Abstract
Purpose
TGF-β is an immunosuppressive cytokine, having direct suppressive activity against conventional CD4+ and CD8+T cells and NK cells, thereby inhibiting tumor immunosurveillance. Here we investigated possible synergy between anti-TGF-β (1D11) and a peptide vaccine on induction of anti-tumor immunity, and the mechanisms accounting for synergistic efficacy.
Experimental Design
The effect of combination treatment with a peptide vaccine and anti-TGF-β was examined in a subcutaneous TC1 tumor model, as well as the mechanisms of protection induced by this treatment.
Results
Anti-TGF-β significantly and synergistically improved vaccine efficacy as measured by reduction in primary tumor growth, although anti-TGF-β alone had no impact. The number of tumor antigen-specific CTL with high functional avidity as measured by IFN-γ production and lytic activity was significantly increased in vaccinated mice by TGF-β neutralization. Although TGF-β is known to play a critical role in CD4+Foxp3+ Treg cells, Treg depletion/suppression by an anti-CD25 mAb (PC61) prior to tumor challenge did not enhance vaccine efficacy, and adding anti-TGF-β did not affect Treg numbers in lymph nodes or tumors or their function. Also, TGF-β neutralization had no effect on IL-17-producing T cells, which are induced by TGF-β and IL-6. Absence of type II NKT cells, which induce myeloid cells to produce TGF-β was not sufficient to eliminate all sources of suppressive TGF-β. Finally, the synergistic protection induced by anti-TGF-β vaccine augmentation was mediated by CD8+ T cells since anti-CD8 treatment completely abrogated the effect.
Conclusions
These results suggest that TGF-β blockade may be useful for enhancing cancer vaccine efficacy.
Keywords: TGF-β, vaccine, CD8+ T cells
Statement of translational relevance.
Blockade of negative regulation of immunity is a key to the success of cancer immunotherapy. However, frequently single agent treatment of cancer with an inhibitor of negative regulation does not always induce a clinical response. TGF-β has been shown to be a potent immunosuppressive cytokine produced by both tumor cells and immune cells associated with immune suppression. We investigated the possibility of synergy between anti-TGF-β and a peptide vaccine on induction of anti-tumor immunity and the mechanisms accounting for synergistic efficacy. The combination treatment of anti-TGF-β and the vaccine synergistically induced anti-tumor immunity to control subcutaneous tumor growth in a mouse model, while anti-TGF-β alone did not show any effect. As we are currently testing a human monoclonal anti-TGF-β in a phase I clinical trial, this study provides the pre-clinical basis for a phase II trial of anti-TGF-β with a cancer vaccine.
Introduction
Accumulating evidence suggests that the immune system is tightly regulated by a network of cellular and cytokine mediators that reduce collateral damage by dampening what might otherwise be an overexuberant inflammatory response. However, tumors exploit this network to evade immunosurveillance so it has been hypothesized that inhibiting components of the network responsible for immunosuppression might be an effective strategy for the generation of more effective immunotherapies for cancers.
TGF-β is a pleiotropic cytokine that plays a role in processes such as neoangiogenesis and immunosuppression. In the context of immunosuppression, TGF-β directly suppresses activation and maturation of innate and adaptive immune cells including CD4+ and CD8+ T cells, NK cells, macrophages, and DCs (1-6). In addition, TGF-β has been shown to play a critical role in the differentiation and induction of immunosuppressive CD4+Foxp3+ Treg cells (7). Furthermore, TGF-β together with IL-6 induces differentiation of IL-17 producing T cell subsets called Th17 and Tc17 cells (8). Although the roles of these IL-17-producing cells in a tumor setting is still unclear, some studies suggest that they contribute to better tumor survival (9).
TGF-β is very abundant in tumor-bearing individuals. An inverse correlation has been found between the level of TGF-β in peripheral blood of cancer patients and disease prognosis (10). Tumors themselves have been considered to be a major source of TGF-β but there is also increasing evidence that cells of the immune system can also serve as a critical source of immunosuppressive TGF-β (1, 11). For example, CD1d-restricted type II NKT cells produce IL-13 which in turn stimulates CD11b+Gr-1+ myeloid derived suppressor cells (MDSCs) to produce TGF-β which inhibits CD8+ T cell-mediated tumor immunosurveillance. In this pathway, depletion of either CD1d-restricted NKT cells or IL-13, could block TGF-β production by MDSCs (1, 12, 13), which has been thought to contribute to tumor metastasis (11). Therefore, targeting TGF-β with an antagonist has the potential to impact multiple pathways responsible for dampening immune responses, and thus it is a very attractive target to enhance tumor immunity.
However, monotherapy with a TGF-β antagonist may not induce an adequate immune response. As we learned with some antagonists of negative immune regulators in clinical trials, such as anti-CTLA4, those reagents may produce serious side effects without stimulating adequate tumor immunosurveillance (14). This might also be the case with TGF-β antagonists since it is known that animals with genetic defects in TGF-β production or TGF-β receptor signaling are seriously impaired and have significantly shorter lifespans compared to their wild-type counterparts (15-19). To reduce potential toxicity it is reasonable to consider combination of the antagonist with other reagents that synergize with the antagonist, an approach frequently used in conventional therapies for the treatment of cancers. In an immunotherapeutic setting, this may be a combination of the antagonist with T cell-adoptive therapy or vaccines.
1D11 is a murine anti-TGF-β monoclonal antibody, which neutralizes all three mammalian isoforms of TGF-β (20). Previous studies have demonstrated that the 1D11 has minimal effects on immune effector mechanisms in normal animals and no overt toxicities have been noted, even after long-term administration (21). This may in part be due to the paracrine nature of TGF-β and the relative lack of penetration into some tissue compartments by such a relatively large molecule. Likewise, the human equivalent of 1D11, designated GC-1008, has been shown to be well-tolerated in subhuman primate chronic toxicity studies (S. Lonning and J. McPherson unpublished observations). Additionally, GC-1008 is now in a clinical trial in cancer patients and reports provided thus far indicate that the antibody is well-tolerated (22). This somewhat surprising lack of serious toxicity observed with 1D11 and GC-1008 is presumably due to a lack of accessibility of these antibodies to TGF-β in its homeostatic roles in vivo.
In this study, we have examined whether TGF-β neutralization can potentiate immune responses to a CTL-inducing vaccine and whether the resulting immunopotentiation inhibits tumor growth in a TC1 tumor model. Results demontstated that, in contrast to monotherapy with the anti-TGF-β which did not have an impact on tumor growth in this tumor model, although it has in some others (1), the anti-TGF-β significantly enhanced the efficacy of a peptide vaccine by inducing an increased number of tumor antigen-specific CTL with high functional avidity, which is critical for the effective elimination of tumors.
