Abstract
4′-Phosphopantetheinyl transferases (PPTs) catalyze the transfer of 4′-phosphopantetheine (4-PP) from coenzyme A to a conserved serine residue of their protein substrates. In humans, the number of pathways utilizing the 4-PP post-translational modification is limited and may only require a single broad specificity PPT for all phosphopantetheinylation reactions. Recently, we have shown that one of the enzymes of folate metabolism, 10-formyltetrahydrofolate dehydrogenase (FDH), requires a 4-PP prosthetic group for catalysis. This moiety acts as a swinging arm to couple the activities of the two catalytic domains of FDH and allows the conversion of 10-formyltetrahydrofolate to tetrahydrofolate and CO2. In the current study, we demonstrate that the broad specificity human PPT converts apo-FDH to holoenzyme and thus activates FDH catalysis. Silencing PPT by small interfering RNA in A549 cells prevents FDH modification, indicating the lack of alternative enzymes capable of accomplishing this transferase reaction. Interestingly, PPT-silenced cells demonstrate significantly reduced proliferation and undergo strong G1 arrest, suggesting that the enzymatic function of PPT is essential and nonredundant. Our study identifies human PPT as the FDH-modifying enzyme and supports the hypothesis that mammals utilize a single enzyme for all phosphopantetheinylation reactions.
Keywords: Enzymes/Covalent Regulation, Enzymes/Dehydrogenase, Metabolism/Fatty Acid, Metabolism/Folate, Protein/Domains, Protein/Post-translational Modification, Vitamins and Cofactors
Introduction
Folate derivatives function as coenzymes in reactions involving the transfer of one-carbon units and participate in key metabolic processes, including nucleotide biosynthesis and the regeneration of methionine from homocysteine (1). The intracellular folate pool consists of several major reduced folate forms, and the overall balance between different folates is maintained by numerous enzymes that incorporate one-carbon groups into the pool, convert these groups within the pool, or utilize them in biosynthetic reactions. One of the folate enzymes, 10-formyltetrahydrofolate (10-fTHF)2 dehydrogenase (FDH, aldehyde dehydrogenase 1L1, EC 1.5.1.6), oxidizes the formyl group of 10-fTHF to yield tetrahydrofolate (THF) and CO2. This reaction requires NADP+ and proceeds through a complex multistep mechanism (2). FDH is expressed in most tissues and is particularly abundant in the liver and kidney; in the former it is present at the level of about 1% of the total soluble cytosolic protein (3).
The FDH substrate, 10-fTHF, is used as a formyl group donor in two steps of de novo purine biosynthesis (4, 5). The excess of 10-fTHF, not required for this pathway, can be utilized by FDH to replenish the THF pool, thus making folate available for other reactions of one-carbon metabolism. It has been proposed that the FDH reaction serves as a regulator of intracellular purine levels, preventing the excessive flux of one-carbon groups into this pathway (6). Several other metabolic functions have also been attributed to FDH, including storage of intracellular folate in the form of THF (7); detoxification of intracellular formic acid by removing it as CO2 (8); and regulation of overall cellular methylation (9). FDH knock-out is not lethal, but mice lacking the enzyme exhibit low hepatic levels of THF and diminished reproductive efficiency (10). Recent studies have also implicated FDH as a potential contributor in the pathogenesis of neural tube defects (11). Of note, FDH is drastically and ubiquitously down-regulated in human cancers (6), a finding that implies a role for the enzyme in controlling cellular proliferation.
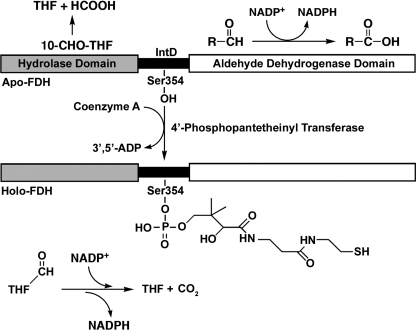
FDH is a tetramer of identical 99-kDa (902 amino acid residues) subunits (12). Each subunit consists of three distinct domains and is the product of the fusion of three unrelated genes (13). The amino-terminal domain (Nt-FDH, residues 1–310) shares a structural and functional similarity with two other enzymes that use 10-fTHF as a substrate, methionyl-tRNA formyltransferase and glycinamide ribonucleotide formyltransferase (14). This domain contains the folate binding site and can hydrolyze 10-fTHF to produce THF and formate (15). The carboxyl-terminal domain (Ct-FDH, residues 401–902) has ∼50% identity to other aldehyde dehydrogenases and shares the general fold of class 1 and 2 aldehyde dehydrogenases (16). By itself, Ct-FDH carries an NADP+-dependent aldehyde dehydrogenase activity and catalyzes the oxidation of short chain aldehydes into their respective carboxylic acids (13). The two catalytic domains of FDH are connected by an intermediate domain (IntD, residues 310–400) which belongs to a family of carrier proteins (17). It was recently demonstrated that this region of FDH is post-translationally modified by the covalent attachment of 4′-phosphopantetheine (4-PP) at serine 354 (18). It has been proposed that the 4-PP prosthetic group functions as a swinging arm, in a manner similar to that of acyl carrier proteins (ACPs), to transfer the formyl group of 10-fTHF from the Nt-FDH to the Ct-FDH where it is oxidized to CO2 (Fig. 1). Thus, the intermediate domain couples two catalytic activities to produce a novel enzymatic reaction (18).
