Abstract
In semen, proteolytic peptide fragments from prostatic acid phosphatase can form amyloid fibrils termed SEVI (semen-derived enhancer of viral infection). These fibrils greatly enhance human immunodeficiency virus (HIV) infectivity by increasing the attachment of virions to target cells. Therefore, SEVI may have a significant impact on whether HIV is successfully transmitted during sexual contact. Here, we demonstrate that surfen, a small molecule heparan sulfate proteoglycan antagonist, inhibits both SEVI- and semen-mediated enhancement of HIV type 1 infection. Surfen interferes with the binding of SEVI to both target cells and HIV type 1 virions but does not deaggregate SEVI fibrils. Because SEVI can increase HIV infectivity by several orders of magnitude, supplementing current HIV microbicide candidates with SEVI inhibitors, such as surfen, might greatly increase their potency.
Introduction
HIV2 is primarily a sexually transmitted disease. Worldwide, estimates suggest that the majority of all HIV infections are acquired through sexual contact. This includes sexual transmission from male to female, from male to male, and from female to male. In all these routes of infection, semen is either the vehicle carrying HIV (in the case of male-to-female and male-to-male transmission) or is often present during the infection process (in the case of female-to-male transmission).
Recently, semen has been reported to enhance HIV infection (1). Fractionation of semen from healthy donors led to the identification of a factor that can enhance HIV infection up to 105-fold in cell culture when viral inocula are limiting. This factor, termed SEVI (semen-derived enhancer of viral infection), corresponds to amyloid fibrils composed of internal 34–40 amino acid proteolytic fragments from prostatic acid phosphatase (PAP), a protein present at a concentration of ∼1–2 mg/ml in semen (1, 2). The predominant peptide fragment, PAP-(248–286), has eight basic residues, rendering it very cationic (isoelectric point = 10.21). The positively charged SEVI fibrils bind to both target cells and HIV virions and augment infection by increasing physical contact between these two components (1, 3), much in the same way that synthetic cationic polymers promote retrovirus attachment to target cells (4).
Although we demonstrated that the cationic nature of SEVI is important for its pro-attachment effects (3), the surface components of target cells that interact with SEVI remained unknown. We previously observed that anionic polymers, such as heparin sulfate, interfere with the binding of SEVI to target cells (3). This led us to hypothesize that the fibrils may bind target cells by interacting with cell-surface heparan sulfate proteoglycans (HSPG), naturally occurring anionic carbohydrate polymers that are closely related in structure to heparin sulfate.
We therefore sought to examine whether antagonists of HSPG might inhibit the viral enhancing activity of SEVI. Bis-2-methyl-4-amino-quinolyl-6-carbamide (surfen), a recently identified small molecule antagonist of HSPG, represented an intriguing candidate. First described in 1938 (5), surfen was reported to have anti-inflammatory and anti-bacterial activity (6, 7). Its anti-HSPG effects on cells include blocking the binding of FGF2 and the fibronectin Hep-II domain and inhibiting HSPG-dependent infection by HSV-1 (8).
We now report that surfen counteracts SEVI-mediated enhancement of HIV-1 fusion to and infection of host cells. Surfen not only antagonizes the interaction between SEVI- and HSPG-expressing target cells but also between SEVI and HIV-1 virions. Importantly, we observed that surfen also inhibits the ability of semen to enhance HIV-1 infection. Incorporation of surfen or surfen-like compounds, which target semen-derived viral enhancement factors, should be considered in the development of HIV microbicides, provided these compounds can be safely used.
EXPERIMENTAL PROCEDURES
Cell Culture
293T and TZM-bl cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, l-glutamine, penicillin (50 units/ml), and streptomycin (50 μg/ml). The CHO and pgs-A745 stable transfectants (a gift from P. Bieniasz, Aaron Diamond Center, New York) were cultured in F-12 medium supplemented with 10% fetal bovine serum, l-glutamine, penicillin (50 units/ml), and streptomycin (50 μg/ml). Primary CD4+ T cells were isolated from buffy coats by Ficoll-Paque density gradient centrifugation, followed by negative selection with CD14+ microbeads and positive selection with CD4+ microbeads (Miltenyi Biotec).
Use of SEVI and Semen
Synthetic PAP-(248–286) (ViroPharmaceuticals) and N-terminally FITC-labeled PAP-(248–286) (GenScript) were dissolved in phosphate-buffered saline at a concentration of 10 mg/ml and converted into SEVI amyloid fibrils by overnight agitation at 37 °C at 1,400 rpm using an Eppendorf Thermomixer. Pooled semen (obtained from the University of California, San Francisco, Fertility Clinic) was divided into aliquots and frozen at −20 °C.
Viral Production
β-Lactamase-Vpr (BlaM-Vpr) chimeric CCR5-tropic 81A viruses used in the fusion assays were produced by FuGENE-mediated transfection of 293T cells with DNA proviral expression plasmids as described (9). CCR5-tropic 81A viruses used in the TZM-bl infectivity assays were produced by transfecting 293T cells with a DNA proviral expression plasmid. Luciferase-expressing viruses used in the CHO/pgs-A745 infectivity assays were produced by transfecting 293T cells with DNA plasmid pNL4–3.Luc.R-E- pseudotyped with 92HT599.24 (a dual-tropic subtype B envelope). Two days after transfection, supernatants were clarified by sedimentation and titered for p24Gag content by anti-p24Gag enzyme-linked immunosorbent assay (ELISA) (PerkinElmer Life Sciences).