Materials and Methods
Mice
C57BL/6 mice were purchased from Animal Production Colonies, Frederick Cancer Research Facility, NCI (Frederick, MD, USA). CD1d gene knockout mice on a C57BL/6 genetic background were generously provided by Dr. Albert Bendelac (University of Chicago) (23) and maintained at the animal facility of NCI. Animal care was in accordance with the guidelines of the NIH Animal Research Advisory Committee.
Tumor model
TC1 is a C57BL/6-derived lung epithelial cell line transfected with human papilloma virus (HPV) 16 E6 and E7 genes (24). The cells were maintained in RPMI1640 containing 10% FCS with 100 μg/ml G418 (Sigma, MO). Twenty-thousand cells were injected subcutaneously into the right flank of syngeneic mice. Four days after tumor injection, the mice were immunized s.c. at the base of the tail with 100 μg of Human Papilloma Virus (HPV)16 E749-57 peptide (RAHYNIVTF) (25), 50 nmol of hepatitis B virus core (HBVc)128-140 helper peptide (TPPAYRPPNAPIL) (26), and 1 μg of mouse GM-CSF (Peptrotech, Rocky Hill, NJ) emulsified in incomplete Freund’s adjuvant IFA, (Sigma) Some mice were treated with anti-TGF-β (clone 1D11, 100 μg/shot) by intraperitoneal injection every other day as indicated and in some experiments, the mice were treated with two doses of anti-CD25 mAb (clone PC61, 1 mg/shot) i.v. on 5 and 3 days prior to tumor challenge.
Flow cytometry
Fluorescent protein labeled monoclonal antibodies against CD8, CD3, CD4, CD25, Foxp3, IFN-γ, IL-17, Gr-1, CD11b were purchased from eBioscience, San Diego, CA. HPV16 E749-57 peptide loaded H-2Db tetramer was made by the NIH tetramer core facility. To stain for intracellular IFN-γ staining, spleen cells or lymph node cells from tumor draining lymph nodes isolated from tumor injected mice were stimulated overnight in culture medium containing 10 μg/ml of brefeldin A (BD Biosciences, San Diago, CA) with naïve spleen cells pulsed with either 100 nM, 0.1 nM or 0 nM of E749-57 peptide. To stain for intracellular IL-17, cells from tumor draining lymph nodes were stimulated with PMA (20 ng/ml, Sigma) and ionomycin (1 μM, Sigma) overnight in the culture medium with brefeldin A. The fluorescence of stained cells was measured by FACSCalibur or LSRII (BD Bioscience), and data were analyzed by Flowjo (Tree Star).
Preparation of tumor infiltrating lymphocytes
Following tumor resection, 6-10 pooled tumors were minced in RPMI1640 medium and mashed on a nylon screen to make into a single cell suspension. The mononuclear cells were separated by using Lymphocyte M medium (Cederlane). After washing, the cells were stained with antibodies and were analyzed by flow cytometry.
In vivo CTL assay
Naïve spleen cells were prepared from C57BL/6 mice. The cells were incubated with either 1 μM or 10 nM of E749-57 peptide or vehicle for 2 hr at 37 °C. After washing, the cells were allowed to sit in PBS at room temperature for 15 min and incubated with 5 μM, 0.5 μM or 0.05μM of CFSE (Invitrogen, Carlsbad, CA) for 15 min at room temperature. After washing, the three different target cells were mixed in a 1:1:1 ratio, and then the 3 × 106 of mixed cells were injected i.v. into tumor-bearing mice 18 days after tumor injection (14 days after vaccination). The next day, spleen cells or tumor draining lymph node cells were harvested and residual CFSE labeled cells were analyzed by flow cytometry. To evaluate the tumor antigen-specific lytic activity, the ratios of residual cell numbers were taken between E749-57 peptide-pulsed targets and vehicle-treated target cells.
Treg suppression assay
Single cell suspensions from pooled tumor draining lymph nodes were used for the Treg suppression assay. After enrichment of T cells by using T cell enrichment columns (R&D systems Inc., Minneapollis, MN) CD8+ T cells were depleted by the MACS system (Miltenyi Biotec Inc, Auburn, CA) to obtain enriched CD4+ T cells. CD4+CD25+ Treg cells were positively selected by the MACS system with a combination of biotin-labeled anti-CD25 (clone 7D4) and anti-biotin magnetic beads (27). The negative fraction was considered as CD4+CD25− T cells to use as responders in the assay. Thy 1.2 negative spleen cells were used as accessory cells. Fifty thousand CD4+CD25− T cells with 5 × 104 accessory cells and variable numbers of Treg were stimulated with 0.5 μg/ml of anti-CD3 (BD Bioscience). To examine CD4+CD25− T cell proliferation in vitro, 5 μCi /ml of 3H-thymidine was added during the final 8 hours of a 72-hour culture. At the end of the culture the 3H-thymidine incorporation was evaluated with a MicroBeta counter (Wallac, Perkin Elmer, Wellesley, MA, USA). Percent suppression was determined by the following formula. % suppression = 100 × (1-cpm of culture with Treg/cpm of culture without Treg).
Statistical analysis
Data were analyzed by the nonparametric Mann-Whitney test, the Student’s t-test or the least square regression test using JMP software (SAS Institutes, Cary, NC)
Results
Blocking TGF-β synergistically enhances the effect of an anti-tumor peptide vaccine
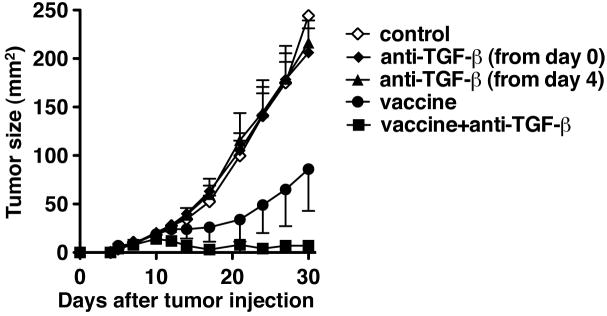
TC1 is a transfected lung epithelial cell tumor expressing the HPV16 E6 and E7 oncogenes. E749-57 peptide RAHYNIVTF is a well-characterized CTL epitope that can be presented by H-2 Db (25). First we examined the ability of the E749-57 peptide to induce an anti-tumor response in TC1 tumor-bearing mice (Fig 1). C57BL/6 mice were challenged s.c. with 2 × 104 TC1 on the right flank. Four days after the challenge, when tumors became palpable, the mice were immunized s.c. with the E749-57 peptide emulsified in incomplete Freund’s adjuvant along with an HBV core derived CD4+ T cell-epitope and GM-CSF. As reported previously (25), the peptide vaccine significantly slowed down the growth of tumors. Using this tumor model, we first examined the effect of an anti-TGF-β antibody on tumor growth. Anti-TGF-β alone did not affect tumor growth regardless of the timing to start the treatment (from the time of tumor challenge or 4 days after tumor challenge). In contrast, the mice that received both the vaccine and anti-TGF-β had significantly reduced tumor burden even compared with the group of mice receiving the vaccine alone. In experiments in which tumors were measured over 40 days, 6 out of 15 mice (40 %) in vaccine + anti-TGF-β group remained tumor free for at least 55 days after tumor challenge; 1 out of 15 mice (7 %) was tumor free in the vaccine alone group. These results indicated that anti-TGF-β synergistically enhances the effect of an anti-tumor vaccine (p=0.0054 by least squares regression test).
