Abstract
The Type I Diabetes Genetics Consortium (T1DGC) has collected thousands of multiplex and simplex families with type I diabetes (T1D) with the goal of identifying genes involved in T1D susceptibility. These families have been genotyped for the HLA class I and class II loci and, recently, for a genome-wide panel of single-nucleotide polymorphisms (SNPs). In addition, multiple SNPs in specific candidate genes have been genotyped in these families in an attempt to evaluate previously reported T1D associations, including the C883A (Pro–Thr) polymorphism in exon 2 of TCF7, a T-cell transcription factor. The TCF7 883A allele was associated with T1D in subjects with T1D not carrying the high-risk HLA genotype DR3/DR4. A panel of 11 SNPs in TCF7 was genotyped in 2092 families from 9 cohorts of the T1DGC. SNPs at two positions in TCF7 were associated with T1D. One associated SNP, C883A (rs5742913), was reported earlier to have a T1D association. A second SNP, rs17653687, represents a novel T1D susceptibility allele in TCF7. After stratification on the high T1D risk DR3/DR4 genotype, the variant (A) allele of C883A was significantly associated with T1D among non-DR3/DR4 cases (transmission =55.8%, P =0.004; OR =1.26) but was not significantly associated in the DR3/DR4 patient subgroup, replicating the earlier report. The reference A allele of intronic SNP rs17653687 was modestly associated with T1D in both DR3/DR4 strata (transmission =54.4% in DR3/DR4; P =0.03; transmission =52.9% in non-DR3/DR4; P =0.03). These results support the previously reported association of the non-synonymous Pro–Thr SNP in TCF7 with T1D, and suggest that other alleles at this locus may also confer risk.
Keywords: polymorphism, transcription factor, Th1, type I diabetes
Introduction
Type I diabetes (T1D) is an autoimmune disease involving destruction of the insulin-producing cells of the pancreas, resulting in dysfunctional glucose homeostasis and the requirement for exogenous insulin. The genetic component of T1D is significant (λs =15) with, by far, the strongest contribution (40–50%) coming from the HLA region.1,2 Although the HLA-DR and HLA-DQ-encoding loci are the major determinants of genetic risk for T1D, association studies have revealed that multiple genes within the HLA region contribute to T1D risk.3–9 In addition, linkage and association studies have identified many T1D susceptibility regions and genes outside the HLA region.10–12 Of the genes identified by association, some have been detected by genome-wide approaches13 whereas others have been identified in candidate gene studies, based on biological plausibility of the gene and/or of the specific polymorphism. Unlike the genome-wide association scans, many of the candidate gene studies investigated a limited of number of single-nucleotide polymorphisms (SNPs) and samples and, consequently, had modest statistical power. In some cases, the reports of T1D association were discordant.
To address the issue of limited statistical power in many of the published candidate gene association studies, the Type I Diabetes Genetics Consortium (T1DGC), an international collaboration that has collected thousands of T1D families (multiplex and simplex),14 undertook genotyping and analysis of multiple SNPs per gene in a variety of candidate genes reported to be associated with T1D. This project involved SNPs in 21 different genes (the T1DGC Rapid Response Project). One of the candidate studied in the Rapid Response Project was the HMG box transcription factor family member TCF7.15 Expression of TCF7 is limited to T cells and NK cells16 in which the gene product binds β-catenin and promotes mRNA expression of Th1-specific loci, including IL12-Rβ2.17 The initial association study for variants in TCF7 with T1D risk reported the association of a single SNP, referred to at the time as C883A, by Transmission Disequilibrium Test (TDT) analysis of genotyping data from 283 Caucasian, multiplex families from the Human Biological Data Interchange (HBDI) repository. The TCF7 C883A SNP is located at the position indicated by rs5742913; however, the polymorphism is reported as a C–T change (dbSNP). The C–A polymorphism at this position has the alternate designation ss93257904; however, throughout this manuscript, the C–A SNP will be referred to as rs5742913.
