Abstract
Smooth muscle activities are regulated by inositol 1,4,5-trisphosphate (InsP3)-mediated increases in cytosolic Ca2+ concentration ([Ca2+]c). Local Ca2+ release from an InsP3 receptor (InsP3R) cluster present on the sarcoplasmic reticulum is termed a Ca2+ puff. Ca2+ released via InsP3R may diffuse to adjacent clusters to trigger further release and generate a cell-wide (global) Ca2+ rise. In smooth muscle, mitochondrial Ca2+ uptake maintains global InsP3-mediated Ca2+ release by preventing a negative feedback effect of high [Ca2+] on InsP3R. Mitochondria may regulate InsP3-mediated Ca2+ signals by operating between or within InsP3R clusters. In the former mitochondria could regulate only global Ca2+ signals, whereas in the latter both local and global signals would be affected. Here whether mitochondria maintain InsP3-mediated Ca2+ release by operating within (local) or between (global) InsP3R clusters has been addressed. Ca2+ puffs evoked by localized photolysis of InsP3 in single voltage-clamped colonic smooth muscle cells had amplitudes of 0.5–4.0 F/F0, durations of ∼112 ms at half-maximum amplitude, and were abolished by the InsP3R inhibitor 2-aminoethoxydiphenyl borate. The protonophore carbonyl cyanide 3-chloropheylhydrazone and complex I inhibitor rotenone each depolarized ΔΨM to prevent mitochondrial Ca2+ uptake and attenuated Ca2+ puffs by ∼66 or ∼60%, respectively. The mitochondrial uniporter inhibitor, RU360, attenuated Ca2+ puffs by ∼62%. The “fast” Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acted like mitochondria to prolong InsP3-mediated Ca2+ release suggesting that mitochondrial influence is via their Ca2+ uptake facility. These results indicate Ca2+ uptake occurs quickly enough to influence InsP3R communication at the intra-cluster level and that mitochondria regulate both local and global InsP3-mediated Ca2+ signals.
Introduction
Smooth muscle functions to regulate many activities including blood flow in vascular blood vessels, peristaltic motion in the gastrointestinal tract, and rhythmic contractions of the uterus during labor. Transient increases in the cytosolic Ca2+ concentration ([Ca2+]c)2 provides the major trigger for contraction to regulate these activities. Other smooth muscle cell functions are also regulated by Ca2+ including transcription, growth, and apoptosis. Stimuli acting on the cell may increase [Ca2+]c by influx of the ion from the extracellular medium, release from intracellular stores or both. The sarcoplasmic reticulum (SR) is an intracellular Ca2+ storage organelle and contains two Ca2+ release channels, the ryanodine receptor (RyR) and the inositol 1,4,5-trisphosphate receptor (InsP3R). Release from RyR is triggered by a small influx of Ca2+ across the plasma membrane in a process known as Ca2+-induced Ca2+-release, which occurs in cardiac and skeletal muscle or in smooth muscle when the SR contains excessive levels of Ca2+ (1–3). In non-contracting cell types and smooth muscle, Ca2+ release from the SR occurs predominantly via InsP3R and is triggered by hormonal stimulation of G protein-coupled receptors.
InsP3R are not distributed uniformly throughout the SR but exist as clusters composed typically of 25–60 channels (4, 5). Ca2+ release from a cluster of InsP3R, a Ca2+ puff, is attributed to the activation of a small number of InsP3R within the cluster (4, 5). Ca2+ puffs are spatially localized Ca2+ transients that are short in duration and are considered the elementary building blocks of Ca2+ release from InsP3R (6–10). Ca2+ puffs have been observed in several cell types including Xenopus oocytes, rat basophilic leukemia, glial, PC12, and smooth muscle cells where the localized release forms microdomains of [Ca2+]c, which exceed that of the bulk cytoplasm (6–8, 10–12). Propagation of the Ca2+ response through the cell occurs when Ca2+ released from one InsP3R cluster diffuses to other neighboring clusters and, in the presence of InsP3, activates them in a Ca2+-induced Ca2+-release-like process (7, 8, 13, 14). Thus, activation of InsP3R may generate a variety of Ca2+ responses that include localized Ca2+ puffs or cell-wide (global) responses in the form of Ca2+ oscillations and propagating Ca2+ waves.
Mitochondria, in addition to their ATP generating facility, take up Ca2+ from the cytosol. Global InsP3-mediated Ca2+ release is modulated by mitochondrial Ca2+ uptake in smooth muscle and other cell types, which include HeLa cells, Xenopus oocytes, PC12 cells, and cultured oligodendrocytes (15–20). Mitochondrial Ca2+ uptake and the increase in mitochondrial Ca2+ concentration ([Ca2+]mit), which normally occurs during global InsP3-mediated increases in [Ca2+]c is prevented by depolarizing the mitochondrial membrane potential (ΔΨM) in HeLa and rat basophilic leukemia-2H3 mast cells (21, 22). Inhibition of mitochondrial Ca2+ uptake has various effects on global InsP3-mediated Ca2+ release. Mitochondrial Ca2+ uptake may negatively regulate InsP3-mediated Ca2+ release in cultured hepatocytes and astrocytes. Here the Ca2+ wave amplitude or velocity or both increased when mitochondrial Ca2+ uptake was prevented by depolarizing ΔΨM (23, 24). In other cell types mitochondrial Ca2+ uptake positively affects InsP3-mediated Ca2+ release so that preventing uptake inhibited Ca2+ responses in smooth muscle, astrocytes, and HeLa cells and increasing mitochondrial Ca2+ uptake augmented InsP3-mediated Ca2+ wave amplitude and velocity in Xenopus oocytes (15–17, 19, 20, 25).
The differences in mitochondrial regulation of global InsP3-mediated Ca2+ release in various tissues may arise from the localization of sites of mitochondrial Ca2+ uptake relative to sites of SR Ca2+ release. Proximity of sites of mitochondrial Ca2+ uptake to InsP3R clusters may determine whether Ca2+ uptake prevents either inactivation or complete activation of InsP3R. The localization of mitochondria will also determine whether the organelle may only regulate the global signal (e.g. waves) alone or may additionally regulate local (e.g. puffs) Ca2+ signals. For example, localization of mitochondria between InsP3R clusters will enable the organelle to regulate global but not local Ca2+ signals. On the other hand, mitochondria positioned at InsP3R clusters will enable the organelle to regulate local and global Ca2+ signals. To determine whether mitochondria regulate local or global Ca2+ signals, the effect of depolarizing ΔΨM, or inhibiting the mitochondrial uniporter, to prevent mitochondrial Ca2+ uptake, on localized release of Ca2+ from InsP3R clusters (Ca2+ puffs) has been examined. InsP3-mediated Ca2+ puffs were evoked by localized flash photolysis of caged InsP3 in colonic smooth muscle cells. Ca2+ puff sites were uncoupled by using EGTA to prevent Ca2+ release from one InsP3R cluster activating adjacent InsP3R clusters and generate a global Ca2+ wave. Inhibition of mitochondrial Ca2+ uptake attenuated the magnitude of Ca2+ puffs as well as global InsP3-mediated Ca2+ signals. These results indicate that mitochondrial Ca2+ uptake influences the InsP3R communication at the intra-cluster level by regulating the amount of Ca2+ released from a cluster of InsP3R before the released Ca2+ diffuses between InsP3R clusters. Mitochondrial Ca2+ uptake appears to prevent a negative feedback effect of high [Ca2+]c on InsP3R activity within a cluster to prolong Ca2+ release from the SR. Support for this conclusion was found in experiments which show the “fast” Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) also prolonged Ca2+ release via InsP3R clusters. Together, these results indicate that sites of mitochondrial Ca2+ uptake are located in proximity to InsP3R to influence the local and global response to InsP3-mediated Ca2+ release.
