Abstract
We found that factor H (FH) exists in porcine seminal plasma. Purified FH strongly inhibited serum alternative pathway complement activation against lipopolysaccharide. The molecular weight, pI, and heparin-binding activity of the purified protein were different from those of purified FH from porcine serum. The complement regulatory activity of seminal plasma FH was ∼2-fold stronger than that of serum FH. Treatment of purified serum FH with sialidase and N-glycosidase F gave almost the same results as those of seminal plasma FH. The deletion of sialic acid from the carbohydrate chains of both FHs contributed to heparin-binding and complement regulatory activities. Results of reverse transcriptase-PCR, Western blot analysis, and immunohistochemistry showed that seminal plasma FH is mainly secreted from epithelial cells of the seminal vesicle in male genital tracts. FH was also detected in the outer acrosomal region of ejaculated sperm by immunofluorescence staining, and found that the purified FH from the sperm membrane has the same complement regulatory activity as that of seminal plasma FH. The ejaculated sperm possessing FH in the outer acrosomal region considerably evaded complement attack. We also found that there is strong complement activity in fluids from female genital tract ducts. These findings indicate that FH bound to the outer acrosomal region and soluble FH play important roles in protecting sperm against complement attack in male and female genital tracts.
Keywords: Blood Coagulation/Complement, Immunology, Reproduction, Complement, Spermatozoa, Factor H, Genital Tract, Porcine, Seminal Plasma
Introduction
The complement system is essential for the host defense system. The system is activated through three major pathways named classical, alternative, and lectin pathways. Those pathways are composed of over 30 proteins in serum and on cell membranes. Those proteins play important roles in complement activation and as self-protective molecules (1–3). The complement pathways are controlled by complement regulators such as membrane cofactor protein (MCP)2 (CD46) (4), decay accelerating factor (DAF) (5–7), and CD59 (8). Both MCP and DAF also play important roles in protection of sperm from complement attack of the male or female genital tracts (9). Complement components such as C3 and C4 have also been reported to be present in seminal plasma (10–12).
Furthermore, some studies have shown strong anti-complement activity in seminal plasma (13, 14) and suggested that diminished complement activity is associated with spermatozonal abnormalities and infertility (15, 16). However, anti-complement protein(s) in seminal plasma other than DAF, MCP, CD59, and clusterin (17) have not been identified.
Complement regulatory protein factor H (FH) as β1H globulin were first identified from human serum by Nilsson and Müeller-Eberhard (18). The protein is well known as an essential down-regulator of the alternative pathway (AP) in animal serum. The protein acts as a cofactor for soluble factor I (FI)-mediated cleavage of the complement C3b to iC3b in animal plasma (19–21). It has also been reported that FH regulates complement activation on the cell membrane via its binding to heparin and sialic acid (22). Notably, it has been shown that some human diseases such as atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, and age-related macular degeneration are caused by serum FH synthesized from the mutated gene (23–25).
During purification of genital angiotensin-converting enzyme (26), we found that FH (accession number CAC81999) was present in PSP (porcine seminal plasma) and tried to purify it to characterize its properties. The existence and function of FH in PSP have not been reported. Here, we describe the biochemical characteristics of PSP FH and its expression and localization in the male genital tract. We discuss the roles of FH in male and female genital tracts.
EXPERIMENTAL PROCEDURES
Materials
Porcine (Sus scrofa) seminal plasma was kindly provided by Okayama Prefecture Center for Animal Husbandry (Okayama, Japan) and stored at −30 °C until use. Porcine organs were purchased from two local slaughterhouses (Tokyo Shibaura Zouki, Tokyo, and Ikeda Food Company, Second Central Municipal Wholesale Market, Kyoto, Japan). Q-Sepharose, Superdex 200 pg, Hi-Trap heparin HP, CNBr-activated Sepharose, Protein G-Sepharose, DYEnamic ET terminal cycle sequencing kit, enhanced chemiluminescence (ECL) kit, anti-rabbit IgG horseradish peroxidase-linked whole antibody, and anti-mouse IgG horseradish peroxidase-linked whole antibody were obtained from GE Healthcare. Matrex Gel Red A was purchased from Amicon (Lexington, MA). Mouse polyclonal anti-C5b-9 antibody was purchased from Abcam (Cambridge, UK). Histofine (SAB-Po kit) was purchased from Nichirei Co. Ltd. (Tokyo, Japan). TRIzol reagent and Alexa Fluor 488 goat anti-rabbit IgG were purchased from Invitrogen. Transcriptor First-strand cDNA Synthesis Kit and Taq DNA polymerase were products of Roche Diagnostics. Polyvinylidene difluoride membranes were purchased from Bio-Rad. Bacterial lipopolysaccharide (LPS) was purchased from Sigma. Mouse anti-porcine CD46 antibody (6D8/9) was obtained from Serotec (Oxford, United Kingdom).
Polyacrylamide Gel Electrophoresis
Slab gel electrophoresis was carried out on 12.5% polyacrylamide gels according to the method described by Laemmli (27). Proteins in the gel were stained with Coomassie Brilliant Blue R-250.
Purification of FH from PSP
All purification steps were performed at 4 °C unless otherwise specified, and 1 mm EDTA and phenylmethylsulfonyl fluoride were added to all buffers. PSP (∼500 ml) was dialyzed overnight against 20 mm Tris-HCl buffer, pH 7.5. The dialysate was centrifuged at 8,000 × g for 45 min to remove the precipitate. The supernatant was applied onto a Q-Sepharose column (6 × 10 cm) equilibrated with 20 mm Tris-HCl buffer, pH 7.5. After extensively washing the column with equilibration buffer, the proteins were eluated with a linear gradient from 0 to 0.5 m NaCl. Fractions containing FH activity were collected and concentrated with solid ammonium sulfate (80% saturation). The precipitate was dissolved in a minimum volume of 20 mm phosphate buffer, pH 6.8, and dialyzed overnight against the same buffer.
The dialysate was applied onto a Matrex Gel Red A column (2.5 × 20 cm) equilibrated with 20 mm phosphate buffer, pH 6.8, and these fractions containing FH activity were collected. The fraction was dialyzed overnight against 20 mm phosphate-buffered saline, pH 8.0. The dialysate was applied at a flow rate of 0.4 ml/min onto a Superdex 200 pg column (2.6 × 60 cm) equilibrated with the above buffer in fast protein liquid chromatography system. The FH fraction was collected and dialyzed overnight against PBS. The sample solution was used in subsequent experiments. FH concentration was determined by the single radial immunodiffusion method as described by Mancini et al. (28).
Preparation of Anti-FH Antibody and Immunoaffinity Column
Purified FH from PSP was used to raise a polyclonal antibody against rabbits. Briefly, 150 μg of purified FH in 20 mm Tris-HCl buffer containing 0.15 m NaCl, pH 7.5, was used for the initial injection, and then five booster injections were given every 10 days. For initial injection, the antigen solution was mixed with an equal volume of complete adjuvant, and incomplete adjuvant was used for subsequent injections. Antibody preparation was carried out at the Research Center for Animal Life Science of Shiga University of Medical Science under the institutional guidelines for animal use. The antisera were collected, and anti-FH antibody was purified from antisera using an FH ligand column. Purified anti-FH antibody was used for immunohistochemical staining, immunoblot analysis, and preparation of an immunoaffinity column. Purified antibody (7.5 mg) was coupled to a CNBr-activated Sepharose 4B (GE Healthcare) according to the manufacturer's instructions.
