Abstract
Ionic currents underlie the firing patterns, excitability, and synaptic integration of neurons. Despite complete sequence information in multiple species, our knowledge about ion channel function in central neurons remains incomplete. This study analyzes the potassium currents of an identified Drosophila flight motoneuron, MN5, in situ. MN5 exhibits four different potassium currents, two fast-activating transient ones and two sustained ones, one of each is calcium activated. Pharmacological and genetic manipulations unravel the specific contributions of Shaker and Shal to the calcium independent transient A-type potassium currents. α-dendrotoxin (Shaker specific) and phrixotoxin-2 (Shal specific) block different portions of the transient calcium independent A-type potassium current. Following targeted expression of a Shaker dominant negative transgene in MN5, the remaining A-type potassium current is α-dendrotoxin insensitive. In Shal RNAi knock down the remaining A-type potassium current is phrixotoxin-2 insensitive. Additionally, barium blocks calcium-activated potassium currents but also a large portion of phrixotoxin-2-sensitive A-type currents. Targeted knock down of Shaker or Shal channels each cause identical reduction in total potassium current amplitude as acute application of α-dendrotoxin or phrixotoxin-2, respectively. This shows that the knock downs do not cause upregulation of potassium channels underlying other A-type channels during development. Immunocytochemistry and targeted expression of modified GFP-tagged Shaker channels with intact targeting sequence in MN5 indicate predominant axonal localization. These data can now be used to investigate the roles of Shaker and Shal for motoneuron intrinsic properties, synaptic integration, and spiking output during behavior by targeted genetic manipulations.
INTRODUCTION
The blend and localization of voltage- and calcium-dependent ion channels determine the resting membrane potential, input impedance, synaptic integration, and firing patterns of a neuron during behavior. The largest and most diverse families of ion channels are voltage- and ligand-gated potassium channels. Voltage-gated potassium channels consist of four α-subunits, typically occurring as homotetramers, but the possible formation of heterotetramers further increases potassium current diversity (Hille 2001). The first four potassium channel genes cloned were the Drosophila Shaker-related genes: Shaker, Shal, Shab, and Shaw (for review, see Salkoff et al. 1992). The vertebrate counterparts are Kv1 (Shaker-related), Kv4 (Shal-related), Kv3 (Shaw-related), and Kv2 (Shab-related) (Baranauskas et al. 1999; Salkoff et al. 1992).
Despite complete sequence and functional data from heterologous expression systems, our knowledge of which potassium channels underlie which currents in Drosophila central neurons remains incomplete, and thus the electrophysiological and behavioral functions of potassium channels remain elusive. Therefore this study uses the genetic model system Drosophila to unravel the channels mediating potassium currents in an individually identified motoneuron. MN5 is one of the five motoneurons innervating the dorsal longitudinal flight muscle (Consoulas et al. 2002; Ikeda and Koenig 1988). Its activity pattern during behavior (Harcombe and Wyman 1977; Levine and Wyman 1973) and its postembryonic development have been described (Consoulas et al. 2002). This study aims to characterize potassium currents of MN5 and to determine the specific contributions of Shaker and Shal channels to these currents. This will provide a framework for investigating the roles of specific ion channels for the development of motoneurons (Duch et al. 2008) and for the behavior of intact animals using targeted genetic manipulations.
In Drosophila, Shaker and Shal encode channels conducting transient A-type like potassium currents, whereas Shab and Shaw channels conduct slowly activating sustained potassium currents (Covarrubias et al. 1991; Wei et al. 1990). Shaker channels are localized to the axons of central neurons (Rogero et al. 1997) and to motoneuron axon terminals (Jan and Jan 1997; Lee et al. 2008; Ueda and Wu 2006). Shal underlies A-type currents in the central processes of embryonic (Baines and Bate 1998) and larval Drosophila motoneurons (Choi et al. 2004), whereas isolated Drosophila mushroom body neurons display Shaker and Shal currents (Gasque et al. 2005).
We describe the potassium currents of MN5 by in situ patch-clamp recordings from the intact adult ventral nerve cord. Genetic and pharmacological tools reveal that both Shal and Shaker encode the A-type current. Immunocytochemistry indicates strong axonal localization of Shaker channels. Specific genetic knock down for Shaker and Shal channels do not cause upregulation of other potassium currents, so that this approach can be used to study the functions of these channels in shaping intrinsic properties, synaptic integration, and motor output.
METHODS
Animals
Drosophila melanogaster were reared in standard plastic vials with cotton stoppers on a yeast-cornmeal-syrup-agar diet at 25°C with a 12-h light/dark regimen. All fly lines carrying chromosomes with UAS-RNAi or dominant negative constructs derived from a W1118 background. The RNAi fly line was obtained from the Vienna Fly Stock Center. The dominant negative knock down line and the C380-GAL4, UAS-mCD8-GFP;;ChaGAL80 fly line were obtained from Dr. S. Sanyal (Emory University, Atlanta, GA). All fly lines were crossed to C380-GAL4, UAS-mCD8-GFP;;ChaGAL80 (Sanyal et al. 2003) to allow visualization of the DLM flight motoneurons MN1-5 (Duch et al. 2008) but to suppress expression in cholinergic interneurons. We use a single dominant negative knock down of Shaker [UAS-Sh(DN)] (Mosca et al. 2005) and RNAi knock down for Shal. Control lines are homozygous W1118 crossed to homozygous C380-GAL4, UAS-mCD8-GFP;;ChaGAL80. In the adult ventral nerve cord, the C380-GAL4 driver drives expression in a few motoneurons, including the DLM motoneurons, and in few unidentified interneurons (Duch et al. 2008). All experiments were conducted with female flies 1–3 days after eclosion except in the flight assay (Fig. 1 C) in which 1- to 3-day-old adult males were used to avoid variability due to egg load.
Fig. 1.
Properties of MN5. A: schematic drawing of dorsal view of the adult Drosophila ventral nerve cord (VNC; pn, prothoracic neuromere; msn, mesothoracic neuromere; mtn, metathoracic neuromere; an, abdominal neuromere). MN5 is located in the msn close to the dorsal surface, with its cell body contralateral to the axonal projection. The dendrites cover a large area of the msn. B: passive relative current transfer in MN5. Color code demarks the somatic amplitude of a steady-state current injected into any given neurite normalized to the somatic amplitude of a similar current injection into the soma. Scale bar from 0.2 (cyan) to 1 (red). C: representative firing pattern of MN5 as recorded extracellularly from the target muscle fiber of MN5 during stationary flight of an intact animal. D and E: total potassium currents as elicited by voltage steps from −90 to +20 mV in 10-mV increments from a holding potential of −90 mV in TTX containing saline (D) and after bath application of 500 μM cadmium (E). F: calcium-dependent currents are revealed by subtraction of E from D. G: command voltage protocol. Note that the time calibration for the protocol (G) is different from the current traces. H–J: current kinetics at different temperatures. Currents were evoked by the voltage protocol shown in G at 25°C (H), 21°C (I), and 17°C (J). Times to peak are indicated for each temperature. Current amplitudes decrease with decreasing temperature (see dotted lines).
Preparation
Wings and legs were cut, and the fly was then pinned dorsal side up in a silicone elastomer (Sylgard)-coated petri dish and submerged in normal saline (composition in mM: 128 NaCl, 2 KCl, 1.8 CaCl2, 4 MgCl2, 5 HEPES, and ∼35 sucrose depending on the osmolality of the solution). pH was adjusted to 7.25 with 1 M NaOH. Osmolality was adjusted to 290 mOsm with sucrose. The animal was dissected along the dorsal midline, and the large dorsal longitudinal flight muscles were stretched laterally and pinned to expose gut, esophagus, and the ventral nerve cord (VNC) underneath. After removal of the gut and the esophagus, the VNC was exposed. The head was removed to facilitate electrode access to the mesothoracic neuromere. For rapid saline exchange during experiments, the volume of the recording chamber was minimized by placing a plexiglas ring (7 mm ID) around the dissected animal and gluing it to the dish with petrolatum (volume of recording chamber was ∼200 μl). The preparation was then mounted onto an upright fixed stage Zeiss Axioskop 2 FS plus fluorescence microscope and viewed with a 40× water-immersion objective.
To facilitate access to MN5 with the patch pipette, the ganglionic sheath was focally removed with a large patch pipette (0.5 MΩ tip resistance) filled with 2% protease in buffer. This was done under visual control of the flight motoneurons by fluorescent excitation of mCD8-GFP. After protease treatment, the preparation was rinsed with 60 ml normal saline for 10 min.
Electrophysiology
Following protease treatment and rinsing, one of the two available MN5s was recorded from with a patch pipette (tip-resistance: 5.8–6.5 MΩ) pulled from borosilicate glass (1.5 mm OD, 1.0 mm ID without filament from World Precision Instruments) with a vertical pipette puller (Narishige). For potassium current recordings, electrodes were filled with normal internal solution with the following composition (in mM): 140 Kgluconate, 2 MgCl2, 2 Mg-ATP, 10 HEPES, 11 EGTA, glucose to adjust osmolality to 300 mOsm. pH was adjusted to 7.25 with KOH. To show the fast time course of the transient calcium current in MN5, we conducted calcium current recordings with the following internal solution (in mM): 120 CsCl, 20 TEA-Br, 0.5 4-AP, 2 ATP-Mg, 1.1 EGTA, and 10 HEPES. Osmolality was adjusted to 300 mOsm with glucose. pH was adjusted to 7.25 with CsOH. For calcium current measurement, the following external solution was used (in mM): 93 NaCl, 5 KCl, 1.8 CaCl2, 1.8 BaCl2, 30 TEA-Cl, 5 HEPES, and ∼35 sucrose depending on the osmolality. Osmolality was adjusted to 290 mOsm with sucrose, pH was adjusted to 7.25 with NaOH. All whole cell currents were recorded from the intact MN5 located in the mesothoracic neuropil of the ventral ganglion of adult Drosophila (Figs. 1A and 7). Recordings were allowed to stabilize for 2–5 min prior to the first voltage protocol and remained stable for 30 min to 90 min. Potassium currents did not run down over a time period of 90 min as confirmed by repeating the same voltage protocol under identical recording conditions multiple times during the first 90 min after breaking into the cell. Therefore subtraction routines could be performed following pharmacological manipulations. Calcium currents were recorded within the first 5 min after break-in. No testing on potential subsequent calcium current run down was conducted. Patch-clamp recordings were carried out at 24°C with an Axopatch 200B patch-clamp amplifier (Molecular Devices), digitized at 20 kHz (digidata 1322A, Molecular Devices), filtered through a 5-kHz low-pass Bessel filter, and recorded with pCLAMP 10.2 software (Molecular Devices).
