Abstract
Age of host and transplantation-site microenvironment influence the tumorigenic potential of neoplastically transformed liver epithelial cells. Tumorigenic BAG2-GN6TF rat liver epithelial cells consistently form tumors at ectopic sites, but differentially express tumorigenicity or hepatocytic differentiation in the liver depending on host age and route of cell transplantation into the liver. Direct inoculation into host livers concentrates tumor cells locally, resulting in undifferentiated tumors near the transplantation site in both young (3-month-old) and old (18-month-old) rats. Transplantation-site tumors regress within 1 month in the livers of young rats, but grow progressively in old rats. However, inoculation of cells into the spleen distributes transplanted cells individually throughout the liver, resulting in hepatocytic differentiation by tumor cells with concomitant suppression of their tumorigenicity in young rats. When transplanted into livers of old rats by splenic inoculation, or when young hepatic-transplant recipients are allowed to age, hepatocytic progeny of BAG2-GN6TF cells proliferate to form foci, suggesting that the liver microenvironment of old rats incompletely regulates the proliferation and differentiation of tumor cell-derived hepatocytes. Upon removal from the liver, BAG2-GN6TF-derived hepatocytes revert to an undifferentiated, aggressively tumorigenic phenotype. We posit that the spectrum between normal differentiation and malignant potential of these cells reflects the dynamic interaction of the specific transformation-related genotype of the cells and the characteristics of the tissue microenvironment at the transplantation site. Changes in the tissue milieu, such as those that accompany normal aging, may determine the ability of a genetically aberrant cell to produce a tumor.
Tumorigenesis is a complex molecular and cellular process that culminates in the growth of aberrant cells in the context of a specific tissue microenvironment in which the growth of normal cells is tightly controlled. Target cells undergoing neoplastic transformation incur multiple genetic aberrations that enable them to grow autonomously in a regulative tissue microenvironment. The role of age as a major risk factor for the development of cancer (1, 2) has been hypothesized to reflect the time required for a target cell to accumulate a sufficient number and variety of genetic abnormalities to enable it to grow autonomously (3, 4). Although genetic malfunction is an essential feature of neoplastic cells, age-dependent changes in the elements of the tissue microenvironment that are involved in regulating growth of parenchymal cells may also have an important role in the increasing frequency of cancer with advancing age (5, 6). We recently have developed an experimental system with which to analyze the interacting roles of cellular genotype and tissue microenvironment in the development of tumors, including age dependency of tumor development (7, 8). This experimental system employs the intrahepatic (i.h.) transplantation of aneuploid BAG2-GN6TF liver epithelial cells. These cells produce tumors in 100% of neonatal rat hosts (9) and a high percentage of adult hosts (8) with short latency when transplanted to s.c. or i.p. sites. Our previous studies have shown that in young rats i.h. transplants of BAG2-GN6TF cells, which deposit the aneuploid cells in one location in the liver, rapidly produce small tumors at the site of inoculation. However, these tumors regress within 1 month of their formation (8). In contrast, when BAG2-GN6TF cells are transplanted i.h. into old rats they quickly produce expanding liver tumors that kill the host rat (7). These results appear to support the hypothesis that elements of the liver microenvironment can regulate the proliferation and differentiation of genetically aberrant parenchymal cells, that aneuploid tumorigenic cells may be normalized by epigenetic influences, and that the regulatory potency of the liver microenvironment declines with advancing age.
In the present study we examine the epigenetic influences of microenvironments on the phenotypic plasticity of BAG2-GN6TF cells when the cells are subjected to different microenvironments (liver and nonhepatic sites) in young and old animals, as well as the stability of the differentiated phenotype when cells are recovered from the liver and transplanted s.c. or i.p. Our results show that the microenvironment of the liver induces BAG2-GN6TF cells to adopt morphologic and functional features of hepatocytic differentiation. When transplanted to extrahepatic tissue sites the cells are aggressively tumorigenic and form undifferentiated tumors. Maintenance of hepatocytic differentiation and suppression of tumorigenic potential in BAG2-GN6TF cells requires sustained epigenetic (microenvironmental) stimuli. Furthermore, age-associated changes in the liver microenvironment result in the abnormal proliferation of the hepatocytic progeny of transplanted BAG2-GN6TF cells. When the differentiated hepatocyte-like progeny of transplanted BAG2-GN6TF cells are recovered from the regulatory (suppressive) liver microenvironment, they revert to an undifferentiated neoplastic phenotype. This study indicates that elements of the liver microenvironment profoundly affect the phenotype expressed by genetically aberrant liver epithelial cells and that alterations in the tissue milieu, such as those that accompany advancing age, modify the capacity of the liver microenvironment to regulate the differentiation and tumorigenic potential of neoplastically transformed liver epithelial cells.
MATERIALS AND METHODS
Cell Lines and Cell Transplantations.