The data were stratified on the genotype HLA–DR3/DR4, with rationale that the expected modest effect of this TCF7 polymorphism might be more difficult to discern in the presence of the high-risk HLA genotype than in its absence. The minor (A) allele of the nonconservative polymorphism Pro–Thr (C883A) was associated with T1D in the subset of T1D cases who do not have the heterozygous DR3/DR4-DQB1*0302 genotype (referred to hereafter as simply DR3/DR4), which is known to confer very high T1D risk. No T1D association was seen in patients who were already high risk because of the presence of the DR3/DR4 genotype. The variant (A) allele showed overtransmission overall (54.8%), greater overtransmission (57.4%) to the non-DR3/4 cases, and significant overtransmission from fathers to affected children (64.1%, P< 0.007). Significant over-transmission was also observed to male affected children, and the presence of at least one copy of the A allele was significantly associated with young age of T1D onset (P =0.036). The previous report also included data for one additional TCF7 SNP (A383T, rs244656) as well as five SNPs within 5 Mb of C883A in the 5q31 region (CSF2 I117T, rs25882; IL13 C4045T, intron 13, rs1295686; IL13 R111Q, rs20541; IL4 C582T, -590, rs2243250; IL9 T113M, rs2969885).15
In the T1DGC Rapid Response project, 11 SNPs within TCF7 were genotyped using the Sequenom iPlex technology on the T1DGC collection of T1D trios (Table 1). For rs5742913, original data analysis showed no copies of the T allele and suggested that the SNP was monomorphic in the tested sample set. Re-analysis of the data for the presence of the A allele at the rs5742913 position, however, revealed a T1D association for the A allele, thus replicating the previous finding. The data presented here also reveal an apparent T1D association for a TCF7 intronic SNP (rs17653687) that has not been reported earlier.
Table 1.
Cohorts used for the study
| Region | Cohort | N pedigrees | N pedigrees MHC | Not DR3/DR4 trios | DR3/DR4 trios | Age of onset mean | s.d. | n T1D patients age of onset | Num Peds with age of onset |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AP | 169 | 118 | 130 | 109 | 10.34 | 7.94 | 366 | 169 |
| 2 | DAN | 130 | 94 | 106 | 85 | 14.99 | 11.23 | 293 | 130 |
| 2 | EUR | 428 | 329 | 429 | 232 | 11.83 | 8.26 | 893 | 428 |
| 4 | HBDI | 424 | 413 | 540 | 342 | 12.28 | 8.67 | 937 | 415 |
| 4 | JOS | 71 | 53 | 56 | 52 | 11.83 | 7.53 | 148 | 71 |
| 4 | NA | 295 | 217 | 263 | 172 | 8.76 | 6.62 | 637 | 295 |
| 5 | BDA | 393 | 0 | 0 | 0 | 12.60 | 9.76 | 853 | 391 |
| 5 | SAR | 74 | 52 | 51 | 52 | 12.75 | 8.71 | 150 | 74 |
| 5 | UK | 108 | 91 | 93 | 90 | 8.22 | 5.29 | 242 | 108 |
| Total | 2092 | 1367 | 1668 | 1134 |
Abbreviations: MHC, major histocompatibility complex; T1D, type I diabetes.
Proportions of DR3/DR4 vs non-DR3/DR4 are shown, as well as average age of onset in each cohort.
Results
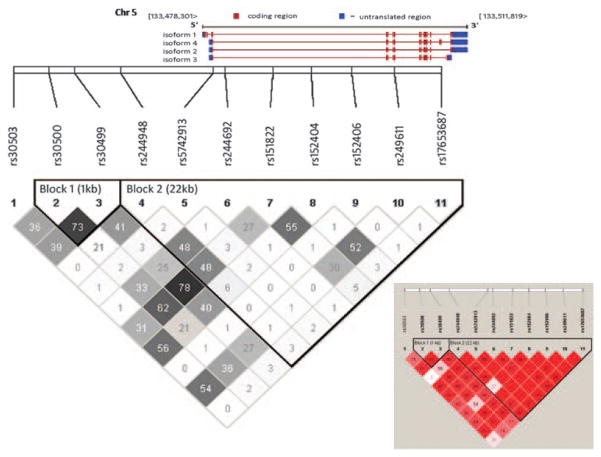
Genotyping in TCF7 was performed for 11 SNPs in 9 different cohorts using the Sequenom platform. The geographic origin and the number of the families in each cohort genotyped in this study are shown in Table 1. The names, positions, and variant allele frequencies for these 11 SNPs, as well as the proportion of genotype calls and the deviation from Hardy Weinberg equilibrium is provided in Table 2. One SNP, rs30500, showed a deviation from Hardy Weinberg equilibrium (P =0.03) based on comparing the observed and expected frequency of heterozygotes among parents, as well as the lowest proportion of genotype calls (96.2%). The other SNPs, including the two T1D-associated SNPs, all had genotype call rates of greater than 97.9% and did not deviate significantly from Hardy Weinberg equilibrium. The pattern of linkage disequilibrium (r2) for TCF7 is shown in Figure 1. Two haplotype blocks are revealed with strong LD within each block.