EXPERIMENTAL PROCEDURES
Cell Isolation
Male guinea pigs (350–500 g) were humanely killed by cervical dislocation followed by immediate exsanguination in accordance with the guidelines of the Animal (Scientific Procedures) Act UK 1986. A segment of intact distal colon (∼5 cm) was transferred to oxygenated (95% O2, 5% CO2) physiological saline solution comprising (mm): 118.4 NaCl, 25 NaHCO3, 4.7 KCl, 1.13 NaH2PO4, 1.3 MgCl2, 2.7 CaCl2, and 11 glucose (pH 7.4). Following removal of the mucosa and longitudinal muscle layer from the tissue, single smooth muscle cells, largely from circular muscle, were enzymatically dissociated (16). All experiments were carried out at room temperature (20 ± 2 °C).
Electrophysiology
Cells were voltage-clamped using conventional tight-seal whole cell recording methods (26). The extracellular solution contained (mm): 80 sodium glutamate, 40 NaCl, 20 tetraethylammonium chloride, 1.1 MgCl2, 3 CaCl2, 10 HEPES, and 30 glucose (pH 7.4 with NaOH). The pipette solution contained (mm): 85 Cs2SO4, 20 CsCl, 1 MgCl2, 30 HEPES, 3 MgATP, 2.5 pyruvic acid, 2.5 malic acid, 1 NaH2PO4, 5 creatine phosphate, 0.5 guanosine phosphate, and 0.025 caged inositol 1,4,5-trisphosphate (InsP3) trisodium salt. Pyruvic acid and malic acid were present to maintain mitochondrial activity. The high concentration of HEPES was to ensure pH control during mitochondrial depolarization. Creatine phosphate and ATP were to maintain [ATP] during the experiments. Whole cell currents were measured using an Axopatch 200B (Axon Instruments, Union City, CA), low-pass filtered at 500 Hz (8-pole Bessel filter; Frequency Devices, Haverhill, MA), digitally sampled at 1.5 kHz using a Digidata interface and pClamp (version 8; Axon Instruments) and stored for analysis. In the majority of experiments, as specified under “Results,” EGTA (250 μm to 1 mm) was added to the pipette solution to buffer the [Ca2+]c. EGTA was added to uncouple InsP3R clusters (27). The slow binding kinetics (Kon = ∼5 μm−1 s−1) of the buffer prevents EGTA from significantly influencing the release of Ca2+ within a cluster (28). In other experiments, as specified under “Results,” BAPTA (250 μm) replaced EGTA in the pipette solution to buffer the [Ca2+]c. The fast binding kinetics of BAPTA (Kon = 100–1000 μm−1 s−1) influences Ca2+ release within an InsP3R cluster (28).
Imaging
Single, freshly isolated colonic smooth muscle cells were loaded with the Ca2+-sensitive dye fluo 3 acetoxymethylester (AM) (10 μm) and wortmannin (10 μm; to prevent contraction) for at least 20 min before the start of the experiment and then allowed to settle for 10 min. 30 min was sufficient to allow intracellular esterases to hydrolyze the AM moiety. Two-dimensional [Ca2+]c images were obtained using a wide-field digital imaging system. Single cells were illuminated at 488 nm (bandpass 14 nm) from a monochrometer (Polychrome IV, T.I.L.L. Photonics, Martinsried, Germany) and imaged through an oil-immersion objective (×40 UV 1.3 NA; Nikon UK, Surrey, United Kingdom). Excitation light was passed via a fiber optic guide through a 485-bandpass (15 nm) filter and a fieldstop diaphragm and reflected off a 505-nm long-pass dichroic mirror. Emitted light was guided through a 535-nm barrier filter (bandpass 45 nm) to an intensified, cooled, frame transfer CCD camera (Pentamax Gen IV, Roper Scientific, Trenton, NJ) operating in “virtual chip” mode with program WinView 32 (Roper Scientific, Trenton, NJ). Full-frame images (160 × 160 pixels), with a pixel size of 720 nm at the cell, were acquired at 50 frames/s. In some experiments the software program Metafluor (Molecular Devices, Wokingham, UK) was used to obtain longer periods of data acquisition. In these experiments sampling rates of ∼10 frames/s were used during and 1 frame/s between Ca2+ transients. Ca2+ imaging data were recorded on a personal computer. Electrophysiological measurements and imaging data were synchronized by recording, on pClamp, a transistor transistor logic output from the CCD camera, which reported its readout status together with the electrophysiological information.
In some experiments, cells were loaded with the Ca2+-sensitive dye fluo 4-AM (10 μm) and the ΔΨM-sensitive dye tetramethylrhodamine ethyl ester (TMRE) (10 nm) so that [Ca2+]i and ΔΨM, respectively, could be imaged near simultaneously (15). Here cells were illuminated at 475 and 560 nm, respectively, and light was passed via a fiber optic guide through a dual 483/553-nm bandpass filter (bandpass 15 and 20 nm, respectively), through an ND4 neutral density filter, and reflected off a custom-made dual long-pass dichroic mirror (transmissive in ranges 505–540 and 577–640 nm, and reflective from 490 to below 300 nm; Chroma, Rockingham, VT). The emitted light was guided through a dual bandpass 518/594-nm barrier filter (bandpass 25 and 18 nm, respectively) to the CCD camera.
Localized Flash Photolysis
The output of a xenon flashlamp (Rapp Optoelecktronic, Hamburg, Germany), used to uncage InsP3, was passed through a UG-5 filter to select ultraviolet light, focused, and merged into the excitation light path through a fiber optic bundle and long-pass dichroic mirror at the lens part of the epi-illumination attachment of the microscope. The diameter of the fiber optic together with the lens magnification determined the area (spot size ∼20 or ∼125 μm) of InsP3 photolysis (29). A photolysis region of ∼20 μm diameter was used to evoke InsP3-mediated Ca2+ release to allow for greater flexibility in photolysis spot placement relative to the patch clamp electrode to prevent uncaging of InsP3 within the patch pipette. The output intensity of the flash lamp was altered in the Ca2+ puff experiments to between 20 and 100% (0.025–0.19 milliwatts) of the maximum output to control the amount of InsP3 that was uncaged and determined empirically in each cell.
Data Analysis
Images were analyzed using the program Metamorph 7.1.3 (Molecular Devices, Wokingham, UK). Fluorescence images were initially background subtracted and smoothed using a median average of 3 × 3 pixels. Changes in fluorescence were expressed as ratios (F/F0 or ΔF/F0) of fluorescence counts (F) relative to baseline (control) values (taken as 1) before stimulation (F0). The average baseline value over the 100 frames occurring before flash photolysis of caged InsP3 was subtracted from peak height (ΔF/F0). Full width at half-maximum amplitude (FWHD) was used to determine the duration of the puff at half its peak value. The time to peak of the Ca2+ puff was measured as the time required to increase from 10 to 90% of maximal peak amplitude. The decay of the Ca2+ puff was measured as the time required to decline from 90 to 10% of maximal amplitude. The delay in the onset of the Ca2+ puff after photolysis of InsP3 was measured as the time required for [Ca2+]c to increase by 0.2 F/F0 from baseline values.
