Abstract
The receptive field organization of nociceptive neurons suggests that noxious information may be encoded by population-based mechanisms. Electrophysiological evidence of population coding mechanisms has remained limited. However, psychophysical studies examining interactions between multiple noxious stimuli can provide indirect evidence that neuron population recruitment can contribute to both spatial and intensity-related percepts of pain. In the present study, pairs of thermal stimuli (35°C/49°C or 49°C/49°C) were delivered at different distances on the leg (0, 5, 10, 20, 40 cm) and abdomen (within and across dermatomes) and subjects evaluated pain intensity and perceived spatial attributes of stimuli. Reports of perceived pain spreading to involve areas that were not stimulated (radiation of pain) were most frequent at 5- and 10-cm distances (χ2 = 34.107, P < 0.0001). Perceived connectivity between two noxious stimuli (filling-in) was influenced by the distance between stimuli (χ2 = 16.756, P < 0.01), with the greatest connectivity reported at 5- and 10-cm separation distances. Spatial summation of pain occurred over probe separation distances as large as 40 cm and six dermatomes (P < 0.05), but was maximal at 5- and 10-cm separation distances. Taken together, all three of these phenomena suggest that interactions between recruited populations of neurons may support both spatial and intensity-related dimensions of the pain experience.
INTRODUCTION
At the level of the spinal cord, nociceptive information is processed by both wide dynamic range (WDR) neurons and nociceptive-specific (NS) neurons. Both classes of nociceptive neurons have receptive fields (RFs) that are not homogeneously sensitive. For example, the central RF zone is more sensitive than peripheral portions of the RF (Price et al. 1978). Accordingly, neurons within a given population, during a single noxious stimulus, have different degrees of activation, depending on the position of their RF in relation to the stimulus. Given the substantial overlap of RFs of different nociceptive neurons, this graded RF organization would be predicted to allow the recruitment of neurons with progressive increases in stimulus intensity (Coghill et al. 1993; Price et al. 1978) or attentional demands (Oshiro et al. 2007; Quevedo and Coghill 2007a). A large number of neurons can be recruited rostrocaudally by noxious thermal stimuli and this recruitment can extend over several segments of the spinal cord with sufficiently intense stimuli (Coghill et al. 1991, 1993). Thus such neuron recruitment may represent one critical dimension of a population code for nociceptive information. However, the subjective availability of this information remains in question. Some authors have suggested that such population coding mechanisms may be more relevant to motor withdrawal reflexes and that population-based information may be too complex to permit signal extraction at thalamocortical levels (Craig 2003).
Although the total population output appears to be used to code intensity of pain, other dimensions of the population response may contribute to the spatial perception of noxious stimuli. For example, activation of neurons that are distant from the epicenter of population activity may contribute to the perception of spatial location and/or size of stimuli. Accordingly, the spatial radiation of pain may also represent a perceptual correlate of population recruitment mechanisms. Radiation of pain is a mismatch between the perceived and actual location of stimulation in which the sensation of pain spreads outward from the location of stimulation. Radiation has been reported to occur across different sensory modalities (Green 1977, 1978; Green and Flammer 1989; Higashiyama and Hayashi 1993; Price et al. 1978). Radiation of pain has been attributed to the activation of peripheral zones of neighboring RFs (Price et al. 1978). However, radiation of pain, by itself, does not provide conclusive evidence that population recruitment can contribute to spatial perception of pain. For example, labeled lines hypotheses of pain localization would indicate that activation of neurons with small RFs would produce well-localized pain sensations, whereas activation of neurons with large RFs would produce sensations of pain arising from a large area.
If radiation occurs from population recruitment during stimulation of a single site, then overlapping recruitment from two adjacent noxious stimuli would be predicted to contribute to two additional phenomena. First, this overlapping recruitment could produce a sensation of pain in the nonstimulated region between stimuli. Such a percept would be analogous to the phenomenon of filling-in that has been found in the visual, auditory, and somatosensory modalities (Cohen and Legargasson 2005; Cohen et al. 2003; Conway et al. 2005; Hsieh and Tse 2006; Komatsu et al. 2002; Liu et al. 2004; Mendola et al. 2006; Micheyl et al. 2003; Motoyoshi 1999; Valmaggia and Gottlob 2002; Welchman and Harris 2003). Second, this overlapping recruitment would also increase the total output of the neuronal population activated by both stimuli and would be well suited to supporting spatial summation of pain (SSP) intensity—the increasing perception of pain magnitude with increases in the stimulated area. Thus if radiation and filling-in are representative of spatial dimensions of population recruitment and interactions, then maximal SSP would be predicted to occur at stimulus separation distances where radiation and filling-in are maximal. To test these predictions, subjects were recruited to evaluate the perceived number of stimuli (one or two), the perception of connectivity (connected or disconnected), and intensity of pairs of noxious thermal stimuli separated by different separation distances (0, 5, 10, 20, and 40 cm) on the legs and within and across dermatomes on the abdomen.
METHODS
Subjects
All subjects participating in this study (six males, seven females) were healthy, pain- and drug-free volunteers between 20 and 29 yr (average, 24.1 yr). All subjects gave written, informed consent acknowledging that they would experience experimental painful stimuli, that all methods and procedures were clearly explained, and that they were free to withdraw from the experiment at any time without prejudice. All procedures were approved by the Institutional Review Board of Wake Forest University School of Medicine.
Stimulation paradigms
All thermal stimuli were delivered with TSA II devices (Medoc, Ramat Yishai, Israel) using 16 × 16-mm stimulus probe(s). All stimuli were 5 s in duration and used rise and fall rates of 4°C/s. Stimuli were delivered to the leg or abdomen by a single probe or by two probes simultaneously. To test the interaction between information from potential noxious stimuli, two paradigms were used in the present experiments.
The “distance-based paradigm” was used on the medial area of the leg. Paired stimuli were separated by 0, 5, 10, 20, and 40 cm on the left leg. When stimuli were separated by 0, 5, or 10 cm, they were delivered on both the lower leg and the thigh. Thus SSP between shorter (0, 5, 10 cm) and larger (20 and 40 cm) distances can be directly compared while minimizing the influence of different sensitivity along the leg. The leg was the area used in this experiment because it was possible to test the influence of distance (≤40 cm) between two stimuli within a single dermatome or only across adjacent dermatomes. Importantly, there are minimal differences in SSP when noxious stimuli are delivered within dermatomes versus when they are delivered across adjacent dermatomes (Nielsen and Arendt-Nielsen 1997).
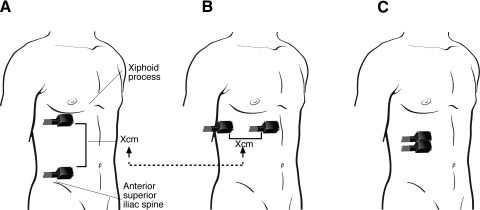
To assess SSP across several dermatomes the “dermatome-based paradigm” was used on the ipsilateral abdomen. Probes were separated vertically using anatomical references (xiphoid process and anterior superior iliac spine) to standardize the dermatomes tested across subjects (Fig. 1 A). Using those references, stimuli were delivered across five or six dermatomes (T6 at superior level and T11/T12 at inferior level). In within-dermatomes trials, probes were placed horizontally to stimulate the same dermatome (Fig. 1B). Also, there were trials that paired stimuli were delivered together (0 cm apart) (Fig. 1C). Predeterminate distances were not possible to be used in this experiment because there was a considerable discrepancy between subjects' height and a fixed distance between stimuli could cross a variable number of dermatomes across subjects. It is possible that during the horizontal trials the two stimuli were not confined to a single dermatome. However, as noted earlier, delivery of stimuli to adjacent dermatomes produces the same magnitude of SSP as that when they are delivered within a single dermatome (Nielsen and Arendt-Nielsen 1997). During the whole experiment, subjects lay down on a bed.