Table 2.
TCF7 SNPs studied
| Marker | Name | Position | HW P value founders | % Call rate | Number of trios | MAF | Alleles (major:minor) |
|---|---|---|---|---|---|---|---|
| 1 | rs30503 | 133465245 | 0.36 | 97.9 | 1541 | 12.7% | C:G |
| 2 | rs30500 | 133467775 | 0.03 | 96.2 | 1492 | 18.4% | C:T |
| 3 | rs30499 | 133469625 | 0.08 | 99.4 | 1584 | 23.5% | A:G |
| 4 | rs244948 | 133472960 | 0.16 | 98.6 | 1554 | 13.6% | G:A |
| 5 | rs5742913 (ss93257904) | 133479582 | 0.72 | 98.6 | 1568 | 11.6% | C:A |
| 6 | rs244692 | 133480434 | 0.16 | 98.5 | 1563 | 8.3% | A:G |
| 7 | rs151822 | 133483860 | 0.08 | 99.3 | 1576 | 23.1% | T:C |
| 8 | rs152404 | 133486613 | 0.13 | 98.8 | 1564 | 14.7% | T:C |
| 9 | rs152406 | 133489485 | 1.00 | 99.6 | 1597 | 5.2% | G:A |
| 10 | rs249611 | 133492556 | 0.88 | 99.6 | 1586 | 8.5% | A:G |
| 11 | rs17653687 | 133495899 | 0.19 | 98.5 | 1555 | 16.2% | A:G |
Abbreviations: MAF, minor allele frequency; SNP, single-nucleotide polymorphism.
Designations for the two single nucleotide polymorphisms that showed type I diabetes association are shown in bold.
Figure 1.
Map of the 11 TCF7 SNPs genotyped for this study, with haplotype blocks and LD values shown.
A TDT analysis of these 11 SNPs indicated that two SNPs (rs 5742913 and rs17653687) had significant transmission deviations (P =0.022 and 0.020) in the total family data (Table 3). For rs5742913 (C883A), the variant A allele was overtransmitted, as reported earlier15 whereas for rs17653687, the variant G allele was under-transmitted. Parental Disequilibrium Test (PDT) analysis (Table 3) of these (unstratified) data also indicated significant association of these two SNPs (P =0.011 and 0.007, respectively). The data were stratified into two groups based on HLA genotype to replicate the previously published results. The strata of TDT analysis are based on cases carrying the high-risk DR3/DR4 genotype (Table 4) and those not carrying the high-risk genotype (non-DR3/DR4, Table 5). For the high-risk DR3/DR4 cases, the C883A SNP (rs5742913) is not significantly associated with T1D risk (transmission of the variant A allele =51.8%, P =0.440) whereas the rs17653687, in the same haplotype block, shows a marginally significant overtransmission (54.4%, P =0.048) of the reference (A allele (Table 4)). Similar results were obtained by PDT analysis for rs5742913 (P =0.277) and for rs17653687 (P =0.026).
Table 3.