Fig. 7.
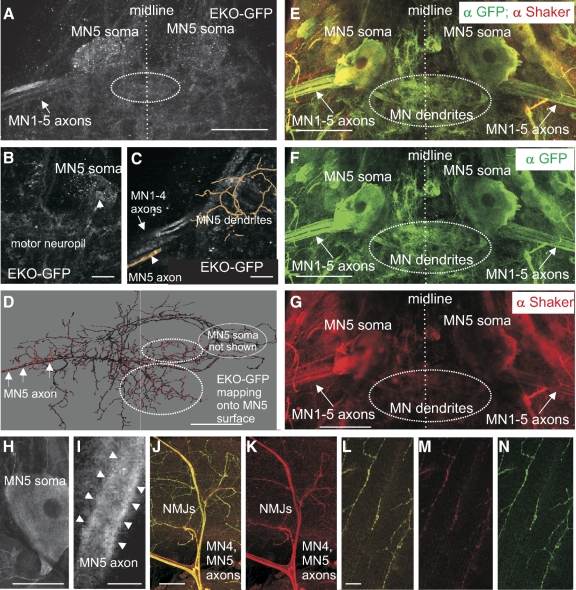
Localization of Shaker channels. A–D: EKO-GFP expression under the control of C380-GAL4;;ChaGAL80. A: representative projection view of confocal image stacks encompassing the dorsal part of the mesothoracic neuromere that contains the somata of both MN5s, the dendritic fields of MN1-5 (see dotted white oval), and the proximal axons of MN1-5 entering the left anterior dorsal mesothoracic nerve. Dotted line indicates ganglionic midline. White arrow points to EKO-GFP-positive axons of MN1-5. B: representative optical section demonstrates punctuated EKO-GFP label throughout MN5 soma, and weak, diffuse EKO-GFP label in the motor neuropil. Arrowhead points to somatic EKO-GFP label close to MN5 nucleus. C: single optical section of EKO-GFP staining superimposed on part of a geometric MN5 reconstruction containing some MN5 dendrites and part of the proximal axon (arrowhead). Strong EKO-GFP label is detected in MN1-4 axons (arrow), and MN5 axon reconstruction covers EKO-GFP label in MN5 axon (arrowhead). D: a representative MN5 surface reconstruction (soma omitted) is color coded for the EKO-GFP staining intensity that is co-localized with MN5 arborization. Brighter colors indicate higher EKO-GFP staining intensity. Arrows point to MN5 axon. EKO-GFP-positive dendritic arbors are marked by dotted white ovals. E–L: double immunostainings for GFP expressed under the control of C380-GAL4;;ChaGAL80 and Drosophila Shaker protein. E–G: representative projection views from cofocal image stacks (E, overlay; F, anti-GFP, green; G, anti Shaker, red) encompassing the dorsal part of the mesothoracic neuromere that contains the somata of both MN5s, the dendritic fields of MN1-5 (dotted white oval), and the proximal axons of MN1-5 entering the anterior dorsal mesothoracic nerves on both sides. Dotted line indicates ganglionic midline. White arrow points to Shaker-positive axons of MN1-5. H and I: single optical sections through the Shaker-positive soma (H) and axon (I) of MN5. Arrowheads indicate immunostaining localization to the membrane of the axon. J and K: representative field of view of MN4 and MN5 axonal arborizations on their target muscle. Yellow shows GFP and Shaker co-localization (J), and red shows the Shaker-positive label only (K). L and M: representative selective enlargements of MN5 terminal axon arborizations on the DLM (L, overlay of anti-GFP and anti-Shaker immunostaining; M, Shaker staining only, red; N, GFP staining only, green). Scales for A, D, and E–G are 50 μm, scales for B are 20 μm, scales for H and I are 10 μm, scales for J and L are 5 μm.
It seemed reasonable to choose temperatures that reflect those of the flies' natural habitat. However, others have recorded transient currents at 5 or 16°C (Wang and Wu 1996). A mathematical fluid transfer model that considers temperature effects on ion movements but not on channel protein properties predicted only minor effects of temperature on the activation speed of transient potassium channels [i.e., a difference of 0.009 ms for time to peak of I(A) between 16 and 24°C at a command potential of +20 mV]. To test whether fast activation and inactivation kinetics of A-type currents depended strongly on temperature, test recordings were conducted at 17, 21, and 25°C from the same neurons. Saline of these three different temperatures was applied via the perfusion system, and bath temperature was controlled with a micro-probe thermometer.
Before approaching the cell, the offset potential was zeroed manually while application of positive pressure avoided dilution of the pipette tip with saline. After obtaining a gigaohm seal, fast capacitive artifacts were compensated manually in cell attached mode at a holding potential of −70 mV. Whole cell configuration was achieved with gentle negative pressure. Recordings were carried out with serial resistances between 9 and 13 MΩ as read from the Axopatch 200B amplifier serial resistance adjustment. Serial resistances >15 MΩ (i.e., access resistance larger than twice the pipette tip resistance) did not allow recordings of the fast transient potassium currents in MN5. Whole cell capacitance was determined using the Axopatch 200B capacitance compensation (C-slow). Voltage errors caused by series resistance were predicted at 98%, and compensated for by 40–50% at a time constant of 2 μs. Input resistance was calculated from the linear slope of the I-V relationship at subthreshold command potentials and subtracted off-line.
Pharmacology
Potassium current measurements were performed in normal saline with tetrodotoxin (TTX; 100 nM, from a 1 mM stock solution in buffer) to block fast sodium currents. For measurements of total potassium currents, calcium currents were not blocked. The resulting error was estimated to be <10% of the potassium current amplitude because calcium current amplitudes in MN5 and other Drosophila motoneurons (Worrel and Levine 2008) are smaller by an order of magnitude than potassium current amplitudes. Cadmium (500 μM in normal saline) was bath applied via a gravitation perfusion system to block calcium and calcium-activated potassium currents. In some experiments, calcium was replaced by barium in an equimolar concentration. Peptide drugs for specific potassium channels were pipetted as stock solutions directly into the bath solution to obtain the desired working concentrations in the bath: α-dendrotoxin (DTX; 200 nM working concentration). DTX is a specific blocker for Shaker-mediated A-type potassium channels (Wu et al. 1989). Phrixotoxin-2 was used at a working concentration of 1 μM and acts as a Shal-specific blocker in Drosophila neurons (Gasque et al. 2005).
Data analysis
Appropriate voltage protocols, blockers, and off-line subtraction routines were used to isolate the currents. A-type potassium currents were isolated by subtracting the currents elicited by the voltage steps from a holding potential of −20 mV from the currents evoked from a holding potential of −90 mV. Calcium-activated currents were isolated by subtraction of currents measured in cadmium containing saline from currents measured from the same neuron in normal saline. The fast transient calcium current was not fully separated from the capacitance artifact at the onset of the command voltage pulse. To visualize the transient calcium current, the capacitance artifact was subtracted off-line by adding the capacitance artifact occurring at the end of the 200-ms command voltage pulse to the beginning of the pulse. Time to peak was measured by calculating the time between command onset and peak amplitude of the transient current. To create inactivation plots, 100-ms-long prepulses from −90 to +20 mV from a holding potential of −90 mV were applied before a 200-ms test pulse to +10 mV. All patch-clamp recordings were analyzed with clampfit 10.2 software (pCLAMP 10.2; Molecular Devices) and Excel 4.0 (Microsoft). Traces were exported as enhanced metafiles into Corel Draw 10 or 13 for the production of figures. Statistical significance was tested by paired or unpaired Student's t-test or ANOVA (as indicated) using Excel 4.0 (Microsoft) and Statistica 5.5 (StatSoft, Tulsa, OK).
Passive multicompartment model
Theoretic modeling of the passive dendritic tree structure of MN5 was conducted in NEURON. The import of the reconstructed geometries of MN5 into NEURON was done as described earlier (Evers et al. 2005). As values for the passive electrical properties of MN5, we used values commonly applied for vertebrate and invertebrate neurons (Meseke et al. 2009) (specific membrane capacity, Cm = 1.15*10−6 F*cm−2, axoplasmic resistance Ra = 90 Ω*cm, passive leak conductance g_pas = 0.0001667 Ω−1 cm−2). These values were consistent with measured values of whole cell input resistance and whole cell capacitance as recorded from the soma of MN5. The sole purpose of multicompartment models in this study was to determine the relative current transfer from dendritic and axonal regions to the soma of MN5 (see Cuntz et al. 2007).
Immunocytochemistry and intracellular staining
For immunostaining of GFP and Shaker potassium channels, flies were dissected in saline as for recordings (see preceding text). Then specimen were fixed in 4% paraformaldehyde (in 0.1 M PBS) for 50 min, washed six times for 20 min with PBS, washed with PBS-TritonX (0.3%) for 1 h, and washed with PBS-TritonX (0.5%) three times for 1 h. Then primary antibodies (rabbit anti-GFP, Invitrogen, 1:400) and goat anti-Shaker (Santa Cruz Biotechnology, 1:100) were applied overnight at 4°C in PBS-TritonX (0.1%). Incubation was followed by washing eight times for 30 min with PBS. Secondary antibodies (Cy2 goat anti-rabbit; Cy5 donkey anti-goat, both from Jackson) were applied at a concentration of 1:500 each in PBS and incubated overnight at 4°C. After washing eight times for 30 min with PBS, preparations were dehydrated in an ascending ethanol series (50, 70, 90, and 100%, 10 min each), and cleared and mounted in methylsalicylate.