BAG2-WB and BAG2-GN6TF cells have been described previously (9). Cell transplant recipients consisted of age-controlled virgin male Fischer 344 rats at 3 months (young rats) and 18 months (old rats) of age (obtained from the Aged Rat Colony of the National Institute on Aging, Harlan–Sprague–Dawley), and German-strain Fischer 344 rats, which are deficient for dipeptidyl peptidase IV (DPPIV), at 3 months of age (generously provided by Sanjeev Gupta, Marion Bessin Liver Research Center, Albert Einstein College of Medicine, New York). All animal studies were carried out in accordance with guidelines put forth by the National Institutes of Health and the Institutional Animal Care and Use Committee of the University of North Carolina.
BAG2-GN6TF cells (5 × 106 cells/0.2 ml in physiological saline) were transplanted into host rats by direct injection into the median lobe of the liver (7–9) or by injection into the dorsal tip of the spleen (9). Animals were euthanized 7, 14, 28, or 85 days after transplantation. One group of 3-month-old rats that received BAG2-GN6TF cells was examined 14 months after transplantation. Age-matched controls consisted of rats that were not surgically manipulated or that received transplants of the parental nontransformed BAG2-WB cells.
Enzyme Histochemistry and Immunohistochemistry.
Formalin-fixed liver and tumor tissues were processed and hematoxylin/eosin (H&E)-stained paraffin sections were prepared. For analysis of β-galactosidase (β-gal) enzyme activity, tissue was frozen in OCT freezing medium, liver cryosections (10-μm thick) were prepared, and sections were incubated in an 5-bromo-4-chloro-3-indolyl β-d-galactoside-containing substrate (8, 9). DPPIV enzyme activity was detected as described (10, 11). All staining reactions included control sections from livers of DPPIV+ and DPPIV− rats to ensure the specificity and quality of the histochemical reaction. Control tissues were obtained from unmanipulated American- and German-strain Fischer 344 rats. Bile canalicular ATPase was detected as described (12). Colocalization of DPPIV and bile canalicular ATPase was accomplished by first performing the DPPIV staining reaction, washing the reacted tissue sections in PBS, and then immediately processing the tissue for ATPase activity. All tissue sections were counterstained with Mayers hematoxylin or methyl green.
Expression of albumin and transferrin were examined immunohistochemically in frozen sections that had been stained to detect β-gal or DPPIV activity (9, 11). Rabbit anti-rat albumin antibodies and rabbit anti-rat transferrin antibodies (Cappel) were used at a 1:200 dilution. Horseradish peroxidase-conjugated goat anti-rabbit IgG (Dako) served as the secondary antibody. Sections were developed in a diaminobenzidine substrate (Vector Laboratories).
Recovery of BAG2-GN6TF Cells from Livers of Transplant Recipients.
Livers of host rats that had received transplants of BAG2-GN6TF cells 1, 4, or 14 months earlier were dissociated by using standard collagenase perfusion techniques (13). Primary liver cells were plated onto plastic tissue culture dishes and maintained in Richter’s improved minimal essential medium (14). The medium was changed every 3 days until colonies of epithelial cells became apparent (10–14 days). Epithelial colonies displaying the characteristic morphology of BAG2-GN6TF cells were isolated by using cloning rings and expanded. The origin of the recovered cell lines was established by evaluating the clonal cell lines for β-gal expression and neomycin resistance (9). The ability of individual recovered cell lines to resist the cytotoxic effects of neomycin was determined by culturing cells in growth medium containing 400 μg/ml G418 (GIBCO/BRL). Cell lines exhibiting viability after 14 days in medium containing G418 were considered to be neomycin-resistant.
Isolation of Genomic DNA and Neo-PCR Analysis.
Amplification of the coding sequence of the neomycin resistance (NeoR) gene by PCR was used to determine the presence of BAG2-retroviral DNA within genomic DNA samples isolated from the recovered BAG2-GN6TF cell lines or from paraffin-embedded BAG2-GN6TF tumors. Genomic DNA was isolated from cultured cells by using standard procedures and from paraffin-embedded tissue samples by using the Puregene DNA isolation kit, as described by the manufacturer (Gentra Systems). Neo-PCR was accomplished by using synthetic oligonucleotide primers complementary to DNA sequences located in the coding region of the NeoR gene (sense: 5′-CATCGCCATGGGTCACGACG; antisense: 5′-GGGCGAAGTGCCGGGGCAGG). Thirty-five cycles of amplification with the Thermus aquaticus polymerase were performed by using 96°C for denaturation, 60°C for primer annealing, and 72°C for elongation. As a control, the actin gene was PCR-amplified by using commercial primers (Research Genetics, Huntsville, AL).
Phenotypic Characterization of Recovered BAG2-GN6TF Cells.
Saturation densities of recovered cell lines were determined in monolayer culture (15). Anchorage-independent growth was assayed as described (16). Tumorigenicity assays were performed as described previously (14). Pregnant Fischer 344 rats at 14 days of gestation were obtained from Charles River Laboratories. Recovered BAG2-GN6TF cell lines were transplanted to s.c. or i.p. sites of 1-day-old neonatal rats (at 1 × 106 cells per animal). Latency was recorded as the time required for the formation of tumors 1 cm in diameter.
