Abstract
Germ cell apoptosis is crucial for spermatogenesis and can be triggered by various stimuli, including intratesticular hormone deprivation. This study proposes a role for insulin-like growth factor binding protein-3 (IGFBP-3) in male germ cell apoptosis. Groups of adult Sprague-Dawley male rats received one of the following treatments for 5 days: (i) daily intratesticular (IT) injections with saline (control); (ii) a single subcutaneous injection of the gonadotropin-releasing hormone antagonist (GnRH-A), acyline, on day 1 and a daily IT injection of saline; (iii) daily IT injection of IGFBP-3; and (iv) a GnRH-A injection on day 1 and a daily IT injection of IGFBP-3. Germ cell apoptosis increased significantly after IGFBP-3 or GnRH-A treatment which was further enhanced by the combined treatment. After co-immunoprecipitation with BAX antibody, IGFBP-3 association with BAX was demonstrated in total and mitochondrial fractions but not in the cytosol of testis extracts. BAX-associated IGFBP-3 expression was increased in mitochondria after treatment compared with control, which was confirmed by an IGFBP-3 enzyme-linked immunosorbent assay. Dot blot studies further validated the BAX-IGFBP-3 binding in vitro. IGFBP-3 as well as BAX induced release of cytochrome c and DIABLO from isolated testicular mitochondria in vitro. IGFBP-3, when combined with an ineffective dose of BAX, triggered release of these proteins from isolated mitochondria at a 4-fold lower dose than IGFBP-3 alone. Our data demonstrate that the IGFBP-3 and BAX interaction activates germ cell apoptosis via the mitochondria-dependent pathway. This represents a novel pathway regulating germ call homeostasis that may have significance for male fertility and testicular disease.
Introduction
Programmed germ cell death can occur spontaneously during spermatogenesis, or it can be induced in a cell-specific manner by a variety of apoptotic stimuli. These stimuli include experimental male contraceptive approaches such as gonadotropin-releasing hormone antagonist (GnRH-A)2 or exogenous administration of testosterone that act to suppress gonadotropins and intratesticular testosterone (1–3). Modulating germ cell death and survival may have significant therapeutic potential for male infertility, contraception, and germ cell cancer.
Using a rat model of intratesticular hormone deprivation, we have previously shown that preleptotene and pachytene spermatocytes and round spermatids at mid stages (VII-VIII) are the most susceptible germ cells to apoptosis after hormone deprivation in the rat (2–4). We have extended our experimental systems from rodents to monkeys (5, 6) and more recently, to humans (7). Across this spectrum of phylogenies, we demonstrated that germ cell apoptosis plays an important role in the organized regression of spermatogenesis after hormone deprivation.
The signaling events leading to apoptosis can be divided into two major pathways, involving either mitochondria (intrinsic) or death receptors (extrinsic) (8–10). In earlier studies, we showed that the mitochondria-dependent intrinsic pathway serves as the key signal for germ cell apoptosis across species after intratesticular hormonal deprivation (3, 4, 6, 11). In subsequent studies, we have shown that the activation of the p38 mitogen-activated protein kinase, through changes in the BAX/BCL-2 rheostat in the mitochondria, activates the intrinsic pathway signaling and promotes male germ cell apoptosis in response to a lack of hormonal support (6, 11).
Insulin-like growth factor binding protein-3 (IGFBP-3) is a pluripotent molecule that acts through multiple mechanisms (12). In addition to its role as a binding protein to the proliferative factor IGF-I, emerging evidence from our laboratories indicates that IGFBP-3 plays an important role in tumor suppression (12–16). We have further shown that IGFBP-3 induces apoptosis by rapidly internalizing into cells and interacting with the nuclear retinoid X receptor α (RXRα) (17) and its binding partner Nur77 (18, 19).
Our recent oligonucleotide microarray analysis showed up-regulation of Igfbp3 gene expression in men when germ cell apoptosis was induced by intratesticular hormonal deprivation (20). However, the role of IGFBP-3 and its signaling pathway in regulating testicular germ cell apoptosis is not known. This study elucidates the possible intracellular mechanisms of IGFBP-3 action in the induction of male germ cell apoptosis. Our data indicate that IGFBP-3, via binding to BAX, activates the mitochondria-dependent pathway and triggers male germ cell apoptosis.
EXPERIMENTAL PROCEDURES
Animals and Experimental Protocol
Adult 60-day-old male Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) and housed in a standard animal facility under controlled temperature (22 °C) and photoperiod of 12 h of light and 12 h of darkness with free access to food and water. Groups of four young adult (2-month-old) rats received the following treatment for 5 days: (i) control, daily intratesticular saline injection; (ii) GnRH-A (acyline, 30 mg/kg of body weight, a gift from Dr. Richard Blye, NICHD/National Institutes of Health) subcutaneous injection on day 1 and daily intratesticular saline injection; (iii) IGFBP-3 (50 μg, gift from Insmed Corp., Richmond, VA), daily intratesticular injection; and (iv) GnRH-A + IGFBP-3, GnRH-A injection on day 1 and daily intratesticular injection of 50 μg of IGFBP-3. All rats were killed on day 6. As an additional control experiment, six adult 60-day-old male Sprague-Dawley rats were treated with subcutaneous injections of vehicle (n = 3) or GnRH-A (n = 3). There was no intratesticular injection in these six rats. These six rats were also killed on day 6 and used as a negative control for the intratesticular injection process.
