Abstract
Although locomotion is known to be generated by networks of spinal neurons, knowledge of the properties of these neurons is limited. Using neonatal transgenic mice that express enhanced green fluorescent protein (EGFP) driven by the c-fos promoter, we visualized EGFP-positive neurons in spinal cord slices from animals that were subjected to a locomotor task or drug cocktail [N-methyl-d-aspartate, serotonin (5-HT), dopamine, and acetylcholine (ACh)]. The activity-dependent expression of EGFP was also induced in dorsal root ganglion neurons with electrical stimulation of the neurons. Following 60–90 min of swimming, whole cell patch-clamp recordings were made from EGFP+ neurons in laminae VII, VIII, and X from slices of segments T12 to L4. The EGFP+ neurons (n = 55) could be classified into three types based on their responses to depolarizing step currents: single spike, phasic firing, and tonic firing. Membrane properties observed in these neurons include hyperpolarization-activated inward currents (29/55), postinhibitory rebound (11/55), and persistent-inward currents (31/55). Bath application of 10–40 μM 5-HT and/or ACh increased neuronal excitability or output with hyperpolarization of voltage threshold and changes in membrane potential. 5-HT also increased input resistance, reduced the afterhyperpolarization (AHP), and induced membrane oscillations, whereas ACh reduced the input resistance and increased the AHP. In this study, we demonstrate a new way of identifying neurons active in locomotion. Our results suggest that the EGFP+ neurons are a heterogeneous population of interneurons. The actions of 5-HT and ACh on these neurons provide insights into the neuronal properties modulated by these transmitters for generation of locomotion.
INTRODUCTION
It is well established that the basic rhythm and pattern of locomotion is generated by a system of spinal neurons that make up the central pattern generator (CPG) for locomotion (Brown 1911; Grillner 1981). Numerous studies have been undertaken to identify neurons that make up the CPG. It was initially suggests that the rat CPG is anatomically localized to the L1/L2 spinal segments (Cazalets et al. 1995, 1996), but subsequent studies suggest that the hindlimb network is distributed throughout the lumbar and supralumbar spinal cord (Cina and Hochman 2000; Cowley and Schmidt 1997; Kjaerulff et al. 1994; Kremer and Lev-Tov 1997; Nishimaru and Kudo 2000; Nishimaru et al. 2000; Palmer et al. 2001). Early efforts to examine the properties of rodent neurons active during locomotion included intracellular and blind patch recordings (Kiehn et al. 1996; MacLean et al. 1995), tetrode recordings (Tresch and Kiehn 1999), and calcium imaging (Nakayama et al. 2002) from the isolated neonatal rat spinal cord.
Recently techniques have become available to identify specific interneurons in reduced spinal cord preparations such that their physiological and pharmacological properties can be examined. For example, it has been shown that the activity of ascending commissural cells can be modulated by the neurotransmitters serotonin (5-hydroxytryptamine, 5-HT) and acetylcholine (ACh) (Carlin et al. 2006; Zhong et al. 2006a). Similarly, descending commissural cells are excited by 5-HT and are rhythmically active during fictive locomotion in vitro (Zhong et al. 2006b). Populations of ventral interneurons, including EphA4 (Butt et al. 2005; Kullander et al. 2003), Dbx1 (Lanuza et al. 2004), En1 (Gosgnach et al. 2006), Chx10 (Crone et al. 2008), and Sim (Zhang et al. 2008) expressing neurons have been shown to have discrete roles in locomotion. HB9-expressing interneurons may also play a role in rhythm generation (Brownstone and Wilson 2008; Hinckley et al. 2005; Wilson et al. 2005). A discrete group of cholinergic medial partition neurons located near the central canal appears to terminate on motoneurons and control their excitability during locomotor activity by reducing the action potential hyperpolarization (Miles et al. 2007). Some of these genetically defined neurons can now be visualized in spinal cord slices through the use of fluorescent reporter genes. Nevertheless, few of these neurons have been extensively characterized in terms of their electrophysiological properties and their response to neurotransmitters that produce fictive locomotion for instance.
These efforts to define locomotor neurons in the spinal cord differ from the present study by virtue of the fact that they employed anatomical and genetic features for identification of the neurons. In contrast we use a marker of neural activity—enhanced green fluorescent protein (EGFP) expression driven by the c-fos promoter—to identify neurons active during a locomotor task in mice. In a recent study (Dai et al. 2005b), we have demonstrated that locomotion induces cfos expression in decerebrate cat spinal neurons. Here we use a degradable form of EGFP to minimize any labeling from activity prior to a locomotor task and then target these neurons for study in spinal cord slices. We characterize the electrophysiological properties of EGFP-positive neurons (“locomotor neurons”) in lamina VII, VIII, and X of T12–L4 segments using whole cell patch-clamp techniques. Previous studies demonstrated that 5-HT is necessary and ACh is sufficient to produce locomotor-like activity in isolated spinal cords of neonatal rats and mice (Cowley and Schmidt 1994; Hochman et al. 1994a; Jiang et al. 1999; Kiehn and Kjaerulff 1996; MacLean et al. 1995; Smith and Feldman 1986, 1987; Smith et al. 1988). Further studies show that several types of 5-HT receptors (5-HT1, 5-HT2, and 5-HT7) are essential in inducing locomotor-like movements in neonatal rat and mouse spinal cord (Beato and Nistri 1998; Bracci et al. 1998; Cazalets et al. 1992, 1995; Hochman et al. 2001; Lui and Jordan 2005; Madriaga et al. 2004) and in adult rat and mouse transected spinal cord (Landry and Guertin 2004; Landry et al. 2006; Ung et al. 2008). We therefore examined the modulation of intrinsic membrane properties of the EGFP+ neurons by these two neuromodulators.
We demonstrate that activation of neurons from the cfos-EGFP mice results in robust expression of EGFP sufficient for identification of these neurons and report heterogeneous anatomical and electrophysiological properties and responses to 5-HT and ACh. Preliminary reports of some of this work have been published (Brownstone et al. 2002; Dai et al. 2004).