Summarized results are expressed as mean ± S.E. of n cells. A paired or unpaired Student's t test was applied to the raw data, as appropriate; p < 0.05 was considered significant.
Drugs and Chemicals
Drugs were applied by addition to the extracellular solution. Concentrations in the text refer to the salts, where appropriate. Fluo 3-AM, fluo 4-AM, and TMRE were purchased from Invitrogen and caged InsP3-trisodium salt from SiChem GmbH (Bremen, Germany). All other reagents were purchased from Sigma.
RESULTS
In voltage-clamped, single smooth muscle cells flash photolysis of caged InsP3 (25 μm) at 60-s intervals produced transient elevations in the free intracellular Ca2+ concentration ([Ca2+]c) throughout the photolysis region (i.e. global increases) (Fig. 1). Upon photolysis of InsP3, [Ca2+]c, measured by ΔF/F0, increased to 3.91 ± 0.63 (n = 6). The rise in [Ca2+]c from InsP3 occurs exclusively as a result of InsP3R activity; InsP3-mediated Ca2+ release does not activate further Ca2+ release via RyR (30, 31).
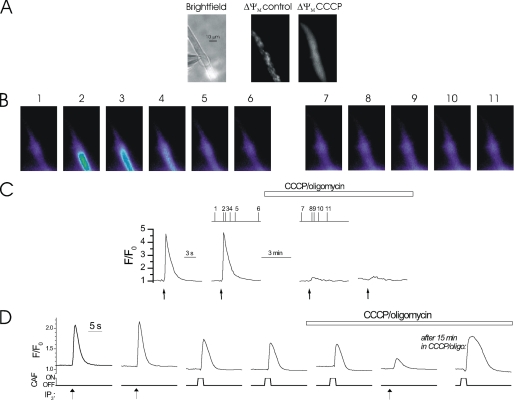
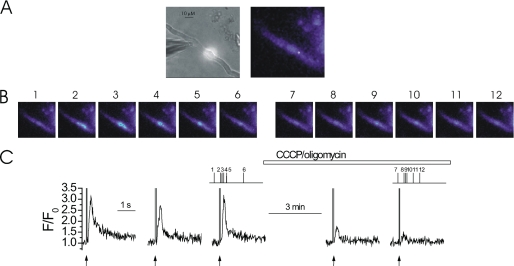
FIGURE 1.
Mitochondrial depolarization decreased the magnitude of InsP3-mediated Ca2+ release. At −70 mV, locally photolyzed caged InsP3 (25 μm) (↑, C) in a ∼20 μm diameter region (A, bright spot in left-hand panel, see also whole cell electrode, left side) evoked approximately reproducible rises in Ca2+ in a freshly isolated colonic myocyte (B and C). Depolarization of ΔΨM by the protonophore CCCP (1 μm) (used with oligomycin; 6 μm) inhibited InsP3-mediated Ca2+ increases (B and C). [Ca2+]c and ΔΨM are shown near simultaneously using fluo 4 and TMRE, respectively. Mitochondria appeared as punctuate areas of fluorescence because of their ΔΨM and were imaged before (A, middle panel) and after (A, right-hand panel) superfusion of CCCP. When ΔΨM was depolarized TMRE dissipated and redistributed throughout the cell. The [Ca2+]c images (B) are derived from the time points indicated by the corresponding numbers in C. [Ca2+]c changes in B are expressed by color; dark blue, low and yellow, high [Ca2+]c. Flash photolysis of InsP3 (↑, C) at ∼60-s intervals generated approximately comparable [Ca2+]c increases (C). InsP3 continued to be photolyzed at ∼60-s intervals during superfusion of CCCP and oligomycin to depolarize ΔΨM, which inhibits mitochondrial Ca2+ uptake (horizontal bar; C). Measurements were made from a 3 × 3 pixel box located within the flash area. Mitochondrial depolarization with CCCP plus oligomycin did not alter SR Ca2+ content: RyR activation by caffeine (CAF, 100 μm, applied by localized pressure ejection) evoked comparable Ca2+ release before, immediately after, and 15 min after superfusion of CCCP plus oligomycin (1 and 6 μm, respectively), despite inhibition of Ca2+ increases evoked by InsP3 (D).
Mitochondria accumulate Ca2+ in these cells following release of the ion via IP3R (15). The contribution of mitochondrial Ca2+ uptake to the magnitude of global InsP3-mediated SR Ca2+ release was examined. Mitochondrial Ca2+ uptake was prevented by collapsing the mitochondrial electrochemical (ΔΨM) gradient using the protonophore CCCP. Because, in CCCP, the mitochondrial ATPase operates in reverse direction oligomycin was also included to inhibit the F1F0-ATPase and prevent ATP depletion. ATP (3 mm) and phosphocreatine (5 mm) were present in the patch pipette filling solution. When mitochondrial Ca2+ uptake was prevented in CCCP and oligomycin (1 and 6 μm, respectively), the InsP3-mediated Ca2+ increase was inhibited (see Fig. 1) (15, 16). The InsP3-mediated Ca2+ increase was 3.91 ± 0.63 ΔF/F0 in control and 1.33 ± 0.47 ΔF/F0 (n = 6) after CCCP and oligomycin (i.e. 34% of control (p < 0.05)). The inhibition of the IP3-mediated Ca2+ release is unlikely to be explained by a reduction in the SR Ca2+ content by CCCP and oligomycin. The SR Ca2+ content, as assessed by the extent of Ca2+ release by the RyR agonist caffeine was unaffected by CCCP plus oligomycin (Fig. 1D). RyR and IP3R share access to a single common Ca2+ store in these cells (32). Together, mitochondrial Ca2+ uptake regulates global InsP3-mediated Ca2+ release.
To generate a global increase in Ca2+, Ca2+ released via one cluster of InsP3R activates other adjacent InsP3R clusters in a Ca2+-induced Ca2+-release-like manner to summate into a cell-wide Ca2+ rise (8, 33, 34). Mitochondria may control the global rise in [Ca2+]c evoked by InsP3 by regulating the Ca2+ signal that propagates among InsP3R clusters. Alternatively mitochondria may regulate the Ca2+ signal, which occurs within a single InsP3R cluster. In the former, mitochondria may only regulate global Ca2+ signals. In the latter, both local and global signals will be controlled by mitochondrial activity. The question arises do mitochondria influence InsP3-mediated release by operating within or between InsP3R clusters?
To address this question, mitochondrial regulation of the amplitude and duration of Ca2+ release from a single cluster of InsP3R was examined. Ca2+ puffs, the fluorescence manifestation of the discrete release of Ca2+ from a single cluster of InsP3R, were evoked by low energy (∼20–50% of maximum) flash photolysis of caged InsP3 (25 μm). Ca2+ puffs differed from a global rise in [Ca2+]c in that they were localized events that occurred within a small region of the flash area and their duration was much shorter than for a global release of Ca2+. In contrast, when there was a global rise, [Ca2+]c increased uniformly throughout the flash area. The following criteria identified Ca2+ rises as “puffs”: a change in peak amplitude of between 1 and 3 ΔF/F0, time to peak of 50 to 100 ms, and a duration at half-maximum amplitude (FWHD) of 100 to 200 ms. These criteria are derived from those previously reported in smooth muscle, Xenopus oocytes, and HeLa cells (7, 10, 13, 14, 35).