Genetic association between TCF7 SNPs and T1D in all pedigrees regardless of MHC genotype
| Marker | Over transmitted allele | is | T:U | Trans (%) | χ2 | TDT P value | Odds ratio | 95% CI | PTDT P value |
|---|---|---|---|---|---|---|---|---|---|
| rs30503 | C | Major | 675:623 | 52.0 | 2.083 | 0.1489 | 1.08 | (0.97–1.20) | 0.0774 |
| rs30500 | C | Major | 852:800 | 51.6 | 1.637 | 0.2008 | 1.07 | (0.96–1.17) | 0.1304 |
| rs30499 | A | Major | 1125:1062 | 51.4 | 1.815 | 0.1779 | 1.06 | (0.97–1.15) | 0.1266 |
| rs244948 | G | Major | 696:679 | 50.6 | 0.21 | 0.6466 | 1.03 | (0.92–1.13) | 0.6921 |
| rs5742913 | A | Minor | 728:643 | 53.1 | 5.27 | 0.0217 | 1.13 | (1.01–1.25) | 0.0112 |
| rs244692 | A | Major | 468:439 | 51.6 | 0.927 | 0.3356 | 1.07 | (0.93–1.21) | 0.4014 |
| rs151822 | T | Major | 1088:1064 | 50.6 | 0.268 | 0.6049 | 1.02 | (0.93–1.11) | 0.4978 |
| rs152404 | C | Minor | 762:750 | 50.4 | 0.095 | 0.7576 | 1.02 | (0.91–1.12) | 0.9597 |
| rs152406 | G | Major | 311:302 | 50.7 | 0.132 | 0.7162 | 1.03 | (0.87–1.20) | 0.7808 |
| rs249611 | G | Minor | 491:488 | 50.2 | 0.009 | 0.9236 | 1.01 | (0.88–1.14) | 0.8754 |
| rs17653687 | A | Major | 868:774 | 52.9 | 5.381 | 0.0204 | 1.12 | (1.01–1.23) | 0.0066 |
Abbreviations: PTDT, parental TDT; SNP, single-nucleotide polymorphism; T1D, type I diabetes; TDT, Transmission Disequilibrium Test.
Designations for the two SNPs that showed T1D association are shown in bold. Data indicating significant association in the unstratified sample set are shown in bold.
Table 4.
Genetic association between TCF7 SNPs and T1D in trios where the affected offspring carried the HLA-DR3/DR4 genotype
| Marker | Over transmitted allele | is | T:U | Trans (%) | χ2 | TDT P value | Odds ratio | 95% CI | PTDT P value |
|---|---|---|---|---|---|---|---|---|---|
| rs30503 | G | Minor | 229:224 | 50.6 | 0.055 | 0.8143 | 1.02 | (0.85–1.22) | 0.9265 |
| rs30500 | C | Major | 268:255 | 51.2 | 0.323 | 0.5697 | 1.05 | (0.88–1.24) | 0.5498 |
| rs30499 | G | Minor | 347:335 | 50.9 | 0.211 | 0.6459 | 1.04 | (0.89–1.20) | 0.7078 |
| rs244948 | G | Major | 223:214 | 51.0 | 0.185 | 0.6668 | 1.04 | (0.86–1.25) | 0.6388 |
| rs5742913 | A | Minor | 223:207 | 51.9 | 0.595 | 0.4404 | 1.08 | (0.89–1.30) | 0.2767 |
| rs244692 | A | Major | 149:139 | 51.7 | 0.347 | 0.5557 | 1.07 | (0.85–1.35) | 0.6051 |
| rs151822 | C | Minor | 343:316 | 52.0 | 1.106 | 0.2929 | 1.09 | (0.93–1.26) | 0.421 |
| rs152404 | C | Minor | 267:233 | 53.4 | 2.312 | 0.1284 | 1.15 | (0.96–1.36) | 0.2022 |
| rs152406 | A | Minor | 102:78 | 56.7 | 3.2 | 0.0736 | 1.31 | (0.97–1.75) | 0.0784 |
| rs249611 | G | Minor | 171:148 | 53.6 | 1.658 | 0.1978 | 1.16 | (0.92–1.43) | 0.2709 |
| rs17653687 | A | Major | 270:226 | 54.4 | 3.903 | 0.0482 | 1.19 | (1.00–1.42) | 0.0263 |
Abbreviations: PTDT, parental TDT; SNP, single-nucleotide polymorphism; T1D, type I diabetes; TDT, Transmission Disequilibrium Test.