Flow Cytometry
Fluorescein isothiocyanate (FITC), phycoerythrin, or allophycocyanin-conjugated antibodies specific for CD3, CD4, and CCR5 were purchased from BD Biosciences. Anti-heparan sulfate antibody (10E4 epitope) was obtained from Weikagaku Biobusiness Corp. and was stained with a secondary rat anti-mouse IgM (BD Biosciences). Cells were analyzed on a BD FACSCalibur and analyzed with FlowJo software (Treestar).
Virion Fusion Assay
Virion fusion was performed similar to methods described (3, 9). For target cell pretreatment with SEVI, 5 × 105 CD14−CD4+ cells were pretreated with SEVI (31.25 μg/ml) for 1 h at 37 °C in the presence of the indicated concentration of surfen (obtained from the Open Chemical Repository in the Developmental Therapeutic Program at the NCI, National Institutes of Health (NSC12155) and stored in DMSO at a concentration of 30 mm). Where indicated, chloroquine (Sigma) was added as a structurally related but inactive surfen control. A SEVI concentration of 31.25 μg/ml was chosen because similar quantities of fibril-forming PAP fragments have been isolated from semen, suggesting that this concentration may be physiologically relevant (1, 3). After pretreatment, cells were washed twice with medium and added to β-lactamase-containing 81A virions (200 ng/ml p24Gag). Alternatively, for virion pretreatment with SEVI, 81A virions (200 ng/ml p24Gag) were pretreated with SEVI (31.25 μg/ml) for 1 h at 37 °C in the presence of the indicated concentration of surfen and then added to 5 × 105 CD14−CD4+ target cells. In all instances, viral fusion was allowed to proceed for 4 h at 37 °C, after which the cells were loaded overnight with CCF2, a fluorescent substrate of β-lactamase. Cells were then stained with surface antibodies, fixed with 2% paraformaldehyde, and analyzed on a BD LSRII flow cytometer. Flow cytometric data were analyzed with FlowJo software (Treestar).
Infectivity Assays
For infection of TZM-bl cells, CCR5-tropic HIV-1 (20 ng/ml p24Gag) was pretreated for 5 min with diluted SEVI or semen in the presence or absence of surfen. Where indicated, chloroquine was added as a structurally related but inactive surfen control. Pretreated virions (20 μl) were added to TZM-bl cells (104/well in 96-well flat bottom plates) in 280 μl of medium. To minimize toxic effects mediated by prolonged exposure of semen to target cells (1), the medium was replaced after 2 h. Infection was assayed 3 days later by quantitating β-galactosidase activity with a Gal-Screen kit (Applied Biosystems). Background signals obtained from uninfected cells were subtracted from values obtained from infected cells.
For infection of CHO and pgs-A745 cells, luciferase-expressing HIV-1 (10 ng/ml p24Gag) was pretreated for 5 min with the indicated concentration of SEVI. Surfen was added where appropriate. Pretreated virions (20 μl) were then added to CHO or pgs-A745 transfectants (4 × 103/well in 96-well flat bottom plates) in 280 μl of medium. Medium was replaced after 2 h, and infection was assayed 3 days later by measuring luciferase activity (Promega).
Cytotoxicity Assay
TZM-bl cells (104/well) were treated with semen in the presence or absence of surfen. A semen concentration of 3.3% was used, as this corresponds to the 50% semen pretreatment concentration that was used in the infectivity assays (because upon infection of target cells, the 50% semen solution was diluted 15-fold to give a final concentration of 50/15 = 3.3%). Medium was replaced after 2 h, and viability was measured 3 days later using the CellTiter-Glo luminescent cell viability assay (Promega). As a positive control for cytotoxicity, cells were treated with the same concentration of semen for 3 days without medium replacement and then assayed for viability.
Fluorescence Microscopy
FITC-labeled SEVI (20 μg/ml) was pretreated with the indicated concentration of surfen or chloroquine for 30 min and then incubated for 1 h at 37 °C with 2.5 × 104 TZM-bl cells seeded in an 8-well LabTek chamber (Nunc). Cells were washed three times prior to imaging with a laser scanning confocal microscope (Leica CS SP5) using an excitation wavelength of 488 nm and a ×20 air objective. These studies were performed at 37 °C in a 5% CO2 atmosphere. Image analysis was performed using Volocity (Improvision) or Zeiss image processing software.
Virus Pulldown Assay
81A virions (100 ng/ml p24Gag) were incubated with SEVI (50 μg/ml) in the presence of the indicated concentrations of surfen for 3 h at 37 °C. The fibrils were then collected by centrifugation at 13,000 rpm for 5 min, and HIV-1 content in the supernatant and pellet was assayed by anti-p24Gag ELISA (PerkinElmer Life Sciences). To assess a potential direct interaction between SEVI and surfen, SEVI (50 μg/ml) was pretreated with the indicated concentrations of surfen for 1 h at 37 °C. The samples were subsequently centrifuged and washed twice. SEVI fibrils were then pelleted and resuspended with HIV-1 virions (100 ng/ml p24Gag) and incubated for 3 h at 37 °C. Finally, samples were centrifuged, and HIV-1 levels in the supernatant and pellet were determined by anti-p24Gag ELISA as described above. Results are reported as the fraction of p24Gag pelleted by the fibrils relative to input (defined as the cumulative amount of p24Gag in the supernatant and pellet).
