Abstract
Farnesyl pyrophosphate (FPP), a key intermediate in the mevalonate pathway and protein farnesylation, can act as an agonist for several nuclear hormone receptors. Here we show a novel mechanism by which FPP inhibits wound healing acting as an agonist for glucocorticoid receptor (GR). Elevation of endogenous FPP by the squalene synthetase inhibitor zaragozic acid A (ZGA) or addition of FPP to the cell culture medium results in activation and nuclear translocation of the GR, a known wound healing inhibitor. We used functional studies to evaluate the effects of FPP on wound healing. Both FPP and ZGA inhibited keratinocyte migration and epithelialization in vitro and ex vivo. These effects were independent of farnesylation and indicate that modulation of FPP levels in skin may be beneficial for wound healing. FPP inhibition of keratinocyte migration and wound healing proceeds, in part, by repression of the keratin 6 gene. Furthermore, we show that the 3-hydroxy-3-methylglutaryl-CoA-reductase inhibitor mevastatin, which blocks FPP formation, not only promotes epithelialization in acute wounds but also reverses the effect of ZGA on activation of the GR and inhibition of epithelialization. We conclude that FPP inhibits wound healing by acting as a GR agonist. Of special interest is that FPP is naturally present in cells prior to glucocorticoid synthesis and that FPP levels can be further altered by the statins. Therefore, our findings may provide a better understanding of the pleiotropic effects of statins as well as molecular mechanisms by which they may accelerate wound healing.
Keywords: Cell/Epithelial, Diseases, Hormones/Steroid, Lipid/Cholesterol, Receptors/Steroid/Thyroid, Tissue/Organ Systems/Skin, Statins, Wound Healing
Introduction
Cutaneous wound healing is a complex biological process that is mediated through a network of signaling pathways that coordinate multiple cellular processes including migration and proliferation, ultimately leading to barrier restoration (1, 2). Keratinocytes are biologically equipped to maintain the integrity of the epidermal barrier (2, 3). Restoration of the epidermis through epithelialization is an important component of wound healing and is often used as its defining parameter. Keratinocytes are the first cells to respond to injury, and after the epidermal barrier is broken they become activated and express keratins 6, 16, and 17 (4, 5). Glucocorticoids (GCs)2 are known inhibitors of wound healing (6). We have previously shown that GCs inhibit wound healing and epithelialization through modulation of diverse physiological processes including metabolism, migration, cell proliferation, differentiation, and inflammation (7). The actions of GCs are mediated by glucocorticoid receptor (GR). Binding of GCs to the ligand-binding domain (LBD) of GR elicits a conformational change, leading to dimerization and nuclear translocation of the ligand-receptor complex. In the nucleus GR interacts with glucocorticoid response elements (GRE) on promoters of regulated genes and recruits various co-activators or co-repressors to regulate expression of target genes (8). We previously reported that GC-mediated inhibition of epithelialization occurs through a complex molecular mechanism that involves four GR monomers, the arginine methyltransferase CARM1, and β-catenin as co-repressors in the context of the keratin 6/16 (K6/16) promoters (7, 9–11). In addition, in a recent study we have shown that farnesyl pyrophosphate (FPP) can bind to and activate a subset of nuclear hormone receptors including GR (12). This prior study was limited to an analysis of the effect of activation of a Gal4-GR-LBD chimera using transient transfection. However, the effectiveness of FPP to target endogenous promoters via GR as well as functional impact and biological consequences of GR-FPP interaction has never been studied.
FPP is considered a branch point intermediate in the mevalonate pathway, essential for synthesis of cholesterol and isoprenylated cellular metabolites. Because of the importance of sterols in barrier formation, the skin epidermis is a major site of cholesterol biosynthesis (13), and the mevalonate pathway has a central role in this process. This pathway involves the formation of mevalonic acid by HMG-CoA reductase (rate-limiting step), which is then converted to geranyl-PP, a precursor of FPP. FPP has two fates: it is converted to squalene, a precursor of cholesterol, or is involved in the farnesylation of a variety of proteins including small GTP-binding proteins like Rho, Ras, and Rac (14–16). Interestingly, studies have shown that barrier disruption and release of different growth factors and hormones can affect the activity of HMG-CoA reductase (17, 18). The mevalonate pathway is the target for statins, which act through inhibition of HMG-CoA reductase. Although inhibition of cholesterol synthesis and reduction in lipid levels are clinically important uses of the statins, they also show additional cholesterol-independent effects. These cholesterol-independent pleiotropic actions of statins involve immunomodulatory effects, decrease of oxidative stress, anabolic effects on bone, and stimulation of fracture healing (19–22) and wound healing (23–25).
The molecular mechanisms underlying the pleiotropic effects of statins are not well understood. In addition to effects on cholesterol synthesis, statins can reduce the activity of the small GTP-binding proteins Rho, Ras, and Rac, where proper membrane localization and function are dependent on isoprenylation (26). However, the finding that FPP can act as an agonist for several nuclear hormone receptors including GR may explain, in part, the pleiotropic effects of statins as well as certain actions of farnesylation inhibitors in cells (12).
In the current study we show that FPP, by acting as a ligand for GR, mediates inhibition of keratinocyte migration, epithelialization, and wound healing. We found that elevation of endogenous FPP by the squalene synthetase inhibitor zaragozic acid A (ZGA) (27, 28) or addition of FPP to the media of cells activates GC signaling pathways in primary human epidermal keratinocytes (HEK), leading to inhibition of keratinocyte migration and inhibition of epithelialization and wound closure. These effects are independent of isoprenylation. Furthermore, we show that mevastatin not only reverses inhibition of epithelialization by ZGA or FPP but also enhances the epithelialization rate in acute wounds. We show that the inhibitory effects of FPP on migration and epithelialization are mediated, in part, by FPP-GR-mediated repression of the K6 promoter. In summary, we have found that FPP, an intermediate product of the mevalonate pathway, can inhibit wound healing by acting as an agonist for GR ex vivo and in vitro. Identification of FPP as an inhibitor of wound healing may provide new therapeutic avenues for treatment of wound healing disorders.
EXPERIMENTAL PROCEDURES
Human Skin Organ Culture Wound Model and Histology
Specimens of normal human skin were obtained from reduction surgery according to an approved Institutional Research Board protocol. Wounds were created using 3-mm punch biopsies through the reticular dermis as previously described (29). They were maintained at the air-liquid interface using Dulbecco's modified Eagle's medium (Invitrogen) with antibiotics and antimycotics and stripped bovine serum. The wounds were left untreated or treated with either dexamethasone (DEX) (1 μm), FPP (10 μm), mevalonate (10 μm), or ZGA (50 μm) (all from Sigma) as indicated. Four days after the treatment, the tissues were fixed and processed for paraffin embedding. Seven-μm sections were cut and stained with hematoxilin and eosin. Staining was analyzed using a Nikon Eclipse E800 microscope, and the digital images were collected using SPOT camera advanced program. The wounds were quantified by planimetry as described previously (9, 30).