In initial experiments Ca2+ puffs were observed infrequently and over only a narrow range of [InsP3] above which they were rapidly summated to produce a global rise in Ca2+ response. To overcome this restricted range and evoke Ca2+ puffs consistently, a low concentration of the slow Ca2+ buffer, EGTA, was added to the cells via the patch pipette. EGTA prevents InsP3-mediated Ca2+ puffs from coalescing into a global Ca2+ rise because the buffer prevents the ion from reaching neighboring InsP3R clusters (27). As a first step in these experiments, the effect of [EGTA] (between 250 μm and 1 mm) on Ca2+ puffs was examined. At [EGTA] (250–500 μm) Ca2+ puffs of a similar magnitude to those reported elsewhere and in non-buffered cells were measured (Fig. 2). Above 500 μm EGTA significantly attenuated the amplitude of Ca2+ puffs and, in some cells, they could not be evoked. EGTA (300 μm) at a concentration similar to that used in experiments examining Ca2+ puffs in Xenopus oocytes was used in the present study to enable Ca2+ puffs to be evoked and measured (27, 36). EGTA (300 μm) neither affects the magnitude nor slows the kinetics of Ca2+ puffs (27, 37).
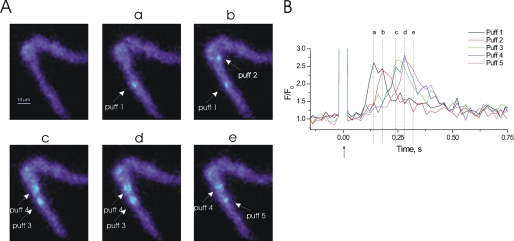
FIGURE 2.
Ca2+ puffs may occur with different latencies of onset after photolysis of InsP3. At −70 mV, localized photolysis of caged InsP3 (25 μm) (↑, B) in a ∼20-μm diameter region triggered several Ca2+ puffs (A and B). Five localized Ca2+ puffs were evoked within the photolysis site; the time of onset for each Ca2+ puff was variable. Ca2+ puff 1 occurred 160 ms after photolysis of InsP3 and was located 22.5 μm away from Ca2+ puff 2, which occurred 200 ms after photolysis. The measurement of delay in Ca2+ puff onset after photolysis of InsP3 was determined by how long it took for the [Ca2+]c to increase by 0.2 F/F0 from baseline. The [Ca2+]c images (A) are derived from the time points indicated by the corresponding lowercase letters in B. [Ca2+]c changes in A are expressed by color: dark blue, low and light blue, high [Ca2+]c. Measurements were made from 3 × 3 pixel boxes located at the center of each puff (not shown). To record Ca2+ puffs single colonic myocytes were buffered using EGTA (300 μm). This did not affect the amplitude, nor duration of the puff but did allow for puffs to be recorded using a larger range of concentrations of InsP3. The large increase in fluorescence at time 0 is the flash artifact.
Characteristics of Ca2+ Puffs
In each cell there were typically one to five individual puffs evoked within the photolysis region (∼20 μm diameter). The latency between photolysis of caged InsP3 and onset of the Ca2+ puff, peak amplitude, time to peak, FWHD, and decay time were measured (Table 1). The amplitude of Ca2+ puffs varied between 0.5 and 4.0 ΔF/F0 and averaged 1.94 ± 0.24 ΔF/F0 (n = 36 from 12 cells). The average time to peak (10–90% interval) of Ca2+ puffs was 57 ± 8 ms, FWHD was 112 ± 16 ms, and the decay time (90–10% interval) was 267 ± 40 ms. Ca2+ puffs also had variable latency between the time of photolysis of InsP3 and when the rise in [Ca2+]c occurred (Fig. 2). In the representative cell shown in Fig. 2, five Ca2+ puffs occurred after photolysis of InsP3. Fig. 2A, a and b, show two Ca2+ puffs (puff 1 and puff 2) that are 22.5 μm apart; the peak of Ca2+ puff 1 preceded that of puff 2 by 40 ms. The majority of cells analyzed displayed 1–2 puff sites and the average onset latency was ∼64 ± 12 ms (n = 36 from 12 cells).
TABLE 1.
Comparison of the amplitude, time to peak, full width at half-maximum amplitude, decay time, and latency of onset of Ca2+ puffs under different conditions
The time to peak and decay time were determined from the changes that occurred between 10 and 90% of the peak ΔF/F0 values.
| Condition | Amplitude, ΔF/F0 | Time to peak (10–90%) | FWHD | Decay time (90-10%) | Latency of onset | n |
|---|---|---|---|---|---|---|
| ms | ||||||
| EGTA buffered | 1.94 ± 0.24 | 57 ± 8 | 112 ± 16 | 267 ± 40 | 64 ± 12 | 36 from 12 cells |
| BAPTA buffered | 1.53 ± 0.54 | 1486 ± 156 | 1208 ± 334 | 4324 ± 435 | 12 from 4 cells | |
| Not buffered | 1.01 ± 0.17 | 419 ± 141 | 481 ± 76 | 1066 ± 279 | 3 from 3 cells | |
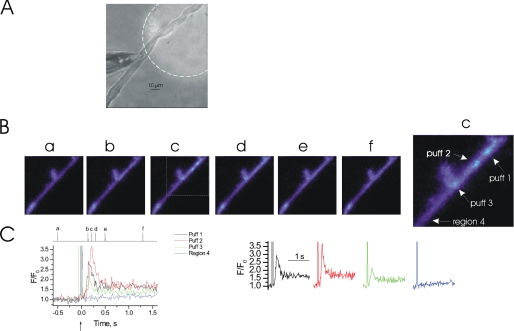
Ca2+ puffs at various sites were not of fixed and constant size but had a continuum of amplitudes presumably due to activation of a different number of InsP3R within different clusters (13, 38). In the representative cell shown in Fig. 3 a large photolysis region (125 μm diameter) was used to evoke InsP3-mediated Ca2+ release and generated puffs of various amplitudes at different sites. There were three individual Ca2+ puffs (labeled puff 1, 2, and 3); the largest increase in [Ca2+]c occurred at puff 2. The increases in [Ca2+]c, which occurred at the other sites are unlikely to be explained by diffusion of Ca2+ from puff 2 (Fig. 3, B and C). If the increases in [Ca2+]c were due to diffusion, then a gradual decrease in the maximum change in [Ca2+]c would be expected to have occurred from the site of Ca2+ release, the rate of Ca2+ increase would have decreased, and there would be a delay in the onset of the Ca2+ increase. At a location situated outside the flash site area, where InsP3 was not photoreleased, no increase in [Ca2+]c occurred (region 4). Although puffs at different sites had various amplitudes, within a site Ca2+ puffs could be reproducibly evoked and had consistent amplitudes (see Figs. 5C, 6C, and 7C). In the remaining experiments a photolysis region of ∼20 μm diameter was used to evoke InsP3-mediated Ca2+ release to allow for greater flexibility in photolysis spot placement relative to the patch clamp electrode to prevent uncaging of InsP3 within the patch pipette.
FIGURE 3.
