What is cell polarity?
Polarity in physics is defined as 'that quality or condition of a body in virtue of which it exhibits opposite or contrasted properties or powers, in opposite or contrasted parts or directions' [1]. Examples of polarized physical systems include magnets and batteries. In biology, polarity refers to the asymmetric distribution of subcellular components, resulting in an asymmetric cell morphology, behavior or function. In other words, in a polarized cell one region looks or acts differently from other regions of the cell. Prominent examples of polarized cell types are neurons and epithelial cells.
What is planar cell polarity?
Epithelial tissues are monolayers of cells that serve as barriers between different environments. Epithelia display two types of polarity: apical-basal polarity and planar cell polarity (PCP; also called tissue polarity). Apical-basal polarity refers to the asymmetry of epithelial cells along their cross-sectional axis, with the apical surface facing the external environment or lumen of a tissue and the basal surface contacting other cells (Figure 1a). Because of the barrier function of epithelia, the apical surface of an epithelial monolayer encounters a different environment than the basal surface. These two compartments have specialized properties that allow them to function in their respective contexts. For example, the apical surface of the intestine secretes enzymes into the lumen to aid in digestion and pumps ions to regulate lumen acidity, while the basal surface contains proteins that facilitate interactions with the underlying extracellular matrix.
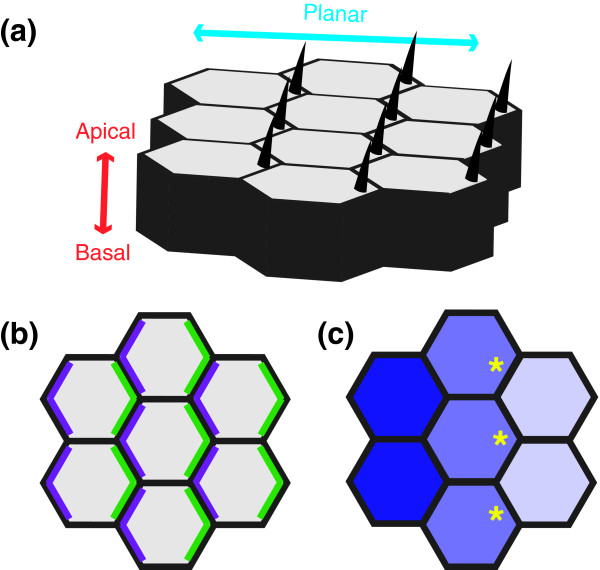
Figure 1.
Planar cell polarity. (a) Epithelial tissues display apical-basal and planar polarity. Hair structures are generated at the apical (top) surface and are absent from the basal (bottom) surface, demonstrating asymmetry along the apical-basal axis. Planar polarity is evident from the fact that the hairs are placed at the distal (right) side of each cell's apical surface and point in a distal direction. (b) Generating planar polarity through asymmetric protein localization. Schematic depicting a bird's-eye or planar view of the apical surface of the wing. The proximal (left) and distal (right) sides of each cell are defined by specific Frizzled system proteins, depicted in purple and green, respectively. (c) Generating planar polarity through protein gradients. In the Drosophila wing, the cadherin Dachsous (blue) is expressed in a decreasing gradient from proximal to distal (left to right). As a result, the cadherin Fat, which is also present in cell membranes, is predicted to be more active at the distal cell surface (yellow asterisks), where it encounters less inhibitory Dachsous protein on the adjacent cell.
Planar polarity refers to asymmetries within the plane of an epithelium. To find an example of planar polarity, simply look down at the surface of your arm. The hairs all point in one direction (more or less), demonstrating a coordinated asymmetry in the plane of the tissue. In addition to generating the obviously patterned organization of anatomical structures such as arm hair, planar polarity also regulates the shape and dimension of tissues during the major morphogenetic events of early development.
How do cells become planar polarized?
