7), lacking in a terminal tyrosine residue, does not aggregate. These results suggest a pivotal role for the C-terminal tyrosine residue in stabilizing the aggregation state of this peptide. It is speculated that interactions between the lysine and tyrosine side chains of consecutive strands in an anti-parallel arrangement, e.g. via cation-π interactions, are responsible for the stabilization of the resulting fibrils. These results offer considerations and insight for the de novo design of self-assembling oligopeptides for biomedical and biotechnological applications, and highlight the usefulness of VCD as a tool to probe amyloid fibril formation.
The self-assembly of polypeptides and proteins has become the subject of intense research activities for various reasons. First, an understanding of the underlying mechanism is necessary to develop diagnostic and therapeutic strategies for a variety of diseases, including Alzheimer’s, Huntington’s, Creutzfield-Jacob, and Parkinson’s disease.1 These diseases are believed to result from the misfolding and subsequent aggregation of specific proteins to form rigid fibrils. These fibrils then deposit in tissues to form insoluble plaques.2 Second, polypeptide/protein self-aggregation results in the formation of novel supramolecular structures, e.g. hydrogels, which are of biomedical and biotechnological relevance.3
Elucidating the mechanism of the self-assembly of peptides and proteins is of fundamental importance.4 However, the rules governing self-aggregation are still debated. Strong experimental evidence suggests that the aggregation process does not primarily reflect the conformational propensities of a peptide’s amino acid residues.5 The pivotal role of aliphatic and aromatic residues for the aggregation of even small peptides suggests that aggregation is strongly driven by hydrophobic forces.5,6 Gazit and co-workers demonstrated the importance of aromatic residues for self-aggregation, but it is unclear whether this is due to aromaticity or to the propensity of these peptides to adopt β-sheet favoring conformations.7 In this regard, conformational disorder has been suggested to be a prerequisite for fibrillation.4,8,9
In this communication we report the fibril formation of an 8-residue alanine-based oligopeptide, namely Ac-A4KA2Y-NH2 (AKY8). Oligoalanines of this size typically exhibit a statistical coil structure in aqueous solution, with a predominant sampling of polyproline II (PPII) conformations.10 PPII has been hypothesized to be a prerequisite for polypeptide/protein aggregation.11 However, our results compel us to attribute the self-aggregation of this peptide to favorable stacking interactions between side chains of different strands.