Designations for the two SNPs that showed T1D association are shown in bold. Data indicating significant association in the DR3/DR4 subset are shown in bold.
Table 5.
Genetic association between TCF7 SNPs and T1D in trios where the affected offspring did not carry the HLA-DR3/DR4 genotype
| Marker | Over transmitted allele | is | T:U | Trans (%) | χ2 | TDT P value | Odds ratio | 95% CI | PTDT P value |
|---|---|---|---|---|---|---|---|---|---|
| rs30503 | C | Major | 336:283 | 54.3 | 4.538 | 0.0332 | 1.19 | (1.01–1.39) | 0.015 |
| rs30500 | C | Major | 403:377 | 51.7 | 0.867 | 0.3519 | 1.07 | (0.92–1.23) | 0.2787 |
| rs30499 | A | Major | 546:485 | 53.0 | 3.609 | 0.0575 | 1.13 | (0.99–1.27) | 0.051 |
| rs244948 | G | Major | 335:327 | 50.6 | 0.097 | 0.7559 | 1.02 | (0.87–1.19) | 0.8487 |
| rs5742913 | A | Minor | 345:273 | 55.8 | 8.388 | 0.0038 | 1.26 | (1.07–1.48) | 0.0075 |
| rs244692 | A | Major | 223:209 | 51.6 | 0.454 | 0.5006 | 1.07 | (0.88–1.28) | 0.6053 |
| rs151822 | T | Major | 534:475 | 52.9 | 3.45 | 0.0633 | 1.12 | (0.99–1.27) | 0.0442 |
| rs152404 | T | Major | 378:335 | 53.0 | 2.593 | 0.1073 | 1.13 | (0.97–1.30) | 0.0465 |
| rs152406 | G | Major | 146:133 | 52.3 | 0.606 | 0.4364 | 1.10 | (0.86–1.38) | 0.4429 |
| rs249611 | A | Major | 241:217 | 52.6 | 1.258 | 0.2621 | 1.11 | (0.92–1.33) | 0.13 |
| rs17653687 | A | Major | 399:355 | 52.9 | 2.568 | 0.1091 | 1.12 | (0.97–1.29) | 0.0307 |
Abbreviations: PTDT, parental TDT; SNP, single-nucleotide polymorphism; T1D, type I diabetes; TDT, Transmission Disequilibrium Test.
Designations for the two SNPs that showed T1D association are shown in bold. Data indicating significant association in the non-DR3/DR4 subset are shown in bold.
For the non-DR3/DR4 cases, however, the result was reversed, with the variant (A) allele of the C883A SNP significantly overtransmitted (transmission =55.8%, P =0.004) and the reference (A) allele of rs17653687 not significantly overtransmitted (transmission =52.9%; P =0.109). TDT analysis of the non-DR3/DR4 cases identified an additional, marginally significant association of the reference (C) allele of rs30503 (transmission =52%; P =0.033). Analyses of the data by PDT also demonstrated the significant overtransmission of the variant 883A allele of rs5742913 (P =0.008) to non-DR3/DR4 cases. PDT analyses also revealed several nominally significant T1D associations, including rs30503 (P =0.015), consistent with the TDT analysis, but also several SNPs (rs30499, P =0.051; rs151822, P =0.044; rs 152404, P =0.047, and rs17653697, P =0.031) that were not significantly T1D associated based on TDT analyses (Table 5). For all these SNPs, the reference allele is overtransmitted, unlike the C883A SNP, in which the variant A allele is overtransmitted. Given the strong LD (Figure 1) among these SNPs, the overtransmission of the reference allele at these SNPs may reflect the protective effect of the variant allele at one of these SNPs, the strongest effect being the intronic rs17653687 SNP. The C883A SNP in exon 2 is the only TCF7 SNP, from the set tested here, in which the variant allele is over transmitted. This overtransmission is seen overall and in the non-DR3/DR4 patient subset but is not seen in the subset of cases with the high-risk DR3/DR4 genotype.