METHODS
The animal protocol was approved by the Dalhousie University Animal Care Committee, the University of Manitoba Central Animal Care Services and conformed to the standards of the Canadian Council on Animal Care.
Production of the cfos-EGFP transgenic mice
C-fos PROMOTER-pd2EGFP-1.
The cfos-EGFP mouse line was produced in the McMahon lab in the following manner: plasmid p301-602 (Gilman et al. 1986) was cut with HindIII and ligated to an EcoRI adaptor. The plasmid was then cut with BamHI to release a 700-bp EcoRI/HindIII-BamHI fragment that encompassed the mouse c-fos promoter (Accession No. V00727, nucleotides 1–600 plus about 100 unsequenced base pairs upstream), including the following response elements: CRE, AP1, SIE, SRE. The gel-purified EcoRI-HindIII-BamHI fragment was cut with EcoRI and ligated into EcoRI-BamHI cut pd2EGFP-1 vector. DH5′ alpha subcloning efficiency (Gibco) cells were transfected with the c-fos promoter-GFP plasmid following manufacturer's instructions (calcium phosphate). The c-fos promoter-GFP plasmid was purified (InVitrogen, SNAP Midi Prep), cut with EcoRI and AflII, releasing a 1.7-kb base-pair fragment that was subsequently gel purified. Twenty micrograms of EcoRI-AfIII fragment was purified by cesium chloride gradient without ethidium bromide and then extensively dialyzed against TEN buffer (1 l, 6 times) at 4°C.
Transgenic mice were generated by the University of Kentucky Transgenic Mouse Facility. Transgene DNA was microinjected (5 ng/μl) into the pronuclei of fertilized B6C3F1 hybrid mouse embryos (Harlan) and implanted into pseudopregnant females. Mice were screened for the transgene both by dot-blot analysis and PCR of genomic DNA isolated from the tail at the time of weaning. A homozygous transgenic line was then bred.
Dorsal root ganglion neuron (DRG) dissociation
DRG were harvested from postnatal (P4–P13) mice. Harvesting of these cells used the same surgical procedure as described previously to harvest the spinal cord (Carlin et al. 2000). Once the spinal cord was removed from the spinal column, fine forceps were used to pull the ganglia from between the vertebrae. This first part of the procedure was performed in ice-cold dissecting artificial cerebrospinal fluid (ACSF). Once removed from the animal, the ganglia were placed in a conical tube containing room-temperature HEPES-based ACSF solution with 2.5% trypsin (Invitrogen, Carlsbad, CA). The trypsin was allowed 20 min to work before the digested tissue was rinsed (×3) with fresh HEPES ACSF containing bovine serum albumin (Sigma). The tissue was then triturated with a plastic pipette and plated directly into the glass-bottomed recording chamber. HEPES-based ACSF contents were (in mM) 140 NaCl, 3 KCl, 10 HEPES, 2 MgCl2, 10 glucose, and 2 CaCl2, pH = 7.4.
Imaging of acutely dissociated DRG
Fluorescent images were captured using a Hamamatsu image capture and processing package (Hamamatsu, Bridgewater, NJ). This included a C2400 CCD camera, a camera controller, ARGUS 20 image processor, and HPS frame grabber and software. The images were acquired at regular intervals of 1–2 min. Frames (512) were accumulated with the ARGUS software, and images were stored on a Pentium class computer. The fluorescent light shutter was closed when not acquiring images. Using control cells alone or unstimulated cells in the field of view (n = 4; see results) the illumination process itself did not cause any detectable increase in fluorescence expression. This is in contrast to the use of a long-pass filter (XF-02; Omega Optical), which caused an increase in GFP expression in proportion to the rate of illumination (data not shown). This latter increase in fluorescence signal is likely due to UV stimulated c-fos expression. Intensity measurements were preformed on stored images with the ARGUS software. Intensity difference was calculated using defined areas in the cell of interest and a reference cell or background.
Preparation of slices and patch-clamp recordings
Experiments were carried out on neonatal (P4-15) cfos-EGFP mice. A locomotor task (walking or swimming) was induced in the animals prior to preparation of slices. The walking protocol was used for only older mice (>P8). With this protocol, a small amount of mother mouse's fur was used to attract the animals. The fur was put into a microcentrifuge chamber. The chamber was then placed near the nose of the animals and moved slowly to attract the animals for walking. The swimming protocol was designed for younger mice of P4–P8. Animals at this age were usually tiny in size and physically weak for long walking (60–90 min) with body weight. Therefore this protocol was used to induce locomotor task in >90% of the animals (n = 64: 44 for patch-clamp experiments and 20 for confocal images) in this study. A length of 1-cm-wide paper tape is prepared for suspending the animal by making the portion of tape that touches the animal nonadhesive with a 4-cm-long piece of the same tape placed sticky side to sticky side. This tape is then placed around the animal's thorax, and the distal ends are joined over the animal's back, leaving ample length of tape to allow the attachment of a clamp for suspension of the animal over a beaker of water. The tape allows the mouse full range of motion in all limbs as well as normal breathing. The ends of the tape are placed in a clamp attached to a calibrated microdrive. The water bath is held at 26–30°C (usually 28°) using a hotplate and thermometer. The microdrive is used to lower the mouse into the water bath. The clamp is situated on the tape in such a manner as to assure that the head is maintained above the surface of the water at all times. On being positioned with its limbs in the water, the mouse immediately begins swimming. During the first 5–10 min, the mouse is allowed to habituate to the water bath. During this time, the animal may attempt to escape the water. If this occurs, the experimenter lifts the animal out of the water for a few seconds and then places it back in. This is repeated a few times until the mouse becomes used to the water and ceases all signs of struggling. At this point, the animal begins to swim steadily for as long as its limbs are kept below the surface of the water. This “swimming task” continues for 60–90 min. Maintaining the temperature within the proper range is a key element to the success of the task. If the water bath is too warm, the mouse may fall asleep. If it is too cold, the animal will struggle. The duration of the task must be ≥60 min to assure that the locomotor activity is sufficient to induce c-fos expression in the locomotor neurons of the spinal cord and consequent expression of the EGFP protein in these neurons. The EGFP allows the locomotor neurons activated by the task to be visualized later in spinal cord slices taken from this mouse. The reason for using swimming task is that this new task is much more efficient in inducing locomotion-like movement in young animals than the walking task. No overt difference in expression of EGFP+ neurons was found between the walking and swimming slices.