Fig 1.
Anti-TGF-β (1D11) synergistically enhanced the efficacy of a CTL-inducing peptide tumor vaccine. TC1 cells (2× 104) were injected s.c. in the right flank of C57BL/6 mice on day 0. Some mice were treated with 100μg of 1D11 every other day from the time of tumor challenge (filled diamonds) or day 4 after tumor challenge (filled triangles) until the end of the experiment. Some mice were given a vaccine consisting of HPV16 E749-57 peptide (100 μg) emulsified in IFA together with 50 nmol of HBVc128-140 and 1 μg of mouse GM-CSF s.c. 4 days after tumor injection (filled circle). Some mice given the vaccine were also treated with 1D11 (100 μg) i.p. every other day from the time of immunization until 30 days after tumor challenge (filled squares). Each data point represents mean ± SD. p=0.032 by Mann-Whitney test between vaccine alone group and vaccine+ TGF-β group on day 30. The synergy between the vaccine and anti-TGF-β was confirmed on day 30 by least squares regression test (p=0.0054). All groups had 5 mice each except for the control group. which had 4 mice. These experiments were repeated at least twice with comparable results.
Anti-TGF-β enhances vaccine-induced CD8+ CTL responses
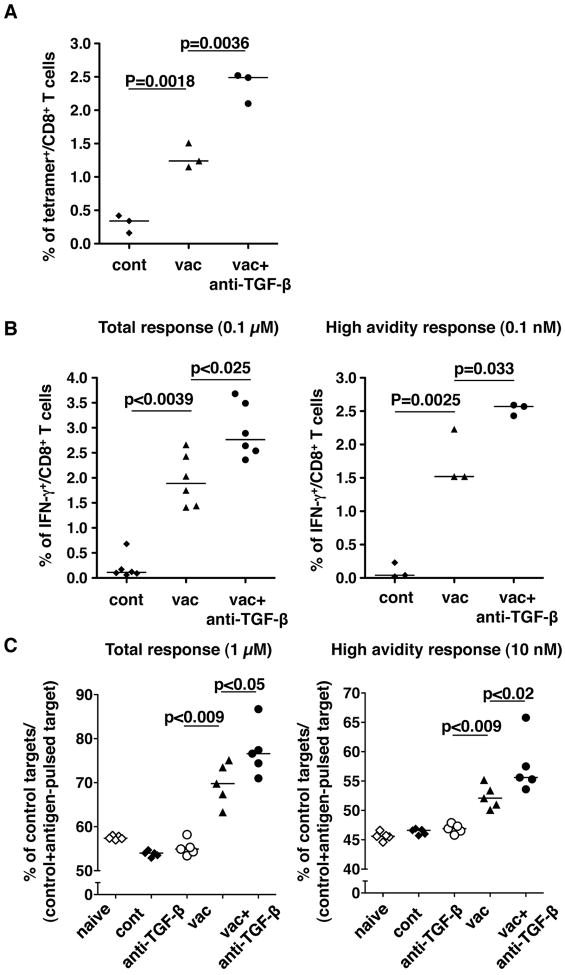
The E749-57 peptide incorporated into the vaccine is known to induce CD8+ CTL responses. To understand the effect of anti-TGF-β treatment on CTL induction by the vaccine in tumor-bearing mice, we first examined the number of E749-57 reactive CD8+ CTL by using Db-tetramer loaded with E749-57 (Fig. 2A). Four days after tumor challenge, the mice were injected with either the vaccine alone or with anti-TGF-β mAb. Two weeks after immunization (18 days after tumor challenge), peptide-specific CD8+ T cells in spleens were measured by the tetramer binding assay. Control mice which were inoculated with the tumor without receiving the vaccine, had a very low frequency of tetramer-reactive CD8+ cells (<0.4 % of CD8+ cells) suggesting that the tumors themselves are not sufficient to induce E749-57-specific CTLs, even though the tumor cells express the E7 protein. Although the number of mice tested was small, tumor-bearing mice that received the peptide vaccine alone appeared to have a significantly higher frequency of tetramer-reactive CD8+ T cells than did control mice. The combination treatment with the vaccine and anti-TGF-β induced a significantly higher frequency of tetramer+ CD8+ T cells than those in the mice that received the vaccine alone, consistent with the result that the anti-TGF-β treatment synergistically reduced tumor size in vivo. We further investigated the function of E749-57-specific CTL by intracellular IFN-γ staining (Fig. 2B). The spleen cells were stimulated overnight with T-depleted spleen cells that were pulsed with two different concentrations of E749-57 peptide. With a high (0.1 μM) concentration of the peptide, CD8+ cells from the mice that received both the vaccine and anti-TGF-β had the highest frequency of IFN-γ+ cells when compared to the vaccine alone group which in turn had a higher frequency of IFN-γ+ cells than those in the control group. When spleen cells were stimulated with a low concentration (0.1 nM) of peptide pulsed on APCs, the cells from mice that received both the vaccine and anti-TGF-β mAb showed the highest frequency of IFN-γ+ cells among the three groups. These results indicated that anti-TGF-β along with the vaccine not only synergistically enhances the total CTL response against the E749-57 peptide but more importantly increases the absolute number of high avidity CTL against tumor antigen, which has been reported to be important in tumor rejection (28-31).
Fig 2.
