Abstract
Globally, tuberculosis (TB) continues to exact an unacceptably high toll of disease and death among children, particularly in the wake of the HIV epidemic. Increased international travel and immigration have seen childhood TB rates increase even in traditionally low burden, industrialised settings, and threaten to facilitate the emergence and spread of multi-drug resistant strains. While intense scientific and clinical research efforts into novel diagnostic, therapeutic and preventative interventions have focused on TB in adults, childhood TB has been relatively neglected. However, children are particularly vulnerable to severe disease and death following infection, and those with latent infection become the reservoir of disease reactivation in adulthood, fueling the future epidemic. Further research into the epidemiology, immune mechanisms, diagnosis, treatment and prevention of childhood TB is urgently needed. Advances in our understanding of TB in children would provide wider insights and opportunities to facilitate efforts to control this ancient disease.
Keywords: Paediatrics, Tuberculosis, Diagnostics, Therapeutics, Prevention, Immune response, Future research priorities, Children, Epidemiology
Introduction
Since the declaration by the WHO of a ‘global TB emergency’ in 1993, a wealth of publications has addressed important aspects of the burden, management and control of tuberculosis (TB). In general, however, the emphasis has been on adult disease. By contrast, paediatric TB has been relatively neglected, mainly due to greater challenges in diagnosis and the lower priority traditionally afforded to children by TB control programmes. As a result both research and surveillance data in the field of childhood TB have been greatly limited. Nevertheless, with roughly a million cases estimated globally each year,1-4 and a much higher risk of severe disease and death among young children than adults, paediatric TB remains a public health emergency. This is particularly evident in developing countries with poor public health infrastructure. Priorities for future research should therefore enhance collaborations between developing and developed nations. Furthermore, by providing insights into current rates of transmission and circulating strains, TB in children remains a sentinel indicator of the effectiveness of TB control programmes. This review addresses some of the unique features of TB in children; summarises existing and novel diagnostic, therapeutic and preventative measures; and outlines important areas of future research.
Epidemiology
Transmission, exposure and infection
As in adults, infection with Mycobacterium tuberculosis (MTB) usually occurs by inhalation of tubercle bacilli in aerosolised respiratory droplets derived from an infectious case of pulmonary TB. Risk of infection is therefore dependent on the probability, duration and proximity of exposure to an infectious case, and on the infectiousness of the source.5-10 This is usually an adult with cavitatory pulmonary disease, although older children may also contribute to transmission.7, 11 Social factors, community TB prevalence and age determine where exposure is most likely to occur and may vary between communities.2, 10, 12 A household source is most commonly implicated for young children;10, 13, 14 older children are increasingly likely to be infected outside the household.10, 13, 15, 16 Poverty, poor housing, urban environments and overcrowding are all associated with increased transmission.3, 5, 9, 12, 17-24
Transmission within a community is measured by the Annual Risk of Infection (ARI).25 Infection rates rise with increased exposure in toddlers, around the ages of school entry and with increased social mobility in late teens and early adulthood.10 ARI is traditionally estimated using childhood tuberculin surveys, although this has limitations due to the poor specificity of the tuberculin skin test (TST), particularly where Bacille Calmette Guerin (BCG) vaccine is given at birth and non-tuberculous mycobacteria (NTM) are endemic. T-cell based interferon gamma release assays (IGRAs) may offer a more specific alternative,26, 27 but have not yet found a use in this context due to their cost, ethical concerns about venepuncture in healthy children,25 and uncertainty about the significance of a positive result for later development of active disease.
From infection to disease
Differences in the pathophysiology and clinical presentation of TB in children make diagnosis more challenging than in adults,28 and definitions of latent infection and disease are less clear cut.29 Nevertheless, following infection several factors appear to influence the balance of risk between latent TB infection (LTBI) or progression to active disease, including age10, 20 and nutritional,30 vaccination31 and immune status.20, 32-34 Children are at much higher risk of progression to active disease than adults.35 This risk is greatest for infants and children under 2 years of age.29, 36 Active surveillance data from the pre-chemotherapy era suggest the majority of children developed radiological abnormalities following infection, including 60-80% of children under 2 years; however <10% of these were notified, suggesting disease was controlled by the host immune response in most cases.10 This has implications for case definitions based on radiological findings. Overall the risk of disease was highest among infants and in late teens, with the lowest risk between 5 and 10 years - the so-called “safe school years”29 (table 1). Most disease occurred in the first year following infection.10, 29 Thus because disease in young children reflects recent infection, rather than secondary reactivation, the paediatric disease burden potentially provides a useful measure of current transmission within a community,12, 37 including multi-drug resistant (MDR) 12 38 and extensively drug resistant (XDR) strains. Untreated LTBI provides the seeds of the epidemic for the next generation.
Table 1. Risk of pulmonary and extra-pulmonary disease in children following infection with MTB.
Adapted from Marais et al. 2004 29
| Risk of disease following primary infection (%) | ||||
|---|---|---|---|---|
| Age at infection (years) | Disseminated/TBM* | Pulmonary TB | No disease | Comments |
| <1 | 10-20 | 30-40 | 50 | High rates of morbidity and mortality |
| 1-2 | 2-5 | 10-20 | 75-80 | |
| 2-5 | 0.5 | 5 | 95 | |
| 5-10 | <0.5 | 2 | 98 | “Safe school years” |
| >10 | <0.5 | 10-20 | 80-90 | Effusions or adult-type pulmonary disease |
TBM = TB meningitis
Impact of the HIV epidemic
The impact of the Human Immunodeficiency Virus (HIV) epidemic on the burden of childhood TB has been less well characterised than for adults.39 However the observed shift in disease burden to younger adults it has caused suggests that children are at particularly high risk of exposure as well as disease.40-42 Reported prevalences of HIV co-infection among children with TB range from <5% in industrialized settings to over 50% in some high burden African settings.2, 40, 42, 43 However it is often difficult to draw reliable inferences about the effect of HIV on TB incidence or risk from these observational data due to ascertainment bias (children with HIV are more likely to be investigated for TB); diagnostic bias (diagnosis is unreliable and is affected by HIV status); incomplete ascertainment of HIV status; and because denominator population data on the proportion of all children infected with HIV are usually lacking. Nevertheless an increased TB incidence and poorer outcome have been observed among HIV infected children in a variety of settings44-48 including an estimated 20 fold increased TB incidence associated with HIV infection in a study from South Africa.44 Methodological constraints in some studies may explain why this has not been a universal finding.40, 42, 49
Estimating the global disease burden
Poor case ascertainment, a lack of resources for active case finding in most settings, and limited paediatric surveillance data from TB control programmes all hamper efforts to define accurately the global burden of childhood TB.2, 23 50 Until recently under the WHO Directly Observed Treatment, Short Course (DOTS) strategy only smear positive cases have been reported for children,3, 51 yet smears are seldom performed in many high burden settings and most disease in children is smear negative. Disease burden estimates, derived using estimates of the proportion of cases that are smear positive by age, suggest that children accounted for nearly 900,000 (11 %) cases globally in 2000.2, 39 As in adults the majority of cases occurred in 22 high burden countries, where a combination of high transmission rates and a large proportion of the population under the age of 15 years mean children account for up to 25-40% of cases, with incidence rates for paediatric TB ranging from 60-600 per 100,000 per year.2, 23, 52 Soaring rates of childhood TB have also been reported in Eastern Europe in the wake of the explosive TB epidemic which followed the break up of the Soviet Union.53 Even traditionally low-burden countries have seen a rise in cases, mainly due to immigration from TB endemic areas. In most countries of Western Europe and North America, where children account for 4-7% cases, paediatric incidence rates vary from about 1 to 15 per 100,000 per year, although much higher rates are observed in some cities, notably London.43, 53
Limited survillance data prevent reliable estimates of the contribution of TB to childhood mortality. Nevertheless, pneumonia is the commonest cause of childhood death globally54 and TB is an important cause of pneumonia in many settings44, 55 so may contribute significantly to global childhood deaths. A necropsy study in Zambia found evidence of TB in 18% of HIV-positive and 26% of HIV-negative children dying of pneumonia.56
More robust regional data on the epidemiology of childhood TB are urgently needed to define the true burden of disease, and to characterise current transmission rates and circulating strains. Recent WHO guidance recommending reporting of all cases of childhood TB (smear positive, smear negative and extrapulmonary) in two age bands (0-4 and 5-14 years) takes an important step in this direction.57
Pathophysiology of TB in children
Following infection children have a higher risk not only of progression to disease,35 but also of extrapulmonary dissemination and death.58 Infants have a particularly high morbidity and mortality from TB.59 While many factors including host genetics, microbial virulence and underlying conditions that impair immune competence (e.g. malnutrition and HIV infection) determine the outcome of infection, it is likely that the high rate of progressive TB seen in young children is largely a reflection on the immaturity of the immune response.
Differences in childhood immune responses to TB
The alveolar macrophage is the first line of defense in the innate immune response to TB and plays a critical role in amplifying the response to infection. Studies in the animal and human host have consistently demonstrated reduced microbial killing60, 61 and diminished monocyte recruitment to the site of infection in infants compared to adults.62 Thus impairment of innate pulmonary defenses in the neonate and infant may allow mycobacteria to overwhelm the effects of the innate immune system prior to the initiation of an antigen-specific immune response.