Immunocytochemistry and Immunohistochemistry
HEK were grown on coverslips to 70% confluence. The cells were incubated for 24 h in a basal serum-free medium custom made without hydrocortisone (Invitrogen). The cells were then incubated with the following concentrations for 24 h alone or in the combination indicated in the figures: DEX (1 μm), FPP (10 μm), ZGA (50 μm), and mevastatin (10 μm). The cells were then fixed in acetone-methanol (1:1) for 2 min, permeabilized with 0.1% Triton X-100 for 10 min and incubated overnight at 4 °C with anti-Ser(P)211 GR antibody (gift from Dr. M. Garabedian, New York University School of Medicine) (31) and visualized using a secondary fluorescein (AlexaFluor 488) anti-rabbit antibody (Invitrogen). The incubated wounded tissue was fixed in 10% formalin overnight and embedded in paraffin.
Seven-μm-thick sections were dewaxed in xylene, rehydrated, and washed with 1× phosphate-buffered saline (PBS). For antigen retrieval, paraffin sections were heated in a 95 °C water bath in target retrieval solution (DAKO Corporation, Carpinteria, CA) and washed. The sections were blocked with 5% bovine serum albumin (Sigma) in 1× PBS for 30 min. Incubation with antibody against K6 (Gift from Dr. P. Coulombe, Johns Hopkins University) (32) was carried out in 5% bovine serum albumin overnight at 4 °C. The slides were then rinsed in PBS and incubated with Alexa Fluor 488-conjugated goat anti-rabbit antibody (Invitrogen) for 1 h at room temperature. All of the coverslips and slides were mounted with mounting medium containing propidium iodide (Vector Laboratories, Burlingame, CA) to visualize the cell nucleus.
Keratinocyte Migration Assay and Scratch Test
Primary HEK were grown to 80% confluence as described above. Twenty-four hours before the experiment, the cells were switched to the basal medium described above. Prior to the study, the cells were treated with 8 μg/ml mitomycin C (ICN, Irvine, CA) for 1 h and washed with basal media. Scratches were then performed as described previously (9). Keratinocytes were incubated with or without DEX (1 μm), epidermal growth factor (EGF) (25 ng/ml) (Invitrogen), ZGA (50 μm), EGF and ZGA simultaneously, B581 (0.7 μm), or RU486 (10 μm) as indicated for 48 h. The cells were photographed immediately after introducing the scratch. After 48 h, the same fields were rephotographed, and cell migration was quantified as previously described (9, 10). Fifteen measurements were taken for each experimental condition, and the distance the cells migrated into the scratch area was quantified using Sigma Scan 5.0 software (Systat Software Inc., San Jose, CA). Three images were analyzed per condition, per time point, and the averages and standard deviations were calculated.
Western Blots
Extracts for immunoblotting were prepared from a subconfluent 10-cm plate of normal HEK treated with DEX (1 μm), FPP (10 μm), RU486 (10 μm), co-treatment DEX/RU486, FPP/RU486, or an equal volume of ethanol vehicle 4 h prior to lysis. The cells were placed on ice, washed twice with PBS, and lysed in 0.5 ml of modified radioimmune precipitation assay buffer containing 50 mm HEPES, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm NaF, 1% Triton X-100, 10% glycerol, and additional protease and phosphatase inhibitors (1 mm phenylmethylsulfonyl fluoride, 20 mm glycerophosphate, 8 mm sodium pyrophosphate, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, and 1 μg/ml aprotinin (Roche Applied Science) through fine needle aspiration. The lysates were centrifuged at 12,000 rpm for 15 min at 4 °C. The soluble supernatants were normalized for total protein concentration using the Bradford protein assay, and the samples were stored at −20 °C. The cell extracts were boiled for 5 min in Laemmli sample buffer, separated by 10% SDS-PAGE, and transferred to nitrocellulose membrane (VWR, Batavia, IL) on 100 V for 1 h in Tris/glycine transfer buffer. The membranes were blocked for 30 min in 5% bovine serum albumin in blocking solution (TBS, pH 7.4) at room temperature and then incubated in blocking solution with primary antibody at 4 °C overnight using 1:1000 of serum for anti-c211 antibody. The membranes were washed three times for 5 min with TBS and 0.1% Triton X-100 and twice with TBS and incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibody (Santa Cruz). The blots were then washed three times for 5 min with TBS and 0.1% Triton X-100 and developed using Super Signal West Pico Chemiluminescent substrate (Pierce) and exposed on x-ray film (Eastman Kodak Co. Bio Max MR-Film) according to the manufacturer's instructions. For loading control we used anti-GAPDH antibody (Santa Cruz). Western blot quantification was done using Total Lab Program (Non-linear Dynamics Inc.).
Plasmids and Transient Transfections
pK6CAT has been described previously (9, 11), and a GRE-CAT reporter that is stimulated by GC was a gift from Dr. P. Chambon. Normal HEKs were split into six-well plates at 1 × 105 cells/well and grown to 60% confluence. Twenty-four h prior to transfection, the cells were washed and transferred to basal serum-free medium custom made without hydrocortisone (Invitrogen). Three μg of plasmid DNA were transfected using FuGENE 6 reagent (Roche Applied Science) following the commercial protocol. The cells were then incubated for 48 h in the presence or absence of DEX (1 μm), FPP (10 μm), ZGA (50 μm), or RU486 (10 μm) (Sigma). Cell extracts to be used for CAT assay were normalized by total protein determined by protein assay (Bio-Rad). 30 μg of protein were used for each reaction. CAT assays were performed using FastCat (Molecular Probes, Eugene, OR) following a commercial protocol. CAT assay values were quantified by Fluor Imager 575 (Molecular Dynamics, Piscataway, NJ). All of the results were generated from three independent experiments and were performed in duplicate.