Intracellular staining of MN5 was conducted with neurobiotin as described previously (Duch et al. 2008). Double staining for GFP-tagged ion channels and MN5 was conducted by combining the anti-GFP immunostaining protocol (see preceding text) with intracellular neurobiotin (Vector Laboratories) staining. MN5 was filled, fixed, and incubated with primary anti-GFP antibody (rabbit anti-GFP, 1:400 in PBS). Incubation and washing was conducted as described in the preceding text, and secondary Cy2 goat anti-rabbit antibody (1:500 in PBS) was incubated simultaneously with Cy3-streptavidin (1:1,000 in PBS, Invitrogen) overnight at 4°C.
Image acquisition
All images were acquired with a 40× oil-immersion objective (NA 1.0) with a Leica SP2 confocal laser scanning microscope at a resolution of 1,024 × 1,024 pixels and z-step width between 0.3 and 0.7 μm. Cy2 was excited with an argon laser at 488 nm and detected between 500 and 530 nm. Cy3 was excited with a Krypton laser at 568 nm and detected between 575 and 620 nm, and Cy5 was excited with a helium neon laser at 633 nm and detected between 640 and 700 nm. Cy2 and Cy5 were excited and detected simultaneously, but Cy3 was scanned in sequential scan mode with excitation switching between each frame. At the moderate laser and detector intensities needed no cross-talk between the three channels was observed. All images were saved as tiff stacks and further processed with AMIRA 4.1.1 (Mercury Systems) and Corel Draw 13.
RESULTS
The flight motoneuron, MN5, innervates the two most dorsal fibers of the dorsal longitudinal wing depressor muscle (DLM) (Consoulas et al. 2002; Ikeda and Koenig 1988). MN5 is a large unipolar motoneuron with its soma located in the mesothoracic neuromere of the ventral nerve cord (VNC), contralateral to its target muscle (Fig. 1A). As is the case with most insect motoneurons, the soma does not generate action potentials but is mostly passive (Duch and Levine 2000; Duch et al. 2008; Meseke et al. 2009). The dendrites of MN5 span large parts of the dorsal mesothoracic motor neuropil (Fig. 1A), and the total membrane surface of all central processes of MN5 comprises ∼13,000 μm2 (Duch et al. 2008). Whole cell capacitance, Cm, as measured from the soma of MN5 is 127 ± 16 (SD) pF. This also reflects ∼13,000 μm2 total membrane surface, assuming 1 μF per cm2 total membrane surface. Therefore although large dendritic trees can cause inaccuracies in measurements of Cm, our electrophysiological estimates of Cm match measurements of total membrane surface area based on MN5 geometric reconstructions from confocal image stacks (Fig. 1A) (Duch et al. 2008).
Passive multicompartment models tested for the relative current transfer from all central processes to the soma (see Cuntz et al. 2007). These models were not intended to provide exact estimates of how much current from distal processes can be seen in somatic voltage-clamp recordings but, instead, served as estimates as to whether some compartments of MN5 were electrotonically much better connected to the soma than others. In such models, every dendrite, the axon, and the soma were injected with steady-state rectangular current pulses, and the resulting current as measured from the soma of the model neuron was divided by the current resulting from a similar current injection directly into the soma. The resulting ratio indicated the relative current transfer from each segment to the soma of MN5. In Fig. 1B, each segment is color coded for this ratio. The model suggests that the relative current transfer to the soma is slightly better for most of the dendrites than for the axon. In addition, many of the dendrites show similar current transfer ratios to the soma. However, the distal dendrites show a slightly lower relative current transfer, at similar values as the axon (Fig. 1B). This indicates that voltage-clamp recordings from the soma may not overestimate specific compartments of MN5 but likely reflect most of the membrane of central MN5 processes (see discussion).
Earlier studies have shown that MN5 exhibits tonic firing at steady frequencies during flight in intact animals (Harcombe and Wyman 1977) and that tonic firing frequencies are altered in response to changing force demands as during lift (Gordon and Dickinson 2006). Extracellular recordings of MN5 from its target muscle fiber during restrained flight in an intact fly confirmed that MN5 exhibits tonic firing at 8–15 Hz without doublets or triplets of action potentials during stationary flight behavior (Fig. 1C) (see also Harcombe and Wyman 1977). The purpose of this study was to analyze the potassium currents of MN5 and to determine which channels underlie transient voltage-dependent potassium currents.
Total outward currents
The outward currents were examined in saline that contained TTX to block transient sodium currents. MN5 exhibits prominent outward currents on voltage steps from −90 to +20 mV in 10-mV increments from a holding potential of −90 mV (Fig. 1D). As in most other invertebrate neurons, these outward currents are carried by potassium and can be blocked by intracellular application of cesium (140 mM) and extracellular application of TEA (30 mM). By contrast, 4-AP (≤4 mM) blocked only a small portion of the transient outward current in MN5. Fast, transient potassium current can be mostly separated from the capacitance artifact (Fig. 1, C–J). However, in some recordings, the settling time following the capacitance artifact might have slightly increased the variability of quantitative measurements of times to peak and peak current amplitudes. For better visualization, artifacts have been removed in most following figures. The potassium currents are composed of at least two transient currents, one of which is calcium dependent and two sustained components, one calcium independent, and the other one calcium dependent (Fig. 1, E and F). Bath application of cadmium (500 μM) blocks all calcium current and therefore is used to isolate calcium-independent potassium outward currents (Fig. 1E). These comprise a fast-activating, fast-inactivating A-type like potassium current, I(A), and a delayed rectifier like potassium current, I(K). See in the following text for further characterization of I(A) and I(K) (Figs. 2, 3). Subtracting the calcium-independent potassium currents (Fig. 1E) from the total outward currents (Fig. 1D) reveals the calcium-dependent potassium outward currents (Fig. 1F). The result of this subtraction also contains calcium currents, but calcium current amplitude is by an order of magnitude smaller than calcium activated potassium current amplitude in MN5, so that the latter can be isolated by subtraction without revealing any inward current and only a minor error in the outward current shape and amplitude (Fig. 1F). Calcium-activated potassium currents in MN5 comprise a fast-activating, fast-inactivating current, ICF, and a slower noninactivating current, ICS. The terms ICF and ICS are according to Zhong and Wu (1991). The fast ICF and the slow ICS are clearly separated by their different activation time courses (Fig. 1F). Furthermore, ICF activates faster than I(A) (Fig. 1, E and F; see also Fig. 3Ci).
Fig. 2.
Noninactivating potassium currents in control animals. A–C: representative current traces of sustained potassium currents. Currents in A and B are elicited by voltage steps from −90 to +20 mV in 10-mV increments from a holding potential of −20 mV (D). Subtraction of B from A reveals a calcium-dependent, sustained potassium current (C). E and F: voltage-current relationships of sustained currents after normalization to cell size (E) and with respect to total amplitudes (F). Amplitudes are determined from the sustained portion of potassium currents elicited from a holding potential of −90 mV (see inset in E). The calcium-independent component activates around −50 mV (black squares), whereas the calcium-dependent portion activates around −20 mV (light gray triangles). Error bars represent SE.
Fig. 3.
Transient potassium currents in control animals. A–C: transient potassium currents were electrically isolated by subtracting current traces elicited from a holding potential of −20 mV from traces evoked from a holding potential of −90 mV. A and B: electrically isolated total (A) and calcium-independent total transient currents (B) do not completely inactivate (see right arrow in A). C: calcium-dependent transient potassium current as revealed by subtraction of B from A. Inset Ci: a representative transient calcium current evoked by a command voltage step from −90 to −50 mV. D: current-voltage relationship of transient potassium currents after normalization to cell size (D) and with respect to total amplitude (E).  , the calcium-independent transient potassium current; ⧫, the calcium dependent portion of the transient current shown in C. Current amplitudes for I-V plots in D and E are measured from peak currents (see left ↓ in A). Inset Ei: the total transient potassium current as recorded from 57 different animals (
, the calcium-independent transient potassium current; ⧫, the calcium dependent portion of the transient current shown in C. Current amplitudes for I-V plots in D and E are measured from peak currents (see left ↓ in A). Inset Ei: the total transient potassium current as recorded from 57 different animals ( ). ▪, average; error bar represents SD. F: voltage-dependent activation and inactivation of the calcium-independent [I(A),
). ▪, average; error bar represents SD. F: voltage-dependent activation and inactivation of the calcium-independent [I(A),  ] and the calcium-dependent transient potassium current [ICF, ⧫]. G: times to peak of the calcium-independent (
] and the calcium-dependent transient potassium current [ICF, ⧫]. G: times to peak of the calcium-independent ( ) and the calcium-dependent A-type current (⧫) over command potentials from −50 to +20 mV. Asterisks indicate statistical significance (unpaired t-test; * indicate P < 0.01). Error bars represent SE.
) and the calcium-dependent A-type current (⧫) over command potentials from −50 to +20 mV. Asterisks indicate statistical significance (unpaired t-test; * indicate P < 0.01). Error bars represent SE.
The activation kinetics of fast-activating potassium currents were <1.5 ms at 24°C (see also following text), and thus faster than A-type currents recorded previously from larval Drosophila motoneurons (Choi et al. 2004). However, in Drosophila muscle, it has been demonstrated that decreases of the bath temperature by only 8°C has significant effects on potassium current amplitude and activation speed (Wang and Wu 1996). Therefore we recorded potassium outward currents in TTX containing saline at 25, 21, and 17°C (Fig. 1, H–J). Lowering the temperature from 25 to 21°C caused a reduction in current amplitude by ∼25% and doubled the time to peak (1.05 ms at 25°C, 2.2 ms at 21°C; Fig, 1, L and J). Lowering the temperature from 21 to 17°C further reduced the current amplitude by ∼20% and increased nearly doubled the time to peak (2.2 ms at 21°C, 3.7 ms at 17°C). All following experiments are conducted at 24°C because this represents a temperature at which flies show frequent flight motor behavior in their natural environment.