Antigen presentation by dendritic cells (DC), the major antigen-presenting cell (APC) in the lung, and the efficiency with which naïve T cells respond to antigen, also appears less effective in infants and may contribute to the delay in initiating an appropriate antigen-specific response, resulting in development of active disease. Blood derived DCs are functionally immature at birth relative to adult DCs and continue to express a less differentiated phenotype throughout early childhood.63 Some studies also suggest that neonatal APCs lack the capacity to deliver important Th1 polarising signals to T-cells. Their capacity to synthesise interleukin (IL)-12, a key APC-derived cytokine, matures slowly during childhood64 and neonatal, monocyte-derived DCs have a specific defect in IL-12p35 expression.65 IL-12 is critical for the initial phases of Th1 polarisation and also for maintaining the efficiency of the interferon (IFN)-γ transcription machinery in Th1 effector cells.65
Neonatal CD4 cells appear intrinsically deficient in their capacity to express Th1 effector function, partially attributed to hypermethylation of the proximal promoter of the IFN-γ gene,66 resulting in a highly restricted pattern of IFN-γ response to a variety of stimuli.67, 68 CD154 (CD40 ligand) expression is also significantly reduced compared with adult cells.69
These findings of generally impaired cell-mediated immune responses in the neonate and young children raise the question of whether antigen-specific immune responses to mycobacteria are equally affected. Delayed type hypersensitivity (DTH) to purified protein derivative (PPD) may be absent in up to 40% of HIV negative children presenting with extrapulmonary TB,70 compounding the difficulties of diagnosis in young children. However, studies measuring responses to neonatal vaccination with M. bovis BCG demonstrate potent Th1 responses, possibly related to the potent APC-activating properties of BCG vaccine.71 Indeed while the long term efficacy of BCG vaccination may be limited, it does offer protection against disseminated disease in infants and young children. The risk of serious and potentially devastating disease is nevertheless still high in the first two years of life, underscoring the need for a better understanding of the determinants of host protection particularly in this vulnerable age group.
Host genetic susceptibility to TB
On the background of the developing immune system in children, a combination of host, bacterial, and environmental factors72-74 contribute to the immunological responses to MTB. Genetic as well as acquired defects in host immune response pathways greatly increase the risk of progressive disease.75-77 Results from genome wide linkage studies suggest that TB disease susceptibility is highly likely to be polygenic, with contributions from many minor loci.78 A large number of TB susceptibility markers have been identified from candidate gene studies as ‘disease-causing’ genes including TIRAP, HLA DQB1, VDR, IL-12β, IL12Rβ1, IFN-γ, SLC11A1 and MCP-1. These are summarised in table 2. However, to date the greatest evidence to support an underlying genetic basis for TB has come from the discovery of single gene defects predisposing to disseminated and often lethal mycobacterial disease. Most observations were initially made in children with reduced ability to activate macrophage antimycobacterial mechanisms through defects in the IFN-γ 76, 77, 79 / IL-1280 pathway resulting in severe mycobacterial infection. Since these original descriptions, mutations in five susceptibility genes have been described 81 confirming that upregulation of the macrophage through the IL-12/23-IFN-γ pathway is a fundamental step in the containment of infection with mycobacteria.
Table 2. Examples of candidate genes and susceptibility to TB disease.
| Candidate gene | Type of study | Population/Region/Country | Reference | Comment |
|---|---|---|---|---|
| HLA DQB1 | Case control | Cambodia | Goldfeld172 Delgado173 |
|
| IFN-γ | Case control and family based study | South African | Rossouw175 |
|
| IFN-γR1 | Case control | West African | Cooke176 |
|
| IL-12β | Case control | Hong Kong Chinese | Tso177 |
|
| IL-12Rβ1 | Family based study | Morocco | Remus178 |
|
| TIRAP | Case control | UK, Vietnam, west & east African | Khor179 |
|
| SLC11A1 | Linkage | Canadian Aboriginal | Greenwood180 |
|
| MCP-1 | Case control | Mexico, Korea | Flores-Villaneuva181 |
|
| VDR | Case control | Gujerati Indian | Wilkinson89 |
|
HLA DQB1 - Major histocompatability complex, class II, DQ beta 1
MCP-1 - Monocyte Chemoattractant Protein-1
SLC11A1 - Solute Carrier Family 11, member 1
VDR - Vitamin D Receptor
TIRAP - Toll-Interleukin 1 Receptor Domain Containing Adaptor Protein
R- Receptor
Whilst there is a growing adult literature on the role of candidate genes from this pathway, data from children is scarce. This is surprising given the marked differences in TB pathophysiology in children, which may also reflect differences in genetic factors. Further studies of TB genetics in well-defined paediatric populations are therefore needed.
HIV
No other factor has fuelled the global TB epidemic more than the HIV co-epidemic. Studies demonstrating a higher risk of TB among HIV infected children44-47 highlight the essential role of cell mediated immunity (CMI) in preventing mycobacterial dissemination.82 Poor CMI in HIV co-infection often results in disseminated disease, especially in advanced stages of HIV-infection, resulting in poorer survival compared to HIV-negative children.48 Risk of active TB in HIV co-infected children is related to both CD4 count and more indirectly also to viral load.83 Conversely, restoration of cellular immunity with anti-retroviral therapy partially reverses TB susceptibility.84-87
Nutrition
Several observational studies from adults and children show an association between malnutrition and TB,30 although proving the direction of a causal link is challenging as TB in itself causes wasting. Diagnosis is further complicated by frequently false negative TST in malnutrition, reverting to positivity only once nutrition has improved. Nevertheless these observational data, coupled with experimental animal data and impaired CMI observed in malnutrition, support its role as a risk factor for childhood TB.30 A single intervention trial in New York in the 1940s found an odds ratio of 5.6 for development of TB among household contacts on placebo compared with those on vitamin and mineral supplements during a five years follow up.88 However the effect of differing types and degrees of malnutrition, and the population attributable risk due to malnutrition in communities where both are endemic, remain to be defined.
Among micronutrients vitamin D deficiency has been most extensively studied, and shown to be associated with TB in UK immigrants.89 Its active metabolite 1alpha, 25-dihydroxy-vitamin D modulates the host response to TB infection in numerous ways including the induction of antimicrobial peptides such as Cathelicidin LL-37. 90-93
Host-Pathogen interactions
Along with host immune responses, mycobacteria themselves have equally evolved as strong players in the battle for containment or dissemination.
Mycobacterial genetic variability, including Large Sequence Polymorphism (LSP) and Single Nucleotide Polymorphism (SNP),94-96 allows survival adaptation to environmental challenges. Six phylogeographical lineages of MTB have been defined by LSPs.97 Each appears to have evolved to adapt to specific ethnic and geographical host populations, as a result of host-pathogen compatibility. 97-99 This may have implications for TB control and the development and evaluation of new vaccines in different geographical areas.
The relationship between MTB strain genotype and clinical manifestation of disease is poorly documented in children. A study in the Western Cape Province of South Africa demonstrated that the Beijing and Haarlem genotype families are significantly associated with drug resistant TB in children.100 The high prevalence of Beijing and Latin American Mediterranean (LAM) strains in children reflects considerable transmission of these genotype families in this setting.100, 101
Genetic markers of virulence and transmissibility,102 and the ability to modulate host cellular immunity have been described for the W/Beijing strain, HN878.103-105 Similarly the East African-Indian lineage is characterized by an LSP conferring an immune subverting phenotype that contributes to its persistence and outbreak potential of this lineage.106 Strain differences in immunogenicity may result in reduced detection by TST107 as documented in a London school contact tracing investigation - an extremely worrying phenomenon which may lead to underestimates of the true global burden of TB and underscores the need for new diagnostics.
Most studies of strain-specific responses are derived from adult TB cases, and it remains to be established whether results are equally applicable to children. Further research to characterise strain differences in pathogenicity and induction of immune responses should include children as well as adults.
Clinical spectrum of disease
The clinical spectrum of childhood TB also reflects differences in the balance between the pathogen and the host immune response, with more severe disease resulting from either poor or ‘over-exuberant’ attempts to contain the disease. Many cases of primary TB infection in children are asymptomatic, self-healing and remain completely unnoticed or accidentally discovered at a later stage.10 In previously healthy children it remains largely unknown what determines the differences in the host/pathogen interactions that leads to successful containment as opposed to progressive disease, however age and immunodeficiency are important factors. Thus while an exuberant immune response in immunocompetent adolescents tends to result in adult-type, cavitating disease,108 in young children and/or HIV co-infection poor CMI is thought to allow unrestrained proliferation of bacilli with progressive parenchymal lung damage (with or without cavity formation)109 and dissemination (figure 1).110 While dissemination can occur to almost any site, TB meningitis (TBM) is one of the commonest consequences of extrapulmonary TB and develops three to six months after primary infection.111 It is also the most severe and potentially devastating form of childhood TB with mortality or significant long term neurological sequelae occurring in almost 50% of cases.112 Anatomical differences in children also modify the presentation of TB compared with adults. Complications arising from enlarging lymph nodes and small airways are common in children less than five years of age.29, 113 Post-primary TB can result in upper-lobe pulmonary consolidation and cavitation with highly infectious patients, more likely to be seen in older children.
Figure 1.
