Abstract
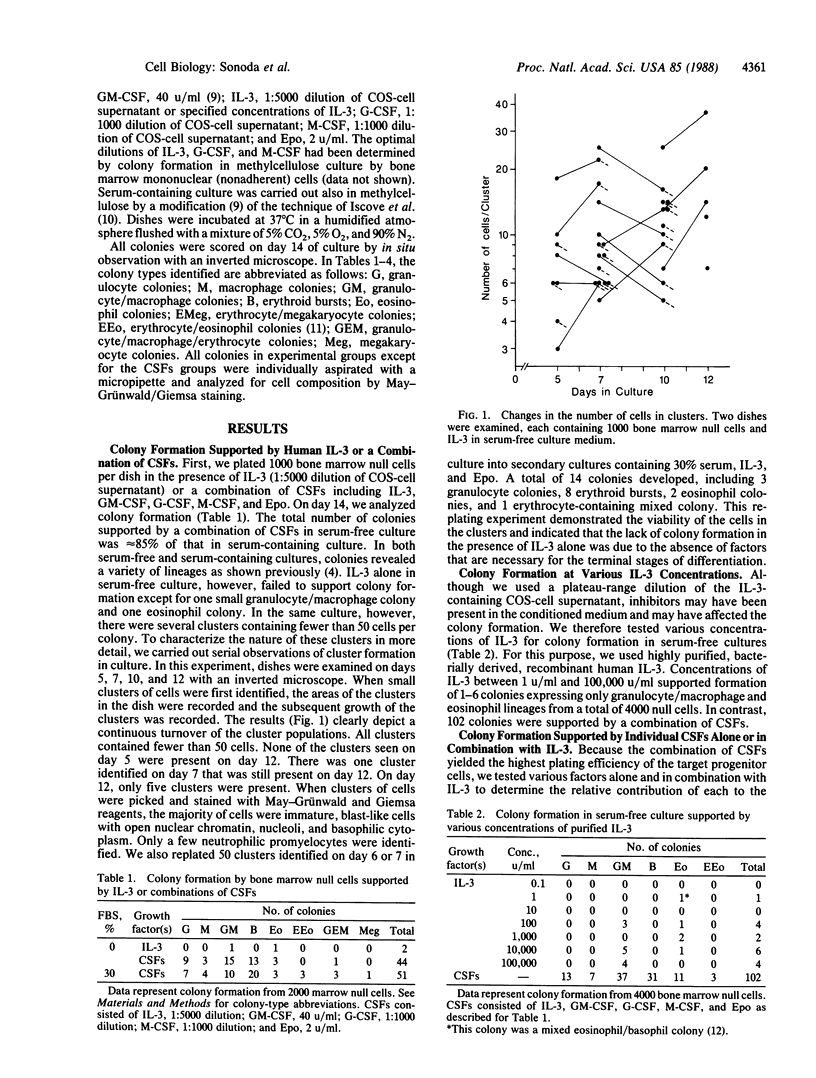
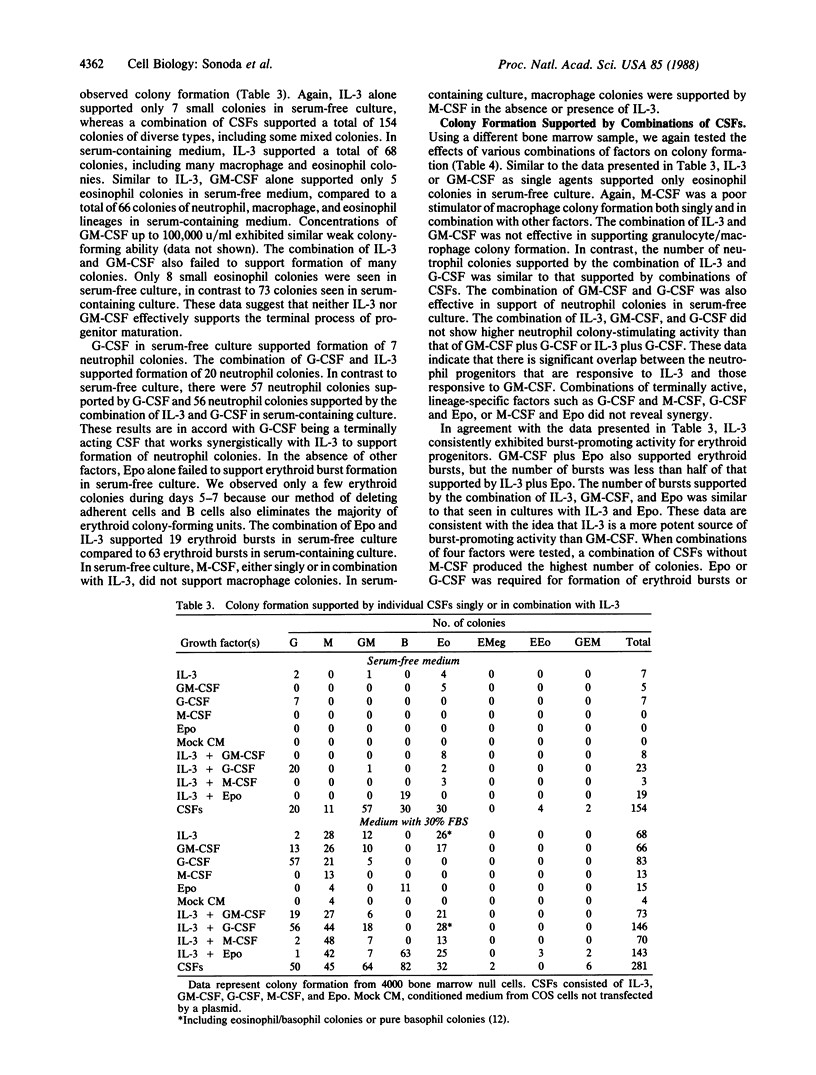
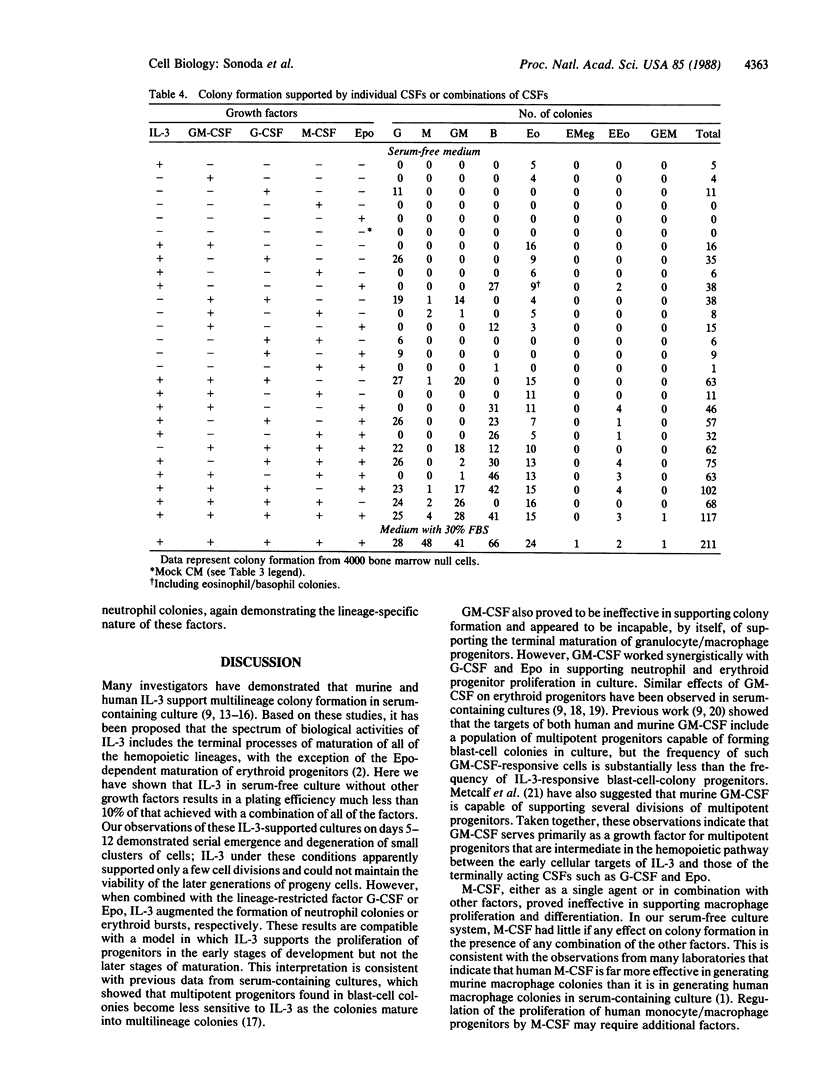
We have used a serum-free culture system for enriched human hemopoietic progenitors to analyze the developmental stages and lineage specificities of the human hemopoietic colony-stimulating factors. None of the individual factors alone efficiently supported hemopoietic colony formation. Neither interleukin 3 nor granulocyte/macrophage-colony-stimulating factor alone or in combination effectively supported proliferation of progenitor cells. However, when combined with granulocyte-colony-stimulating factor or erythropoietin, these factors yielded neutrophil colonies or erythroid bursts, respectively. Serial observations of interleukin 3-supported cultures revealed sequential emergence and subsequent degeneration of clusters of cells. These observations suggest that the primary targets of interleukin 3 and granulocyte/macrophage-colony-stimulating factor are multipotent progenitors at the early stages of development rather than cells in the terminal process of maturation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannistra S. A., Rambaldi A., Spriggs D. R., Herrmann F., Kufe D., Griffin J. D. Human granulocyte-macrophage colony-stimulating factor induces expression of the tumor necrosis factor gene by the U937 cell line and by normal human monocytes. J Clin Invest. 1987 Jun;79(6):1720–1728. doi: 10.1172/JCI113012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Donahue R. E., Emerson S. G., Wang E. A., Wong G. G., Clark S. C., Nathan D. G. Demonstration of burst-promoting activity of recombinant human GM-CSF on circulating erythroid progenitors using an assay involving the delayed addition of erythropoietin. Blood. 1985 Dec;66(6):1479–1481. [PubMed] [Google Scholar]
- Donahue R. E., Wang E. A., Kaufman R. J., Foutch L., Leary A. C., Witek-Giannetti J. S., Metzger M., Hewick R. M., Steinbrink D. R., Shaw G. Effects of N-linked carbohydrate on the in vivo properties of human GM-CSF. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):685–692. doi: 10.1101/sqb.1986.051.01.081. [DOI] [PubMed] [Google Scholar]