Thioflavin T Interaction Assays
SEVI (5 mg/ml) was agitated with the indicated concentration of surfen at 1,400 rpm for 1 h at 37 °C. The samples were then diluted to 250 μg/ml SEVI, and thioflavin T (Sigma) was added at final concentration of 5 μm. Changes in fluorescence (excitation 440 nm, emission 482 nm) resulting from SEVI amyloid fibrils binding to thioflavin T were assayed in triplicate using a PerkinElmer Life Sciences Ls-5B luminescence spectrometer.
Electron Microscopy
SEVI (0.5 mg/ml) was incubated with 10, 50, or 100 μm surfen, or the equivalent amount of DMSO, for 3 h at 37 °C. Fibrils were then collected by centrifugation, washed once with phosphate-buffered saline, and resuspended in 50 μl of phosphate-buffered saline for electron microscopy. Samples were prepared for imaging as described (3).
RESULTS
Surfen Inhibits SEVI-mediated Enhancement of HIV-1 Entry into Target Cells
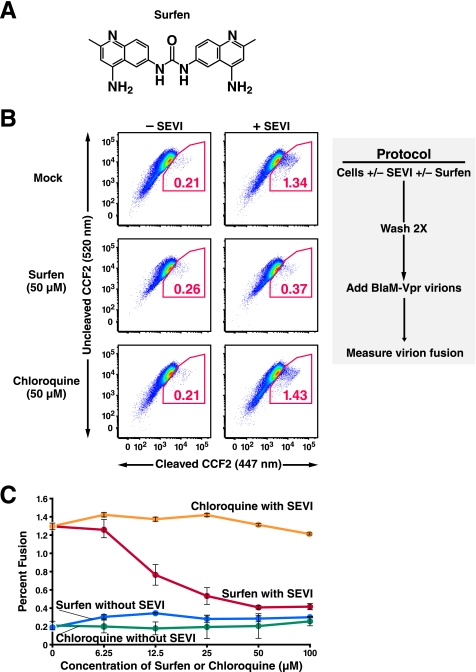
We have previously demonstrated that pretreatment of target cells with SEVI enhances their fusion to HIV-1 virions (3). This enhancement may be mediated by the interaction of the anionic surface of HSPG on the cells with the cationic surface of SEVI, as treatment of SEVI with anionic polymers such as heparin sulfate abrogates the SEVI enhancement (3). Because surfen (Fig. 1A) was recently reported to be an antagonist of HSPG (8), we tested whether it would interfere with the ability of SEVI to enhance HIV-1 virion fusion to primary CD4+ T cells using a fusion assay developed by our laboratory (9). CD4+ T cells were pretreated with SEVI in the absence or presence of surfen and then incubated with β-lactamase-Vpr (BlaM-Vpr)-expressing CCR5-tropic 81A virions. We utilized CCR5-tropic virions in our studies because sexual transmission of HIV-1 occurs predominantly with the CCR5-tropic HIV-1 variants. Fusion was measured by the spectral shift of cleaved CCF2, a fluorescent substrate of β-lactamase.
FIGURE 1.
Surfen blocks SEVI-mediated enhancement of HIV-1 fusion to primary CD4+ T cells. A, structure of surfen. B, effect of surfen and chloroquine on HIV-1 fusion to CD14−CD4+ T cells. Target cells were treated with 31.25 μg/ml SEVI in the presence of DMSO or 50 μm surfen or chloroquine as indicated. Cells were then washed twice before infection with BlaM-Vpr-containing 81A virions (200 ng/ml p24Gag), and fusion was assessed 4 h later. Values within the plots reflect the percentages of cells that fused with virions. Results are gated on CD3+CD4+ cells. These data are representative of three independent experiments performed with cells from three different donors. C, dose-response curves of surfen and chloroquine on SEVI-mediated enhancement of HIV-1 fusion to CD14−CD4+ T cells. CD14−CD4+ cells were treated with 31.25 μg/ml SEVI and the indicated concentrations of surfen or chloroquine. Virion fusion was assessed as described in B of this figure. Error bars reflect standard deviations for triplicate determinations, and the data are representative of three independent experiments. Representative primary flow cytometric data for this graph are presented in supplemental Fig. 1.
Consistent with previous results (3), we found that in the absence of surfen, addition of SEVI enhanced fusion of HIV-1 to CD4+ cells by more than 6-fold. However, if the cells were pretreated with 50 μm surfen prior to the addition of SEVI, only a 1.4-fold increase in fusion was observed (Fig. 1B). These effects of surfen appeared specific because pretreatment of cells with the structurally related chloroquine, another aminoquinoline, did not inhibit SEVI-mediated enhancement of HIV-1 fusion.
Surfen induced a dose-dependent inhibition of SEVI action at concentrations greater than 6.25 μm with a maximal inhibitory plateau occurring at 50–100 μm (Fig. 1C and supplemental Fig. 1). Of note, when surfen was added to cells in the absence of SEVI, HIV-1 fusion was not inhibited (Fig. 1, B and C, and supplemental Fig. 1), suggesting that surfen was acting directly on SEVI and not compromising the infectivity of the HIV-1 virions.
To further investigate the effect of surfen on the interaction between SEVI and the virions, we incubated 81A virions simultaneously with SEVI and increasing concentrations of surfen, and we then infected target cells with these treated virions. Similar to the results obtained when surfen was added during SEVI pretreatment of target cells, addition of surfen to SEVI during virion pretreatment also inhibited SEVI enhancement activity (supplemental Fig. 2). Higher concentrations of surfen were necessary for inhibition in the target cell pretreatment experiment, likely because the treated cells were washed, thereby removing unbound surfen before exposure to HIV-1 virions.