FIGURE 1.
Domain structure of FDH and catalytic activities of apo- and holoenzyme. The amino-terminal domain of FDH catalyzes the hydrolysis of 10-fTHF to THF and formate; the carboxyl-terminal aldehyde dehydrogenase domain catalyzes the oxidation of short chain aldehydes to their respective carboxylic acids. 10-fTHF dehydrogenase catalysis requires the full-length holoenzyme, including the 4-PP prosthetic group covalently attached to serine 354 of the carrier protein-related intermediate domain.
ACPs are members of the family of carrier proteins that contain the 4-PP modification and are involved in fatty acid, polyketide, and nonribosomal protein biosynthesis (19). To date, human proteins shown to have the 4-PP modification include cytosolic fatty acid synthase (FAS) and mitochondrial ACP (20) as well as FDH (18). In addition, α-aminoadipate semialdehyde dehydrogenase, an enzyme of the lysine degradation pathway, could be a subject for this type of modification (21). The addition of 4-PP to a protein is catalyzed by 4-PP transferases (PPTs). These enzymes use coenzyme A as the donor of the 4-PP group, which is attached to a conserved serine residue of the carrier protein (22, 23). PPTs have been classified into three different groups on the basis of their structural organization (24). So far, only one PPT gene has been identified in the human genome. It encodes a 329-residue monomeric enzyme that exhibits broad substrate specificity (25). This cytosolic enzyme is capable of modifying the ACP domain of FAS and mitochondrial ACP (20) and has been also implicated in the modification of α-aminoadipate semialdehyde dehydrogenase (21). It is not clear, however, whether additional PPTs are present in humans. In this study, we demonstrate that FDH is a substrate for human PPT and that PPT siRNA silencing in a human cell line results in loss of the ability to modify FDH and produce active enzyme. These results give further support to the hypothesis that a single PPT is responsible for all 4-PP modifications in humans.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Purification of PPT
Using available sequence data for PPT mRNA (GenBank accession no. 20357567), PCR primers (forward, 5′-ATG GTT TTC CCT GCC AAA C-3′; reverse, 5′-TCA TGA CTT TGT ACC ATT TC-3′) were designed to amplify full-length PPT cDNA from a human heart cDNA library (Clontech). The PCR product was cloned into a linearized pCR2.1 plasmid using a TA Cloning kit (Invitrogen). The fragment encoding PPT was subcloned into the pRSET-B expression vector immediately downstream of the His6 tag. The construct was confirmed by DNA sequencing at Medical University of South Carolina Nucleic Acid Analysis Facility.
Expression of PPT was carried out in Escherichia coli as described elsewhere (25). Briefly, the PPT/pRSET construct was transformed into E. coli (BL21(DE3)-codon plus strain; Stratagene), the cultures were grown to A600 = 0.6, and expression was induced by the addition of 1 mm isopropyl 1-thio-β-d-galactopyranoside. After induction, the culture was allowed to grow for 4 h at 20 °C with constant shaking. The protein was purified from lysed cells using a PrepEase His-Tagged Protein Purification kit (USB Corp.).
Cloning, Expression, and Purification of FDH
Human FDH cDNA was amplified by PCR from human liver cDNA library (Clontech) and cloned into a pCR2.1 plasmid. The FDH coding sequence was further subcloned into a pRSET vector to generate a construct for expression of full-length enzyme with a His6 tag at the amino terminus (hFDH/pRSET). The enzyme was expressed in E. coli (BL21(DE3)-codon plus strain) at 20 °C and purified on a 5-fTHF affinity column as described previously (18).
Site-directed Mutagenesis
The construct for expression of truncated FDH was obtained by site-directed mutagenesis to introduce a stop codon at residue 408 (mutagenic primer, 5′-GCA GCA TTG ACT AAG TGG AAT GGC-3′; reverse primer, 5′-GCC ATT TCC ACT TAG TCA ATG CTG C-3′). To produce a protein that could not be derivatized at the 4-PP modification site, an additional S354A mutation was also introduced by site-directed mutagenesis (forward primer, 5′-CAG GGG CCG CGG CTG TGG ACG TTG-3′; reverse primer, 5′-CAA CGT CCA CAG CCG CGG CCC CTG-3′).