Tissue Preparation and Subcellular Fractionation
Both control and experimental animals were injected intraperitoneally with heparin (130 IU/100 g body weight) 15 min before a lethal intraperitoneal injection of sodium pentobarbital (100 mg/kg of body weight) to facilitate testicular perfusion using a whole body perfusion technique (2). After perfusion with saline, one testis was removed and weighed. Portions of testicular parenchyma were snap frozen in liquid N2 and stored at −80 °C for subcellular fractionation and Western blotting.
Mitochondrial and cytosolic fractions were prepared as described in our prior studies (4, 6, 21, 22). Briefly, saline-perfused testes were homogenized using a Dounce homogenizer in HEPES buffer (0.25 m sucrose, 50 mm HEPES, 10 mm NaCl, 10 mm EDTA, 2 mm dithiothreitol) supplemented with protease inhibitors (Complete Protease Inhibitors; Roche Applied Science). The crude homogenates were centrifuged at 1000 × g for 10 min at 4 °C, and the resultant supernatant was centrifuged at 10,000 × g for 15 min at 4 °C to sediment the low speed fraction containing mainly mitochondria. The mitochondria were washed twice in HEPES buffer and pelleted. The cytosolic fractions were isolated following centrifugation of the 10,000 × g supernatant fraction at 20,000 × g for 60 min at 4° C. The resulting supernatant was the cytosolic fraction. The purity of the cytosolic and mitochondrial fractions was validated by Western blotting using antibodies to actin (1:2000; Sigma- Aldrich) and cytochrome c oxidase subunit IV (1:500; Molecular Probes), respectively.
Co-immunoprecipitation and Western Blot Analysis
Co-immunoprecipitation of IGFBP-3 with BAX (sc-493; Santa Cruz Biotechnology, Santa Cruz, CA) in the total, cytosol, and mitochondrial fractions was performed using the ExactaCruzTM F kit (Santa Cruz Biotechnology). Briefly, following incubation of the antibody against BAX with the species-specific immunoprecipitation (IP) matrix, the mitochondrial fraction in the matrix was pelleted via microcentrifugation at maximum speed for 30 s at 4 °C and washed twice with 500 μl of phosphate-buffered saline. After the final wash of the IP antibody-IP matrix complex, 500 μg of total, cytosolic, or mitochondrial fractions of testis lysates was added to the pelleted matrix and incubated at 4 °C on a rotator overnight. The mixture was again pelleted by microcentrifuge at maximum speed for 30 s at 4 °C, washed three times with radioimmune precipitation assay lysis buffer, and resuspended in 40 μl of reducing 2× electrophoresis sample buffer (Santa Cruz Biotechnology). After boiling samples for 3 min, a quick spin was performed to pellet the IP matrix, and the supernatant was loaded onto a gel to continue with electrophoresis. Bands were visualized using the corresponding horseradish peroxidase-conjugated ExactaCruzTM F reagents and the enhanced chemiluminescence solutions per the manufacturer's specifications (Amersham Biosciences).
Western blotting was performed as described previously (4, 6, 21, 22). In brief, proteins were separated on a 4–12% SDS-polyacrylamide gel with MES or MOPS purchased from Invitrogen at 150 V. Gel was transferred on a Immunoblot polyvinylidene difluoride membrane (Bio-Rad) overnight at 4 °C. Membrane was blocked in blocking solution (0.3% Tween 20 in Tris-buffered saline and 10% nonfat dry milk) for 1 h at room temperature and then probed using goat polyclonal anti- IGFBP-3 (1:200, R&D Systems, Inc., Minneapolis, MN) overnight at 4 °C with constant shaking. After three 10-min washes in 0.3% Tween 20 in Tris-buffered saline, membrane was then incubated with anti-goat (Santa Cruz Biotechnology) IgG-horseradish peroxidase secondary antibody at a 1:1000 dilution. All antibodies were diluted in blocking buffer. For immunodetection, membrane was washed three times in 0.3% Tween 20 in Tris-buffered saline wash buffer, incubated with enhanced chemiluminescence solutions per the manufacturer's specifications (Amersham Biosciences), and exposed to Hyperfilm ECL (Denville Scientific, Inc., Metuchen, NJ). Band intensities were determined using Quantity One software from Bio-Rad. Nonreducing Western blotting was also performed as described above except for the absence of sample heating and reducing agents.
Rodent IGFBP-3 ELISA
Goat anti-mouse IGFBP-3 IgG, biotinylated goat anti-mouse IGFBP-3 antibody, mouse IGFBP-3 protein standard and streptavidin-horseradish peroxidase conjugate were purchased from R&D Systems. Super block blocking buffer, o-phenylenediamine dihydrochloride, and hydrogen peroxide substrate were purchased from Pierce. The mouse/rat IGFBP-3 assay has a sensitivity of 0.2 ng/ml and does not cross-react with mIGF-I, mIGF-II, mIGFBP-1, or mIGFBP-2. The ELISA uses a polyclonal anti-mouse IGFBP-3 antibody, which has a very high degree of cross-reactivity with rat IGFBP-3. 96-well microtiter plates were coated with goat anti-mouse IGFBP-3 IgG at 0.3 μg/well in 100 μl of 50 mm sodium bicarbonate, pH 9.5, incubated 3 h at room temperature on a shaker, washed three times with 300 μl/well wash buffer (phosphate-buffered saline, 0.05% Tween 20) followed by two washes with blocking buffer. Recombinant mouse IGFBP-3 protein standard was diluted in sample buffer (phosphate-buffered saline, 0.1% Tween 20, and 10% normal goat serum) in concentrations ranging from 0 to 50 ng/ml. Samples were appropriately diluted with assay buffer prior to assay. Standards, controls, or diluted samples (50 μl/well) and 50 ng/well biotinylated goat anti-mouse IGFBP-3 IgG (in 50 μl of assay buffer) were incubated overnight at room temperature on shaker. The wells were washed three times with wash buffer followed by the addition of streptavidin-horseradish peroxidase conjugate (100 μl/well in phosphate-buffered saline, 5% Tween 20) and incubated further for 20 min at room temperature. After four washes, 100 μl of o-phenylenediamine dihydrochloride (1 mg/ml in hydrogen peroxide substrate) was added to each well and incubated for 10–20 min. The reaction was stopped by the addition of 50 μl of 2 n H2SO4, and the absorbance was determined at 490 nm in a plate reader (Molecular Design, Sunnyvale, CA). The standard curve was analyzed using four-parameter logistic curvefit.