Anti-TGF-β increased tumor antigen specific CD8+ T cell responses induced by the CTL-inducing peptide vaccine. TC1 cells (2 × 104) were injected s.c. in the right flank of C57BL/6 mice. Four days after tumor challenge, some mice were immunized with the peptide vaccine as described in Fig 1. Some vaccinated mice were also treated i.p. with anti-TGF-β (1D11) every other day from the time of immunization. A and B. Eighteen days after tumor injection (14 days after vaccination), all mice were euthanized and spleen cells were prepared. A. The spleen cells were stained with anti-CD3, anti- CD8 and E749-57-loaded H-2Db tetramer and analyzed by flow cytometry. The proportions of tetramer+ cells were determined among CD3+CD8+ cells. B. The spleen cells were stimulated with spleen cells of naïve C57BL/6 mice pulsed with either 0.1 μM or 0.1 nM of the E749-57 peptide. After overnight culture, the cells were stained with anti-CD3, anti-CD8 and anti-IFN-γ by the intracellular cytokine staining method described in the Materials and Methods. The proportion of IFN-γ+ cells was determined among CD3+CD8+ cells. C. Eighteen days after tumor injection (14 days after vaccination), all mice were injected i.v. with a mixture of naïve C57BL/6 spleen cells labeled with different concentrations of CFSE and pulsed with different concentrations of E749-57 peptides as described in Materials and Methods. Sixteen hours after the spleen cell injection, spleen cells were prepared, and residual CFSE+ cells were analyzed by flow cytometry. Each symbol represents one data point. Medians are shown as bars. p-values by Student’s t-test (A and right panel of B, where the data distribution is consistent with Gaussian) or Mann-Whitney (left panel of B and C) test are indicated. These experiments were repeated at least twice with comparable results.
We next examined the lytic function of vaccine-induced CTL in vivo in tumor-challenged mice (Fig. 2C). Two weeks after immunization (18 days after tumor challenge), lytic activity was measured by injecting a mixture of three target cells pulsed with 1 μM or 10 nM of E7 peptide or unpulsed labeled with different concentrations of CFSE (see Materials and Methods section). After 18 hrs, the residual target cells in either spleens or tumor draining lymph nodes were measured by flow cytometry. In tumor-challenged mice with no treatment and with anti-TGF-β treatment alone, all three target cells were recovered in similar proportions, no different from those observed in the target cell mixture prior to the injection. This result suggested that there was no significant tumor antigen-specific lytic activity in those mice. In mice that received the vaccine alone, the target cells pulsed with E7 peptides were significantly reduced in their proportion among CFSE-labeled cells recovered. Mice that received both the vaccine and anti-TGF-β showed further reduction of residual peptide-pulsed target cells, pulsed with either the high or low concentration of peptide, indicating that the combination of the vaccine and anti-TGF-β induces more CTL with high (as well as low) functional avidity that can lyse tumor cells with fewer antigenic peptide-MHC complexes on their surface. Thus, the combination of the vaccine and anti-TGF-β antibody synergistically induces a higher magnitude of CTL response which includes a greater absolute number of tumor antigen-specific CTLs with high avidity as well as low avidty.
Enhancement of vaccine efficacy by anti-TGF-β may not be due to the suppression of CD4+CD25+ T cells
It has been reported that TGF-β plays critical roles in the induction and the function of CD4+CD25+Foxp3+ Treg cells. Since it has been suggested that Treg cells contribute to the immune suppression that occurs in cancer patients (32), we hypothesized that the enhancement of the vaccine efficacy by anti-TGF-β treatment may be due to the suppression of induction and/or immunosuppressive activity of Treg cells in tumor-challenged mice.
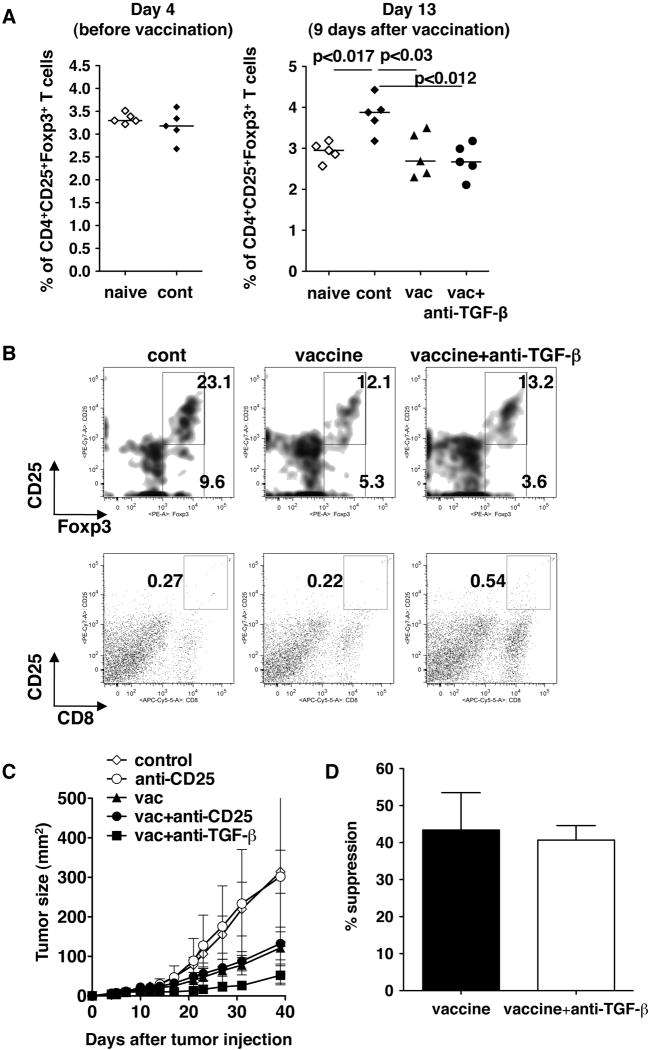
To test this hypothesis, we first examined the number of Treg cells in draining lymph nodes of tumors from different groups of mice (Fig. 3A). There was no increase of the CD4+CD25+Foxp3+ T cell proportion in the draining lymph nodes on day 4 after tumor challenge (at the time of vaccination) compared to naïve mice. Nine days after vaccination (13 days after tumor challenge), there was a significant increase of the CD4+CD25+Foxp3+ T cell proportion in the mice without vaccination. Although the proportion of the cells in vaccinated mice was significantly lower than that of unvaccinated mice, there was no difference in the proportion between vaccine alone and vaccine with anti-TGF-β groups. We also examined the number of Treg cells and activated CD8+ T cells (CD8+CD25+) in tumors (Fig. 3B). Consistent with the results of tumor draining lymph nodes, the proportion of CD4+CD25+Foxp3+ T cells in tumors of vaccinated mice was lower than in those of control mice. However, there was no difference between vaccinated mice with or without anti-TGF-β treatment (Fig.3 upper panels). In contrast, there was an increased number of activated CD8+ T cells in tumors of mice treated with the vaccine+anti-TGF-β (Fig. 3B lower panels). Ratios between CD8+CD25+ cells and CD4+CD25+Foxp3+ T cells were 0.012, 0.018, 0.041 for control, vaccine alone, vaccine + anti-TGF-β respectively. These results suggest that there is a higher ratio of activated CD8+ T cells to Treg in mice treated with vaccine + anti-TGF-β.