The procedure for the preparation of spinal cord slices was as previously reported (Carlin et al. 2000). Transverse slices were cut at thickness of 180–250 μm from T12 to L4 segments and remained in the recovering ACSF for ≥1 h before the patch-clamp recordings. The slices were then transferred to a recording chamber mounted in the stage of an upright Olympus BX50 microscope fitted with differential interference contrast (DIC) optics and epifluorescence. The chamber was perfused with recording ACSF at rate of 2 ml/min, bubbled with 95% O2-5%CO2. The EGFP-positive neurons were identified at ×40 magnification using epifluorescence with a narrow band GFP cube (Omega XF-37). The neurons were then visualized using infrared illumination and images collected (Hamamatsu camera controller C2400 and ARGUS image processor). The visualized neurons were patched (electrode resistances: 3–6 MΩ). A MultiClamp 700A patch-clamp amplifier, Digidata 1322A A/D converter, Minidigi 1A, and pClamp (9.0) software (all from Molecular Devices) were used for data acquisition. Whole cell patch recordings were made in current-clamp mode with bridge balance and capacitance compensation and in voltage-clamp mode with series resistance compensation by 70–80%. A constant hyperpolarizing current from 0 to 100 pA was injected into some of the cells to maintain the resting membrane potential at about −60 mV or lower during the recordings. The recording protocols were repeated two to three times in each condition (control, drugs, and washout etc.) with ∼30 s apart between two successive recordings. Normally recordings were made in 2–8 min following drug application and repeated for ∼10 min before switching to new conditions. To have the recordings from healthy cells, the time for intracellular recording was usually <40 min. Data were low-pass filtered at 3 kHz and sampled at 10 kHz. The data were analyzed using self-written codes with Igor Pro (4.0) and Axon Clampfit (9.0). Student's t-test and ANOVA were performed with statistical significance defined as P < 0.05. Results are shown as means ± SD.
Confocal images
Slices of both control and locomotion mice were cut at 100–150 μm and fixed for 20 min with 4% paraformaldehyde. They were then mounted on slides, allowed to air dry, and fitted with coverslips with Vectashield mounting medium (Vector Laboratories H-1000). Slides were scanned and photographed with step of 1.5 μm and thickness of 15–50 μm using confocal laser scanning microscope (Olympus Fluoview 2.1). The thickness of slices for scanning was chosen appropriately to avoid image saturation while the clear distribution of EGFP-positive cells was preserved.
Measurement of cell membrane properties
The membrane properties measured and calculated in this study include current threshold (Ith), voltage threshold (Vth), resting membrane potential (Em), input resistance (Rin), membrane time constant (τm and τ1), whole cell capacitance (Cm), slope of frequency/current (F-I) relation, action potential (AP) height and width, and afterhyperpolarization (AHP) depth, and half-decay time. The Ith was determined by step currents with 0.5-s duration and 50 pA for each step. The minimum step that could evoke a single spike or repetitive firing was taken as the Ith. The Vth was defined as the membrane potential at which the rising rate of dV/dt >10 mV/ms. The Vth reported in this paper was measured from the first spike of firings evoked by a 2-s triangular current with amplitude of 0.5–1.5 nA. The resting membrane potential was monitored throughout the recordings using Axon Minidigi 1A. The reported Em in this paper was calculated from the membrane potentials averaged over 100 ms prior to the step currents used for determining the Ith. The measurements of AP and AHP properties were based on an averaged spike from the first one to five spikes evoked by Ith. The Vth was used as reference value to measure the AP height and width and AHP depth. The half-decay time of AHP was measured from the time of AHP peak to the time of half AHP decaying from the peak. Step currents with duration of 2 s and step of 50 pA were injected into the cells to determine the F-I relations. The instantaneous firing frequency was calculated as the reciprocal of the interspike interval (ISI) for the first, second, and steady-state (ss) spikes. The ss firing frequency was calculated as a mean rate of firings over the last 1 s duration of the firings in the tonic firing neurons (type 3) or over the last 5–10 spikes in the neurons with phasic firing (type 2). In a few of the type 2 cells that fired only a few spikes, the averaged rate of firing for all spikes was taken as the ss firing frequency. A linear regression was applied to the F-I relations for calculation of the slopes. The slopes calculated from the F-I relations of the first, second, and ss spikes were defined as slope 1, slope 2, and slope SS, respectively.
A 1-s hyperpolarizing step current with step of −20 to −50 pA was injected into the cells. The voltage deflection with no or minimum activation of h-current was averaged over the first 0.5 s starting from the peak deflection of the membrane potential. The Rin was calculated by the mean value of the voltage divided by the amplitude of the corresponding step current. The time constants (τm and τ1) were determined by fitting a double decaying exponential function with form of Y = y0 + A1*Exp (V/τm) + A2*Exp(V/τ1) to the averaged voltage responses to a train of five pulses (0.5 ms, −1 nA, 250-ms interval). The whole cell capacitance was calculated by the formula: Cm = {τm*L/[Rin*Tanh(L)]}, where L = π/(τm/ τ1 − 1)1/2.
In addition to the measurement of the preceding membrane properties, persistent inward current (PIC) and hyperpolarization activation inward current (Ih) were also tested in the present study. The PIC was recorded in voltage clamp by applying a slow voltage bi-ramp with duration of 5–10 s, peak of 0–30 mV, and holding potential of −70 mV (Lee and Heckman 2001). Ih was recorded in current clamp with the same protocol for Rin recording.