Dot Blot Assessment
Targeted proteins and respective controls were spotted onto nitrocellulose membrane (Pierce) at 1 μl/spot and then allowed to dry. After blocking the nonspecific sites by 0.2% I-block (Applied Biosystems, Foster City, CA) for 3 h at room temperature, the nitrocellulose membranes were incubated with tested peptides (5 μg/ml) as detailed below overnight at 4 °C with gentle rocking. After three 10-min washes in 0.3% Tween 20 in Tris-buffered saline, the nitrocellulose membranes were incubated with the primary antibody (anti-IGFBP-3, 1:200 or alternatively anti-BAX, 1:500) overnight 4 °C with gentle rocking. Following three 10-min washes in 0.3% Tween 20 in Tris-buffered saline, the membranes were then incubated with secondary antibody (anti-goat antibody, 1:1000, or alternatively anti-rabbit antibody, 1:2000; Santa Cruz Biotechnology) for 1 h at room temperature with gentle rocking. For immunodetection, membranes were washed three times in 0.3% Tween 20 in Tris-buffered saline, incubated with enhanced chemiluminescence solutions per the manufacturer's specifications (Amersham Biosciences), and exposed to Hyperfilm ECL. The peptides used for dot blots included: Humanin (HN, a known binding partner of IGFBP-3 (23); obtained from GeneMed Synthesis Inc., San Antonio, TX), BCL-2 (fragment; obtained from Santa Cruz Biotechnology), BAX (mouse full long sequence; obtained from ProSpec-Tany TechnoGene Ltd., Rehovot, Israel), and bovine serum albumin (BSA, obtained from Sigma-Aldrich), IGFBP-3. The reverse experiment protein dots were: HN, BSA, IGFBP-3; HN mutant (24) (HN-C8P, a non-BAX-binding mutant obtained from GeneMed Synthesis, Inc.), and BAX.
Mitochondria Purification and Cytochrome c and DIABLO Release Assays
Purification of mitochondria and protein release assays were performed by differential centrifugation as described previously (24, 25). In brief, testes were homogenized in HM buffer (10 mm HEPES, pH 7.4, 250 mm mannitol, 10 mm KCl, 5 mm MgCl2, 1 mm EGTA) containing 1 mm phenylmethylsulfonyl fluoride and a mixture of protease inhibitors (Roche Applied Science). The homogenate was centrifuged twice at 1000 × g for 5 min to remove nuclei and debris, and the resulting supernatant was centrifuged at 10,000 × g for 10 min to sediment the low speed fraction containing mitochondria. The mitochondria were washed twice with the HM buffer and resuspended in fractionation buffer and used within 2 h.
For detection of cytochrome c and DIABLO release, 10 μl of mitochondria (about 50 μg) was added into a final volume of 50 μl of HM buffer containing BAX or IGFBP-3 or BAX together with IGFBP-3 peptide (mixture was preincubated at 30 °C for 15 min) and was incubated further at 30 °C for 45 min. Samples were then pelleted by centrifugation and resuspended in a volume of HM buffer equal to the volume of supernatant collected and analyzed by immunoblotting using cytochrome c (1:2000; Santa Cruz Biotechnology) or SMAC/DIABLO (1:5000; Calbiochem) antibodies as described above.
To determine whether BAX and IGFBP-3 can bind to each other during the 15 min preincubation, these peptides were mixed at two concentrations as follows: lower concentrations of 5 ng/50 μl BAX and 100 ng/50 μl IGFBP-3 (which we used in the mitochondria protein release experiment) and higher concentrations of 500 ng/50 μl BAX and 600 ng/50 μl IGFBP-3 (which is the equal-molar concentration of BAX and IGFBP-3 peptide) and incubated at 30 °C for 15 min or 15 min plus 45 min with mitochondria (about 50 μg/50 μl). The mixture was subjected to Western blot analyses using antibody to IGFBP-3 and under nonreducing conditions to test the association of BAX with IGFBP-3.
Statistical Analysis
Statistical analyses were performed using the SigmaStat 2.0 Program (Jandel Cooperation, San Rafael, CA). The Student-Newman-Keuls test after one-way repeated measures analysis of variance was used for statistical significance. Differences were considered significant if p < 0.05.