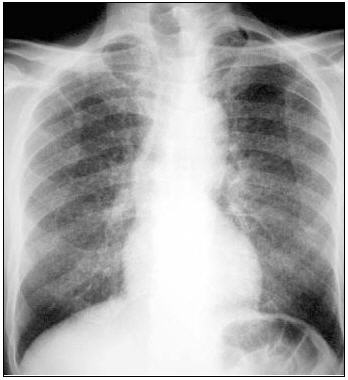
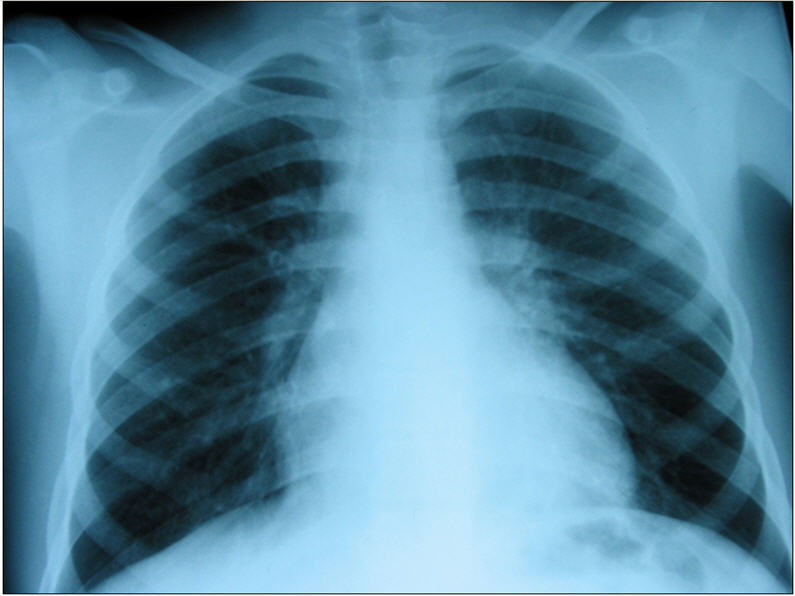
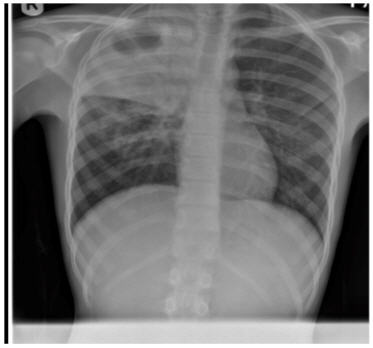
Radiological presentation of TB in childhood. (a) miliary TB; (b) hilar lymphadenopathy; (c) cavitating lung disease (adult type).
HIV infection often mimics TB associated signs and symptoms, such as weight loss, failure to thrive and chronic pulmonary symptoms, corroborating the diagnostic difficulties (reviewed in references45, 49) In turn, the treatment of HIV with ART can result in unmasking signs and symptoms of underlying LTBI or active TB in the form of immune reconstitution disease (IRD).87
Diagnosis of TB in children
Diagnostic difficulties pose the greatest challenge to childhood TB management.114 TB is often not considered in the differential diagnosis in children, especially in low endemic settings. TB can mimic many common childhood diseases, including pneumonia, generalised bacterial and viral infections, malnutrition and HIV. However the main impediment to the accurate diagnosis of active TB is the paucibacillary nature of the disease in children. Younger children also produce smaller amounts of sputum, which is usually swallowed rather than expectorated. Bacteriological samples may be collected by conducting early morning gastric washings, a fairly unpleasant procedure that requires hospital admission and overnight-fast for up to three consecutive nights. Consequently bacteriological confirmation is the exception rather than the rule with only 10-15 % of sputum samples revealing acid fast bacilli (AFB) and culture remaining negative in around 70% of cases with probable TB.115 Without a definitive diagnosis treatment is therefore often initiated on clinical judgment, aided by algorithms based on exposure history, clinical features, chest x-ray (CXR) and TST.116, 117 Several approaches have been taken to improve the diagnosis -
a) Improving specimens obtained
Sputum induction by nebulised hypertonic saline has been reported to have a higher yield than gastric washings;115 however, feasibility of this technique has not been tested outside hospital settings and infection control procedures must be strictly adhered to. The string method,118 like gastric washings, obtains swallowed specimens from the stomach and claims to be better tolerated, but diagnostic studies in children are lacking.
b) Improving bacteriological detection, including rapid resistance analysis
Recent advances in bacteriological and molecular methods for the detection of MTB in patient samples aim to identify drug-resistance in parallel with detection of MTB. These include the Microscopic Observation Drug Susceptibility assay (MODS),119 more sensitive PCR techniques120 or phage-based tests such as FASTPlaque.121 This represents laudable progress, particularly in the context of increasing drug resistance. Calorimetric culture systems such as the TK medium122 and electronic-nose technology123 are also under investigation. Among adults MODS appears to be at least as sensitive as gold standard liquid culture methods.119 Data comparing its performance in children is more limited, but MODS has been evaluated in a paediatric hospital setting and found to be more sensitive than solid media in one study.124 Data validating other new methods in paediatric specimens are also lacking, yet performance may be affected by the paucibacillary nature of childhood TB. The lowest limit of detection of TB by the electronic nose for example has been reported to be 104 CFU/ml of sputum for example which is just within the range of the expected bacillary burden in paediatric specimens.123 Validation of these assays on paediatric samples is a research priority.
c) Improving immunological diagnosis
In addition to the traditional TST, which is known to lack both sensitivity and specificity, blood based assays have recently become available. These T-cell assays rely on stimulation of host blood cells with MTB specific antigens and measure production of IFN-γ. Numerous published studies compare the two available commercial assays, T Spot TB (Oxford Immunotec) and Quantiferon-Gold IT (Cellestis), with the TST for both detection of active disease and LTBI. 27, 125 T-cell assays have proven to be more specific than the TST,7, 126 but are yet unable to distinguish between active disease and LTBI. Interpretation therefore remains dependent on the clinical context. Few studies present paediatric data however none have provided an assessment of age-related performance of these assays, and reservations remain regarding their performance in very young children and in immunocompromised populations, such as those with HIV.127, 128
The TST has also been found to be a very poor indicator of TB infection in young children with HIV. Although studies from South Africa129 indicated increased sensitivity of the T Spot assay compared to TST, the data were not stratified by CD4 count. The costs and technical demands of IGRA assays however will probably limit their wider use in resource-poor settings, where better tests are most needed.
Antibody-profiling in blood 130, 131 or antigen-detection in urine have been attempted by many groups,132 mainly in adult patients. A recent review of serological tests concluded that commercial antibody detection tests for extrapulmonary TB have no role in clinical care or case detection.133
The search for novel biomarkers in blood or urine that can reliably distinguish active from latent TB in children with and without other co-infections remains an important global goal. Well-defined cohorts of paediatric patients in TB-endemic and non-endemic settings will be essential for initial screening and future validation of such potential markers. In the meantime, the diagnosis of TB in children in resource-poor countries continues to rely on practical algorithms, which lack standard symptom definitions and adequate validation.116 This poses an increased challenge in the context of HIV infection.
Diagnosis of latent infection in children
LTBI, in children as in adults, lacks a diagnostic gold standard. The diagnosis is usually pursued after a documented household exposure, or to evaluate if chemoprophylactic therapy is indicated in the context of immunosuppression. In this setting, pre-existing MTB specific host immune responses are measured to confirm previous infection. Data in adults have confirmed that IGRA are more sensitive and specific than the TST 27, 125 in this context. Preliminary data suggest IGRA also perform better in children but age-related data are still sparse. Longitudinal studies assessing their positive predictive value for the development of active TB are required in both TB-endemic and low-incidence countries, as the continued exposure in TB endemic settings might yield very different results, compared to the “one-off” exposure more typically encountered in non-endemic countries.
Treatment of TB in children
The aim of anti-TB treatment (ATT) in adults and children alike is to cure the patient of TB, reduce spread to others and avoid the development of drug resistance within the community. For the first time in three decades there is a promising pipeline of new anti-TB agents at various stages of development, and several have already entered clinical trials.134
Clinical trials of ATT are usually carried out in adults with microbiologically proven pulmonary TB, allowing objective microbiological case definitions and treatment outcomes. The difficulty achieving a clear microbiological diagnosis in the majority of paediatric cases severely hampers trials in children, as microbiological case definitions and treatment endpoints are impractical. As a result few randomised controlled trials have been conducted in children to establish optimum ATT regimens134 and current treatment guidelines are largely inferred from adult data. Furthermore, although first line drugs have scarcely changed for over three decades, there is still a lack of pharmacokinetic studies in children, particularly in the context of HIV infection and malnutrition.
TB treatment consists of two phases - an intensive phase, using a combination of bactericidal drugs to kill the rapidly growing bacilli and a continuation phase using fewer drugs to eradicate the slower growing persistent bacilli.135 National recommendations still vary considerably in treatment duration and drug regimens used.136 In keeping with studies in adults, observational data in children suggest that for drug-susceptible pulmonary TB (PTB), 6 months’ isoniazid and rifampicin combined with 2 months’ pyrazinamide initially has a 99 % cure rate; however quadruple therapy with the addition of ethambutol or streptomycin in the intensive phase is generally recommended where there is high risk of INH-drug resistance. Although some authorities have recommend longer treatment courses for TBM, disseminated TB or bone TB, evidence of their superiority is lacking. The adjunctive use of steroids in TBM has been shown to reduce death and severe disability.137 DOTS may also improve treatment outcomes in paediatric settings.138
Fixed dose combination tablets contribute to increased adherence to treatment regimens, but the significant differences in absorption, distribution and excretion of pharmacological agents in children of various ages might require dose-adjustments. Existing fixed dose combinations contain 4-6 mg of isoniazid per kilogram, but published pharmacokinetic studies in children support a standard dose of 10 mg/kg or even 20 mg/kg isoniazid as younger children acetylate this drug more quickly.139 Pyrazinamide similarly has a lower half-life in children140 who also have lower peak serum levels of ethambutol than adults.141 Equally toxicity may vary in children, both in a beneficial and negative fashion. This emphasizes the importance of specific paediatric pharmacokinetic studies of all ATT, new and old.
Treatment of TB in HIV co-infected children
HIV co-infection is increasingly common in TB endemic areas, often requiring simultaneous therapy. Pharmacokinetic interactions between ATT (in particular rifampicin) and ART, and similar side-effects profiles of many of the drugs pose special challenges. Latest WHO recommendations advise starting ART once ATT is established (after a period of 2-8 weeks) for all WHO clinical stage 4 HIV-infected children and stage 3 children with advanced or severe immunosuppression; for children in WHO clinical stage 3 with mild or no immunosuppression, ART may be deferred until 6 months of ATT are completed.142 On-going prospective trials involving adults and children in TB/HIV endemic countries, wish to inform future guidelines for the ideal timing of the initiation of anti-retroviral therapy (ART) in patients with HIV receiving TB therapy. Unpublished results from prospective trials show that high mortality is associated with TB in advanced stages of HIV-disease in children who do not receive ART promptly. However, these findings have to be considered in the light of developing IRD, which is particularly common in this group. Further research is required to improve our understanding of IRD in children.87
Where available, therapeutic drug monitoring (TDM) should be undertaken when children are receiving concomitant ART and ATT. TDM data from ethnically similar children in resource-rich countries may in the future inform dosing recommendations in resource-poor settings where TDM is not available.