Determination of N-terminal Amino Acid Sequence
The purified FH was subjected to 10% SDS-PAGE, and the protein on the gel was blotted onto a polyvinylidene difluoride membrane. The protein bands on the membrane were cut out and sequenced using an Applied Biosystems Model 492cLC Protein Sequencer equipped with an online phenylthiohydantoin analyzer (ABI 140D Microgradient Delivery System, Foster City, CA).
Deglycosylation of FH by PNGase F
The N-linked glycans of the purified FH from PSP and PS (porcine serum) were removed with N-glycosidase F (PNGase F, Enzymic In-solution N-Deglycosylation kit) under reducing and denaturing conditions according to the instructions of the manufacturer (Sigma). Briefly, 40 μg of freeze-dried FH was resuspended in 90 μl of reaction buffer. FH was further denatured by addition of octyl β-d-glucopyranoside and β-ME and then incubation at 100 °C for 10 min. After adding a protease inhibitor mixture (Calbiochem) and 5 μl of PNGase F solution (500 units/ml), the mixture was incubated at 37 °C overnight. After boiling, the sample was subjected to SDS-PAGE analysis followed by staining with Coomassie Blue. Furthermore, PNGase F-treated PS FH for the complement regulatory assay was prepared under nondenaturing conditions.
Sialidase Treatment of FH from PS
The sialic acid of the purified PS FH was removed by sialidase treatment. About 1 mg of freeze-dried PS FH was suspended in 1 ml of 0.1 m sodium acetate buffer, pH 5.5, containing 4 mm CaCl2. After adding protease inhibitors (Complete Mini EDTA-free, Roche) and 100 μl of sialidase solution (type VIII, Sigma, 10 units/ml), the mixture was incubated at 37 °C overnight. Sialidase-treated PS FH was then purified by heparin-affinity column chromatography and used for complement regulatory assay.
Western Blot Analysis
Proteins from male genital tissues were extracted with lysis buffer (50 mm Tris-HCl containing 0.15 m NaCl, 0.1% SDS, 1% sodium cholate, 1% Nonidet P-40, 1 mm EDTA, and 0.5 mm phenylmethylsulfonyl fluoride, pH 7.4). Twenty mg of the protein was separated using 10.5% SDS-polyacrylamide gels. Proteins were then transferred to polyvinylidene difluoride membranes, and the membranes were probed with affinity purified anti-FH antibody (1:1000 (v/v)) and anti-GAPDH antibody (1:5000 (v/v)). After treating the membranes with anti-rabbit IgG horseradish peroxidase-linked secondary antibody (1:1000 (v/v)), bound antibodies were detected using enhanced chemiluminescence and imaged on an LAS-4000 luminescent image analyzer (Fujifilm Life Science USA, Stamford, CT).
Determination of AP Complement Activity by Enzyme-linked Immunosorbent Assay
PS diluted in EGTA/gelatin veronal buffer (VBS containing 10 mm EGTA, 0.05% Tween 20, and 0.1% gelatin, pH 7.5) was preincubated in LPS-coated microtiter plates (Maxisorp, Nunc, Roskille, Denmark) for 5 min at 37 °C. MgCl2 was then added at a final concentration of 5 mm before incubation for 1 h at 37 °C. Complement activity was assessed using a monoclonal antibody to direct membrane attack complex, followed by detection of the monoclonal antibody binding using horseradish peroxidase-conjugated anti-mouse IgG antibodies. ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), Roche) substrate (absorbance at 405 nm) was used to estimate the presence of peroxidase antibodies. The absorbance was measured using a plate reader (Multiscan JX, Thermo Lab Systems, Yokohama, Japan). In the cases of determination of the complement regulatory activities of PS FH and PSP FH on AP, the percent purities of FHs used were >98% as judged by SDS-PAGE.
Isolation of RNA, cDNA Library Synthesis, and RT-PCR
Total RNA was isolated from porcine testis, epididymis caput, epididymis corpus, epididymis cauda, prostate, seminal vesicle, and liver using a High Pure RNA tissue kit (Roche) according to the manufacturer's recommendations. Reverse transcription was performed for 30 min at 42 °C using Superscript II reverse transcriptase (Invitrogen), oligo(dT) primer, and 5 μg of total RNA. PCR analysis was performed using 1 μl of first strand cDNA as a template or 1 μl of total RNA in RT-minus control reaction as a control with a 2720 thermal cycler (Applied Biosystems). Primers used for amplification were as follows: 5′-GAA AAA ATT CCA TGT TCT GA-3′ (SCR15–20-F) and 5′-TCC ACA CGT AGG ATA GGC CA-3′ (SCR15–20-R) for FH, and 5′-CCG TGG GTT GTT AAC TTG CC-3′ (β-actin-F) and 5′-CTG CAG CAC TTT ATT TCC TC-3′ (β-actin-R) for β-actin primers. PCR products were resolved on 1% agarose gels.
Immunohistochemical Staining of Various Porcine Genital Tract Tissues
Most of the adult porcine genital tract tissues including the testis, epididymis, seminal vesicle, and prostate were obtained within 5 min after anesthetic death by sodium pentobarbital. The tissue specimens were fixed in 10% neutral buffered formalin and embedded in paraffin and then cut into 5-μm thick sections. Immunohistochemical staining of these tissue sections was carried out as described previously (29), and their images were taken using a Nikon Microphot FXA microscope (Tokyo, Japan).
Immunofluorescence Staining of Testicular, Epididymal, and Ejaculated Sperm
Spermatozoa were collected from the testis, epididymis, and ejaculated fluids and washed three times with PBS. The pellets supported on an aminopropyltriethoxysilane-coated slide glass (Matsunami Glass, Osaka, Japan) were fixed with methyl alcohol for 1 h at room temperature. After blocking with PBS containing 1% bovine serum albumin (1% bovine serum albumin-PBS), the specimen was reacted with 1:1000 diluted anti-FH antibody in 1% bovine serum albumin-PBS for 1 h at room temperature and then washed several times with PBS followed by incubation with 1:1000 diluted Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody (Invitrogen) in 1% bovine serum albumin-PBS for 1 h at room temperature. After washing three times with PBS, sperm were coverslipped with Vectashield and 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Each specimen was observed under an inverted fluorescent microscope (Olympus, Tokyo, Japan).
Purification of FH from Ejaculated Sperm Membrane
Ejaculated sperm (9 × 108) were washed three times with PBS. Washed sperm were mixed with PBS containing 2% sodium cholate (Dojindo Laboratories, Kumamoto, Japan) and rotated for 15 min at 4 °C. The mixture containing sperm was centrifuged at 7,000 × g for 30 min. The supernatant was applied onto an FH immunoaffinity column. The column was washed with PBS containing 2% sodium cholate and then washed with PBS containing 0.5 m NaCl. FH was eluted with 0.1 m glycine-HCl buffer, pH 3.0, and fractions were immediately neutralized to pH 7.5 with 1 m Tris-HCl buffer, pH 9.0.