RESULTS
IGFBP-3 and GnRH-A Induce Apoptosis of Male Germ Cells
We have shown previously that IGFBP-3 treatment resulted in a 4-fold increase of germ cell apoptosis at stages XIV-I and a more modest increase of apoptosis occurred at stages VII-VIII (26). Consistent with our previous observations, GnRH-A induced marked increased in apoptosis exclusively at stages VII-VIII. Addition of IGFBP-3 to GNRH-A treatment doubled the increased apoptosis at stages VII-VIII without affecting stages XIV-I (see “Opposing Roles of Insulin-like Growth Factor Binding Protein 3 and Humanin in the Regulation of Testicular Germ Cell Apoptosis” in Ref. 26).
BAX-associated IGFBP-3 Increases after GnRH-A and/or IGFBP-3 Treatment in Total and Mitochondrial Fractions of Testicular Extracts
Western Blot Analyses of IGFBP-3 in Total, Cytosol, and Mitochondria of Testis Homogenates
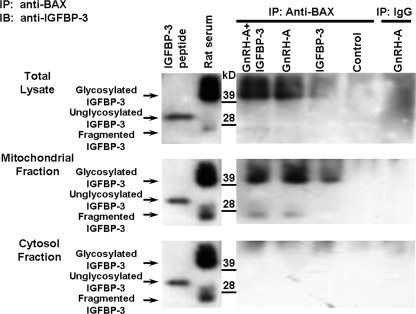
To determine the role of IGFBP-3 and its relationship with BAX in male germ cell apoptosis, we first examined the IGFBP-3/BAX interaction in testicular homogenates and in cytosol or mitochondria of rats treated with IGFBP-3 alone or in combination with GnRH-A. IGFBP-3 protein expression was not detected by immunoblotting in total, cytosol, or mitochondrial fractions without BAX co-immunoprecipitation (supplemental Fig. 1). The testicular homogenates (total, mitochondria, and cytosol fractions) were then precipitated by anti-BAX antibody (supplemental Fig. 2). After co-immunoprecipitation with BAX antibody, IGFBP-3 (endogenous glycosylated protein; molecular mass ∼40–42 kDa) was detected in total (Fig. 1, top panel) and mitochondria (Fig. 1, middle panel) fractions and not in cytosol (Fig. 1, bottom panel) in groups treated with IGFBP-3, GnRH-A, and the combination, but not in the untreated rats (control). IGFBP-3 fragments (molecular mass 20–24 kDa) were found in the mitochondrial fractures after treatment with GnRH-A and GnRH-A plus IGFBP-3 groups. To show that these changes were not related to intratesticular injections, we demonstrated that the same changes in IGFBP-3 were present in GnRH-A-treated animals without intratesticular injections (supplemental Fig. 3).
FIGURE 1.
Endogenous IGFBP-3 interacts with BAX in the rat testis. Western blots for IGFBP-3 after IP of the testis homogenates with anti-BAX or IgG (negative control for the IP step) are shown. Rats were treated with vehicle (Control), GnRH-A, IGFBP-3 peptide, or GnRH-A plus IGFBP-3 peptide, as described under “Experimental Procedures.” Anti-BAX antibody was used to immunoprecipitate IGFBP-3. Synthetic IGFBP-3 peptide was used as positive control for unglycosylated IGFBP-3 (molecular mass ∼30 kDa), and rat serum was shown as positive control for glycosylated IGFBP-3 (molecular mass ∼40–42 kDa) (right panels). IGFBP-3 fragments (molecular mass ∼ 20–24 kDa) were detected only in serum and mitochondrial fractions after GNRH-A treatments. Endogenous IGFBP-3 expression was not detected after IP with rabbit IgG in GnRH-A-treated testis tissue (IP-IgG, right lane, negative control for IP). After co-immunoprecipitation with anti-BAX antibody, endogenous IGFBP-3 (glycosylated IGFBP-3, molecular mass ∼40–42 kDa) was detectable by Western blotting with anti-IGFBP-3 after in vivo treatment with GnRH-A with or without IGFBP-3 in testis total lysate (top panel) or mitochondrial fraction (middle panel), but not detected in the control group. Endogenous IGFBP-3 was not detectable in testis cytosol fraction (bottom panel) in control and all treated animal groups.
IGFBP-3 Measurement by ELISA in Mitochondrial Fractions
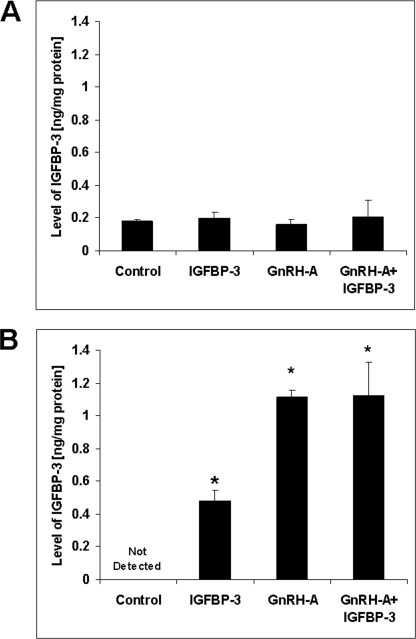
IGFBP-3 and BAX interaction was also quantitatively demonstrated by ELISA. Substantiating the results of the Western blot analyses, IGFBP-3 protein was very low (close to the detection limit of the assay) in samples before immunoprecipitation with BAX (Fig. 2A). Fig. 2B shows the amount of IGFBP-3 that was co-immunoprecipitated with BAX. Importantly, there is no detectable IGFBP-3 in the basal control state, but we found significantly higher levels of mitochondrial BAX-associated IGFBP-3 in the IGFBP-3, GnRH-A, and GnRH-A + IGFBP-3 treatment groups (p < 0.05 in all groups) compared with saline-treated rats (no detectable level), demonstrating increased BAX-associated IGFBP-3 in mitochondria with these treatments. These increased levels of IGFBP-3 were coincident with increase in germ cell apoptosis (26).