Treatment of LTBI
Treatment of LTBI, also known as chemoprophylaxis, is important to prevent future disease activation. The fact that over 50% of hospitalized children with culture-confirmed TB have a reported close TB contact and do not receive chemoprophylaxis, is an indication of the important missed opportunities using existing public health interventions. For the last 20 years WHO guidelines recommend all children under 5 years in close contact with an infectious (usually smear positive) case receive 6 months isoniazid once active disease has been excluded. Isoniazid monotherapy for 6-9 months has been proven to reduce the TB risk in exposed children by >90% with good adherence.143 More recent studies suggest that 3 months of combined isoniazid and rifampicin are equally effective.144
In a recent study with very short follow-up, continuous isoniazid prophylaxis for HIV-infected children without documented evidence of latent infection, but living in an environment of high exposure, has also been shown to reduce overall morbidity and mortality from TB and other infections.145 Further trials in HIV-infected children receiving ART are ongoing. Recommendations for chemoprophylaxis will continue to differ in TB-endemic and non-endemic settings, because of the perceived risk of exposure. Whilst most paediatricians in Europe and North America would advocate chemoprophylaxis for HIV infected, TB-exposed children only, this needs to be interpreted with caution if the exposure is potentially ongoing or recurrent, and the ability to distinguish LTBI from active disease is limited. In this context, many colleagues in TB-endemic settings are reluctant to place children on chemoprophylaxis because of the potential emergence of resistant strains, if indeed the child has active disease instead of LTBI.
Drug resistant TB
The emergence and spread of resistant TB poses a serious threat to TB control. Rates of single MDR (resistance to both isoniazid and rifampicin) including XDR (also resistant to fluoroquinolones and at least one second-line injectable agent such as amikacin, kanamycin and/or capreomycin) are rising in many parts of the world, particularly the former Soviet Union (e.g. 28% isolates in Kazakhstan)146 and southern Africa.147 Mass immigration from these countries contributes to the increasing incidence observed in Western Europe (currently <1% isolates).53, 146
Drug resistance originates mainly in adult patients with high bacillary loads who receive inadequate ATT or are poorly compliant with treatment. Acquisition of resistance rarely occurs in children due to the paucibacillary nature of their disease; overall children may also be subject to less selection pressure from ATT therapy. Thus most resistance in children is due to primary transmission of a resistant organism, and MDR- / XDR-TB rates in children reflect community transmission rates. Diagnosis requires a high index of suspicion as the culture yield in children makes definitive microbiological confirmation difficult. Resistance should be suspected if an index case has known resistant TB; the child shows initial improvement on ATT and then deteriorates; or there is no response to initial treatment. Acquired resistance is well described in HIV co-infected adults previously treated for TB, possibly due to malabsorption of ATT.148 The presence of acquired resistance in the paediatric population is reported and in particular children with TB/HIV co-infection should be closely monitored.149
Treatment of MDR-TB should be discussed with a TB expert and needs to be guided by the resistance profile of isolated strains in individual cases. Current guidelines recommend using at least four drugs to which the patient is naïve including an injectable and a fluoroquinolone, in an initial phase of at least 6 months; followed by at least three of the most active and best tolerated drugs in a 12-18 month continuation phase. Standardised regimens have been developed for settings where drug susceptibility testing is not available.150 Six classes of second-line drugs (SLDs) are available151 but experience in children is limited for the majority and multi-centre paediatric trials are needed. Under optimum circumstances MDR-TB responds well to appropriate therapy. However delays in diagnosis and treatment, adherence issues, and a lack of child-friendly formulations and strategies for DOTS all frequently complicate management and contribute to a high morbidity and mortality.152, 153
WHO currently recommends avoidance of chemoprophylaxis in cases of contact with known MDR-TB and to observe for 2 years if clinically well. Children with latent MDR-TB infection become the reservoir for future transmission following disease reactivation in adulthood, emphasizing the need to further research and improved management of MDR-TB infection in children, both at the clinical and operational level.
Control and Prevention
BCG Vaccination
Several large-scale randomized clinical trials, performed in different settings worldwide, have suggested a protective efficacy of BCG vaccination against pulmonary TB ranging from 0 to 80%.31 This observed variability in vaccine efficacy has been attributed to numerous factors,154 including strain specific immunogenicity, technique of vaccine administration,155 age at vaccination, genetic differences between populations,156 host nutritional factors, host co-infection by parasites,157 exposure to environmental mycobacteria and genetic variation in MTB strains.97 Overall protective efficacy is estimated to be about 50%.31, 158 However, the greatest effect appears to be in preventing severe disseminated disease in young children, including TBM and miliary TB.31, 159, 160 The longevity of protection is less clear. A meta-analysis of early trials suggested that protective immunity lasts less than 10 years,161 however data published more recently suggests protection may persist for 50-60 years.162
WHO guidelines recommend administration of BCG soon after birth to all infants in countries with a high TB prevalence. Additional protection by revaccination with BCG has not been demonstrated.163 To date, the efficacy of the BCG vaccination has not been determined in HIV infected individuals in whom the immune responses to BCG may be reduced,164 although this is the subject of ongoing trials. Due to the risk of disseminated BCG disease which may rarely complicate use of this live vaccine in immunocompromised individuals, BCG vaccination is no longer recommended in children known to be HIV-infected.164, 165 In practice, this has had little impact in HIV-endemic countries, where the HIV-status of the baby is rarely established at birth, the usual time of BCG vaccination.
Development of new vaccines
The global commitment of WHO and the Stop TB166, 167 campaign has spurred on the efforts of the international research community to develop a more effective anti-TB vaccine by the year 2015.167 The new vaccine candidates currently undergoing pre-clinical and clinical trials are listed in Table 3. In view of the proven efficacy of existing BCG vaccine in preventing disseminated TB in children and reducing child mortality,160, 168 two conceptually different strategies have been pursued: firstly, the development of ‘priming vaccines’, which, it is hoped, will replace BCG by providing better and longer protection; secondly, the design of ‘booster vaccines’ to boost pre-existing BCG-derived immunity. Novel vaccines currently under development all use a “booster-strategy” after priming with BCG in infancy.169 As the current candidates are progressing through phase I and II trials, including studies in HIV-infected individuals and age-de-escalation, it is most likely that more than one vaccine will progress into phase 3.170
Table 3. Novel TB vaccines presently in clinical trials or expected to be in clinical trials within the next 12 months.
| Vaccine Name | Construct | Principal | Progress |
|---|---|---|---|
| H1IC | Recombinant fusion protein of ESAT-6 and AG85B in IC31 adjuvant | Subunit vaccine | Completed Phase 1 in the Netherlands, ongoing Phase 1 in Ethiopia 182 |
| H1LTK | Recombinant fusion protein of ESAT6 and AG85B in LTK adjuvant, intranasal vaccine | Subunit vaccine | In Phase 1 in the UK 183 |
| MTb72f | Recombinant fusion protein of Rv1196 and Rv0125 plus immunostimulant in oil-in-water emulsion | Subunit vaccine | In Phase 1 in Switzerland 184 |
| Aeras402 | Recombinant fusion protein of AG85A, AG85B, TB10.4 coupled with replication-deficient adenovirus-35 | Live, non-replicating vector | Completed Phase 1 in USA, Ongoing Phase 1 in South Africa 185 |
| MVA-85A | Recombinant AG85A expressed through replication-deficient modified vaccinia Ankara | Live, non-replicating vector | Completed phase 1 in UK, Gambia, South Africa, Starting phase 2 in same settings 186 |
| HyVac 4 | Recombinant fusion protein of AG85A plus TB10.4 | Subunit vaccine | Expected to start Phase 1 in 2008 187 |
| VPM1002 | Recombinant BCG vaccine expressing listeriolysin | Live attenuated | Expected to start Phase 1 in 2008 188 |
New research is directed at the development of a multistage TB vaccine containing latency antigens, an attractive concept, which is actively being pursued.171 Such a vaccine could be used as a booster vaccine with the goal of preventing new infections in those uninfected with MTB and to prevent reactivation in those with LTBI. Unfortunately, the lack of reliable correlates of protective immunity currently remains a major obstacle to predict vaccine efficacy in all TB vaccine trials for both adults and children.
Conclusion: future research and public health priorities
Despite real progress made by the WHO DOTS strategy in recent years, the global TB epidemic remains an ugly blot on the international public health landscape. TB in children presents particularly difficult challenges, but research priorities and advances in paediatric TB research59 may also provide wider insights and opportunities for TB control. Some important research priorities are summarized in Table 4. While a new vaccine to prevent TB is the ultimate goal, better diagnostics would probably represent the next most important step forward, and as such require urgent prioritisation. A reliable diagnostic tool would not only improve individual case management, but also provide a more robust case definition for much needed drug and vaccine trials, and for studies of TB epidemiology and correlates of protective immunity in childhood. Regional data on the epidemiology of childhood TB would in turn help to inform public health policy by providing a window on current transmission and the effectiveness of control strategies and by identifying children with LTBI for chemoprophylaxis to limit the future propagation of the epidemic.
Table 4. Future research and public health priorities for childhood TB.
| Epidemiology |
|
| Host factors |
|
| Pathogen factors |
|
| Diagnostics |
|
| Treatment |
|
| Prevention |
|
Footnotes
Conflict of Interest
The authors do not have any conflicts of interest
Search strategy and selection criteria:
Data for this review were identified by searches of PubMed and EMBASE. References from relevant articles and documentation from organisations such as the World Health Organisation were also included. The search criteria focused predominantly on paediatrics; however some adult literature was included where this was relevant or illustrated important differences between paediatric and adult tuberculosis.