Assay of 10-Formyltetrahydrofolate Dehydrogenase Activity
10-fTHF dehydrogenase activity was measured spectrophotometrically as described previously (13). Briefly, 10-formyldideazafolate was used as a substrate in the assay in the presence of 100 μm NADP+. Absorbance was monitored at 295 nm and used to calculate the specific activity. The assays were conducted at 30 °C in a 1-cm quartz cuvette using a Shimadzu 2401PC double-beam spectrophotometer.
Activation of Apo-FDH by PPT
Purified recombinant FDH (5 μm) was incubated with 1.4 μm purified recombinant PPT, 100 μm CoA, 70 mm MgCl2, and 70 mm Tris-HCl, pH 7.5, at room temperature. At different time points (0–240 s), the reaction was quenched by the addition of 80 mm EDTA, and FDH activity was assayed. To determine the effect of PPT concentration on FDH reactivation, 0.008–10 μm PPT was added to a mixture of 4 μm FDH, 100 μm CoA, 50 mm MgCl2, and 50 mm Tris-HCl, pH 7.5. After incubation at room temperature for 1 min, the reaction was quenched by the addition of EDTA, and FDH activity was assayed.
Fluorescent Labeling of FDH by PPT
The fluorescent reporter, a fluorescein maleimide derivative of CoA, was synthesized as described previously (18, 26). The reporter (20 μl) was added to 34 μg of purified recombinant FDH or its mutants expressed in E. coli and 32 μg of recombinant PPT in a 200-μl solution containing 20 mm MgCl2 and 100 mm Tris-HCl, pH 7.8. The mixture was incubated at room temperature, and 20-μl aliquots were removed at corresponding time points, immediately quenched by the addition of EDTA, and subjected to SDS-PAGE. Immediately following electrophoresis, the gel was imaged under UV light and then stained with Coomassie Blue. A digital image of the gel exposed to UV irradiation was used to quantify the percent of labeling using ImageJ software.
siRNA Silencing of PPT in Cell Culture
A549 cells (1.7 × 105) were transfected with 50 nm of StealthTM RNA interference (Invitrogen) duplex (targeting the PPT sequence 5′-ACC UGA GCU GCA AGU UGG AAU UGA U-3′) using Lipofectamine 2000 reagent (Invitrogen). Scrambled StealthTM RNA interference was used as a negative control. PPT mRNA levels were evaluated using reverse transcription (RT)-PCR. Total RNA for RT-PCR was isolated using RNA Easy Mini kit (Qiagen). The RT reaction was performed with an oligo(dT)18 primer using AdvantageTM RT-for-PCR kit (Clontech). PCR was carried out using PPT-specific primers (forward primer, 5′-ATG GTT TTC CCT GCC AAA CGG-3′; reverse primer, 5′-TCA TGA CTT TGT ACC ATT TCG-3′) and Pfu-Turbo DNA polymerase (Stratagene). PPT protein levels were evaluated by immunoblot assays using a rabbit polyclonal antiserum (1:10,000) generated against pure recombinant human PPT.
Fluorescent Labeling of FDH by Cell Lysate
Cell lysates were obtained by mechanical disruption of a 100-μl suspension of 6 × 106 HEK293 or A549 cells using a Dounce homogenizer in the presence of a 10-μl protease inhibitor mixture (Sigma). 150 μl of 50% glycerol was added to the mixture, and insoluble content was removed by centrifugation at 13,000 × g for 5 min. In a total volume of 55 μl, 7 μg of recombinant FDH was incubated with 20 μl of cell lysate, 4 μg of PPT, or water in the presence of 10 μl of fluorescent reporter in Tris-HCl, pH 7.5, containing 50 mm MgCl2. Reaction mixtures were incubated for 1 h at 37 °C.
Cell Proliferation, Cell Cycle, and Apoptosis Assays
Cell viability was assessed using an MTT cell proliferation assay (Promega). Cells were plated at a density of 5 × 103 cells/100 μl of medium in 96-well format, and MTT was added at the specified time points. Plates were further processed according to the manufacturer's instructions. Absorbance at 570 nm was read using a Wallace 1420 multilabel counter (PerkinElmer Life Sciences). Cell cycle analysis was performed using propidium iodide staining/FACS. Specifically, 1.0 × 106 cells were washed and pelleted. Pellets were resuspended in 200 μl of cold phosphate-buffered saline, fixed by the addition of 4 ml of 70% ethanol, and incubated at −20 °C overnight. Following centrifugation, samples were resuspended in 500 μl of phosphate-buffered saline containing 40 μg/ml propidium iodide and 100 μg/ml RNase, incubated for 30 min at 37 °C, and samples were submitted for analysis by flow cytometry. Apoptosis was detected by annexin V labeling using an annexin V-FLUOS staining kit (Roche Applied Science). Annexin V/propidium iodide-stained cells were detected by flow cytometry carried out at the Hollings Cancer Center Flow Cytometry and Cell Sorting Facility on a Becton Dickinson FACSCalibur. Data analysis was performed using CellQuest and Mod Fit software (Becton Dickinson).