Discussion
Many published association studies of candidate gene polymorphisms are limited in statistical power and, consequently, the reports of T1D association are often discordant. The extensive T1DGC collection of families has enabled replication analyses of a variety of reported associations (T1DGC Rapid Response Project). TDT and PDT analyses of the large combined dataset indicate that two SNPs (rs5742913 and rs17653687) in TCF7, a gene encoding a T-cell transcription factor, are significantly associated with T1D (Table 3). After stratification on the T1D high-risk HLA-DR3/DR4 genotype, the variant A allele of C883A (Pro–Thr) (rs5742913) is associated with T1D only in the non-DR3/DR4 patients, consistent with the initial report of TCF7 association15 whereas the rs17653687, an intronic polymorphism, shows a modest overtransmission of the reference allele, A, in both DR3/DR4 and non-DR3/4 patients. The association with T1D of the A allele of the C883A polymorphism, previously reported15 and replicated in this study, illustrates the value of stratifying T1D association data on the high-risk genotype HLA-DR3/DR4. A recent study of a variety of T1D candidate genes18 concluded that TCF7 could not be excluded as a T1D candidate gene, reporting evidence of association of rs5742913/C883A in a large case/control cohort (P =0.0005) but no association in a family collection (P =0.3486). This report, however, did not find evidence for increased T1D association of the TCF7 SNP in individuals with low HLA risk.
The role of polymorphism in the HLA class I and class II genes in T1D risk is likely to reflect the specificity of peptide binding and presentation by the HLA proteins, whereas polymorphism in other T1D susceptibility genes, such as PTPN22, CTLA4, and TCF7, may involve the overall level of T-cell activation. TCF7 is a T-cell transcription factor that activates expression of IL-1217 and has multiple isoforms. Resting T cells preferentially express inhibitory TCF7 isoforms and T-cell activation changes the isoform balance in favor of stimulatory TCF7 isoforms.19 The Pro–Thr polymorphism (rs5742913) at nucleotide position 883 of exon 2 is a non-conservative change that could potentially affect TCF7 function. As TCF7 upregulates genes in the Th1 pathway and the A allele (Thr) is associated with a Th1 disease, T1D, one possible explanation for the T1D association would be that the Thr variant has increased activity and, consequently, tilts the Th1/Th2 balance toward the Th1 pathway of T-cell differentiation.
On the basis of the LD patterns and the observed disease association patterns (Table 5), the associated SNP rs5742913 is a plausible causal polymorphism for T1D susceptibility. For the intron SNP rs17653687, the variant allele G appears to be ‘protective’ (OR <1.0 and undertransmitted). The association of this SNP cannot be attributed to LD with the C883A SNP. Additional studies will be required to validate this association and to explore effects on isoform production and other potential functional mechanisms.
In summary, these data support the hypothesis that TCF7 is involved in genetic risk to T1D, although the associations observed for both the intronic SNP rs17653687 and the nonsynonymous change encoded by rs5742913 are relatively modest (odds ratios ≤1.26). This is in stark contrast with the very large effects seen with class II haplotypes in the HLA region.
Materials and methods
Subjects
The T1DGC assembled a collection of 2295 affected sib-pair families for genotyping in this evaluation of candidate genes published earlier as containing variants associated with T1D. The samples and description of the families are provided in the T1DGC web site (http://www.t1dgc.org) and contain samples from nine cohorts. Details of the sample, quality control, and other aspects of the data can be found in this volume (Brown et al.20). Age of onset data were also included in the cohort and study data but were not used in the current data analyses reported here.
Genotyping
SNP genotyping was performed by the Broad Institute Center for Genotyping and Analysis (http://www.broad.mit.edu/gen_analysis/genotyping/). Aliquots of the T1DGC source 96-well plates were adjusted to 5–10 ng ml−1 in water in new 96-well plates. The iPLEX Gold chemistry of Sequenom’s MassARRAY platform (San Diego, CA, USA) was used for genotyping of all TCF7 SNPs as part of the larger set of T1DGC Rapid Response Project. Sequenom’s SpectroDesigner software was used for SNP assay design, and SpectroTyper 4.0 was used to call genotypes automatically, and followed by manual review. SNPs were also genotyped on the Illumina GoldenGate chemistry but not reported here.