Search terms for both review papers and research articles included the following in various combinations: ‘Paediatrics’, ‘Children’, ‘Tuberculosis’, ‘Mycobacteria’, ‘Mycobacterium tuberculosis’, ‘Diagnostics’, ‘Epidemiology’, ‘Immune response’, ‘HIV’, ‘Multidrug resistance’, ‘BCG vaccination’, ‘Management’, ‘Childhood TB’, ‘Ontogeny’, ‘Treatment’, ‘Therapeutics’, ‘Phylogeography’, ‘TB drug clinical trials’, ‘Malnutrition’, ‘Clinical presentation’, ‘Pathology’, ‘Genetics’. Reference lists of these articles were then searched to identify further relevant articles.
References
- 1.Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990-2000. Bull World Health Organ. 1994;72(2):213–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004 May;8(5):636–47. [PubMed] [Google Scholar]
- 3.Raviglione MC, Snider DE, Jr., Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995 Jan 18;273(3):220–6. [PubMed] [Google Scholar]
- 4.Walls T, Shingadia D. Global epidemiology of paediatric tuberculosis. J Infect. 2004 Jan;48(1):13–22. doi: 10.1016/s0163-4453(03)00121-x. [DOI] [PubMed] [Google Scholar]
- 5.Almeida LM, Barbieri MA, Da Paixao AC, Cuevas LE. Use of purified protein derivative to assess the risk of infection in children in close contact with adults with tuberculosis in a population with high Calmette-Guerin bacillus coverage. Pediatr Infect Dis J. 2001 Nov;20(11):1061–5. doi: 10.1097/00006454-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Brailey M. A Study of Tuberculosis infection and mortality in the children of tuberculous households. Am J Hygiene. 1940;31:1–43. doi: 10.2105/ajph.30.7.816. SecA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewer K, Deeks J, Alvarez L, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003 Apr 5;361(9364):1168–73. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 8.Grzybowski S, Barnett GD, Styblo K. Contacts of cases of active pulmonary tuberculosis. Bull Int Union Tuberc. 1975;50(1):90–106. [PubMed] [Google Scholar]
- 9.Lienhardt C, Sillah J, Fielding K, et al. Risk factors for tuberculosis infection in children in contact with infectious tuberculosis cases in the Gambia, West Africa. Pediatrics. 2003 May;111(5 Pt 1):e608–14. doi: 10.1542/peds.111.5.e608. [DOI] [PubMed] [Google Scholar]
- 10.Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004 Mar;8(3):278–85. [PubMed] [Google Scholar]
- 11.Curtis AB, Ridzon R, Vogel R, et al. Extensive transmission of Mycobacterium tuberculosis from a child. N Engl J Med. 1999 Nov 11;341(20):1491–5. doi: 10.1056/NEJM199911113412002. [DOI] [PubMed] [Google Scholar]
- 12.Marais BJ, Obihara CC, Warren RM, Schaaf HS, Gie RP, Donald PR. The burden of childhood tuberculosis: a public health perspective. Int J Tuberc Lung Dis. 2005 Dec;9(12):1305–13. [PubMed] [Google Scholar]
- 13.Miller FJW, Seal RME, Taylor MD. Tuberculosis in Children. London, UK: J & A Churchill Ltd; 1963. [Google Scholar]
- 14.Nakaoka H, Lawson L, Squire SB, et al. Risk for tuberculosis among children. Emerg Infect Dis. 2006 Sep;12(9):1383–8. doi: 10.3201/eid1209.051606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaaf HS, Michaelis IA, Richardson M, et al. Adult-to-child transmission of tuberculosis: household or community contact? Int J Tuberc Lung Dis. 2003 May;7(5):426–31. [PubMed] [Google Scholar]
- 16.Verver S, Warren RM, Munch Z, et al. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet. 2004 Jan 17;363(9404):212–4. doi: 10.1016/S0140-6736(03)15332-9. [DOI] [PubMed] [Google Scholar]
- 17.Bates I, Fenton C, Gruber J, et al. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infect Dis. 2004 May;4(5):267–77. doi: 10.1016/S1473-3099(04)01002-3. [DOI] [PubMed] [Google Scholar]
- 18.Cantwell MF, McKenna MT, McCray E, Onorato IM. Tuberculosis and race/ethnicity in the United States: impact of socioeconomic status. Am J Respir Crit Care Med. 1998 Apr;157(4 Pt 1):1016–20. doi: 10.1164/ajrccm.157.4.9704036. [DOI] [PubMed] [Google Scholar]
- 19.Drucker E, Alcabes P, Bosworth W, Sckell B. Childhood tuberculosis in the Bronx, New York. Lancet. 1994 Jun 11;343(8911):1482–5. doi: 10.1016/s0140-6736(94)92588-7. [DOI] [PubMed] [Google Scholar]
- 20.Guwatudde D, Nakakeeto M, Jones-Lopez EC, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003 Nov 1;158(9):887–98. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lockman S, Tappero JW, Kenyon TA, Rumisha D, Huebner RE, Binkin NJ. Tuberculin reactivity in a pediatric population with high BCG vaccination coverage. Int J Tuberc Lung Dis. 1999 Jan;3(1):23–30. [PubMed] [Google Scholar]
- 22.Saiman L, San Gabriel P, Schulte J, Vargas MP, Kenyon T, Onorato I. Risk factors for latent tuberculosis infection among children in New York City. Pediatrics. 2001 May;107(5):999–1003. doi: 10.1542/peds.107.5.999. [DOI] [PubMed] [Google Scholar]
- 23.van Rie A, Beyers N, Gie RP, Kunneke M, Zietsman L, Donald PR. Childhood tuberculosis in an urban population in South Africa: burden and risk factor. Arch Dis Child. 1999 May;80(5):433–7. doi: 10.1136/adc.80.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enarson DA, Wang JS, Dirks JM. The incidence of active tuberculosis in a large urban area. Am J Epidemiol. 1989 Jun;129(6):1268–76. doi: 10.1093/oxfordjournals.aje.a115246. [DOI] [PubMed] [Google Scholar]
- 25.Rieder H. Annual risk of infection with Mycobacterium tuberculosis. Eur Respir J. 2005 Jan;25(1):181–5. doi: 10.1183/09031936.04.00103804. [DOI] [PubMed] [Google Scholar]
- 26.Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007 Jan;11(3):1–196. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- 27.Pai M, Riley LW, Colford JM., Jr. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004 Dec;4(12):761–76. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 28.Shingadia D, Novelli V. Diagnosis and treatment of tuberculosis in children. Lancet Infect Dis. 2003 Oct;3(10):624–32. doi: 10.1016/s1473-3099(03)00771-0. [DOI] [PubMed] [Google Scholar]
- 29.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004 Apr;8(4):392–402. [PubMed] [Google Scholar]
- 30.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004 Mar;8(3):286–98. [PubMed] [Google Scholar]
- 31.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. Jama. 1994 Mar 2;271(9):698–702. [PubMed] [Google Scholar]
- 32.Barnes PF, Bloch AB, Davidson PT, Snider DE., Jr. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991 Jun 6;324(23):1644–50. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 33.Chen ZW. Immunology of AIDS virus and mycobacterial co-infection. Curr HIV Res. 2004 Oct;2(4):351–5. doi: 10.2174/1570162043351147. [DOI] [PubMed] [Google Scholar]
- 34.Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998 Mar;157(3 Pt 1):679–91. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 35.Beyers N, Gie RP, Schaaf HS, et al. A prospective evaluation of children under the age of 5 years living in the same household as adults with recently diagnosed pulmonary tuberculosis. Int J Tuberc Lung Dis. 1997 Feb;1(1):38–43. [PubMed] [Google Scholar]
- 36.Rieder HL. Epidemiology of tuberculosis in children. Annales Nestle. 1997;55(1):1–9. [Google Scholar]
- 37.Bloch AB, Snider DE., Jr. How much tuberculosis in children must we accept? Am J Public Health. 1986 Jan;76(1):14–5. doi: 10.2105/ajph.76.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaaf HS, Marais BJ, Hesseling AC, Gie RP, Beyers N, Donald PR. Childhood drug-resistant tuberculosis in the Western Cape Province of South Africa. Acta Paediatr. 2006 May;95(5):523–8. doi: 10.1080/08035250600675741. [DOI] [PubMed] [Google Scholar]
- 39.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003 May 12;163(9):1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 40.Graham SM, Coulter JB, Gilks CF. Pulmonary disease in HIV-infected African children. Int J Tuberc Lung Dis. 2001 Jan;5(1):12–23. [PubMed] [Google Scholar]
- 41.Mukadi YBDCKM. Special challenges of tuberculosis in HIV-infected children. Annales Nestle. 1997;55(1):35–41. [Google Scholar]
- 42.Coovadia HM, Jeena P, Wilkinson D. Childhood human immunodeficiency virus and tuberculosis co-infections: reconciling conflicting data. Int J Tuberc Lung Dis. 1998 Oct;2(10):844–51. [PubMed] [Google Scholar]
- 43.Nelson LJ, Schneider E, Wells CD, Moore M. Epidemiology of childhood tuberculosis in the United States, 1993-2001: the need for continued vigilance. Pediatrics. 2004 Aug;114(2):333–41. doi: 10.1542/peds.114.2.333. [DOI] [PubMed] [Google Scholar]
- 44.Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis. 2000 Jul;31(1):170–6. doi: 10.1086/313925. [DOI] [PubMed] [Google Scholar]
- 45.Mukadi YD, Wiktor SZ, Coulibaly IM, et al. Impact of HIV infection on the development, clinical presentation, and outcome of tuberculosis among children in Abidjan, Cote d’Ivoire. Aids. 1997 Jul 15;11(9):1151–8. doi: 10.1097/00002030-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Gutman LT, Moye J, Zimmer B, Tian C. Tuberculosis in human immunodeficiency virus-exposed or -infected United States children. Pediatr Infect Dis J. 1994 Nov;13(11):963–8. doi: 10.1097/00006454-199411000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Jeena PM, Pillay P, Pillay T, Coovadia HM. Impact of HIV-1 co-infection on presentation and hospital-related mortality in children with culture proven pulmonary tuberculosis in Durban, South Africa. Int J Tuberc Lung Dis. 2002 Aug;6(8):672–8. [PubMed] [Google Scholar]
- 48.Palme IB, Gudetta B, Bruchfeld J, Muhe L, Giesecke J. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Infect Dis J. 2002 Nov;21(11):1053–61. doi: 10.1097/00006454-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 49.Marais BJ, Graham SM, Cotton MF, Beyers N. Diagnostic and management challenges for childhood tuberculosis in the era of HIV. J Infect Dis. 2007 Aug 15;196(Suppl 1):S76–85. doi: 10.1086/518659. [DOI] [PubMed] [Google Scholar]