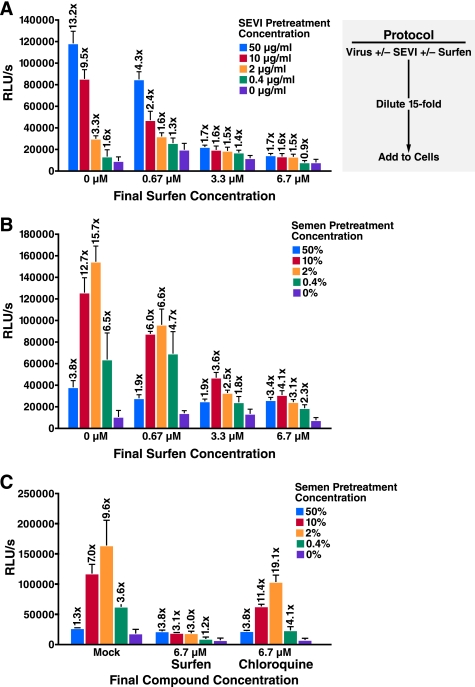
Surfen Inhibits SEVI- and Semen-mediated Enhancement of HIV-1 Infection
Next, we tested whether SEVI-mediated enhancement of productive HIV-1 infection is also inhibited by surfen. We added SEVI to HIV-1 virions in the presence or absence of surfen and then diluted the SEVI-treated viral particles 15-fold before adding them to TZM-bl cells, a reporter cell line that expresses β-galactosidase under a Tat-inducible promoter (10, 11). In agreement with previously published results (1, 3), addition of SEVI to HIV-1 81A virions enhanced viral infection in a dose-dependent manner; pretreating virions with 50 μg/ml SEVI, the highest concentration tested, increased infection 13.2-fold, whereas lower concentrations of SEVI induced lower levels of enhancement (Fig. 2A, columns on the far left). If the SEVI-exposed virions were treated with surfen at a final concentration of 3.3 or 6.7 μm, enhancement of HIV infection was almost completely abolished at all concentrations of SEVI that were tested (Fig. 2A). Intermediate effects were obtained when surfen was added at a final concentration of 0.67 μm. Surfen also inhibited the enhancement of infection obtained when target cells rather than virions were pretreated with SEVI (data not shown).
FIGURE 2.
Surfen inhibits SEVI- and semen-mediated enhancement of HIV-1 infection. 81A virions were pretreated for 5 min with the indicated concentration of SEVI (A) or semen (B) in the presence of 0, 10, 50, or 100 μm surfen. The samples were then diluted 15-fold (to final surfen concentrations of 0, 0.67, 3.3, or 6.7 μm as indicated) and added to TZM-bl cells. Medium was replaced after 2 h, and cells were assayed for Tat-inducible β-galactosidase activity 3 days later. The numbers above the bars indicate the n-fold infectivity enhancement relative to infection measured in the absence of SEVI or semen. C, comparing the effects of surfen and chloroquine on semen-mediated enhancement of HIV-1 infection. Samples were treated similar to B of this figure. RLU/s, relative light units/s. Shown are average values (± S.D.) of triplicate measurements from one of three independent experiments that yielded similar results.
To examine the effect of surfen in a more physiological context, we next tested whether surfen similarly blocks semen-mediated enhancement of viral infection. For these studies, we pretreated the HIV-1 virions with the indicated concentrations of semen with or without surfen. Target cells were then exposed to the pretreated virions for 2 h, after which the medium was replaced and infection was allowed to proceed for 3 days. In the absence of surfen, semen enhanced HIV-1 infection in a dose-dependent manner (Fig. 2B, columns on the far left). In agreement with published results, a sharp decrease in semen-mediated enhancement of HIV-1 infectivity was observed with 50% semen due to toxic effects on the cells (1). Similar to its effects on synthetic SEVI, surfen also inhibited semen-mediated enhancement of HIV-1 infection. Final surfen concentrations of 3.3 or 6.7 μm decreased maximal enhancement activity from almost 16- to ∼4-fold, whereas a final surfen concentration of 0.67 μm modestly inhibited semen enhancement (Fig. 2B). As a specificity control, we further demonstrated that in contrast to surfen, chloroquine did not efficiently block SEVI- or semen-mediated enhancement of HIV-1 infection (Fig. 2C and data not shown).
Prolonged in vitro exposure to high levels of semen results in cell death (1). To ensure that the observed inhibitory effects were not the result of high toxicity obtained when surfen is combined with semen, we examined the viability of TZM-bl cells in the presence of surfen and semen. As shown in supplemental Fig. 3, surfen did not decrease the viability of cells treated with semen under conditions used for the infectivity assays. In contrast, TZM-bl cells continuously incubated for 3 days with semen, a condition toxic to cells (1), displayed diminished cell viability.