InsP3-mediated Ca2+ puffs may occur in multiple regions simultaneously. At −70 mV, localized photolysis of caged InsP3 (25 μm) (↑, C) in a 125-μm diameter region (A, bright spot highlighted with a dotted line, see also whole cell electrode, left side) triggered Ca2+ puffs in an EGTA (300 μm)-buffered colonic myocyte (B and C). The Ca2+ puffs were of various amplitudes and occurred near simultaneously in different regions of the flash photolysis site. [Ca2+]c did not increase in areas (region 4) located outside the photolysis site. The [Ca2+]c images (B) are derived from the time points indicated by the corresponding lowercase letters in C. [Ca2+]c changes in B are expressed by color: dark blue, low and light blue, high [Ca2+]c. Measurements were made from 3 × 3 pixel boxes located at the center of each puff (not shown). The large increase in fluorescence at time 0 is the flash artifact.
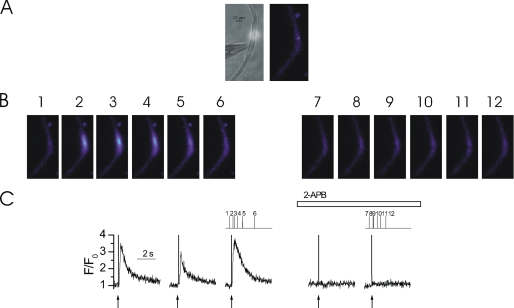
FIGURE 5.
Ca2+ puffs are inhibited by 2-APB. At −70 mV, locally photolyzed caged InsP3 (25 μm) (↑, C) in a ∼20-μm diameter region (A, bright spot in left-hand panel, see also whole cell electrode, left side) evoked Ca2+ puffs in an EGTA (300 μm)-buffered colonic myocyte (B and C). A second and third photolysis of InsP3, each at ∼60-s intervals, at the same site generated an approximately comparable [Ca2+]c increase (C). Superfusion of 2-APB (100 μm) abolished InsP3-mediated Ca2+ puffs within ∼60 s (B and C). The [Ca2+]c images (B) are derived from the time points indicated by the corresponding numbers in C. [Ca2+]c changes in B are represented by color: dark blue, low and light blue, high [Ca2+]c. Measurements were made from a 3 × 3 pixel box (A, right-hand panel, white square). The large increase in fluorescence at time 0 is the flash artifact.
FIGURE 6.
Depolarization of ΔΨM with CCCP/oligomycin inhibits Ca2+ puffs. At −70 mV, locally photolyzed caged InsP3 (25 μm) (↑, C) in a ∼20-μm diameter region (A, bright spot in left-hand panel, see also whole cell electrode, left side) evoked Ca2+ puffs in an EGTA (300 μm)-buffered colonic myocyte (B and C). Note: there are two individual Ca2+ puff sites in response to photorelease of InsP3; one site releases Ca2+ just before the other site. Flash photolysis of InsP3 every ∼60 s generated approximately comparable [Ca2+]c increases (C). Superfusion of CCCP and oligomycin (1 and 6 μm, respectively) while continuing to photolyze InsP3 at ∼60 intervals, decreased the amplitude of InsP3-mediated Ca2+ puffs (B and C). The [Ca2+]c images (B) are derived from the time points indicated by the corresponding numbers in C. [Ca2+]c changes in B are expressed by color: dark blue, low and light blue, high [Ca2+]c. Measurements were made from a 3 × 3 pixel box (A, right-hand panel, white square). The large increase in fluorescence at time 0 is the flash artifact.
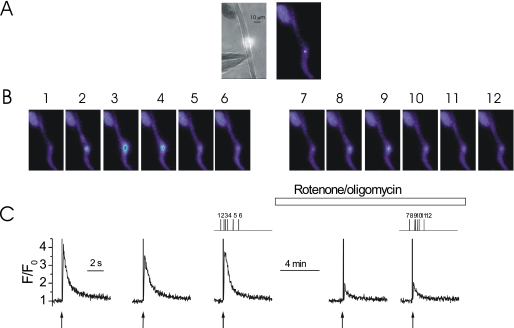
FIGURE 7.
Depolarization of ΔΨM with rotenone inhibits Ca2+ puffs. At −70 mV, locally photolyzed caged InsP3 (25 μm) (↑, C) in a ∼20-μm diameter region (A, bright spot in left-hand panel, see also whole cell electrode, left side) evoked Ca2+ puffs in an EGTA (300 μm)-buffered colonic myocyte (B and C). Flash photolysis of InsP3 every ∼60 s generated approximately comparable [Ca2+]c increases (C). Superfusion of rotenone and oligomycin (5 and 6 μm, respectively), whereas continuing to photolyze InsP3 at ∼60-s intervals, decreased the amplitude of InsP3-mediated Ca2+ puffs (B and C). The [Ca2+]c images (B) are derived from the time points indicated by the corresponding numbers in C. [Ca2+]c changes in B are expressed by color: dark blue, low and light blue, high [Ca2+]c. Measurements were made from a 3 × 3 pixel box (A, right-hand panel, white square). The large increase in fluorescence at time 0 is the flash artifact.
Ca2+ puffs could be evoked in the absence of the Ca2+ buffer EGTA. However, there was substantial variation in response to the same photolysis strength and Ca2+ puffs could only be evoked over a narrow range of [InsP3] and flash intensities presumably because, in the absence of EGTA, Ca2+ could evoke release at neighboring InsP3R clusters. Ca2+ puffs in non-buffered cells were evoked by low energy flash photolysis of a low concentration (6.25 μm) of caged InsP3. In the representative cell, a single Ca2+ puff was evoked in response to photolysis of InsP3 (Fig. 4). [Ca2+]c was increased at this location by 0.88 ΔF/F0. The average puff amplitude in non-buffered myocytes was 1.01 ± 0.17 ΔF/F0 (n = 3). The average time to peak (10–90% interval) was 419 ± 141 ms, FWHD was 481 ± 76 ms, and the decay time (90–10% interval) was 1066 ± 279 ms (Table 1). The rise and decay of Ca2+ puffs from non-buffered cells were both slower than that measured in EGTA-buffered cells suggesting that EGTA facilitates diffusion of Ca2+ away from an InsP3R cluster presumably by capturing the ion (37). Increasing the flash lamp intensity, which liberates a greater amount of InsP3, triggered a global rise in Ca2+ (Fig. 4). The global [Ca2+]c increase, evoked by the greater release of InsP3, was 3.48 ΔF/F0.
FIGURE 4.
InsP3-mediated Ca2+ puffs and global Ca2+ increases. At −70 mV, locally photolyzed caged InsP3 (6.25 μm) (↑, B) in a ∼20-μm diameter region (A, bright spot in left-hand panel, see also whole cell electrode, left side) evoked a Ca2+ puff that was preceded by a Ca2+“blip”-like event (A and B). Flash photolysis of caged InsP3 in the area is indicated by the circle, in A each ∼60 s generated Ca2+ puffs (B, black line). When photolysis energy was increased to photolyze more InsP3 a larger amount of Ca2+ release occurred throughout the region (B, red line). The Ca2+ puff was overlaid (B, right panel) for comparison. The [Ca2+]c images (A) are derived from the time points indicated by the corresponding numbers in B. [Ca2+]c changes in A are expressed by color: dark blue, low and light blue, high [Ca2+]c. Measurements were made from a 3 × 3 pixel box located at the center of each Ca2+ release event (not shown). The large increase in fluorescence at time 0 is the flash artifact.