Statistical analyses
A TDT21 was carried out on each of the markers as implemented by Haploview 4.1.22 The transmission proportions were used to compute odds ratios and 95% confidence intervals as described earlier.23 The parental TDT method, as implemented by Haploview 4.1, was also used as a family-based test of genetic association. The PDT incorporates parental phenotypes and, specifically, the parental genotype–phenotype correlation terms.24 This model is based on the between- within-sibship association model using a liability-threshold-model approach. The incorporation of parental phenotypes can considerably increase power, as compared with the standard transmission/disequilibrium test and equivalent quantitative tests, while providing both significant protection against stratification and a means of evaluating the contribution of stratification to positive results. This methodology enables the extraction of more information from existing family-based collections that are currently being genotyped and analyzed by use of standard approaches.
For pedigrees, full DRB1-DQB1 typing was available.25 T1D patients were stratified into those carrying DR3/DR4, defined here as carrying one DRB1*0301-DQB1* 0201 haploytpe and one DRB1*0401/02/04/05/08-DQB1*0302/04 or DQB1*0201 haplotype. All other patients were categorized as non-DR3/DR4.
Acknowledgments
We are grateful to all the T1D patients and family members who contributed samples and to all the participating T1DGC investigators and sites, listed at www.t1dgc.org. This research uses resources provided by the Type I Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute of Allergy and Infectious Diseases (NIAID), the National Human Genome Research Institute (NHGRI), the National Institute of Child Health and Human Development (NICHD), and the Juvenile Diabetes Research Foundation International (JDRF) and supported by U01 DK062418. We thank John Todd and Joanna Howson for careful reading of the manuscript and valuable discussion. Genotyping was performed at the Broad Institute Center for Genotyping and Analysis is supported by grant U54 RR020278 from the National Center for Research Resources.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Risch N. Assessing the role of HLA-linked and unlinked determinants of disease. Am J Hum Genet. 1987;40:1–14. [PMC free article] [PubMed] [Google Scholar]
- 2.Concannon P, Erlich HA, Julier C, Morahan G, Nerup J, Pociot F, et al. Type 1 diabetes: evidence for susceptibility loci from four genome-wide linkage scans in 1,435 multiplex families. Diabetes. 2005;54:2995–3001. doi: 10.2337/diabetes.54.10.2995. [DOI] [PubMed] [Google Scholar]
- 3.Noble JA, Valdes AM, Bugawan TL, Apple RJ, Thomson G, Erlich HA. The HLA class I A locus affects susceptibility to type 1 diabetes. Hum Immunol. 2002;63:657–664. doi: 10.1016/s0198-8859(02)00421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noble JA, Valdes AM, Thomson G, Erlich HA. The HLA class II locus DPB1 can influence susceptibility to type 1 diabetes. Diabetes. 2000;49:121–125. doi: 10.2337/diabetes.49.1.121. [DOI] [PubMed] [Google Scholar]
- 5.Valdes AM, Erlich HA, Noble JA. Human leukocyte antigen class I B and C loci contribute to Type 1 Diabetes (T1D) susceptibility and age at T1D onset. Hum Immunol. 2005;66:301–313. doi: 10.1016/j.humimm.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cucca F, Dudbridge F, Loddo M, Mulargia AP, Lampis R, Angius E, et al. The HLA-DPB1-associated component of the IDDM1 and its relationship to the major loci HLA-DQB1, -DQA1, and -DRB1. Diabetes. 2001;50:1200–1205. doi: 10.2337/diabetes.50.5.1200. [DOI] [PubMed] [Google Scholar]