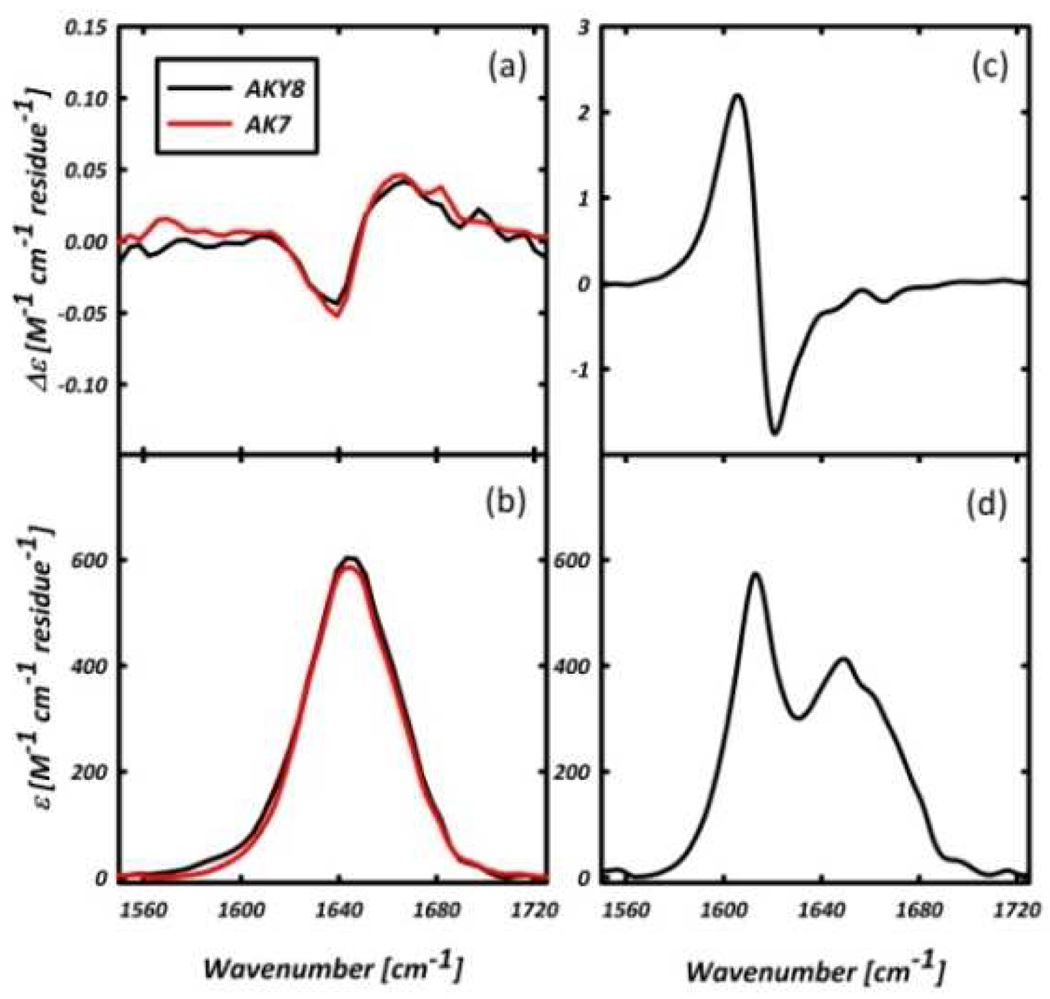
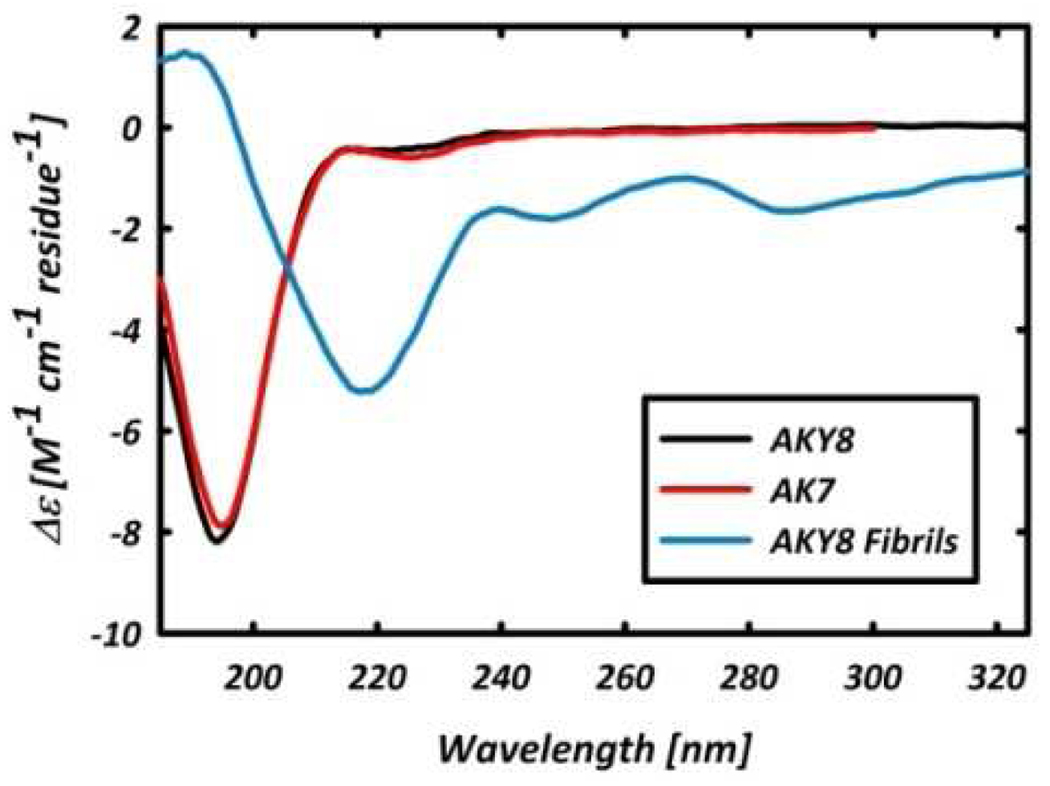
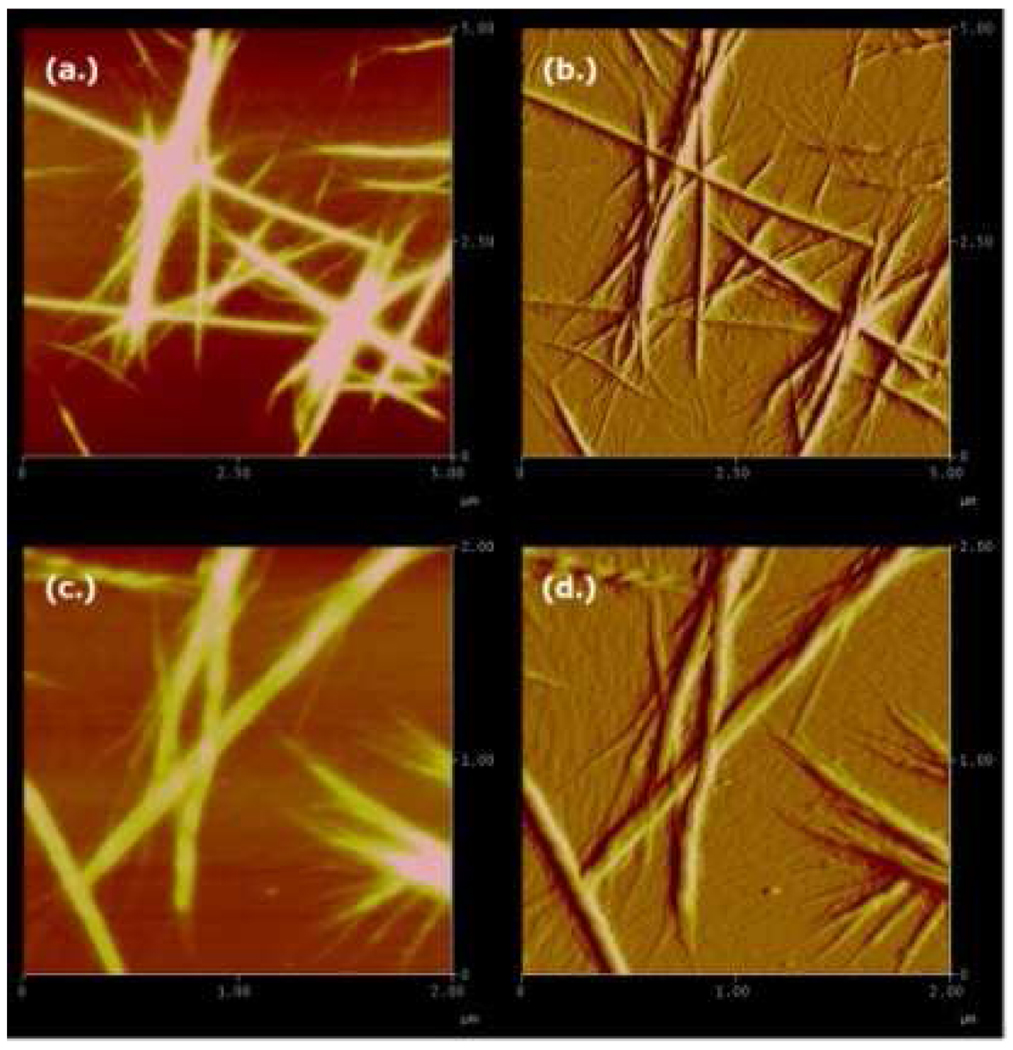
Figure 1 compares the amide I’ region of the FTIR and VCD spectra of AKY8 and the reference peptide Ac-A4KAA-NH2 (AK7) in D2O, at peptide concentrations of ~20 and ~50 mM for AKY8 and AK7, respectively. The negative couplet in the VCD spectra of the two peptides measured immediately after dissolution (Figure 1(a)) are identical, and indicative of a statistical ensemble of conformations, with a predominance of PPII-like conformations.12 The UV-CD spectra of AKY8 and AK7, displayed in Figure 2, corroborate this notion. Overnight incubation of both peptides, however, results in the formation of a gelatinous solution for AKY8, but not for AK7. Representative AFM images of the resultant AKY8 solution depict rather large fibrils with heights of 22 – 24 nm (Figure 3). We found that the AKY8 fibrils bind congo red, an indication that they are of the amyloid type.
Figure 1.
(a.) VCD and (b.) FTIR of monomeric AKY8 and AK7 in D2O. (c.) FTIR and (d.) VCD spectra of AKY8 fibril solution in D2O after overnight incubation at acidic pH.
Figure 2.
UV-CD spectra of monomeric AKY8 (black) and AK7 (red), and AKY8 fibril solution (blue)
Figure 3.