Fig. 1.
Stimulated areas in the abdomen. A: using anatomical references, pairs of stimuli were delivered across dermatomes. The superior probe was positioned 5 cm below the xiphoid process in an area approximating the T6 dermatome, whereas the inferior probe was positioned 5 cm above the iliac spine in the vicinity of the T11/T12 dermatome. B: the distance “X” was used to place the probes in a horizontal orientation where spatial summation was evaluated within dermatomes. C: pairs of stimuli were also delivered side by side to evaluate the influence of separation of stimuli during spatial summation of pain (SSP) (B vs. C).
To eliminate effects due to visual input, a screen blocked the subjects' view of the stimulated area during all trials during the second training series and in the experimental trials. Two elastic bands (width: 2.5 cm) were placed by the same experimenter to control the pressure exerted on the thermodes. Importantly, the elastic bands were present on the skin for the entire duration of the experiment (regardless of whether one or two probes were in contact with the skin) to give similar tactile stimulation during all trials. Paired stimuli were temporally synchronized electronically and monitored on a digital chart recorder (PowerLab/4sp; ADInstruments, Castle, Australia). The stimulus probes were calibrated and adjusted independently to ensure (quantitatively and temporally) synchronized stimulus delivery of identical stimulus temperatures. To further reduce confounds resulting from potentially slight differences in stimulus delivery between probes, the probe positions (proximal/distal) were switched halfway through each experimental session.
To minimize sensitization or habituation, stimuli were delivered to marked sites in a predetermined spatial fashion and each area was stimulated only once. Moreover, both stimulus intensities and probe separation distances were randomized to avoid order effects.
Two temperatures were used in all experimental trials: 35°C as baseline and 49°C as the noxious thermal stimulus. The 49°C stimulus temperature was chosen since, frankly, noxious stimuli elicit a high frequency of reports of pain radiation (Price et al. 1978). Paired stimuli were delivered in multiple combinations: 1) on the legs: 49°C proximal/49°C distal (49°Cp/49°Cd), 49°C proximal/35°C distal (49°Cp/35°Cd), and 35°C proximal/49°C distal (35°Cp/49°Cd); 2) on the abdomen across dermatomes: 49°C superior/49°C inferior (49°Cs/49°Ci), 49°C superior/35°C inferior (49°Cs/35°Ci), and 35°C superior/49°C inferior (35°Cs/49°Ci); and 3) on the abdomen within dermatomes: 49°C medial (closer to the midline)/49°C lateral (further from the midline) (49°Cm/49°Cl), 49°C medial/35°C lateral (49°Cm/35°Cl), and 35°C medial/49°C (35°Cs/49°Ci).
Single 49°C stimuli were also applied at all stimulated sites along the leg (or abdomen) to control for differences in sensitivity across body regions and to evaluate possible interactions between stimuli (noxious and innocuous) such as SSP. An 30-s interval between any two consecutive stimuli was used to avoid long-term suppression or sensitization of nociceptive afferents (Price and Dubner 1977). Three (paired) or four (single) trials were used for each condition (distance × combination of probes). To further minimize risks of habituation or sensitization, data were acquired on 2 separate days and trials on both body sites were divided equally across days, with each session lasting <2 h.
Psychophysical assessment and training
Pain intensity and pain unpleasantness were rated with separate mechanical visual analog scales (VAS) (Price et al. 1983, 1994; Rosier et al. 2002). These 15-cm-long sliding scales were anchored with the words “no pain sensation”–“the most intense pain imaginable” and “not unpleasant at all”–“the most unpleasant imaginable.” After subjects slid the scale to the appropriate level that corresponded to their actual pain perception, pain ratings were quantified by a labeled numeric index (0–10 range) on the back of the scale (out of the subjects' view). In the first training series, a single probe delivering different temperatures (from 35 to 49°C) was used to give subjects experience in rating pain intensity and pain unpleasantness. In the second training series, subjects were trained to respond to the tasks that were used during the experiment. Pairs of noxious stimuli separated by 10 cm were delivered using only the two temperatures (35 and 49°C) used in the experiment. Before stimulation, subjects were instructed to rate overall pain intensity first. Next, subjects were asked to classify the connectivity of the pain from the two stimuli as follows: 1) separated (two independent stimuli), 2) connected (two distinct stimuli interconnected to each other), or 3) if they felt only one stimulus (apparently only one probe was activated). This training series allowed subjects to gain experience in providing one rating (overall) for two stimuli delivered simultaneously and in performing the connectivity rating.
In the present experiment, a primary focus was spatial summation of pain. Thus pain intensity was assessed using a standard method (one rating for both stimuli) (Defrin and Urca 1996; Defrin et al. 2003; Nielsen and Arendt-Nielsen 1997; Price et al. 1989). It was important to ask subjects to rate overall pain without dividing their attention between two stimuli. We have previously demonstrated that when subjects are instructed to divide their attention between two stimuli and rate them separately, pain inhibition is produced and spatial summation is abolished (Quevedo and Coghill 2007a,b).
Statistical analyses
Radiation of pain was assessed using the reports of connectivity during 35°C/49°C and 49°C/35°C stimulus pairs. The frequency of trials for which subjects reported connection and two separate stimuli was compared with the frequency of reports in which subjects perceived only one probe using the chi-squared (χ2) analysis. This procedure allowed radiation to be assessed in an indirect fashion and did not require subjects to actively attend to a body region to provide a rating. Thus this indirect assessment is not subject to directed attention (and visual components) to the neutral probe location that can occur during active ratings and is able to hold potential confounds of expectation relatively constant (subjects always expect two stimuli, even when only one probe is on the skin).
To analyze the relationship between frequency of radiation and interindividual differences in pain sensitivity, a radiation index (percentage of trials with reports of radiation) was created from trials of pairs of noxious and neutral stimuli (49 and 35°C). This index number was created by attributing a value of 1 when subjects reported that the two sites of pain were disconnected or reported that the two sites were connected during pain. A value of 0 was attributed when subjects reported that only one probe was on. The within-subjects mean of this binarized response was then calculated. Pain sensitivity was calculated by within-subjects mean of responses to single 49°C stimuli. The relationship between these two variables was assessed by regression analysis.
To test the hypothesis that there is an optimal distance for the perception of connectivity between the two stimuli, the frequency of the trials for which subjects reported connectivity between the two stimuli was compared with the frequency when subjects reported the other two possibilities (separated and one probe) using the χ2 analysis.
The two assessed aspects of pain (intensity and unpleasantness) were highly similar, so, for clarity, analyses of SSP are focused only on pain intensity. For each subject, VAS ratings were first averaged across the three to four presentations of each condition (stimuli × distance). Comparisons between pain ratings from pairs of 49°C/49°C and single control stimuli (49°C) were used to assess spatial summation. The average of 49°Cp/49°Cd ratings was compared across different distances using repeated-measures ANOVAs to determine the optimal distance for SSP. Similar analyses were used to assess within- versus between-dermatome spatial summation on the abdomen. To compare SSP across sensitive and insensitive subjects, the pain ratings during pairs of 49°C/49°C stimuli were normalized by dividing them by the ratings from single 49°C stimuli. Linear regression analysis was used to determine whether percentage spatial summation was related to individual differences in pain sensitivity. Also, during pairs of 49°C/49°C stimuli, a repeated-measures ANOVA was used to determine whether there was a relationship between the perception of connectivity and SSP.
RESULTS
There were no differences in pain ratings between 35°Cp/49°Cd and 49°Cp/35°Cd on the legs (P = 0.5) and also in the 35°Cs/49°Ci and 49°Cs/35°Ci and 35°Cm/49°Cl and 49°Cm/35°Cl abdomen (P = 0.8). For this reason both conditions were analyzed as one rating for each body region for subsequent analyses.