To ensure Ca2+ puffs arose from InsP3-mediated Ca2+ release the effect of the InsP3R blocker 2-aminoethoxydiphenyl borate (2-APB) was examined. In this series of experiments EGTA (300 μm) was again used to facilitate the measurement of consistent Ca2+ puffs. Approximately reproducible Ca2+ puffs were evoked by photolysis of caged InsP3 (25 μm) (Fig. 5). Ca2+ puffs were abolished within 60 s by 2-APB (100 μm) (Fig. 5). Ca2+ puff amplitude was 2.04 ± 0.32 ΔF/F0 before and 0.25 ± 0.02 ΔF/F0 (n = 3) (p < 0.05) after application of 2-APB. Together these results suggest the localized Ca2+ increases evoked by InsP3R in EGTA-buffered colonic myocytes are Ca2+ puffs and suitable for the examination of the influence of mitochondrial Ca2+ uptake on intra-cluster dynamics during InsP3-mediated SR Ca2+ release.
Mitochondrial Control of Ca2+ Puffs
To determine whether or not mitochondria modulate InsP3-mediated Ca2+ signaling by operating within or between InsP3R clusters, the effect of inhibition of mitochondrial Ca2+ uptake on Ca2+ puffs was examined. The slow Ca2+ chelator EGTA (300 μm), as before, was used to prevent Ca2+ released from one InsP3R cluster from activating neighboring InsP3R clusters and generating a global Ca2+ rise. Ca2+ puffs were evoked by localized flash photolysis of caged InsP3 at 60-s intervals (Fig. 6). When the cell was superfused with CCCP (1 μm; and oligomycin, 6 μm), to depolarize ΔΨM and inhibit mitochondrial Ca2+ uptake, Ca2+ puff amplitude decreased to 34% of control (1.34 ± 0.17 ΔF/F0 before and 0.46 ± 0.05 ΔF/F0 after CCCP, n = 5, p < 0.05; Fig. 6). Rotenone (5 μm; and oligomycin, 6 μm) a complex 1 inhibitor, which also depolarizes the ΔΨM and prevents mitochondrial Ca2+ uptake, inhibited SR Ca2+ release. Ca2+ puff amplitude was decreased to 40% of its original amplitude in rotenone (Fig. 7). Thus, Ca2+ puff amplitude was 2.02 ± 0.36 ΔF/F0 before and 0.82 ± 0.15 ΔF/F0 after rotenone application (n = 4, p < 0.05). Direct inhibition of mitochondrial Ca2+ uptake by Ru360 (10 μm) also inhibited Ca2+ puff amplitude to 38% of control values (from 1.32 ± 0.40 in control to 0.51 ± 0.03 in Ru360 ΔF/F0, n = 9 puffs from 3 cells, p < 0.05). These results suggest that Ca2+ released from a cluster of InsP3R is taken up by mitochondria before it diffuses to other neighboring clusters, i.e. mitochondrial Ca2+ uptake is involved in regulating InsP3R Ca2+ release at the intra-cluster level.
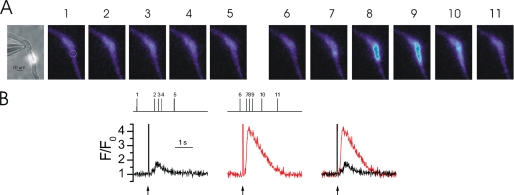
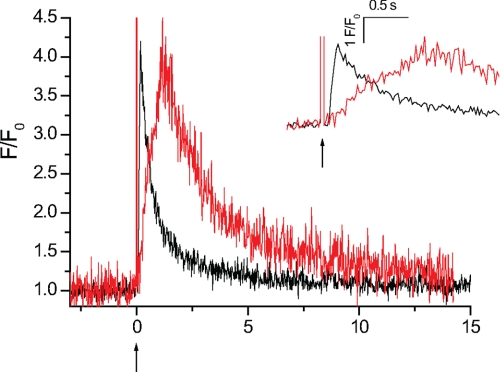
The results suggest that mitochondrial Ca2+ uptake prolongs Ca2+ release and occurs rapidly to influence the kinetics of Ca2+ release within an InsP3R cluster. To test this proposal further the cell was buffered with fast Ca2+ chelator BAPTA. Although BAPTA has a similar Ca2+ binding affinity to EGTA its faster binding kinetics (Kon = 100–1000 μm−1 s−1) allows BAPTA to capture the ion while within an InsP3R cluster in a way similar to how mitochondrial Ca2+ uptake is proposed to function. A Ca2+ puff evoked in an EGTA-buffered myocyte is compared with a Ca2+ release event of the same magnitude in a BAPTA-buffered myocyte (Fig. 8). Although the average peak amplitude (1.53 ± 0.54 ΔF/F0 in BAPTA) was similar to that in EGTA, the average time to peak (1486 ± 156 ms; n = 12 from 4 cells), a measure of the time course of release, was significantly prolonged in BAPTA-buffered myocytes (Table 1). These results suggest that restricting the [Ca2+]c change at an InsP3R cluster prolongs the time course of InsP3-meditated Ca2+ release. These results are consistent with those in Xenopus oocytes; BAPTA prolonged the duration of InsP3-mediated Ca2+ release and Ca2+ release events were no longer spatially discrete (28). This experiment further supports that mitochondrial Ca2+ uptake influences both global and local InsP3-mediated Ca2+ signals.
FIGURE 8.
Localized InsP3-mediated Ca2+ release in BAPTA- and EGTA-buffered myocytes. When myocytes are buffered with the fast Ca2+ chelator BAPTA, Ca2+ release is significantly prolonged. A Ca2+ puff in an EGTA (300 μm)-buffered myocyte (black trace) is compared with a Ca2+ release event of a comparable magnitude in a BAPTA (250 μm)-buffered myocyte (red trace). Myocytes were voltage-clamped at −70 mV and caged InsP3 (25 μm) (↑) was locally photolyzed in a ∼20-μm diameter region (not shown). The rate of rise, a measure of the time course of Ca2+ release, was significantly increased when compared with an EGTA-buffered myocyte. The inset shows an expanded time scale to illustrate the differences in rate of rise of the Ca2+ events in the EGTA (black line)- and BAPTA (red line)-buffered myocytes. Measurements were made from 3 × 3 pixel boxes located at the center of each Ca2+ release event (not shown). The large increase in fluorescence at time 0 is the flash artifact.
DISCUSSION
InsP3-sensitive Ca2+ release initiates at discrete sites on the SR. These sites contain a few tens of receptors from which the local increase in [Ca2+] is termed a puff. Ca2+ puffs are spatially restricted events and of short duration. Puffs are considered elementary release events and may interact and coalesce to generate a global release in Ca2+. In the present study Ca2+ puffs, evoked by photorelease of caged InsP3, occurred in multiple regions of the cell either near simultaneously or after various latencies of onset. Ca2+ puffs at different locations had various amplitudes and durations. Yet, at an individual site Ca2+ puff amplitude was relatively constant in response to a given [InsP3]. Ca2+ puffs occurred by the release of Ca2+ from InsP3R. In support, puffs were blocked by the InsP3R inhibitor 2-APB.
In smooth muscle, global InsP3-mediated Ca2+ release is modulated by mitochondrial Ca2+ uptake. InsP3-evoked Ca2+ release decreased when the driving force for mitochondrial Ca2+ uptake was collapsed by depolarization of ΔΨM with either CCCP or rotenone (15, 16, 25). How mitochondria regulate InsP3-mediated Ca2+ signals was examined here. If Ca2+ uptake occurred at an InsP3R cluster mitochondria would influence local Ca2+ signaling at the level of a puff. Alternatively, if Ca2+ uptake occurred between clusters then mitochondria would influence global but not local Ca2+ signals. These two possibilities were addressed by examining the influence of mitochondria on local Ca2+ signals. When mitochondrial Ca2+ uptake was prevented by depolarizing ΔΨM with CCCP or rotenone, Ca2+ puff amplitude decreased by 66 or 60%, respectively. When mitochondrial Ca2+ uptake was prevented directly by the uniporter antagonist Ru360, Ca2+ puff amplitude decreased by 62%. These results suggest that mitochondria regulate Ca2+ release by acting within an IP3R cluster.