Chromatin Immunoprecipitation Assays
Chromatin immunoprecipitation assays were performed with HEK untreated or treated with DEX (1 μm) or FPP (10 μm) for 24 h, as described (33, 34). Keratinocytes were fixed using cross-linking buffer (50 mm HEPES, pH 8.0, 1% formaldehyde, 1 mm EDTA, 0.5 mm EGTA, and 100 mm NaCl) for 10 min at room temperature. The cross-link was quenched using 0.125 mol of glycine, and the cells were rinsed twice with cold PBS, collected by centrifugation, resuspended, and nutated in buffer I (50 mm HEPES, pH 8.0, 1 mm EDTA, 10% glycerol, 5% Nonidet P-40, and 0.25% Triton X-100) for 10 min at 4 C. The nuclei were pelleted by centrifugation and then washed in Buffer II (10 mm Tris, pH 8.0, 1 mm EDTA, 0.5 mm EGTA, and 200 mm NaCl) for 10 min at room temperature. The nuclei were pelleted by centrifugation and resuspended in radioimmune precipitation assay buffer (10 mm Tris, pH 8.0, 1 mm EDTA, 0.5 mm EGTA, 140 mm NaCl, 5% glycerol, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 1% Triton X-100). The samples were sonicated on ice nine times for 20 s each time at 60-s intervals. Cell debris was removed by centrifugation at 12,000 × g for 10 min. Protein-DNA complexes were immunoprecipitated using 5 μg of anti-GR (N499) antibody (33), which was a gift from Dr. I. Rogatsky, or 2 μg of rabbit IgG (Santa Cruz). Immunoprecipitated purified chromosomal DNA was used for PCR amplification with the following primers: K6 forward, ATGCAGGTGTGAATCTCACTATTTGTAAAGCC; and K6 reverse, AGGAATCGGACTCCAGTAGCAGC. One percent of the input chromatin was processed and used for PCR amplification in parallel. PCRs were carried out for 35 cycles, and the products were resolved on 2% agarose gels and visualized by ethidium bromide staining.
RESULTS
FPP Activates GR in Primary Keratinocytes
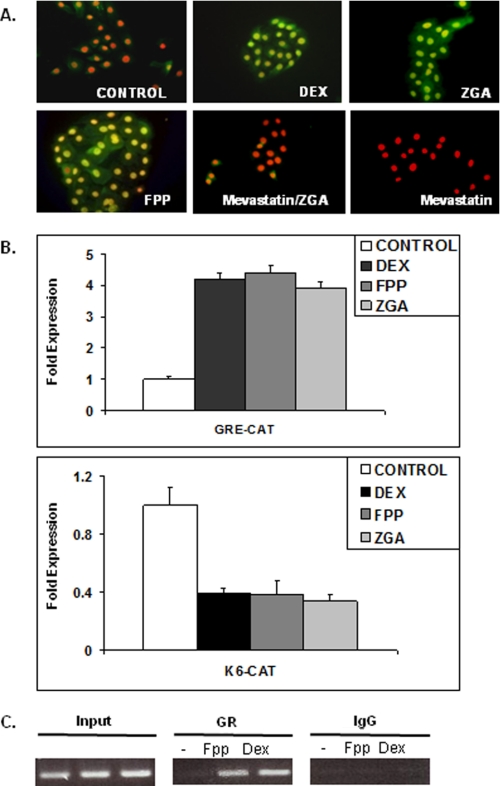
FPP can enter cells in culture and act as a ligand for GR (12). To further confirm that FPP activates GR in human keratinocytes, we used immunocytochemistry to determine the localization of ligand-activated GR. It was previously shown that phosphorylation of Ser211 is fully dependent on the binding of an agonist DEX to GR (31) and that the presence of Ser(P)211-GR in the cell nucleus is associated with the DEX-mediated nuclear translocation of total cytosolic GR (Fig. 1A). Also, the transcriptional activity of GR correlated with the amount of Ser(P)211-GR, suggesting that Ser211 phosphorylation is a biomarker for ligand activated GR in vivo (31, 35). Primary human keratinocytes were incubated with either DEX, ZGA (which elevates FPP in cells by blocking its conversion to squalene) (12, 27, 28), or FPP, which were added to the medium for 24 h. Control cells received no additions or just mevastatin. We also pretreated cells with mevastatin for 2 h and then incubated the cells with ZGA. Localization and activation of GR was determined by using anti-Ser(P)211 GR antibody that recognizes ligand-induced phosphorylation at Ser211 (31). Weak Ser(P)211 GR immunoreactivity was observed in the cytoplasm and nucleus of untreated cells (Fig. 1A). As expected, prominent Ser(P)211 GR staining was evident in the nuclei of cells treated with DEX (Fig. 1A). Similarly, prominent Ser(P)211 GR staining was detected in the nuclei of cells treated with either ZGA or FPP (Fig. 1A). Pretreatment with mevastatin, which blocks formation of FPP, abolished GR activation by ZGA (Fig. 1A). These findings indicate that FPP can act as a GR agonist, which results in GR activation and nuclear localization in primary human keratinocytes.
FIGURE 1.
FPP mediates activation and function of GR in human keratinocytes. A, FPP activates GR. Primary human keratinocytes stained with anti-Ser(P)211 GR antibody reveal localization and activation of GR. This antibody recognizes ligand-induced phosphorylation at Ser211. The nuclei were visualized by propidium iodide staining. Weak Ser(P)211 GR immunoreactivity was observed in the cytoplasm and nuclei of untreated cells. In contrast, strong signal was evident in the nuclei of all treated cells: DEX, FPP, and ZGA, which elevates endogenous FPP levels. Mevastatin abolishes ZGA-induced activation of GR by preventing accumulation of endogenous FPP. B, FPP activates GR-mediated transcriptional regulation in keratinocytes. Transfection experiments of primary human keratinocytes with the K6-CAT and GRE-CAT reporters are shown. The data are presented as relative CAT activity, a measure of actual CAT activity normalized for total protein. The results show that DEX, ZGA, or FPP treatment lead to repression of the K6 promoter. Similar to DEX, FPP or ZGA also stimulated the GRE-CAT reporter. C, FPP targets the K6 promoter through GR. HEK were either untreated (−) or treated with 1 μm DEX or 10 μm FPP. Similar amounts of genomic DNA (Input) was used in each treatment. K6 promoter sequences were amplified by PCR after immunoprecipitation with anti-GR antibody or rabbit IgG.