RESULTS
Direct Inoculation of BAG2-GN6TF Cells into the Liver.
Direct inoculation of BAG2-GN6TF tumor cells into the hepatic parenchyma resulted in the formation of tumors at the transplantation site in all animals (young and old), as we reported previously (8). In the livers of 18-month-old rats the tumors were large (≈1 cm in diameter) and displayed an undifferentiated morphology that was indistinguishable from the undifferentiated spindle-cell tumors that form after the transplantation of BAG2-GN6TF cells to extrahepatic (s.c. and i.p.) tissue sites of young (3-month-old) or old (18-month-old) rats (Fig. 1 A and B). Likewise, i.h. tumors developing in young rats exhibited an undifferentiated morphology, but in contrast to the i.h. tumors of old rats, tumors in young animals regressed within 1 month (8). I.h. tumors resulting from direct inoculation of BAG2-GN6TF cells into the liver weakly expressed albumin and transferrin and contained some cells that were diffusely DPPIV+ (data not shown). Tumor cells that weakly expressed these hepatocyte-specific proteins usually were located near the tumor/parenchyma border, suggesting that the adjacent normal parenchyma affected the phenotype of the tumor cells.
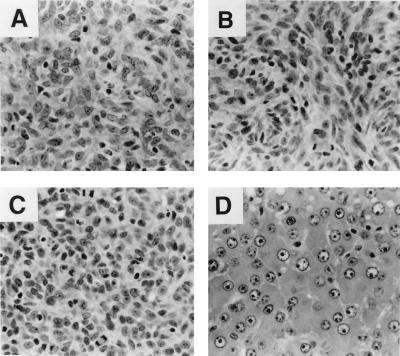
Figure 1.
Histology of BAG2-GN6TF tumors. (A) Undifferentiated spindle-cell tumor that formed after transplantation of BAG2-GN6TF cells to the s.c. site of an 18-month-old rat (harvested 85 days after cell transplantation, H&E). (B) Undifferentiated spindle-cell tumor produced by direct injection of BAG2-GN6TF cells into the liver of an 18-month-old rat (harvested 28 days after cell transplantation, H&E). (C) S.c. tumor formed after transplantation of BAG2-GN6TF cells recovered from the liver of a host rat 14 months after transplantation (H&E). (D) Well differentiated HCC that arose in an 18-month-old rat 85 days after transplantation of BAG2-GN6TF cells into the liver by injection of cells into the spleen (H&E).
Inoculation of BAG2-GN6TF Cells into the Spleen.
Dispersal of BAG2-GN6TF cells throughout the hepatic parenchyma was accomplished by inoculating cells into the spleens of host rats. Under these conditions no i.h. tumors formed in young adult rats, but β-gal+ progeny of BAG2-GN6TF cells were detected in all transplant recipients as single or as small groups of hepatocyte-like cells (Table 1 and Fig. 2A). The identity of tumor cell-derived hepatocyte progeny in livers of host rats was confirmed by transplanting BAG2-GN6TF cells into livers of DPPIV− rats and assaying for DPPIV enzymatic activity. When transplanted into livers of DPPIV− rats by splenic inoculation, DPPIV+ hepatocytic progeny of the transplanted BAG2-GN6TF cells were readily identified against a background of DPPIV− host hepatocytes (Fig. 2 B and C). The DPPIV+ hepatocytes in hepatic plates were comparable to adjacent host hepatocytes in size and morphology. Close physical contact between the differentiated progeny of the transplanted tumor cells and host hepatocytes was demonstrated by colocalization of DPPIV staining and bile canalicular ATPase activity in hybrid bile canaliculi (Fig. 2 B and C). Immunohistochemical stains showed that DPPIV+ hepatocyte-like BAG2-GN6TF cells expressed both albumin (Fig. 2C) and transferrin (data not shown). β-Gal+ hepatocytes also expressed albumin or transferrin as demonstrated by immunostaining (data not shown). BAG2-GN6TF-derived hepatocytes were identified in host rats at all time points examined after transplantation, including the earliest time point examined (7 days after transplantation). Thus, tumor cells differentiated rapidly after their introduction into the liver microenvironment.
Table 1.
Frequency of hepatocytic differentiation (single cells), hepatocyte focus formation, and liver tumor development by BAG2-GN6TF cells after transplantation into the livers of syngeneic hosts by injection into the spleen
| Time*, days | Young adult rats (3 months old)
|
Old rats (18 months old)
|
||||
|---|---|---|---|---|---|---|
| Single cells | Hepatocyte foci† | Liver tumors | Single cells | Hepatocyte foci† | Liver tumors | |
| 7 | 3/3 | 0/3 | 0/3 | 5/5 | 4/5 | 0/5 |
| 14 | 3/3 | 0/3 | 0/3 | 6/6 | 4/6 | 0/6 |
| 21 | 3/3 | 0/3 | 0/3 | 3/3 | 3/3 | 0/3 |
| 28 | 3/3 | 0/3 | 0/3 | 3/3 | 3/3 | 0/3 |
| 85 | 3/3 | 0/3 | 0/3 | 5/5 | 5/5 | 1/5‡ |
| 440§ | 5/5 | 4/5 | 0/5 | ND | ND | ND |
ND, not determined.