Sustained potassium currents
The noninactivating potassium currents (Fig. 2) can be isolated by voltage steps from a holding potential of −20 mV (voltage steps from −90 to +20 mV in 10-mV increments, Fig. 2D), where all transient voltage-dependent potassium current components are inactivated. This electrically isolates a potassium outward current consisting of ICS and I(K) (Fig. 2A). The calcium-independent component, I(K) can be separated by bath application of cadmium (500 μM) to block calcium influx (Fig. 2B). Subtraction of I(K) (Fig. 2B) from the total outward current elicited from a holding potential of −20 mV (A) reveals ICS (C). The current-voltage relationships for I(K) and ICS are shown as mean current amplitudes normalized to cell size (Fig. 2D) and as mean total current amplitudes (E). I(K) activates at potentials around −50 mV. By contrast, ICS activates at significantly more positive membrane potentials (about −20 mV; Fig. 2, E and F, ANOVA, P < 0.05). The slopes of the current-voltage curves are similar for I(K) and ICS (Fig. 2, E and F).
Transient potassium currents
Electrical isolation of the voltage dependent A-type like currents (Fig. 3) is accomplished by subtracting current traces elicited from a holding potential of −20 mV from traces evoked from a holding potential of −90 mV. For both holding potentials, the command voltage steps are set between −90 and +20 mV in 10-mV increments. The resulting total transient potassium current is shown in Fig. 3A. It consists of two components that can be separated into one calcium-independent current [I(A), Fig. 3B) and one calcium-dependent portion (ICF; C) by bath application of 500 μM cadmium and subtraction of calcium independent (Fig. 3B) from the total transient potassium current (A). ICF activates and inactivates faster than I(A) (Fig. 3, B and C). Both I(A) and ICF show a rapidly inactivating component but both do not completely inactivate (Fig. 3, A, see right ↓, B, and C) within the duration of the command pulses (200 ms). Therefore I(A) and ICF each contain a fast and a slower inactivating component (Fig. 3, B and C). However, for ICF, the slower component might also reflect a portion of ICS, which in turn might have been caused by different activation amplitudes of ICS between holding potentials of −20 and −90 mV. It is clear that both protocols activate I(K) similarly, but it remains unclear whether this is also the case for ICS because we have not studied the properties of MN5 calcium currents in detail.
The voltage-current relationships of I(A) and ICF peak currents show that both currents activate at potentials between −60 and −50 mV (Fig. 3, D and E). Therefore the activation voltage of ICF (Fig. 3, D and E) is ∼30 mV more hyperpolarized than that of the slow calcium-dependent ICS (Fig. 2, E and F). By contrast, the calcium-independent currents, I(A) and I(K), both activate at about −50 mV. The I-V plots for ICF and I(A) show means ± SE (Fig. 3, D and E). To visualize the overall variability of the dataset, the total transient potassium current at a command voltage of +20 mV is shown for 57 individual MN5 recordings (Fig. 3Ei).
Due to the extensive dendritic field of MN5 (Fig. 1, A and B) command voltage steps more positive than +20 mV require to inject large-amplitude currents very fast through the patch pipette (>4 nA/ms), which often results in unstable recording conditions. Therefore command voltages more positive than +20 mV are omitted from this study. Consequently, not all A-type like potassium channels were fully activated at maximum depolarizing voltage commands; this resulted in incomplete saturation of the activation curve (Fig. 3F). Therefore the half-maximal activation voltages for A-type potassium currents in MN5 lack some accuracy. However, fitting the activation data of I(A) and ICF with first-order Boltzmann relationships yields half-maximal activation voltages of −12.1 ± 1.4 mV for I(A) and −22.34 ± 2.02 mV for ICF (Fig. 3F, see dotted line). Steady-state inactivation was measured by applying prepulses of 100-ms duration from −90 to +20 mV in 10-mV increments followed by a 200-ms lasting voltage step to +10 mV. First-order Boltzmann fits of the inactivation curves show that ICF is half inactivated at −49.33 ± 0.24 mV, whereas half inactivation for I(A) is −40.02 ± 0.33 mV (Fig. 3F, see ···). In summary, half-maximum activation and inactivation of I(A) is ∼10 mV more depolarized than that of ICF.
Both transient potassium currents that are present in MN5 have fast activation kinetics at our recording temperature of 24°C (see methods). Times to peak were measured by calculating the duration from pulse onset to peak amplitude for each particular current. At the activation voltage of −50 mV, time to peak for I(A) is 2.42 ± 0.31 ms and 1.36 ± 0.23 ms for ICF. Time to peak decreases between command voltages of −50 and −20 mV and remains constant at command voltages more depolarized than −20 mV. Time to peak at a command voltage of +20 mV is 1.46 ± 0.23 ms for the calcium-independent I(A) and 1.00 ± 0.25 ms for ICF (Fig. 3G) current. Differences in time to peak of I(A) and ICF are significant (paired Student's t-test, P < 0.05). The fast time to peak for ICF raises the question whether MN5 contains a calcium current that activates fast and hyperpolarized enough to activate ICF by voltage-dependent calcium influx. A transient I(Ca) was recorded in saline containing TEA and TTX and with an internal solution containing cesium, TEA, and 4-AP (see methods). Command voltage steps from −90 to +20 mV in 10-mV increments revealed a transient I(Ca), which activated around −50 mV (not shown) and had a time to peak of 0.76 ± 0.16 ms. A representative example trace of transient calcium inward current in response to a command voltage step from −90 to −50 mV is shown as inset in Fig. 3C (Fig. 3Ci). In summary, I(Ca) in MN5 activates at more hyperpolarized membrane potentials than ICF, so that activation voltage and activation time constant are consistent with the fast activation times of ICF. I(Ca) was completely abolished by bath application of 500 μM cadmium (not shown).
Shaker channels mediate 40% of the total transient potassium outward current
To investigate which genes underlie transient voltage-activated potassium currents in Drosophila MN5, we used pharmacological agents and targeted genetic manipulations. The two candidate genes thought to underlie I(A) in Drosophila are Shal and Shaker. We have previously demonstrated that dominant negative knock down of Shaker potassium channels increases the intrinsic excitability of MN5 (Duch et al. 2008). The green mamba toxin, DTX, specifically blocks Kv1 (Shaker-related) potassium channels in nanomolar concentrations in vertebrates (for review, see Harvey 2001; Harvey and Robertson 2004) as well as in Drosophila (Wu et al. 1989). In MN5 DTX blocked a large portion of I(A). I(A) was electrically isolated by subtracting current traces elicited from a holding potential of −20 mV from traces evoked from a holding potential of −90 mV (see preceding text) in cadmium (500 μM) containing saline (Fig. 4 A). Application of DTX (200 nM) abolished a large portion of the transient I(A) and also some of the slower inactivating component of I(A) (Fig. 4B, compare with Figs. 1 and 3). To further test whether DTX is selective for Shaker channels, it was applied following targeted genetic knock down of Shaker channels in MN5 by expressing a dominant negative transgene for Shaker (SDN) (Mosca et al. 2005) under the control of C380-GAL4. As genetic controls, C380-GAL4 females were crossed with W1118 males; this is the genetic background for flies carrying the SDN transgene. In controls, DTX eliminated a large portion of the I(A) (Fig. 4C, example traces at −10 mV, gray with DTX). By contrast, following genetic knock down of Shaker potassium channels, I(A) was strongly reduced, and DTX had no effect on the remaining potassium current (Fig. 4D, example traces at −10 mV, gray with DTX).
Fig. 4.
Shaker current in MN5. A: representative I(A) as isolated by bath application of cadmium. B: a large portion of I(A) is blocked by application of 200 nM DTX. C and D: representative I(A) current traces for command potentials of −10 mV in control (C) and following expression of a Shaker dominant negative transgene (SDN) in MN5 (D) in normal saline (black traces) and after application of 200 nM DTX (gray traces). In control animals, DTX reduces A-type current amplitude (C). In SDN, the total transient current amplitude is decreased as compared with control animals (C and D) but not affected by DTX application (D). E: current-voltage relationship of transient currents normalized to cell size in control animals (black lines) and SDN (light gray symbols). Shown are I-V curves in normal saline (control animals: black diamonds; SDN: light gray diamonds) and after application of 200 nM DTX (control animals: black squares; SDN: light gray squares). F: relative amplitude of the transient potassium current that remains after application of 200 nM DTX in control animals (dark gray bars) and SDN (light gray bars) with respect to total transient current evoked in normal saline. In control animals, DTX reduces the current by 35–40%, but it has no effect following SDN expression. Asterisks indicate statistical significance (ANOVA P < 0.001). G: representative DTX-sensitive current from a control animal as revealed by subtraction of current in DTX from current in TTX containing saline. Error bars in E represent SE and SD in F.
The current-voltage relationships for the total transient potassium currents (Fig. 4E) were compared among four experimental groups: in control animals and in animals carrying a dominant negative knock down for Shaker channels (SDN). Both were recorded in TTX containing saline before and after DTX application. Activation voltages were similar for all four groups (Fig. 4E). DTX reduced the peak current amplitudes over all command potentials in controls but not in MN5 expressing a dominant negative knock down for Shaker (Fig. 4E). In fact, the current-voltage relationship of the transient current after DTX application in control animals was similar to those observed for SDN with or without DTX (Fig. 4E). This demonstrates that acute DTX application and expression of a dominant negative transgene for Shaker throughout postembryonic development were similarly effective in eliminating Shaker-mediated currents. It further showed that expression of SDN during development did not result in compensatory expression of other channels that mediate A-type potassium currents.
In SDN animals, nearly 100% of the total transient potassium outward current remained after DTX application. This was true for all command potentials investigated. In control animals, ≤60% of the total transient potassium current remained after bath application of DTX (Fig. 4F). This indicates that Shaker underlies 40% of the total transient potassium outward current. Differences in amplitude of the remaining current after acute DTX treatment between control and SDN animals were significant over all command voltages investigated (Fig. 4F, unpaired Student's t-test, P < 0.05). Subtraction of I(A) before and after DTX application revealed the Shaker-mediated I(A) in MN5 (Fig. 4G).
In summary, in the wild-type MN5, Shaker comprises 40% and ICF comprises 30% of the total transient outward current. Shaker currents can be blocked by DTX, and ICF can be blocked by cadmium. Further experiments were carried out to determine which channels underlie the remaining 30% of the total transient potassium current.