Solutions and chemicals
The dissecting ACSF contained (in mM) 25 NaCl, 188 sucrose, 1.9 KCl, 1.2 NaH2PO4, 10 MgSO4, 26 NaHCO3, 1.5 kynurenic acid, and 25 glucose. The recovering ACSF contained (in mM) 118 NaCl, 4.5 KCl, 1.2 NaH2PO4, 10 MgSO4, 26 NaHCO3, 1.0 CaCl2, 1.5 kynurenic acid, and 25 glucose. The recording ACSF contained (in mM) 130 NaCl, 4.5 KCl, 1.25 NaH2PO4, 1.25 MgCl2, 26 NaHCO3, 2.5 CaCl2, and 10 glucose. The pH of these solutions was ∼7.4 when bubbled with 95% O2-5%CO2.
The intracellular solution contained (in mM) 150 KMeSO4, 10 NaCl, 10 HEPES, 0.1 EGTA, 3 Mg-ATP, and 0.3 GTP. Alexa 594 (∼3%; Invitrogen Canada) or Lucifer yellow (∼1%) was added to the intracellular solution in some experiments to study the morphology of the recorded neurons. All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise noted.
RESULTS
The present study is based on the experimental results from intracellular recordings of 72 spinal neurons (60 EGFP+ and 12 EGFP−) from 44 cfos-EGFP mice of P4-15; data of 6 DRG cells from P4–P13 cfos-EGFP mice; and confocal images from 20 cfos-EGFP mice of P4-15. The details of the results are presented in the following text.
EGFP expression in acutely dissociated DRG cells
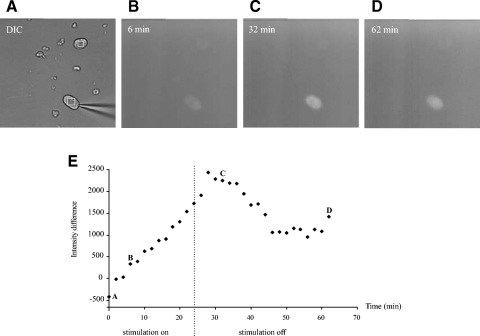
The activity-dependent nature of the c-fos-driven EGFP expression was confirmed using acutely dissociated dorsal root ganglion neurons. These neurons were chosen because of their high degree of viability during the dissociation process as well as a previous report of c-fos expression in these cells due to trains of action potentials (Fields and Nelson 1994). Once dissociated, these cells were patch clamped and repeatedly depolarized under voltage-clamp conditions (Vh = −60 mV, test pulse to 0 mV × 500 ms × 0.2 Hz) while fluorescence intensity measurements were taken at regular intervals. Using this procedure, stimulated cells were seen to fluoresce more intensely during stimulation (n = 5/6). In a number of experiments, multiple cells were included in the field of view, and the difference of intensities was recorded for stimulated and unstimulated cells. As illustrated in Fig. 1 (A–D), the fluorescence intensity of the stimulated cell can be seen to increase dramatically, while it is unchanged in the unstimulated cell. The accompanying graph (Fig. 1E) demonstrates that the fluorescence increase corresponds to the period of stimulation, and after a short delay, the intensity decreases with cessation of the stimulation. The rapidity of these fluorescence changes is consistent with the expression of a fos-β-galactosidase fusion protein that showed increased expression in as little as 15 min poststimulation in transfected neuroblastoma cells (Schilling et al. 1991). The decay of fluorescence intensity was slow and could continue for hours before returning to baseline. These experiments demonstrate that neurons from the c-fos-EGFP animals used in this study express EGFP under conditions that should increase the expression of c-fos.
Fig. 1.
Enhanced green fluorescent protein (EGFP) expression in acutely dissociated dorsal root ganglion cells. Dissociated cells were patch clamped and repeatedly depolarized under voltage-clamp conditions while measurements of fluorescence intensity difference were taken at regular intervals. A: multiple cells were included in the field of view, and the difference of intensities were recorded for stimulated and unstimulated cells. B: the fluorescence intensity of the stimulated cell was increased after 6-min stimulation while it was unchanged in the unstimulated cell. C: the fluorescence intensity of the stimulated cell reached a peak even after termination of the stimulation. After a short delay, the intensity decreased. D: the decay of fluorescence intensity appears to be a biphasic decay process, an initial fast phase and a 2nd slower phase. The 2nd phase could take hours to return to baseline. E: plot of fluorescence intensity difference over stimulation time. The stimulation started at time 0 and terminated at time 24 min (- - -). Three time intervals (B–D) were chosen to show the changes in fluorescence intensity in B–D, respectively.
EGFP expression in spinal cord slices in vitro
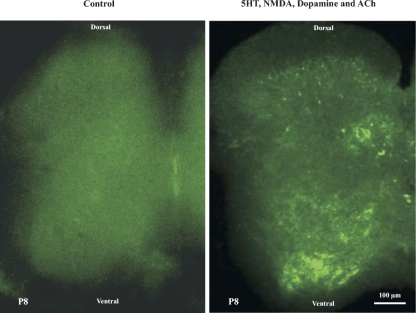
Previous studies have shown that certain neurotransmitters applied directly to the isolated spinal cord of the neonatal rat or mouse can induce locomotor-like activity (see Kiehn and Butt 2003 for review). To determine whether EGFP expression could be evoked in vitro by these same agents in slices from the cfos-EGFP transgenic mice, we applied a mixture of 30 μM 5-HT, NMDA, dopamine, and ACh to slices of 150 μm thickness taken from the T12–-L4 segments of P8 cfos-EGFP transgenic mice. After 2 h, control and drug-treated slices were fixed and examined using a laser scanning confocal microscope. The number and intensity of EGFP+ cells were dramatically increased in the drug-treated slices. The intensity of the EGFP+ neurons was increased by the drug cocktail to a degree that necessitated that the sensitivity (gain) of the camera had to be reduced by 20–30% of control to avoid saturation of the images. As Fig. 2 shows qualitatively, the drug cocktail induced a dramatic increase in the EGFP detectable in neurons in most laminae of the spinal cord. A pronounced increase in EGFP expression occurred in the ventral horn, in lamina VII, VIII, and IX, and around the central canal (lamina X). Motoneurons as well as interneurons displayed increased fluorescence, showing that many cells in the presumed locomotor areas of the spinal cord including the ventromedial region (see Kiehn and Kjaerulff 1998; Kjaerulff and Kiehn 1996) are activated during the chemical stimulus and that these cells can be detected with the EGFP reporter. These experiments demonstrate that the c-fos-EGFP mouse model can be used to reveal neurons responsive to bath application of drugs in slice preparations.