Radiation to the neutral probe
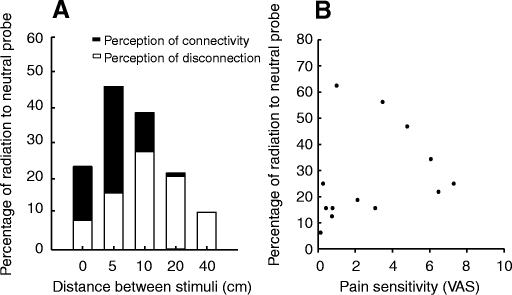
On the leg, during pairs of 35°C/49°C and 49°C/35°C, subjects frequently reported pain from both probes when only one delivered a noxious stimulus, suggesting a radiation from the noxious stimulus to the neutral probe (Fig. 2 A). This effect (radiation) involves all trials where subjects reported that pain from the probes was either connected or disconnected (when one probe was neutral). There was a significant effect of distance between probes on the frequency of reports on pain arising from the neutral probe (χ2 = 34.107, P < 0.0001). Painful sensations from the neutral probe were reported in 23% of the trials at 0 cm (disconnected perception of 8% and connected perception of 15%), 47% of the trials at 5-cm (disconnected perception of 16% and connected perception of 31%), 39% of the trials at 10-cm (disconnected perception of 27% and connected perception of 12%), 22% of the trials at 20-cm (disconnected perception of 21% and connected perception of 1%), and 9% of the trials at 40-cm (disconnected perception of 9% and connected perception of 0%) separation distances.
Fig. 2.
The perception of radiation of pain and pain sensitivity. A: during 35°C/49°C stimulus pairs, subjects reported pain from the neutral probe (35°C) at all distances. The most frequent reports of pain radiation occurred at separation distances of 5 and 10 cm. B: individual differences in the perceptions of radiation of pain were not influenced by individual differences in pain sensitivity.
To determine whether sensitive subjects had higher degrees of radiation, we used a regression analysis to assess the relationship between pain sensitivity (as defined by ratings of single 49°C stimuli) and percentage of perception of radiation (mean of three to four ratings/condition). No significant correlation between these two factors was found during pairs of 35°C/49°C and 49°C/35°C (r2 = 0.17; P = 0.1) pairs of stimuli (Fig. 2B).
On the abdomen, when probes were separated, radiation of pain was reported in 40% of the trials and this was similar within (horizontal arrangement of probes) and across dermatomes (vertical arrangement of probes). When probes were placed side by side, subjects reported that both probes were activated in 38% of the trials. There was no significant relationship between the position of the probes (vertical vs. horizontal vs. side by side) and the frequency of reports on pain arising from the neutral probe (χ2 = 0.132, P < 0.7).
Perceived connectivity between stimuli
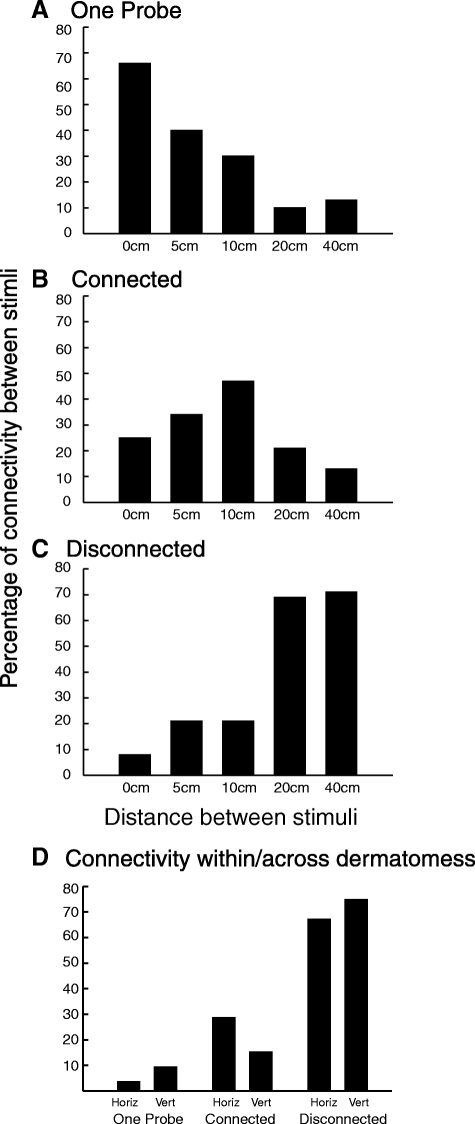
On the leg, the perception of connectiveness (separated, connected, and one probe) was significantly influenced by the distance between probes (χ2 = 107.129, P < 0.0001) during pairs of 49°C/49°C stimuli (Fig. 3). Only one probe was reported to be activated during 66% of trials at 0-cm, 40% of trials at 5-cm, 31% of trials at 10-cm, 10% of trials at 20-cm, and 13% of trials at 40-cm separation distances (χ2 = 57.352, P < 0.0001) (Fig. 3A). Subjects reported that they perceived two connected sites (connected perception) in 24% of trials at 0-cm, 35% of trials at 5-cm, 46% of trials at 10-cm, 21% of trials at 20-cm, and 14% of trials at 40-cm separation distances (χ2 = 16.756, P < 0.01) (Fig. 3B). Subjects felt two distinct sites of pain and no connection between them (separated perception) in 7% of trials at 0-cm, 21% of trials at 5-cm, 21% of trials at 10-cm, 69% of trials at 20-cm, and 71% of trials at 40-cm separation distances (χ2 = 89.457, P < 0.0001) (Fig. 3C). There were trials that subjects reported not being able to discriminate the specific site of pain: 3% of trials at 0-cm, 4% of trials at 5-cm, 2% of trials at 10-cm, and 2% of trials at 40-cm separation distances.
Fig. 3.
The relationship between spatial perceptions of pairs of noxious stimuli (49°C/49°C) and stimulus separation distance on the leg (A–C) and within and across dermatomes on the abdomen (D). A: frequency of reports of only one probe activated. B: frequency of reports that the perceived pain was not restricted to the area under the activated probes but extended to connect the 2 stimuli. The highest reports of connectivity were found at 10 cm (χ2 = 17.149, P < 0.01). These reports are consistent with the filling-in phenomenon found in other systems. C: frequency of reports of 2 separate painful stimuli. D: connectivity perceived during pairs of noxious stimuli delivered on the abdomen. There was no difference on the perception of connectivity, irrespective of whether the stimuli were delivered within or across dermatomes (χ2 = 3.7, P < 0.1).
On the abdomen, the perception of connectivity was not significantly influenced by the position of probes (within and across dermatomes) (χ2 = 3.7, P < 0.1) during pairs of 49°C/49°C stimuli (Fig. 3D). During the within-dermatomes condition (horizontal condition), subjects felt two distinct sites of pain and no connection between them (separated perception) in 29% of trials, two stimuli connected (connected perception) in 67% of trials, and only one stimulus (one probe perception) in 4% of the trials. During the across-dermatomes condition, subjects felt two distinct sites of pain and no connection between them (separated perception) in 15% of trials, two stimuli connected (connected perception) in 75% of trials, and only one stimulus (one probe perception) in 10% of the trials. When probes were positioned side by side, subjects felt two distinct sites of pain and no connection between them (separated perception) in 31% of trials, two stimuli connected (connected perception) in 21% of trials, and only one stimulus (one probe perception) in 48% of the trials. The perception of connectiveness (separated, connected, and one probe) was significantly influenced by the distance (side by side vs. separated) between probes (χ2 = 46.6, P < 0.0001) during pairs of 49°C/49°C stimuli.