- 50.Brent AJ, Anderson ST, Kampmann B. Childhood tuberculosis: out of sight, out of mind? Trans R Soc Trop Med Hyg. 2007 Nov 8; doi: 10.1016/j.trstmh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC, WHO Global Surveillance and Monitoring Project Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. Jama. 1999 Aug 18;282(7):677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 52.Murray CJ, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990 Mar;65(1):6–24. [PubMed] [Google Scholar]
- 53.Walls T, Shingadia D. The epidemiology of tuberculosis in Europe. Arch Dis Child. 2007 Aug;92(8):726–9. doi: 10.1136/adc.2006.102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005 Mar 26 1;Apr 26 1;365(9465):1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 55.Scott JA, Brooks WA, Peiris JS, Holtzman D, Mulhollan EK. Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest. 2008 Apr;118(4):1291–300. doi: 10.1172/JCI33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chintu C, Mudenda V, Lucas S, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet. 2002 Sep 28;360(9338):985–90. doi: 10.1016/S0140-6736(02)11082-8. [DOI] [PubMed] [Google Scholar]
- 57.WHO Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2006. cited; Available from: http://libdoc.who.int/hq/2006/WHO_HTM_TB_2006.371_eng.pdf. [PubMed]
- 58.Jacobs RF, Starke JR. Tuberculosis in children. Med Clin North Am. 1993 Nov;77(6):1335–51. doi: 10.1016/s0025-7125(16)30197-3. [DOI] [PubMed] [Google Scholar]
- 59.WHO . A Research Agenda for Childhood Tuberculosis. Geneva: World Health Organisation; 2007. [Google Scholar]
- 60.Holt PG. Postnatal maturation of immune competence during infancy and childhood. Pediatr Allergy Immunol. 1995 May;6(2):59–70. doi: 10.1111/j.1399-3038.1995.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 61.Jackson JC, Palmer S, Wilson CB, et al. Postnatal changes in lung phospholipids and alveolar macrophages in term newborn monkeys. Respir Physiol. 1988 Sep;73(3):289–300. doi: 10.1016/0034-5687(88)90051-5. [DOI] [PubMed] [Google Scholar]
- 62.Zeligs BJ, Nerurkar LS, Bellanti JA. Chemotactic and candidacidal responses of rabbit alveolar macrophages during postnatal development and the modulating roles of surfactant in these responses. Infect Immun. 1984 May;44(2):379–85. doi: 10.1128/iai.44.2.379-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006 Feb;74(2):1106–12. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Upham JW, Lee PT, Holt BJ, et al. Development of interleukin-12-producing capacity throughout childhood. Infect Immun. 2002 Dec;70(12):6583–8. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goriely S, Vincart B, Stordeur P, et al. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001 Feb 1;166(3):2141–6. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 66.White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002 Mar 15;168(6):2820–7. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 67.Rowe J, Macaubas C, Monger TM, et al. Antigen-specific responses to diphtheria-tetanus-acellular pertussis vaccine in human infants are initially Th2 polarized. Infect Immun. 2000 Jul;68(7):3873–7. doi: 10.1128/iai.68.7.3873-3877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kampmann B, Tena-Coki G, Anderson S. Blood tests for diagnosis of tuberculosis. Lancet. 2006 Jul 22;368(9532):282. doi: 10.1016/S0140-6736(06)69064-8. author reply 282-3. [DOI] [PubMed] [Google Scholar]
- 69.Nonoyama S, Penix LA, Edwards CP, et al. Diminished expression of CD40 ligand by activated neonatal T cells. J Clin Invest. 1995 Jan;95(1):66–75. doi: 10.1172/JCI117677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Weert EM, Hartgers NM, Schaaf HS, et al. Comparison of diagnostic criteria of tuberculous meningitis in human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis J. 2006 Jan;25(1):65–9. doi: 10.1097/01.inf.0000183751.75880.f8. [DOI] [PubMed] [Google Scholar]
- 71.Marchant A, Goetghebuer T, Ota MO, et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol. 1999 Aug 15;163(4):2249–55. [PubMed] [Google Scholar]
- 72.Lienhardt C, Bennett S, Del Prete G, et al. Investigation of environmental and host-related risk factors for tuberculosis in Africa. I. Methodological aspects of a combined design. Am J Epidemiol. 2002 Jun 1;155(11):1066–73. doi: 10.1093/aje/155.11.1066. [DOI] [PubMed] [Google Scholar]
- 73.Meya DB, McAdam KP. The TB pandemic: an old problem seeking new solutions. J Intern Med. 2007 Apr;261(4):309–29. doi: 10.1111/j.1365-2796.2007.01795.x. [DOI] [PubMed] [Google Scholar]
- 74.Bennett S, Lienhardt C, Bah-Sow O, et al. Investigation of environmental and host-related risk factors for tuberculosis in Africa. II. Investigation of host genetic factors. Am J Epidemiol. 2002 Jun 1;155(11):1074–9. doi: 10.1093/aje/155.11.1074. [DOI] [PubMed] [Google Scholar]
- 75.Levin M, Newport M. Understanding the genetic basis of susceptibility to mycobacterial infection. Proc Assoc Am Physicians. 1999 Jul-Aug;111(4):308–12. doi: 10.1046/j.1525-1381.1999.99242.x. [DOI] [PubMed] [Google Scholar]
- 76.Newport MJ, Huxley CM, Huston S, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996 Dec 26;335(26):1941–9. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 77.Kampmann B, Hemingway C, Stephens A, et al. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest. 2005 Sep;115(9):2480–8. doi: 10.1172/JCI19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hill AV. Aspects of genetic susceptibility to human infectious diseases. Annu Rev Genet. 2006;40:469–86. doi: 10.1146/annurev.genet.40.110405.090546. [DOI] [PubMed] [Google Scholar]
- 79.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, et al. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet. 1999 Apr;21(4):370–8. doi: 10.1038/7701. [DOI] [PubMed] [Google Scholar]
- 80.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998 May 29;280(5368):1432–5. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 81.Ottenhoff TH, Verreck FA, Hoeve MA, van de Vosse E. Control of human host immunity to mycobacteria. Tuberculosis (Edinb) 2005 Jan-Mar;85(1-2):53–64. doi: 10.1016/j.tube.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 82.Tena GN, Young DB, Eley B, et al. Failure to control growth of mycobacteria in blood from children infected with human immunodeficiency virus and its relationship to T cell function. J Infect Dis. 2003 May 15;187(10):1544–51. doi: 10.1086/374799. [DOI] [PubMed] [Google Scholar]
- 83.Elenga N, Kouakoussui KA, Bonard D, et al. Diagnosed tuberculosis during the follow-up of a cohort of human immunodeficiency virus-infected children in Abidjan, Cote d’Ivoire: ANRS 1278 study. Pediatr Infect Dis J. 2005 Dec;24(12):1077–82. doi: 10.1097/01.inf.0000190008.91534.b7. [DOI] [PubMed] [Google Scholar]
- 84.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006 Sep;43(1):42–6. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 85.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. Aids. 2005 Dec 2;19(18):2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 86.Kampmann B, Tena-Coki GN, Nicol MP, Levin M, Eley B. Reconstitution of antimycobacterial immune responses in HIV-infected children receiving HAART. Aids. 2006 Apr 24;20(7):1011–8. doi: 10.1097/01.aids.0000222073.45372.ce. [DOI] [PubMed] [Google Scholar]
- 87.Walters E, Cotton MF, Rabie H, Schaaf HS, Walters LO, Marais BJ. Clinical presentation and outcome of Tuberculosis in Human Immunodeficiency Virus infected children on anti-retroviral therapy. BMC Pediatr. 2008 Jan 11;8(1):1. doi: 10.1186/1471-2431-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Downes J. An experiment in the control of tuberculosis among Negroes. Milbank Mem Fund Q. 1950 Apr;28(2):127–59. [PubMed] [Google Scholar]
- 89.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000 Feb 19;355(9204):618–21. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 90.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004 Sep 1;173(5):2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 91.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007 Jul 15;176(2):208–13. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 92.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007 Jun 1;178(11):7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 93.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007 Mar;103(3-5):793–8. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 94.Fleischmann RD, Alland D, Eisen JA, et al. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J Bacteriol. 2002 Oct;184(19):5479–90. doi: 10.1128/JB.184.19.5479-5490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsolaki AG, Hirsh AE, DeRiemer K, et al. Functional and evolutionary genomics of Mycobacterium tuberculosis: insights from genomic deletions in 100 strains. Proc Natl Acad Sci U S A. 2004 Apr 6;101(14):4865–70. doi: 10.1073/pnas.0305634101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kato-Maeda M, Bifani PJ, Kreiswirth BN, Small PM. The nature and consequence of genetic variability within Mycobacterium tuberculosis. J Clin Invest. 2001 Mar;107(5):533–7. doi: 10.1172/JCI11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gagneux S, DeRiemer K, Van T, et al. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2869–73. doi: 10.1073/pnas.0511240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gagneux S, Small PM. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis. 2007 May;7(5):328–37. doi: 10.1016/S1473-3099(07)70108-1. [DOI] [PubMed] [Google Scholar]