Fig. 2.
EGFP expression in spinal cord slices in vitro. A cocktail of 30 μM serotonin (5-HT), N-methyl-d-aspartate (NMDA), dopamine, and ACh was applied to slices of 150 μm thickness taken from the T12–L4 segments of P8 cfos-EGFP transgenic mice. After 2 h, control (left) and drug-treated slices (right) were fixed and examined using a laser scanning confocal microscope. The drug cocktail induced a dramatic increase in the EGFP detectable in neurons in most laminae of the spinal cord. A pronounced increase in EGFP expression occurred in the ventral horn, in lamina VII, VIII, and IX, and around the central canal (lamina X).
In vivo locomotor activity induces EGFP expression
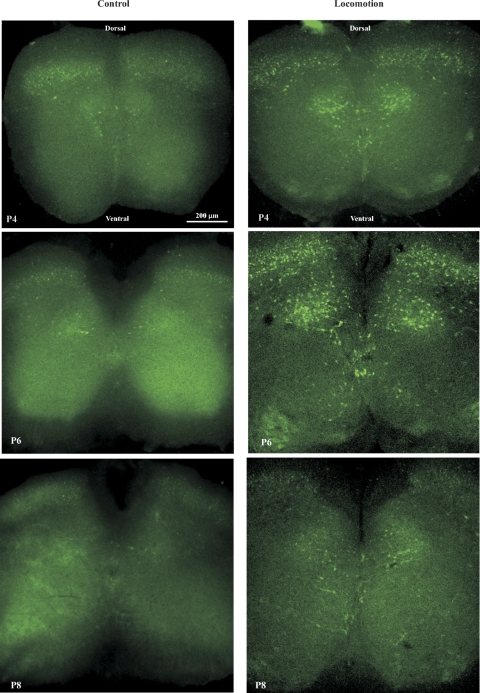
To determine whether these animals could be used to study neurons activated following locomotion, locomotor activity (swimming) was induced in intact cfos-EGFP transgenic mice for a period of 60–90 min. Slices prepared and fixed from these animals were compared with slices from control littermates. Figure 3 shows confocal images from control (left) and locomotion (right) groups from P4 (top), P6 (middle), and P8 (bottom) mice. In the ventral horn, numerous cells were labeled with EGFP in lamina VII, VIII, and X in the locomotion group, whereas only a few cells were labeled in the same region in the control group. Thus it is clear that the swimming task induced EGFP expression in neurons in the locomotor area of the ventral horn. In this study, lamina VII, VIII and X were set as target areas from which the EGFP+ neurons were selected for patch-clamp recordings.
Fig. 3.
Target areas for EGFP+ neurons for patch-clamp recordings. Locomotor activity was induced in GFP mice at age of P4–8. Slices were cut from spinal cords T12–L4. Photos were taken at ×10 magnification using Olympus confocal laser scanning microscope (see methods for details). Left: control; right: locomotion. Top: slices from P4 mice; middle: from P6; bottom: from P8. Control slices show that most of the EGFP-labeled cells are located in dorsal horn areas from laminas I–VI. A few of cells are also labeled near central canal area (laminar X). These EGFP-labeled cells are reduced with animal age. The locomotion slices show that in addition to the cells labeled in dorsal horn areas, a lot of cells are labeled in laminas VII, VIII and X. These labeled cells appear constantly with less correlation to the animal ages compared with the control ones. The EGFP-positive cells in laminas VII, VIII, and X are targeted for patch-clamp recordings.
EGFP-positive cells were also seen in dorsal horn areas in both control and locomotion groups. This is not surprising, as dorsal horn neurons are expected to be activated and express Fos during both the locomotor task (Dai et al. 2005b) and the surgical procedures themselves (Coggeshall 2005). Neurons in the dorsal horn were not targeted in this study, but it is striking that a group of neurons in the medial part of the dorsal horn (laminae I–VI) was activated due to the locomotor task. Recent observations demonstrate rhythmically active neurons in this region during locomotor activity (J. M. Wilson, E. Blagovechtchenski, and R. M. Brownstone, unpublished data). Figure 3 also shows that the EGFP-labeled cells are reduced with age in both control and locomotion groups. However, the labeled cells appear constantly with less correlation to the animal ages compared with the control ones. The dramatic increase in EGFP-labeled cells in locomotion groups validates this animal model for the present study.
Electrophysiological properties of EGFP-positive neurons in the ventral horn
The results presented in this study were based on the data collected from 55 neurons sampled in laminae VII, VIII, and X. Of the 55 neurons, 43 neurons were EGFP positive and 12 were non-EGFP positive. The 55 neurons were selected for data analysis based on the criteria that resting Em ≈ (or >) −60 mV, AP overshoot > (or ≈) 0 mV, and Rin > 150 MΩ.