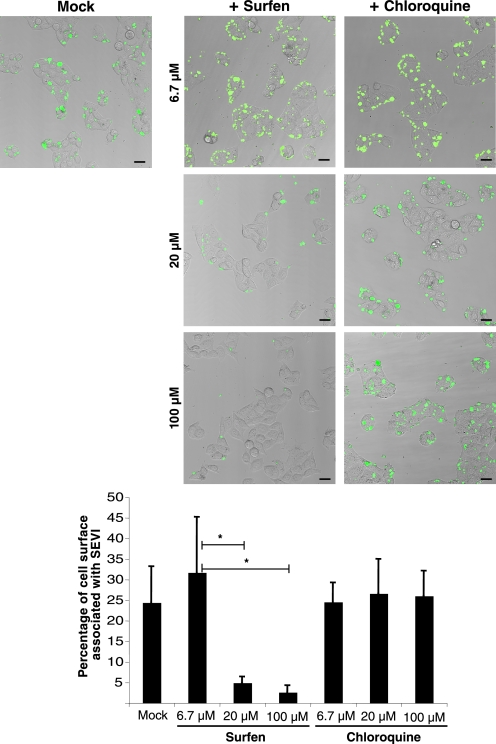
High Concentrations of Surfen Are Necessary to Inhibit SEVI Binding to Target Cells
To determine whether surfen, as a reported HSPG antagonist, exerts its effect on SEVI by inhibiting the interaction of SEVI with HSPG-expressing target cells, we utilized laser scanning confocal microscopy to visualize the interaction of FITC-labeled SEVI with TZM-bl cells. In the absence of surfen, SEVI interacted extensively with the cells (Fig. 3, left panel). Conversely, when SEVI was pretreated with 100 μm surfen and subsequently incubated with target cells, SEVI binding was almost completely abolished. In the presence of 20 μm surfen, binding was partially reduced, and in the presence of 6.7 μm surfen, binding was similar to the control sample lacking surfen. In contrast to surfen, chloroquine did not diminish SEVI binding to the cells (Fig. 3; lower magnification images are presented in supplemental Fig. 4). Interestingly, the concentration of surfen necessary to inhibit the interaction of SEVI with TZM-bl cells was higher than that necessary to inhibit SEVI-mediated enhancement of HIV-1 infection. For example, although surfen at a final concentration of 6.7 μm was sufficient to almost completely eliminate SEVI enhancement of HIV-1 infection in TZM-bl cells (Fig. 2A), surfen at a concentration of 6.7 μm did not diminish SEVI binding to the same target cells (Fig. 3 and supplemental Fig. 4). Therefore, concentrations of surfen that impaired SEVI enhancement of viral infectivity were not sufficient to block SEVI binding to target cells, suggesting that surfen may exert its inhibition through a mechanism other than interfering with SEVI binding to HSPG on target cells.
FIGURE 3.
Surfen inhibits the binding of SEVI to host cells. Top, FITC-labeled SEVI was either DMSO-treated (image on left) or treated with the indicated concentrations of surfen or chloroquine (images on right). The SEVI was then incubated with TZM-bl cells, washed, and imaged by laser scanning confocal microscopy. Shown are merged images of phase contrast (gray) and FITC-SEVI (green). Black bar, 30 μm. Bottom, quantification of the percentage of cell surface area associated with SEVI was carried out on 3–5 independent images for each condition. *, p < 0.05 (two-tailed t test). Lower magnification images of the same samples are presented in supplemental Fig. 4. Results are representative of one of five independent experiments.
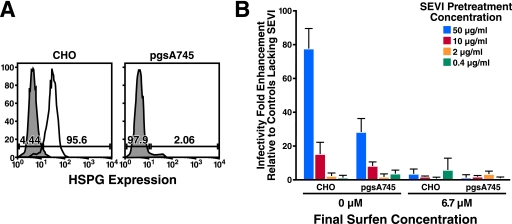
SEVI Enhancement of HIV-1 Infection of HSPG-deficient Cells Is Inhibited by Surfen
To examine whether surfen can antagonize SEVI activity independent of HSPG, we set out to determine whether SEVI can enhance HIV infection in a cell line completely devoid of cell surface HSPG, and if so whether surfen inhibits this enhancement. For these studies, we took advantage of the CHO cell line derivative pgs-A745, which does not express the enzyme xylosyltransferase and therefore lacks cell-surface HSPG (12). We obtained CHO and pgs-A745 cells stably transfected with human CD4 and CCR5, and a Y261C mutant of cyclin T1 that can support HIV Tat function (13). Comparable cell-surface expression of CD4 and CCR5 was detected in the CD4/CCR5-transfected CHO and pgs-A745 cells (data not shown). However, the CHO transfectants expressed cell-surface HSPG, but the pgs-A745 transfectants did not (Fig. 4A).
FIGURE 4.
Surfen blocks SEVI-mediated enhancement of HSPG-deficient pgs-A745 cells. A, expression of HSPG on CHO and pgs-A745 cells. CD4- and CCR5-expressing CHO and pgs-A745 cells were stained with an anti-heparan sulfate antibody and a secondary rat anti-mouse IgM. Unstained controls are shown as gray shaded histograms. B, luciferase-encoding HIV-1 virions were pretreated for 5 min with the indicated concentrations of SEVI in the absence or presence of 100 μm surfen, and then diluted 15-fold (to give a final surfen concentration of 6.7 μm) upon infection of the CHO or pgs-A745 transfectants. Medium was replaced after 2 h, and cells were assayed for luciferase activity 3 days later. Shown are average values (± S.D.) of triplicate measurements from one of three independent experiments that yielded similar results.