RESULTS
Cloning, Expression, and Purification of Human PPT
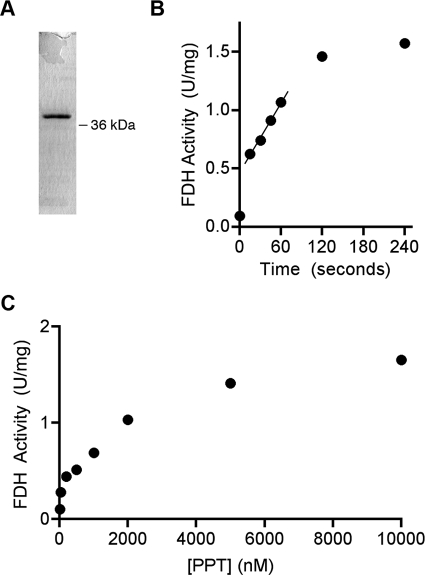
We have cloned PPT from a cDNA library generated using human heart mRNA. The PPT coding fragment was inserted into a pRSET vector in such a way that the expressed protein would have a His6 tag at the amino terminus separated from the first amino acid of PPT by a linker of four amino acid residues (Gly-Met-Ala-Ser). This construct was used for expression in E. coli as described under “Experimental Procedures.” Recombinant PPT was expressed mostly as a soluble protein. The protein was purified from cell lysate to near homogeneity using metal affinity chromatography (Fig. 2A).
FIGURE 2.
Activation of FDH by purified recombinant human PPT. A, SDS-PAGE (Coomassie-stained) of recombinant human PPT (His6-tagged, expressed in E. coli) purified by metal affinity chromatography. B, time dependence of apo-FDH activation by the purified PPT (1.4 μm). 5 μm apo-FDH (purified recombinant enzyme expressed in E. coli) was used for each time point. C, activation of apo-FDH by different concentrations of PPT. 4 μm apo-FDH was incubated for 1 min with increasing concentrations of PPT. In all experiments, 100 μm CoA was used. Activation of FDH was measured by reconstituted 10-fTHF dehydrogenase activity as described under “Experimental Procedures.”
PPT Modifies FDH at Serine 354 and Activates Its Catalysis
Our previous studies have shown that recombinant FDH, expressed in E. coli, lacks dehydrogenase activity toward 10-fTHF but retains hydrolase and aldehyde dehydrogenase activities (18). This phenomenon occurs because of the lack of modification of FDH with 4-PP in bacterial cells (18). Therefore, this recombinant FDH is a suitable substrate for FDH-specific PPT because it exists as an apoenzyme. To study whether human PPT can phosphopantetheinylate FDH, recombinant PPT was incubated with E. coli-expressed, recombinant FDH in the presence of CoA and MgCl2. It was observed that recombinant PPT enables the FDH enzyme to accomplish 10-fTHF dehydrogenase catalysis. Activation of FDH proceeded in a time-dependent manner (Fig. 2B). Maximum enzymatic activation was achieved in about 2 min, and the FDH activity did not change further. To determine the effective concentrations of PPT toward FDH as a substrate, FDH activation was evaluated (as reconstituted dehydrogenase activity) after a 1-min incubation using variable amounts of PPT and an excess of CoA (Fig. 2C). These experiments indicated that FDH phosphopantetheinylation proceeds efficiently at low micromolar concentrations of both proteins; a nonlinear regression analysis of our data revealed that 1.22 μm PPT can modify 50% of FDH under the reaction conditions.
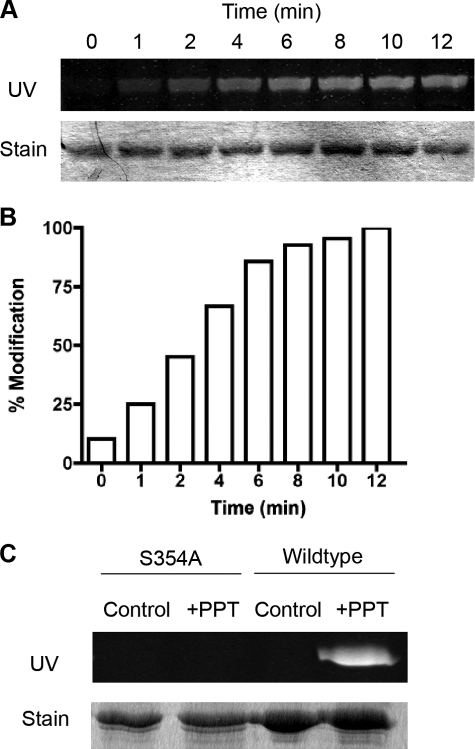
To demonstrate directly the ability of PPT to modify FDH covalently, a CoA-derived fluorescent reporter was synthesized as described previously (26). This reporter allows for the study of proteins undergoing phosphopantetheinylation and consists of the fluorescent label, fluorescein maleimide, attached to the sulfhydryl group of the 4-PP part of CoA (18). E. coli-expressed FDH was incubated with recombinant PPT in the presence of the fluorescent reporter and MgCl2, and this reaction was quenched at various time points using EDTA to chelate Mg2+ ions from the solution as they are required for the PPT reaction to proceed. SDS-PAGE and UV irradiation demonstrated incorporation of the fluorescent label into FDH in a time-dependent manner (Fig. 3, A and B). Maximum labeling of recombinant FDH was achieved after 12 min.