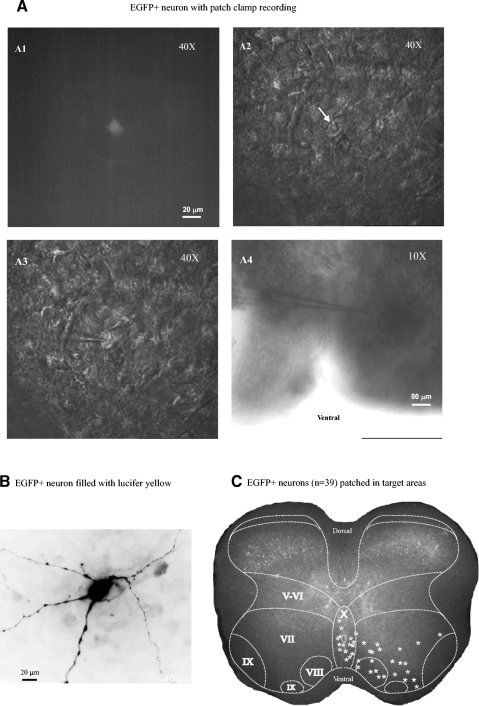
An example of an EGFP-positive neuron patched in the target area is shown in Fig. 4 A. The fluorescence image of EGFP could be visualized on the monitor (Fig. 4A1). The same cell is shown in an infrared image (Fig. 4A2, arrow) and patched with a glass pipette electrode (A3). This cell was located in lamina VIII as shown in a low-power (×10) infrared image (Fig. 4A4). Figure 4B shows an EGFP+ neuron filled with Lucifer yellow after intracellular recordings. The image displays the detailed morphology of the neuron (round soma with >4 stem dendrites). Figure 4C is an illustration of the laminar distribution of 39 EGFP+ neurons patched in the target area for which the low-power images of the electrode placement were available. Cells located in the left side of the slice were mirrored onto right side.
Fig. 4.
EGPF+ neurons with patch-clamp recording. A1: an EGFP-positive neuron was seen from a ×40 water-immersion lens with EGFP fluorescence cube. A2: the same cell (marked with white arrow) was observed in infrared image. A3: the cell was patched with a glass pipette electrode. A4: the same cell was shown in a low-power (×10) lens. The electrode position indicated the location of the cell in laminar VIII. B: an EGFP+ neuron was filled with lucifer yellow. The image shows the detailed morphology of the neuron: round soma with >4 stem dendrites. C: a laminar distribution of 39 EGFP+ neurons patched for recordings (shown as asterisk) was shown in the target area lamina VII, VIII, and X.
Three types of EGFP+ neurons
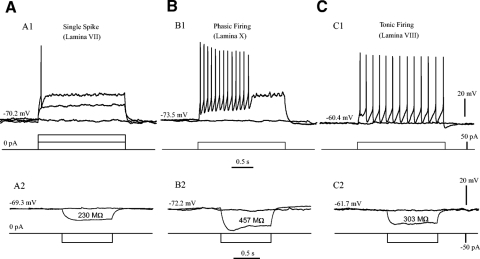
The EGFP+ neurons in lamina VII, VIII, and X could be classified into three types based on their firing patterns in response to depolarizing step currents: single spike (type 1), phasic firing (type 2), and tonic firing (type 3). The similar firing pattern has been reported in rat spinal neurons in ventral horn (Szucs et al. 2003; Theiss and Heckman 2005; Theiss et al. 2007), superficial horn (Prescott and De Koninck 2002), and deep dorsal horn (Hochman et al. 1997). In response to a depolarizing step current, neurons of type 1 elicited only one to two spikes (Fig. 5 A1); the type 2 fired briefly with firing duration <1 s (B1); the type 3 fired repetitively for a few or tens of seconds (C1). In addition to differences in firing patterns, the three types of EGFP+ neurons also showed differences in some electrophysiological properties, such as current threshold and input resistance (Fig. 5). In Fig. 5A1 a single spike was elicited by a step current of 100 pA for 2-s duration in a type 1 cell in lamina VII. The Rin was calculated as 230 MΩ for this cell (Fig. 5A2). With half the amount of the step current (50 pA), brief firing was evoked in a type 2 cell in lamina X (Fig. 5B1), which had a Rin of 457 MΩ (Fig. 5B2). The same amount of current produced tonic firing in a type 3 cell in lamina VIII (Fig. 5C1). The Rin was 303 MΩ for this cell (Fig. 5C2).
Fig. 5.
Three types of EGFP+ neurons. The EGFP+ neurons were classified into 3 types based on their firing patterns (A1–C1, top) in response to depolarizing step currents (2 s, 50 pA for each step; A1–C1, bottom). The passive membrane responses (A2–C2, top) to hyperpolarizing step currents (1 s, −50 pA for each step; A2–C2, bottom) were used to calculate input resistance by Ohm law. A: cells of single spike (type 1). A1: 2 step currents were injected into a type 1 cell and only one single spike was elicited by the 2nd step (2 s, 100 pA). A2: a hyperpolarizing step current (1 s, −50 pA) was injected into the same cell, and the voltage deflection was used to calculate the Rin (230 MΩ). B: cells of phasic firing (type 2). B1: a 50-pA step current evoked a brief firing in a type 2 cell for ∼0.6 s. B2: the cell membrane potential was hyperpolarized by a −50-pA step current, and the Rin was calculated as 457 MΩ in this cell. C: cells of tonic firing (type 3). C1: the tonic firing in a type 3 cell was evoked by a 50 pA depolarizing step current. C2: the hyperpolarization of membrane potential was produced by a −50-pA step current. Rin = 303 MΩ in this cell.