FIGURE 2.
IGFBP-3 was detected in testicular mitochondrial fraction by ELISA only after anti-BAX IP. A, rats were treated with vehicle (Control), GnRH-A, IGFBP-3, or GnRH-A plus IGFBP-3 peptide as described under “Experimental Procedures.” IGFBP-3 levels measured by ELISA were very low and not different in testicular mitochondrial fractions without anti-BAX co-immunoprecipitation after the various treatments. B, quantitative measurements of IGFBP-3 in testicular mitochondrial fractions after anti-BAX co-immunoprecipitation showed no detectable IGFBP-3 in the basal control state and significant increases in IGFBP-3 after treatment with GnRH-A, IGFBP-3, or combination of both peptides compared with control group. Values are mean ± S.D. (error bars) (n = 4 testis homogenates). *, p < 0.05.
Direct Interaction between IGFBP-3 and BAX
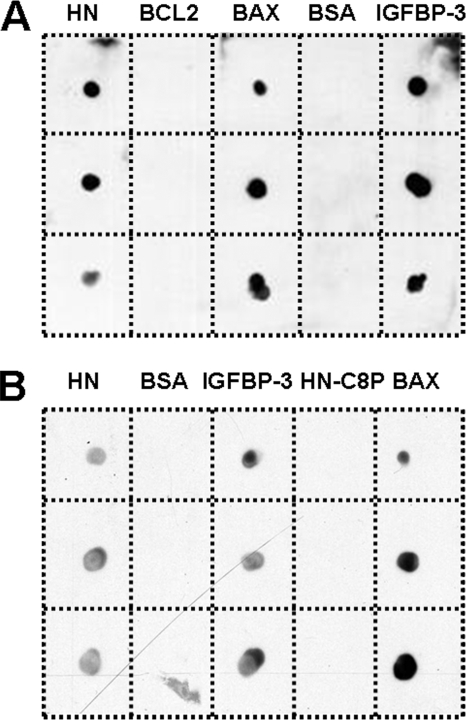
To validate further our new finding that IGFBP-3 interacts directly with BAX in testicular mitochondria, we performed dot blot experiments where increasing concentrations of HN (a prosurvival factor and known binding partner of IGFBP-3 and BAX) (23, 24), BCL-2 (prosurvival factor structurally related to BAX), BAX, BSA, and IGFBP-3 were spotted on a membrane and then incubated sequentially with IGFBP-3, goat anti- IGFBP-3 antibody, anti-goat secondary antibody, and ECL Plus reporting reagent. Fig. 3A clearly demonstrates that the system can detect IGFBP-3 and that IGFBP-3 interacts with HN (positive control), itself, and BAX but not with BCL-2 and BSA (negative controls). In Fig. 3B, HN, BSA, IGFBP-3, HN-C8P (a non-BAX-binding mutant of HN) (24) and BAX were spotted on the membrane and then incubated with BAX, rabbit anti-BAX antibody, anti-rabbit secondary antibody, and ECL Plus reporting reagent. This complementary experiment using anti-BAX confirmed binding of BAX to IGFBP-3, and HN, whereas HN mutant (HN-C8P) showed absence of interaction (Fig. 3B).
FIGURE 3.
BAX and IGFBP-3 interaction in dot blot experiments. A, the peptides used for dot blots against anti-IGFBP-3 included HN (a known binding partner of IGFBP-3 used as positive control, 2, 5, and 10 nmol), BCL-2 (fragment, 1, 2, and 5 μg), BAX (mouse full long sequence, 1, 2, and 5 μg), and BSA (used as negative control, 1, 2, and 5 μg), IGFBP-3 (used here as positive control for anti-IGFBP-3 antibody, 1, 2, and 5 μg). B, the reverse experiment protein dots versus with anti-BAX were: HN (a known binding partner of BAX used here as positive control, 2, 5, and 10 nmol), BSA (used here as negative control, 1, 2, and 5 μg); IGFBP-3 (1, 2, and 5 μg); HN mutant (HN-C8P, a non-BAX-binding mutant; 2, 5, and 10 nmol), and BAX (used here as positive control for anti-BAX antibody, 1, 2, and 5 μg). Both dot blots confirmed the interaction between BAX and IGFBP-3.