Fig 3.
The effect of anti-TGF-β on vaccine efficacy is not due to blocking Treg cells. TC1 cells (2 × 104) were injected s.c. in the right flank of C57BL/6 mice. Four days after tumor challenge, some mice were immunized with the peptide vaccine as described in Fig 1. Some immunized mice were also treated i.p with 1D11 (100 μg) every other day from the time of immunization for two weeks. A. Four days or 13 days after tumor injection, tumor draining lymph node cells were recovered and stained with anti-CD3, anti-CD4, anti-CD25 and anti-Foxp3. The proportions of CD3+CD4+CD25+Foxp3+ cells were determined by flow cytometry. Each symbol represents one data point. Median are shown as bars. B. Sixteen days after tumor challenge, tumor infiltrating Tregs were examined by flow cytometry. Tumor infiltrating lymphocytes were recovered as described in the Materials and Methods section, stained with anti-CD3, anti-CD4, anti-CD8, anti-CD25 and anti-Foxp3. Presented density plots were gated on the CD3+CD4+ population (upper row). Presented pseudo dot plots represented entire population (lower row). C. Some mice were treated with anti-CD25 (1 mg) five and three days before tumor injection. The experiment was terminated on day40 after tumor challenge. Each data point represents mean ± SD. All groups had 5 mice each. D. Eleven days after tumor challenge, tumor draining lymph node cells were used to examine Treg suppressive activity. Fifty thousand CD4+CD25− cells with 5 × 104 accessory cells with/without 1.25 × 104 CD4+CD25+ Treg cells were stimulated with 0.5 μg/ml anti-CD3 for 72 hr. Cell proliferation was measured by 3H-thymidine incorporation, The % suppression was determined as described in the Materials and Methods section. These experiments were repeated at least twice with comparable results.
Although no significant impact of anti-TGF-β treatment was observed on the proportion of CD4+CD25+Foxp3+ T cells in tumor draining lymph nodes, this did not exclude the possibility that anti-TGF-β alters the immunosuppressive function of these cells. Therefore, we next depleted CD4+CD25+ cells in vivo by treating mice with anti-CD25 (clone PC61) prior to tumor challenge to test whether anti-CD25 gives a similar impact on the vaccine efficacy (Fig 3C). Despite the fact that anti-CD25 cannot completely deplete CD4+CD25+Foxp3+ Treg cells, it has been shown that this treatment can significantly block the immunosuppressive effect of Treg cells in vivo to enhance anti-tumor immunity. However, anti-CD25 treatment did not show any impact on vaccine efficacy, in contrast with anti-TGF-b β treatment. We also examined the effect of anti-TGF-β treatment on the suppressive activity of Treg cells in tumor draining lymph nodes of vaccinated tumor-bearing mice (Fig 3D). The activity of Treg cells to suppress proliferation of CD4+CD25− T cells was not affected by anti-TGF-β treatment. Taken together, we concluded that enhancement of vaccine efficacy by anti-TGF-β treatment is not due to the suppression of Treg cells.
Enhancement of vaccine efficacy by anti-TGF-β may not be due to the suppression of IL-17-producing T cell-induction
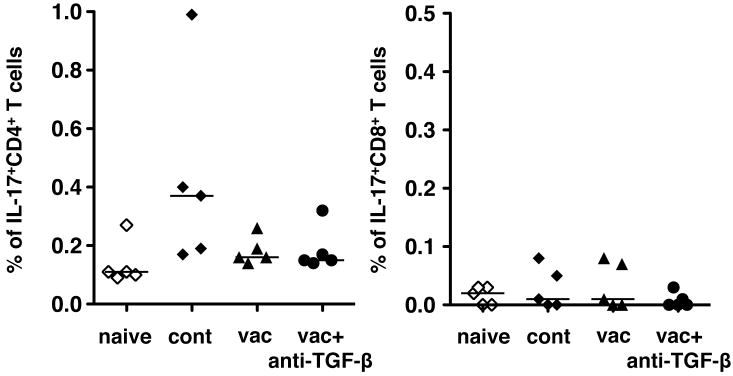
TGF-β also has been shown to play a critical role to induce IL-17 producing T cells, both CD4+ and CD8+. Recently there have been reports demonstrating that TGF-β contributes to the induction of IL-17 producing CD8+ T cells in tumor-bearing mice and that IL-17 produced by these CD8+ T cells acts as a pro-survival factor for tumor cells (9). Therefore, it is possible that anti-TGF-β treatment may prevent induction of IL-17 producing CD8+ T cells specific for tumor antigen incorporated in the vaccine to contribute to survival of tumors in vivo. Thus, we examined the number of IL-17 producing T cells in tumor draining lymph nodes 18 days after tumor challenge (Fig 4). There were only a few IL-17 producing cells detected in both CD4+ and CD8+ T cell compartments of tumor draining lymph nodes. As the vaccine treatment with/without anti-TGF-β did not increase or decrease the already low number of IL-17 producing cells, it is unlikely that the effect of anti-TGF-β could have been mediated by an alteration in IL-17-producing cells.
Fig. 4.
Anti-TGF-β with the peptide vaccine does not affect IL-17 production by T cells. TC1 cells (2 × 104) were injected s.c. in the right flank of C57BL/6 mice. Four days after tumor challenge, some mice were immunized with the peptide vaccine as described in Fig 1. Some immunized mice were also treated i.p. with 1D11 (100 μg) every other day from the time of immunization throughout the experiment. Eighteen days after tumor injection (14 days after vaccination), all mice were euthanized and tumor draining lymph node cells were harvested and stained for intracellular IL-17 together with surface CD3, CD4, CD8 staining. Median are shown as bars. These experiments were repeated at least twice with comparable results.