Two subtypes of EGFP+ neurons were observed in our experiments based on their firing properties. One displayed a “slow” type of repetitive firing (Szucs et al. 2003) and the other a “delayed” type of phasic firing (Hochman et al. 1997; Prescott and De Koninck 2002). For simplicity, we classified the slow type (n = 3) as type 3 and delayed type (n = 2) as type 2. Table 1A summarizes the membrane properties of 55 neurons (43 EGFP+ and 12 EGFP−) sorted in laminae VII, VIII, and X. No significant difference is found between the EGFP+ and EFGP− neurons in the membrane properties shown in the table. Laminar distributions of these neurons predicted differences only in input resistance and whole cell capacitance. The neurons in lamina X displayed the highest Rin and lowest C, whereas the cells in lamina VII had the lowest Rin and highest Cm. These results may suggest a graded distribution of the increased size in EGFP+ neurons from central cannel (lamina X) to ventral areas (lamina VII and VIII). The majority of EGFP+ neurons in lamina VII were type 1 or type 3, whereas lamina VIII neurons tended to be type 3. Lamina X neurons were of all three types. Thus the EGFP+ neurons located in the targeted laminae did not differ dramatically in terms of membrane properties or types, but the differences in Rin and Cm suggest that a detailed analysis of cell morphology may yield new ways of classifying locomotor neurons in the ventral horn.
Table 1A.
Membrane properties of the EGFP+ neurons classified in laminar distribution
| Lamina VII (19) | Lamina VIII (13) | Lamina X (23) | Significance | EGFP+ (43) | EGFP− (12) | Total (55) | |
|---|---|---|---|---|---|---|---|
| Em, mV | −65.5 ± 6 | −69.9 ± 6 | −69.7 ± 8 | NS | −68.6 ± 8 | −72 ± 12 | −69.3 ± 9 |
| Tth, pA | 89.5 ± 51 | 111.5 ± 58 | 91.3 ± 49 | NS | 98.7 ± 56 | 87.5 ± 31 | 96.4 ± 52 |
| Rin (MΩ) | 283.5 ± 124 | 321.6 ± 141 | 480.6 ± 252 | * | 378.3 ± 217 | 356.8 ± 171 | 374.9 ± 207 |
| τm, ms | 21.0 ± 16 | 19.7 ± 9 | 24.8 ± 13 | NS | 20.2 ± 12 | 28.7 ± 13 | 22.0 ± 13 |
| τ1, ms | 4.7 ± 2 | 4.4 ± 2 | 3.8 ± 2 | NS | 4.1 ± 2 | 4.6 ± 2 | 4.2 ± 2 |
| Cm, pF | 145.1 ± 67 | 124.7 ± 52 | 88.7 ± 43 | * | 107.7 ± 50 | 128.8 ± 76 | 115.8 ± 59 |
| Vth, mV | −45.8 ± 11 | −38.5 ± 10 | −37.3 ± 11 | NS | −39.5 ± 8 | −42.9 ± 17 | −40.1 ± 11 |
| AP height, mV | 49.8 ± 14 | 42.6 ± 11 | 49.1 ± 11 | NS | 46.2 ± 13 | 53.1 ± 11 | 47.7 ± 12 |
| AP width, ms | 3.6 ± 1 | 3.2 ± 1 | 4.2 ± 1 | NS | 3.7 ± 1 | 4.0 ± 1 | 3.8 ± 1 |
| AHP depth, mV | 13.4 ± 6 | 17.3 ± 8 | 14.2 ± 6 | NS | 14.5 ± 6 | 14.1 ± 6 | 14.4 ± 6 |
| AHP 1/2 decay, ms | 46.3 ± 34 | 47.4 ± 30 | 53.1 ± 41 | NS | 49.4 ± 33 | 47.2 ± 46 | 48.9 ± 36 |
| Slope 1, Hz/nA | 175.9 ± 74 | 165.4 ± 168 | 204.8 ± 93 | NS | 189.4 ± 118 | 126.1 ± 47 | 179.7 ± 112 |
| Slope 2, Hz/nA | 143.5 ± 68 | 117.8 ± 93 | 147.1 ± 55 | NS | 141.5 ± 72 | 101.2 ± 33 | 135.3 ± 70 |
| Slope SS, Hz/nA | 122.3 ± 66 | 82.2 ± 30 | 112.8 ± 43 | NS | 109.8 ± 52 | 98.8 ± 28 | 107.4 ± 50 |
| Type 1 | 7 | 2 | 4 | 10 | 3 | 13 | |
| Type 2 | 2 | 2 | 11 | 9 | 6 | 15 | |
| Type 3 | 10 | 9 | 8 | 24 | 3 | 27 |
Parentheses enclose n values. Values are means ± SD. EGFP, enhanced green fluorescent protein.
, significant difference with P < 0.005 performed by single-factor ANOVA for the cells' laminar distributions. NS: no significant difference.
We also sorted the data according to the cell types to show the difference in membrane properties. Because there is no significant difference between the EGFP+ and EFGP− neurons, we put both groups together. The results are summarized in Table 1B. In general, type 1 neurons tended to possess the smallest Rin and AHP and the biggest Ith and Cm, whereas type 2 neurons had the minimum Ith and Cm and the maximum Rin, τm, and AP width. The type 2 neurons also showed a steeper slope of the F-I relation than that of type 3 neurons. The properties of type 3 neurons generally fell between those of types 1 and 2 expect for AP and AHP, where type 3 neurons had the minimum AP width and the maximum AHP height and half decay time.
Table 1B.