To examine whether SEVI enhances HIV infection in these cells, we incubated luciferase-encoding HIV-1 in the presence or absence of SEVI and infected the CHO and pgs-A745 cells. Infection was assayed 3 days later by measuring luciferase activity in cell lysates. We observed that SEVI enhanced HIV infection in both the CHO and pgs-A745 cells, although the enhancing effect was more pronounced in the CHO cells (Fig. 4B, left panels). The diminished SEVI enhancing effect in the pgs-A745 cells may be the result of the decreased ability of these mutant cells to bind SEVI (supplemental Fig. 5).
We then tested whether surfen inhibited SEVI enhancement activity in the CHO and pgs-A745 cells. When surfen was added to the SEVI-treated virions at a final concentration of 6.7 μm (a concentration that inhibited SEVI enhancement in the TZMbl cells (Fig. 2A)), SEVI enhancing activity was abolished in both the CHO and pgs-A745 cells (Fig. 4B, right panels). These results suggest that surfen can inhibit SEVI enhancement in a manner that is independent of cell-surface HSPG.
Surfen Inhibits the Binding of HIV-1 Virions to SEVI
Because surfen inhibits SEVI enhancement of HIV-1 infection in a cell line that lacks HSPG expression, the inhibitory effects of surfen are not due solely to antagonism of target cell HSPG. Since addition of surfen to SEVI during virion pretreatment inhibited SEVI enhancement activity (Fig. 2 and supplemental Fig. 2), we considered the possibility that the inhibitory effect of surfen was also mediated by interfering with the interaction between SEVI and HIV-1 virions. We tested whether surfen inhibits the binding of SEVI to HIV-1 using a binding assay that measures the association of SEVI fibrils with HIV-1 virions. In this assay, SEVI is incubated with HIV-1 virions and then collected by low speed centrifugation. HIV-1 virions associated with the pelleted SEVI are then quantified by p24Gag ELISA (3).
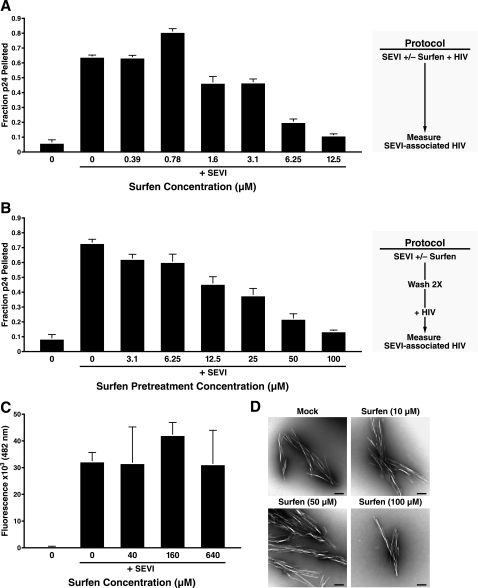
We observed, using this assay, that surfen inhibited the binding of HIV-1 to SEVI fibrils in a dose-dependent manner (Fig. 5A). Of note, the concentrations of surfen necessary to inhibit the interaction between SEVI and virions were lower than those necessary for inhibiting the interaction between SEVI and target cells. For example, although 12.5 μm surfen was sufficient to almost completely abolish the ability of SEVI to associate with HIV-1 virions (Fig. 5A), 20 μm surfen still allowed for significant interactions between SEVI and target cells (Fig. 3 and supplemental Fig. 4).
FIGURE 5.
Surfen inhibits the binding of SEVI to HIV-1 in the absence of de-aggregating the fibrils. A, 50 μg/ml SEVI was incubated with HIV-1 81A (100 ng/ml p24Gag) in the presence of the indicated concentration of surfen. The fibrils were then centrifuged after 3 h, and the absolute amounts of p24Gag in the pellet and supernatant were determined by ELISA. The 1st lane is a negative control in which both surfen and SEVI were omitted. Values are the mean ± S.D. from one of three experiments that yielded similar results. B, 50 μg/ml SEVI was treated with the indicated concentration of surfen and then washed twice. The pretreated SEVI was then incubated with HIV-1 81A (100 ng/ml p24Gag) for 3 h. After centrifugation, the absolute amounts of p24Gag in the pellet and supernatant were determined by ELISA. The 1st lane is a negative control in which both surfen and SEVI were omitted. Values are the mean ± S.D. from one of three experiments that yielded similar results. C, SEVI was incubated with the indicated concentration of surfen and mixed with 5 μm thioflavin T, and emission at 482 nm was recorded. The 1st lane is a negative control in which SEVI was omitted. Results are representative of data obtained from two independent experiments. Shown are average values (± S.D.) of triplicate measurements. D, electron micrograph of SEVI fibrils incubated with the indicated concentrations of surfen. Black bar, 200 nm.
We then tested whether surfen blocks the interaction between SEVI and HIV-1 by interacting directly with the SEVI fibrils. We pretreated SEVI fibrils with surfen to allow any interaction between the two components to occur. After washing the surfen-treated SEVI to remove any unbound surfen, we resuspended the SEVI in the presence of HIV-1 and measured the amount of fibril-associated HIV-1 after centrifugation. We found, using this two-step binding assay, that pretreating SEVI with surfen inhibited its ability to subsequently associate with HIV-1 (Fig. 5B). The concentration of surfen needed for inhibition was higher than that necessary when the HIV-1, surfen, and SEVI were all added simultaneously (Fig. 5A). This observation might be explained because, in the two-step binding assay, unbound surfen was washed out after the SEVI pretreatment, whereas in the single-step binding assay, surfen was present in the samples throughout the entire assay. A direct interaction between surfen and SEVI was further supported by the observation that SEVI fibrils could remove surfen from solution. In particular, when SEVI was added to surfen and then removed by centrifugation, a diminished level of surfen remained in the supernatant relative to a control sample in which surfen was mock-treated in the absence of SEVI (supplemental Fig. 6).