Spatial summation of pain and effect of distance on the leg
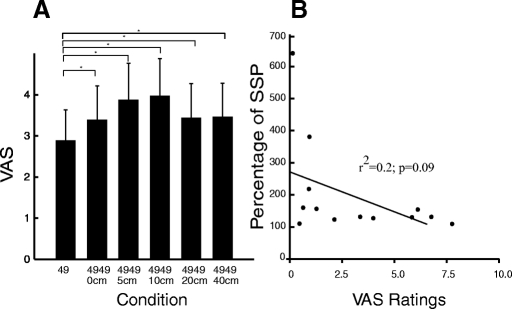
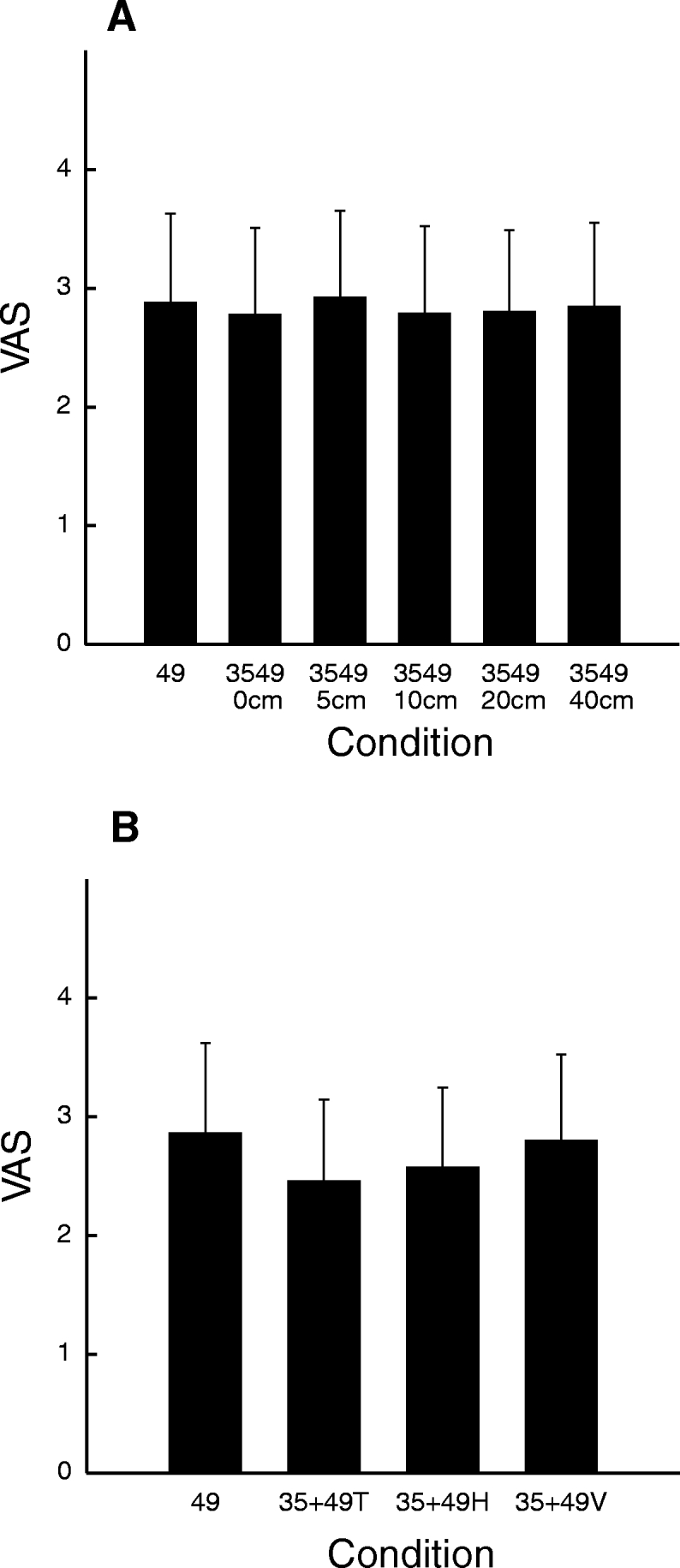
There was a significant nonlinear effect of distance between probes on pain intensity ratings when both probes were in the noxious range (49°C/49°C) (P < 0.05) (Fig. 4 A). When compared with other stimulus distances, SSP was more pronounced at 5-cm (P < 0.05) and 10-cm (P < 0.01) separation distances. The magnitude of SSP was not reliably different among stimuli delivered at 0, 20, and 40 cm (P = 0.8), although pain intensity ratings were still significantly greater than those evoked by a single 49°C stimulus (P < 0.01).
Fig. 4.
SSP at different distances. A: on the leg, SSP was found ≤40-cm distance between stimuli (P < 0.05) and was maximal at 10 cm (P < 0.01). There was no difference in SSP at 0-, 20-, and 40-cm separation distances (P = 0.8). B: pain sensitivity and SSP. There was no correlation between individual differences in pain sensitivity and SSP during pairs of noxious stimuli.
There was a trend for the degree of SSP to be inversely related to pain sensitivity. Using data from the 10-cm distance where SSP was greatest, relatively insensitive subjects tended to exhibit greater SSP than that in highly sensitivity subjects (r2 = 0.2; P = 0.09) (Fig. 4B). During pairs of 49°C/49°C stimuli, there was no relationship between the perception of connectivity and SSP (P = 0.1).
Spatial summation of pain within and across dermatomes
Spatial summation of pain was similar within (horizontal condition [H]) and across (vertical condition [V]) dermatomes on the abdomen (Fig. 5). Pairs of 49°C/49°C stimuli were rated greater than single 49°C stimuli both across dermatomes (vertical separation of five to six dermatomes) and within dermatomes (horizontal separation equal to vertical separation) (P < 0.05). There was no difference in SSP between both conditions, despite the fact that probes in the across-dermatome condition were five to six dermatomes apart (P = 0.5) (Fig. 5). However, ratings for 49°C/49°C within- and 49°C/49°C across-dermatome conditions were >49°C/49°C in the together condition (T; stimuli were applied side by side) (P < 0.05 and P < 0.01, respectively). During the together condition there was a nonsignificant trend for ratings to be greater than those evoked by single 49°C stimuli (P = 0.08). During pairs of 49°C/49°C stimuli, there was no relationship between the perception of connectivity and SSP (P = 0.1).
Fig. 5.
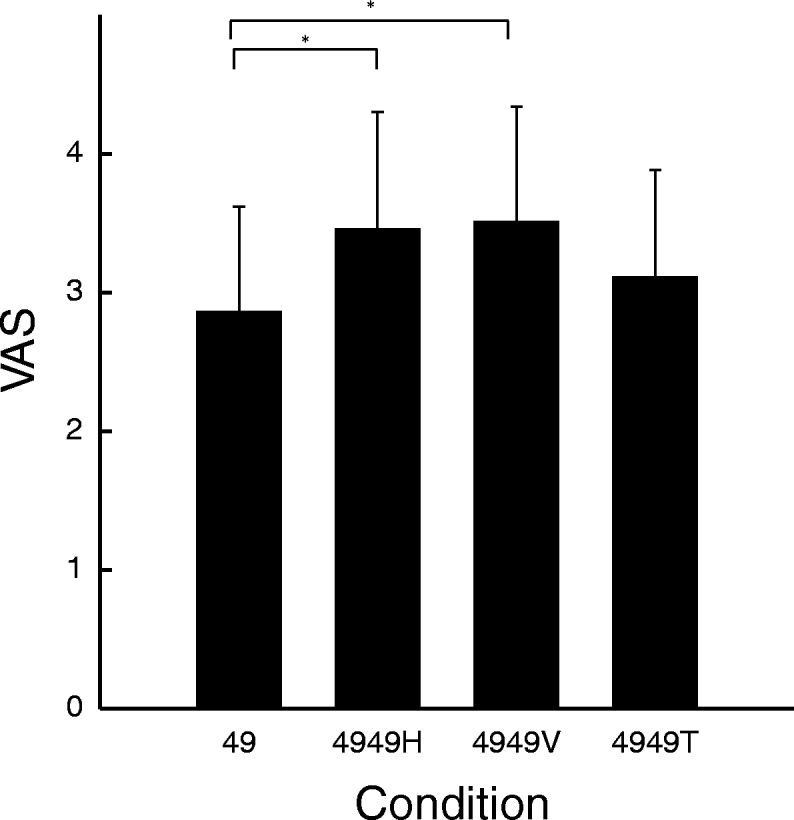
SSP on the abdomen. SSP was present when stimuli were delivered ≤6 dermatomes apart from each other. There was no difference between and across dermatomes (P = 0.5). However, SSP was greater when stimuli were separated vertically and horizontally than when they were side by side (P < 0.01 and P < 0.05, respectively).
