Abstract
The Ca2+/calmodulin-dependent protein phosphatase calcineurin is a key mediator in antigen-specific T cell activation. Thus, inhibitors of calcineurin, such as cyclosporin A or FK506, can block T cell activation and are used as immunosuppressive drugs to prevent graft-versus-host reactions and autoimmune diseases. In this study we describe the identification of 2,6- diaryl-substituted pyrimidine derivatives as a new class of calcineurin inhibitors, obtained by screening of a substance library. By rational design of the parent compound we have attained the derivative 6-(3,4-dichloro-phenyl)-4-(N,N-dimethylaminoethylthio)-2-phenyl-pyrimidine (CN585) that noncompetitively and reversibly inhibits calcineurin activity with a Ki value of 3.8 μm. This derivative specifically inhibits calcineurin without affecting other Ser/Thr protein phosphatases or peptidyl prolyl cis/trans isomerases. CN585 shows potent immunosuppressive effects by inhibiting NFAT nuclear translocation and transactivation, cytokine production, and T cell proliferation. Moreover, the calcineurin inhibitor exhibits no cytotoxicity in the effective concentration range. Therefore, calcineurin inhibition by CN585 may represent a novel promising strategy for immune intervention.
Keywords: Calcium/Calcineurin, Enzymes, Enzymes/Inhibitors, Immunology/Humoral response/Immunosupressor/Immunophilin, Immunology/Humoral response/NFAT, Phosphorylation/Phosphatases/Serine-Threonine, Phosphorylation/Serine/Threonine, Protein/Drug Interactions
Introduction
The activation and the precise interplay between signaling pathways are crucial for the successful initiation and progression of the immune response as a reaction of an antigen contact. In T cells the stimulation of the T cell receptor by a specific antigen leads to a calcium release from the intracellular stores and to a calcium release-activated Ca2+channel-mediated calcium influx into the cytoplasm which activates calmodulin and thereby the Ser/Thr-protein phosphatase calcineurin (1). Consequently, calcineurin represents a bottleneck in T cell receptor signaling and allows the modulation of T cell activation by low molecular compounds, such as cyclosporin A (CsA)2 or tacrolimus (FK506) (2). The cyclic undecapeptide CsA and the macrolid FK506 bind to and inhibit the phosphatase activity of calcineurin only after interaction with their respective peptidyl prolyl cis/trans isomerases (PPIases), cyclophilins (Cyp), and FK506-binding proteins (FKBP) through a gain-of-function mechanism (3, 4). Based on their particular characteristic to bind the immunosuppressive drugs CsA and FK506, members of the Cyp and FKBP family of PPIases were also termed immunophilins (5). Among the many known PPIases, the most abundant isoforms, Cyp18 and FKBP12, were identified as major intracellular acceptor proteins for CsA and FK506, respectively. The PPIase activity of both enzymes is strongly inhibited by the immunosuppressive drugs (6), leading to many of serious side effects (e.g. nephrotoxicity, neurotoxicity, hypertension, fibrosis) that were observed in the prevention and therapy of graft-versus-host reactions or autoimmune diseases (7–10). Therefore, CsA derivatives, such as [DAT-Sar]3CsA, were synthesized to obtain cyclophilin-independent calcineurin inhibitors (11). However, [DAT-Sar]3CsA still inhibited PPIase activity of Cyp18 in the nanomolar concentration range, albeit the [DAT-Sar]3CsA·Cyp18 complex did not exhibit immunosuppressive properties. Furthermore, there are some CsA derivatives, such as [MeVal]4CsA, that exclusively inhibit cyclophilins without affecting calcineurin or suppressing the immune response. Besides the intracellular calcium level, the enzymatic activity of calcineurin is regulated by other proteins or oligopeptides, such as DSCR1/2 (12), Cain/Cabin (13, 14), CHP (15), Carabin (16), AKAP79 (17), and the autoinhibitory domain of calcineurin itself (18). However, these endogenous inhibitors and A238L, a protein from African swine fever virus (19), do not penetrate the cell membrane and exhibit only a limited proteolytic stability. Both circumstances exclude their possible application as drugs in a clinical therapy. The natural compounds okadaic acid and microcystin LR were identified by screening tests as potent inhibitors of protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) and weak inhibitors of calcineurin (20, 21). Moreover, chemical modification of known protein phosphatase 1 and 2A inhibitors, such as cantharidin and endothall, resulted in derivatives with higher specificity for calcineurin (22). Nevertheless, all synthesized compounds were still able to inhibit the PP1 and PP2A and, therefore, many signal transduction pathways in the cell. Moreover, pyrethroid insecticides were described in the literature as potent calcineurin inhibitors, but recent studies are at variance, showing no calcineurin inhibition of this class of compounds (23). Tyrosine kinase inhibitors from tyrphostin type exhibit a calcineurin inactivating potency in the lower micromolar range but lack calcineurin specificity as well (24). In addition to the anti-HIV-1 replication inhibitory properties of ring-substituted benzothiophen-2-carboxamides, a significant reduction of calcineurin activity was measured (25). For the dihydroisobenzofuran dibefurin, a microbial metabolite of the strain AB 1650I-759, an IC50 of 46 μm was determined for calcineurin inhibition (26). Although this natural compound has shown an influence in the mixed lymphocyte reaction assay, the specificity of inhibition remains unknown. The polyphenolic aldehyde gossypol from cotton seed was identified by screening of a substance library inhibiting calcineurin activity with an IC50 value of 15 μm (27). However, this compound also inactivates other cellular enzymes, such as dehydrogenases, protein kinases, steroidogenic adrenal enzymes, cathepsin L, and topoisomerase II. Recently, the natural flavonol kaempferol was identified in vitro as a noncompetitive calcineurin inhibitor with an IC50 value of 52 μm (28).
In this study we describe the identification of 2,6-diphenyl-substituted pyrimidine derivatives as a new class of calcineurin inhibitors by screening of a compound library. The directed side chain modification of the parent compound led to a derivative, CN585, exhibiting potent calcineurin inhibition and no cytotoxic side effects. Accordingly, CN585 does not inhibit other Ser/Thr protein phosphatases or PPIases. Furthermore, the inhibitor showed strong immunosuppressive activity in peripheral blood mononuclear cells (PBMC) and Jurkat cells by inhibiting calcineurin-dependent dephosphorylation of NFAT (nuclear factor of activated T cells), intracellular cytokine production, and T cell proliferation.
EXPERIMENTAL PROCEDURES
Materials
The expression of human calcineurin in Escherichia coli and the following purification procedure were performed as described (29). Recombinant PP1 (rabbit muscle α-isoform) and calmodulin were purchased from MERCK, and PP2C (human α-isoform) was from Biomol. PP2A was isolated from porcine brain and purified to homogeneity as published (30). The recombinant protein kinase A from bovine was purchased from New England Biolabs. Human Cyp18, FKBP12, and Pin1 were prepared as described elsewhere (31, 32). The biotinylated and non-biotinylated RII peptide were synthesized by solid peptide synthesis. All cell culture media and supplements were purchased from BioWest.
Compound Library
For the identification of new calcineurin inhibitors, a lead discovery library from Hans-Knöll Institut (Jena, Germany) with 3000 synthetic, semisynthetic, and natural compounds was screened. The screening was performed in a 96-well format using a scintillation proximity assay (27). For increasing the inhibitory potency and decreasing the toxicity of the initial lead, a lead optimization library with 32 pyrimidine derivatives was synthesized. Identity and purity of all compounds were controlled by high performance liquid chromatography, mass spectrometry, and 1H NMR.
Phosphorylation and Purification of RII Peptide
The biotinylated and nonbiotinylated 19-mer RII peptides derived from the regulatory subunit RII of bovine protein kinase A (DLDVPIPGRFDRRVSVAAE-OH) were phosphorylated at the serine residue using the catalytic subunit of protein kinase A. The reaction mixture for the phosphorylation of the biotinylated RII peptide contained 600 μm RII peptide, 100 μCi of [γ-33P]ATP, 650 μm ATP, and 17.5 kilounits of protein kinase A in a final volume of 30 μl of buffer A (50 mm Tris/HCl, pH 7.5, 20 mm MgCl2, 1 mm dithiothreitol). The mixture was incubated at 30 °C for 6 h. The nonbiotinylated RII peptide was phosphorylated using 1 mm RII peptide, 1 mm ATP, and 50 kilounits of protein kinase A in a final volume of 100 μl buffer A. The mixture was incubated at 30 °C for 6 h. Complete stoichiometric phosphorylation was verified by mass spectrometry.