To exclude the possibility that surfen interfered with the binding of SEVI to HIV-1 by deaggregating the fibrils, we examined the effect of surfen on the fibrillar state of SEVI. Adding surfen to SEVI did not affect its interaction with thioflavin T, a dye that selectively binds amyloid fibrils (Fig. 5C). Electron microscopy confirmed the presence of fibrils in SEVI samples containing surfen (Fig. 5D). Collectively, the data suggest that surfen directly binds SEVI fibrils and prevents its subsequent association with HIV-1 virions.
DISCUSSION
In this study, we identify surfen as a small molecule inhibitor of SEVI enhancement activity. We examined surfen because of its reported role as an antagonist of HSPG (8) and our hypothesis that SEVI primarily interacts with cells by binding to cell-surface HSPG. We show here that surfen blocks the binding of SEVI to target cells. However, to our surprise, the inhibitory effect of surfen did not appear to be solely the result of antagonizing the interaction of SEVI with target cell HSPG because of the following: 1) concentrations of surfen necessary for inhibiting SEVI-mediated enhancement of HIV-1 infection were lower than those necessary for inhibiting SEVI binding to target cells, and 2) surfen blocked SEVI-mediated enhancement of HIV-1 infection in HSPG-deficient cells. Upon further investigation, we found that surfen interacts directly with SEVI and that surfen-laden SEVI was impaired in its ability to bind virions. We favor a model where surfen inhibits SEVI-mediated enhancement of HIV infection by blocking the interactions of SEVI with both target cells as well as the virions.
The ability of surfen to directly interact with SEVI was surprising in light of the fact that both are cationic molecules and therefore would be predicted to repel one another. It is possible that hydrophobic ring stacking between the fibrils and the aminoquinoline moieties of surfen mediates this interaction. It has also been suggested that surfen can form stacked structures (8), which may explain its ability to interact with the SEVI amyloid fibrils. Of note, not all aminoquinolines can inhibit SEVI-mediated enhancement of viral infection, as suggested by the lack of inhibitory activity of chloroquine.
Significantly, surfen blocked the ability of semen to enhance HIV-1 infection, raising the possibility that surfen or other related compounds might prove useful as components of a multifunctional microbicide. Current HIV-1 microbicide candidates only target the activity of the virions. In the presence of SEVI or semen, these microbicides have greatly reduced potency.3 It is logical to consider identifying compounds that not only target the virus but also host factors present in semen that enhance HIV infection. If proven safe, such a combination of microbicides might display markedly increased effectiveness.
We previously demonstrated that anionic polymers, such as heparin sulfate, can block SEVI binding to both HIV-1 and target cells (3). However, the basis of this inhibition appears to be purely electrostatic and therefore different from the inhibition observed with surfen. We found that anionic polymers that were highly sulfated (and therefore had a high effective concentration of negative charges) were most potent at blocking SEVI activity, presumably by forming strong electrostatic interactions. Unlike surfen, anionic polymers also appear to have direct inhibitory activity against HIV-1 virions (14–16). Because of this direct antiviral activity, anionic polymers have already been tested as HIV microbicides in clinical trials. Unfortunately, the outcomes of those trials were for the most part unfavorable. A large scale trial of the anionic polymer Carraguard suggested that this compound provided no protection against HIV acquisition when used as a vaginal microbicide (17). Worse yet, the results of a phase III clinical trial with another anionic polymer, cellulose sulfate, showed that women who received the compound had a higher rate of HIV acquisition relative to the placebo arm (18). On a more positive note, a clinical trial with yet another anionic polymer, Pro-2000, suggested a 30% reduction in HIV infection, but unfortunately, the results were not statistically significant (19).
One possible reason why anionic polymers have not fared well in preventing HIV acquisition is that these polymers might have induced the expression of inflammatory chemokines that recruited HIV-susceptible target cells to the genital mucosa. Inflammation also promotes T cell activation and dendritic cell maturation, processes that increase permissivity for HIV infection. The high availability of target cells as a result of inflammation may have offset any beneficial effects of the anionic polymers in preventing HIV infection. Interestingly, surfen has been reported to be anti-inflammatory through its ability to inhibit the binding of the anaphylatoxin C5a to its receptor (6). Therefore, surfen may prove useful not only as an inhibitor of SEVI activity but also as a means to limit the inflammatory response that can recruit HIV-susceptible target cells to the genital mucosa.
In addition to enhancing HIV-1 infection, SEVI and semen can increase the infectivity of other viruses. It was recently demonstrated that the infectivity of XMRV, a virus that replicates in the human prostate, is enhanced by SEVI (20). Because the precursor of SEVI is produced in abundance by the prostate, XMRV infection can potentially be suppressed by SEVI inhibitors, such as surfen. Microbicides incorporating inhibitors against SEVI and other naturally occurring viral enhancement factors may therefore prove useful in limiting the spread of a variety of viral pathogens.