Enhancement of vaccine efficacy by anti-TGF-β may not be due to the suppression of NKT cell-induced -TGF-β production by MDSC
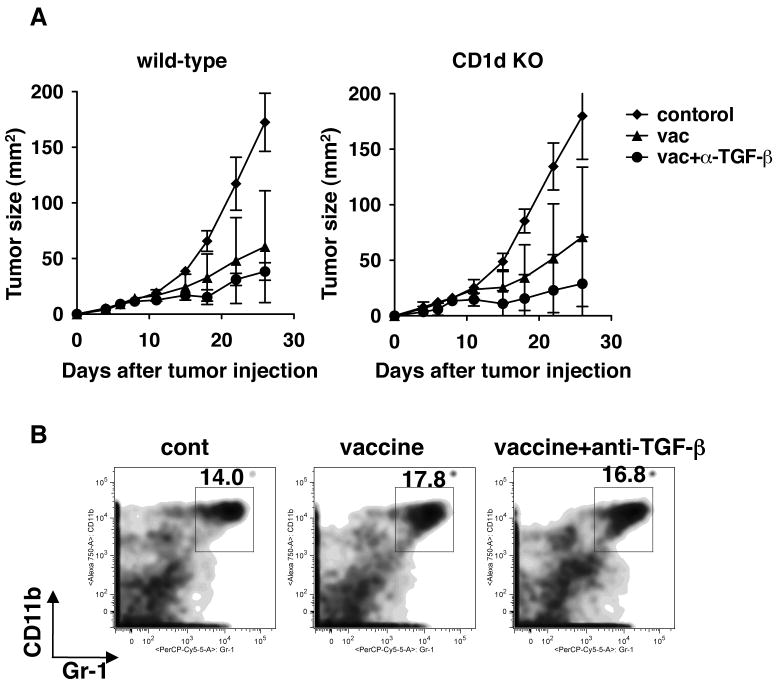
We have previously reported that in some mouse tumor models, IL-13 produced by CD1d-restricted type II NKT cells in tumor-challenged mice induces TGF-β production by MDSCs to suppress anti-tumor CD8+ T cells (1, 12). To assess the role of this pathway we challenged mice with TC1 tumors and immunized both wild-type and CD1 KO mice with the peptide vaccine. Since CD1d is necessary for NKT cell-development, CD1d KO mice lack all CD1-restricted NKT cells including type II NKT cells. As shown in Fig. 5A, vaccine efficacy was not enhanced in NKT cell-deficient mice compared to wild-type mice. In contrast, anti-TGF-β enhanced the vaccine efficacy in CD1d KO mice as it did in NKT cell-intact wild-type mice. In addition, the numbers of myeloid-derived suppressor cells (MDSC), which were defined as Gr-1+CD11b+ cells, did not differ among the tumors from untreated, vaccine alone and vaccine+anti-TGF-β treated mice (Fig 5B). Therefore, blockade of the type II NKT cell-IL-13-MDSC-TGF-β pathway was not sufficient to eliminate all the sources of suppressive TGF-β.
Fig. 5.
The effect of anti-TGF-β on vaccine efficacy is not due solely to blocking TGF-β resulting from NKT cell-mediated immunosuppression. TC1 cells (2 × 104) were injected s.c. in the right flank of C57BL/6 or CD1d KO mice. Four days after tumor challenge, some mice were immunized with the peptide vaccine as described in Fig 1. Some immunized mice were also treated i.p. with 1D11 (100 μg) every other day from the time of immunization for two weeks. A. Tumor size was followed for 28 days. Each data point was shown as mean ± SD. All groups had 5 mice each. p<0.03 by Mann-Whitney test between vaccine group and vaccine + anti-TGF-β group on day 26. B. Tumor infiltrating CD11b+Gr-1+ cells were examined in tumors from wild-type mice 16 days after tumor challenge. Tumor infiltrating lymphocytes prepared as in Fig 3B were stained with antibodies against CD11b and Gr-1. These experiments were repeated at least twice with comparable results.
CD8+ T cells are necessary for the protection induced by a combination between anti--TGF-β and the vaccine
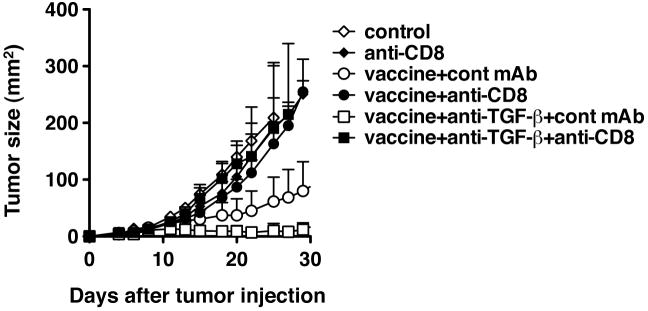
To confirm the enhanced anti-tumor response that was observed in the tumor-bearing mice that received both the vaccine and anti-TGF-β is mediated by CD8+ CTL, we depleted CD8+ cells in vivo. As shown in Figure 6, anti-CD8 treatment at the time of vaccination completely abrogated the protection, and the tumor growth curve was almost superimposable on that of control mice, indicating that the protection induced by the peptide vaccine and anti-TGF-β mAb was CD8-dependent as well as that induced by the vaccine alone.
Fig. 6.
The protection induced by the combination of anti-TGF-β and the vaccine is mediated by CD8+ T cells. TC1 cells (2 × 104) were injected s.c. in the right flank of C57BL/6 mice. Four days after tumor challenge, some mice were immunized with the peptide vaccine as described in Fig 1. Some immunized mice were also treated i.p. with 1D11 (100 μg) every other day from the time of immunization for two weeks. Some mice were also treated with anti-CD8 after immunization as indicated. p<0.03 by Mann-Whitney test between vaccine+cont mAb group and vaccine+anti-CD8 group. All groups had 5 mice each. p<0.03 by Mann-Whitney test between vaccine+anti-TGF-β+cont mAb group and vaccine+ TGF-β+anti-CD8 group on day 29. Each data point was shown as mean ± SD. These experiments were repeated at least twice with comparable results.
Discussion
Here we demonstrated that the blockade of TGF-β by a monoclonal antibody (1D11) and a CTL-inducing peptide vaccine synergistically induced anti-tumor immunity in a subcutaneous TC1 tumor model, supporting our hypothesis that removing negative regulators of the immune system enhances tumor vaccine efficacy. These results are consistent with a study with a recombinant Listeria vaccine (33) and anti-TGF-β antibody (2G7), a recent publication with a recombinant adenoviral vaccine and SM16, a small type I TGF-β receptor kinase inhibitor that blocks SMAD phosphorylation (34) and a peptide vaccine with 1D11 in glioma model (Ueda et al., manuscript submitted along with this manuscript).