Both peptides were separated from ATP by a 1 ml of RP-C2 clean-up extraction column (Amchro). The column was equilibrated using 1 ml of 70% (v/v) ethanol, 1 ml 70% (v/v) acetonitrile followed by 3 ml of H2O. After application of the phosphorylation mixture, the column was washed with 3 ml of H2O, and the peptide was eluted with 500 μl of 70% (v/v) acetonitrile then lyophilized and dissolved in H2O before use.
Calcineurin Phosphatase Assay Using 33P-Labeled RII Phosphopeptide
Calcineurin activity was measured using the scintillation proximity assay as described previously (33, 34). In brief, 300 nm calmodulin and 14 nm calcineurin were preincubated in buffer B (40 mm Tris/HCl, pH 7.5, 100 mm NaCl, 6 mm MgCl2, 1 mm CaCl2, 500 μm Tris-(2-carboxyethyl)-phosphin-hydrochloride (TCEP), 100 μg/ml bovine serum albumin) for 10 min at room temperature in a 96-well microtiter plate (Costar). After this, 10 pmol of 33P-labeled RII phosphopeptide was added to each well in a total assay volume of 100 μl and incubated for different time periods at 30 °C. Then 90 μl of the reaction mixture were transferred into a scintillation well coated with streptavidin (PerkinElmer Life Sciences), and the biotinylated RII peptide was allowed to bind onto streptavidin for 20 min at room temperature. The wells were washed twice, and RII peptide-associated 33P was measured in a MicroBeta top counter (Wallac). Initial rates were calculated from the linear plots of the concentration of the dephosphorylated peptide against incubation time. To ensure initial kinetics, dephosphorylation was followed only 10% toward completion.
To determine the inhibition mode of inhibitors, biotinylated RII phosphopeptide was used at constant concentrations of 100 nm, whereas the nonbiotinylated RII phosphopeptide was added in the concentration range between 2.0 and 20 μm. This RII phosphopeptide mixture exhibits a Km value of 20 μm as determined by a Lineweaver-Burk plot (27). The calcineurin used exhibits a kcat/Km value for RII phosphopeptide of 19.7 mm−1 s−1 (35). Initial rates of calcineurin activity were determined at varying RII phosphopeptide concentrations in the presence of different inhibitor concentrations (0–20 μm). The inhibition mode and the Ki value of the inhibitor were obtained from Dixon Plot.
The reversibility was tested by plotting Vmax versus the concentration of calcineurin. Therefore, various calcineurin concentrations (4–28 nm) were incubated with 300 nm calmodulin, 1–10 μm inhibitor, and 100 nm 33P-labeled RII phosphopeptide at 30 °C. The inhibitor acts reversible when all straight lines exhibit a common point of intersection. An alternative approach was the removal of inhibitor from enzyme-inhibitor complex by dialysis. A preincubated mixture of 15 nm calcineurin and 40 μm CN585 in assay buffer containing 100 nm calmodulin was preincubated for 30 min. The mixture and a non-inhibited calcineurin control were placed in 3-kDa MicroDialyzer (Pierce) and dialyzed against buffer B at 4 °C. Phosphatase activities of the dialyzed enzyme or enzyme-CN585 complex were determined using RII phosphopeptide substrate.
The putative calcineurin binding site for CN585 was identified by kinetic measurements and curve simulation. Two theoretical binding curves were calculated by using DynaFit software (36) according to a model of identical binding sites and a model of different binding sites for CN585 and Cyp18·CsA. The simulated curves were compared with the measured simultaneous inhibition of calcineurin by CN585 and Cyp18·CsA.
Calcineurin Phosphatase Assay Using p-Nitrophenyl Phosphate (pNPP)
200 nm calcineurin were preincubated with different concentrations of CN585 or DMSO as controls for 15 min in buffer C (50 mm Tris/HCl, pH 8.0, 30 mm MgCl2, 5 mm CaCl2, 100 μg/ml bovine serum albumin, 0.5 mm dithiothreitol). The reaction was initiated by the addition of 10 mm pNPP. The release of p-nitrophenolate caused by calcineurin-catalyzed hydrolysis of pNPP was measured at 405 nm by a Dynatec MR7000 microtiter plate reader (Chantilly) at room temperature for 30 min. Initial slope k values of the reactions were determined by linear regression and compared with DMSO control.
Measurement of Other Ser/Thr-Protein Phosphatases
The activities for the protein phosphatases PP2A and PP2C were measured with RII phosphopeptide, as described for calcineurin. To determine PP2C activity, 30 mm MgCl2 was added in buffer B. PP1 was assayed by using 32P-labeled phosphorylase a as substrate. The protein phosphatase activities were adjusted to 20% dephosphorylation of the substrates.
Measurement of PPIase Activity
PPIase activity was determined with oligopeptide substrates using the protease-coupled assay as described elsewhere (6). Typically, experiments were performed with PPIase concentrations in the low nm range. The effect of the compounds on the PPIase activity was calculated from the remaining activity after preincubation of the enzymes and the compounds for 30 min at 22 °C.
Coprecipitation Experiments Using CN778 Affinity Matrix
For preparation of the affinity matrix, 1 mg of CN778 was preincubated with 400 μl of streptavidin-Sepharose (GE Healthcare) overnight at 25 °C under gentle agitation. The beads were washed 5 times, resuspended in PBS, and incubated with 2 μg of calcineurin, calcineurin subunits, or 150 μl of Jurkat cell lysate (5 mg/ml) in the presence or absence of 5 μm CsA·Cyp18 or FK506·FKBP12 complexes for 3 h at 4 °C. Subsequently the beads were washed with PBS, boiled in SDS sample buffer, and separated by SDS-PAGE. The separated proteins were blotted onto nitrocellulose membrane and probed with anti-calcineurin antibody (Sigma).
Cell Culturing
Jurkat cells were obtained from DSMZ Braunschweig (Germany) and cultured in RPMI 1640 supplemented with 10% (v/v) fetal calf serum and 2 mm l-glutamine in a humidified incubator at 37 °C and 5% (v/v) CO2. Stock solutions of all compounds were in DMSO and added to a final concentration of 0.5% (v/v) DMSO per sample.
Cytotoxicity Measurements
To test the cytotoxicity of the 2,6-diaryl pyrimidine derivatives, 5 × 106 Jurkat cells/ml were incubated for 24 h with 20 μm concentrations of each compound. Additionally, DMSO treated samples were used as controls. The cytotoxicity was determined by flow cytometry using the Guava ViaCount Kit (Guava Technologies, Hayward, CA).
NFAT-Luciferase Reporter Gene Assay
Jurkat cells were transfected with a NFAT-luciferase reporter plasmid (Stratagene) by electroporation and cultured for 16 h. The cells were preincubated with the compounds for 30 min and then stimulated with 2 μm ionomycin and 100 nm PMA for 5 h. After cell lysis, the level of extracted luciferase was determined by bioluminescence measurement using the luciferase assay system (Promega). Additionally, pcDNA-lacZ was cotransfected, and β-galactosidase activity was measured as the internal standard.
Intracellular Cytokine Production
Human PBMC from healthy volunteers were isolated by Ficoll density gradient centrifugation (37). For the experiments 5 × 106 cells/ml were preincubated with different concentrations of CN585, 15 μm CN675, 1 μm [MeVal]4CsA, or 1 μm CsA at 37 °C for 30 min in a 24-well plate. The cells were stimulated with 2 μm ionomycin and 100 nm PMA for 5 h with 5 μg/ml brefeldin A for the last 3 h. PBMC were fixed in PBS with 2% (m/v) paraformaldehyde for 20 min, permeabilized with 0.5% (m/v) saponin in PBS supplemented with 1% (v/v) fetal calf serum, incubated with anti- interleukin-2 (IL-2) fluorescein isothiocyanate-conjugated antibody, and analyzed by flow cytometry (FACSort, BD Biosciences).