Determination of AP Complement Activity on Epididymal and Ejaculated Sperm
Ejaculated and epididymis cauda sperm (2.4 × 105) washed with PBS were coated overnight on a 96-well immunoplate. Nonspecific binding was blocked with PBS containing 0.1% porcine serum albumin. PS diluted with gelatin veronal buffer/EGTA without Tween 20 was put into each of the wells coated with sperm. AP complement activation and detection of complement binding were performed as described above.
Detection of AP Complement Activities in Fluids from Male and Female Genital Tracts and Seminal Plasma
Fluids from the epididymis caput, corpus, and cauda, and seminal plasma in the porcine genital tract ducts and the fluids from porcine female genital tract ducts such as the vagina, uterus, corunu uterus, and ovary in the non-mating stage (season) were separately obtained from one institute and two local slaughterhouses with their courtesy. AP complement activity assay was carried out as described above.
RESULTS
FH Purification and Molecular Weight of the Purified FH
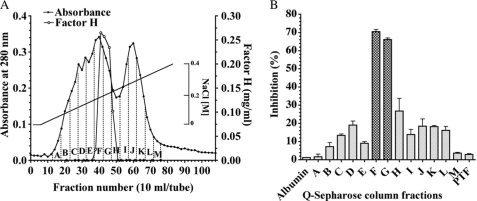
FH was purified from ∼500 ml of PSP by Q-Sepharose, Matrex Red A, and Superdex 200 pg (in fast protein liquid chromatography) columns. Table 1 shows representative results. The protein was purified ∼274-fold with a 22.0% yield over the dialysis fraction. As shown in Fig. 1B, complement regulatory activities were detected in fractions F, G, and H and PSP FH as a protein was contained in these fractions (Fig. 1A).
TABLE 1.
Purification of FH from porcine seminal plasma
| Step | Volume | Total absorbance (280 nm)a | Factor Hb | Yield | Purification |
|---|---|---|---|---|---|
| ml | mg | % | -fold | ||
| Dialysis | 550 | 10,302 | 30 | 100 | 1 |
| Q-Sepharose | 175 | 142 | 28 | 93 | 67 |
| Matrex Red A | 280 | 42 | 16 | 52 | 128 |
| Superdex 200 | 48 | 8.4 | 6.7 | 22 | 274 |
a Protein concentration was measured by absorbance at 280 nm in a 1-cm light path, and 1 mg of protein was defined as the concentration required to yield an absorbance of 1.4 (E280 nm0.1% = 1.4).
b The concentration of factor H was determined by the method of Mancini (28).
FIGURE 1.
Detection of AP component regulatory protein(s) in PSP using a Q-Sepharose column. A, Q-Sepharose column chromatography. Fractions of 10 ml were collected and the concentration of FH determined as described under “Experimental Procedures.” Absorbance is indicated at 280 nm (filled circles), FH (open circles). B, complement regulatory activity of Q-Sepharose fractions. Q-Sepharose fractions designated A to M and the pass-through fraction (PTF) were dialyzed extensively against Milli-Q water and freeze-dried. The dried-up proteins were dissolved with gelatin veronal buffer. The assay of complement regulatory activity was performed using LPS-coated microtiter plates. Complement activity is presented as percent of the control level, which was measured as the complement activity added to purified FH (250 μg/well). Purified porcine albumin (250 μg/well) was used as a negative control. Each value is the mean ± S.E. from triplicate experiments.
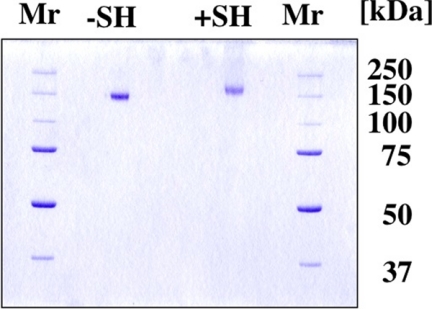
The purified FH at the final step migrated as a single band on SDS-PAGE in the presence or absence of β-ME (Fig. 2). The overall yield of FH was ∼6.7 mg from 500 ml of PSP. The molecular weights (Mr) of the purified protein were 140,000 in the absence and 155,000 in the presence of β-ME on 10.5% SDS-PAGE. The concentration of FH in PSP and PS was determined to be 14.8 and 58.6 mg/100 ml, respectively, by the single radial immunodiffusion method (28).
FIGURE 2.
Polyacrylamide gel electrophoresis of FH purified from PSP. Three μg of purified FH was subjected to 10.5% SDS-PAGE in the absence (lane 1) or presence (lane 2) of β-ME, followed by Coomassie Brilliant Blue R-250 staining. Precision Plus protein unstained standards (Bio-Rad) were also used for estimation of molecular weights. Molecular size of the standard is indicated on the right.
Comparisons of Molecular Weight, pI, Heparin-binding Activity, and Complement Regulatory Activity of FHs from PSP and PS
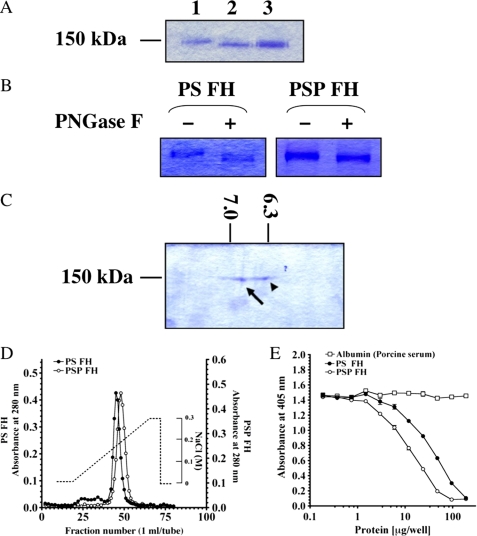
We compared the molecular weight, pI, heparin-binding activity, and complement regulatory activity of FH from PSP with those of FH from PS. Molecular weight of the purified PS FH was 144,000, and the protein was 4 kDa larger than that of PSP FH on SDS-PAGE (Fig. 3A). The same result was obtained by Western blotting and immunoprecipitation with affinity purified anti-FH antibody from PS and PSP (data not shown). As shown in Fig. 3B, deglycosylation of both FHs by PNGase treatment showed that both FHs migrated to the same position on SDS-PAGE. Furthermore, pI values of PSP FH and PS FH were 6.90 and 6.35, respectively, from two-dimensional electrophoresis (Fig. 3C). Comparison of the strengths of binding to a heparin column (Fig. 3D) showed that PSP FH bound to the column more strongly than PS FH. Complement regulatory activity of PSP FH against AP complement activation was 2-fold stronger than that of PS FH (Fig. 3E).
FIGURE 3.