A common set of planar polarity genes has been shown to direct planar polarity in different contexts. These genes and their encoded proteins fall into two classes based on their genetic and molecular properties: the Frizzled system and the Fat system. The Frizzled system consists of the cell-surface proteins Frizzled, Flamingo and Van Gogh and the associated cytosolic factors Dishevelled, Diego and Prickle [2-6]. These components, also known as the core PCP proteins, promote cell polarity in part by adopting an asymmetric localization [2-6]. In the Drosophila wing epithelium, which produces a planar polarized pattern of distally directed hairs, the core PCP proteins localize to proximal or distal cell boundaries (Figure 1b). An asymmetric distribution of proteins related to Frizzled, Flamingo, Van Gogh, Dishevelled, and Prickle is also observed in some vertebrate tissues that display planar polarity [7,8]. The Fat system of planar polarity consists of the atypical cadherins Fat and Dachsous and the Golgi kinase Four-jointed [9,10]. No asymmetric distribution of these proteins has been reported.
How is planar polarity coordinated between cells?
A characteristic property of planar polarity systems is that polarity information in one cell can be transmitted to adjacent cells, a property that helps to align cells with their immediate neighbors. As a result, disrupting the Frizzled system in one cell can cause polarity disruptions up to several cell diameters away. For example, wild-type wing cells point their hairs toward cells that lack Frizzled and away from cells that lack Van Gogh, suggesting that cells can monitor the activity of their neighbors and orient their polarity accordingly [2,3]. These results have led to the longstanding idea that Frizzled - a well-known receptor for Wnt ligands - is active in a large-scale gradient that organizes planar polarity across hundreds of cells. However, there is currently no direct evidence for a gradient of Frizzled expression or activity, and the obvious candidates for generating such a gradient, the Wnt ligands, do not appear to be required for planar polarity in Drosophila.
In contrast, the Dachsous cadherin and the Four-jointed kinase are expressed in gradients in several tissues that display planar polarity. Dachsous protein at the surface of one cell can bind to Fat on the neighboring cell, an interaction that is thought to inhibit Fat activity [9,10]. Therefore, a gradient of Dachsous is predicted to result in lower Fat activity on the proximal side of each cell, even though the distribution of Fat is uniform (Figure 1c). Notably, flattening or reversing these gradients is sufficient to reorient planar cell polarity, suggesting that the Fat system could provide global direction to the Frizzled pathway [4,5,11]. Consistent with this idea, Frizzled signaling is altered in the absence of Dachsous and Fat and is required for the effects caused by removing Dachsous activity [12,13].
However, other evidence indicates that the Fat system can act independently of the Frizzled pathway to regulate planar polarity. For example, loss of function or ectopic expression of Fat pathway proteins in the Drosophila abdomen can reorient cell polarity even in tissues that lack Frizzled, and cells mutant for both Dachsous and Flamingo are more defective than cells completely lacking either protein alone, suggesting that these components function at least partly in parallel [14]. A resolution of this controversy will require identification of the signals that act downstream of Fat, to determine whether these signals regulate the level or localization of Frizzled activity or if they lead to a distinct cellular response.
How do planar polarity pathways affect tissue structure?
During development, many tissues increase in length and simultaneously narrow in width through polarized cell movements, cell shape changes, and oriented cell divisions [3]. The Frizzled pathway is required for a subset of these elongation events, including elongation by mesenchymal cells in the Xenopus notochord and the zebrafish dorsal midline [15-17]. Frizzled and Fat are also required for elongation by epithelial cells during the development of the Drosophila wing, the mouse neural tube, and the mouse kidney [18-20].
Although planar polarity pathways regulate elongation in both mesenchymal and epithelial tissues, the cell behaviors that lead to elongation in these contexts appear to be different. Epithelial cells remain interconnected by adherens junctions throughout tissue elongation, while mesenchymal cells are less tightly adherent and display classical migratory behavior. Therefore, planar polarity mechanisms can regulate a range of cell behaviors that contribute to tissue structure and organization. Planar polarity during body axis elongation in the Drosophila embryo does not require key players in the Frizzled PCP system [21]. This suggests that new molecular systems that govern planar polarity remain to be discovered. The guidance systems used in different contexts may reflect the types of spatial cues available, the speed required for cell polarization, and the downstream effectors that need to be mobilized to generate specific properties of tissue organization.