FPP-GR Targets and Represses Expression of the K6 Promoter
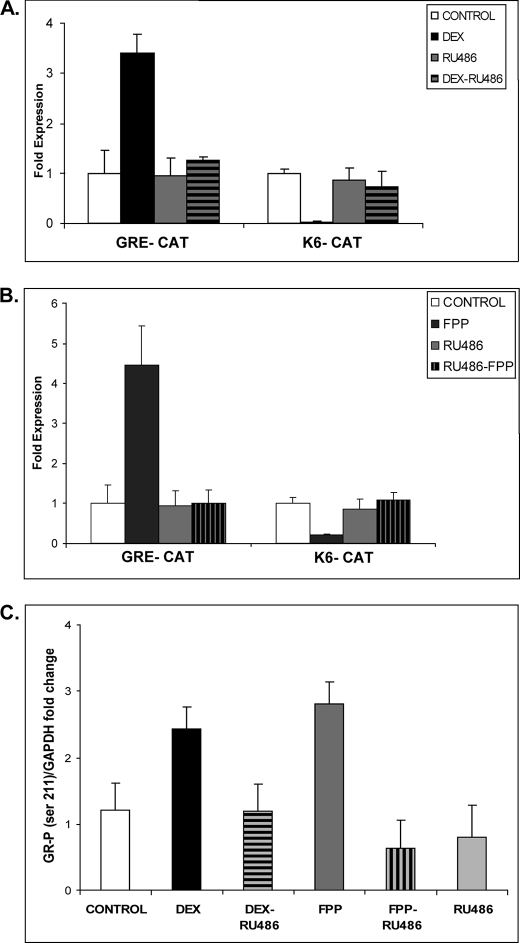
To determine whether FPP-mediated activation of GR leads to transcriptional regulation of target genes in keratinocytes, we utilized transient transfections and CAT assays. We have previously shown that GC through GR mediates repression of the K6 and K16 promoters (10, 11) Thus, we analyzed whether DEX, FPP, or ZGA affects K6 promoter activity in transfection experiments. We used a GRE-CAT reporter (containing a consensus glucocorticoid response element), which is stimulated by GC as a positive control. As expected from prior studies, we found that DEX stimulates the activity of GRE-CAT while it represses the activity of the K6 promoter (Fig. 1B). The addition of 10 μmol of RU486, a GR antagonist, to the cells inhibited stimulation of GRE-CAT by DEX or FPP and reversed the inhibition of the K6 promoter-CAT reporter, further indicating that the FPP response we are studying acts through GR (Fig. 2, A and B). Interestingly, treatment with either DEX or FPP led to increased levels of Ser(P)211-GR in HEK as observed with immunoblotting, and this effect was readily reversed with the addition of RU486 (Fig. 2C). To further confirm that FPP acts as a GR agonist in the context of the K6 promoter in vivo, we carried out chromatin immunoprecipitation assays using polyclonal antibodies to GR or IgG as a negative control and primers amplifying a 122-base pair PCR product from −143 to −21 relative to the K6 transcription start (10). The cells were incubated in the presence or absence of FPP for 24 h, and chromatin fragments containing identical amounts of total genomic DNA (input) were used for the immunoprecipitations (Fig. 1C). Normalized to control IgG, both DEX and FPP yielded an enrichment of the K6-containing fragment when antibody to GR was used for the immunoprecipitation (Fig. 1C). We concluded that FPP-GR binds to the K6-GRE and mediates its repression.
FIGURE 2.
FPP-activated GR is transcriptionally active. A and B, transfection experiments of HEK with the K6-CAT and GRE-CAT reporters are shown. The data are presented as relative CAT activity, a measure of actual CAT activity normalized to total protein. The results show that DEX and FPP treatment lead to repression of the K6 promoter and stimulation of the GRE-CAT reporter. The effect of both DEX and FPP was reversed with RU486 antagonist of GR. C, quantitative analysis of immunoblotting with anti-Ser(P)211 GR antibody normalized to GAPDH and plotted as fold change in GR phosphorylation. HEK were incubated in the presence or absence of 1 μm DEX, 10 μm FPP, 10 μm RU486, or a combination for 4 h. Both DEX and FPP induce an increase in Ser211 GR phosphorylation, which was reversed with RU486.
FPP Inhibits Keratinocyte Migration, Whereas Statins Reverse It
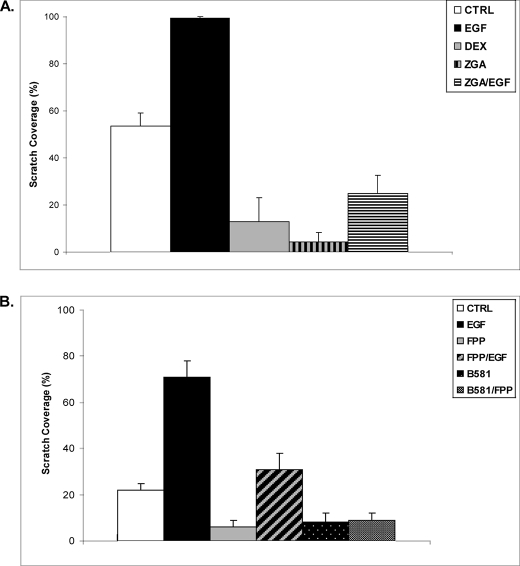
To establish the biological significance of these findings, we focused on wound healing because we have shown previously that the GC-GR complex targets the K6 promoter, inhibits keratinocyte migration, and blocks EGF-mediated stimulation of migration (10). To determine whether FPP exerts similar effects, we utilized the keratinocyte migration scratch assay. Primary human keratinocytes were “wounded” by a scratch and incubated with DEX, EGF, ZGA, or EGF/ZGA. Keratinocytes that received no addition served as a control. Keratinocyte migration was monitored for 48 h after the scratch, and cell migration was quantified (Fig. 3A). 48 h after the scratch, keratinocytes in control plate covered 54% of the scratched area. As expected, DEX blocked keratinocyte migration, reducing their migration to 13%, whereas keratinocytes incubated in the presence of EGF completely covered the scratch after 48 h (100% closure). ZGA treatment blocked keratinocyte migration, decreasing the extent of closure to 4%. Similarly, ZGA blocked EGF-stimulated keratinocyte migration, reducing it from 100% (EGF) to 25% (ZGA + EGF). We concluded that FPP inhibits keratinocyte migration, similarly to GCs. In addition, FPP dominantly blocks keratinocyte migration in the presence of EGF (Fig. 3B).
FIGURE 3.
FPP blocks keratinocyte migration. A, ZGA and DEX inhibit keratinocyte migration in the wound scratch assay. EGF treatment stimulated keratinocyte migration, and after 48 h cells covered the scratch completely. When present together, ZGA blocks EGF-stimulated keratinocyte migration. The average coverage of the scratch widths in percentage is presented by the histograms. B, HEK were wounded by scratch and incubated with or without, EGF, FPP, EGF + FPP, B581, or B581 + FPP. Keratinocyte migration was monitored for 48 h. FPP shows the same inhibitory effect on keratinocyte migration as ZGA. Interestingly, treatment either with B581 alone or with FPP had the same effect, indicating that enhanced protein farnesylation mediated through increased FPP in cells does not inhibit migration. CTRL, control.