Comparison of molecular weight, pI, heparin-binding activity, and regulation of AP complement activation between FHs from PSP and PS. A, SDS-PAGE of purified FHs from PS and PSP. Three μg each of purified FH from PS (lane 1), PSP (lane 2), and mixed FHs from PSP and PS (lane 3) was loaded on SDS-PAGE and stained with Coomassie Brilliant Blue R-250. B, deglycosylation of both FHs by PNGase F. Purified FHs from PS and PSP were treated with PNGase F (lanes 2 and 4) or not-treated with PNGase F (lanes 1 and 3). These samples were electrophoresed on 7.5% SDS-PAGE and stained with Coomassie Brilliant Blue R-250. C, two-dimensional electrophoresis of purified FHs from PSP and PS. A mixture of 4 μg of each FH from PSP and PS was loaded and stained with Coomassie Brilliant Blue R-250. pI values of FH from PSP (arrow) and PS (arrowhead) were 6.90 and 6.35, respectively. D, comparison of heparin-binding activities of PSP and PS FHs. Approximately 3 mg of purified FH from PSP (open circles) and the same amount of purified FH from PS (filled circles) were applied onto a HiTrapTM heparin column. Each protein was eluted with a linear gradient from 0 to 0.3 m NaCl. Similar results were obtained from at least three separate experiments. E, AP complement regulatory activities of FHs from PSP (open circles) and PS (filled circles). The assay was carried out on an LPS-coated microtiter plate. Porcine albumin (open squares) was used as a negative control. Similar results were obtained from at least three separate experiments. Each value is the mean ± S.E. from triplicate experiments.
Effect of Glycosidase Treatment on Molecular Weight, pI, Heparin-binding Activity, and Complement Regulatory Activity of PS FH
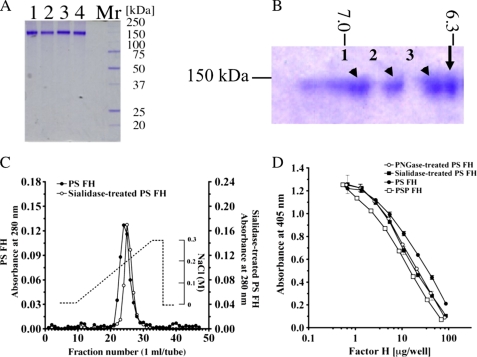
To confirm the effect of carbohydrate chains containing sialic acid on heparin-binding and complement regulatory activities of PS FH, we compared those activities of non-treated PS FH with those of glycosidase-treated PS FH. Mr values of PNGase F-treated PS FH and sialidase-treated PS FH were 140,000 and 142,000 (Fig. 4A), respectively. Sialidase-treated PS FH showed triple spots of pI values (6.92 (1), 6.82 (2), and 6.40 (3), respectively) in two-dimensional electrophoresis (Fig. 4B). Comparison of the strengths of binding to a heparin column (Fig. 4C) showed that sialidase-treated PS FH bound to the column more strongly than non-treated PS FH. Complement regulatory activities of PNGase and sialidase-treated PS FH against AP complement activation were 1.2- and 1.5-fold stronger, respectively, than that of non-treated PS FH (Fig. 4D).
FIGURE 4.
Comparison of molecular weight, pI, heparin-binding activity, and regulation of AP complement activation between PS FH and glycosidase-treated PS FH. A, SDS-PAGE of PNGase F-treated PS FH (lane 1), sialidase-treated PS FH (lane 2), PS FH (lane 3), and PSP FH (lane 4). Five μg of each FH was loaded and stained with Coomassie Brilliant Blue R-250. These proteins were applied to complement regulatory assay. B, two-dimensional electrophoresis of a mixture of purified PS FH (arrow) and sialidase-treated purified PS FH (arrowhead). A mixture of 5 μg of each FH was loaded, electrophoresed, and stained with Coomassie Brilliant Blue R-250. PS FH was a single spot and the pI was 6.31. Sialidase-treated purified PS FH showed triple spots, and the pI values were 6.92 (1), 6.82 (2), and 6.40 (3), respectively. C, comparison of heparin-binding activities of PS FH and silidase-treated purified PS FH. Approximately 1 mg of purified PS FH (filled circles) and the same amount of sialidase-treated purified PS FH (open circles) were applied onto a HiTrapTM heparin column. Each protein was eluated with a linear gradient from 0 to 0.3 m NaCl. D, AP complement regulatory activities of PS FH (filled circles), PNGase F-treated PS FH (open circles), sialidase-treated PS FH (filled squares), and PSP FH (open squares). The assay was carried out on an LPS-coated microtiter plate. Each value is the mean ± S.E. from triplicate experiments.
Regulation of AP Complement Activation by FH-free PSP
FH-free PSP was prepared by passing PSP through an anti-FH immunoaffinity column. PSP was also passed through a Sepharose 4B column as a control.
There was a significant difference between complement regulatory activities of FH-free PSP and PSP (Fig. 5). The regulatory activity of PSP was significantly suppressed by removing FH.
FIGURE 5.
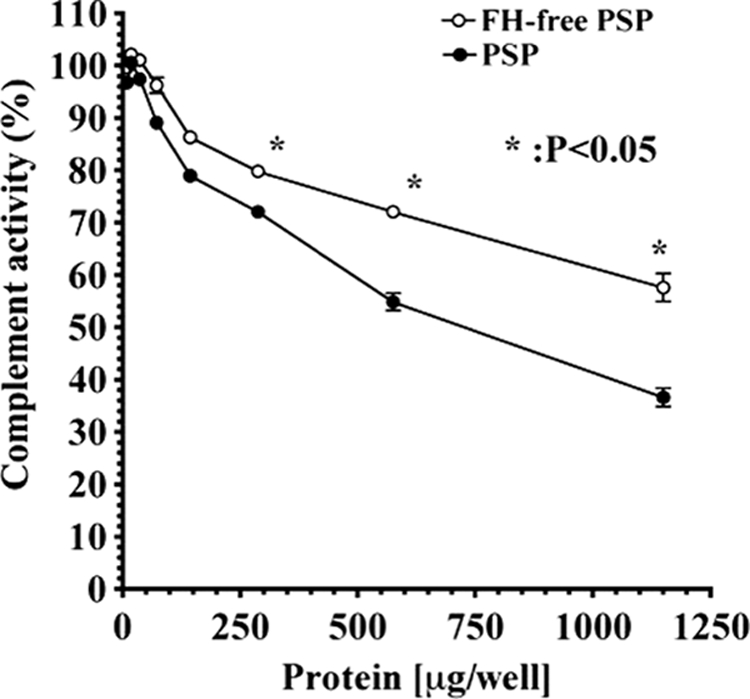
Regulation of AP complement activation by PSP and FH-free PSP. The assays were carried on LPS-coated microtiter plates as described under “Experimental Procedures.” Complement activity is presented as percent of the control level, which was measured as complement activity without the addition of FH-free PSP and PSP. Similar results were obtained from at least three separate experiments. Each value is the mean ± S.D. from triplicate experiments. *, p < 0.05, FH-free PSP was significant compared with PSP. FH-free PSP (open circles) and PSP (filled circles) containing FH.
Expression and Localization of FH Protein in Genital Tract Tissues
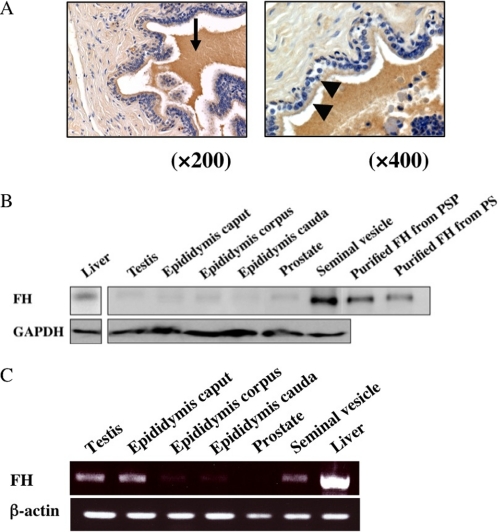
To determine the genital tract tissues in which FH was secreted and expressed, we performed immunohistochemical analysis of porcine genital tract tissues, including the testis, epididymis, seminal vesicle, and liver, with affinity purified anti-FH antibody. FH was strongly stained in epithelial cells of the seminal vesicle and its fluid (Fig. 6A), and was stained very faintly in interstitial cells of the testis but not in the prostate (data not shown).