Shal channels mediate 30% of the total transient potassium outward current
Bath application of the Kv4 (Shal-related) selective spider toxin phrixotoxin-2 (1 μM) (Gasque et al. 2005) reduced the amplitude of the total transient potassium current (Fig. 5, A and B). The Shaker selective blocker DTX further reduced the phrixotoxin-2-insensitive current (Fig. 5C), suggesting that both Shal and Shaker contribute to I(A). Additional application of cadmium (500 μM) to the same recording abolished most of the remaining potassium current (Fig. 5D). Therefore the total transient potassium current is almost completely abolished by Shaker, Shal, and calcium current blockade. The phrixotoxin-2-sensitive current was revealed by subtraction and is fast activating/fast inactivating (Fig. 5E). These pharmacological data indicate that Shal mediates ∼30% of the total transient potassium current in MN5 (see quantification in Fig. 5I, differences in current amplitudes before and after phrixotoxin-2 in control animals are significant, paired Student's t-test, P < 0.01). The contribution of Shal channels was further investigated by targeted RNAi knock down for Shal expressed in MN5 under the control of C380-GAL4. Following Shal RNAi knock down, I(A), as recorded in cadmium-containing saline (Fig. 5F), was reduced by the Shaker-specific toxin DTX (G) but not by the Shal-specific toxin phrixotoxin-2 (H). Phrixotoxin-2 also had no effect on potassium currents of Shal RNAi knock down animals as recorded in saline without cadmium or DTX (not shown). Therefore phrixotoxin-2 reduced I(A) in control animals but not following targeted RNAi knock down for Shal. This shows that Shal RNAi knock down selectively eliminates phrixotoxin-2-sensitive I(A). The DTX-sensitive I(A) that remains in Shal RNAi knock down has a longer time to peak as compared with controls (Fig. 5A). This is statistically significant (1.46 ± 0.23 ms in controls, 4.03 ± 1.94 ms following Shal RNAi knock down, P < 0.01, Student's t-test). Current-voltage relationships for controls, controls in phrixotoxin-2, and Shal RNAi knock down are shown in Fig. 5I. Differences in current amplitudes before and after phrixotoxin-2 in control animals (paired Student's t-test, P < 0.01) and between current amplitudes as recorded in saline in control animals and Shal RNAi knock down animals are significant (unpaired Student's t-test, P < 0.01). Shal RNAi reduced the total transient potassium current amplitude by 30% as compared with controls. Similarly, acute pharmacological block of Shal by phrixotoxin-2 application to control neurons reduced the total transient potassium current amplitude by 30%. This demonstrates that acute phrixotoxin-2 application and expression of Shal RNAi throughout postembryonic development were similarly effective in eliminating Shal-mediated currents. It further demonstrated that expression of Shal RNAi during development did not cause compensatory expression of other channels that mediate A-type potassium currents. However, compensatory changes of other currents cannot be excluded.
Fig. 5.
Shal current in MN5. A–D: total potassium currents as recorded from a representative control animal in TTX (A), and after the sequential application of phrixotoxin-2 (1 μM; B), DTX (200 nM, C), and cadmium (500 μM, D). E: representative phrixotoxin-2-sensitive current from a control animal as revealed by subtraction of current in phrixotoxin-2 from current in TTX containing saline. F–H: representative total potassium currents as recorded following Shal RNAi knock down in MN5. I(A) (F; measured in TTX and 500 μM cadmium saline) is strongly reduced by application of DTX (G). Bath application of phrixotoxin-2 has no effect (H). I: current-voltage relationship of transient potassium currents in control (black diamonds) and Shal RNAi knock down animals (gray diamonds) in TTX and after application of phrixotoxin-2 in control animals (back squares). Error bars represent SE. Values in TTX containing saline are significantly different as compared with phrixotoxin-2 application to control animals (paired Student's t-test, P < 0.01) and to TTX containing saline in Shal RNAi knock down animals (unpaired Student's t-test, P < 0.05 for −20 mV and P < 0.01 for −10 mV through +20 mV).
Barium blocks a large portion of phrixotoxin-2-sensitive outward current
Barium acts as a competitive blocker for a variety of potassium channels (Hurst et al. 1996). In addition, barium does not activate calcium-dependent potassium currents. In MN5, the blockade of all calcium-activated potassium current by bath application of cadmium causes a reduction in transient potassium current by ∼30% (Figs. 1 and 3). By contrast, replacing external calcium with barium (1.8 mM) resulted in a 60% reduction of the total transient potassium current (Fig. 6 E). Note that 60% of the total transient potassium current amplitude equals to the sum of ICF and Shal-mediated current. Therefore we tested whether barium acted also as a blocker for voltage-dependent potassium channels in MN5. Voltage-activated potassium outward currents were isolated by bath application of cadmium (Fig. 6A). Barium further reduced the amplitude of calcium-independent potassium currents (Fig. 6B). Most of the calcium-independent potassium current that remained after cadmium and barium application was blocked by bath application of DTX (200 nM, Fig. 6C) to the same preparation. This indicated that barium blocked Shal channels. However, even after barium and DTX, a small transient outward current remained (Fig. 6C), which was sensitive to phrixotoxin-2 (D). Therefore barium did not block all current that was sensitive to the Shal-specific blocker phrixotoxin-2. Quantification of these data as I-V plots indicates that barium blocks Shal currents but likely not Shaker currents (Fig. 6F). The amplitude of I(A) as recorded in cadmium containing saline (Fig. 6F, black squares) was reduced by 60% by bath application of DTX (Fig. 6F, black circles). Bath application of barium reduced I(A) to a lesser extent (Fig. 6F, gray squares) than cadmium and DTX application. Bath application of DTX to barium containing saline eliminated almost all I(A) (Fig. 6F, gray triangles), demonstrating that almost all of the current left in barium was DTX-sensitive Shaker current. The small remaining current after barium and DTX is consistent with the finding that a small phrixotoxin-2-sensitive current component remains after barium application (Fig. 6, C and D). However, we cannot exclude the possibility that barium might not be completely selective for Shal but may also block a small portion of Shaker-mediated current.
Fig. 6.
Barium blocks Shal potassium currents. A–D: total potassium currents in control animals elicited by voltage steps from −90 to +20 mV in 10-mV increments from a holding potential of −90 mV in TTX. A: I(A) as revealed after application of 500 μM cadmium. B: same as in A but with barium added to the bath (1.8 mM). C: addition of 200 nM DTX to the same recording abolished most of the remaining current. D: phrixotoxin-2 (1 μM) abolished the remaining small transient current. E: relative peak current amplitudes in TTX containing, calcium-free, barium containing saline with respect to the current amplitude in TTX and calcium containing control saline. F: current-voltage relationship of peak current amplitudes normalized to cell size in 500 μM cadmium [I(A); black squares], after application of cadmium and 200 nM DTX (dark gray circles), in calcium-free solution with barium as a substitute (medium gray squares), and with application of 200 nM DTX to calcium-free, barium containing solution (light gray triangles). Error bars represent SE.
Shaker channels are localized predominantly to the axon
Why do two different genes code for I(A) in the same neuron? One possibility is that Shaker and Shal channels are localized to different cellular compartments. In many vertebrate neurons, Kv1 channels (Shaker-like) are predominantly axonal and have a specific axonal targeting domain (Gu et al. 2003), whereas dendritic locations have been reported for Kv4 channels (Shal) (Chen et al. 2006; Johnston et al. 2000). Similarly, in some neurons in the Drosophila brain immunocytochemical data suggest that Shaker potassium channels are targeted to the axons (Rogero et al. 1997), and electrophysiological data demonstrate the presence of Shaker channels in the axon terminals of larval Drosophila motoneurons (Lee et al. 2008; Ueda and Wu 2006). We tested for a possible axonal localization of Shaker channels in MN5 with two approaches. First, we expressed a modified GFP-tagged Shaker channel transgene with altered voltage sensitivity but intact targeting sequence (EKO-GFP) (White et al. 2001) in MN5 under the control of C380-GAL4;;ChaGAL80 (Fig. 7, A–D). Second, immunostaining for native Drosophila Shaker channels was analyzed in relation to the expression of GFP in MN1-5 under the control of C380-GAL4;;ChaGAL80 (Fig. 7, E–L).
Representative projection views from confocal image stacks following EKO-GFP expression under the control of C380-GAL4;;ChalGAL80 reveal prominent label in the axons of the flight motoneurons, MN1-5 (Fig. 7A, arrow). In addition, the large cell bodies of MN5 on both sides show GFP label in such preparations (Fig. 7, A and B, MN1-4 cell bodies are out of focus in this projection view). Careful inspection of single optical section (not shown) indicated that somatic label was not restricted to the membrane but that EKO-GFP was localized throughout the cytosol and around the nucleus (Fig. 7B, arrowhead). Therefore somatic label might represent the production of the channel protein. No positive label was detected along the primary neurite connecting the soma and the axon. However, some diffuse EKO-GFP label was detected in the flight motoneuron neuropil (Fig. 7A, see dotted circle) and in some nonidentified neuropil areas. Intracellular fills and geometric reconstruction of MN5 (Duch et al. 2008) were conducted in the same preparations to test for co-localization with EKO. Superimposing single optical sections of EKO-GFP label onto part of a representative MN5 reconstruction revealed prominent localization to the axon of MN5. The MN5 axon reconstruction overlays with axonal EKO-GFP label, and neighboring MN1-4 axons show bright EKO-GFP label (Fig. 7C, see arrows). We have previously published methods for co-localization analyses of immunolabeled structures and reconstructed dendritic surfaces (Duch and Mentel 2004; Evers et al. 2005; Meseke et al. 2009; Schmitt et al. 2004). In the representative example in Fig. 7D, the intensity of EKO-GFP that is localized within the boundaries of the MN5 reconstruction is depicted as a color code on the geometric MN5 reconstruction with brighter colors representing higher EKO-GFP staining intensities. The MN5 soma is omitted for better visualization. Most prominent EKO-GFP localization within MN5 is found in the axon (Fig. 7D, see arrows), but some label is also detected in proximal dendrites (Fig. 7D, see dotted circles). Optical resolution of the confocal images was 120 × 120 × 310 nm. Therefore we are confident that EKO-GFP localization in the axon of MN5 (diameter, 1–2 μm) can be detected within the limits of optical resolution. By contrast, for EKO-GFP mapping to small dendrites (0.4–0.8 μm diam) optical resolution limits make it impossible to decide unambiguously whether the label is caused by protein localization within the dendrites or within neighboring processes. Another problem with targeted expression of GFP tagged proteins under the control of a nonendogenous promoter, like C380, is potential mis-targeting as might be caused by false expression times or dosages. Therefore we conducted immunocytochemistry for native Drosophila Shaker channels as a second approach (Fig. 7, E–N).