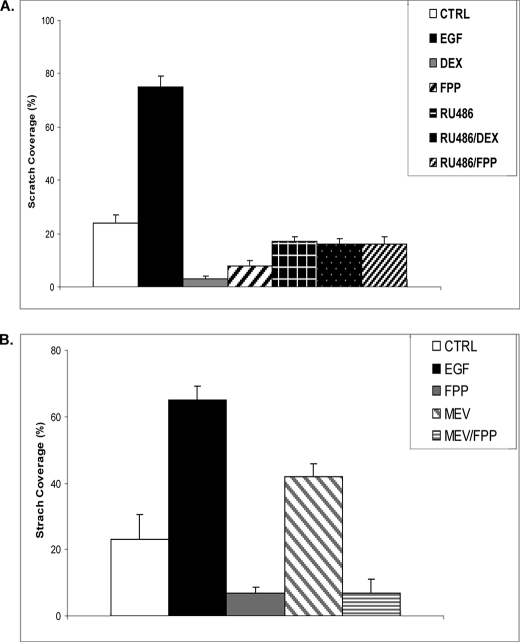
In cells two molecules of FPP condense to form squalene, a precursor of cholesterol. FPP is involved in the farnesylation of a variety of proteins including small GTPases such as Ras. We previously reported that ZGA or inhibitors of farnesylation (e.g. B581), which increases the levels of FPP in cells, leads to activation of several nuclear receptors (12). To test whether ZGA-mediated inhibition of migration occurred via an effect of FPP on GR, through enhanced farnesylation, or as a result of inhibition in sterol synthesis, we performed the study shown in Fig. 3B. Primary human keratinocytes were wounded by scratch and incubated with either EGF, FPP, EGF/FPP, B581, or B581/FPP, and migration was monitored and quantified after 48 h. As found with ZGA (Fig. 3A), FPP inhibited keratinocyte migration. Interestingly, treatment with B581 by itself or with FPP had the same inhibitory effect, supporting the notion that FPP-mediated inhibition of keratinocytes migration is due to activation of GR rather than to enhanced protein farnesylation. To confirm that FPP inhibition of HEK migration is exerted through activation of GR, we performed the additional migration assay shown in Fig. 4A. HEK after scratch were incubated in presence or absence of DEX, EGF, FPP, DEX/RU486, or FPP/RU486. RU486 almost completely restored inhibition of HEK migration mediated by either DEX or FPP. Interestingly, mevastatin treatment enhanced HEK migration 2-fold compared with control, and co-treatment with FPP not only abolished this stimulatory effect but further inhibited migration in a similar manner to FPP (Fig. 4B). These results support the notion that FPP inhibits HEK migration via GR and that statins may prevent this inhibition by blocking the endogenous FPP formation.
FIGURE 4.
FPP inhibits HEK migration, whereas mevastatin promotes it. A, DEX (1 μm) and FPP (10 μm) inhibit keratinocyte migration in the wound scratch assay. When present together, RU486 (10 μm) reverses both DEX and FPP inhibition of keratinocyte migration. B, mevastatin (MEV, 10 μm) increases keratinocyte migration in the wound scratch assay. Simultaneous treatment with FPP (10 μm) abolishes the effect of mevastatin and leads to a similar inhibition of migration as a FPP treatment alone. EGF treatment served as a positive control (CTRL) in both experiments. As expected, it stimulated keratinocyte migration. The average coverage of the scratch widths in percentage is presented by the histograms.
Statins Promote Wound Healing and Reverse FPP-mediated Inhibition of Epithelialization and Wound Closure
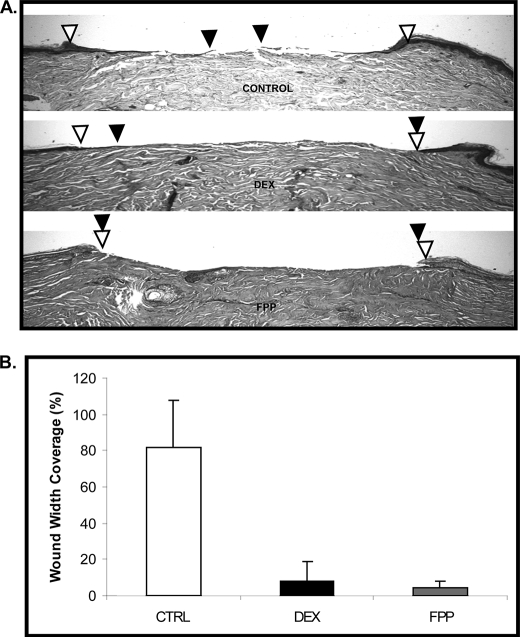
To test whether FPP-mediated inhibition of keratinocyte migration correlates with inhibition of epithelialization and wound healing, we utilized an established human skin organ culture wound model (29). Normal human skin was wounded using 3-mm punch biopsy, and the tissues were maintained at the air-liquid interface in the presence or absence of DEX or FPP. Four days after the treatment, organ cultures were processed for paraffin embedding and stained with hematoxilin and eosin for the evaluation of epithelialization. We observed epithelialization in untreated, control wounds as indicated by black arrows pointing at the migration front (Fig. 5A). As expected, topical treatment with DEX inhibited epithelialization, i.e. the wound edges remained almost at the same initial position 4 days after the treatment. Similarly, topical treatment with FPP completely inhibited epithelialization because the wound edges remained at the same position after 4 days of FPP treatment. The experiments were repeated three times in triplicates using human skin obtained from three different donors and quantified (Fig. 5B). We concluded that exogenously added FPP inhibits epithelialization during acute wound healing.
FIGURE 5.
FPP inhibits epidermal wound healing. A, FPP completely inhibited epithelialization in a human skin organ culture model. Topical DEX treatment delayed epithelialization when compared with control (CTRL) untreated skin. The open arrowheads indicate wound edges after initial wounding, whereas solid arrowheads point to the epithelialized edges of the migrating fronts 4 days after the wounding. B, the experiment shown in Fig. 5 was repeated three times in triplicate using human skin obtained from three different donors. Quantification of wound epithelialization using planimetry is shown.