AFM images of AKY8 fibrils. (a.) height and (b.) amplitude images of a 5 µm × 5 µm area. (c.) height and (d.) amplitude images of a 2 µm × 2 µm area. AFM images were acquired in air.
The IR spectrum of the fibril solution (Figure 1(d)) shows a band at ~1616 cm−1, indicative of an antiparallel β-sheet conformation. The corresponding UV-CD spectrum (Figure 2), which shows a minimum at ~220 nm, also indicates a β-sheet conformation.13 The broad IR band centered at ~1650 cm−1 is blue-shifted by ~7 cm−1 with respect to the amide I’ band of the monomer, and coincides with the amide I’ bands in the isotropic and anisostropic Raman spectra of AKY8 (data not shown). This suggests that both IR and Raman bands should be assigned to a distorted β-sheet conformation, in which the two Raman active modes become IR active. On the contrary, the IR and VCD spectra of AK7 do not indicate any aggregation, even after overnight incubation.
The most peculiar observation in this study is the VCD spectrum of the AKY8 fibril solution, which exhibits a drastically enhanced positive couplet, with an inflection point at 1616 cm−1. It is nearly 2 orders of magnitude larger in intensity than the initial VCD spectrum of un-aggregated AKY8 in this region (Figure 1). Enhanced VCD signals in the amide I’ region have recently been reported for lysozyme and insulin fibrils.14
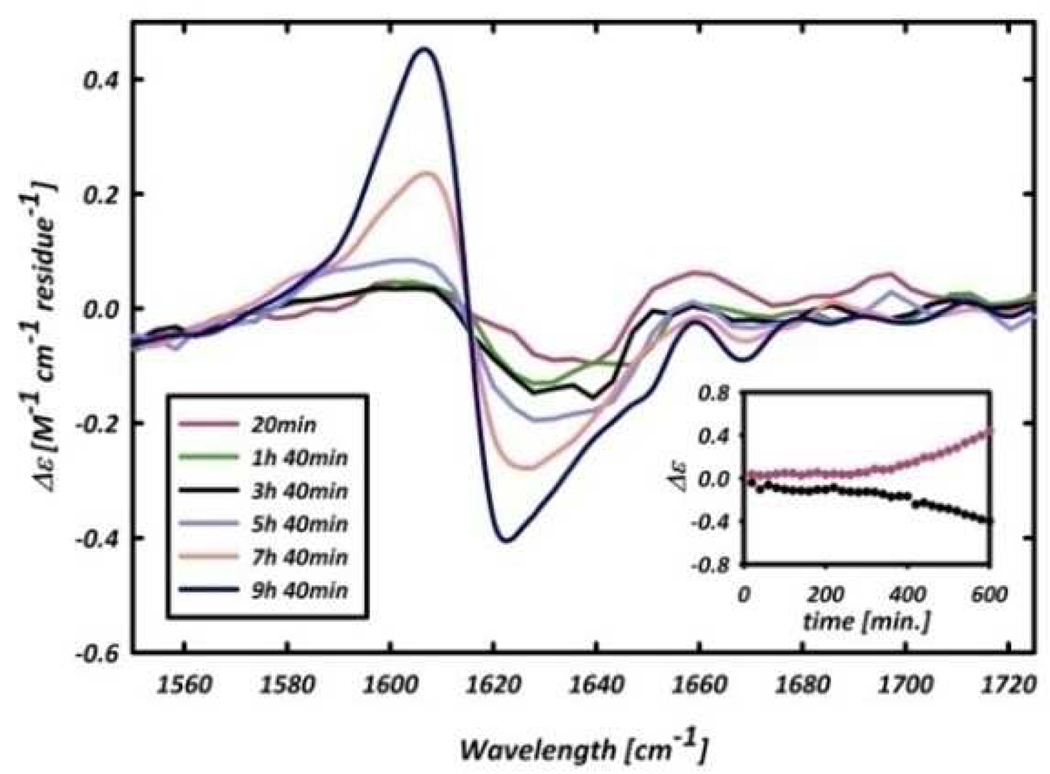
The giant VCD signal can conveniently be used to probe the kinetics of the aggregation process. Figure 4 shows the formation of the enhanced couplet as a function of time for the first 10 h of sample incubation. The inset shows the VCD intensity of the maximum (red) and minimum (black) of the emerging couplet. A lag time is observed prior to the fibril growth, which is typical for fibrillogenesis,15 leading us to conclude that the enhanced VCD signal is a direct probe of the fibrillization process, rather than of the early β-sheet formation. The UV-CD spectrum of the AKY8 fibril solution (Figure 2) shows clear circular dichroism in the region of the tyrosine side chain absorption, which is absent in the respective spectrum of the monomer. This, and the inability of AK7 to aggregate, clearly proves that tyrosine is pivotal for the observed self-aggregation of AKY8. Even though the role of aromatic residues in aggregation processes is well documented,6 our result is very surprising, since it was not expected that a single tyrosine positioned at the C-terminal could aggregate a peptide which does not have other aggregation promoting sources. The IR spectrum of the AKY8 fibril solution indicates an antiparallel β- sheet conformation, so that interactions between the tyrosine residues in adjacent strands of the same sheet can be ruled out. Instead, we propose that sheet formation is caused by cation-π interactions between the lysine and tyrosine side chains to yield the following out-of-register arrangement:
AAAAKAAY
YAAKAAAA
AAAAKAAY
YAAKAAAA