FIGURE 3.
Fluorescent labeling of apo-FDH by recombinant PPT. A, SDS-PAGE of recombinant apo-FDH incubated with recombinant PPT and fluorescent reporter for different periods of time. Upper panel, fluorescence of the gel after UV irradiation. Lower panel, Coomassie stain of the same gel. B, quantification of the fluorescence from A. C, fluorescence labeling of wild-type and S354A mutant apo-FDH by recombinant PPT. Upper panel, fluorescence under UV irradiation of SDS-PAGE. Lower panel, Coomassie stain of the same gel.
It has been reported previously that FDH is modified with 4-PP at serine 354. To confirm that PPT modifies FDH at this specific residue, PPT was incubated with an S354A mutant enzyme composed of Nt-FDH and IntD (Nt-FDH/IntD) in the presence of the fluorescent reporter and MgCl2 (Fig. 3C). As a control, wild-type Nt-FDH/IntD was incubated with recombinant PPT in a separate reaction mixture. These experiments revealed incorporation of the fluorescent label into wild-type apoenzyme but not S354A mutant FDH, thus confirming that PPT modifies FDH at this specific serine residue.
PPT Silencing Prevents Modification of Recombinant FDH by Mammalian Cell Lysate
Our previous studies have shown that lysate from Sf9 insect cells can modify recombinant FDH with the CoA-derived fluorescent reporter (18). To demonstrate that mammalian cells can accomplish this modification, recombinant FDH was incubated with lysates from HEK293 and A549 cells in the presence of the reporter. The results of this experiment showed that extracts from both cell lines are able to modify a significant portion of FDH with the fluorescent reporter (Fig. 4A).
FIGURE 4.
PPT siRNA silencing prevents apo-FDH fluorescent labeling. A, modification of FDH using a CoA-derived fluorescent reporter was determined after incubation of E. coli-expressed FDH with PPT, water (control), or cell-free extracts from HEK293 or A549 cells. Reaction mixtures were subjected to SDS-PAGE, and the gel was exposed to UV light irradiation (upper panel) and then stained with Coomassie Blue (lower panel). B, levels of PPT mRNA in A549 cells measured by RT-PCR at different times after siRNA transfection. Samples were normalized by the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). C, levels of PPT protein upon siRNA transfection in the same cells detected by Western blotting. The time post-transfection is indicated. Levels of actin are shown as loading control. Time point 0 in B and C shows levels in nontreated cells. D, lysates from A549 cells transfected with PPT-specific (-PPT) or scrambled siRNA (Scr) were incubated with recombinant FDH in the presence of fluorescent reporter and MgCl2. Fluorescent modification of a His-tagged mutant of FDH (Nt-FDH+IntD), truncated at residue 408, was also evaluated.
It has been proposed that there is only one enzyme responsible for all phosphopantetheinylation reactions in mammals (20). To test this hypothesis, we used an siRNA approach to knockdown PPT. PPT silencing was efficient, lasting from 24 to 96 h post-transfection, with a strong decrease in both PPT mRNA and protein levels (Fig. 4, B and C). To test whether other cellular enzymes can modify FDH, lysate from PPT-silenced A549 cells was incubated with E. coli-expressed FDH and the fluorescent reporter (Fig. 4D). These experiments revealed that depletion of PPT leads to decreased covalent modification of FDH by the cell extracts. This finding suggests that PPT is the only mammalian enzyme responsible for activating FDH and that silencing PPT is sufficient to inhibit phosphopantetheinylation of FDH.
PPT siRNA Induces Growth Arrest in A549 Cells
We have also evaluated the effect of PPT silencing on cellular proliferation. MTT proliferation assays have demonstrated that cells with depleted PPT have a decreased proliferation rate: at 96 h post-transfection ∼50% fewer viable cells were observed in PPT siRNA-transfected preparations than in control preparations transfected with scrambled siRNA (Fig. 5A). Strong G0/G1 arrest became noticeable in PPT-deficient cells as early as 24 h post-transfection (Fig. 5B). However, an annexin V assay revealed that PPT-silenced cells did not undergo apoptosis (Fig. 5C).