Anti-Shaker was combined with anti-GFP immunostaining in flies expressing GFP under the control of C380-GAL4;;ChaGAL80 to reveal the locations of MN5 dendrites, soma, and axon (Fig. 7, E and F). Prominent Shaker immunopositive label was detected in the axons of MN1-5 (Fig. 7, E and G, see arrows). High-magnification single optical sections indicated that Shaker was localized to the membrane of the axonal compartment (Fig. 7I, see arrowheads). In addition, some Shaker label was found in the MN5 somata. Careful inspection of single optical sections indicated that Shaker label was not restricted to the somatic membrane (Fig. 7H) but instead throughout the somatic cytosol. However, light microscopy resolution is not sufficient to rule out a possible localization of Shaker channels to the soma membrane of MN5. In correspondence with the data on EKO-GFP localization (Fig. 7, A, B, and D), some diffuse Shaker positive staining was found in the neuropil where the dendrites of MN1-5 are located (Fig. 7G, see dotted circle). As with EKO-GFP label in the neuropil, optical resolution is not high enough to decide unambiguously whether Shaker is localized to some flight motoneuron dendrites or to other processes in close proximity. However, immunostainings as well as EKO-GFP expression data clearly demonstrate localization of Shaker channels to the proximal axonal compartment of MN5 but not to the primary neurite between the soma and the axon. Large-diameter dendrites were also devoid of Shaker label in both approaches. Therefore Shaker channels are clearly localized to the proximal axonal compartment of MN5. We further tested whether the axon contained Shaker channels all the way from its proximal part in the CNS to the target muscle. In fact, all axonal arbors of MN1-5 were Shaker immunopositive throughout the nerve and over their target muscle (DLM, Fig. 7, J and K). Moreover, all MN1-5 terminals on the DLM show Shaker immunopositive staining (Fig. 7, L–N). Therefore both approaches strongly indicate axonal targeting of Shaker channels, but additional localization to some dendrites cannot be excluded.
DISCUSSION
This study revealed at least four different potassium currents in the identified adult Drosophila motoneuron, MN5, two are calcium independent and two calcium dependent. This is reminiscent to the potassium current complement previously described in a variety of insect (e.g., Byerly and Leung 1988; Gasque et al. 2005; Heidel and Pflüger 2006; Wüstenberg et al. 2004; Xu et al. 2005) and vertebrate neurons (for review, see e.g., Coetzee et al. 1999; Maffie and Rudy 2008). Potassium current densities are in a similar range to what has been found in cultured cytokinesis arrested Drosophila giant neurons (Peng and Wu 2007a,b). Pharmacological and genetic approaches identified the genes underlying I(A). We did not use mutations for potassium channels but targeted knock down expressed in a subset of motoneurons and a small number of unidentified neurons under the control of C380-GAL4;; ChaGAL80 (Duch et al. 2008). The rationale was that mutants affect all cells during all stages of development, including neurons presynaptic to MN5 as well as its target muscle and thereby might cause more potential secondary effects than the targeted expression of transgenes in a subset of neurons. The effectiveness of the dominant negative knock down for Shaker has been tested previously (Duch et al. 2008; Mosca et al. 2005), and its selectiveness has been further confirmed pharmacologically in this study. We also confirmed the selective knock down of Shal RNAi pharmacologically. Following Shal RNAi expression in MN5 potassium currents that are sensitive to the Shal-specific blocker phrixotoxin-2 are abolished, but calcium-dependent and DTX-sensitive potassium currents are present at normal amplitudes (see following text). The results are summarized schematically in Fig. 8 and discussed in the following text. Identification of the genes responsible for the adult I(A) in MN5 sets the framework for unraveling its functions during development (Duch et al. 2008) and during behavior.
Fig. 8.
Relative contributions of Shaker and Shal to I(A) in MN5. Bars indicate percentage (y axis) of total transient potassium current in MN5 following different genetic and pharmacological manipulations. Gradient bars indicate currents mediated by at least two genes. From left to right: left bar shows that 30% of the total transient potassium current is calcium dependent (light gray part of 1st bar), and 70% is calcium independent [I(A), medium to dark gray gradient part of first bar]. Middle bar shows that 60% of I(A) and thus 40% of the total transient potassium current are DTX sensitive and are eliminated in Shaker dominant negative knock down (SDN, dark gray part of 2nd and 3rd bars). The remaining 60% can be blocked by barium (light to medium gray part of 2nd bar). Right bar shows that half of the barium-sensitive current, and thus 30% of the total transient potassium current, are eliminated in cadmium (ICF, light gray part of 3rd bar). The other half of the barium-sensitive (but calcium independent) current, and thus 30% of the the total transient potassium current [or 40% of I(A), respectively], is blocked by phrixotoxin-2 and abolished in Shal RNAi knock down (medium gray part of 3rd bar).
How much of the cell can be seen?
In situ recordings of large neurons are susceptible to inadequate space clamp, i.e., in many preparations it is not clear how much of the total current is reflected in somatic recordings (Heckman et al. 2003; Lee and Heckman 1996). Several lines of evidence indicate that our somatic voltage-clamp recordings reflect currents originating from large parts of the somatodendritic domain and the proximal axon of MN5. First, whole cell capacitance (127 ± 16 pF) as measured in voltage clamp matches the surface of MN5 (13,000 μm2) as determined by geometric reconstruction from confocal image stacks (assuming 1 μF/cm2 membrane) (Duch et al. 2008). Second, fast Shaker potassium currents can be recorded in voltage clamp from the soma, although immunocytochemistry and the expression of GFP-tagged channels indicate that the majority of these channels is located in the axon of MN5 (see following text). Third, passive multicompartment models indicate that most dendrites are electrotonically not further away from the soma than the axon. Fourth, recordings of fast currents required serial resistances <15 MΩ. For smaller embryonic and larval Drosophila motoneurons, series resistance of >50 MΩ is sufficient to record fast currents (Baines and Bate 1998; Choi et al. 2004). Therefore we are confident that the currents measured in this study result from channels located throughout large parts of the dendrites and the proximal axon. However, we cannot exclude the possibility that the measurements might be dominated by currents from proximal parts of MN5, or that the voltage sensitivity is affected by channel location. In addition, we know that knock down of potassium channels causes dendritic overgrowth in adult (Duch et al. 2008) and larval Drosophila motoneurons (Hartwig et al. 2008), which in turn might cause slightly altered current properties due to changes in passive electrical dendritic tree structure.
Shaker and Shal mediate I(A) in MN5
The two genes encoding Drosophila I(A) are Shaker and Shal (Covarrubias et al. 1991; Wei et al. 1990). The Shaker-selective toxin DTX blocks 40% of the total transient potassium current, consisting of I(A) and ICF. This equals 60% block of I(A) (Fig. 8). Genetic knock down of Shaker channels results in a similar reduction of I(A), and DTX does not further reduce current amplitude in the knock down. This demonstrates that Shaker channels comprise 60% of I(A) in MN5.
Thirty percent of the total transient potassium current, and thus 40% of I(A), were blocked by the Shal-selective toxin phrixotoxin-2 (Gasque et al. 2005). RNAi knock down of Shal resulted in a similar reduction of I(A). The remaining I(A) in Shal RNAi knock down was DTX sensitive, whereas phrixotoxin-2 did not further reduce I(A) in these animals.
Therefore Shaker channels underlie 60% and Shal channels underlie 40% of I(A) as recorded from the soma of MN5 (Fig. 8). The total transient potassium current is reduced by 30% by phrixotoxin-2 or by Shal knock down and by 40% by DTX or by Shaker knock down, demonstrating that mixed Shaker/Shal I(A) makes up 70% of the total transient potassium current (Fig. 8). This matches the percentage of I(A) measured in cadmium. Therefore several different genetic and pharmacological results support the relative contributions of Shaker, Shal, and ICF to the transient potassium currents in MN5 (Fig. 8).
Shaker channels are expressed in Drosophila photoreceptors (Hardie 1991). Both Shal and Shaker channels are present in cultured Drosophila mushroom body neurons (Gasque et al. 2005). By contrast, Shal has been suggested to carry I(A) in embryonic (Baines and Bate 1998; Tsunoda and Salkoff 1995) and larval Drosophila motoneurons (Choi et al. 2004). In this study, we find both Shaker and Shal currents in an adult flight motoneuron. Therefore adult flight and larval crawling motoneurons might use different channel combinations for transient potassium currents, which might reflect their different functions in slow crawling and fast flight movements.
In addition to I(A), we also found fast-activating, fast-inactivating calcium-dependent potassium current in MN5 (ICF). Although the identification of the channels is beyond the scope of this study, there are three potential candidate genes that might code for this current: slowpoke (slo), SK, and eag. The vertebrate SK channel blockers apamin and scyllatoxin (Strøbæk et al. 2000) have no effect on the transient calcium activated potassium current in MN5 (data not shown), but it remains unclear whether these are effective blockers for Drosophila SK channels. The slo channel blockers charybdotoxin and iberiotoxin (Derst et al. 2003) had no effect on MN5 calcium-activated potassium current (data not shown), but it remains unclear whether these toxins block all possible splice variants of slo channels in Drosophila. Earlier studies in the Drosophila DLM flight muscle showed that eag hypomorph mutants cause a decrease in multiple potassium currents, including the calcium-dependent ones (Zhong and Wu 1991), but it remains unclear whether eag might also play a role in flight motoneuron calcium-activated potassium current. Further combined genetic and pharmacological analysis as demonstrated for Sh and Shal in this study, will be necessary to identify the genes coding for ICF in adult Drosophila flight motoneurons.