- 99.Hirsh AE, Tsolaki AG, DeRiemer K, Feldman MW, Small PM. Stable association between strains of Mycobacterium tuberculosis and their human host populations. Proc Natl Acad Sci U S A. 2004 Apr 6;101(14):4871–6. doi: 10.1073/pnas.0305627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marais BJ, Victor TC, Hesseling AC, et al. Beijing and Haarlem genotypes are overrepresented among children with drug-resistant tuberculosis in the Western Cape Province of South Africa. J Clin Microbiol. 2006 Oct;44(10):3539–43. doi: 10.1128/JCM.01291-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nicol MP, Sola C, February B, Rastogi N, Steyn L, Wilkinson RJ. Distribution of strain families of Mycobacterium tuberculosis causing pulmonary and extrapulmonary disease in hospitalized children in Cape Town, South Africa. J Clin Microbiol. 2005 Nov;43(11):5779–81. doi: 10.1128/JCM.43.11.5779-5781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lopez B, Aguilar D, Orozco H, et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol. 2003 Jul;133(1):30–7. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Manca C, Reed MB, Freeman S, et al. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect Immun. 2004 Sep;72(9):5511–4. doi: 10.1128/IAI.72.9.5511-5514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manca C, Tsenova L, Bergtold A, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci U S A. 2001 May 8;98(10):5752–7. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reed MB, Domenech P, Manca C, et al. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature. 2004 Sep 2;431(7004):84–7. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]
- 106.Newton SM, Smith RJ, Wilkinson KA, et al. A deletion defining a common Asian lineage of Mycobacterium tuberculosis associates with immune subversion. Proc Natl Acad Sci U S A. 2006 Oct 17;103(42):15594–8. doi: 10.1073/pnas.0604283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson ST, Williams AJ, Brown JR, et al. Transmission of Mycobacterium tuberculosis undetected by tuberculin skin testing. Am J Respir Crit Care Med. 2006 May 1;173(9):1038–42. doi: 10.1164/rccm.200509-1526OC. [DOI] [PubMed] [Google Scholar]
- 108.Marais BJ, Gie RP, Hesseling AH, Beyers N. Adult-type pulmonary tuberculosis in children 10-14 years of age. Pediatr Infect Dis J. 2005 Aug;24(8):743–4. doi: 10.1097/01.inf.0000173305.04212.09. [DOI] [PubMed] [Google Scholar]
- 109.Madhi SA, Huebner RE, Doedens L, Aduc T, Wesley D, Cooper PA. HIV-1 co-infection in children hospitalised with tuberculosis in South Africa. Int J Tuberc Lung Dis. 2000 May;4(5):448–54. [PubMed] [Google Scholar]
- 110.Hussey G, Chisholm T, Kibel M. Miliary tuberculosis in children: a review of 94 cases. Pediatr Infect Dis J. 1991 Nov;10(11):832–6. doi: 10.1097/00006454-199111000-00008. [DOI] [PubMed] [Google Scholar]
- 111.Donald PR, Schoeman JF. Tuberculous meningitis. N Engl J Med. 2004 Oct 21;351(17):1719–20. doi: 10.1056/NEJMp048227. [DOI] [PubMed] [Google Scholar]
- 112.Thwaites GE, Tran TH. Tuberculous meningitis: many questions, too few answers. Lancet Neurol. 2005 Mar;4(3):160–70. doi: 10.1016/S1474-4422(05)01013-6. [DOI] [PubMed] [Google Scholar]
- 113.Marais BJ, Donald PR, Gie RP, Schaaf HS, Beyers N. Diversity of disease in childhood pulmonary tuberculosis. Ann Trop Paediatr. 2005 Jun;25(2):79–86. doi: 10.1179/146532805X45665. [DOI] [PubMed] [Google Scholar]
- 114.Marais BJ, Pai M. Recent advances in the diagnosis of childhood tuberculosis. Arch Dis Child. 2007 May;92(5):446–52. doi: 10.1136/adc.2006.104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005 Jan 8-14;365(9454):130–4. doi: 10.1016/S0140-6736(05)17702-2. [DOI] [PubMed] [Google Scholar]
- 116.Marais BJ, Gie RP, Hesseling AC, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics. 2006 Nov;118(5):e1350–9. doi: 10.1542/peds.2006-0519. [DOI] [PubMed] [Google Scholar]
- 117.Starke JR. Diagnosis of tuberculosis in children. Pediatr Infect Dis J. 2000 Nov;19(11):1095–6. doi: 10.1097/00006454-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 118.Chow F, Espiritu N, Gilman RH, et al. La cuerda dulce--a tolerability and acceptability study of a novel approach to specimen collection for diagnosis of paediatric pulmonary tuberculosis. BMC Infect Dis. 2006;6:67. doi: 10.1186/1471-2334-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006 Oct 12;355(15):1539–50. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sarmiento OL, Weigle KA, Alexander J, Weber DJ, Miller WC. Assessment by meta-analysis of PCR for diagnosis of smear-negative pulmonary tuberculosis. J Clin Microbiol. 2003 Jul;41(7):3233–40. doi: 10.1128/JCM.41.7.3233-3240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kalantri S, Pai M, Pascopella L, Riley L, Reingold A. Bacteriophage-based tests for the detection of Mycobacterium tuberculosis in clinical specimens: a systematic review and meta- analysis. BMC Infect Dis. 2005;5(1):59. doi: 10.1186/1471-2334-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kocagoz T, O’Brien R, Perkins M. A new colorimetric culture system for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2004 Dec;8(12):1512. author reply 1513. [PubMed] [Google Scholar]
- 123.Fend R, Kolk AH, Bessant C, Buijtels P, Klatser PR, Woodman AC. Prospects for clinical application of electronic-nose technology to early detection of Mycobacterium tuberculosis in culture and sputum. J Clin Microbiol. 2006 Jun;44(6):2039–45. doi: 10.1128/JCM.01591-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oberhelman RA, Soto-Castellares G, Caviedes L, et al. Improved recovery of Mycobacterium tuberculosis from children using the microscopic observation drug susceptibility method. Pediatrics. 2006 Jul;118(1):e100–6. doi: 10.1542/peds.2005-2623. [DOI] [PubMed] [Google Scholar]
- 125.Ferrara G, Losi M, D’Amico R, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet. 2006 Apr 22;367(9519):1328–34. doi: 10.1016/S0140-6736(06)68579-6. [DOI] [PubMed] [Google Scholar]
- 126.Arend SM, Thijsen SF, Leyten EM, et al. Comparison of two interferon-gamma assays and tuberculin skin test for tracing tuberculosis contacts. Am J Respir Crit Care Med. 2007 Mar 15;175(6):618–27. doi: 10.1164/rccm.200608-1099OC. [DOI] [PubMed] [Google Scholar]
- 127.Clark SA, Martin SL, Pozniak A, et al. Tuberculosis antigen-specific immune responses can be detected using enzyme-linked immunospot technology in human immunodeficiency virus (HIV)-1 patients with advanced disease. Clin Exp Immunol. 2007 Nov;150(2):238–44. doi: 10.1111/j.1365-2249.2007.03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rangaka MX, Wilkinson KA, Seldon R, et al. Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007 Mar 1;175(5):514–20. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- 129.Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004 Dec 18-31;364(9452):2196–203. doi: 10.1016/S0140-6736(04)17592-2. [DOI] [PubMed] [Google Scholar]
- 130.Gennaro ML. Immunologic diagnosis of tuberculosis. Clin Infect Dis. 2000 Jun;30(Suppl 3):S243–6. doi: 10.1086/313868. [DOI] [PubMed] [Google Scholar]
- 131.Laal S, Skeiky YAW. Immune based methods. In: Cole STEK, McMurray DN, Jacobs WR, editors. Tuberculosis and the tubercle bacillus. Washington, DC: ASM Press; 2005. pp. 71–83. [Google Scholar]
- 132.Boehme C, Molokova E, Minja F, et al. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans R Soc Trop Med Hyg. 2005 Dec;99(12):893–900. doi: 10.1016/j.trstmh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 133.Steingart KR, Henry M, Laal S, et al. A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Thorax. 2007 Oct;62(10):911–8. doi: 10.1136/thx.2006.075754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Donald PR. The assessment of new anti-tuberculosis drugs for a paediatric indication. Int J Tuberc Lung Dis. 2007 Nov;11(11):1162–5. [PubMed] [Google Scholar]
- 135.Schaaf HS, Marais BJ, Whitelaw A, et al. Culture-confirmed childhood tuberculosis in Cape Town, South Africa: a review of 596 cases. BMC Infect Dis. 2007 Nov 29;7(1):140. doi: 10.1186/1471-2334-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guidance for national tuberculosis programmes on the management of tuberculosis in children. Chapter 2: anti-tuberculosis treatment in children. Int J Tuberc Lung Dis. 2006 Nov;10(11):1205–11. [PubMed] [Google Scholar]
- 137.Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997 Feb;99(2):226–31. doi: 10.1542/peds.99.2.226. [DOI] [PubMed] [Google Scholar]
- 138.Sharma S, Sarin R, Khalid UK, Singla N, Sharma PP, Behera D. The DOTS strategy for treatment of paediatric pulmonary tuberculosis in South Delhi, India. Int J Tuberc Lung Dis. 2008 Jan;12(1):74–80. [PubMed] [Google Scholar]
- 139.Schaaf HS, Parkin DP, Seifart HI, et al. Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Arch Dis Child. 2005 Jun;90(6):614–8. doi: 10.1136/adc.2004.052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu M, Starke JR, Burman WJ, et al. Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy. 2002 Jun;22(6):686–95. doi: 10.1592/phco.22.9.686.34067. [DOI] [PubMed] [Google Scholar]
- 141.Donald PR, Maher D, Maritz JS, Qazi S. Ethambutol dosage for the treatment of children: literature review and recommendations. Int J Tuberc Lung Dis. 2006 Dec;10(12):1318–30. [PubMed] [Google Scholar]
- 142.WHO Considerations for infants and children coinfected with tuberculosis and HIV. Antiretroviral therapy of HIV in infants and children: towards universal access. WHO. 2006:43–8. [Google Scholar]
- 143.Hsu KH. Thirty years after isoniazid. Its impact on tuberculosis in children and adolescents. Jama. 1984 Mar 9;251(10):1283–5. doi: 10.1001/jama.251.10.1283. [DOI] [PubMed] [Google Scholar]
- 144.Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis. 2005 Mar 1;40(5):670–6. doi: 10.1086/427802. [DOI] [PubMed] [Google Scholar]
- 145.Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. Bmj. 2007 Jan 20;334(7585):136. doi: 10.1136/bmj.39000.486400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Euro T. Surveillance of tuberculosis in Europe. Report on tuberculosis cases notified in 2005. 2005. cited; Available from: http://www.euroTB.org.