FIGURE 5.
Effects of PPT siRNA silencing on A549 cells. A, cell proliferation evaluated by MTT assays. B, cell cycle assessed by FACS analysis of propidium iodide-stained cells 24 h after transfection with scrambled or PPT siRNA. C, apoptosis in PPT siRNA-transfected cells examined by annexin V assays.
DISCUSSION
The two catalytic subunits of FDH communicate via an intermediate domain that is a structural and functional homolog of carrier proteins (2). Like other carrier proteins, its characteristic feature is a modification with a 4-PP group, which takes place at serine 354 in the case of FDH. In FAS, the prosthetic group works as a swinging arm, transferring acyl substrates between the different catalytic sites involved in fatty acid biosynthesis. In the mechanism of FDH catalysis, 4-PP transfers a formyl group from the amino-terminal hydrolase domain to the carboxyl-terminal aldehyde dehydrogenase domain, thus coupling the two catalytic activities into one reaction (18). Covalent modification of proteins with 4-PP, originating from CoA, is an enzymatic process that is catalyzed by 4-PP transferases. The enzyme responsible for the phosphopantetheinylation of FDH has not been reported previously; however, only one PPT has currently been identified in mammals (20). In the present study, we have demonstrated that the previously reported human PPT, which modifies acyl carrier proteins, is also responsible for phosphopantetheinylating FDH.
We have shown previously that recombinant FDH, expressed in bacteria, lacks 4-PP modification and is therefore incapable of catalyzing the 10-fTHF dehydrogenase reaction (18). At the same time, E. coli-expressed FDH retained full hydrolase and aldehyde dehydrogenase activity (Fig. 1). In our experiments, incubation of this enzyme with purified recombinant human PPT in the presence of CoA and Mg2+ restored 10-fTHF dehydrogenase activity. Using a CoA-derived fluorescent reporter, we have also directly demonstrated that recombinant PPT can modify FDH in vitro. Phosphopantetheinylation by PPT occurs specifically at serine 354 of FDH because replacement of this serine with alanine prevented this covalent modification.
Although this finding demonstrated that FDH is a substrate for PPT, it did not exclude the possibility that other mammalian enzymes could catalyze the phosphopantetheinylation. Indeed, based on the discovery of multiple PPTs in E. coli, it was proposed that individual carrier proteins were phosphopantetheinylated by target-specific PPTs (27). Further analysis of prokaryotic PPTs led to their classification into three distinct classes. Class 1 PPTs, homotrimers with active sites located at the junction between each subunit (28), modify bacterial carrier proteins associated with fatty acid and polyketide synthesis (29). Class 2 PPTs are exemplified by the Sfp enzyme of Bacillus subtilis, which is capable of recognizing a broad spectrum of carrier proteins (24). Although class 2 PPTs are monomers consisting of two domains, structural comparison between class 1 and 2 PPTs has demonstrated that they have comparable domain architecture despite the lack of sequence similarity (30). Class 3 PPTs are distinct in that they constitute a specific enzymatic domain integrated within a larger, multifunctional protein, and the PPT of FAS2 in Saccharomyces cerevisiae is the prototypical example (31).
Lower organisms often encode multiple PPTs; yeast, for instance, contain representatives of all three classes (20). However, some organisms appear to utilize only one PPT to accomplish their phosphopantetheinylation reactions (20). Thus, mammals may not require multiple PPTs because they lack nonribosomal peptide and polyketide synthesis. In agreement with this paradigm, there is only one PPT in humans (20), which is functionally and structurally similar to the broad-specificity Sfp enzyme of B. subtilis (25). The existence of additional mammalian PPTs, however, could not be excluded completely. Importantly, the results of our PPT knockdown experiments not only support the role of the enzyme in FDH modification but also indicate that this PPT is the only enzyme with this type of activity in humans.
It would be expected that FDH and other carrier proteins have a common consensus motif if they are modified by the same enzyme. Such a motif though is not apparent from the sequence analysis. The recently solved crystal structure of human PPT in complex with the ACP domain of FAS provides an explanation of how the enzyme can recognize different targets. It appears that PPT target specificity relies on the overall shape of the ACP domain rather than on interactions with particular peptide motif (25). In the crystal structure, PPT interacts with ACP over a large hydrophobic interface. Interestingly, replacement of three hydrophobic residues, situated immediately downstream of the modified serine (Leu2157, Met2158, and Val2160), to alanine reduces the Km by at least 3 orders of magnitude, indicating that this hydrophobic patch is important for PPT substrate recognition. Although the corresponding motif in FDH is different from in ACP (VDVV versus LMSV, respectively), the overall hydrophobicity of the region is preserved and thus might be important for PPT binding.