Targeted knock down of Shaker or Shal in MN5 do not cause upregulation of other channels underlying transient potassium currents
In both vertebrate and invertebrate neurons, perturbations of neuronal excitability can be compensated for by homeostatic regulation (Marder and Goaillard 2006; Turrigiano and Nelson 2004). Such mechanisms can potentially occur in genetic knock downs. In cultured Drosophila giant neurons, calcium channel mutations cause upregulation of Shaker, whereas calcium channel levels remain unaltered in potassium channel mutants (Peng and Wu 2007b). At the larval Drosophila neuromuscular junction, mutations for Shaker channels cause upregulation of slowpoke calcium-activated potassium current (Lee et al. 2008). By contrast, in MN5 dominant negative Shaker knock down and RNAi knock down for Shal clearly result in reduced total current amplitudes of I(A) as recorded from the soma. Furthermore, the reduction of total current amplitude that is caused by genetic knock down matches precisely the acute pharmacological block of Shaker or Shal currents. Therefore genetic knock down of Shaker or Shal currents as recorded from the soma are not compensated by upregulation of other transient potassium currents. Note that C380-GAL4 drives expression in MN5 after ∼25 h of pupal development, so that no transgene expression occurs during embryonic and larval life. However, compensatory regulation at the MN5 neuromuscular junction might occur in these knock downs. Although we did not test for potential changes in calcium or sodium currents, we know that knock down of Shaker alters the adult MN5 excitability (Duch et al. 2008). Therefore genetic perturbations of I(A) in the central compartments of MN5 is not fully compensated during adult development. This makes SDN and Shal RNAi useful tools to investigate the functions of these channels in adult motoneurons.
Shaker channels are probably located predominantly to the axon
Expression of GFP-tagged modified Shaker channels with intact targeting sequence (EKO-GFP) (White et al. 2001) in MN5 and immunostaining for native Shaker channels both demonstrate predominant axonal localization. However, both approaches also revealed Shaker-positive signal in the soma and potentially in some dendritic processes. Because the channels are made in the soma, it is not clear whether somatic Shaker channels are functional. In contrast to most vertebrate neurons the somatic membrane of insect motoneurons is typically passive (Duch and Levine 2000; Duch et al. 2008; Meseke et al. 2009). Furthermore, Shaker channels are not localized to the primary neurite connecting the soma and the proximal axon of MN5, which shows strong Shaker immunostaining and EKO-GFP localization. This indicates that Shaker channels that are made in the soma are targeted specifically to the axon but not to more proximal parts of the primary neurite. It seems unlikely that a Shaker signal in the proximal axonal compartment reflects transport to more distal processes because immunostaining intensities were similar along all axonal processes, from proximal to the axon terminals on the muscle, which are known to contain functional Shaker currents (Lee et al. 2008; Mosca et al. 2005).
Expression of EKO-GFP in MN5 and immunocytochemistry indicated some Shaker channel localization to dendrites. However, labeling intensities were much lower in dendrites as compared with the axon, and many dendrites were devoid of Shaker positive signal. Therefore these data are not sufficient to conclude a dendritic Shaker localization. Note also that the expression of GFP-tagged channels under the control of a nonendogenous promoter might cause some false localization and that optical resolution is not high enough to distinguish between Shaker immunolabel on MN5 dendrites and other neural arbors in close proximity. Therefore our data clearly support axonal Shaker localization, but potential additional dendritic localization cannot be excluded. This is consistent with axonal Shaker channel targeting in Drosophila brain neurons (Rogero et al. 1997), and electrophysiological data demonstrating the presence of Shaker channels in motoneuron axon terminals (Lee et al. 2008; Ueda and Wu 2006). Similarly, in lobster stomatogastric ganglion Shaker is localized to axons, whereas Shal is located in the somata (Baro et al. 2000). In many vertebrate neurons, Kv1 channels (Shaker-like) are predominantly axonal and have a specific axonal targeting domain (Gu et al. 2003), whereas dendritic localization has been reported for Kv4 channels (Shal) (Chen et al. 2006; Johnston et al. 2000). A dendritic localization of Shal channels seems plausible, but cannot be tested immunocytochemically, because Drosophila Shal antibodies are not available.
Barium blocks calcium-independent potassium channels in MN5
Barium is commonly used to replace calcium in the external solution to avoid movements, especially in Drosophila larval preparations. However, it may also act as a competitive potassium channel blocker (Hurst et al. 1996). In MN5, barium clearly reduces the amplitude of calcium-independent I(A) as demonstrated by addition of barium to cadmium containing external solution. Most of the I(A) remaining in cadmium and barium is blocked by the Shaker-specific drug, DTX, indicating that barium acts as a blocker for non-Shaker, and thus Shal channels. However, a small current remaining in barium and DTX can be blocked by phrixotoxin-2, indicating that barium is less effective than phrixotoxin-2. However, we cannot exclude the possibility that barium might also block a small component of Shaker current, although large-amplitude DTX-sensitive currents remain after barium application.
Properties and possible functions of transient potassium currents in MN5
Activation and inactivation voltages of I(A) and ICF are within common ranges found in other invertebrate (Heidel and Pflüger 2006; Kloppenburg et al. 1999a,b) and vertebrate neurons (Hsiao et al. 2009; Jerng and Pfaffinger 2008; Maffie and Rudy 2008; Pak et al. 1991; Persson et al. 2005; Watanabe et al. 2002; Wu and Barish 1992). However, if measured at temperatures matching those commonly found in the flies′ natural habitat, MN5 exhibits Shaker and Shal currents with faster activation and inactivation times than those reported in other studies carried out at lower temperatures. This is not a contradiction because our data confirm earlier work in muscle (Wang and Wu 1996) that lower temperatures strongly reduce activation speed and current amplitude of I(A). Moreover, A-type like potassium currents in acutely isolated Drosophila larval neurons (Xu et al. 2005) and in hippocampal pyramidal neurons are similarly fast (Johnston et al. 2000). The activation time kinetics of muscle and neuronal A-type currents can be modulated by auxiliary subunits, such as the Hyperkinetic (Hk) subunit for Shaker channels (Wang and Wu 1996; Yao and Wu 1999).
Functionally, in MN5 the fast transient potassium currents are good candidates for preventing doublets or triplets of spikes during tonic firing as occurring during flight (Harcombe and Wyman 1977) (see also Fig. 1C), especially when localized to the proximal axon as proposed for Shaker channels in this study and for Kv1 channels in neocortical neurons (Goldberg et al. 2008). If the Kv1/Kv4 potassium channel targeting was conserved, a somatodendritic localization of Shal would be reminiscent to what is reported for many vertebrate and also some crustacean neurons. This would indicate different functions of Shaker and Shal channels in MN5. In pyramidal neurons, dendritic Kv4 channels are crucial in controlling the amplitude of back-propagating action potentials (Chen et al. 2006; Johnston et al. 2000; Migliore et al. 1999), whereas calcium-activated potassium currents mediate membrane repolarization after spiking and afterhyperpolarization (Lancaster and Adams 1986; Shao et al. 1999; Storm 1987). Although the specific functions of these currents in MN5 are not yet known, the identification of the underlying channel genes, paired with targeted expression of knock downs that are not subject to homeostatic upregulation of other potassium currents in flight motoneurons, provide strong tools. Recording MN5 in situ and extracellularly during flight of intact animals will allow us to determine the specific contributions of different potassium currents for spiking output and synaptic input integration during behavior.
GRANTS
This work was supported by the German Research Foundation (DFG, RY 117/1-1) to S. Ryglewski, and funds from the Arizona State University to S. Ryglewski and C. Duch are gratefully acknowledged.
ACKNOWLEDGMENTS
We thank Dr. R. B. Levine (Tucson, AZ) for fruitful discussions during the course of experiments and for helpful comments on the manuscript, Dr. M. Herrera-Valdez (Arizona State University) for calculating theoretical temperature effects on time to peak for ionic currents in a fluid transfer model, S. Berger (Arizona State University) for computational models on passive current transfer in the MN5 dendritic tree as presented in Fig. 1B, and J. Boerner for help with Fig. 1A.