- Gregory C. J. Erythropoietin sensitivity as a differentiation marker in the hemopoietic system: studies of three erythropoietic colony responses in culture. J Cell Physiol. 1976 Oct;89(2):289–301. doi: 10.1002/jcp.1040890212. [DOI] [PubMed] [Google Scholar]
- Hara H., Ogawa M. Erythropoietic precursors in mice under erythropoietic stimulation and suppression. Exp Hematol. 1977 Mar;5(2):141–148. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Iscove N. N., Guilbert L. J., Weyman C. Complete replacement of serum in primary cultures of erythropoietin-dependent red cell precursors (CFU-E) by albumin, transferrin, iron, unsaturated fatty acid, lecithin and cholesterol. Exp Cell Res. 1980 Mar;126(1):121–126. doi: 10.1016/0014-4827(80)90476-0. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Iscove N. N. The role of erythropoietin in regulation of population size and cell cycling of early and late erythroid precursors in mouse bone marrow. Cell Tissue Kinet. 1977 Jul;10(4):323–334. doi: 10.1111/j.1365-2184.1977.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Koike K., Ihle J. N., Ogawa M. Declining sensitivity to interleukin 3 of murine multipotential hemopoietic progenitors during their development. Application to a culture system that favors blast cell colony formation. J Clin Invest. 1986 Mar;77(3):894–899. doi: 10.1172/JCI112387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Ogawa M., Ihle J. N., Miyake T., Shimizu T., Miyajima A., Yokota T., Arai K. Recombinant murine granulocyte-macrophage (GM) colony-stimulating factor supports formation of GM and multipotential blast cell colonies in culture: comparison with the effects of interleukin-3. J Cell Physiol. 1987 Jun;131(3):458–464. doi: 10.1002/jcp.1041310319. [DOI] [PubMed] [Google Scholar]