Ca2+ exerts positive and negative feedback effects on IP3R. Positive feedback, which increases Ca2+ release, occurs over lower concentrations of the ion (∼<500 nm), whereas negative feedback dominates at higher [Ca2+]. Mitochondria because of their low affinity for the ion may limit only the negative feedback effect of Ca2+ on the IP3-mediated Ca2+ release. In support of the occurrence of negative feedback the fast Ca2+ chelator BAPTA prolonged Ca2+ release in a way similar to how mitochondrial Ca2+ uptake appears to function to increase the InsP3-mediated Ca2+ release. Together the results suggest mitochondrial Ca2+ uptake influences the amount of Ca2+ released from a cluster of InsP3R and as a result will regulate both local and global InsP3-mediated Ca2+ signals.
The question arises as to how mitochondria, by lowering [Ca2+] near IP3R, increases the [Ca2+]c derived from IP3R activity. Large increases in [Ca2+]c (∼1 μm) may generate a persistent reduction in activity in IP3R, which resembles channel inactivation (29, 40). When IP3R falls into this inactivated-like state Ca2+ release is terminated for many (up to 30) seconds (29, 40). Mitochondria by removing Ca2+ near IP3R may prevent the ion from reaching a concentration high enough to induce the persistent inactivation of the channel. Ca2+ release may persist when mitochondria maintain a slightly lower [Ca2+] near IP3R.
Other support for close proximity between sites of mitochondrial Ca2+ uptake and sites of SR Ca2+ release has come predominantly from experiments that measure increases in both [Ca2+]c and mitochondrial Ca2+ concentration ([Ca2+]mit) during InsP3-mediated Ca2+ release (11, 17, 18, 41). In rat basophilic leukemia-2H3 mast cells buffering [Ca2+]c with EGTA (100 μm) decreased the global [Ca2+]c signal yet did not prevent an increase in [Ca2+]mit (22). This result suggests that InsP3R clusters are located to within 100 nm of sites of mitochondrial Ca2+ uptake. It may be that there are structural tethers that keep sites of mitochondrial Ca2+ uptake and SR Ca2+ release in close proximity (42). Indeed, structural evidence provides support for close association between mitochondria and SR membranes. Close associations between the SR and mitochondrial membranes have been noted in several cell types (21, 22, 43–45). For example, in unstimulated tracheal smooth muscle the majority of mitochondria completely enveloped and form multiple junctions with at least one contact point within ∼22 nm of the SR (43, 45). In HeLa cells there are also close contacts between the endoplasmic reticulum and mitochondria (5–20% mitochondrial surface) (21). Immunogold labeling of thin sections of Purkinje neurons demonstrated close associations between mitochondria and InsP3R in stacked endoplasmic reticulum cisternae (46, 47). It is in these regions of close contact that Ca2+ released from the SR is proposed to be taken up by mitochondria via the Ca2+ uniporter (11, 48).
Although there is close proximity between SR and mitochondria, Ca2+ uptake may either positively or negatively regulate local InsP3-mediated Ca2+ release. In oligodendrocyte progenitor cells, as in the present study, mitochondrial Ca2+ uptake positively affected (increased) InsP3-mediated Ca2+ release. Depolarizing ΔΨM decreased the number of cells that exhibited methacholine-evoked Ca2+ puffs and shortened the duration of Ca2+ puffs in those cells that still responded (49). However, the changes in puff characteristics were attributed to a decrease in the amount of agonist-mediated InsP3 produced after ΔΨM depolarization rather than feedback regulation of InsP3R activity. Changes in InsP3 concentration are an unlikely explanation for the present findings in smooth muscle because the concentration of inositide was increased by photolysis of caged InsP3 rather than being generated by an agonist.
Alternatively, there may be a negative relationship between mitochondrial function and Ca2+ puff amplitude when InsP3R clusters are in close proximity to sites of mitochondrial Ca2+ uptake. In rat basophilic leukemia-2H3 mast cells, although sites of mitochondrial Ca2+ uptake are located near InsP3R, inhibition of mitochondrial Ca2+ uptake increased InsP3-mediated Ca2+ release (22). In Xenopus oocytes the majority of mitochondria are located an average of ∼2.3 μm from Ca2+ puffs sites so that they are separated by a distance where presumably they do not take up Ca2+ during a Ca2+ puff (44). Yet, there are also a small subset of mitochondria that are in close proximity (<1.25 μm) to Ca2+ release sites. Upon sustained InsP3 application, both Ca2+ puffs and Ca2+ wave initiation occurred less frequently at sites where mitochondria were in close proximity to the SR suggesting that this population of mitochondria function to prevent Ca2+ release (i.e. mitochondria support a negative feedback influence of InsP3-mediated Ca2+ release) (44). Interestingly, the amplitude of the global InsP3-evoked Ca2+ response decreased when mitochondrial Ca2+ uptake was prevented in Xenopus oocytes (i.e. in this case mitochondria appear to provide a positive feedback influence on Ca2+ release) (20). Initially, the effects of mitochondria on local and global signals may appear contradictory. Perhaps in Xenopus oocytes mitochondria may influence inter-cluster (positive feedback) as well as intra-cluster (negative feedback) communication.
Both the positive and negative effects of ΔΨM depolarization on InsP3-mediated Ca2+ release were prevented when cells were buffered using BAPTA (23, 39). These results suggest mitochondrial effects on InsP3-mediated Ca2+ release are exerted via Ca2+ uptake. For example, uncoupling InsP3R clusters with BAPTA prevented the potentiation of InsP3-mediated Ca2+ release, which occurred upon depolarizing ΔΨM in hepatocytes (23). BAPTA also restored InsP3-mediated Ca2+ release, which had decreased after depolarizing ΔΨM in baby hamster kidney-21 cells (39). In the present study BAPTA prolonged InsP3-mediated Ca2+ release. In both smooth muscle and Xenopus oocytes BAPTA appears to operate by binding to Ca2+ within an InsP3R cluster and preventing intra-cluster negative feedback to prolong the SR Ca2+ release (28). At the concentration used (250 μm) BAPTA should “capture” Ca2+ ∼70–200 nm from the release site (28). Thus BAPTA, which both prolongs InsP3-mediated Ca2+ release and prevents ΔΨM depolarization from affecting InsP3-mediated Ca2+ release, supports a direct role of mitochondrial Ca2+ uptake in influencing InsP3R communication at the intra-cluster level.
In conclusion, in colonic smooth muscle sites of mitochondrial Ca2+ uptake influence InsP3-mediated Ca2+ release within an InsP3R cluster. Presumably close contacts between mitochondria and InsP3R act to prevent negative feedback inhibition of Ca2+ release at InsP3R clusters. The interaction between mitochondria and SR will modulate Ca2+ signaling mechanisms from local Ca2+ puffs to global Ca2+ oscillations and waves.
This work was supported by Wellcome Trust Grant 078054/Z/05/Z and British Heart Foundation Grant PG/08/066.