Can defects in planar polarity cause human disease?
Planar polarity is not only a complex biological process that integrates basic cell biology, cell-cell communication and dynamic changes in cell and protein interactions over time, but it is also directly relevant to human disease. Of note, some of the defects in mice mutant for planar polarity pathways appear to resemble specific human pathologies. Disrupting the Frizzled or Fat systems causes defects in closure of the mouse neural tube [7]. Neural tube defects are common congenital birth defects in humans, and mutations in VANGL1, a human homolog of Van Gogh, have been identified in patients with familial and sporadic neural tube defects [22]. Mice mutant for Diego- and Fat-related proteins have abnormal kidney development, resulting in a phenotype that resembles human polycystic kidney disease [23]. Mutations in homologs of Fat, Frizzled, Dishevelled, Flamingo and Van Gogh cause abnormal cochlear development in mice [8]. It will be of interest to determine whether abnormal planar polarity signaling is associated with similar conditions in humans.
How do you measure planar polarity?
Planar polarity can be measured by evaluating polarized cell behavior or morphology. For example, alterations in the striking pattern of Drosophila wing hairs have been used to identify genes that affect the planar polarity of the underlying cells, and alterations in embryo morphology can be used to assay planar polarized cell movements during tissue elongation. Planar polarity can also be measured directly by quantifying the localization of asymmetrically distributed proteins. Immunohistochemistry and live imaging of fluorescent reporters can be used to visualize proteins in their tissue context and evaluate their distribution. To quantify the extent of cell polarization, the strategy is to analyze protein localization in fluorescent images and calculate the ratio of fluorescence intensity between regions of the cell where the protein is present and regions where it is weakly localized or absent (Figure 2c). The fluorescence ratio provides a quantitative measure of asymmetric protein distribution.
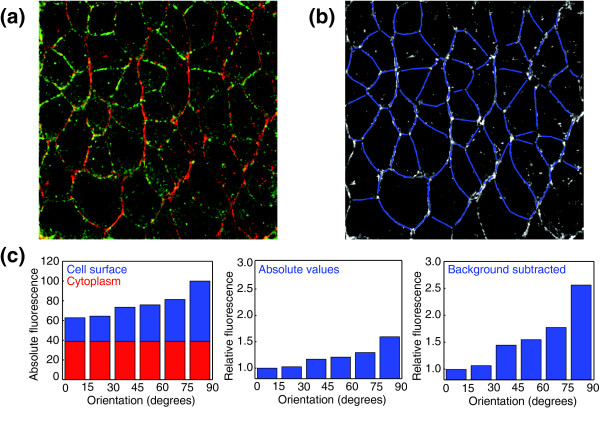
Figure 2.
Quantitative analysis of planar cell polarity. (a) Myosin II is planar polarized in the epidermis during elongation of the Drosophila embryo. Myosin II (red) localizes to vertical interfaces between anterior and posterior cells and Par3 (green) localizes to horizontal interfaces. Anterior is to the left and ventral is down in this image and in (b). (b) All cell interfaces in the image (red channel from (a)) were manually outlined in blue in order to quantify the orientation and mean fluorescence intensity of each interface.(c) The red channel in (a) and the blue lines in (b) were used to quantify the distribution of myosin II. Cell interfaces were grouped by orientation into 15° intervals. The absolute mean fluorescence intensity was quantified for each interval (left panel, sum of blue and red bars). Background was measured as the mean fluorescence intensity of the cytoplasm (left panel, red bars). Relative edge intensities were calculated using the raw data (center panel) or background correction (right panel). Values shown are relative to the mean fluorescence of horizontal interfaces (0-15°). The fold increase in myosin II at vertical interfaces (75-90°) in this example is 1.6 without background correction and 2.6 with background correction.
Why would you want to quantify planar polarity?