Absence of increased pain during combined 49°C/35°C stimulation
There was no difference in pain ratings between single 49°C stimuli and pairs of 49°C/35°C stimuli on the leg (P = 0.8) (Fig. 6 A) or on abdomen (P = 0.2) (Fig. 6B). Also, subjects reported that they were unaware of whether there were one or two probes on the skin. Moreover, 8 of 13 subjects reported at least one time that there was connection between two perceived stimuli when only one probe was placed on the skin.
Fig. 6.
Pain intensity was not modulated by the presence of a neutral probe. There was no difference between a single 49°C stimulus and combined 49 and 35°C stimuli. Thus the presence of a thermal neutral probe did not increase pain intensity.
DISCUSSION
Population-based mechanisms of nociceptive processing in the CNS remain poorly understood due to lack of information about how large numbers of nociceptive neurons respond to incoming afferent information. The present psychophysical data provide strong, yet indirect evidence that neuron recruitment contributes importantly to spatial dimensions of pain. This recruitment of neurons can support interactions between multiple stimuli and can produce different perceptions such as radiation, spatial summation, and filling-in.
Radiation
During clinical evaluation, the reported area of pain does not always reflect the origin of nociceptive information (de Leeuw et al. 1995a,b; Kreiner and Okeson 1999; Naranjo Hernandez et al. 1992). This mismatch between the site and source of pain can provoke equivocation on diagnosis and, in consequence, treatment failure (Farella et al. 2002; Harris et al. 1993; Okeson and Falace 1997). Experimentally, mislocation of somatosensory information has been reported for noxious (Price et al. 1978) and innocuous thermal (Green 1977, 1978; Taus et al. 1975), electrical (Hardy et al. 1967; Higashiyama and Hayashi 1993), chemical (Green and Flammer 1989), and tactile (Culver 1970; Green and Flammer 1989) stimuli.
Radiation of pain has been typically studied by asking subjects to rate the perceived intensity of a neutral stimulus that is delivered simultaneously with a separate noxious stimulus (Green 1977, 1978; Higashiyama and Hayashi 1993). This procedure allows direct assessment of spatial radiation but also involves cognitive factors such as directed attention to the potential stimulated sites. In the present investigation, the perception of connectivity was used to indirectly determine whether pain was perceived at both stimulus locations. When pairs of 35°C + 49°C stimuli were delivered and subjects reported feeling two noxious stimuli that were either connected or disconnected, this indicates that noxious stimuli were perceived at the neutral temperature probe (Fig. 2). Importantly, this procedure requires no directed attention specifically to the neutral probe, as other paradigms require. Spatial attention during sensory stimulation modulates interactions between separate stimuli (Quevedo and Coghill 2007b). When subjects are required to divide their attention between two simultaneous stimuli or direct their attention to one of them, SSP can be abolished (Quevedo and Coghill 2007a) or facilitated (Quevedo and Coghill 2007b), respectively. Thus the attentional set is an important determinate of the final perception of pain.
Another cognitive factor that would influence radiation is the expectation of pain from the presence of a contact stimulator on the body surface (Carlsson et al. 2000; Green 1978; Johnson et al. 1998; Koyama et al. 2005; McCaul and Malott 1984; Miron et al. 1989; Mullen and Suls 1982; Sawamoto et al. 2000; Suls and Fletcher 1985; Tracey et al. 2002). However, due to the constant presence of the elastic bands that held the probes in position and because the probes were hidden from the subjects' view, subjects were not able to distinguish the number of probes on the skin and were not aware if there were one or two areas that would receive a painful stimulus. Thus the influence of probe number on expected pain intensity and/or location was minimized. Consistent with this observation, pain from pairs of 49°C/35°C stimuli on the leg and on abdomen was not distinguished from that produced by a 49°C stimulus delivered by a single probe. Moreover, the observed radiation cannot be explained by expectation alone because radiation varies across probe separation distances, whereas the probability of probe activation was maintained constant during all trials using paired stimuli.
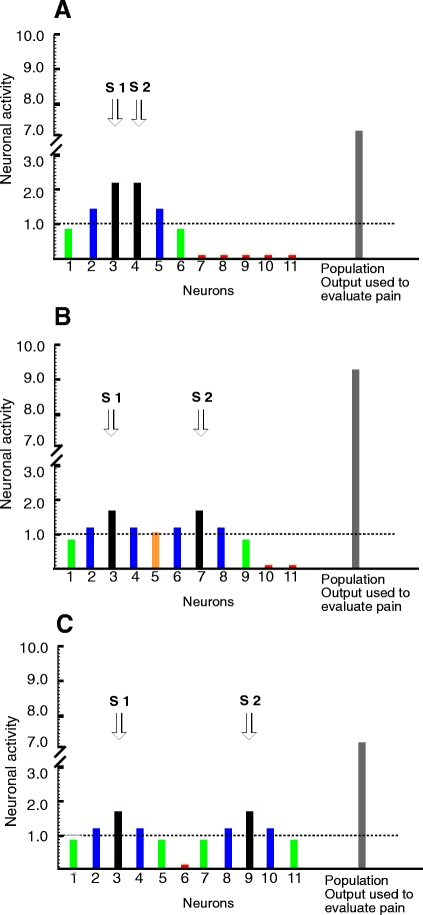
The observed radiation of pain is consistent with the concept that intensely noxious stimuli recruit activity over a widely distributed population of neurons (Coghill et al. 1991). Sufficiently intense activation of neurons outside of the epicenter of the activated population may give rise to the perceptual experience that pain is spreading from the stimulated area (Fig. 7).
Fig. 7.