- 147.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006 Nov 4;368(9547):1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 148.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007 Aug 15;196(Suppl 1):S86–107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 149.Soeters M, de Vries AM, Kimpen JL, Donald PR, Schaaf HS. Clinical features and outcome in children admitted to a TB hospital in the Western Cape--the influence of HIV infection and drug resistance. S Afr Med J. 2005 Aug;95(8):602–6. [PubMed] [Google Scholar]
- 150.WHO Guidelines for the programmatic management of drug-resistant tuberculosis. 2006. cited; Available from: http://whqlibdoc.who.int/publications/2006/9241546956_eng.pdf.
- 151.WHO WHO Treatment of tuberculosis: guidelines for national programmes. 2003. cited; Available from: http://whqlibdoc.who.int/hq/2003/WHO_CDS_TB_2003.313_eng.pdf.
- 152.Schaaf HS, Shean K, Donald PR. Culture confirmed multidrug resistant tuberculosis: diagnostic delay, clinical features, and outcome. Arch Dis Child. 2003 Dec;88(12):1106–11. doi: 10.1136/adc.88.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Drobac PC, Mukherjee JS, Joseph JK, et al. Community-based therapy for children with multidrug-resistant tuberculosis. Pediatrics. 2006 Jun;117(6):2022–9. doi: 10.1542/peds.2005-2235. [DOI] [PubMed] [Google Scholar]
- 154.Behr MA, Wilson MA, Gill WP, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999 May 28;284(5419):1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 155.Davids V, Hanekom WA, Mansoor N, et al. The effect of bacille Calmette-Guerin vaccine strain and route of administration on induced immune responses in vaccinated infants. J Infect Dis. 2006 Feb 15;193(4):531–6. doi: 10.1086/499825. [DOI] [PubMed] [Google Scholar]
- 156.Bellamy R. Genetic susceptibility to tuberculosis. Clin Chest Med. 2005 Jun;26(2):233–46. vi. doi: 10.1016/j.ccm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 157.Malhotra I, Mungai P, Wamachi A, et al. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999 Jun 1;162(11):6843–8. [PubMed] [Google Scholar]
- 158.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995 Nov 18;346(8986):1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 159.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993 Dec;22(6):1154–8. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 160.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006 Apr 8;367(9517):1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 161.Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. 1998 Mar;2(3):200–7. [PubMed] [Google Scholar]
- 162.Aronson NE, Santosham M, Comstock GW, et al. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study. Jama. 2004 May 5;291(17):2086–91. doi: 10.1001/jama.291.17.2086. [DOI] [PubMed] [Google Scholar]
- 163.Rodrigues LC, Pereira SM, Cunha SS, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005 Oct 8;366(9493):1290–5. doi: 10.1016/S0140-6736(05)67145-0. [DOI] [PubMed] [Google Scholar]
- 164.Hesseling AC, Cotton MF, Marais BJ, et al. BCG and HIV reconsidered: moving the research agenda forward. Vaccine. 2007 Sep 4;25(36):6565–8. doi: 10.1016/j.vaccine.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 165.Hesseling AC, Marais BJ, Gie RP, et al. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine. 2007 Jan 2;25(1):14–8. doi: 10.1016/j.vaccine.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 166.WHO The Global Plan to Stop TB, 2006-2015. Annex 1 Methods used to estimate costs, funding and funding gaps. 2006. cited; Available from: http://whqlibdoc.who.int/publications/2006/924159487X_eng.pdf.
- 167.WHO Stop TB. cited; Available from: www.stoptb.org/
- 168.Roth A, Garly ML, Jensen H, Nielsen J, Aaby P. Bacillus Calmette-Guerin vaccination and infant mortality. Expert Rev Vaccines. 2006 Apr;5(2):277–93. doi: 10.1586/14760584.5.2.277. [DOI] [PubMed] [Google Scholar]
- 169.Doherty TM, Dietrich J, Billeskov R. Tuberculosis subunit vaccines: from basic science to clinical testing. Expert Opin Biol Ther. 2007 Oct;7(10):1539–49. doi: 10.1517/14712598.7.10.1539. [DOI] [PubMed] [Google Scholar]
- 170.Snider DE., Jr. Ethical issues in tuberculosis vaccine trials. Clin Infect Dis. 2000 Jun;30(Suppl 3):S271–5. doi: 10.1086/313872. [DOI] [PubMed] [Google Scholar]
- 171.Andersen P. Vaccine strategies against latent tuberculosis infection. Trends Microbiol. 2007 Jan;15(1):7–13. doi: 10.1016/j.tim.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 172.Goldfeld AE, Delgado JC, Thim S, et al. Association of an HLA-DQ allele with clinical tuberculosis. Jama. 1998 Jan 21;279(3):226–8. doi: 10.1001/jama.279.3.226. [DOI] [PubMed] [Google Scholar]
- 173.Delgado JC, Baena A, Thim S, Goldfeld AE. Aspartic acid homozygosity at codon 57 of HLA-DQ beta is associated with susceptibility to pulmonary tuberculosis in Cambodia. J Immunol. 2006 Jan 15;176(2):1090–7. doi: 10.4049/jimmunol.176.2.1090. [DOI] [PubMed] [Google Scholar]
- 174.Lombard Z, Dalton DL, Venter PA, Williams RC, Bornman L. Association of HLA-DR, -DQ, and vitamin D receptor alleles and haplotypes with tuberculosis in the Venda of South Africa. Hum Immunol. 2006 Aug;67(8):643–54. doi: 10.1016/j.humimm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 175.Rossouw M, Nel HJ, Cooke GS, van Helden PD, Hoal EG. Association between tuberculosis and a polymorphic NFkappaB binding site in the interferon gamma gene. Lancet. 2003 May 31;361(9372):1871–2. doi: 10.1016/S0140-6736(03)13491-5. [DOI] [PubMed] [Google Scholar]
- 176.Cooke GS, Campbell SJ, Sillah J, et al. Polymorphism within the interferon-gamma/receptor complex is associated with pulmonary tuberculosis. Am J Respir Crit Care Med. 2006 Aug 1;174(3):339–43. doi: 10.1164/rccm.200601-088OC. [DOI] [PubMed] [Google Scholar]
- 177.Tso HW, Lau YL, Tam CM, Wong HS, Chiang AK. Associations between IL12B polymorphisms and tuberculosis in the Hong Kong Chinese population. J Infect Dis. 2004 Sep 1;190(5):913–9. doi: 10.1086/422693. [DOI] [PubMed] [Google Scholar]
- 178.Remus N, El Baghdadi J, Fieschi C, et al. Association of IL12RB1 polymorphisms with pulmonary tuberculosis in adults in Morocco. J Infect Dis. 2004 Aug 1;190(3):580–7. doi: 10.1086/422534. [DOI] [PubMed] [Google Scholar]
- 179.Khor CC, Chapman SJ, Vannberg FO, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet. 2007 Apr;39(4):523–8. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Greenwood CM, Fujiwara TM, Boothroyd LJ, et al. Linkage of tuberculosis to chromosome 2q35 loci, including NRAMP1, in a large aboriginal Canadian family. Am J Hum Genet. 2000 Aug;67(2):405–16. doi: 10.1086/303012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Flores-Villanueva PO, Ruiz-Morales JA, Song CH, et al. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J Exp Med. 2005 Dec 19;202(12):1649–58. doi: 10.1084/jem.20050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Agger EM, Rosenkrands I, Olsen AW, et al. Protective immunity to tuberculosis with Ag85B-ESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine. 2006 Jun 29;24(26):5452–60. doi: 10.1016/j.vaccine.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 183.Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, Andersen P. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol. 2006 Nov 1;177(9):6353–60. doi: 10.4049/jimmunol.177.9.6353. [DOI] [PubMed] [Google Scholar]
- 184.Skeiky YA, Alderson MR, Ovendale PJ, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004 Jun 15;172(12):7618–28. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 185.Havenga M, Vogels R, Zuijdgeest D, et al. Novel replication-incompetent adenoviral B-group vectors: high vector stability and yield in PER.C6 cells. J Gen Virol. 2006 Aug;87(Pt 8):2135–43. doi: 10.1099/vir.0.81956-0. [DOI] [PubMed] [Google Scholar]
- 186.McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004 Nov;10(11):1240–4. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 187.Dietrich J, Aagaard C, Leah R, et al. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. 2005 May 15;174(10):6332–9. doi: 10.4049/jimmunol.174.10.6332. [DOI] [PubMed] [Google Scholar]
- 188.Conradt P, Hess J, Kaufmann SH. Cytolytic T-cell responses to human dendritic cells and macrophages infected with Mycobacterium bovis BCG and recombinant BCG secreting listeriolysin. Microbes Infect. 1999 Aug;1(10):753–64. doi: 10.1016/s1286-4579(99)80077-x. [DOI] [PubMed] [Google Scholar]