Green Fluorescent Protein-NFAT Translocation Assay
HeLa cells were transfected with a pEGFP-NFAT2–421 plasmid by electroporation (Amaxa). Ten hours after the transfection procedure, the cells were incubated for 45 min with CN585, CN675, and CsA as the positive control and [MeVal]4CsA as the negative control. The cells were stimulated by the addition of 2 μm ionomycin, 100 nm PMA for 1 h, paraformaldehyde-fixed, and stained with 4′,6-diamidino-2-phenylindole. All fluorescence images were recorded with a cooled CCD camera upon Nikon TE200 fluorescence microscope and analyzed by analySIS software (Soft Imaging System) (38).
Proliferation of PBMC
Human PBMC were labeled with 5 μm 5,6-carboxyfluorescein diacetate succinimidyl ester and incubated with the compounds as indicated 20 min at 37 °C. Afterward the PMBC (2 × 106 cells/ml) were stimulated with plate-bound anti-CD3 plus anti-CD28 antibodies (BD Pharmingen) and cultured for 5 days. All samples were directly analyzed on viable cells by flow cytometry (FACSort). The portion of proliferating cells was determined by using ModfitLT software (Verity Software).
RESULTS
CN585 Potently Inhibits Calcineurin Activity
To identify new calcineurin inhibitors that can act as potent immunosuppressive drugs, a compound library of synthetic low molecular mass compounds was screened by determining the inhibition of calcineurin-catalyzed dephosphorylation of RII phosphopeptide. In the first screening round we identified 4-(N,N-dimethylamino-propylamino)-2,6-diphenyl-pyrimidine (CN391) as a potential calcineurin inhibitor, with an IC50 value of 30 μm. Based on this compound, several derivatives were synthesized and tested for their inhibitory efficacy in the RII phosphopeptide-based calcineurin activity assay (Table 1). With the applied synthesis strategy several derivatives were obtained with an improved inhibitory potency against calcineurin. However, some derivatives exhibited no (CN675 and CN448) or a weaker (CN676) calcineurin inhibition compared with the parent compound CN391. Thus, derivatives possessing chlorine atoms as R4 and R5 and sulfur and nitrogen as R1 (CN584, CN585) showed an improved calcineurin inhibitory potency, whereas an oxygen atom at position R1 did not increase inhibitory efficacy (CN392). Furthermore, compounds lacking a tertiary amino group at position R2 exhibit higher IC50 values.
TABLE 1.
Structures of the synthesised 2,6-diaryl-substituted pyrimidine derivatives
1 Phosphatase assay with RII phosphopeptide as substrate (S.D. ≤ 10%).
2 Cytotoxicity of the 20 μm compound against Jurkat cells after 24 h of incubation (propidium iodide-permeable cells, DMSO as control; S.D. ≤ 10% of triplicates).
3 n.i., measured calcineurin inhibition at 20 μm was ≤3%.
To develop novel immunosuppressive drugs on the basis of calcineurin inhibition that exhibit no adverse effects, cytotoxicity of the synthesized pyrimidine derivatives was tested in Jurkat cells using the Guava ViaCount assay kit. In comparison to CN391, CN585 showed no significant cytotoxic effect with a survival rate of 94 ± 10% but was still able to inhibit calcineurin activity with an IC50 of 3.5 μm. Because CN585 exhibited the best inhibition/cytotoxicity ratio, the compound was selected for further characterization.
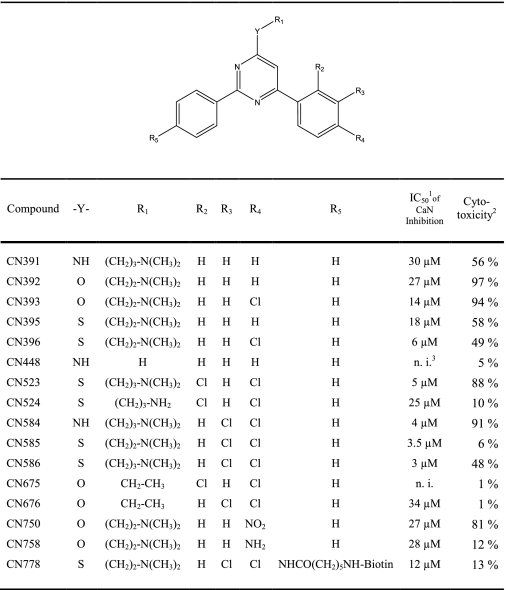
In addition to the standard phosphatase assay with RII phosphopeptide (Fig. 1A), the influence of CN585 on calcineurin was measured using the small artificial substrate pNPP (Fig. 1B). In contrast to the RII phosphopeptide assay, CN585 showed only minor inhibition of calcineurin-catalyzed hydrolysis of pNPP. Moreover, the same extent of decrease was observed for CN675, which was used as negative control in all inhibition and cell experiments, as CN675 does not inhibit calcineurin activity against RII phosphopeptide and has exhibited no cytotoxicity.
FIGURE 1.
Influence of CN585 and CN675 on calcineurin phosphatase activity. Calcineurin activity was measured in phosphatase assays using 33P-labeled RII phosphopeptide (A) or pNPP (B) as substrates in the presence of increasing concentrations of CN585 (closed circle) or CN675 (open circle). The data presented are the percent activity of a DMSO-containing control as means ± S.D. of three independent measurements.
Calcineurin Inhibition by CN585 Is Calmodulin-independent, Reversible, and Non-competitive
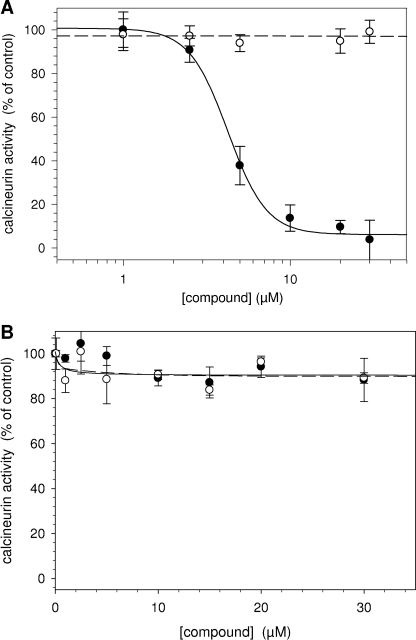
To test whether the calcineurin inhibition by CN585 is mediated by interfering with the Ca2+/calmodulin-dependent activation, the calcineurin activity was monitored in the presence of CN585 and various concentrations of calmodulin. As shown in Fig. 2, calmodulin concentrations in the range of 50–200 nm had no influence on the IC50 value of calcineurin inhibition.
FIGURE 2.
Effect of calmodulin on CN585-mediated calcineurin inhibition. Calcineurin inhibition by various CN585 concentrations was measured in the presence of 50 nm (square), 100 nm (diamond), and 200 nm (triangle) calmodulin using the RII phosphopeptide substrate. Initial rates of calcineurin activity were plotted against the inhibitor concentration. The data presented are the means ± S.D. of three independent experiments.
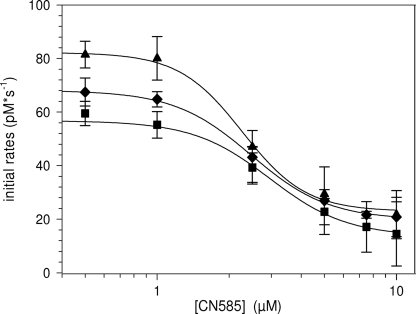
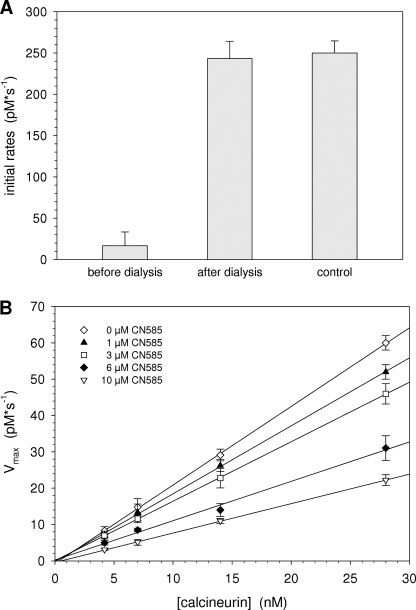
To exclude an irreversible inhibition of calcineurin by an inhibitor-induced covalent chemical modifications or protein denaturation, a dialysis experiment was performed. A preincubated mixture of calcineurin and CN585 was dialyzed in a MicroDialyzer with a 3-kDa cutoff membrane against buffer A at 4 °C. Aliquots of dialyzed samples were assayed using RII phosphopeptide substrate. Moreover, reference activity was determined in a calcineurin sample treated similarly but lacking CN585. As shown in Fig. 3A, the inhibition was completely reversible. Additionally, a kinetic analysis using different concentrations of calcineurin and CN585 was performed (Fig. 3B). Therefore, Vmax was plotted versus calcineurin concentration for each CN585 concentration. As revealed, the straight lines have a common point of intersection, and the slopes of the lines decrease with increasing CN585 concentration, indicating a reversible inhibition type.