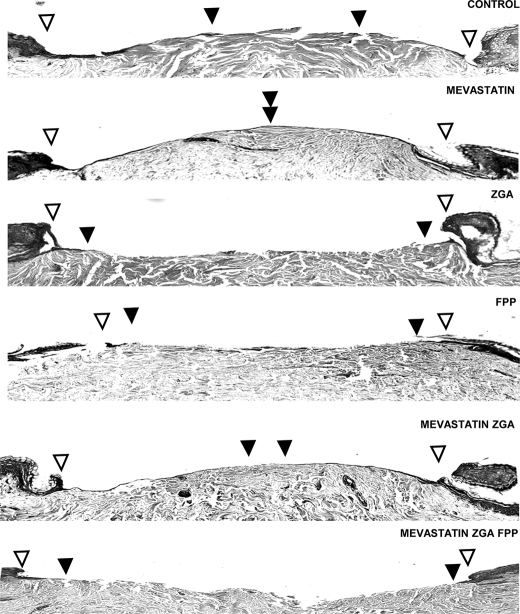
We examined next whether alteration in endogenous FPP levels affects wound healing. Normal human skin was subjected to 3-mm punch biopsy, and the tissues were maintained at the air-liquid interface and incubated with either mevastatin, ZGA, FPP, mevastatin/ZGA, or mevastatin/ZGA/FPP (Fig. 6). We observed epithelialization in untreated, control wounds as indicated by black arrowheads pointing at the migration front. Mevastatin-treated specimens, which would decrease the levels of endogenous FPP, significantly promoted epithelialization and wound closure as compared with the untreated wound. Topical treatment with ZGA completely inhibited epithelialization. This effect likely reflects the accumulation of endogenous FPP because: 1) co-treatment with mevastatin reversed the inhibitory effect of ZGA on wound healing and 2) addition of exogenous FPP to cells incubated with mevastatin + ZGA restored inhibition of epithelialization. This finding also indicates that the inhibitory effect of ZGA is not related to decreased production of sterols but rather results from an increase in FPP levels in cells.
FIGURE 6.
FPP inhibits epithelialization in skin organ culture, whereas mevastatin reverses this inhibition and promotes wound closure. Open arrowheads indicate wound edges after initial wounding, whereas solid arrowheads point at the epithelialized edges of the migrating fronts 4 days after wounding. Mevastatin improved epithelialization by decreasing endogenous levels of FPP. Both FPP and ZGA treatment completely inhibited epithelialization when compared with control untreated skin, whereas mevastatin reversed the inhibitory effect of ZGA.
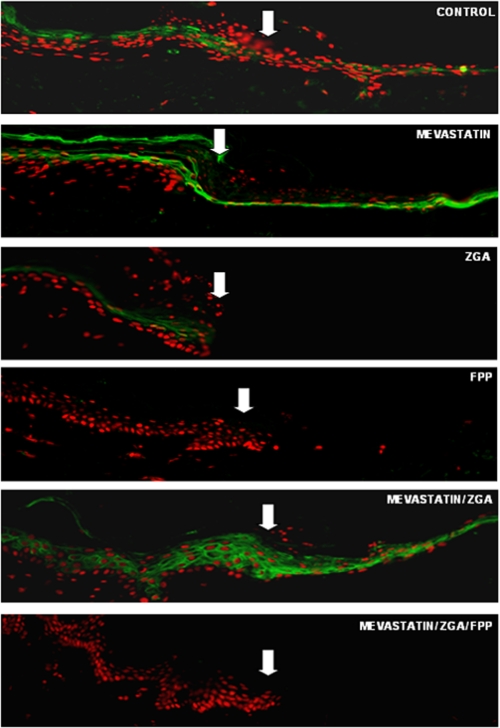
We have shown previously that GCs through GR inhibit keratinocyte migration and wound epithelialization in part by targeting expression of the early markers of wound healing, K6 and K16 (9, 10). In this study we have shown that both ZGA and FPP can act similarly to GC and repress K6 transcription. To test whether suppression of K6 participates in the inhibition of keratinocyte migration and epithelialization, acute wounds were maintained at the air-liquid interface in the absence or presence of either mevastatin, ZGA, FPP, mevastatin/ZGA, or mevastatin/ZGA/FPP. The tissues were harvested 4 days post-wounding, and sections were stained with keratin 6-specific antibody (Fig. 7). In control skin, K6 was present both at the wound margin and in epithelial tongue. Treatment with mevastatin induced expression of K6 at the wound edge and epithelial tongue, whereas FPP and ZGA not only inhibited wound healing, they also abolished expression of K6 at the wound margin. Co-treatment with mevastatin reversed the inhibitory effect of ZGA on K6 levels at the wound margin and in the epithelial tongue, whereas addition of exogenous FPP restored the inhibition. We conclude that decreasing FPP levels may be beneficial for wound healing and that, at appropriate concentrations, cholesterol synthesis inhibitors may promote epithelialization and wound closure.
FIGURE 7.
Altering endogenous levels of FPP modulates epithelialization through inhibition of K6, whereas mevastatin reverses it. Shown is immunolocalization of K6 (green) at the wound edge and epithelial tongue after injury to epidermis in the human skin organ culture model. The white arrows indicate the initial wound edges. Mevastatin induces the expression of K6 at the wound edge. Both ZGA and FPP reduce the levels of K6 at the wound margin and inhibit epithelialization. Mevastatin reverses the effect of ZGA because of an upstream block in mevalonate pathway. The addition of exogenous FPP restores inhibition of wound healing and K6 expression.
DISCUSSION
In the current study we report novel findings that may directly impact treatment of patients suffering from wound healing disorders. We found that FPP, a product of cholesterol synthesis pathway, inhibits epithelialization and wound closure by acting as GR agonist. Conversely, we show that statins promote wound healing. The finding that FPP inhibits keratinocyte migration and epithelialization introduces a new paradigm shift, because the activation of GR does not depend on the presence of glucocorticoids. These findings provide novel mechanisms of inhibition of wound healing and open new therapeutic approaches.
Several lines of evidence strongly support the notion that FPP inhibits wound healing by acting through GR rather than through an effect on protein farnesylation. First, incubation of cells with FPP leads to nuclear translocation of GR (Fig. 1A). Second, DEX and FPP similarly stimulate or inhibit gene expression depending on the gene promoter (Fig. 1B). Third, DEX or FPP incubation leads to the binding of GR on the K6 gene promoter as determined by chromatin immunoprecipitation assays (Fig. 1C). In addition, RU486 inhibits stimulation by GR (FPP or DEX) on a reporter containing a positive GRE and reverses the DEX- or FPP-mediated inhibition of a negatively regulated gene (K6) (Fig. 2). In a scratch assay, keratinocyte migration was similarly inhibited by DEX or by elevation of endogenous FPP (ZGA) (Fig. 3). FPP or B581, a protein farnesylation inhibitor that increases FPP levels in cells, similarly inhibited keratinocyte migration (Fig. 2B). Thus, it is unlikely that mevastatin acts to enhance keratinocyte migration through inhibition of protein farnesylation. Our studies do not support the notion that the effect of mevastatin on stimulating keratinocyte migration is through an effect of inhibiting cholesterol synthesis. Thus, both ZGA (which inhibits cholesterol synthesis but elevates FPP) and FPP (which would increase cholesterol synthesis) inhibit keratinocyte migration. Similar effects on epidermal wound healing with FPP, ZGA, DEX, and mevastatin were found in a human skin organ culture model (Figs. 5–7), which closely mimics wound healing in vivo. Taken together, our findings indicate that the stimulation of wound healing by mevastatin results from a decrease in cellular FPP levels and not from a decrease in cholesterol synthesis or protein farnesylation.