FIGURE 6.
Localization and expression of porcine FH. A, immunohistochemical localization of FH in porcine genital tract tissues. Epithelial cells (arrowhead) and secreted fluid (arrow) in the seminal vesicle were stained, but connective tissue was not stained. Original magnifications: A, left, ×200, and right, ×400. Nuclei were stained with hematoxylin (purple). B, Western blot analysis of various male genital tract tissues. Twenty μg of lysate from each tissue homogenate with RIPA (50 mm Tris-HCl, pH 7.4, 150 mm NaCl 0.1% SDS, 1% sodium cholate, 1% Nonidet P-40, 1 mm EDTA, 0.5 mm phenylmethylsulfonyl fluoride) was immunoblotted with affinity purified anti-FH antibody (upper panel) and anti-GAPDH antibody (lower panel). Lanes from left to right refer to tissue lysates from: liver, testis, epididymis caput, epididymis corpus, epididymis cauda, prostate, and seminal vesicle; and GAPDH was employed as an internal standard. Purified FHs from PSP (eight lane) and PS (far right lane) were used as positive controls. C, expression of FH mRNA in various male genital tract tissues. Twenty-five μg of each tissue was homogenated, and purified RNA was obtained as described under “Experimental Procedures.” RT-PCR products were separated in 1.5% agarose. Lanes from left to right refer to mRNA from: testis, epididymis caput, epididymis corpus, epididymis cauda, prostate, seminal vesicle, and liver. β-Actin was used as an internal control. Liver was used as a positive control.
Detection of FH protein was also performed by immunoblot analyses to confirm whether or not the immunohistochemical stained protein was FH. An ∼140-kDa protein band was detected in the seminal vesicle and a 144-kDa protein band was detected in the liver using the affinity purified anti-porcine FH antibody (Fig. 6B). In the male genital tracts, the density of the band originating from the seminal vesicle was stronger than that in other tissues such as the testis, epididymis, and prostate.
Moreover, based on the fact that FH consists of 20 modules termed short consensus repeats (SCR), which are composed of 60 amino acid residues, RT-PCR of SCR15–20 was performed using total RNA preparations from the porcine testis, epididymis, prostate, and seminal vesicle. Amplified PCR products were detected in the male genital tract tissues except the prostate. Large quantities of PCR products were detected in the testis, epididymis caput, and seminal vesicle. Each product was a single band of 1,200 base pairs (Fig. 6C). Sequencing of the corresponding cDNAs indicated that they are identical to those reported previously (30) (accession number AJ278470). There is a discrepancy between the PCR product and FH protein expression data. β-Actin was used as an internal control because of its abundant expression.
Localization of FH on Testicular, Epididymal Sperm, and Ejaculated Sperm Membrane
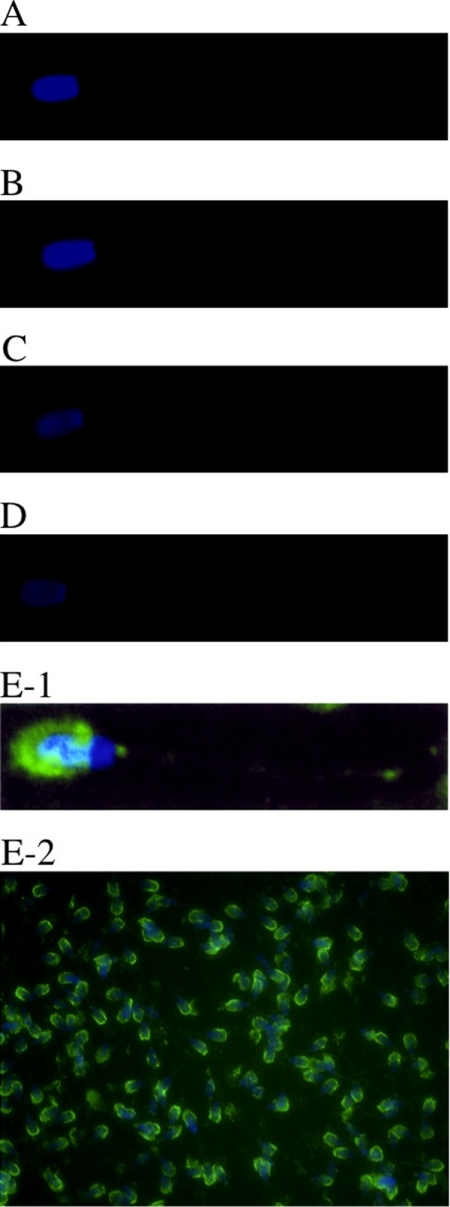
We performed immunofluorescence staining to determine the localization of FH on sperm obtained from testis, epididymis caput, corpus, and cauda, and ejaculated sperm.
It was found that FH exists on the ejaculated sperm (Fig. 7, E-1 and E-2). The outer acrosomal region of the ejaculated sperm was strongly stained (Fig. 7, E-1 and E-2). Sperm in the testis, epididymis caput, corpus, and cauda were scarcely stained (Fig. 7, A–D). FH was not detected on ejaculated sperm in the absence of anti-FH antibody (data not shown).
FIGURE 7.
Immunofluorescence staining of FH on the sperm membrane. Sperm obtained from the testis (A), epididymis caput (B), epididymis corpus (C), and epididymis cauda (D) and ejaculated sperm (E-1 and E-2) on a poly-l-lysine-coated slide glass were fixed and stained with affinity purified anti-FH antibody followed by Alexa 488-conjugated goat anti-rabbit IgG. The outer acrosomal region of ejaculated sperm was strongly stained (green). The tails of sperm obtained from testis, epididymis caput, corpus, and cauda tissues were very faintly stained. Original magnifications: A–E-1, ×800, and E-2, ×200. Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue).
Regulation of AP Complement Activation by Purified FH from Ejaculated Sperm
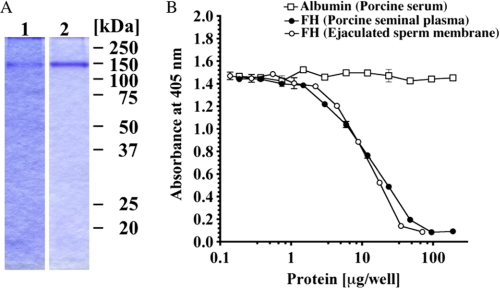
To investigate complement regulatory activity of FH on the ejaculated sperm membrane, we purified FH from ejaculated sperm by detergent extraction and by using immunoaffinity column. The overall yield of FH was ∼435 μg from 9.12 × 108 ejaculated sperm, and the purified protein migrated as a single band at 140 kDa on SDS-PAGE (Fig. 8A). Purified FH from the ejaculated sperm membrane regulated the AP complement activation in a dose-dependent manner by 50% of the control level at the concentration of 10 μg/100 μl. There was no difference between the regulatory activities of FH originating from the sperm membrane and PSP FH (Fig. 8B).