FIGURE 3.
Calcineurin inhibition by CN585 is reversible. A, a preincubated mixture of 15 nm calcineurin and 40 μm CN585 in assay buffer containing 100 nm calmodulin was used to examine the reversibility of inhibition in a dialysis experiments. The mixture and a non-inhibited calcineurin control were placed in a 3-kDa MicroDialyzer and dialyzed. Initial rates of calcineurin activity of the dialyzed enzyme or CN585-enzyme complex were determined using RII phosphopeptide substrate. B, additionally, initial rates of 4–28 nm calcineurin preincubated with calmodulin, 1–10 μm CN585, and 33P-labeled RII phosphopeptide were measured at 30 °C. Vmax was plotted versus the concentration of calcineurin. In the case of a reversible inhibitor, all straight lines have a common point of intersection. The data shown are the means ± S.D. of three independent experiments.
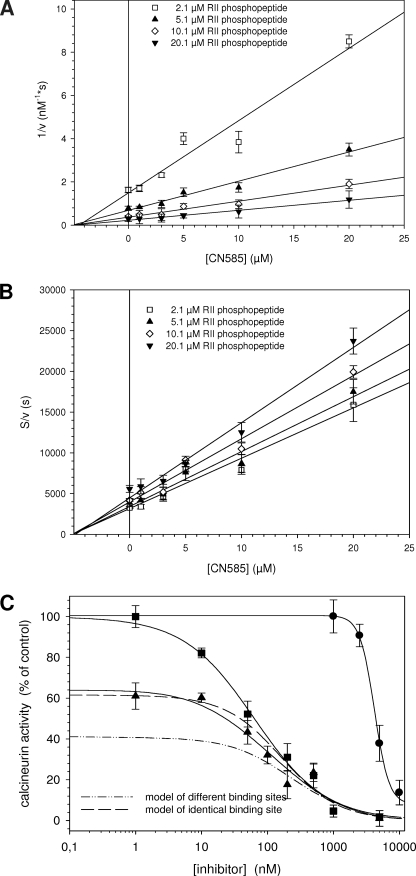
To determine the Ki value and the mode of inhibition, a series of kinetic experiments with a mixture of biotinylated and non-biotinylated RII phosphopeptide was performed (Fig. 4). The Ki value of 3.8 ± 0.3 μm obtained from the Dixon plot was similar to the determined IC50 value of 3.5 ± 0.4 μm at 100 nm substrate, as shown in Fig. 1A. Furthermore, a non-competitive type of inhibition can be inferred from the Dixon plot (Fig. 4A) (66). To clearly distinguish between the non-competitive and the mixed, uncompetitive, and competitive inhibition type, we additionally plotted [S]/v against the inhibitor concentration as described by Cornish-Bowden (39). Also in this plot (Fig. 4B), the intersection point occurs on the abscissa, indicating a simple non-competitive inhibition.
FIGURE 4.
Analysis of the inhibition type and putative calcineurin binding site. Inhibition type of CN585 was evaluated using substrate concentration from 2.1 to 20.1 μm RII phosphopeptide mixture containing 100 nm biotinylated and 0.5–20 μm nonbiotinylated RII phosphopeptide. The inhibition mode and the Ki value of CN585 were determined by plotting 1/v against CN585 (Dixon plot) (A) and by plotting [S]/v against CN585 (B). C, to obtain indications about the CN585 binding site on calcineurin, the phosphatase was simultaneously inhibited with CN585 and increasing concentrations of the Cyp18·CsA complex. Measurements were performed using 33P-labeled RII phosphopeptide, 3 μm CN585, 10 μm CsA, and various Cyp18 concentrations. Because of the high affinity between Cyp18 and CsA, the concentration of the Cyp18·CsA complex is identical to the applied Cyp18 concentration. Moreover, by using DynaFit software, two theoretical binding curves were calculated according to a model of identical binding sites (long dash) and a model of different binding sites (dash-dot-dot) of CN585 and Cyp18·CsA. The shape of the measured curves (solid line) clearly indicates a binding on identical or overlapping binding sites of both inhibitors. Moreover, the concentration-dependent inhibition of calcineurin by Cyp18·CsA and CN585 alone is also displayed in the figure. All experimental data presented are the means ± S.D. of three independent experiments.
CN585 Binding onto Calcineurin
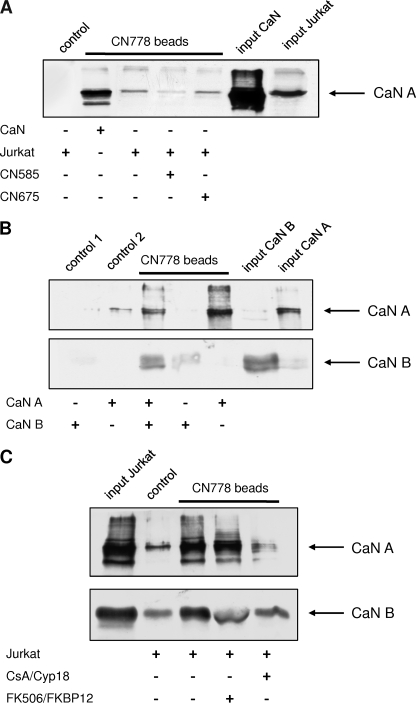
To encircle the putative CN585 binding site, the calcineurin activity was measured in the simultaneous presence of 3 μm CN585 and 10 μm CsA at varying Cyp18 concentrations (Fig. 4B). These conditions allowed testing whether the inhibitors bind to either identical or independent binding sites. Calcineurin was inhibited more efficiently in the presence of both compounds compared with Cyp18·CsA alone. However, the residual calcineurin activity should be different for both inhibition models where a lower degree of inhibition can be expected for competition at identical binding sites (Fig. 4C, long dash) compared with independent binding sites (dash-dot-dot). Therefore, both curves were simulated using DynaFit software and compared with the experimental data. A close fit of the experimental data and the calculated curve for identical binding sites can be observed, indicating that CN585 and Cyp18·CsA share similar or the same calcineurin binding site. Next, we were interested in whether the regulatory B subunit of calcineurin is necessary for CN585 binding. Therefore we performed binding assays with the isolated catalytic subunit, calcineurin A, and the regulatory subunit calcineurin B. These experiments revealed that the inhibitor matrix interacts predominantly with the calcineurin A/calcineurin B heterodimer and the isolated catalytic subunit of calcineurin (Fig. 5B). The B subunit shows only very weak interaction. Moreover, the Cyp18·CsA complex, but not the FKBP12·FK506 complex, interferes with calcineurin binding to the matrix, indicating that the Cyp18·CsA complex shares common or overlapping binding sites on the catalytic subunit of calcineurin (Fig. 5C).
FIGURE 5.
Coprecipitation of recombinant and endogenous calcineurin using CN778 affinity matrix. A, biotinylated CN585 derivative, CN778, was immobilized onto streptavidin-Sepharose. Beads were incubated with recombinant calcineurin or Jurkat cell lysate in the presence and absence of CN585 or CN675 for 3 h at 4 °C. Recombinant calcineurin (CaN) and endogenous calcineurin of Jurkat cells interacts with the affinity matrix. CN585 interferes with calcineurin binding, whereas the non-inhibitory compound CN675 has no effect on calcineurin interaction. B, binding of calcineurin subunits onto the CN778 matrix is shown. The catalytic subunit of calcineurin A (CaN A) and the regulatory subunit B (CaN B) were incubated separately or together with the immobilized CN778. The regulatory subunit shows no interaction with the inhibitor, whereas the catalytic subunit interacts with the inhibitor matrix. C, shown is competition of calcineurin interaction to CN585 by CsA·Cyp18 and FK506·FKBP12 complexes. CN778 affinity matrix was incubated with Jurkat cell lysate, and after several washing steps, CsA·Cyp18 and FK506·FKBP12 complexes were added. In the case of CsA·Cyp18, calcineurin binding to the beads was strongly diminished, whereas the FK506·FKBP12 had no effect on calcineurin interaction. Biotin-saturated streptavidin-Sepharose was used as control. Subsequently, the matrix was washed with PBS and boiled in SDS sample buffer, and bound proteins were separated by SDS-PAGE. The proteins were blotted onto nitrocellulose membrane, probed with anti-calcineurin antibody, and analyzed by ECL reaction.