In vitro studies document that FPP can directly bind to and recruit co-activators to the GR LBD (12). However, these prior studies were limited to an analysis of the effect of activation of a Gal4-GR-LBD chimera using transient transfection. In this current study, we have extended our research to endogenous levels of GR to examine the effect of FPP on a fundamentally important biologic effect on the processes involved in wound healing in keratinocytes and skin. We have previously shown that GC-mediated inhibition of migration and epithelialization occurs in part through repression of K6/16, early markers of keratinocyte activation (7, 9–11). In this study we also show that FPP inhibits keratinocyte migration and epithelialization through a similar mechanism involving repression of K6. Both ZGA and FPP mimic GC inhibition of keratinocyte migration and epithelialization, and these effects appear to be independent of the synthesis of cholesterol and farnesylation.
Although the full biological implications of these findings remain to be elucidated, they raise a number of interesting possibilities. FPP is an important branch point intermediate in the mevalonate pathway, essential for synthesis of sterols and isoprenylated cellular metabolites. We have previously shown that FPP can act as a ligand for several nuclear receptors that may participate in mediating some of the pleiotropic effects of statins (12). One of the well documented pleiotropic effects is improved tissue repair (21, 22, 24, 25, 36–38). Simvastatin was also recently shown to improve wound healing in diabetic mice and their normoglycemic littermates (24). However, increased doses of statins could have completely opposite effects because of antiproliferative, antimigratory, and proapoptotic actions (23, 39–42). Similarly, we show here that topical application of mevastatin promotes wound closure. Interestingly, when a 10-fold higher concentration was used, it inhibited wound epithelialization (data not shown). Such effects of higher doses may originate from complete inhibition of sterol synthesis, which would influence cell membrane function and lead to a decrease in cell migration. Taken together, our findings indicate that FPP may be an important regulator of wound healing, and thus, modulation of endogenous concentrations by different physiologic states or drugs may control epithelialization and clinical outcome.
In the current study we found that increasing endogenous FPP levels leads to activation of the GR pathway and consequent inhibition of HEK migration and wound healing. Moreover, this effect was independent of modulation of isoprenylation and sterologenesis and could be reversed by mevastatin treatment. Of special interest, mevastatin alone was capable of enhancing epithelialization compared with control samples. Thus, it is possible that statin treatment could elicit beneficial effects on wound healing in humans. However, the efficacy of such therapy depends on whether sufficient levels of FPP occur in the skin in vivo or in pathological processes such as chronic wounds, to mediate effects through GR. It is well documented that growth factors, hormones, and barrier disruption can induce epidermal HMG-CoA reductase activity (18, 43, 44). Of further interest, a study of FPP levels in liver before and after ZGA administration indicates a basal level of ∼5 and 140 μm post-ZGA administration (27). In our study, the addition of just 10 μm FPP to cell culture medium is sufficient to activate GR and cause profound effects on keratinocyte migration. Therefore, decreasing the basal level of FPP in skin could be beneficial for wound healing in vivo. Further studies are necessary to address this question because topical application of statins may be safe and clinically useful as drugs for the treatment of patients with chronic refractory wounds that do not respond to standard modes of treatment.
Acknowledgments
We thank Dr. Michael Garabedian for the gift of the Ser(P)211 GR antibody, Dr. Pierre Coulombe for K6 antibody, and Dr. Inez Rogatsky for the gift of anti-GR (N499) antibody and helpful suggestions for the chromatin immunoprecipitations experiments. We also thank Dr. Madhu Bargava, Elizabeth Lebrun, and Jonathan Bourne for critical reading and helpful discussion.
This work was supported, in whole or in part, by National Institutes of Health Grant AR45974 (to M. T.-C.), NR08029 (to M. T.-C.), AG030673 (to M. T.-C.), and DK16636 (to H. H. S.). This work was also supported by the Entertainment Industry Foundation (to H. H. S.).
- GC
- glucocorticoid
- FPP
- farnesyl pyrophosphate
- GR
- glucocorticoid receptor
- ZGA
- zaragozic acid A
- HMG
- 3-hydroxy-3-methylglutaryl
- GRE
- glucocorticoid response element(s)
- HEK
- human epidermal keratinocytes
- DEX
- dexamethasone
- PBS
- phosphate-buffered saline
- EGF
- epidermal growth factor
- TBS
- Tris-buffered saline
- CAT
- chloramphenicol acetyltransferase
- K
- keratin.