An additional argument in favor of a single PPT in humans is that PPT silencing has a profound effect on cellular proliferation, resulting in a strong G1 cell cycle arrest. Such an effect should not be expected if the PPT activity was redundant. Although PPT silencing perhaps inhibits all phosphopantetheinylation processes in the cell, the antiproliferative effects are most likely associated with impairment of fatty acid biosynthesis. Indeed, siRNA silencing of FAS or treatment of tumor cells with orlistat (a potent inhibitor of the thioesterase catalysis of FAS) results in a phenotype similar to that observed with knockdown of PPT, including arrest of G1/S progression and inhibition of cellular proliferation (32). Of note, siRNA FAS silencing or orlistat treatment of tumor cells induces apoptosis (33), an effect not observed with PPT silencing in our studies, possibly because of the presence of still modified ACP, and therefore active FAS, in the cell. However, it should be expected that a mouse knockout of PPT would be embryonic lethal, similar to knockout of FAS (34).
Whether limited availability of PPT under normal or pathological conditions could result in the accumulation of inactive apo-FDH is unclear. In E. coli, for example, apo-ACP is undetectable as an intermediate in ACP biosynthesis, suggesting rapid protein modification following translation (35). It is likely that most FDH in the cell also exists in the phosphopantetheinylated form. The enzyme, processed to holo form, could also be targeted by reactive metabolites at the 4-PP group, resulting in inhibition of activity. In this regard, it has been shown in animal studies that acetaminophen treatment or chronic ethanol ingestion decreases the hepatic activity of FDH (36, 37). This was attributed to the inactivation of the enzyme caused by the addition of reactive metabolites to cysteine 707, a critical catalytic residue (36, 38). It is more likely, however, that these adducts are formed with the sulfhydryl group of the 4-PP moiety, which should be easily accessible. Whether FDH, derivatized at its 4-PP group, can be repaired by subsequent removal of inactivated 4-PP and remodification with a fresh group remains a question for future investigation.
Acknowledgments
We thank Kelly Moxley for excellent technical assistance and Dr. Henry Donato for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant DK54388.
- 10-fTHF
- 10-formyltetrahydrofolate
- FDH
- 10-formyltetrahydrofolate dehydrogenase
- THF
- tetrahydrofolate
- Nt
- amino-terminal
- Ct
- carboxyl-terminal
- IntD
- intermediate domain
- 4-PP
- 4′-phosphopantetheine
- PPT
- 4′-phosphopantetheinyl transferase
- ACP
- acyl carrier protein
- FAS
- fatty acid synthase
- siRNA
- small interfering RNA
- RT
- reverse transcription
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- FACS
- fluorescence-activated cell sorter.
REFERENCES
- 1.Wagner C. (1995) in Folate in Health and Disease (Bailey L. B. ed) pp. 23–42, Marcel Dekker, New York [Google Scholar]
- 2.Krupenko S. A. (2009) Chem. Biol. Interact. 178, 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kisliuk R. L. (1999) in Antifolate Drugs in Cancer Therapy (Jackman A. L. ed) Humana Press, Totowa, NJ [Google Scholar]
- 4.Smith G. K., Benkovic P. A., Benkovic S. J. (1981) Biochemistry 20, 4034–4036 [DOI] [PubMed] [Google Scholar]
- 5.Boger D. L., Kochanny M. J., Cai H., Wyatt D., Kitos P. A., Warren M. S., Ramcharan J., Gooljarsingh L. T., Benkovic S. J. (1998) Bioorg. Med. Chem. 6, 643–659 [DOI] [PubMed] [Google Scholar]
- 6.Krupenko S. A., Oleinik N. V. (2002) Cell Growth Differ. 13, 227–236 [PubMed] [Google Scholar]
- 7.Fu T. F., Maras B., Barra D., Schirch V. (1999) Arch. Biochem. Biophys. 367, 161–166 [DOI] [PubMed] [Google Scholar]
- 8.Johlin F. C., Swain E., Smith C., Tephly T. R. (1989) Mol. Pharmacol. 35, 745–750 [PubMed] [Google Scholar]
- 9.Anguera M. C., Field M. S., Perry C., Ghandour H., Chiang E. P., Selhub J., Shane B., Stover P. J. (2006) J. Biol. Chem. 281, 18335–18342 [DOI] [PubMed] [Google Scholar]