Supplementary Material
Acknowledgments
We thank J. Wong at the Gladstone Electron Microscopy Core Facility for generating the electron micrographs; P. Bienasz for providing the CHO and pgs-A745 stable transfectants; members of the Greene laboratory for helpful discussions; G. Howard for editorial assistance; J. Carroll, C. Goodfellow, and A. Wilson for assistance in preparing the figures; and S. Cammack and R. Givens for administrative assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants P01 A1083050-01 (to W. C. G.), 1R01AI067057-01A2 (to F. K.), P30 AI027763 (to University of California San Francisco-GIVI CFAR), and P01 HD40543 WHIN PPG (to W. C. G.) from NICHD. This work was also supported by The Giannini Foundation fellowship (to N. R. R.), the Deutsche Forschungsgemeinschaft (to F. K.), and the WHIN PPG grant (to W. C. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6.
J. Münch and F. Kirchhoff, manuscript in preparation.
- HIV
- human immunodeficiency virus
- ELISA
- enzyme-linked immunosorbent assay
- FITC
- fluorescein isothiocyanate
- HSPG
- heparan sulfate proteoglycans
- PAP
- prostatic acid phosphatase
- SEVI
- semen-derived enhancer of viral infection
- surfen
- bis-2-methyl-4- amino-quinolyl-6-carbamide
- CHO
- Chinese hamster ovary.
REFERENCES
- 1.Münch J., Rücker E., Ständker L., Adermann K., Goffinet C., Schindler M., Wildum S., Chinnadurai R., Rajan D., Specht A., Giménez-Gallego G., Sánchez P. C., Fowler D. M., Koulov A., Kelly J. W., Mothes W., Grivel J. C., Margolis L., Keppler O. T., Forssmann W. G., Kirchhoff F. (2007) Cell 131, 1059–1071 [DOI] [PubMed] [Google Scholar]
- 2.Rönnberg L., Vihko P., Sajanti E., Vihko R. (1981) Int. J. Androl. 4, 372–378 [DOI] [PubMed] [Google Scholar]
- 3.Roan N. R., Münch J., Arhel N., Mothes W., Neidleman J., Kobayashi A., Smith-McCune K., Kirchhoff F., Greene W. C. (2009) J. Virol. 83, 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyoshima K., Vogt P. K. (1969) Virology 38, 414–426 [DOI] [PubMed] [Google Scholar]
- 5.Umber F., Stoerring F. K., Foellmer W. (1938) Klinische Wochenschrift. 17, 443–446 [Google Scholar]
- 6.Lanza T. J., Durette P. L., Rollins T., Siciliano S., Cianciarulo D. N., Kobayashi S. V., Caldwell C. G., Springer M. S., Hagmann W. K. (1992) J. Med. Chem. 35, 252–258 [DOI] [PubMed] [Google Scholar]
- 7.Panchal R. G., Hermone A. R., Nguyen T. L., Wong T. Y., Schwarzenbacher R., Schmidt J., Lane D., McGrath C., Turk B. E., Burnett J., Aman M. J., Little S., Sausville E. A., Zaharevitz D. W., Cantley L. C., Liddington R. C., Gussio R., Bavari S. (2004) Nat. Struct. Mol. Biol. 11, 67–72 [DOI] [PubMed] [Google Scholar]
- 8.Schuksz M., Fuster M. M., Brown J. R., Crawford B. E., Ditto D. P., Lawrence R., Glass C. A., Wang L., Tor Y., Esko J. D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13075–13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavrois M., De Noronha C., Greene W. C. (2002) Nat. Biotechnol. 20, 1151–1154 [DOI] [PubMed] [Google Scholar]
- 10.Platt E. J., Wehrly K., Kuhmann S. E., Chesebro B., Kabat D. (1998) J. Virol. 72, 2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei X., Decker J. M., Liu H., Zhang Z., Arani R. B., Kilby J. M., Saag M. S., Wu X., Shaw G. M., Kappes J. C. (2002) Antimicrob. Agents Chemother. 46, 1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esko J. D., Stewart T. E., Taylor W. H. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 3197–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y. J., Hatziioannou T., Zang T., Braaten D., Luban J., Goff S. P., Bieniasz P. D. (2002) J. Virol. 76, 6332–6343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan L. N., Phelan M., Mallinson M., Norcross M. A. (1991) J. Virol. 65, 1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rider C. C. (1997) Glycoconj. J. 14, 639–642 [DOI] [PubMed] [Google Scholar]
- 16.Witvrouw M., De Clercq E. (1997) Gen. Pharmacol. 29, 497–511 [DOI] [PubMed] [Google Scholar]
- 17.Skoler-Karpoff S., Ramjee G., Ahmed K., Altini L., Plagianos M. G., Friedland B., Govender S., De Kock A., Cassim N., Palanee T., Dozier G., Maguire R., Lahteenmaki P. (2008) Lancet 372, 1977–1987 [DOI] [PubMed] [Google Scholar]
- 18.Van Damme L., Govinden R., Mirembe F. M., Guédou F., Solomon S., Becker M. L., Pradeep B. S., Krishnan A. K., Alary M., Pande B., Ramjee G., Deese J., Crucitti T., Taylor D. (2008) N. Engl. J. Med. 359, 463–472 [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. (2009) Science 323, 996–997 [DOI] [PubMed] [Google Scholar]
- 20.Hong S., Klein E. A., Das Gupta J., Hanke K., Weight C. J., Nguyen C., Gaughan C., Kim K. A., Bannert N., Kirchhoff F., Munch J., Silverman R. H. (2009) J. Virol. 83, 6995–7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.