FIGURE 8.
Complement regulatory activity of FH purified from the ejaculated sperm membrane. A, SDS-PAGE of FH purified from the ejaculated sperm membrane (lane 1). FH was solubilized with 2% sodium cholate/PBS treatment and purified using an anti-FH immunoaffinity column. Purified FH from PSP was loaded as a control (lane 2). B, comparison of complement regulatory activities of FHs from ejaculated sperm membrane (open circles) and PSP (filled circles). The assay was carried out on an LPS-coated plate as described under “Experimental Procedures.” Porcine albumin (open squares) was used as a negative control. Similar results were obtained from at least three separate experiments. Each value is the mean ± S.E. from triplicate experiments.
Assessment of AP Complement Activity against Sperm from the Epididymis Cauda and Ejaculated Sperm
We examined whether the ejaculated sperm are in fact able to evade the complement attack of AP in comparison with sperm from the epididymis cauda. Washed ejaculated sperm and sperm from the epididymis cauda were coated on 96-well immunoplates instead of LPS. Ejaculated sperm and sperm from the epididymis cauda evaded depositing membrane attack complex by 77 and 29% in comparison with that of the LPS level used as a positive control, respectively (Fig. 9). Membrane attack complex was not detected in the absence of sperm (data not shown).
FIGURE 9.
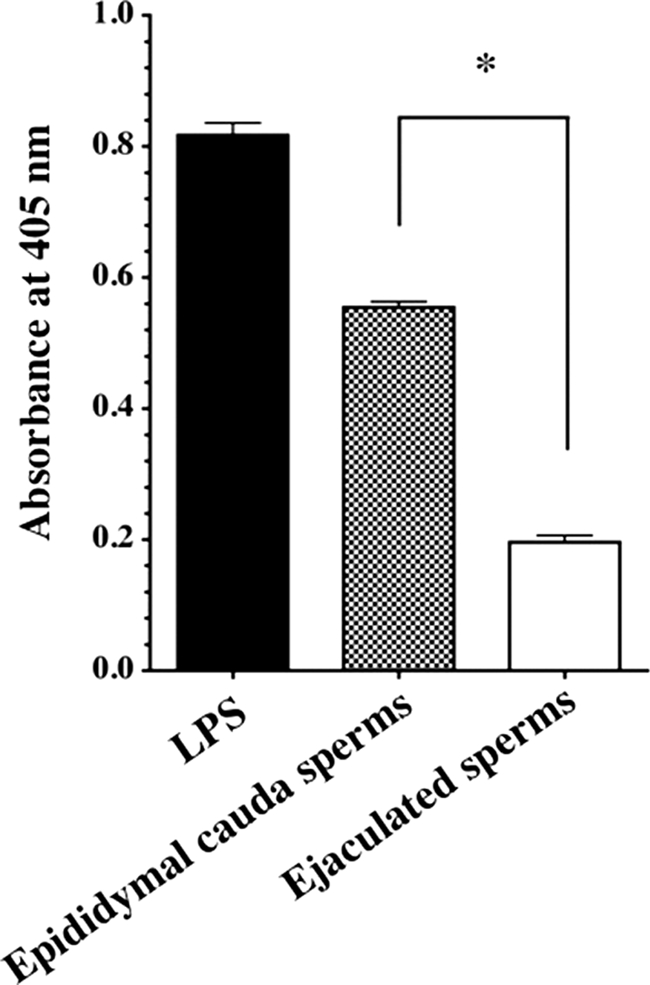
Assessment of AP complement activation on the sperm membrane. AP complement assay was performed using a 96-well immunoplate coated with sperm from the epididymal cauda or ejaculated sperm. LPS was used as a positive control. Similar results were obtained from at least three separate experiments. Each value is the mean ± S.D. from triplicate experiments. *, p < 0.001, ejaculated sperm were significant compared with epididymal sperms.
Detection of AP Complement Activities in Fluids from Male and Female Genital Tracts
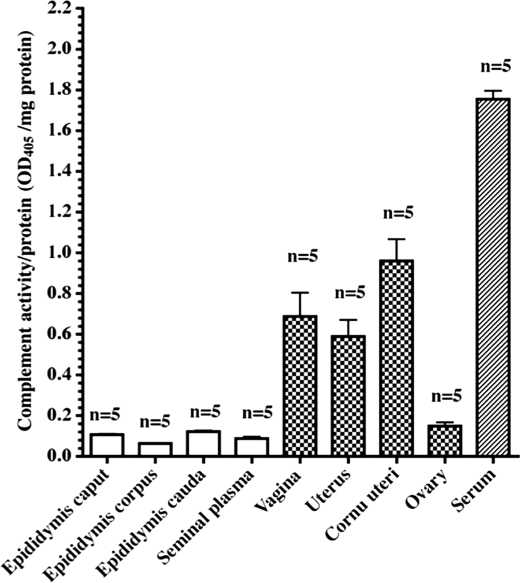
As shown in Fig. 10, AP complement activities were weakly detected in fluids from epididymis caput, corpus, and cauda, and seminal plasma. However, AP complement activities were detected more strongly (6–10-fold) in fluids from some porcine female genital tract ducts such as the vagina, uterus, corunu uterus, and ovary in the non-mating stage (season) than in fluids from male genital tract ducts (Fig. 10). AP complement activities in fluids from male and female genital tract ducts including seminal plasma and ovaries were ∼1:18 and 1:2 to 1:3 weaker, respectively, than that in serum (Fig. 10). Serum was used as a positive control.
FIGURE 10.
AP complement activities in seminal plasma and female genital tracts in non-mating season. AP complement assays of porcine serum, seminal plasma, and fluids from porcine female genital tract ducts such as the vagina, uteri, uterus cornu, and ovarian fluid were carried out on LPS-coated 96-well immunoplates as described under “Experimental Procedures.” Each bar represents the mean ± S.E. for five pigs.
DISCUSSION
Factor H is a regulatory protein of the AP. This protein, which possesses cofactor activity for factor I-mediated cleavage of C3b, competes with factor B in binding to C3b and decreases formation of the alternative pathway C3 convertase (C3bBb) (19, 20). It has been reported that there are membrane-associated anti-complement proteins such as CD46 (MCP), CD55 (DAF), and CD59 in cell-free human and rat seminal plasma (31–33). However, a soluble type of anti-complement protein other than clusterin has not been identified in animal seminal plasma.
We tried to isolate FH from fresh PSP by column chromatographies on Q-Sepharose, Matrex Red A, and Superdex 200 pg. We also characterized its biochemical and biological properties and its role as a regulator in AP.