Days after transplantation.
Clusters of β-gal+ hepatocytes organized into plates separated by sinusoidal spaces.
Two animals survived to the 85-day end point. The liver of one rat contained a well differentiated HCC (see Figure 1D).
Three-month-old rats that received cell transplants by spleen injection were allowed to age naturally for 14 months before determination of the fate of the transplanted tumor cells.
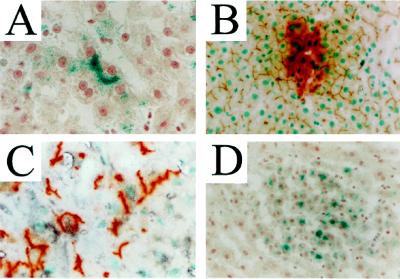
Figure 2.
Hepatocyte differentiation by BAG2-GN6TF cells transplanted to the liver by splenic inoculation. The differentiated progeny of transplanted tumor cells were distinguished from host hepatocytes in livers of syngeneic rats based on their expression of β-gal (blue chromagen, A and D) or DPPIV (red chromagen, B and C). (A) β-Gal+ hepatocyte progeny of BAG2-GN6TF cells in a 3-month-old rat. (B) Colocalization of DPPIV enzyme activity and bile canalicular ATPase activity (brown precipitate) in the liver of young-adult DPPIV− rats containing DPPIV+ hepatocyte progeny of BAG2-GN6TF cells. (C) Immunohistochemical detection of albumin (black precipitate) in DPPIV+ hepatocyte progeny of BAG2-GN6TF cells in 3-month-old rat liver. (D) Foci of β-gal+ hepatocytes in the liver of an 18-month-old rat 14 days after transplantation of BAG2-GN6TF cells to the liver by splenic inoculation. Identical foci of β-gal+ hepatocytes were observed in the livers of rats 14 months after transplantation of BAG2-GN6TF cells into the liver of a 3-month-old rat (data not shown).
Effects of Age on BAG2-GN6TF Cells in the Liver.
After transplantation of BAG2-GN6TF cells into livers of 18-month-old rats by inoculation into the spleen, single and small groups of β-gal+ hepatocyte-like cells were identified in the hepatic parenchyma of all transplant recipients. In addition, foci of multiple hepatocyte-like BAG2-GN6TF progeny also were observed in livers of old transplant recipients (Fig. 2D). Hepatocyte progeny of BAG2-GN6TF cells proliferated to form foci soon after their transplantation into the livers of old rats, as evidenced by the presence of foci of β-gal+ hepatocyte-like cells at early time points after transplantation (7 days after transplantation). Livers of the majority of old transplant recipients harbored β-gal+ hepatocyte foci 7 and 14 days after transplantation and the foci were maintained in the livers of all old rats at the later time points (Table 1). Because old DPPIV− rats are not available, the formation of foci of BAG2-GN6TF cells could only be assessed using the β-gal reporter enzyme. Foci were composed of β-gal+ hepatocyte-like cells that were organized into plates separated by sinusoidal spaces; however, the plates were not smoothly continuous with the host hepatic plates. In contrast to these results with transplanted BAG2-GN6TF cells, β-gal+ hepatocyte foci were never seen in either young or old rats after transplantation of normal BAG2-WB cells (data not shown).
The fate of BAG2-GN6TF cells transplanted into the livers of old rats via the spleen was difficult to determine because of the formation of extrahepatic tumors, presumably from the inadvertent introduction of tumor cells into the i.p. cavity during transplantation. Among one group of 18-month-old rats, five of five developed extrahepatic tumors and three of five required early euthanasia. The range of survival times for these groups of old rats was 35–85 days, and the mean time of survival was 64 ± 20 days. The liver of one of the two rats that survived to the 85-day endpoint contained a large (>2-cm diameter) well differentiated hepatocellular carcinoma (HCC; Fig. 1D). This tumor weakly expressed β-gal (data not shown), indicating its origin from the transplanted tumor cells. Neo-PCR showed that the tumor cells contained the NeoR gene in their genomic DNA (Fig. 3), verifying the origin of this tumor from BAG2-GN6TF cells.
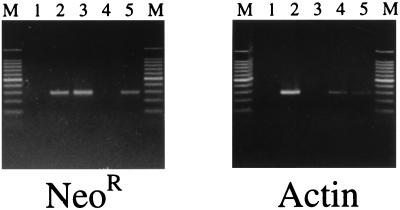
Figure 3.
PCR detection of the neomycin-resistance gene in BAG2-GN6TF-derived HCC. Identical DNA templates were used in the PCR amplification of the NeoR gene and the actin gene. Lanes marked M contain molecular size markers (100-bp ladder). Lanes: 1, no DNA control; 2, DNA template from BAG2-GN6TF cells; 3, pSV2neo plasmid DNA (positive control for NeoR gene); 4, DNA template from paraffin-embedded control rat liver; 5, DNA template from paraffin-embedded HCC (from Fig. 1D).