Hypothetical data from CNS neurons demonstrating the conceptual mechanism of filling-in and SSP. A: pairs of stimuli delivered in close proximity activate a very similar population of neurons. There are increases in the discharge of single neurons that are stimulated in the central areas of their receptive field (RF; black bars) and their activation (∼2.3) is beyond the localization threshold (1.0). Thus these cells contribute to the spatial location of stimuli (dashed line). Since there is no valley between these 2 peaks, the spatial percept is of one contiguous area of pain. Neurons that receive stimulation at intermediary RF zones (blue bars) (∼1.3) are also able to reach the localization threshold, but contribute to the percept that pain radiates from the stimulated area. Other neurons that are stimulated in more peripheral RF zones (green bars) are not able to contribute to spatial location of the stimulated area (∼0.8), but contribute to the total population output and perceived pain intensity. At distant areas, neurons are not activated (red bars). The total output (7.2) from all of this activation gives an afferent signal that is used to process intensity-related information downstream in the system. B: filling-in during population recruitment. When stimuli are separated at an optimal distance, 2 overlapping neuronal populations are recruited. Some neurons (orange bar) that before were not able to reach the localization threshold level are now recruited because they receive low-level input from both stimuli and, accordingly, contribute to filling-in. The neurons that are stimulated in the center of their RFs (black bars) receive afferent input from only one probe and their activation is somewhat diminished (1.7), compared with A, where those neurons are activated by both stimuli. However, in consequence of the greater number of neurons recruited, the total population output is greater (9.4) than when 2 stimuli are placed side by side (A) or placed at greater distances apart (C). Thus SSP is more pronounced. C: when stimuli are located at greater separation distances, each stimulus activates independent populations of neurons that interact minimally with each other. As a result there is no perception of spatial connection between stimuli (filling-in) and SSP is less pronounced than when more neurons are recruited (B). In fact, there is no difference in the population output between A and C (∼7.2). However, the mechanisms that produce spatial summation in both situations are different. In A spatial summation is driven mainly by increased activation of single neurons and in B spatial summation is produced by the increase in the number of neurons recruited. This is in agreement with the present psychophysical data in which SSP was not different in 0-, 20-, and 40-cm separation distances, but was more pronounced at 5- and 10-cm separation distances.
Filling-in
Filling-in is a phenomenon that allows the CNS to interpolate missing information to construct representations of continuous surfaces and demonstrates that physical stimuli presented do not necessary correspond to the final perception (Komatsu et al. 2002). This phenomenon has been reported across visual, auditory, and somatosensory modalities (Cohen and Legargasson 2005; Cohen et al. 2003; Conway et al. 2005; Hsieh and Tse 2006; Komatsu et al. 2002; Liu et al. 2004; Mendola et al. 2006; Micheyl et al. 2003; Motoyoshi 1999; Valmaggia and Gottlob 2002; Welchman and Harris 2003).
In the present study, this perceptual construction of continuous sensation was assessed by asking the subjects how they spatially perceived the overall stimulation during pairs of noxious stimuli. Subjects could report that they perceived only one activated site, two disconnected activated sites, two activated sites that were connected, or they could not make a spatial evaluation. The classification of the sensation as arising from two connected sites is consistent with the filling-in phenomenon (Komatsu et al. 2002). This perception of pain where no stimulus was delivered (Fig. 3B) represents another spatial mismatch between the actual and perceived stimulated areas that would be consistent with population recruitment (Fig. 7).
As with radiation of pain, the observed perception of filling-in is consistent with the concept that noxious stimuli recruit activity over a widely distributed population of neurons (Fig. 7). Thus if two neuronal populations are activated by the two painful stimuli, such that there is an overlap of both populations, neurons in the overlapping region would be ideally positioned to contribute to the production of a final perception that there is connectivity between the two stimuli.
To determine whether the perception of connectivity is found only when stimuli are delivered at the same dermatome or is present when stimuli are delivered to markedly separate dermatomes, subjects evaluated noxious stimuli applied to the abdomen. Subjects reported connectivity between the two stimuli not only when they were delivered to separated dermatomes but also when they were delivered within a single dermatome. This finding provides evidence that filling-in has a central component that might involve several dermatomes. This integration could be mediated at the spinal cord level, in part, by propriospinal interconnections, or could occur at higher levels of the CNS. Filling-in in other sensory systems (visual and auditory) has been found to take place at the cortical level.
Spatial summation of pain
SSP is a classic example of integration between multiple noxious stimuli. The present investigation found SSP over 40-cm distances between stimuli on the leg (Fig. 4A) and up to five to six dermatomes on the abdomen (Fig. 5). The present data indicate that SSP is modulated by the spatial distribution of the stimuli. It was expected that the highest SSP would be seen when probes were closest together (i.e., 0 cm apart) (Defrin and Urca 1996) and that SSP would decrease with increasing probe separation. On the leg, however, pain intensity increased as probe separation increased from 0, to 5, to 10 cm and then decreased as separation of probes was increased to 20 and 40 cm (Fig. 4A). SSP on the abdomen was also greater when stimuli were separated either vertically (across dermatomes) or horizontally (within dermatomes) than when they were placed side by side. In fact, SSP was not significant when stimuli were delivered side by side. Although initially counterintuitive, this finding of nonmonotonic changes in SSP across stimulus separation distances provides further evidence for population recruitment. Given the relatively large receptive field sizes of nociceptive neurons in the CNS, especially some WDR neurons (Hori et al. 1984; Milne et al. 1981), delivery of two noxious stimuli in relatively close proximity would be predicted to activate a neuronal population that would be generally similar to that activated by one stimulus alone (Fig. 7). This scenario is consistent with the relatively weak SSP seen at 0-cm separation distances. However, when stimuli are at an “optimal distance” from each other, each stimulus may activate somewhat different neuronal populations that overlap to some extent (Fig. 7B). The overlap of these populations may enable neurons that normally are not activated by either stimulus to reach threshold and contribute to the final output and/or may produce facilitated responses in neurons that were weakly activated by one stimulus alone. This is demonstrated by neuron number 5, which does not reach threshold by input from either stimulus alone, but, when two stimuli are delivered at an optimal distance, it simultaneously receives sufficient input from both stimuli to become activated (Fig. 7B). Consistent with this notion, spatial summation of pain was optimal at 5- to 10-cm separation distances. These distances were also characterized by the most frequent reports of radiation and filling-in.
Integration across dermatomes has been found using different approaches. For example, SSP was found across neighboring dermatomes (Douglass et al. 1992; Staud et al. 2004) or even bilaterally using the same dermatome (Nielsen and Arendt-Nielsen 1997). In agreement with previous studies, SSP was found within and across dermatomes in the leg and abdomen. Noxious stimuli can be integrated at different levels of the neuraxis (Bouhassira et al. 1992; Gall et al. 1998; Morton et al. 1987, 1988; Wall 1978, 1980; Willer et al. 1989) and this integration includes not only central sites (Andersen et al. 1994; Price 1972; Price et al. 1977) but also peripheral sites (Davis et al. 1993; Defrin et al. 2003; Graven-Nielsen and Mense 2001; Martin et al. 1987) or both central and peripheral sites (Price et al. 1977, 1989). In the present study, there was no difference in spatial summation during stimulation of the abdomen using the same distance between noxious thermal stimuli within and across (five or six dermatomes away) dermatomes (P = 0.5) (Fig. 5). This provides other strong evidence that the integration between stimuli not only can occur at the peripheral level but also can occur centrally. Moreover, on the leg, the greatest SSP and most frequent reports of filling-in were found when probes were separated by 10 cm. Since the receptive fields of primary afferent neurons rarely exceed a diameter of 5 cm, the integration that is responsible for the maximal SSP and filling-in at this distance (10 cm) likely takes place within the CNS. However, the level at which this occurs remains to be elucidated.