CN585 Specifically Inhibits Calcineurin Activity
All four main Ser/Thr protein phosphatases (PP1, PP2A, PP2B, and PP2C) exhibit a high degree of sequence homology and share common structural features in the active site (21). Therefore, the inhibition of the four protein phosphatases by CN585 was tested in phosphatase assays with specific substrates (Table 2). It is noteworthy that CN585 inhibits calcineurin exclusively, whereas no other phosphatase was affected by the inhibitor.
TABLE 2.
Enzymatic activity of the main Ser/Thr-protein phosphatases in the presence of CN585
| Phosphatase activity |
||
|---|---|---|
| 10 μm CN585 | 100 μm CN585 | |
| % of untreated control (S.D. ≤ 10%) | ||
| PP1 | 95 | 91 |
| PP2A | 98 | 103 |
| PP2B (calcineurin) | 14 | 3 |
| PP2C | 102 | 96 |
Because calcineurin inhibition by the immunosuppressive drugs FK506 and CsA is mediated by prior binding to cellular PPIases, we analyzed whether the CN-compound influences the PPIase activity of Cyp18, FKBP12, and Pin1 that represents the prototypic members of the three human PPIase families (40). In contrast to CsA or FK506, the small compound CN585 did not inhibit one of the PPIases (Table 3).
TABLE 3.
PPIase activity of prototypic representatives of the three human PPIase families after preincubation with CN585
| PPIase activity |
||
|---|---|---|
| 10 μm CN585 | 100 μm CN585 | |
| % of untreated control (S.D. ≤ 10%) | ||
| Cyp18 | 104 | 107 |
| FKBP12 | 97 | 93 |
| Pin1 | 104 | 94 |
To test the capability of CN585 to interact with endogenous calcineurin, CN778, a biotinylated derivative of CN585, was synthesized and immobilized onto streptavidin-Sepharose. The CN778 affinity matrix was incubated with recombinant calcineurin or with Jurkat cell lysate in the presence of CN585 or CN675. Subsequently, the proteins bound to the matrix were analyzed by SDS-PAGE and Western blot using an anti-calcineurin antibody. The evaluation of the blot (Fig. 5A) revealed that recombinant and authentic calcineurin bind to the CN778 matrix. The addition of CN585 to the sample significantly reduced the amount of bound calcineurin. In contrast, CN675 had no influence on the calcineurin/CN778 interaction.
CN585 Exhibits Potent Immunosuppressive Activity
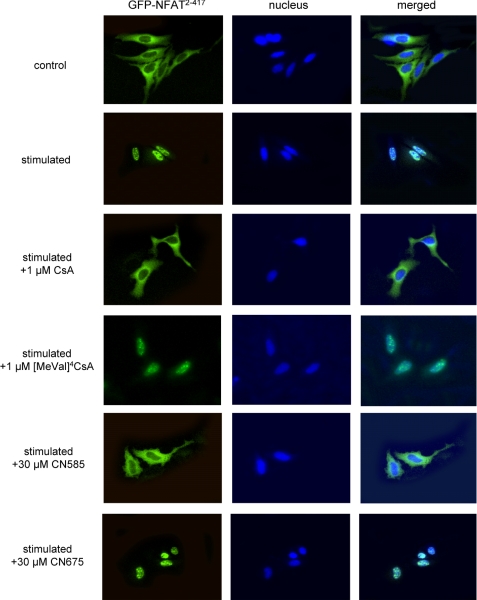
The determination of calcineurin activity in cells is difficult because other Ser/Thr protein phosphatases and alkaline phosphatase are present and interfere with the calcineurin phosphatase assays. Nevertheless, the transcription factor NFAT represents the only substrate that is specifically dephosphorylated by calcineurin (41). The phosphorylated NFAT is localized in the cytosol and undergoes conformational changes upon calcineurin-mediated dephosphorylation. Thereby a nuclear localization sequence becomes accessible, and the affinity to specific DNA sequences increases. Furthermore, NFAT translocates into the nucleus and activates the transcription of genes, such as interleukin-2. To visualize calcineurin activity in cells, HeLa cells were transfected with a NFAT2–417-green fluorescent protein expression plasmid and stimulated with PMA and ionomycin. HeLa cells have a higher cytosol/nucleus ratio and were used instead of T cells in this experiment. As shown in Fig. 6, the truncated NFAT migrated into the nucleus in response to the stimuli. In contrast to the non-immunosuppressive [MeVal]4CsA, the immunosuppressant CsA prevented NFAT translocation. CN585 exhibited a comparable effect on nuclear translocation, whereas CN675, which does not inhibit calcineurin activity, had no influence on NFAT localization.
FIGURE 6.
Influence of CN585 on NFAT translocation in HeLa cells. HeLa cells were transfected with a green fluorescent protein-NFAT2–417 expression plasmid by electroporation. Ten hours after transfection cells were incubated for 45 min with CN585, CN675, or CsA as positive and [MeVal]4CsA as negative controls. Then cells were stimulated with PMA/ionomycin for 1 h, paraformaldehyde-fixed, and 4′,6-diamidino-2-phenylindole-stained. The fluorescence images were recorded with a CCD camera upon Nikon TE200 epifluorescence microscope. The experiment was repeated two times with similar results.
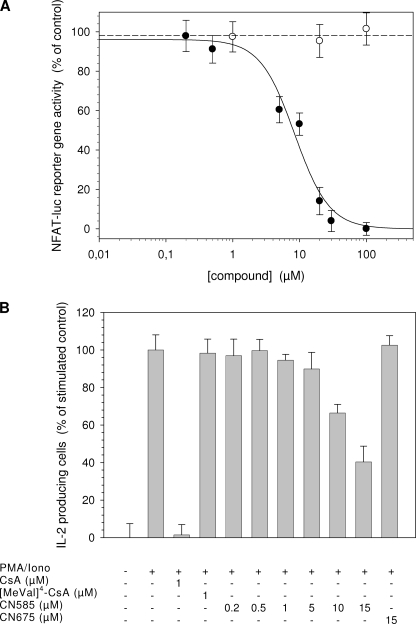
Moreover, the NFAT transactivation activity was investigated in a luciferase reporter gene assay. As shown in Fig. 7A, CN585 decreased NFAT reporter gene activity in a concentration-dependent manner with an IC50 value of 10 ± 3 μm. In contrast, in the presence of CN675, no effects on luciferase level can be observed. Furthermore, a similar behavior was observed for the control [MeVal]4CsA (data not shown).
FIGURE 7.
CN585 decreases NFAT reporter gene activity and reduces intracellular IL-2 production of stimulated PBMC. A, Jurkat cells were transfected with a NFAT-luciferase reporter plasmid by electroporation. The cells were preincubated with the compounds for 30 min and then stimulated with PMA/ionomycin for 5 h. After cell lysis the level of extracted luciferase was determined by using luciferase assay system. Additionally, pcDNA-lacZ was cotransfected, and β-galactosidase activity was measured as internal standard. B, Ficoll-isolated human PBMC (5 × 106 cells/ml) were preincubated with various concentrations of CN585, 15 μm CN675, or 1 μm CsA at 37 °C for 30 min in a 24-well plate. Subsequently, cells were stimulated with PMA/ionomycin (iono) for 5 h and for the last 3 h in the presence brefeldin A. PBMC were fixed in paraformaldehyde, permeabilized with 0.5% saponin in PBS/fetal calf serum, incubated with anti-IL-2 fluorescein isothiocyanate-conjugated antibody, and analyzed by flow cytometry. The data presented are the means ± S.D. of three independent experiments.
Additionally, the influence of CN585 on IL-2 production in T cells was determined after stimulation of PBMC with PMA/ionomycin. The following flow cytometric analysis of intracellular cytokine production allows the discrimination between IL-2-producing and non-producing T cells (42). As shown in Fig. 7B, CsA as well as CN585 application significantly reduced the number of IL-2-producing cells. Moreover, the inhibition by CN585 was concentration-dependent with an IC50 value of 13 ± 2 μm. The control compounds CN675 and [MeVal]4CsA were ineffective in this assay.