Injection-site tumors regressed from the livers of young rats that were maintained for >28 days after cell transplantation (8), enabling examination of the long-term fate of differentiated progeny of the transplanted tumor cells. Single and small groups of β-gal+ progeny of the transplanted cells were identified in livers of all (five of five) transplant recipients 14 months after the transplantation of BAG2-GN6TF cells into 3-month-old rats, and multicellular foci of BAG2-GN6TF progeny were present in four of five old rats in this group (Fig. 2D and Table 1). However, no neoplastic lesions were identified in any of these rats. The β-gal+ hepatocytes and foci observed in this group of old rats expressed both albumin and transferrin (data not shown).
Dedifferentiation of BAG2-GN6TF Progeny upon Removal from Host Livers.
Progeny of BAG2-GN6TF cells that resided in livers of host rats for 1, 4, or 14 months as differentiated hepatocytes were reestablished in cell culture from collagenase-dispersed primary liver cell preparations. In the primary liver cell preparations from these animals, β-gal staining was limited to morphologically identifiable hepatocytes (data not shown). In addition to epithelial cell colonies with normal morphology, which are readily established from normal rat liver (13), colonies of irregularly shaped spindle cells were established from primary liver cell preparations representing each animal that received BAG2-GN6TF cell transplants. Epithelial cell colonies established from the livers of unmanipulated control rats (no BAG2-GN6TF cell transplant) or from the livers of rats that received transplants of untransformed diploid BAG2-WB cells did not exhibit this aberrant morphology. All of the recovered BAG2-GN6TF cell lines isolated 1 and 4 months after cell transplantation expressed neomycin resistance in cell culture, while those isolated 14 months after transplantation did not (Table 2). BAG2-GN6TF clones isolated 1 and 4 months after transplantation expressed strong or moderate levels of β-gal activity, while cell lines reestablished from the livers of rats 14 months after transplantation were weakly positive or, in some cases, negative (Table 2 and Fig. 4). Neo-PCR verified the origin of these cell lines. All recovered cell lines are positive for this genetic marker of the transplanted cells (Table 2).
Table 2.
Characteristics of recovered BAG2-GN6TF cell lines
| Cell line | β-Gal expression | NeoR expression | NeoR PCR | Soft agar growth, % CFE | Saturation density, cells/cm2 | Tumorigenicity, % tumors/rats injected | Tumor latency, days |
|---|---|---|---|---|---|---|---|
| BAG2-GN6TF | + | + | + | 16 ± 2 | 2.8 × 105 | 100 (20/20) | 21 ± 4 |
| R1.1 | + | + | + | 8.9 ± 1.4 | 2.2 × 105 | 100 (10/10) | 27 ± 2 |
| R1.2 | + | + | + | 6.0 ± 0.9 | 2.0 × 105 | 100 (4/4) | 34 ± 2 |
| R1.3 | +/−* | + | + | 10.0 ± 1.3 | 1.8 × 105 | 100 (10/10) | 32 ± 2 |
| R1.4 | + | + | + | 6.0 ± 0.9 | 2.0 × 105 | 100 (7/7) | 39 ± 1 |
| R14.1 | + | − | + | 3.3 ± 0.4 | 1.4 × 105 | 100 (5/5) | 31 ± 2 |
| R14.2 | − | − | + | 4.8 ± 0.3 | 2.0 × 105 | 100 (6/6) | 39 ± 1 |
| R14.3 | +/− | − | + | 5.3 ± 0.5 | 6.7 × 105 | 100 (9/9) | 27 ± 1 |
| R14.4 | − | − | + | 11.3 ± 0.5 | 2.9 × 105 | 100 (5/5) | 22 ± 1 |
| R14.5 | +/− | − | + | 5.8 ± 0.5 | 2.8 × 105 | 100 (8/8) | 29 ± 1 |
| R14.6 | + | − | + | 12.6 ± 0.9 | 3.3 × 105 | 100 (5/5) | 28 ± 1 |
All rats were 3 months old at the time of cell transplant. R1 and R14 cell lines were established from the livers of rats 1 month (n = 2 rats) or 14 months (n = 3 rats) after transplantation of BAG2-GN6TF cells. NeoR expression is expression of antibiotic resistance by cells cultured in the presence of G418. NeoR PCR is detection of the BAG2 retroviral Tn5 neomycin resistance gene by PCR amplification. Column labeled Tumorigencity shows the percentage of animals forming tumors after transplantation to s.c. or i.p. sites of 1-day-old syngeneic rats (tumors formed/animals injected). Tumor latency is the average time to formation of a tumor of 1-cm diameter.
Moderate levels of β-gal activity expressed.
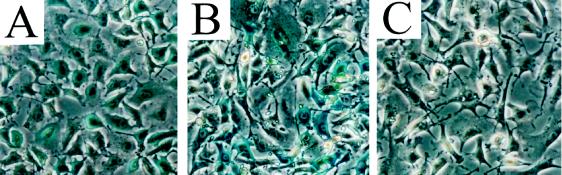
Figure 4.