- 7.Erlich HA, Rotter JI, Chang JD, Shaw SJ, Raffel LJ, Klitz W, et al. Association of HLA-DPB1*0301 with IDDM in Mexican-Americans. Diabetes. 1996;45:610–614. doi: 10.2337/diab.45.5.610. [DOI] [PubMed] [Google Scholar]
- 8.Lie BA, Todd JA, Pociot F, Nerup J, Akselsen HE, Joner G, et al. The predisposition to type 1 diabetes linked to the human leukocyte antigen complex includes at least one non-class II gene. Am J Hum Genet. 1999;64:793–800. doi: 10.1086/302283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nejentsev S, Gombos Z, Laine AP, Veijola R, Knip M, Simell O, et al. Non-class II HLA gene associated with type 1 diabetes maps to the 240-kb region near HLA-B. Diabetes. 2000;49:2217–2221. doi: 10.2337/diabetes.49.12.2217. [DOI] [PubMed] [Google Scholar]
- 10.Bottini N, Gloria-Bottini F, Borgiani P, Antonacci E, Lucarelli P, Bottini E. Type 2 diabetes and the genetics of signal transduction: a study of interaction between adenosine deaminase and acid phosphatase locus 1 polymorphisms. Metabolism. 2004;53:995–1001. doi: 10.1016/j.metabol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Marron MP, Raffel LJ, Garchon HJ, Jacob CO, Serrano-Rios M, Martinez Larrad MT, et al. Insulin-dependent diabetes mellitus (IDDM) is associated with CTLA4 polymorphisms in multiple ethnic groups. Hum Mol Genet. 1997;6:1275–1282. doi: 10.1093/hmg/6.8.1275. [DOI] [PubMed] [Google Scholar]
- 12.Mirel DB, Valdes AM, Lazzeroni LC, Reynolds RL, Erlich HA, Noble JA. Association of IL4R haplotypes with type 1 diabetes. Diabetes. 2002;51:3336–3341. doi: 10.2337/diabetes.51.11.3336. [DOI] [PubMed] [Google Scholar]
- 13.Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 14.Rich SS, Concannon P, Erlich H, Julier C, Morahan G, Nerup J, et al. The Type 1 Diabetes Genetics Consortium. Ann N Y Acad Sci. 2006;1079:1–8. doi: 10.1196/annals.1375.001. [DOI] [PubMed] [Google Scholar]
- 15.Noble JA, White AM, Lazzeroni LC, Valdes AM, Mirel DB, Reynolds R, et al. A polymorphism in the TCF7 gene, C883A, is associated with type 1 diabetes. Diabetes. 2003;52:1579–1582. doi: 10.2337/diabetes.52.6.1579. [DOI] [PubMed] [Google Scholar]
- 16.Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, et al. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- 17.Guler ML, Gorham JD, Dietrich WF, Murphy TL, Steen RG, Parvin CA, et al. Tpm1, a locus controlling IL-12 responsiveness, acts by a cell-autonomous mechanism. J Immunol. 1999;162:1339–1347. [PubMed] [Google Scholar]
- 18.Cooper JD, Smyth DJ, Bailey R, Payne F, Downes K, Godfrey LM, et al. The candidate genes TAF5L, TCF7, PDCD1, IL6 and ICAM1 cannot be excluded from having effects in type 1 diabetes. BMC Med Genet. 2007;8:71. doi: 10.1186/1471-2350-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman CM, Stolberg VR, Chiu BC, Lukacs NW, Kunkel SL, Chensue SW. CCR4 participation in Th type 1 (mycobacterial) and Th type 2 (schistosomal) anamnestic pulmonary granulomatous responses. J Immunol. 2006;177:4149–4158. doi: 10.4049/jimmunol.177.6.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown WM, Pierce JJ, Hilner JE, Perdue LH, Lohman K, Lu L, et al. the Type I Diabetes Genetics Consortium. Overview of the Rapid Response data. Genes Immun. 2009;10(Suppl 1):S5–S15. doi: 10.1038/gene.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM) Am J Hum Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 23.Kazeem GR, Farrall M. Integrating case-control and TDT studies. Ann Hum Genet. 2005;69:329–335. doi: 10.1046/j.1529-8817.2005.00156.x. [DOI] [PubMed] [Google Scholar]
- 24.Purcell S, Sham P, Daly MJ. Parental phenotypes in family-based association analysis. Am J Hum Genet. 2005;76:249–259. doi: 10.1086/427886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57:1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]