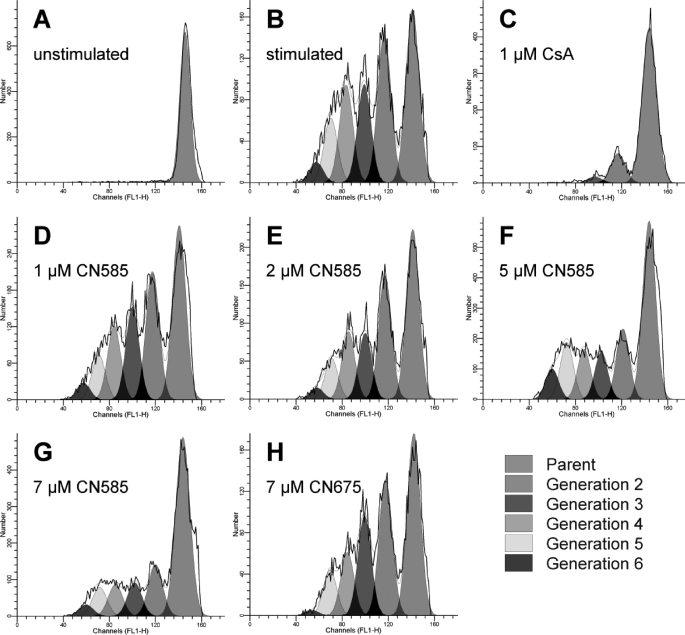
The proliferation of T cells as a result of increased cytokine production is an important process in immune response. Therefore, we tested whether CN585 is able to block the proliferation of anti-CD3/CD28-stimulated T cells. In this experiment human PBMC were labeled with 5,6-carboxyfluorescein diacetate succinimidyl-ester, allowing the discrimination and determination of cell numbers in individual generations of proliferating T cells by flow cytometry (Fig. 8) (43). Therewithal, CN585 decreased the number of proliferating T cells after stimulation with plate-bound anti-CD3/CD28 antibodies in a concentration-dependent manner with an IC50 value of 9.4 ± 1.1 μm.
FIGURE 8.
Influence of CN585 on proliferation of stimulated PBMC. Human PBMC were prepared using Ficoll density gradient, labeled with 5,6-carboxyfluorescein diacetate succinimidyl ester, and incubated with 1 μm CsA, various CN585 concentrations, 7 μm CN675, or DMSO as control. Afterward, the PMBC (2 × 106 cells/ml) were stimulated with plate-bound anti-CD3/anti-CD28 antibodies and cultured for 5 days. All samples were directly analyzed on viable cells by flow cytometry, and individual generations were calculated using ModFitLT software. The experiment was repeated three times with similar results.
DISCUSSION
Besides the potent immunosuppressive drugs CsA and FK506, glucocorticoids and cytotoxic compounds, such as azathioprine or mycophenolate mofetil, are widely used in the prevention of graft-versus-host reactions and in the therapy of serious autoimmune diseases (44). However, these immunosuppressive agents have many serious side effects such as nephrotoxicity (7), hypertension (45), hyperlipidemia (46), neuropathies (47), and an increased risk for diabetes mellitus type II (48). Therefore, the development of new strategies and safer immunosuppressive drugs is absolutely necessary.
Our study describes the development of an immunosuppressive drug that, in contrast to CsA and FK506, only exhibits calcineurin inhibitory activity but no inhibition of immunophilins. Even though the contribution of cyclophilin inhibition to toxic CsA effects is not well understood because most of the studies do not differentiate between calcineurin and cyclophilin inhibition, a specific calcineurin inhibitory drug should exhibit an improved adverse effect profile. For example, it was shown that CsA-caused nephrotoxicity is mediated by inhibition of cyclophilin activity. Hong et al. (49) demonstrated that Cyp18 wild type but not the inactive Cyp18 R55A variant protects nephrons for toxic effects of CsA in transgenic mice. Moreover, it was shown that inhibition of cyclophilin activity but not calcineurin activity leads to myopathies by blocking differentiation and inducing apoptosis at an early stage of muscle differentiation (50). Additionally, CsA-induced endothelial cell damages are mediated by inhibition of cyclophilin activity (51). Caramelo et al. (51) demonstrated that cyclosporin derivatives (e.g. [MeVal]4CsA) that do not inhibit calcineurin cause similar endothelial cell defects as the parent compound CsA. Interstitial and myocardial fibrosis represent other serious adverse effects of CsA that are caused by mechanisms independent of calcineurin inhibition (52). Thereby, CsA treatment increased the concentration and content of collagen in ventricles of transplanted and recipient hearts. CsA-induced gingiva hyperplasia is also connected with increased collagen levels due to the CsA mediated inhibition of collagen phagocytosis (53). The nephro- and myocardial toxicity of CsA represents a big drawback of the substance, as it reduces significantly the survival rates of transplanted organs.
To develop new low molecular weight inhibitors of calcineurin that can act as immunosuppressive drugs, we screened a substance library and identified the pyrimidine derivative CN391 as a calcineurin inhibitory compound. Moreover, by rational design several derivatives were synthesized with the aim of increasing the inhibitory potency and minimizing the cytotoxicity (Table 1). The compound CN585 exhibited the highest inhibition/cytotoxicity ratio and was, therefore, further characterized. CN585 inhibited calcineurin with an IC50 value of 3.5 μm using RII phosphopeptide as the phosphatase substrate (Fig. 1A). Moreover, a reversible inhibition mode was demonstrated by kinetic analysis and dialysis experiments (Fig. 3, A and B). Consequently, CN585 represents the most effective and promising direct low molecular weight inhibitor of calcineurin hitherto described. Other direct calcineurin inhibitors, such as gossypol, tyrphostin derivatives, or ring-substituted benzothiophen-2-carboxamides exhibit slightly weaker inhibition efficacy and lack calcineurin specificity as well.
The identification of pyrimidine derivatives as calcineurin inhibitors was completely new, as there were no previous indications for an interaction of pyrimidine derivatives with protein phosphatases. However, several pyrimidine derivatives were synthesized and investigated, so far exhibiting a broad range of biological activities. For example, N-3-substituted pyrimidinones are potent AT1-selective angiotensin II receptor antagonists (54), and 2- or 4-(4-methylpiperazino) pyrimidines were described as 5-HT2A receptor antagonists (55). The pyrrolo-[2,3] pyrimidines are effective inhibitors of the protein-tyrosine kinase JAK3 (56). Moreover, it was demonstrated that pyridothienopyrimidines and pyrimidine amides are effective antiallergic compounds (57, 58). Several synthesized pyrimidine derivatives exhibit potent antiviral and bacteriostatic properties (59, 60). Furthermore, for aziridino- and acridino pyrimidines a strong cytotoxicity against cancer cells was described (61, 62).
In contrast to many potent protein phosphatase inhibitors, CN585 inhibits calcineurin highly specific without affecting other Ser/Thr protein phosphatases even though the catalytic subunits of the PPP family members exhibit significant sequence homology (63). This specificity for calcineurin can be explained with an inhibitor binding site that is remote from the active site, as shown for the Cyp18·CsA and FKBP12·FK506 complexes exhibiting strong specificity for calcineurin as well. Moreover, in contrast to active site-directed inhibitors, such as okadaic acid, CN585 did not inhibit the calcineurin activity toward the small artificial substrate pNPP, which can enter the active site even when the autoinhibitory domain is bound to the active site (Fig. 1B). It is remarkable that Cyp18·CsA and FKBP12·FK506 complexes do not inhibit calcineurin phosphatase activity toward pNPP (11), as well indicating a similar binding site of CN585. Moreover, like Cyp18·CsA, CN585 inhibits calcineurin non-competitively, also supporting the hypothesis of CN585 binding to a site influencing or overlapping with that of the Cyp18·CsA complex. In fact, the competition experiment and curve simulations (Fig. 4B) support also the hypothesis that both complexes exhibit similarity in the way to block calcineurin-catalyzed dephosphorylation. Experiments using CN778 affinity matrix revealed that the catalytic subunit interacts strongly with the matrix, whereas the regulatory subunit of calcineurin exhibits only very weak binding to the immobilized inhibitor, suggesting that the B subunit is not required for interaction with CN585. However, it cannot be inferred from these results that CN585 does not possess interaction sites on the B subunit, as in the case of the immunophilin-immunosuppressant drug complex, the B subunit is necessary but also not sufficient to mediate interaction with the Cyp18·CsA complex (64).