Additive Effects of IGFBP-3 and BAX on Cytochrome c and DIABLO Release from Mitochondria
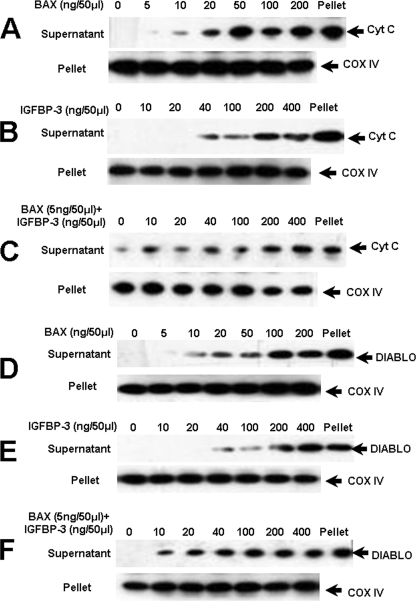
We examined the release of cytochrome c (which in turn induces a series of biochemical reactions that result in caspase activation and subsequent cell death) and DIABLO (a proapoptotic protein released from the mitochondria to block the action of inhibitor of apoptosis proteins) (8–10) from testicular mitochondria fractions by increasing concentrations of IGFBP-3 and BAX, or a combination of a very low, noneffective dose of BAX (5 ng/50 μl) with increasing concentrations of IGFBP-3. As shown in Fig. 4, both BAX at 10 ng/50 μl and IGFBP-3 at 40 ng/50 μl alone could induce release of cytochrome c (panels A and B) and DIABLO (panels D and E). IGFBP-3 at a 4-fold lower dose level (10 ng/μl), when combined with an ineffective dose of BAX (5 ng/50 μl), was able to trigger cytochrome c (panel C) and DIABLO (panel F) release from the mitochondria, demonstrating that IGFBP-3 and BAX cooperate in mitochondrial outer membrane permeabilization. As shown in supplemental Fig. 4A, we incubated BAX and IGFBP-3 for 15 min before the addition of testicular mitochondrial fraction lysates. Western blot analyses under nonreducing conditions showed that BAX and IGFBP-3 peptides bind to each other at higher concentration. This is consistent with our previous dot blot experiment that BAX and IGFBP-3 bind to each other.
FIGURE 4.
Mitochondrial release of cytochrome c and DIABLO induced by IGFBP-3 and BAX. Increasing concentrations of BAX were incubated with adult rat testis mitochondria (50 μg in 50-μl final volume) for 45 min at 30 °C, samples were centrifuged to generate pellets and supernatants that were analyzed by SDS-PAGE/immunoblotting using anti-cytochrome c (Cyt C, panel C) and anti-DIABLO (panel D). Increasing concentrations of IGFBP-3 were incubated with adult rat testis mitochondria. Samples were processed as above (anti-cytochrome c, panel B; and anti-DIABLO, panel E). An ineffective dose of BAX (5 ng/50 μl) was preincubated with increasing concentrations of IGFBP-3 peptide for 15 min at 30 °C in HM buffer before adding 50 μg of rat testis mitochondria. Samples were processed as above (panel C), using antibody against cytochrome c and against DIABLO (panel F). Compared with BAX (panels A and D) or IGFBP-3 (panels B and E) alone, the combination of BAX (5 ng/50 μl, an ineffective dose) with IGFBP-3 (panels C and F) induces release of cytochrome c and DIABLO from testis mitochondrial fractions at a lower concentration suggesting an additive action of the two proteins. Cytochrome c oxidase IV (COX IV) is utilized as a sample loading control.
DISCUSSION
In earlier studies, we have demonstrated that the mitochondria-dependent (intrinsic) pathway is a key signal transduction pathway for male germ cell apoptosis across species (3, 4, 6, 11, 22, 26). In the present study, using both in vitro and in vivo model systems, we provide evidence that the intrinsic pathway in germ cells can be activated by IGFBP-3, via BAX binding at the mitochondria.
IGFBP-3 induces apoptosis in various cancer cells (13–16, 27, 28). In addition to acting as a serum carrier of IGFs and antagonizing IGF action, it has also been demonstrated that IGFBP-3 acts as a direct cell death inducer (29) mediated by the inactivation of antiapoptotic protein, BCL-2, through serine phosphorylation. More recently, it has been shown that IGFBP-3 induces apoptosis by interacting with RXRα (15) and its binding partner Nur77 and promoting translocation to the mitochondria to initiate the apoptotic cascade (17, 18) in prostate cancer cells. Another group demonstrated a proapoptotic role for RXRα/Nur77 heterodimers at the mitochondria, specifically an interaction of Nur77 with BCL-2 converting it from a “protector” to a “killer” (30, 31).
Consistent with an important role of IGFBP-3 in cell death, here we show that IGFBP-3 also plays an important role in testicular germ cell apoptosis either as a single effector or additionally when apoptosis is triggered by hormone deprivation. Supporting evidence for this hypothesis stems from the findings of our recent study involving IGFBP-3 knock-out mice (26). In that study, we examined whether genetic deletion of IGFBP-3 would confer resistance to hormone derivation-induced apoptosis. To induce apoptosis, mice were given a single subcutaneous injection of GnRH-A (acyline, 20 mg/kg of body weight), and the animals were killed 2 weeks after treatment (32). Compared with wild type, a significant (p < 0.001) reduction (76.2–79.7%) in the incidence of germ cell apoptosis was noted in IGFBP-3 knock-out mice (26). This suggests that IGFBP-3 plays a role in germ cell apoptosis.