Expression of β-gal enzyme activity in BAG2-GN6TF cell lines. (A) Parental BAG2-GN6TF cells. (B) A representative clonal cell line established from a primary liver cell dispersion prepared 1 month after transplantation of BAG2-GN6TF cells. (C) Representative clonal cell line established from a primary liver cell dispersion prepared 14 months after transplantation of BAG2-GN6TF cells (weak β-gal expression).
The cell lines established from livers of BAG2-GN6TF transplant recipients exhibited a transformed morphology in vitro that is indistinguishable from the morphology of the parental BAG2-GN6TF cells (Fig. 4). Growth of the recovered cell lines was not inhibited by cellular contact; while some cell lines grew to saturation densities that were reduced relative to BAG2-GN6TF cells, most were comparable to the BAG2-GN6TF cell line (Table 2). All recovered cell lines are capable of anchorage-independent growth, although the colony-forming efficiency (CFE) of some of the lines in soft agar was reduced relative to the parental BAG2-GN6TF cell line (Table 2). Length of residence in the liver as differentiated hepatocytes did not significantly alter the CFE of recovered progeny; the average CFE of cell lines isolated 1 month after transplantation was 7.7 ± 2.0%, while the average CFE of cell lines isolated 14 months after transplantation was 7.2 ± 1.6%. All recovered BAG2-GN6TF cell lines were 100% tumorigenic at s.c. or i.p. transplantation sites of 1-day-old neonatal rats with short latency (Table 2). Tumors that formed after transplantation of the recovered cell lines contain undifferentiated spindle cells, which are indistinguishable from tumors produced by the parental BAG2-GN6TF cells (Fig. 1).
DISCUSSION
Several previous studies have reported that some tumor cells are induced to differentiate in vitro by incorporation of specific chemical and biological agents into the culture medium and that after removal of the differentiating stimulus the tumor cells undergo phenotypic simplification or dedifferentiation (17–20). In addition, studies have documented the participation of teratocarcinoma cells in normal mouse development after their transplantation into the blastocyst (21–26) and regulation of differentiation of other tumor cell types by appropriate embryonic fields (27, 28). These studies suggested that the phenotype expressed by transformed cells is dynamic and subject to normalization in response to epigenetic (microenvironmental) regulation (26). However, examination of the plasticity of the tumorigenic phenotype of transformed epithelial cells after their transplantation into the microenvironment of an adult tissue in vivo has not been reported. We report here that aneuploid tumorigenic rat liver epithelial cells incorporate into host hepatic plates and differentiate morphologically and functionally into hepatocytes after they are transplanted into the liver (by inoculation into the spleen). These same tumor cells express an abnormal phenotype when the microenvironment of the liver is altered either by concentrating tumor cells locally within the liver parenchyma or by natural aging of the liver microenvironment. Furthermore, the hepatocyte-like progeny of BAG2-GN6TF cells revert to an undifferentiated, tumorigenic phenotype immediately upon recovery from the liver and reestablishment in culture. These findings demonstrate retrodifferentiation of tumor cells that had been induced to differentiate by transplantation into a specific tissue microenvironment of the adult rat. Our data indicate that some genetically aberrant cells can differentiate, with concomitant suppression of their tumorigenicity, in response to appropriate (adult) tissue microenvironments in vivo, and that their differentiated progeny require constant, tissue-specific epigenetic regulation to remain phenotypically normal.
Consistent with our previous report (8), direct inoculation of a bolus of BAG2-GN6TF tumor cells into the hepatic parenchyma of rats is followed by formation of i.h. tumors. These data sharply contrast with those obtained when the tumor cells are transplanted into the liver via the spleen, which distributes transplanted cells throughout the liver as single cells and which does not result in the rapid development of hepatic tumors in either young or old rats (Table 1). The different outcomes produced by these two transplantation methods most likely reflect differences in the local tissue microenvironment encountered by the transplanted tumor cells in the liver. Inclusion of tumor cells in a group of similar abnormal cells is associated with their heightened production of and response to autocrine and paracrine growth factors (29, 30). Thus, tumor cells that are concentrated at the site of injection by direct inoculation into the liver may encounter local concentrations of cytokines and growth factors that are not characteristic of the normal liver. Perhaps more important, tumor cells inoculated into the liver as a bolus lack close physical contact with parenchymal cells and other elements of the normal hepatic extracellular matrix that may be necessary for suppression of the neoplastic phenotype of the transplanted tumor cells. Dispersal of tumor cells throughout the hepatic parenchyma (achieved by splenic inoculation) facilitates interaction between the transplanted cells and differentiation-promoting factors in the hepatic microenvironment. Intimate contact between the transplanted BAG2-GN6TF tumor cells and elements in the normal hepatic microenvironment appears to be essential for induction and maintenance of differentiation and suppression of tumorigenicity.