To exclude the possibility that calcineurin inactivation by CN585 is mediated by disturbing the stability or formation of the Ca2+-calmodulin-calcineurin complex, kinetic experiments with different calmodulin concentrations were performed (Fig. 2) revealing independence of the calcineurin inhibition constant from the calmodulin concentration. In contrast to the immunosuppressive drugs, the enzymatic activity of the prototypic PPIases Cyp18, FKBP12, and Pin1 was not influenced by CN585 (Table 3) introducing the compound as a more specific probe for calcineurin function and finally immunosuppression than CsA or FK506. Further advantages of CN585 compared with peptidic or proteinaceous inhibitors are a high cell permeability and proteolytic stability.
To study the immunosuppressive activity of CN585 in cell-based assays, we first investigated whether CN585 binds to endogenous calcineurin, because kinetic analyses were exclusively performed using recombinant enzyme. Therefore, CN778, a biotinylated derivative of CN585, was immobilized and incubated with Jurkat cell lysate in the presence and absence of CN585 or CN675. In this experiment binding of endogenous calcineurin to the CN778 affinity matrix was demonstrated clearly (Fig. 5). Furthermore, only the addition of CN585 interfered with CN778/calcineurin interaction, indicating an identical binding site of both compounds on calcineurin.
For the purpose of evaluating immunosuppressive properties of CN585, we investigated calcineurin inhibition in cells. The determination of cellular calcineurin activity is difficult because calcineurin activity in Jurkat cells is about 3% that of the total phosphatase activity toward RII phosphopeptide (data not shown). Therefore, we examined the immunosuppressive activity of CN585 by monitoring the dephosphorylation of the specific calcineurin substrate NFAT in cell-based assays (41). After calcineurin-catalyzed dephosphorylation, NFAT undergoes conformational changes leading to an accumulation in the nucleus, increased DNA binding, and gene transcription (65). In PMA/ionomycin-stimulated HeLa cells, CN585 reduced the migration of green fluorescent protein-NFAT2–417 into the nucleus significantly. A comparable effect was observed for CsA (Fig. 6). Besides the NFAT translocation assay, a luciferase-coupled NFAT reporter gene assay was used to study CN585 effects on NFAT activation.
This experiment revealed potent immunosuppressive properties of CN585 as well. CN585 and CsA significantly inhibited NFAT transcriptional activation, whereas the calcineurin- inactive compound CN675 does not (Fig. 7A). Moreover, in a cytokine production assay using human PBMC, CN585 reduced the number of IL-2-producing cells after PMA/ionomycin stimulation in a concentration-dependent manner (Fig. 7B). In contrast, CN675 had no effect on intracellular IL-2 production. CN585 also decreased the proliferation of anti-CD3/anti-CD28 antibody-stimulated human T cells concentration-dependently, whereas CN675 was ineffective (Fig. 8).
In this study we describe a novel potent and specific inhibitor of the protein phosphatase calcineurin that does not inhibit other protein phosphatases or PPIases, is cell-permeable, and shows no cytotoxicity against Jurkat cells. Moreover, the compound exhibited potent immunosuppressive properties in different cell assays. Therefore, the pyrimidine derivative CN585 may have the potential to prevent graft-versus-host reactions and suppresses autoimmune diseases.
Acknowledgments
We thank Martina Heidler for excellent technical assistance and Hella Klemens for the preparation of Cyp18, FKBP12, and Pin1.
The work was supported by the Deutsche Forschungsgemeinschaft.
- CsA
- cyclosporin A
- NFAT
- nuclear factor of activated T cells
- Cyp
- cyclophilin
- PPIase
- peptidyl prolyl cis/trans isomerase
- FKBP
- FK506 binding protein
- PP
- protein phosphatase
- pNPP
- p-nitrophenyl phosphate
- IL-2
- interleukin-2
- PBS
- phosphate-buffered saline
- PBMC
- peripheral blood mononuclear cells
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1.Clipstone N. A., Crabtree G. R. (1992) Nature 357, 695–697 [DOI] [PubMed] [Google Scholar]
- 2.Clipstone N. A., Crabtree G. R. (1993) Ann. N. Y. Acad. Sci. 696, 20–30 [DOI] [PubMed] [Google Scholar]
- 3.Schreiber S. L., Crabtree G. R. (1992) Immunol. Today 13, 136–142 [DOI] [PubMed] [Google Scholar]
- 4.Fischer G. (1994) Angew. Chem. Int. Ed. Engl. 33, 1415–1436 [Google Scholar]
- 5.Soldin S. J., Murthy J. N., Donnelly J. G., Chen Y., Goodyear N. (1993) Ther. Drug Monit. 15, 468–471 [DOI] [PubMed] [Google Scholar]
- 6.Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. (1989) Nature 337, 476–478 [DOI] [PubMed] [Google Scholar]
- 7.Hows J. M., Chipping P. M., Fairhead S., Smith J., Baughan A., Gordon-Smith E. C. (1983) Br. J. Haematol. 54, 69–78 [DOI] [PubMed] [Google Scholar]
- 8.Klintmalm G., Bohman S. O., Sundelin B., Wilczek H. (1984) Lancet 2, 950–954 [DOI] [PubMed] [Google Scholar]
- 9.Reis F., Parada B., Teixeira de Lemos E., Garrido P., Dias A., Piloto N., Baptista S., Sereno J., Eufrásio P., Costa E., Rocha-Pereira P., Santos-Silva A., Figueiredo A., Mota A., Teixeira F. (2009) Transplant. Proc. 41, 868–873 [DOI] [PubMed] [Google Scholar]
- 10.Shah A. K. (1999) Clin. Neuropharmacol. 22, 67–73 [DOI] [PubMed] [Google Scholar]
- 11.Baumgrass R., Zhang Y., Erdmann F., Thiel A., Weiwad M., Radbruch A., Fischer G. (2004) J. Biol. Chem. 279, 2470–2479 [DOI] [PubMed] [Google Scholar]
- 12.Fuentes J. J., Genescà L., Kingsbury T. J., Cunningham K. W., Pérez-Riba M., Estivill X., de la Luna S. (2000) Hum. Mol. Genet. 9, 1681–1690 [DOI] [PubMed] [Google Scholar]
- 13.Sun L., Youn H. D., Loh C., Stolow M., He W., Liu J. O. (1998) Immunity 8, 703–711 [DOI] [PubMed] [Google Scholar]
- 14.Lai M. M., Burnett P. E., Wolosker H., Blackshaw S., Snyder S. H. (1998) J. Biol. Chem. 273, 18325–18331 [DOI] [PubMed] [Google Scholar]
- 15.Lin X., Sikkink R. A., Rusnak F., Barber D. L. (1999) J. Biol. Chem. 274, 36125–36131 [DOI] [PubMed] [Google Scholar]
- 16.Pan F., Sun L., Kardian D. B., Whartenby K. A., Pardoll D. M., Liu J. O. (2007) Nature 445, 433–436 [DOI] [PubMed] [Google Scholar]
- 17.Coghlan V. M., Perrino B. A., Howard M., Langeberg L. K., Hicks J. B., Gallatin W. M., Scott J. D. (1995) Science 267, 108–111 [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto Y., Perrino B. A., Soderling T. R. (1990) J. Biol. Chem. 265, 1924–1927 [PubMed] [Google Scholar]