Individual differences in spatial tuning of nociceptive processing
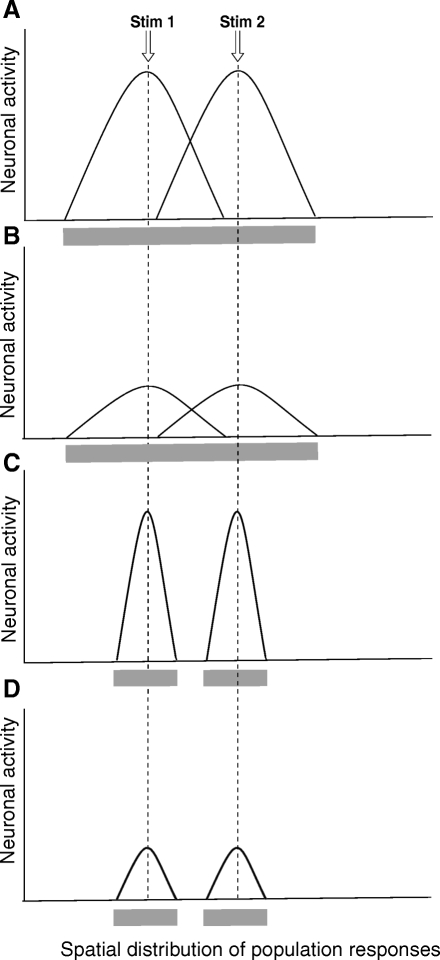
Since 1) the intensity of noxious stimuli influences the radiation of pain (Price et al. 1978), 2) higher noxious temperatures produce greater recruitment of neurons at the spinal level (Coghill et al. 1991), and 3) increasing noxious intensities evoke greater percepts of pain, it is logical to postulate that increasing population recruitment would be associated with greater percepts of pain. Accordingly, it follows that individuals who experience a great deal of pain from a stimulus of a given intensity could be expected to have greater population recruitment and thus greater radiation and SSP than that of individuals in whom the stimulus evoked a lesser experience of pain. However, the present findings indicate that individual differences in pain intensity sensitivity have only a minimal relationship with individual differences of these spatial aspects of pain. Neither the frequency of radiation nor the magnitude of spatial summation was correlated with individual differences in pain sensitivity. Therefore different dimensions of neural population responses appear to be used by supraspinal levels during the construction spatial versus intensity-related subjective experiences (Fig. 8). For example, spatial percepts may be derived from the extent of neuronal activity above a certain threshold, whereas intensity may be derived from the total population output. This distinction is further supported by the different patterns of brain activity evoked during discrimination of pain location versus pain intensity (Oshiro et al. 2007). Thus although studies of individual differences in pain have focused on intensity, individual differences in spatial tuning may represent an important and distinct dimension of the pain experience.
Fig. 8.
Individual differences in sensitivity and the perception of filling-in. Here a hypothetical representation of the activity of populations of CNS nociceptive neurons during pairs of stimuli at an “optimal separation distance” is shown in 4 different situations in which subjects have perception of connectivity independently of their pain sensitivity. The perception of connectivity is due to the overlapping of the population activity between the 2 sites. A: neuronal population distribution of activity for a highly sensitive subject with high connectivity. During pairs of simultaneously noxious stimuli, this subject would perceive a continuous area of pain (gray horizontal bar under the graphic). B: neuronal population distribution of activity for a low-sensitivity subject with high connectivity. During pairs of simultaneously noxious stimuli, similarly to A, this subject would perceive a continuous area of pain (gray horizontal bar under the graphic). C: neuronal population distribution of activity for a highly sensitive subject with low connectivity. During pairs of simultaneously noxious stimuli, this subject would perceive 2 separated areas of pain (2 gray horizontal bars under the graphic). D: neuronal population distribution of activity for a low-sensitivity subject with low connectivity. During pairs of simultaneously noxious stimuli, this subject would perceive 2 separated areas of pain (2 gray horizontal bars under the graphic).
GRANTS
This research was supported by National Institutes of Health Grants R01 NS-39426 and DA-20168.
ACKNOWLEDGMENTS
We thank Dr. Jamir Sarda Jr. for helpful comments on the manuscript.
REFERENCES
- Andersen OK, Jensen LM, Brennum J, Arendt-Nielsen L. Evidence for central summation of C and A delta nociceptive activity in man. Pain 59: 273–280, 1994 [DOI] [PubMed] [Google Scholar]
- Bouhassira D, Villanueva L, Bing Z, Le Bars D. Involvement of the subnucleus reticularis dorsalis in diffuse noxious inhibitory controls in the rat. Brain Res 595: 353–357, 1992 [DOI] [PubMed] [Google Scholar]
- Carlsson K, Petrovic P, Skare S, Petersson KM, Ingvar M. Tickling expectations: neural processing in anticipation of a sensory stimulus. J Cogn Neurosci 12: 691–703, 2000 [DOI] [PubMed] [Google Scholar]
- Coghill RC, Eisenach J. Individual differences in pain sensitivity: implications for treatment decisions. Anesthesiology 98: 1312–1314, 2003 [DOI] [PubMed] [Google Scholar]
- Coghill RC, Mayer DJ, Price DD. The roles of spatial recruitment and discharge frequency in spinal cord coding of pain: a combined electrophysiological and imaging investigation. Pain 53: 295–309, 1993 [DOI] [PubMed] [Google Scholar]
- Coghill RC, Price DD, Hayes RL, Mayer DJ. Spatial distribution of nociceptive processing in the rat spinal cord. J Neurophysiol 65: 133–140, 1991 [DOI] [PubMed] [Google Scholar]
- Cohen SY, Lamarque F, Saucet JC, Provent P, Langram C, LeGargasson JF. Filling-in phenomenon in patients with age-related macular degeneration: differences regarding uni- or bilaterality of central scotoma. Graefes Arch Clin Exp Ophthalmol 241: 785–791, 2003 [DOI] [PubMed] [Google Scholar]
- Cohen SY, Legargasson JF. Adaptation to central scotoma. Part II. Perceptual filling-in phenomenon (in French). J Fr Ophtalmol 28: 1131–1136, 2005 [DOI] [PubMed] [Google Scholar]
- Conway BR, Kitaoka A, Yazdanbakhsh A, Pack CC, Livingstone MS. Neural basis for a powerful static motion illusion. J Neurosci 25: 5651–5656, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 26: 1–30, 2003 [DOI] [PubMed] [Google Scholar]
- Culver CM. Errors in tactile localization. Am J Psychol 83: 420–427, 1970 [PubMed] [Google Scholar]
- Davis KD, Meyer RA, Campbell JN. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. J Neurophysiol 69: 1071–1081, 1993 [DOI] [PubMed] [Google Scholar]
- Defrin R, Ronat A, Ravid A, Peretz C. Spatial summation of pressure pain: effect of body region. Pain 106: 471–480, 2003 [DOI] [PubMed] [Google Scholar]
- Defrin R, Urca G. Spatial summation of heat pain: a reassessment. Pain 66: 23–29, 1996 [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Boering G, Stegenga B, de Bont LG. Radiographic signs of temporomandibular joint osteoarthrosis and internal derangement 30 years after nonsurgical treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 79: 382–392, 1995a [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Boering G, Stegenga B, de Bont LG. TMJ articular disc position and configuration 30 years after initial diagnosis of internal derangement. J Oral Maxillofac Surg 53: 234–242, 1995b [DOI] [PubMed] [Google Scholar]
- Douglass DK, Carstens E, Watkins LR. Spatial summation in human thermal pain perception: comparison within and between dermatomes. Pain 50: 197–202, 1992 [DOI] [PubMed] [Google Scholar]
- Farella M, Michelotti A, Gargano A, Cimino R, Ramaglia L. Myofascial pain syndrome misdiagnosed as odontogenic pain: a case report. Cranio 20: 307–311, 2002 [DOI] [PubMed] [Google Scholar]
- Gall O, Bouhassira D, Chitour D, Le Bars D. Involvement of the caudal medulla in negative feedback mechanisms triggered by spatial summation of nociceptive inputs. J Neurophysiol 79: 304–311, 1998 [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen T, Mense S. The peripheral apparatus of muscle pain: evidence from animal and human studies. Clin J Pain 17: 2–10, 2001 [DOI] [PubMed] [Google Scholar]