- Leary A. G., Ogawa M. Identification of pure and mixed basophil colonies in culture of human peripheral blood and marrow cells. Blood. 1984 Jul;64(1):78–83. [PubMed] [Google Scholar]
- Leary A. G., Yang Y. C., Clark S. C., Gasson J. C., Golde D. W., Ogawa M. Recombinant gibbon interleukin 3 supports formation of human multilineage colonies and blast cell colonies in culture: comparison with recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1987 Nov;70(5):1343–1348. [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Vadas M. A., Lopez A. F., Williamson D. J., Wong G. G., Clark S. C., Wang E. A. Biologic properties in vitro of a recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1986 Jan;67(1):37–45. [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R., Burgess A. W. Direct stimulation by purified GM-CSF of the proliferation of multipotential and erythroid precursor cells. Blood. 1980 Jan;55(1):138–147. [PubMed] [Google Scholar]
- Nakahata T., Spicer S. S., Ogawa M. Clonal origin of human erythro-eosinophilic colonies in culture. Blood. 1982 Apr;59(4):857–864. [PubMed] [Google Scholar]
- Prystowsky M. B., Otten G., Naujokas M. F., Vardiman J., Ihle J. N., Goldwasser E., Fitch F. W. Multiple hemopoietic lineages are found after stimulation of mouse bone marrow precursor cells with interleukin 3. Am J Pathol. 1984 Nov;117(2):171–179. [PMC free article] [PubMed] [Google Scholar]
- Rennick D. M., Lee F. D., Yokota T., Arai K. I., Cantor H., Nabel G. J. A cloned MCGF cDNA encodes a multilineage hematopoietic growth factor: multiple activities of interleukin 3. J Immunol. 1985 Feb;134(2):910–914. [PubMed] [Google Scholar]
- Sieff C. A. Hematopoietic growth factors. J Clin Invest. 1987 Jun;79(6):1549–1557. doi: 10.1172/JCI112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M., Ihle J. N. Permissive role of interleukin 3 (IL-3) in proliferation and differentiation of multipotential hemopoietic progenitors in culture. J Cell Physiol. 1985 Aug;124(2):182–190. doi: 10.1002/jcp.1041240203. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]