- 19.Miskin J. E., Abrams C. C., Goatley L. C., Dixon L. K. (1998) Science 281, 562–565 [DOI] [PubMed] [Google Scholar]
- 20.Bialojan C., Takai A. (1988) Biochem. J. 256, 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohse D. L., Denu J. M., Dixon J. E. (1995) Structure 3, 987–990 [DOI] [PubMed] [Google Scholar]
- 22.Enz A., Zenke G., PomboVillar E. (1997) Bioorg. Med. Chem. Lett. 7, 2513–2518 [Google Scholar]
- 23.Enz A., Pombo-Villar E. (1997) Biochem. Pharmacol. 54, 321–323 [DOI] [PubMed] [Google Scholar]
- 24.Martin B. L. (1998) Biochem. Pharmacol. 56, 483–488 [DOI] [PubMed] [Google Scholar]
- 25.Gualberto A., Marquez G., Carballo M., Youngblood G. L., Hunt S. W., 3rd, Baldwin A. S., Sobrino F. (1998) J. Biol. Chem. 273, 7088–7093 [DOI] [PubMed] [Google Scholar]
- 26.Brill G. M., Premachandran U., Karwowski J. P., Henry R., Cwik D. K., Traphagen L. M., Humphrey P. E., Jackson M., Clement J. J., Burres N. S., Kadam S., Chen R. H., McAlpine J. B. (1996) J. Antibiot. 49, 124–128 [DOI] [PubMed] [Google Scholar]
- 27.Baumgrass R., Weiwad M., Erdmann F., Liu J. O., Wunderlich D., Grabley S., Fischer G. (2001) J. Biol. Chem. 276, 47914–47921 [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Zhou C. L., Lei H., Zhang S. D., Zheng J., Wei Q. (2008) IUBMB Life 60, 549–554 [DOI] [PubMed] [Google Scholar]
- 29.Mondragon A., Griffith E. C., Sun L., Xiong F., Armstrong C., Liu J. O. (1997) Biochemistry 36, 4934–4942 [DOI] [PubMed] [Google Scholar]
- 30.Tung H. Y., Alemany S., Cohen P. (1985) Eur. J. Biochem. 148, 253–263 [DOI] [PubMed] [Google Scholar]
- 31.Tradler T., Stoller G., Rücknagel K. P., Schierhorn A., Rahfeld J. U., Fischer G. (1997) FEBS Lett. 407, 184–190 [DOI] [PubMed] [Google Scholar]
- 32.Schutkowski M., Bernhardt A., Zhou X. Z., Shen M., Reimer U., Rahfeld J. U., Lu K. P., Fischer G. (1998) Biochemistry 37, 5566–5575 [DOI] [PubMed] [Google Scholar]
- 33.Nakayama G. R., Nova M. P., Parandoosh Z. (1998) J. Biomol. Screen. 3, 43–48 [Google Scholar]
- 34.Sullivan E., Hemsley P., Pickard A. (1997) J. Biomol. Screen. 2, 19–23 [Google Scholar]
- 35.Kilka S., Erdmann F., Migdoll A., Fischer G., Weiwad M. (2009) Biochemistry 48, 1900–1910 [DOI] [PubMed] [Google Scholar]
- 36.Kuzmic P. (1996) Anal. Biochem. 237, 260–273 [DOI] [PubMed] [Google Scholar]
- 37.Iwatani Y., Amino N., Mori H., Asari S., Ina K., Ennyu K., Miyai K. (1982) J. Immunol. Methods 54, 31–42 [DOI] [PubMed] [Google Scholar]
- 38.Carruthers N. J., Dowd M. K., Stemmer P. M. (2006) FASEB J. 20, A1123–1124 [Google Scholar]
- 39.Cornish-Bowden A. (1974) Biochem. J. 137, 143–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer G., Aumüller T. (2003) Rev. Physiol. Biochem. Pharmacol. 148, 105–150 [DOI] [PubMed] [Google Scholar]
- 41.Jain J., McCaffrey P. G., Miner Z., Kerppola T. K., Lambert J. N., Verdine G. L., Curran T., Rao A. (1993) Nature 365, 352–355 [DOI] [PubMed] [Google Scholar]
- 42.Thiel A., Radbruch A. (1999) Arthritis Res. 1, 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richter A., Löhning M., Radbruch A. (1999) J. Exp. Med. 190, 1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciancio G., Burke G. W., Miller J. (2000) Expert Opin. Pharmacother. 1, 1307–1330 [DOI] [PubMed] [Google Scholar]
- 45.Rego A., Vargas R., Suarez K. R., Foegh M. L., Ramwell P. W. (1990) J. Pharmacol. Exp. Ther. 254, 799–808 [PubMed] [Google Scholar]
- 46.Moore R., Hernandez D., Valantine H. (2001) Drug Saf. 24, 755–766 [DOI] [PubMed] [Google Scholar]
- 47.Bechstein W. O. (2000) Transpl. Int. 13, 313–326 [DOI] [PubMed] [Google Scholar]
- 48.Jindal R. M., Sidner R. A., Milgrom M. L. (1997) Drug Saf. 16, 242–257 [DOI] [PubMed] [Google Scholar]
- 49.Hong F., Lee J., Piao Y. J., Jae Y. K., Kim Y. J., Oh C., Seo J. S., Yun Y. S., Yang C. W., Ha J., Kim S. S. (2004) Biochem. Biophys. Res. Commun. 316, 1073–1080 [DOI] [PubMed] [Google Scholar]
- 50.Hong F., Lee J., Song J. W., Lee S. J., Ahn H., Cho J. J., Ha J., Kim S. S. (2002) FASEB J. 16, 1633–1635 [DOI] [PubMed] [Google Scholar]
- 51.Caramelo C., Alvarez-Arroyo M. V., Yagüe S., Suzuki Y., Castilla M. A., Velasco L., Gonzalez-Pacheco F. R., Tejedor A. (2004) Nephrol. Dial. Transplant. 19, 285–288 [DOI] [PubMed] [Google Scholar]
- 52.Kolár F., Papousek F., MacNaughton C., Pelouch V., Milerová M., Korecky B. (1996) Mol. Cell. Biochem. 163–164, 253–260 [DOI] [PubMed] [Google Scholar]
- 53.Arora P. D., Silvestri L., Ganss B., Sodek J., McCulloch C. A. (2001) J. Biol. Chem. 276, 14100–14109 [DOI] [PubMed] [Google Scholar]
- 54.Cirillo R., Renzetti A. R., Cucchi P., Guelfi M., Salimbeni A., Caliari S., Castellucci A., Evangelista S., Subissi A., Giachetti A. (1995) Br. J. Pharmacol. 114, 1117–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mokrosz M. J., Strekowski L., Kozak W. X., Duszynska B., Bojarski A. J., Klodzinska A., Czarny A., Cegla M. T., Deren-Wesooek A., Chojnacka-Wojcik E. (1995) Arch. Pharm. (Weinheim) 328, 659–666 [DOI] [PubMed] [Google Scholar]
- 56.Conklyn M., Andresen C., Changelian P., Kudlacz E. (2004) J. Leukoc. Biol. 76, 1248–1255 [DOI] [PubMed] [Google Scholar]
- 57.Ban M., Taguchi H., Katsushima T., Aoki S., Watanabe A. (1998) Bioorg. Med. Chem. 6, 1057–1067 [DOI] [PubMed] [Google Scholar]
- 58.Quintela J. M., Peinador C., Veiga C., González L., Botana L. M., Alfonso A., Riguera R. (1998) Bioorg. Med. Chem. 6, 1911–1925 [DOI] [PubMed] [Google Scholar]
- 59.Mai A., Artico M., Ragno R., Sbardella G., Massa S., Musiu C., Mura M., Marturana F., Cadeddu A., Maga G., La Colla P. (2005) Bioorg. Med. Chem. 13, 2065–2077 [DOI] [PubMed] [Google Scholar]
- 60.Nitta Y., Miyamoto T., Nebashi T., Yoneda F. (1965) Chem. Pharm. Bull. 13, 901–906 [DOI] [PubMed] [Google Scholar]
- 61.Wang W., Ho W. C., Dicker D. T., MacKinnon C., Winkler J. D., Marmorstein R., El-Deiry W. S. (2005) Cancer Biol. Ther. 4, 893–898 [DOI] [PubMed] [Google Scholar]
- 62.Długosz A., Duś D. (1996) Farmaco 51, 367–374 [PubMed] [Google Scholar]
- 63.Barford D. (1996) Trends Biochem. Sci. 21, 407–412 [DOI] [PubMed] [Google Scholar]
- 64.Clipstone N. A., Fiorentino D. F., Crabtree G. R. (1994) J. Biol. Chem. 269, 26431–26437 [PubMed] [Google Scholar]
- 65.Okamura H., Aramburu J., García-Rodríguez C., Viola J. P., Raghavan A., Tahiliani M., Zhang X., Qin J., Hogan P. G., Rao A. (2000) Mol. Cell 6, 539–550 [DOI] [PubMed] [Google Scholar]