- Green BG. Localization of thermal sensation: an illusion and synthetic heat. Percept Psychophys 22: 331–337, 1977 [Google Scholar]
- Green BG. Referred thermal sensations: warmth versus cold. Sens Processes 2: 220–230, 1978 [PubMed] [Google Scholar]
- Green BG, Flammer LJ. Localization of chemical stimulation: capsaicin on hairy skin. Somatosens Mot Res 6: 553–566, 1989 [DOI] [PubMed] [Google Scholar]
- Hardy JD, Wolff HG, Goodell H. Pain Sensations and Reactions New York: Hafner, 1967 [Google Scholar]
- Harris M, Feinmann C, Wise M, Treasure F. Temporomandibular joint and orofacial pain: clinical and medicolegal management problems. Br Dent J 174: 129–136, 1993 [DOI] [PubMed] [Google Scholar]
- Higashiyama A, Hayashi M. Localization of electrocutaneous stimuli on the fingers and forearm: effects of electrode configuration and body axis. Percept Psychophys 54: 108–120, 1993 [DOI] [PubMed] [Google Scholar]
- Hori Y, Lee KH, Chung JM, Endo K, Willis WD. The effects of small doses of barbiturate on the activity of primate nociceptive tract cells. Brain Res 307: 9–15, 1984 [DOI] [PubMed] [Google Scholar]
- Hsieh PJ, Tse PU. Illusory color mixing upon perceptual fading and filling-in does not result in “forbidden colors.” Vision Res 46: 2251–2258, 2006 [DOI] [PubMed] [Google Scholar]
- Johnson MH, Breakwell G, Douglas W, Humphries S. The effects of imagery and sensory detection distractors on different measures of pain: how does distraction work? Br J Clin Psychol 37: 141–154, 1998 [DOI] [PubMed] [Google Scholar]
- Komatsu H, Kinoshita M, Murakami I. Neural responses in the primary visual cortex of the monkey during perceptual filling-in at the blind spot. Neurosci Res 44: 231–236, 2002 [DOI] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci USA 102: 12950–12955, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner M, Okeson JP. Toothache of cardiac origin. J Orofac Pain 13: 201–207, 1999 [PubMed] [Google Scholar]
- Liu T, Slotnick SD, Yantis S. Human MT+ mediates perceptual filling-in during apparent motion. Neuroimage 21: 1772–1780, 2004 [DOI] [PubMed] [Google Scholar]
- Martin HA, Basbaum AI, Kwiat GC, Goetzl EJ, Levine JD. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience 22: 651–659, 1987 [DOI] [PubMed] [Google Scholar]
- McCaul KD, Malott JM. Distraction and coping with pain. Psychol Bull 95: 516–533, 1984 [PubMed] [Google Scholar]
- Mendola JD, Conner IP, Sharma S, Bahekar A, Lemieux S. fMRI measures of perceptual filling-in in the human visual cortex. J Cogn Neurosci 18: 363–375, 2006 [DOI] [PubMed] [Google Scholar]
- Micheyl C, Carlyon RP, Shtyrov Y, Hauk O, Dodson T, Pullvermuller F. The neurophysiological basis of the auditory continuity illusion: a mismatch negativity study. J Cogn Neurosci 15: 747–758, 2003 [DOI] [PubMed] [Google Scholar]
- Milne RJ, Foreman RD, Giesler GJ, Jr, Willis WD. Convergence of cutaneous and pelvic visceral nociceptive inputs onto primate spinothalamic neurons. Pain 11: 163–183, 1981 [DOI] [PubMed] [Google Scholar]
- Miron D, Duncan GH, Bushnell MC. Effects of attention on the intensity and unpleasantness of thermal pain. Pain 39: 345–352, 1989 [DOI] [PubMed] [Google Scholar]
- Morton CR, Du HJ, Xiao HM, Maisch B, Zimmermann M. Inhibition of nociceptive responses of lumbar dorsal horn neurones by remote noxious afferent stimulation in the cat. Pain 34: 75–83, 1988 [DOI] [PubMed] [Google Scholar]
- Morton CR, Maisch B, Zimmermann M. Diffuse noxious inhibitory controls of lumbar spinal neurons involve a supraspinal loop in the cat. Brain Res 410: 347–352, 1987 [DOI] [PubMed] [Google Scholar]
- Motoyoshi I. Texture filling-in and texture segregation revealed by transient masking. Vision Res 39: 1285–1291, 1999 [DOI] [PubMed] [Google Scholar]
- Mullen B, Suls J. The effectiveness of attention and rejection as coping styles: a meta-analysis of temporal differences. J Psychosom Res 26: 43–49, 1982 [DOI] [PubMed] [Google Scholar]
- Naranjo Hernandez A, Rodriguez Lozano C, Ojeda Bruno S. Fibromyalgia syndrome (in Spanish). An Med Interna 9: 95–100, 1992 [PubMed] [Google Scholar]
- Nielsen J, Arendt-Nielsen L. Spatial summation of heat induced pain within and between dermatomes. Somatosens Mot Res 14: 119–125, 1997 [DOI] [PubMed] [Google Scholar]
- Okeson JP, Falace DA. Nonodontogenic toothache. Dent Clin North Am 41: 367–383, 1997 [PubMed] [Google Scholar]
- Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting spatial discrimination of pain. J Neurosci 27: 3388–3394, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Exp Neurol 37: 371–387, 1972 [DOI] [PubMed] [Google Scholar]
- Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 56: 217–226, 1994 [DOI] [PubMed] [Google Scholar]
- Price DD, Dubner R. Neurons that subserve the sensory-discriminative aspects of pain. Pain 3: 307–338, 1977 [DOI] [PubMed] [Google Scholar]
- Price DD, Hayes RL, Ruda M, Dubner R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J Neurophysiol 41: 933–947, 1978 [DOI] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain 3: 57–68, 1977 [DOI] [PubMed] [Google Scholar]
- Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 17: 45–56, 1983 [DOI] [PubMed] [Google Scholar]
- Price DD, McHaffie JG, Larson MA. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J Neurophysiol 62: 1270–1279, 1989 [DOI] [PubMed] [Google Scholar]
- Quevedo AS, Coghill RC. An illusion of proximal radiation of pain due to distally directed inhibition. J Pain 8: 280–286, 2007a [DOI] [PubMed] [Google Scholar]
- Quevedo AS, Coghill RC. Attentional modulation of spatial integration of pain: evidence for dynamic spatial tuning. J Neurosci 27: 11635–11640, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosier EM, Iadarola MJ, Coghill RC. Reproducibility of pain measurement and pain perception. Pain 98: 205–216, 2002 [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci 20: 7438–7445, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Robinson ME, Price DD. Spatial summation of heat pain within and across dermatomes in fibromyalgia patients and pain-free subjects. Pain 111: 342–350, 2004 [DOI] [PubMed] [Google Scholar]
- Suls J, Fletcher B. The relative efficacy of avoidant and nonavoidant coping strategies: a meta-analysis. Health Psychol 4: 249–288, 1985 [DOI] [PubMed] [Google Scholar]
- Taus RH, Stevens JC, Marks LE. Spatial localization of warmth. Percept Psychophys 17: 194–196, 1975 [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci 22: 2748–2752, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmaggia C, Gottlob I. Optokinetic nystagmus elicited by filling-in in adults with central scotoma. Invest Ophthalmol Vis Sci 43: 1804–1808, 2002 [PubMed] [Google Scholar]
- Wall PD. The gate control theory of pain mechanisms. A re-examination and re-statement. Brain 101: 1–18, 1978 [DOI] [PubMed] [Google Scholar]
- Wall PD. The role of substantia gelatinosa as a gate control. Res Publ Assoc Res Nerv Ment Dis 58: 205–231, 1980 [PubMed] [Google Scholar]
- Welchman AE, Harris JM. Is neural filling-in necessary to explain the perceptual completion of motion and depth information? Proc Biol Sci 270: 83–90, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer JC, De Broucker T, Le Bars D. Encoding of nociceptive thermal stimuli by diffuse noxious inhibitory controls in humans. J Neurophysiol 62: 1028–1038, 1989 [DOI] [PubMed] [Google Scholar]