Quantifying the polarized distribution of a protein (or any other biological phenomenon) makes it possible to compare different samples and genotypes using statistical methods. Fluorescence ratios can reveal significant differences in the degree of polarity in different contexts, and thus have advantages over a qualitative plus/minus assessment. The use of fluorescence ratios also has the advantage of detecting planar polarity earlier than is apparent from assaying the cellular outcome of asymmetric protein activity. For example, core PCP proteins are asymmetrically localized in the Drosophila wing several hours before wing hair formation, and polarized movement of vesicles containing Frizzled can be detected even earlier by combining quantitative fluorescence measurements with live-cell imaging [24]. In the Drosophila embryo, cytoskeletal and junctional proteins localize to complementary planar domains within cells before the onset of polarized cell movements during axis elongation. Quantitative analysis revealed that the actin cytoskeleton is the first known structure to become planar polarized in this process [25]. A timeline of the onset of different molecular asymmetries can elucidate the symmetry-breaking events and signaling cascades that establish planar polarity.
Can planar polarity measurements be compared between experiments?
Yes, if this is done carefully. Differences in fixation, antibody penetration, choice of fluorophores or imaging conditions can all affect planar polarity measurements. To account for differences in sample preparation and illumination settings, it is necessary to subtract the background fluorescence before calculating polarity ratios (Figure 2). Background fluorescence should be estimated in the original image, without brightness or contrast adjustments, by calculating the average pixel value of a subcellular compartment where the protein is absent (for example, the cytoplasm when studying cortical proteins) or more conservatively, the mode or most frequent pixel value in the image. Background subtraction makes it possible to combine polarity measurements from multiple images to obtain higher statistical power.
Imaging settings should be set to cover the entire dynamic range of pixel values, avoiding saturated and underexposed pixels. Saturated pixels have the maximum brightness level that the detector can measure, and generally result when the exposure time is too long or the laser power or detector gain are set too high. When more than 5% of the pixels in an image are saturated, the polarity ratio is generally underestimated. Conversely, underexposed pixels with zero brightness level will lead to an overestimation of the polarity ratio. Acquiring 12-bit rather than 8-bit images can help prevent over- or underexposure of images by increasing the dynamic range.
References
- Merriam-Webster Online Dictionary. Merriam-Webster Online 2009. http://www.merriam-webster.com/dictionary/polarity
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/S1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009. in press . [DOI] [PubMed]
- Strutt H, Strutt D. Asymmetric localisation of planar polarity proteins: Mechanisms and consequences. Semin Cell Dev Biol. 2009. in press . [DOI] [PubMed]
- Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Rida PC, Chen P. Line up and listen: Planar cell polarity regulation in the mammalian inner ear. Semin Cell Dev Biol. 2009. in press . [DOI] [PMC free article] [PubMed]
- Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. Bioessays. 2005;27:1218–1227. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- Sopko R, McNeill H. The skinny on Fat: an enormous cadherin that regulates cell adhesion, tissue growth, and planar cell polarity. Curr Opin Cell Biol. 2009;21:717–723. doi: 10.1016/j.ceb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–968. doi: 10.1242/dev.125.5.959. [DOI] [PubMed] [Google Scholar]
- Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/S0092-8674(02)00658-X. [DOI] [PubMed] [Google Scholar]
- Casal J, Struhl G, Lawrence PA. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/S1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 2009. in press . [DOI] [PMC free article] [PubMed]
- Baena-Lopez L, Baonza A, Garcia-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol. 2005;15:1640–1644. doi: 10.1016/j.cub.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of innerear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/S1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M, Kirillova I, De Marco P, Merello E, Hayes JM, Wallingford JB, Drapeau P, Capra V, Gros P. Mutations in VANGL1 associated with neural - tube defects. N Engl J Med. 2007;356:1432–1437. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- McNeill H. Planar cell polarity and the kidney. J Am Soc Nephrol. 2009;20:2104–2111. doi: 10.1681/ASN.2008111173. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic S, Sanny JSP, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]