Data obtained from the transplantation of rat liver epithelial tumor cells into livers of old rats (via injection into the spleen) support our previous suggestion that the regulatory capacity of the liver microenvironment declines as rats age (7, 8). Although transplanted tumor cells respond to the epigenetic, differentiation-promoting signals present in the hepatic microenvironment of old rats, the hepatocyte progeny of the transplanted tumor cells continue to proliferate in the livers of old rats and generate foci of differentiated hepatocyte-like cells. Foci of tumor cell progeny are present in livers of old rats as early as 7 days after transplantation, indicating that hepatocyte progeny of BAG2-GN6TF cells proliferate soon after their introduction into livers of old rats. It is unlikely that the foci represent BAG2-GN6TF cells that incorporated into host hepatic plates as large groups of cells because (i) untransformed BAG2-WB cells do not form such foci after their transplantation into livers of old rats and (ii) BAG2-GN6TF cells do not generate hepatocyte foci when they are transplanted into the livers of young rats. These observations suggest that although the liver microenvironment of old rats can regulate (at least in part) the differentiation of transplanted tumor cells, it is unable to suppress the proliferation of these apparently differentiated cells. Foci of hepatocyte progeny of BAG2-GN6TF cells may represent pretumorigenic lesions that, given enough time, will generate hepatic tumors. In the current study, most of the old rats that received tumor cell transplants did not survive long enough for tumors to develop in the liver, because of the occurrence of extrahepatic tumors. Thus, although the ability of tumor cells to form tumors in the livers of old rats after inoculation into the spleen was not conclusively determined, the finding of a well differentiated HCC in one of these old rats that survived 85 days after transplantation suggests that progeny of BAG2-GN6TF cells that form foci continue to proliferate in the liver of old rats and eventually progress to form tumors.
When young rats with single or small groups of hepatocyte progeny of BAG2-GN6TF cells in their livers were allowed to age for 14 months, foci of β-gal+ hepatocyte-like cells developed. This observation is important for several reasons. First, it demonstrates that genetically aberrant (but phenotypically normal) cells are capable of long-term survival in the liver. Second, it provides additional support for our hypothesis that the regulatory capacity of the liver declines with age. Third, it shows that when the regulatory capacity of the liver microenvironment declines, hepatocytic progeny of BAG2-GN6TF can reenter the cell cycle and proliferate. The absence of foci of BAG2-GN6TF-derived hepatocyte-like cells in the livers of young rats indicates that the proliferation of these cells is suppressed in young livers. It is not until the animal (and, more specifically, the hepatic microenvironment) ages that these quiescent cells acquire the potential to proliferate abnormally. The results of the present study suggest that the hepatocytic progeny of BAG2-GN6TF cells in livers of young rats could give rise to hepatic tumors in hosts that are allowed to attain naturally an advanced age (18–24 months old).
Collagenase dissociation of the livers of BAG2-GN6TF transplant recipients yields isolated epithelial cells that in culture form colonies of cells that have the characteristic morphology of BAG2-GN6TF tumor cells. The recovered BAG2-GN6TF cell lines display multiple phenotypic characteristics in vitro that are indistinguishable from those of the parental tumor cell line, and they are highly tumorigenic after s.c. or i.p. transplantation into syngeneic hosts. We have established numerous epithelial cell lines from normal rat livers (13), but these cell lines have never expressed transformation-related phenotypes in vitro or tumorigenic potential in vivo (unpublished observation). Furthermore, recovery from the liver of hepatocyte-like progeny of transplanted BAG2-WB cells yields nontumorigenic cells that are nearly identical to the cells originally transplanted (13). Our finding that BAG2-GN6TF cells differentiate into hepatocyte-like cells in the liver but revert to an undifferentiated, tumorigenic phenotype upon removal from the liver, combined with the observation that the hepatocyte progeny of transplanted BAG2-GN6TF cells proliferate abnormally in livers of old rats, indicates strongly that the tissue microenvironment has a determining role in mediating the phenotype expressed by these genetically aberrant cells. While genomic alterations undoubtedly play a critical role in neoplastic transformation, disruption of normal homeostatic relations between transformed cells and their tissue microenvironment appears to be equally relevant to tumor formation (6, 31). Since the phenotype expressed by transformed cells is dynamic and potentially regulatable, extracellular forces represent essential determinants of the malignant behavior of a transformed cell.
Acknowledgments
This work was supported by National Institutes of Health Grants CA 64340 and CA 29323. K.D.M. was supported, in part, by National Institutes of Health Training Grant T32 ES07126, and W.B.C. was supported, in part, by an IBM Fund Junior Faculty Development Award.