A typical procedure for purification of FH is summarized in Table 1. FH was finally purified 274-fold with a 22% yield over the dialyzed PSP. The overall yield of FH from ∼500 ml of fresh PSP was ∼6.7 mg. The concentration of FH in PSP determined by the single radial immunodiffusion method (28) was 14.8 mg/100 ml. This value is ¼ less than the concentrations of porcine serum FH (58.6 mg/100 ml) and human serum FH (30–60 mg/100 ml) previously reported (34). This purified protein gave a single band on SDS-PAGE in the absence and presence of β-ME (Fig. 2). The Mr values of the purified FH were 140,000 and 155,000 on SDS-PAGE in the absence and presence of β-ME, respectively. On the other hand, Mr values of FH purified from porcine serum were 144,000 and 159,000 on SDS-PAGE in the absence and presence of β-ME, respectively. pI values of the purified FHs from PSP and PS were 6.90 and 6.35, respectively, in two-dimensional electrophoresis. Although Mr and pI values of the two purified FHs were different, the first N-terminal 20-amino acid sequences of the two FHs were identical. We also compared the inhibitory activity of purified seminal plasma FH with that of purified serum FH. The inhibitory activity of purified seminal plasma FH was stronger (about 2-fold) than that of purified serum FH (Fig. 3E). It has been reported that the N-terminal domain of FH plays an important role in regulation of complement activation and that the C-terminal domain of FH is mainly involved in binding to heparin (22, 36). Therefore, we compared the heparin-binding activities of the two FHs. Heparin-binding activity of purified seminal plasma FH was stronger than that of serum FH, and both purified proteins after PNGase F treatment moved to the same molecular weight position on SDS-PAGE (Fig. 3B). Moreover, heparin-binding activity of sialidase-treated PS FH was stronger than that of non-treated PS FH (Fig. 4C). This result indicates that the differences in heparin-binding activity and pI are due to deletion of sialic acid at glycosylation sites in both FHs. On the other hand, complement regulatory activity of sialidase-treated PS FH was stronger than that of non-treated PS FH. A similar result was also obtained using PNGase F-treated PS FH (Fig. 4D). Therefore, the stronger regulatory activity of purified PSP FH indicates that PSP FH has truncated molecules at the carbohydrate chains including sialic acid and the steric hindrance of the carbohydrates affects the regulatory activity of both FHs. The deletion of sialic acid from the carbohydrate chains of PS FH is thought to contribute to its stronger complement regulatory activity. Generally, sialidase in human (or rat) seminal plasma releases bound sialic acid from the sperm membrane, thereby increasing the concentration of free sialic acid in human (or rat) seminal plasma (37). It has been reported that the concentration of sperm-bound sialic acid gradually decreases from the caput to the cauda epididymis during epididymal migration, and decreased sperm-bound sialic acid concentration contributes to elevation in sperm motility (38). Furthermore, comparison of PSP and FH-free PSP (Fig. 5) showed that the regulatory activity of PSP was significantly decreased by removing FH. These results suggest that FH plays an important role in regulation of complement activation of AP in seminal plasma. In humans and mice, it has been reported that seminal plasma has strong complement regulatory activity due to both MCP and DAF (30, 31). These proteins with complement regulatory activity of an alternative pathway were expressed in the testis and prostate. The former is anchored on the inner acrosomal membrane of sperm and the latter is secreted from the prostate with glycosylphosphatidylinositol anchored on prostasome (39, 40). It has been suggested that the role of prostasome is to transfer proteins to sperm (41). After ejaculation, these complement regulatory proteins are transferred to the sperm to protect against complement attack (42). We attempted to identify the complement regulatory factor(s) against AP in PSP by fractionation of PSP using Q-Sepharose column chromatography. However, no inhibitory or regulatory protein peaks other than that of FH were detected (Fig. 1B).
It has been reported that 60-, 50–55-, and 45-kDa bands of CD46 (MCP) were detected in human testicular tissue lysate and that the 45-kDa band was an inner acrosomal membrane anchoring protein and the 60-kDa band was a human prostasome-specific type protein (43). To evaluate tissue distribution of CD46, using an antibody against CD46, we determined the expression of the protein in porcine genital tract organs, seminal plasma, and sperm lysate. 60- and 45-kDa CD46 was identified in the testis, epididymis, prostate, and seminal vesicle. However, it was revealed that CD46 does not exist in seminal plasma and sperm lysate. CD46 was also not detected inmmunohistochemically in the sperm acrosomal membrane (data not shown). Accordingly, FH, but not CD46, CD55, or CD59, may play important roles in protection of sperm against complement attack of an alternative pathway in porcine genital tracts.
As shown in Fig. 6A, FH was also stained in epithelial cells and fluids of the seminal vesicle. Western blot and RT-PCR analyses also revealed that FH was synthesized in epithelial cells of the seminal vesicle (Fig. 6, B and C) and secreted into fluid of the seminal vesicle. However, it was found that there is a discrepancy between gene and protein expressions. The discrepancy indicates that there may be a translational disorder or degradation of nascent or synthesized FH after normal translation (44, 45).
FH was also found to be localized on ejaculated sperm membranes. To compare the regulatory activity of seminal plasma FH with that of sperm membrane FH, we tried to elute FH from sperm membranes with various reagents. Sperm membrane-bound FH was eluted in turn with 2% sodium cholate = low pH (pH 3.0) > 2% Chaps > 2% octyl glycoside. However, phosphatidylinositol-phospholipase C, neuraminidase, and high ion strength such as 1 m NaCl could not release FH from sperm membrane. Accordingly, it is thought that FH binds to sperm membranes in a hydrophobic binding manner. Comparison of seminal plasma FH and FH eluted from sperm membranes showed that both FHs have the same Mr and complement regulatory activities (Fig. 8). The ejaculated sperm showed ∼3-fold stronger activity than that of the epididymal sperm for regulation of complement activation (Fig. 9). These results indicate that FH on the membrane of ejaculated sperm is able to directly inhibit complement activation. Furthermore, to confirm the function of PSP FH, we determined complement activity in fluids from several male and female genital tract ducts, seminal plasma, and ovary fluid. As shown in Fig. 10, complement activity was weakly detected in fluids from the porcine genital tract ducts and seminal plasma (35, 46, 47). However, the activities in secreted or transudatory fluids of female genital tract ducts were 6–10-fold stronger than those in seminal plasma. This indicates that binding of FH to the sperm outer acrosomal region contributes to the decay of complement attack on sperm advancing into female genital tract ducts. It is therefore thought that these two types of FH play important roles in realization of fertilization in the porcine. Furthermore, the existence of soluble FH in seminal plasma is thought to contribute to self-protection against bacterial infections in the both male and female genital tracts.
Supplementary Material
Acknowledgment
We thank Yukiko Koyama for excellent technical assistance in determination of the N-terminal amino acid sequence of FH.
This work was supported in part by Grant-in-Aid for Scientific Research 19590304 (to I. O.) from the Ministry of Education, Science and Culture of Japan and by the President's Discretionary Fund of Shiga University of Medical Science (to K. T.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- MCP
- membrane cofactor protein
- AP
- alternative pathway
- DAF
- decay-accelerating factor
- FI
- factor I
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- LPS
- lipopolysaccharide
- PBS
- phosphate-buffered saline
- PNGase F
- peptide N-glycosidase F
- PS
- porcine serum
- PSP
- porcine seminal plasma
- RT
- reverse transcriptase
- SCR
- short consensus repeat
- β-ME
- β-mercaptoethanol
- Chaps
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid.