TGF-β has been shown to influence multiple aspects of the tumor microenvironment, including suppression of local immunity. In addition, TGF-β may also play a critical role in negative feedback mechanisms in immune responses to avoid self-destruction caused by over-zealous immune responses. This role is revealed in mice genetically ablated of TGF-β or TGF-β receptor genes (15-19). For example, TGF-β1 KO mice show a severe wasting syndrome and multiple organ failure as a result of autoimmune inflammation (15). Similarly, mice expressing a dominant negative type II TGF-β receptor only on T cells develop a severe autoimmune disease characterized by inflammation in multiple organs and development of autoantibodies (16). In contrast, long-term treatment of mice with anti-TGF-β 1D11 showed no immune dysregulation (21). These differences in terms of phenotype of mice genetically ablated of TGF-β or TGF-β receptor gene and of mice chronically treated with 1D11, which binds only activated TGF-β suggest limited access of 1D11 to active TGF-β in vivo.
Dominant negative type II TGF-β receptor transgenic mice also show strong natural tumor immune responses and reject highly invasive tumors, suggesting that despite the fact that TGF-β suppresses almost all lymphoid populations and myeloid cells such as macrophages and DCs (35), one critical target of TGF-β to support tumor growth is T cells (36). Therefore, we hypothesized that even if anti-TGF-β alone is not enough to facilitate natural tumor immunosurveillance, blockade of TGF-β at the time of T cell induction by a vaccine should enhance vaccine efficacy. The results that anti-TGF-β significantly enhanced vaccine efficacy as well as anti-tumor CD8+ cell activity measured by in vivo CTL assay in the TC1 tumor model demonstrated that this hypothesis was correct at least in this tumor model.
In studies to design a T cell-inducing vaccine, the efficacy should be determined by both quantity (magnitude) and quality of the responses induced. The quality can be represented in multiple ways, such as cytokine profile and functional avidity. The functional avidity of T cells has been shown to play a critical role in tumor immunity as well as in infectious disease settings (30, 37-40). CD8+ T cells with higher functional avidity which can recognize a lower number of antigen-MHC complexes on the target cell surface (37) and kill targets faster (30) have been shown to be more effective for tumor rejection (30, 38-40). Therefore, we asked whether enhancement of vaccine efficacy by anti-TGF-β is due to induction of increased numbers of high functional avidity CD8+ T cells. The results showed that combination treatment induced an increased number of tumor antigen-specific CD8+ T cells with high functional avidity as measured by intracellular IFN-γ staining and in vivo CTL assay. However, the proportion of high functional avidity cells did not seem to change, so the increased absolute number of high functional avidity CD8+ T cells was due to the greater number of antigen-specific CD8+ T cells induced, including both high and low avidity cells. This observation suggests that there is still room for improvement of the vaccines efficacy by means of inducing a better quality of response. Adding reagents that can skew the response toward higher functional avidity may be worth considering. Recently it was suggested that IL-15 incorporated into a vaccine could induce higher functional avidity CD8+ T cells (41). Thus, combination of anti-TGF-β and IL-15 together with the vaccine might be a possible way to further improve vaccine efficacy.
Interestingly while anti-TGF-β has been shown to reduce tumor growth in multiple tumor models (1, 42, 43), anti-TGF-β,. 1D11 alone did not show any impact on growth of the TC1 tumor in vivo. This observation is consistent with a previous study in TC1 tumor model using different anti-TGF-β antibody, 2G7, specific to TGF-β1 (33) and a recent study with 1D11 in a mouse glioma model (Ueda et al., a manuscript submitted along with this manuscript). At least three potential explanations can be given for the differences in anti-TGF-β effect. One is that in the TC1 model, tumors do not use TGF-β as a major mechanism to evade the immune system although we detected 2257±125.4 pg/ml of TGF-β1 produced in a 3 day culture supernatant. This idea may be supported by the results suggesting that at least three immunosuppressive immunological components, Treg cells, IL-17 producing T cells and NKT cells, in which TGF-β plays a role, are not involved in the immune suppression occurring in this tumor model. This idea may also be supported by our unpublished observation that extending the period of anti-TGF-β treatment after vaccination did not improve the clinical outcome compared with treating for only two weeks after the vaccination, suggesting that anti-TGF-β is required only when TGF-β is induced as a negative feedback mechanism to terminate the activation of T cells induced by the vaccine. The second possibility is that the anti-TGF-β antibody that binds only active TGF-β does not have enough access to active TGF-β playing a critical role in tumor growth, so that the activities of TGF-β are not well suppressed in the TC1 model. In fact, while a previous study with anti-TGF-β1, 2G7, and another study with 1D11 (Ueda et al., a manuscript submitted along with this manuscript) agreed with the result of present study, a recent study with orally-administered SM16, a small type I TGF-β receptor kinase inhibitor which may have better distribution in vivo, in the TC1 tumor model showed that a single agent treatment with SM16 significantly slowed tumor growth. The third possibility is that despite the fact that TC1 cells are carrying foreign viral antigens, the tumor cells are not sufficiently immunogenic to induce any T cell immune responses that can be revealed by removing the negative suppressor. Regardless of the reason for not seeing the effect of anti-TGF-β in a single agent treatment, the results presented in the present study strongly suggest that combination of anti-TGF-β with a vaccine is an attractive approach to treat cancers.
As mentioned above, recently Kim et al conducted a study to examine the effect of a small molecule TGF-β receptor kinase inhibitor on adenoviral vaccines (34). In that study, an adenoviral vector expressing HPV16 E7 was used as a vaccine in the TC1 tumor model. Although the overall conclusion from both studies was that a vaccine and TGF-β antagonist synergize to induce anti-tumor immunity, there are several interesting differences between two studies. First, although there was a significant effect of SM16 on tumor growth with a single agent treatment, 1D11 alone did not show any effect on tumor growth. As noted, this may be because of their size and tissue accessibility or their target specificity for receptor vs for activated TGF-β. This difference also made the synergy more striking in the case of 1D11, because simple additivity would have no impact. Second, the distribution of vaccine-induced tumor-specific CD8+ T cells was different. Kim at al, observed a concentration of T cells just in tumors. In the present study, although we did not assess the number of intratumoral T cells, a similar increase of tumor antigen-specific T cells was observed in both spleens and tumor draining lymph nodes. Third, in the present study, the level of E749-57-loaded Db tetramer+ T cells and tumor antigen-specific lytic activity measured by in vivo CTL assay was hardly detected in spleens and tumor draining lymph nodes of untreated tumor-bearing mice, while more than 1% of CD8+ T cells were E749-57-loaded Db tetramer+ in the previous study. These differences may be caused by the differences in the nature of TGF-β/TGF-β receptor antagonists and the vaccines used in each study and may provide important insights when these reagents are translated into the clinic.