It has been shown that cation - π interactions, particularly between a tyrosine and charged lysine, can be important for the stabilization of protein structures.16 The role of such an interaction in peptide aggregation, however, has not yet been proposed. The fibrils revealed by AFM are consistent with the stacking of β- sheet tapes, which could be stabilized by π-stacking between tyrosine residues or by additional cation - π interactions. First simulations of the IR and VCD profile of the amide I band for a very simple two-dimensional model of amide I oscillators, in which a one-dimensional chain is representing a sheet, indicates that efficient packing rather than tilting of the sheet structure might yield the observed enhancement of the VCD signal.
Figure 4.
Temporal evolution of the amide I’ VCD signal of the AKY8 fibril solution. Inset: Intensity of the VCD signal as a function of time at 1604 cm−1 (red) and 1620 cm−1 (black)
Taken together, the results of the present study show that a single tyrosine residue can promote self-aggregation of a short polyalanine peptide. We propose that cation - π interactions and, to a minor extent, π-π interactions serve as the driving force for the aggregation process. Furthermore, the fibrillization gives rise to a large enhancement of the amide I VCD signal, which may reflect a rather compact facial assembly of β-sheets.
Supplementary Material
Acknowledgement
We would like to acknowledge financial support from NSF (CHEM 0804492) to R.S.S and (0849164) to S.D., and from NIH (R01-GM071793) to G.Y.
Footnotes
Supporting Information Available Materials and Methods. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Dobson CM. Trends Biochem. Sci. 1999;24:329. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 2.Glenner GG. N. Engl. J. Med. 1980;302:1283. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- 3.Yokoi H, Kinoshita T, Zhang S. Proc. Natl. Acad. Sci. 2005;102:8414. doi: 10.1073/pnas.0407843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobson CM. Nature. 2003;426:884. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 5.Kim W, Hecht MH. Proc. Natl. Acad. Sci. 2006;103:15824. doi: 10.1073/pnas.0605629103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazit E. Prion. 2007;1:32. doi: 10.4161/pri.1.1.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazit E. FASEB J. 2002;16:77. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 8.Chiti F, Dobson CM. Ann. Rev. Biochem. 2006;75:333. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 9.Rochet JC, Landsbury PT., Jr Curr. Opin. Struct. Biol. 2000;10:60. doi: 10.1016/s0959-440x(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 10.Graf J, Nguyen PH, Stock G, Schwalbe H. J. Am. Chem. Soc. 2007;129:1179. doi: 10.1021/ja0660406. [DOI] [PubMed] [Google Scholar]
- 11.Blanch EW, Morozova-Roche LA, Cochran DAE, Doig AJ, Hecht L, Barron LD. J. Mol. Biol. 2000;301:553. doi: 10.1006/jmbi.2000.3981. [DOI] [PubMed] [Google Scholar]
- 12.Schweitzer-Stenner R. J. Phys. Chem. B. 2009;113:2922. doi: 10.1021/jp8087644. [DOI] [PubMed] [Google Scholar]
- 13.MacPhee CE, Dobson CM. J. Am. Chem. Soc. 2000;122:12707. [Google Scholar]
- 14.Ma S, Cao X, Mak M, Sadik A, Walkner C, Freedman TB, Lednev IK, Dukor RK, Nafie LA. J. Am. Chem. Soc. 2007;129:12365. doi: 10.1021/ja074188z. [DOI] [PubMed] [Google Scholar]
- 15.Frieden C. Prot. Sci. 2007;16:2334. doi: 10.1110/ps.073164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma JC, Dougherty DA. Chem. Rev. 1997;97:1303. doi: 10.1021/cr9603744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.