REFERENCES
- 1.Fujita T., Matsushita M., Endo Y. (2004) Immunol. Rev. 198, 185–202 [DOI] [PubMed] [Google Scholar]
- 2.Miwa T., Song W. C. (2001) Int. Immunopharmacol. 1, 445–459 [DOI] [PubMed] [Google Scholar]
- 3.Walport M. J. (2001) N. Engl. J. Med. 344, 1058–1066 [DOI] [PubMed] [Google Scholar]
- 4.Liszewski M. K., Post T. W., Atkinson J. P. (1991) Annu. Rev. Immunol. 9, 431–455 [DOI] [PubMed] [Google Scholar]
- 5.Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. (1982) J. Immunol. 129, 184–189 [PubMed] [Google Scholar]
- 6.Fujita T., Shinkai Y., Inoue T., Tamura N. (1988) J. Immunol. 64, 369–374 [PMC free article] [PubMed] [Google Scholar]
- 7.Lublin D. M., Atkinson J. P. (1989) Annu. Rev. Immunol. 7, 35–58 [DOI] [PubMed] [Google Scholar]
- 8.Rollins S. A., Sims P. J. (1990) J. Immunol. 144, 3478–3483 [PubMed] [Google Scholar]
- 9.Taylor C. T., Biljan M. M., Kingsland C. R., Johnson P. M. (1994) Hum. Reprod. 9, 907–911 [DOI] [PubMed] [Google Scholar]
- 10.Blenk H., Hofstetter A. (1991) Infection 19, S138–S140 [DOI] [PubMed] [Google Scholar]
- 11.Rahimi A., Sepehri H., Pakravesh J., Bahar K. (1999) Am. J. Reprod. Immunol. 41, 330–336 [DOI] [PubMed] [Google Scholar]
- 12.Boit R., Petzoldt D., Klinga K., Eggert-Kruse W. (2003) Andrologia 35, 93–99 [DOI] [PubMed] [Google Scholar]
- 13.Petersen B. H., Lammel C. J., Stites D. P., Brooks G. F. (1980) J. Lab. Clin. Med. 96, 582–591 [PubMed] [Google Scholar]
- 14.Price R. J., Roberts T. K., Green D., Boettcher B. (1984) Am. J. Reprod. Immunol. 6, 92–98 [DOI] [PubMed] [Google Scholar]
- 15.Tarter T. H., Alexander N. J. (1984) Am. J. Reprod. Immunol. 6, 28–32 [DOI] [PubMed] [Google Scholar]
- 16.Chowdhury N. A., Kamada M., Takikawa M., Mori H., Gima H., Aono T. (1996) Arch. Androl. 36, 109–118 [DOI] [PubMed] [Google Scholar]
- 17.Jenne D. E., Tschopp J. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 7123–7127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilsson U. R., Müeller-Eberhard H. J. (1965) J. Exp. Med. 122, 277–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 3268–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whaley K., Ruddy S. (1976) J. Exp. Med. 144, 1147–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. (1977) J. Exp. Med. 146, 257–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manuelian T., Hellwage J., Meri S., Caprioli J., Noris M., Heinen S., Jozsi M., Neumann H. P., Remuzzi G., Zipfel P. F. (2003) J. Clin. Invest. 111, 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Córdoba S. R., de Jorge E. G. (2008) Clin. Exp. Immunol. 151, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zipfel P. F., Skerka C. (2006) Curr. Opin. Rheumatol. 18, 548–555 [DOI] [PubMed] [Google Scholar]
- 25.Amirlak I., Amirlak B. (2006) Nephrology 11, 213–218 [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi K., Araki H., Sakaue T., Yamamoto Y., Fujiwara M., Nishi K., Ohkubo I. (2007) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 146, 215–226 [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 28.Mancini G., Carbonara A. O., Heremans J. F. (1965) Immunochemistry 2, 235–254 [DOI] [PubMed] [Google Scholar]
- 29.Nishi K., Fukunaga T., Yamamoto Y., Kane M., Tanegashima A., Rand S., Brinkmann B. (1992) Int. J. Legal Med. 105, 75–80 [DOI] [PubMed] [Google Scholar]
- 30.Hegasy G. A., Willhoeft U., Majno S. A., Seeberger H., Zipfel P. F., Hellwage J. (2003) Immunogenetics 55, 462–471 [DOI] [PubMed] [Google Scholar]
- 31.Vanderpuye O. A., Labarrere C. A., McIntyre J. A. (1993) Int. Arch. Allergy Immunol. 101, 376–384 [DOI] [PubMed] [Google Scholar]
- 32.Seya T., Hara T., Matsumoto M., Kiyohara H., Nakanishi I., Kinouchi T., Okabe M., Shimizu A., Akedo H. (1993) Eur. J. Immunol. 23, 1322–1327 [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin P. J., Holland S. J., Taylor C. T., Olah K. S., Lewis-Jones D. I., Hara T., Seya T., Johnson P. M. (1996) J. Reprod. Immunol. 31, 209–219 [DOI] [PubMed] [Google Scholar]
- 34.Sim R. B., DiScipio R. G. (1982) Biochem. J. 205, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris C. L., Mizuno M., Morgan B. P. (2006) Mol. Immunol. 43, 57–67 [DOI] [PubMed] [Google Scholar]
- 36.Schmidt C. Q., Herbert A. P., Kavanagh D., Gandy C., Fenton C. J., Blaum B. S., Lyon M., Uhrín D., Barlow P. N. (2008) J. Immunol. 181, 2610–2619 [DOI] [PubMed] [Google Scholar]
- 37.Turner T. T. (1979) Invest. Urol. 16, 311–321 [PubMed] [Google Scholar]
- 38.du Toit D., Bornman M. S., van Aswegen C. H., du Plessis D. J. (1994) Arch. Androl. 32, 21–23 [DOI] [PubMed] [Google Scholar]
- 39.Kitamura M., Namiki M., Matsumiya K., Tanaka K., Matsumoto M., Hara T., Kiyohara H., Okabe M., Okuyama A., Seya T. (1995) Immunology 84, 626–632 [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson K. L., Holmes C. H. (1994) J. Reprod. Fertil. 102, 419–424 [DOI] [PubMed] [Google Scholar]
- 41.Babiker A. A., Ronquist G., Nilsson U. R., Nilsson B. (2002) Am. J. Reprod. Immunol. 47, 183–192 [DOI] [PubMed] [Google Scholar]
- 42.Babiker A. A., Nilsson B., Ronquist G., Carlsson L., Ekdahl K. N. (2005) Prostate 62, 105–114 [DOI] [PubMed] [Google Scholar]
- 43.Oglesby T. J. (1998) in The Human Complement System in Health and Disease (John E. ed) pp. 355–382, Academic Press, New York [Google Scholar]
- 44.Chabottaux V., Sounni N. E., Pennington C. J., English W. R., van den Brûle F., Blacher S., Gilles C., Munaut C., Maquoi E., Lopez-Otin C., Murphy G., Edwards D. R., Foidart J. M., Noël A. (2006) Cancer Res. 66, 5165–5172 [DOI] [PubMed] [Google Scholar]
- 45.Sienel W., Mecklenburg I., Dango S., Ehrhardt P., Kirschbaum A., Passlick B., Pantel K. (2007) Clin. Cancer Res. 13, 3840–3847 [DOI] [PubMed] [Google Scholar]
- 46.Jin M., Larsson A., Nilsson B. O. (1991) Am. J. Reprod. Immunol. 26, 53–57 [DOI] [PubMed] [Google Scholar]
- 47.Perricone R., Pasetto N., de Carolis C., Vaquero E., Piccione E., Baschieri L., Fontana L. (1992) Clin. Exp. Immunol. 89, 154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.