In the present study, we could not identify a single immunosuppressive mechanism abrogated by anti-TGF-β sufficient to account for the synergistic enhancement of vaccine efficacy, even though we examined several possible mechanisms, such as Treg cells and IL-17-producing cells that can be induced by TGF-β Further, NKT cells alone were not sufficient to account for the source of TGF-β. Although it is possible that other immunological mechanisms involving TGF-β play a critical role in the TC1 tumor model to suppress vaccine efficacy, it may also be possible that it is a combination of multiple mechanisms. Adhesion molecules on endothelial cells are critical for tumor-specific T cells homing to the right place (44, 45). Inhibition of TGF-β signaling can change the expression levels of adhesion molecules (34). Therefore, one effect of anti-TGF-β may be on the tumor microenvironment recruiting T cells induced by the vaccine. This may also depend on the nature of the vaccine combined with anti-TGF-β or on the type of tumors studied, as suppression of Treg cells seemed be a mechanism of action in other studies (33) (Ueda et al., a manuscript submitted along with this manuscript). This issue should be further explored in future studies.
Currently, the human equivalent of 1D11, called GC1008, is in a phase I clinical trial in human cancer patients (22). Similar to the studies with 1D11 in mice, GC1008 showed minimal adverse effects in the patients. The study presented here, in conjuction with the data presented by Ueda et al. (companion manuscript), provide the preclinical basis and rationale for the use of pan-neutralizing anti-TGF-β antibodies in combination with tumor vaccines for the treatment of cancer patients.
Acknowledgments
We would like to thank Drs. Hideho Okada and Kenichi Hanada for suggestions on the protocol of tumor infiltrating lymphocyte preparation. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. National Cancer Institute has a Cooperative Research and Development Agreement with Genzyme Cooperation.
References
- 1.Terabe M, Matsui S, Park J-M, et al. Transforming Growth Factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block Cytotoxic T Lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobie JJ, Wu RS, Kurt RA, et al. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–4. [PubMed] [Google Scholar]
- 3.Cottrez F, Groux H. Regulation of TGF-beta response during T cell activation is modulated by IL-10. J Immunol. 2001;167:773–8. doi: 10.4049/jimmunol.167.2.773. [DOI] [PubMed] [Google Scholar]
- 4.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–9. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 6.Trotta R, Col JD, Yu J, et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol. 2008;181:3784–92. doi: 10.4049/jimmunol.181.6.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 9.Nam JS, Terabe M, Kang MJ, et al. Transforming Growth Factor {beta} Subverts the Immune System into Directly Promoting Tumor Growth through Interleukin-17. Cancer Res. 2008;68:3915–23. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teicher BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res. 2007;13:6247–51. doi: 10.1158/1078-0432.CCR-07-1654. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terabe M, Swann J, Ambrosino E, et al. A nonclassical non-Va14Ja18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–33. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–83. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 15.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-b1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–0. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas PJ, McNeil N, Hilgenfeld E, et al. Transforming growth factor-beta pathway serves as a primary tumor suppressor in CD8+ T cell tumorigenesis. Cancer Res. 2004;64:6524–9. doi: 10.1158/0008-5472.CAN-04-0896. [DOI] [PubMed] [Google Scholar]
- 19.Diebold RJ, Eis MJ, Yin M, et al. Early-onset multifocal inflammation in the transforming growth factor beta 1-null mouse is lymphocyte mediated. Proc Natl Acad Sci U S A. 1995;92:12215–9. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L, Allen JB. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol. 1989;142:1536–41. [PubMed] [Google Scholar]
- 21.Ruzek MC, Hawes M, Pratt B, et al. Minimal effects on immune parameters following chronic anti-TGF-beta monoclonal antibody administration to normal mice. Immunopharmacol Immunotoxicol. 2003;25:235–57. doi: 10.1081/iph-120020473. [DOI] [PubMed] [Google Scholar]
- 22.Morris JC, Shapiro GI, Tan AR, et al. Phase I/II Study of GC1008: A human anti-transforming growth factor-beta (TGFb) monoclonal antibody (MAb) in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC) J Clin Oncol. 2008;26 doi: 10.1371/journal.pone.0090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1- deficient mice. Immunity. 1997;6:459–67. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 24.Hung CF, Cheng WF, Hsu KF, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001;61:3698–703. [PubMed] [Google Scholar]
- 25.Feltkamp MCW, Smits HL, Vierboom MPM, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. EurJImmunol. 1993;23:2242–9. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 26.Sarobe P, Pendleton CD, Akatsuka T, et al. Enhanced in vitro potency and in vivo immunogenicity of a CTL epitope from hepatitis C virus core protein following amino acid replacement at secondary HLA-A2.1 binding positions. J Clin Invest. 1998;102:1239–48. doi: 10.1172/JCI3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton AM. Fractionation of T and B cells using magnetic beads. Curr Protoc Immunol. 2003;Chapter 3(Unit 3 5A) doi: 10.1002/0471142735.im0305as57. [DOI] [PubMed] [Google Scholar]
- 28.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J Immunol. 1999;162:2227–34. [PubMed] [Google Scholar]
- 29.Zeh HJ, 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–94. [PubMed] [Google Scholar]
- 30.Derby MA, Alexander-Miller MA, Tse R, Berzofsky JA. High avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low avidity CTL. J Immunol. 2001;166:1690–97. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- 31.Oh S, Hodge JW, Ahlers JD, Burke DS, Schlom J, Berzofsky JA. Selective induction of high avidity CTL by altering the balance of signals from antigen presenting cells. J Immunol. 2003;170:2523–30. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 32.Nomura T, Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in tumor immunity. Curr Top Microbiol Immunol. 2005;293:287–302. doi: 10.1007/3-540-27702-1_13. [DOI] [PubMed] [Google Scholar]
- 33.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–9. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Buchlis G, Fridlender ZG, et al. Systemic blockade of transforming growth factor-beta signaling augments the efficacy of immunogene therapy. Cancer Res. 2008;68:10247–56. doi: 10.1158/0008-5472.CAN-08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 36.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–22. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 37.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high or low avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. ProcNatlAcadSciUSA. 1996;93:4102–7. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee HG. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–57. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. JImmunol. 1999;162:2227–34. [PubMed] [Google Scholar]
- 40.Zeh HJ, III, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. JImmunol. 1999;162:989–94. [PubMed] [Google Scholar]
- 41.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15R alpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:15154–9. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nam JS, Terabe M, Mamura M, et al. An Anti-Transforming Growth Factor {beta} Antibody Suppresses Metastasis via Cooperative Effects on Multiple Cell Compartments. Cancer Res. 2008;68:3835–43. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Invest. 1993;92:2569–76. doi: 10.1172/JCI116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark RA, Huang SJ, Murphy GF, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–34. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlos TM. Leukocyte recruitment at sites of tumor: dissonant orchestration. J Leukoc Biol. 2001;70:171–84. [PubMed] [Google Scholar]