REFERENCES
- Baines RA, Bate M. Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci 18: 4673–4683, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranauskas G, Tkatch T, Surmeier DJ. Delayed rectifier currents in rat globus pallidus neurons are attributable to Kv2.1 and Kv3.1/3.2 K(+) channels. J Neurosci 1 19: 6394–6404, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro DJ, Ayali A, French L, Scholz NL, Labenia J, Lanning CC, Graubard K, Harris-Warrick RM. Molecular underpinnings of motor pattern generation: differential targeting of shal and shaker in the pyloric motor system. J Neurosci 20: 6619–6630, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L, Leung HT. Ionic currents of Drosophila neurons in embryonic cultures. J Neurosci 8: 4379–4393, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci 26: 12143–12151, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JC, Park D, Griffith LC. Electrophysiological and morphological characterization of identified motor neurons in the Drosophila third instar larva central nervous system. J Neurophysiol 91: 2353–2365, 2004 [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci 868: 233–285, 1999 [DOI] [PubMed] [Google Scholar]
- Consoulas C, Restifo LL, Levine RB. Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J Neurosci 22: 4906–4917, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias M, Wei AA, Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron 7: 763–773, 1991 [DOI] [PubMed] [Google Scholar]
- Cuntz H, Borst A, Segev I. Optimization principles of dendritic structure. Theor Biol Med Model 4: 21, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derst C, Messutat S, Walther C, Eckert M, Heinemann SH, Wicher D. The large conductance Ca2+-activated potassium channel (pSlo) of the cockroach Periplaneta americana: structure, localization in neurons and electrophysiology. Eur J Neurosci 17: 1197–1212, 2003 [DOI] [PubMed] [Google Scholar]
- Duch C, Levine RB. Remodeling of membrane properties and dendritic architecture accompanies the postembryonic conversion of a slow into a fast motoneurons. J Neurosci 20: 6950–6961, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duch C, Mentel T. Activity affects dendritic shape and synapse elimination during steroid controlled dendritic retraction in Manduca sexta. J Neurosci 24: 9826–9837, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duch C, Vonhoff F, Ryglewski S. Dendrite elongation and dendritic branching are affected separately by different forms of intrinsic motoneuron excitability. J Neurophysiol 100: 2525–2536, 2008 [DOI] [PubMed] [Google Scholar]
- Evers JF, Schmitt S, Sibila M, Duch C. Progress in functional neuroanatomy: precise automatic geometric reconstruction of neuronal morphology from confocal image stacks. J Neurophysiol 93: 2331–2342, 2005 [DOI] [PubMed] [Google Scholar]
- Gasque G, Labarca P, Reynaud E, Darszon A. Shal and shaker differential contribution to the K+ currents in the Drosophila mushroom body neurons. J Neurosci 25: 2348–2358, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron 58: 387–400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Dickinson MH. Role of calcium in the regulation of mechanical power in insect flight. Proc Natl Acad Sci USA 103: 4311–4315, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Jan YN, Jan LY. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science 301: 646–649, 2003 [DOI] [PubMed] [Google Scholar]
- Harcombe ES, Wyman RJ. Output pattern generation by Drosophila flight motoneurons. J Neurophysiol 40: 1066–1077, 1977 [DOI] [PubMed] [Google Scholar]
- Hardie RC. Voltage-sensitive potassium channels in Drosophila photoreceptors. J Neurosci 11: 3079–3095, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig CL, Worrell J, Levine RB, Ramaswami M, Sanyal S. Normal dendrite growth in Drosophila motor neurons requires the AP-1 transcription factor. Dev Neurobiol 68: 1225–1242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AL. Twenty years of dendrotoxins. Toxicon 39: 15–26, 2001 [DOI] [PubMed] [Google Scholar]
- Harvey AL, Robertson B. Dendrotoxins: structure-activity relationships and effects on potassium ion channels. Curr Med Chem 11: 3065–3072, 2004 [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci 26: 688–695, 2003 [DOI] [PubMed] [Google Scholar]
- Heidel E, Pflüger HJ. Ion currents and spiking properties of identified subtypes of locust octopaminergic dorsal unpaired median neurons. Eur J Neurosci 23: 1189–1206, 2006 [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes (3rd ed.). Sunderland, MA: Sinaur, 2001 [Google Scholar]
- Hsiao CF, Kaur G, Vong A, Bawa H, Chandler SH. Participation of Kv1 channels in control of membrane excitability and burst generation in mesencephalic V neurons. J Neurophysiol 101: 1407–1418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Toro L, Stefani E. Molecular determinants of external barium block in Shaker potassium channels. FEBS Lett 388: 59–65, 1996 [DOI] [PubMed] [Google Scholar]
- Ikeda K, Koenig JH. Morphological identification of the motor neurons innervating the dorsal longitudinal flight-muscle of Drosophila melanogaster. J Comp Neurol 273: 436–444, 1988 [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol 505: 267–282, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ. Multiple Kv channel-interacting proteins contain an N-terminal transmembrane domain that regulates Kv4 channel trafficking and gating. J Biol Chem 283: 36046–36059, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Magee JC, Poolos NP, Watanabe S, Colbert CM, Migliore M. Dendritic potassium channels in hippocampal pyramidal neurons. J Physiol 1: 75–81, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg P, Kirchhof BS, Mercer A. Voltage activated currents from adult honeybee (Apis mellifera) antennal lobe motor neurons recorded in vivo and in situ. J Neurophysiol 81: 39–48, 1999a [DOI] [PubMed] [Google Scholar]
- Kloppenburg P, Levini RM, Harris-Warrick RM. Dopamine modulates two potassium currents and inhibits the intrinsic firing properties of an identified motor neuron in a central pattern generator network. J Neurophysiol 81: 29–38, 1999b [DOI] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol 55: 1268–1282, 1986 [DOI] [PubMed] [Google Scholar]
- Lee J, Ueda A, Wu CF. Pre- and post-synaptic mechanisms of synaptic strength homeostasis revealed by slowpoke and shaker K(+) channel mutations in Drosophila. Neuroscience 17: 1283–1296, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol 76: 2107–2110, 1996 [DOI] [PubMed] [Google Scholar]
- Levine JD, Wyman RJ. Neurophysiology of flight in wild-type and a mutant Drosophila. Proc Natl Acad Sci USA 70: 1050–1054, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffie J, Rudy B. Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J Physiol 586: 5609–5623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 7: 563–574, 2006 [DOI] [PubMed] [Google Scholar]
- Meseke M, Evers JF, Duch C. Developmental changes in dendritic architecture and synapse location tune single neuron computations to changing behavioral requirements. J Neurophysiol 102: 41–58, 2009 [DOI] [PubMed] [Google Scholar]
- Migliore M, Hoffman DA, Magee JC, Johnston D. Role of an A-type K+ conductance in the back-propagation of action potentials in the dendrites of hippocampal pyramidal neurons. J Comput Neurosci 7: 5–15, 1999 [DOI] [PubMed] [Google Scholar]
- Mosca TJ, Carrillo RA, White BH, Keshishian H. Dissection of synaptic excitability phenotypes by using a dominant-negative Shaker K+ channel subunit. Proc Natl Acad Sci USA 102: 3477–3482, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak MD, Baker K, Covarrubias M, Butler A, Ratcliffe A, Salkoff L. mShal, a subfamily of A-type K+ channel cloned from mammalian brain. Proc Nati Acad Sci USA 88: 4386–4390, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng IF, Wu CF. Differential contributions of Shaker and Shab K+ currents to neuronal firing patterns in Drosophila. J Neurophysiol 97: 780–794, 2007a [DOI] [PubMed] [Google Scholar]
- Peng IF, Wu CF. Drosophila cacophony channels: a major mediator of neuronal Ca2+ currents and a trigger for K+ channel homeostatic regulation. J Neurosci 27: 1072–1081, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AS, Klement G, Almgren M, Sahlholm K, Nilsson J, Petersson S, Arhem P, Schalling M, Lavebratt C. A truncated Kv1.1 protein in the brain of the megencephaly mouse: expression and interaction. BMC Neurosci 6: 65, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogero O, Hämmerle B, Tejedor FJ. Diverse expression and distribution of Shaker potassium channels during the development of the Drosophila nervous system. J Neurosci 17: 5108–5118, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Baker K, Butler A, Covarrubias M, Pak MD, Wei A. An essential ‘set’ of K+ channels conserved in flies, mice and humans. Trends Neurosci 15: 161–166, 1992 [DOI] [PubMed] [Google Scholar]
- Sanyal S, Narayanan R, Consoulas C, Ramaswami M. Evidence for cell autonomous AP1 function in regulation of Drosophila motor-neuron plasticity. BMC Neurosci 4: 20, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S, Evers JF, Duch C, Scholz M, Obermayer K. New methods for the computer-assisted 3-D reconstruction of neurons from confocal image stacks. Neuroimage 23: 1283–1298, 2004 [DOI] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 1: 135–146, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol 385: 733–759, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøbaek D, Jørgensen TD, Christophersen P, Ahring PK, Olesen SP. Pharmacological characterization of small-conductance Ca(2+)-activated K(+) channels stably expressed in HEK 293 cells. Br J Pharmacol 129: 991–999, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda S, Salkoff L. Genetic analysis of Drosophila neurons: Shal, Shaw, and Shab encode most embryonic potassium currents. J Neurosci 15: 1741–1754, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107, 2004 [DOI] [PubMed] [Google Scholar]
- Ueda A, Wu CF. Distinct frequency-dependent regulation of nerve terminal excitability and synaptic transmission by IA and IK potassium channels revealed by Drosophila Shaker and Shab mutations. J Neurosci 26: 6238–6248, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Wu CF. In vivo functional role of the Drosophila hyperkinetic f3 subunit in gating and inactivation of Shaker K+ channels. Biophy J 71: 3167–3176, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 99: 8366–8371, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei A, Covarrubias M, Butler A, Baker K, Pak M, Salkoff L. K+ current diversity is produced by an extended gene family conserved in Drosophila and mouse. Science 248: 599–603, 1990 [DOI] [PubMed] [Google Scholar]
- White BH, Osterwalder TP, Yoon KS, Joiner WJ, Whim MD, Kaczmarek LK, Keshishian H. Targeted attenuation of electrical activity in Drosophila using a genetically modified K(+) channel. Neuron 31: 699–711, 2001 [DOI] [PubMed] [Google Scholar]
- Worrell JW, Levine RB. Characterization of voltage-dependent Ca2+ currents in identified Drosophila motoneurons in situ. J Neurophysiol 100: 868–878, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Tsai MC, Chen ML, Zhong Y, Singh S, Lee CY. Actions of dendrotoxin on K+ channels and neuromuscular transmission in Drosophila melanogaster, and its effects in synergy with K+ channel-specific drugs and mutations. J Exp Biol 147: 21–41, 1989 [DOI] [PubMed] [Google Scholar]
- Wu RL, Barish ME. Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. J Neurosci 12: 2235–2246, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüstenberg DG, Boytcheva M, Grünewald B, Byrne JH, Menzel R, Baxter DA. Current- and voltage-clamp recordings and computer simulations of Kenyon cells in the honeybee. J Neurophysiol 92: 2589–2603, 2004 [DOI] [PubMed] [Google Scholar]
- Xu TX, Gong N, Xu TL. Divalent cation modulation of a-type potassium channels in acutely dissociated central neurons from wide-type and mutant Drosophila. J Neurogenet 19: 87–107, 2005 [DOI] [PubMed] [Google Scholar]
- Yao WD, Wu CF. Auxiliary hyperkinetic beta subunit of K+ channels: regulation of firing properties and K+ currents in Drosophila neurons. J Neurophysiol 81: 2472–2484, 1999 [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Alteration of four identified K+ currents in Drosophila muscle by mutations in eag. Science 252: 1562–1564, 1991 [DOI] [PubMed] [Google Scholar]