The BCL-2 family of proteins governs the mitochondria-dependent pathway for apoptosis (33, 34). One of the intriguing aspects of apoptosis regulation by members of this family is their subcellular localization and translocation. Some BCL-2 family members such as BCL-2 constitutively localize to the mitochondrial membrane whereas others such as BAX translocate from the cytosol to mitochondria early during apoptosis. Furthermore, insertion of BAX into mitochondrial membranes has been shown to play an essential role in releasing cytochrome c from the mitochondrial membrane space to the cytosol in various cell systems (35–37). Data reported in this study show that without BAX immunoprecipitation, IGFBP-3 was not detected or was present in very low concentrations in the testis probably because of a lack of adequate sensitivity of the Western blots and/or ELISA. After immunoprecipitation with BAX, the IGFBP-3 bound to BAX was located mainly in the mitochondria and not in the cytosol of the testis homogenates. Moreover, the significant increase in IGFBP-3 concentrations that was associated with BAX only occurs in apoptotic conditions and only in the mitochondria, as shown by both Western blots and ELISA. BAX-bound IGFBP-3 can be up-regulated by changes in intratesticular testosterone levels and administration of IGFBP-3. Furthermore, we have shown that IGFBP-3 can release cytochrome c and DIABLO from the mitochondria, thereby initiating the apoptosis cascade. IGFBP-3 at 4-fold lower dose levels, when combined with very low concentrations of BAX, was able to trigger cytochrome c and DIABLO release from the mitochondria. Consequently, we speculate that one mechanism by which IGFBP-3 can induce apoptosis is through binding to BAX and translocation of this complex to mitochondria, triggering the intrinsic pathway signaling. We cannot, however, exclude the possibility that IGFBP-3 induces apoptosis through interactions with nuclear proteins such as RXRα and its binding partner Nur77, which rapidly translocate to the mitochondria to initiate the cytochrome c-mediated death pathway (18, 38, 39). Direct BAX binding of IGFBP-3 as well as RXRα/Nur77 translocation with subsequent BCL-2 conversion as described previously may be parallel pathways that serve to amplify the apoptotic signal in the germ cell.
In this study we have also validated a previous observation that BAX binds the neurosurvival factor, HN (24, 25). We have also published that IGFBP-3 binds HN and that they modify each other's actions on apoptosis and survival (23). The exact mechanism of action is still being unraveled and includes both the activation of cell surface receptors as well as intracellular signaling. It is intriguing that these two HN partners/antagonists are now demonstrated to bind each other and that this interaction occurs in the mitochondria, as HN could be transcribed from the 16 S rRNA gene of the mitochondria DNA. Future studies will be needed to determine exactly how these three important cell fate regulators interface in the testis and other cell systems.
In summary, we have demonstrated a new signal transduction pathway for apoptosis in male germ cells involving the physical interaction between IGFBP-3 and BAX at the mitochondria. This interaction between two proapoptotic proteins may be important in the regulation of germ cell homeostasis and may be modulated for the regulation of male fertility and testicular diseases such as germ cell tumors.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- GnRH-A
- gonadotropin-releasing hormone antagonist
- ELISA
- enzyme-linked immunosorbent assay
- HN
- Humanin
- IGF
- insulin-like growth factor
- IGFBP-3
- insulin-like growth factor-binding protein-3
- IP
- immunoprecipitation
- MES
- 2-(N-morpholino)ethanesulfonic acid
- MOPS
- 4-morpholinepropanesulfonic acid
- RXRα
- retinoid X receptor α.
REFERENCES
- 1.Sinha Hikim A. P., Wang C., Leung A., Swerdloff R. S. (1995) Endocrinology 136, 2770–2775 [DOI] [PubMed] [Google Scholar]
- 2.Sinha Hikim A. P., Swerdloff R. S. (1993) Endocrinology 133, 2161–2170 [DOI] [PubMed] [Google Scholar]
- 3.Sinha Hikim A. P., Lue Y., Diaz-Romero M., Yen P. H., Wang C., Swerdloff R. S. (2003) J. Steroid Biochem. Mol. Biol. 85, 175–182 [DOI] [PubMed] [Google Scholar]
- 4.Vera Y., Erkkilä K., Wang C., Nunez C., Kyttänen S., Lue Y., Dunkel L., Swerdloff R. S., Sinha Hikim A. P. (2006) Mol. Endocrinol. 20, 1597–1609 [DOI] [PubMed] [Google Scholar]
- 5.Lue Y., Wang C., Liu Y. X., Hikim A. P., Zhang X. S., Ng C. M., Hu Z. Y., Li Y. C., Leung A., Swerdloff R. S. (2006) J. Clin. Endocrinol. Metab. 91, 539–545 [DOI] [PubMed] [Google Scholar]
- 6.Jia Y., Sinha Hikim A. P., Lue Y. H., Swerdloff R. S., Vera Y., Zhang X. S., Hu Z. Y., Li Y. C., Liu Y. X., Wang C. (2007) Biol. Reprod. 77, 83–92 [DOI] [PubMed] [Google Scholar]