- 10.Champion K. M., Cook R. J., Tollaksen S. L., Giometti C. S. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 11338–11342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anthony T. E., Heintz N. (2007) J. Comp. Neurol. 500, 368–383 [DOI] [PubMed] [Google Scholar]
- 12.Schirch D., Villar E., Maras B., Barra D., Schirch V. (1994) J. Biol. Chem. 269, 24728–24735 [PubMed] [Google Scholar]
- 13.Krupenko S. A., Wagner C., Cook R. J. (1997) J. Biol. Chem. 272, 10266–10272 [DOI] [PubMed] [Google Scholar]
- 14.Chumanevich A. A., Krupenko S. A., Davies C. (2004) J. Biol. Chem. 279, 14355–14364 [DOI] [PubMed] [Google Scholar]
- 15.Krupenko S. A., Wagner C., Cook R. J. (1997) J. Biol. Chem. 272, 10273–10278 [DOI] [PubMed] [Google Scholar]
- 16.Tsybovsky Y., Donato H., Krupenko N. I., Davies C., Krupenko S. A. (2007) Biochemistry 46, 2917–2929 [DOI] [PubMed] [Google Scholar]
- 17.Reuland S. N., Vlasov A. P., Krupenko S. A. (2006) Protein Sci. 15, 1076–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donato H., Krupenko N. I., Tsybovsky Y., Krupenko S. A. (2007) J. Biol. Chem. 282, 34159–34166 [DOI] [PubMed] [Google Scholar]
- 19.Walsh C. T., Gehring A. M., Weinreb P. H., Quadri L. E., Flugel R. S. (1997) Curr. Opin. Chem. Biol. 1, 309–315 [DOI] [PubMed] [Google Scholar]
- 20.Joshi A. K., Zhang L., Rangan V. S., Smith S. (2003) J. Biol. Chem. 278, 33142–33149 [DOI] [PubMed] [Google Scholar]
- 21.Praphanphoj V., Sacksteder K. A., Gould S. J., Thomas G. H., Geraghty M. T. (2001) Mol. Genet. Metab. 72, 336–342 [DOI] [PubMed] [Google Scholar]
- 22.Perham R. N. (2000) Annu. Rev. Biochem. 69, 961–1004 [DOI] [PubMed] [Google Scholar]
- 23.Elovson J., Vagelos P. R. (1968) J. Biol. Chem. 243, 3603–3611 [PubMed] [Google Scholar]
- 24.Mootz H. D., Finking R., Marahiel M. A. (2001) J. Biol. Chem. 276, 37289–37298 [DOI] [PubMed] [Google Scholar]
- 25.Bunkoczi G., Pasta S., Joshi A., Wu X., Kavanagh K. L., Smith S., Oppermann U. (2007) Chem. Biol. 14, 1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Clair J. J., Foley T. L., Schegg T. R., Regan C. M., Burkart M. D. (2004) Chem. Biol. 11, 195–201 [DOI] [PubMed] [Google Scholar]
- 27.Lambalot R. H., Gehring A. M., Flugel R. S., Zuber P., LaCelle M., Marahiel M. A., Reid R., Khosla C., Walsh C. T. (1996) Chem. Biol. 3, 923–936 [DOI] [PubMed] [Google Scholar]
- 28.Parris K. D., Lin L., Tam A., Mathew R., Hixon J., Stahl M., Fritz C. C., Seehra J., Somers W. S. (2000) Structure 8, 883–895 [DOI] [PubMed] [Google Scholar]
- 29.Gehring A. M., Lambalot R. H., Vogel K. W., Drueckhammer D. G., Walsh C. T. (1997) Chem. Biol. 4, 17–24 [DOI] [PubMed] [Google Scholar]
- 30.Chirgadze N. Y., Briggs S. L., McAllister K. A., Fischl A. S., Zhao G. (2000) EMBO J. 19, 5281–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fichtlscherer F., Wellein C., Mittag M., Schweizer E. (2000) Eur. J. Biochem. 267, 2666–2671 [DOI] [PubMed] [Google Scholar]
- 32.Knowles L. M., Axelrod F., Browne C. D., Smith J. W. (2004) J. Biol. Chem. 279, 30540–30545 [DOI] [PubMed] [Google Scholar]
- 33.Knowles L. M., Yang C., Osterman A., Smith J. W. (2008) J. Biol. Chem. 283, 31378–31384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chirala S. S., Chang H., Matzuk M., Abu-Elheiga L., Mao J., Mahon K., Finegold M., Wakil S. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 6358–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackowski S., Rock C. O. (1983) J. Biol. Chem. 258, 15186–15191 [PubMed] [Google Scholar]
- 36.Pumford N. R., Halmes N. C., Martin B. M., Cook R. J., Wagner C., Hinson J. A. (1997) J. Pharmacol. Exp. Ther. 280, 501–505 [PubMed] [Google Scholar]
- 37.Min H., Im E. S., Seo J. S., Mun J. A., Burri B. J. (2005) Alcohol. Clin. Exp. Res. 29, 2188–2193 [DOI] [PubMed] [Google Scholar]
- 38.Mun J. A., Doh E., Min H. (2008) Nutr. Res. Prac. 2, 195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]