- [Ca2+]c
- cytosolic Ca2+ concentration
- InsP3
- inositol 1,4,5-trisphosphate
- InsP3R
- inositol 1,4,5-trisphosphate receptor
- ΔΨM
- mitochondrial membrane potential
- SR
- sarcoplasmic reticulum
- RyR
- ryanodine receptor
- [Ca2+]mit
- mitochondrial Ca2+ concentration
- AM
- acetoxymethylester
- CCCP
- carbonyl cyanide 3-chloropheylhydrazone
- FWHD
- duration at half-maximum amplitude
- 2-APB
- 2-aminoethoxydiphenyl borate
- TMRE
- tetramethylrhodamine ethyl ester
- BAPTA
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid.
REFERENCES
- 1.Taylor C. W., Laude A. J. (2002) Cell Calcium 32, 321–334 [DOI] [PubMed] [Google Scholar]
- 2.Iino M. (1990) J. Gen. Physiol. 95, 1103–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezprozvanny I., Watras J., Ehrlich B. E. (1991) Nature 351, 751–754 [DOI] [PubMed] [Google Scholar]
- 4.Swillens S., Dupont G., Combettes L., Champeil P. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 13750–13755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shuai J., Rose H. J., Parker I. (2006) Biophys. J. 91, 4033–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker I., Yao Y. (1991) Proc. Biol. Sci. 246, 269–274 [DOI] [PubMed] [Google Scholar]
- 7.Bootman M., Niggli E., Berridge M., Lipp P. (1997) J. Physiol. 499, 307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bootman M. D., Berridge M. J., Lipp P. (1997) Cell 91, 367–373 [DOI] [PubMed] [Google Scholar]
- 9.Yao Y., Choi J., Parker I. (1995) J. Physiol. 482, 533–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boittin F. X., Coussin F., Morel J. L., Halet G., Macrez N., Mironneau J. (2000) Biochem. J. 349, 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzuto R., Brini M., Murgia M., Pozzan T. (1993) Science 262, 744–747 [DOI] [PubMed] [Google Scholar]
- 12.Horne J. H., Meyer T. (1997) Science 276, 1690–1693 [DOI] [PubMed] [Google Scholar]
- 13.Sun X. P., Callamaras N., Marchant J. S., Parker I. (1998) J. Physiol. 509, 67–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker I., Yao Y. (1996) J. Physiol. 491, 663–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalmers S., McCarron J. G. (2008) J. Cell Sci. 121, 75–85 [DOI] [PubMed] [Google Scholar]
- 16.McCarron J. G., Muir T. C. (1999) J. Physiol. 516, 149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson P. B., Russell J. T. (1996) J. Biol. Chem. 271, 33493–33501 [DOI] [PubMed] [Google Scholar]
- 18.Drummond R. M., Tuft R. A. (1999) J. Physiol. 516, 139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins T. J., Lipp P., Berridge M. J., Li W., Bootman M. D. (2000) Biochem. J. 347, 593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jouaville L. S., Ichas F., Holmuhamedov E. L., Camacho P., Lechleiter J. D. (1995) Nature 377, 438–441 [DOI] [PubMed] [Google Scholar]
- 21.Rizzuto R., Pinton P., Carrington W., Fay F. S., Fogarty K. E., Lifshitz L. M., Tuft R. A., Pozzan T. (1998) Science 280, 1763–1766 [DOI] [PubMed] [Google Scholar]
- 22.Csordás G., Thomas A. P., Hajnóczky G. (1999) EMBO J. 18, 96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajnóczky G., Hager R., Thomas A. P. (1999) J. Biol. Chem. 274, 14157–14162 [DOI] [PubMed] [Google Scholar]
- 24.Boitier E., Rea R., Duchen M. R. (1999) J. Cell Biol. 145, 795–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swärd K., Dreja K., Lindqvist A., Persson E., Hellstrand P. (2002) Circ. Res. 90, 792–799 [DOI] [PubMed] [Google Scholar]
- 26.Bradley K. N., Flynn E. R., Muir T. C., McCarron J. G. (2002) J. Physiol. 538, 465–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callamaras N., Parker I. (2000) EMBO J. 19, 3608–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dargan S. L., Parker I. (2003) J. Physiol. 553, 775–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarron J. G., MacMillan D., Bradley K. N., Chalmers S., Muir T. C. (2004) J. Biol. Chem. 279, 8417–8427 [DOI] [PubMed] [Google Scholar]
- 30.McCarron J. G., Craig J. W., Bradley K. N., Muir T. C. (2002) J. Cell Sci. 115, 2207–2218 [DOI] [PubMed] [Google Scholar]
- 31.MacMillan D., Chalmers S., Muir T. C., McCarron J. G. (2005) J. Physiol. 569, 533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarron J. G., Olson M. L. (2008) J. Biol. Chem. 283, 7206–7218 [DOI] [PubMed] [Google Scholar]
- 33.McCarron J. G., Chalmers S., Muir T. C. (2008) J. Cell Sci. 121, 86–98 [DOI] [PubMed] [Google Scholar]
- 34.Parker I., Yao Y. (1995) Ciba Found. Symp. 188, 50–60; discussion 60–55 [DOI] [PubMed] [Google Scholar]
- 35.Marchant J. S., Parker I. (1998) Biochem. J. 334, 505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose H. J., Dargan S., Shuai J., Parker I. (2006) Biophys. J. 91, 4024–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith I. F., Wiltgen S. M., Parker I. (2009) Cell Calcium 45, 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas D., Lipp P., Berridge M. J., Bootman M. D. (1998) J. Biol. Chem. 273, 27130–27136 [DOI] [PubMed] [Google Scholar]
- 39.Landolfi B., Curci S., Debellis L., Pozzan T., Hofer A. M. (1998) J. Cell Biol. 142, 1235–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oancea E., Meyer T. (1996) J. Biol. Chem. 271, 17253–17260 [DOI] [PubMed] [Google Scholar]
- 41.Hajnóczky G., Robb-Gaspers L. D., Seitz M. B., Thomas A. P. (1995) Cell 82, 415–424 [DOI] [PubMed] [Google Scholar]
- 42.Csordás G., Renken C., Várnai P., Walter L., Weaver D., Buttle K. F., Balla T., Mannella C. A., Hajnóczky G. (2006) J. Cell Biol. 174, 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai J., Kuo K. H., Leo J. M., van Breemen C., Lee C. H. (2005) Cell Calcium 37, 333–340 [DOI] [PubMed] [Google Scholar]
- 44.Marchant J. S., Ramos V., Parker I. (2002) Am. J. Physiol. Cell Physiol. 282, C1374–C1386 [DOI] [PubMed] [Google Scholar]
- 45.Nixon G. F., Mignery G. A., Somlyo A. V. (1994) J. Muscle Res. Cell Motil. 15, 682–700 [DOI] [PubMed] [Google Scholar]
- 46.Satoh T., Ross C. A., Villa A., Supattapone S., Pozzan T., Snyder S. H., Meldolesi J. (1990) J. Cell Biol. 111, 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mignery G. A., Südhof T. C., Takei K., De Camilli P. (1989) Nature 342, 192–195 [DOI] [PubMed] [Google Scholar]
- 48.Rizzuto R., Bastianutto C., Brini M., Murgia M., Pozzan T. (1994) J. Cell Biol. 126, 1183–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haak L. L., Grimaldi M., Smaili S. S., Russell J. T. (2002) J. Neurochem. 80, 405–415 [DOI] [PubMed] [Google Scholar]