- 7.Wang C., Cui Y. G., Wang X. H., Jia Y., Sinha Hikim A. P., Lue Y. H., Tong J. S., Qian L. X., Sha J. H., Zhou Z. M., Hull L., Leung A., Swerdloff R. S. (2007) J. Clin. Endocrinol. Metab. 92, 3292–3304 [DOI] [PubMed] [Google Scholar]
- 8.Green D. R. (2000) Cell 102, 1–4 [DOI] [PubMed] [Google Scholar]
- 9.Hengartner M. O. (2000) Nature 407, 770–776 [DOI] [PubMed] [Google Scholar]
- 10.Reed J. C. (2000) Am. J. Pathol. 157, 1415–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vera Y., Diaz-Romero M., Rodriguez S., Lue Y., Wang C., Swerdloff R. S., Sinha Hikim A. P. (2004) Biol. Reprod. 70, 1534–1540 [DOI] [PubMed] [Google Scholar]
- 12.Yamada P. M., Lee K. W. (2009) Am. J. Physiol. Cell Physiol. 296, C954–C976 [DOI] [PubMed] [Google Scholar]
- 13.Lee K. W., Cohen P. (2002) J. Endocrinol. 175, 33–40 [DOI] [PubMed] [Google Scholar]
- 14.Ali O., Cohen P., Lee K. W. (2003) Horm. Metab. Res. 35, 726–733 [DOI] [PubMed] [Google Scholar]
- 15.Liu B., Lee K. W., Li H., Ma L., Lin G. L., Chandraratna R. A., Cohen P. (2005) Clin. Cancer Res. 11, 4851–4856 [DOI] [PubMed] [Google Scholar]
- 16.Cohen P. (2006) Endocrinology 147, 2109–2111 [DOI] [PubMed] [Google Scholar]
- 17.Lee K. W., Liu B., Ma L., Li H., Bang P., Koeffler H. P., Cohen P. (2004) J. Biol. Chem. 279, 469–476 [DOI] [PubMed] [Google Scholar]
- 18.Lee K. W., Ma L., Yan X., Liu B., Zhang X. K., Cohen P. (2005) J. Biol. Chem. 280, 16942–16948 [DOI] [PubMed] [Google Scholar]
- 19.Lee K. W., Cobb L. J., Paharkova-Vatchkova V., Liu B., Milbrandt J., Cohen P. (2007) Carcinogenesis 28, 1653–1658 [DOI] [PubMed] [Google Scholar]
- 20.Lue Y., Wang C., Cui Y., Wang X., Sha J., Zhou Z., Xu J., Wang C., Sinha Hikim A. P., Swerdloff R. S. (2009) Biol. Reprod. 80, 484–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha Hikim A. P., Lue Y., Yamamoto C. M., Vera Y., Rodriguez S., Yen P. H., Soeng K., Wang C., Swerdloff R. S. (2003) Endocrinology 144, 3167–3175 [DOI] [PubMed] [Google Scholar]
- 22.Jia Y., Castellanos J., Wang C., Sinha-Hikim I., Lue Y., Swerdloff R. S., Sinha-Hikim A. P. (2009) Biol. Reprod. 80, 771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikonen M., Liu B., Hashimoto Y., Ma L., Lee K. W., Niikura T., Nishimoto I., Cohen P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13042–13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai D., Luciano F., Zhu X., Guo B., Satterthwait A. C., Reed J. C. (2005) J. Biol. Chem. 280, 15815–15824 [DOI] [PubMed] [Google Scholar]
- 25.Luciano F., Zhai D., Zhu X., Bailly-Maitre B., Ricci J. E., Satterthwait A. C., Reed J. C. (2005) J. Biol. Chem. 280, 15825–15835 [DOI] [PubMed] [Google Scholar]
- 26.Lue Y., Swerdloff R. S., Liu Q., Mehta H., Sinha Hikim A. P., Lee K. W., Jia Y., Hwang D., Cobb L. J., Cohen P., Wang C. (2010) Endocrinology, in press [DOI] [PubMed] [Google Scholar]
- 27.Johnson C., Jia Y., Wang C., Lue Y. H., Swerdloff R. S., Zhang X. S., Hu Z. Y., Li Y. C., Liu Y. X., Sinha Hikim A. P. (2008) Biol. Reprod. 79, 806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin S. L., Ferry R. J., Jr., Cohen P. (2003) J. Clin. Endocrinol. Metab. 88, 900–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prieur A., Tirode F., Cohen P., Delattre O. (2004) Mol. Cell. Biol. 24, 7275–7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajah R., Valentinis B., Cohen P. (1997) J. Biol. Chem. 272, 12181–12188 [DOI] [PubMed] [Google Scholar]
- 31.Lin B., Kolluri S. K., Lin F., Liu W., Han Y. H., Cao X., Dawson M. I., Reed J. C., Zhang X. K. (2004) Cell 116, 527–540 [DOI] [PubMed] [Google Scholar]
- 32.Kolluri S. K., Zhu X., Zhou X., Lin B., Chen Y., Sun K., Tian X., Town J., Cao X., Lin F., Zhai D., Kitada S., Luciano F., O'Donnell E., Cao Y., He F., Lin J., Reed J. C., Satterthwait A. C., Zhang X. K. (2008) Cancer Cell 14, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha Hikim A. P., Rajavashisth T. B., Sinha H. I., Lue Y., Bonavera J. J., Leung A., Wang C., Swerdloff R. S. (1997) Biol. Reprod. 57, 1193–1201 [DOI] [PubMed] [Google Scholar]
- 34.Cory S., Adams J. M. (2002) Nat. Rev. Cancer 2, 647–656 [DOI] [PubMed] [Google Scholar]
- 35.Danial N. N., Korsmeyer S. J. (2004) Cell 116, 205–219 [DOI] [PubMed] [Google Scholar]
- 36.Eskes R., Antonsson B., Osen-Sand A., Montessuit S., Richter C., Sadoul R., Mazzei G., Nichols A., Martinou J. C. (1998) J. Cell Biol. 143, 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu S., Narita M., Tsujimoto Y. (1999) Nature 399, 483–487 [DOI] [PubMed] [Google Scholar]
- 38.Antonsson B., Montessuit S., Lauper S., Eskes R., Martinou J. C. (2000) Biochem. J. 345, 271–278 [PMC free article] [PubMed] [Google Scholar]
- 39.Liu B., Lee H. Y., Weinzimer S. A., Powell D. R., Clifford J. L., Kurie J. M., Cohen P. (2000) J. Biol. Chem. 275, 33607–33613 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.