REFERENCES
- 1.Brem H., Tomic-Canic M. (2007) J. Clin. Invest. 117, 1219–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pastar I., Stojadinovic O., Tomic-Canic M. (2008) Surg. Technol. Int. 17, 105–112 [PubMed] [Google Scholar]
- 3.Tomic-Canic M., Magnus S. A., Oscar M. A. (2004) in The Epidermis in Wound Healing (Rovee D. T., Maibach H. I. eds) pp. 25–57, CRC Press LLC, Boca Raton, FL [Google Scholar]
- 4.Tomic-Canic M., Komine M., Freedberg I. M., Blumenberg M. (1998) J. Dermatol. Sci. 17, 167–181 [DOI] [PubMed] [Google Scholar]
- 5.Freedberg I. M., Tomic-Canic M., Komine M., Blumenberg M. (2001) J. Invest. Dermatol. 116, 633–640 [DOI] [PubMed] [Google Scholar]
- 6.Wicke C., Halliday B., Allen D., Roche N. S., Scheuenstuhl H., Spencer M. M., Roberts A. B., Hunt T. K. (2000) Arch. Surg. 135, 1265–1270 [DOI] [PubMed] [Google Scholar]
- 7.Stojadinovic O., Lee B., Vouthounis C., Vukelic S., Pastar I., Blumenberg M., Brem H., Tomic-Canic M. (2007) J. Biol. Chem. 282, 4021–4034 [DOI] [PubMed] [Google Scholar]
- 8.Schaaf M. J., Cidlowski J. A. (2002) J. Steroid Biochem. Mol. Biol. 83, 37–48 [DOI] [PubMed] [Google Scholar]
- 9.Stojadinovic O., Brem H., Vouthounis C., Lee B., Fallon J., Stallcup M., Merchant A., Galiano R. D., Tomic-Canic M. (2005) Am. J. Pathol. 167, 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee B., Vouthounis C., Stojadinovic O., Brem H., Im M., Tomic-Canic M. (2005) J. Mol. Biol. 345, 1083–1097 [DOI] [PubMed] [Google Scholar]
- 11.Radoja N., Komine M., Jho S. H., Blumenberg M., Tomic-Canic M. (2000) Mol. Cell. Biol. 20, 4328–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das S., Schapira M., Tomic-Canic M., Goyanka R., Cardozo T., Samuels H. H. (2007) Mol. Endocrinol. 21, 2672–2686 [DOI] [PubMed] [Google Scholar]
- 13.Feingold K. R., Wiley M. H., Moser A. H., Lau D. T., Lear S. R., Siperstein M. D. (1982) J. Lab. Clin. Med. 100, 405–410 [PubMed] [Google Scholar]
- 14.Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. (1989) Cell 57, 1167–1177 [DOI] [PubMed] [Google Scholar]
- 15.Sheares B. T., White S. S., Molowa D. T., Chan K., Ding V. D., Kroon P. A., Bostedor R. G., Karkas J. D. (1989) Biochemistry 28, 8129–8135 [DOI] [PubMed] [Google Scholar]
- 16.Wolda S. L., Glomset J. A. (1988) J. Biol. Chem. 263, 5997–6000 [PubMed] [Google Scholar]
- 17.Proksch E., Elias P. M., Feingold K. R. (1990) J. Clin. Invest. 85, 874–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris I. R., Höppner H., Siefken W., Farrell A. M., Wittern K. P. (2000) J. Invest. Dermatol. 114, 83–87 [DOI] [PubMed] [Google Scholar]
- 19.Weber M. S., Zamvil S. S. (2008) Curr. Top Microbiol. Immunol. 318, 313–324 [DOI] [PubMed] [Google Scholar]
- 20.Lahera V., Goicoechea M., de Vinuesa S. G., Miana M., de las Heras N., Cachofeiro V., Luño J. (2007) Curr. Med. Chem. 14, 243–248 [DOI] [PubMed] [Google Scholar]
- 21.Bauer D. C. (2003) Osteoporos Int. 14, 273–282 [DOI] [PubMed] [Google Scholar]
- 22.Serin-Kilicoglu S., Erdemli E. (2007) J. Trauma 63, 187–191 [DOI] [PubMed] [Google Scholar]
- 23.Schiefelbein D., Goren I., Fisslthaler B., Schmidt H., Geisslinger G., Pfeilschifter J., Frank S. (2008) J. Biol. Chem. 283, 15479–15490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bitto A., Minutoli L., Altavilla D., Polito F., Fiumara T., Marini H., Galeano M., Calò M., Lo Cascio P., Bonaiuto M., Migliorato A., Caputi A. P., Squadrito F. (2008) Pharmacol. Res. 57, 159–169 [DOI] [PubMed] [Google Scholar]
- 25.Rego A. C., Araujo Filho I., Damasceno B. P., Egito E. S., Silveira I. A., Brandao-Neto J., Medeiros A. C. (2007) Acta Cir. Bras. 22, (Suppl. 1) 57–63 [DOI] [PubMed] [Google Scholar]
- 26.Liao J. K. (2005) Am. J. Cardiol. 96, 24F–33F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller R. K. (1996) Biochim. Biophys. Acta 1303, 169–179 [DOI] [PubMed] [Google Scholar]
- 28.Tong H., Holstein S. A., Hohl R. J. (2005) Anal. Biochem. 336, 51–59 [DOI] [PubMed] [Google Scholar]
- 29.Tomic-Canic M., Mamber S. W., Stojadinovic O., Lee B., Radoja N., McMichael J. (2007) Wound Repair Regen. 15, 71–79 [DOI] [PubMed] [Google Scholar]
- 30.Brem H., Tomic-Canic M., Entero H., Hanflik A. M., Wang V. M., Fallon J. T., Ehrlich H. P. (2007) Exp. Gerontol. 42, 523–531 [DOI] [PubMed] [Google Scholar]
- 31.Wang Z., Frederick J., Garabedian M. J. (2002) J. Biol. Chem. 277, 26573–26580 [DOI] [PubMed] [Google Scholar]
- 32.Paladini R. D., Takahashi K., Bravo N. S., Coulombe P. A. (1996) J. Cell Biol. 132, 381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogatsky I., Zarember K. A., Yamamoto K. R. (2001) EMBO J. 20, 6071–6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takayama S., Rogatsky I., Schwarcz L. E., Darimont B. D. (2006) J. Biol. Chem. 281, 17856–17863 [DOI] [PubMed] [Google Scholar]
- 35.Miller A. L., Webb M. S., Copik A. J., Wang Y., Johnson B. H., Kumar R., Thompson E. B. (2005) Mol. Endocrinol. 19, 1569–1583 [DOI] [PubMed] [Google Scholar]
- 36.Shao H., Tan Y., Eton D., Yang Z., Uberti M. G., Li S., Schulick A., Yu H. (2008) Stem Cells 26, 1376–1384 [DOI] [PubMed] [Google Scholar]
- 37.Skoglund B., Aspenberg P. (2007) BMC Musculoskelet. Disord. 8, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H., Lu D., Jiang H., Xiong Y., Qu C., Li B., Mahmood A., Zhou D., Chopp M. (2008) J. Neurotrauma 25, 130–139 [DOI] [PubMed] [Google Scholar]
- 39.Rabkin S. W., Lodha P., Kong J. Y. (2007) Cardiovasc. Toxicol. 7, 1–9 [DOI] [PubMed] [Google Scholar]
- 40.Li X., Liu L., Tupper J. C., Bannerman D. D., Winn R. K., Sebti S. M., Hamilton A. D., Harlan J. M. (2002) J. Biol. Chem. 277, 15309–15316 [DOI] [PubMed] [Google Scholar]
- 41.Fukuyama R., Fujita T., Azuma Y., Hirano A., Nakamuta H., Koida M., Komori T. (2004) Biochem. Biophys. Res. Commun. 315, 636–642 [DOI] [PubMed] [Google Scholar]
- 42.Weis M., Heeschen C., Glassford A. J., Cooke J. P. (2002) Circulation 105, 739–745 [DOI] [PubMed] [Google Scholar]
- 43.Harris I. R., Farrell A. M., Grunfeld C., Holleran W. M., Elias P. M., Feingold K. R. (1997) J. Invest. Dermatol. 109, 783–787 [DOI] [PubMed] [Google Scholar]
- 44.Jackson S. M., Wood L. C., Lauer S., Taylor J. M., Cooper A. D., Elias P. M., Feingold K. R. (1992) J. Lipid Res. 33, 1307–1314 [PubMed] [Google Scholar]