ABBREVIATIONS
- β-gal
β-galactosidase
- CFE
colony-forming efficiency
- DPPIV
dipeptidyl peptidase IV
- HCC
hepatocellular carcinoma
- i.h.
intrahepatic
- NeoR
neomycin resistance gene
- H&E
hematoxylin/eosin
References
- 1.Newell G R, Spitz M R, Sider J G. Semin Oncol. 1989;16:3–9. [PubMed] [Google Scholar]
- 2.Miller R A. Cancer. 1991;68:2496–2501. doi: 10.1002/1097-0142(19911201)68:11+<2496::aid-cncr2820681503>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Peto R, Roe F J C, Lee P N, Levy L, Clack J. Br J Cancer. 1975;32:411–426. doi: 10.1038/bjc.1975.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peto R, Parish S E, Gray R G. IARC Scientific Publications. Lyon, France: International Agency for Research on Cancer; 1985. , No. 58, pp. 43–53. [Google Scholar]
- 5.Anisimov V N. Semin Oncol. 1989;16:10–19. [PubMed] [Google Scholar]
- 6.Rubin H. Cancer Res. 1985;45:2935–2942. [PubMed] [Google Scholar]
- 7.McCullough K D, Coleman W B, Smith G J, Grisham J W. Cancer Res. 1994;54:3668–3671. [PubMed] [Google Scholar]
- 8.McCullough K D, Coleman W B, Smith G J, Grisham J W. Cancer Res. 1997;57:1807–1813. [PubMed] [Google Scholar]
- 9.Coleman W B, Wennerberg A E, Smith G J, Grisham J W. Am J Pathol. 1993;142:1373–1382. [PMC free article] [PubMed] [Google Scholar]
- 10.Dabeva M, Hwang S-G, Vasa S R G, Hurston E, Novikoff P M, Hixson D C, Gupta S, Shafritz D A. Proc Natl Acad Sci USA. 1997;94:7356–7361. doi: 10.1073/pnas.94.14.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman W B, McCullough K D, Esch G L, Faris R A, Hixson D C, Smith G J, Grisham J W. Am J Pathol. 1997;151:353–359. [PMC free article] [PubMed] [Google Scholar]
- 12.Wachstein M, Meisel E. Am J Clin Pathol. 1955;27:13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Grisham J W, Coleman W B, Smith G J. Proc Soc Exp Biol Med. 1993;204:270–279. doi: 10.3181/00379727-204-43663. [DOI] [PubMed] [Google Scholar]
- 14.Lee L W, Raymond V W, Tsao M-S, Lee D C, Earp H S, Grisham J W. Cancer Res. 1991;51:5238–5244. [PubMed] [Google Scholar]
- 15.Coleman W B, McCullough K D, Esch G L, Civalier C J, Livanos E, Weissman B E, Grisham J W, Smith G J. Mol Carcinog. 1995;13:220–232. doi: 10.1002/mc.2940130405. [DOI] [PubMed] [Google Scholar]
- 16.Tsao M S, Earp H S, Grisham J W. Cancer Res. 1985;45:4428–4432. [PubMed] [Google Scholar]
- 17.Hass R. Eur J Cell Biol. 1992;58:1–11. [PubMed] [Google Scholar]
- 18.Wood K M, Roberts T M. Biochim Biophys Acta. 1993;115:133–150. doi: 10.1016/0304-419x(93)90002-t. [DOI] [PubMed] [Google Scholar]
- 19.Ngyuen H T, Medford R M, Nadal-Ginard B. Cell. 1983;34:281–293. doi: 10.1016/0092-8674(83)90159-9. [DOI] [PubMed] [Google Scholar]
- 20.Hass R. Crit Rev Oncog. 1994;5:349–371. doi: 10.1615/critrevoncog.v5.i4.20. [DOI] [PubMed] [Google Scholar]
- 21.Brinster R L. J Exp Med. 1974;140:1049–1056. doi: 10.1084/jem.140.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mintz B, Illmensee K. Proc Natl Acad Sci USA. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papaioannou V E, McBurney M W, Gardner R L. Nature (London) 1975;258:70–73. doi: 10.1038/258070a0. [DOI] [PubMed] [Google Scholar]
- 24.Illmensee K, Mintz B. Proc Natl Acad Sci USA. 1976;73:549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papaioannou V E, Gardner R L, McBurney M W, Babinet C, Evans M J. J Embryol Exp Morphol. 1978;44:93–104. [PubMed] [Google Scholar]
- 26.Mintz B, Fleischman R A. Adv Cancer Res. 1981;34:211–278. doi: 10.1016/s0065-230x(08)60243-2. [DOI] [PubMed] [Google Scholar]
- 27.Podesta A H, Mullins J, Pierce G B, Wells R S. Proc Natl Acad Sci USA. 1984;81:7608–7611. doi: 10.1073/pnas.81.23.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerschenson M, Graves K, Carson S D, Wells R S, Pierce G B. Proc Natl Acad Sci USA. 1986;83:7307–7310. doi: 10.1073/pnas.83.19.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laderoute K R, Murphy B J, Short S M, Grant T D, Knapp A M, Sutherland R M. Br J Cancer. 1992;65:157–162. doi: 10.1038/bjc.1992.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansbridge J N, Ausserer W A, Knapp M A, Sutherland R M. J Cell Physiol. 1994;161:374–382. doi: 10.1002/jcp.1041610223. [DOI] [PubMed] [Google Scholar]
- 31.Rinehart C A, Torti V A. Mol Carcinog. 1997;18:187–192. [PubMed] [Google Scholar]