Membrane properties of the EGFP+ neurons classified in cell types
| Significance (Student's t-test) |
|||||||
|---|---|---|---|---|---|---|---|
| Type 1 (Single) | Type 2 (Phasic) | Type 3 (Tonic) | Total Mean | Types 1 vs. 2 | Types 2 vs. 3 | Types 1 vs. 3 | |
| n | 13 | 15 | 27 | 55 | |||
| Em, mV | −66.7 ± 5.4 | −70.6 ± 5.3 | −67.4 ± 8.5 | −69.1 ± 9 | † | NS | NS |
| Ith, pA | 115.4 ± 59.1 | 84.6 ± 31.5 | 88.9 ± 56 | 95.5 ± 52 | † | NS | NS |
| Rin, MΩ | 301.2 ± 109.9 | 436.2 ± 195.7 | 372.9 ± 233.5 | 374.9 ± 207 | † | NS | NS |
| τm, ms | 23.4 ± 14.9 | 25.8 ± 10.8 | 20.4 ± 13.4 | 22.3 ± 13 | NS | NS | NS |
| τ1, ms | 4.2 ± 1.6 | 3.9 ± 1.9 | 4.1 ± 2.6 | 4.2 ± 2.3 | NS | NS | NS |
| Cm, pF | 129.7 ± 46 | 104.6 ± 52 | 109.3 ± 53 | 116.7 ± 59 | NS | NS | NS |
| Vth, mV | −43.1 ± 6.1 | −39.0 ± 11.5 | −38.3 ± 8.9 | −40.2 ± 11 | NS | NS | † |
| AP height, mV | 49.9 ± 10.7 | 47.7 ± 11.3 | 47.5 ± 14.2 | 48.1 ± 12.6 | NS | NS | NS |
| AP width, ms | 3.5 ± 0.6 | 4.5 ± 1.3 | 3.3 ± 1.2 | 3.7 ± 1 | † | * | NS |
| AHP depth, mV | 10.7 ± 4.6 | 13.4 ± 6.2 | 16.7 ± 6.1 | 14.5 ± 6 | NS | † | * |
| AHP 1/2 decay, ms | 30.7 ± 22.2 | 46.6 ± 39.4 | 55.5 ± 30.4 | 49.3 ± 36 | NS | NS | * |
| Slope 1, Hz/nA | NA | 197.1 ± 67.6 | 176.1 ± 126.8 | 182.2 ± 112 | NA | NS | NA |
| Slope 2, Hz/nA | NA | 143.7 ± 51.4 | 133.2 ± 77.2 | 136.2 ± 70 | NA | NS | NA |
| Slope SS, Hz/nA | NA | 134 ± 51.8 | 96.9 ± 46.1 | 106.9 ± 50 | NA | † | NA |
| Lamina VII | 7 | 2 | 10 | ||||
| Lamina VIII | 2 | 2 | 9 | ||||
| Lamina X | 4 | 11 | 8 | ||||
: significant difference with P < 0.05;
, significant difference with P < 0.005; NS, not significant difference; NA, not applicable.
The difference in firing patterns did not strictly correspond to the difference in the neuron's laminar location and membrane properties. The type 2 neurons were more concentrated in lamina X (73%, n = 15) than in other areas while neurons of types 1 and 3 appeared evenly distributed in lamina VII, VIII, and X. The functional significance of the condensed distribution of type 2 neurons is unknown. However, type 2 neurons were significantly different from type 1 in Em, Ith, Rin, and AP properties, suggesting that the type 2 neurons are a population of interneurons anatomically, electrophysiologically, and functionally distinct from type 1 neurons. In addition, type 2 neurons also showed a significant difference from type 3 in F-I relations, AP, and AHP properties. Some studies in rat spinal dorsal horn neurons suggested that the type 2 and 3 cells might be two extremes of one heterogeneous cell population (Szucs et al. 2003). The present study does not rule out this possibility. However, because the type 2 and 3 neurons tended to be similar in passive properties (Em, Rin, Cm, etc) but different in active properties (AP, AHP, F-I relations), and laminar distribution, it seems likely that these two types of neurons are functionally different. Significant differences in Vth and AHP were also observed between type 1 and 3 cells, suggesting that these two types of neurons should be dynamically as well as functionally different as well. In summary, based on electrophysiological properties and location, at least three distinct types of neurons can be identified in the ventral horn by their expression of EGFP following a locomotor task.
Membrane currents mediated by multiple channels
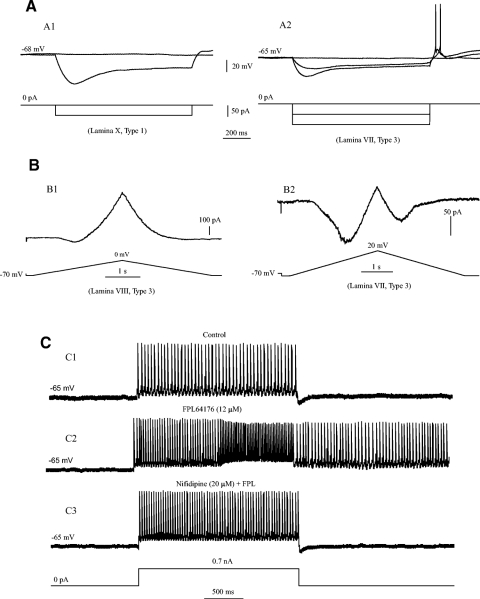
Hyperpolarization-activated inward current (Ih) was tested in this study. An example is shown in Fig. 6 A (and Fig. 5B2). The Ih was activated in a type 1 neuron in lamina X by a step current of −50 pA and 1-s duration (Fig. 6A1). Some neurons also exhibited postinhibitory rebound after termination of the step current. Figure 6A2 shows that two hyperpolarizing step currents with −50 pA for each step were injected into a type 3 neuron in lamina VII. A small Ih was activated by the first step current with a spike elicited by the postinhibitory rebound. The second step current produced a large Ih with an earlier elicitation of the rebound spike. Ih was investigated in 67 neurons, (55 EGFP+ and 12 EGFP−). Ih was observed in 52% of the total neurons (35/67), 53% of the EGFP+ neurons (29/55), and 50% of the EGFP− neurons (6/12). Eleven of the 29 EGFP+ neurons that displayed Ih exhibited the postinhibitory rebound. This accounted for 38% of the EGFP+ neurons displaying Ih (11/29) and 20% of the all 55 EGFP+ neurons (11/55). No correlation was found between Ih expression and neuronal type or laminar distribution.
Fig. 6.
Membrane currents observed in EGFP+ neurons. A1: a hyperpolarization-activated inward current (Ih) was activated by a step current of −50 pA with 1-s duration injected into a type 1 cell in lamina X. A2: the same currents were activated with same current injection in a type 3 cell in lamina VII. Spikes elicited from postinhibitory rebounds were observed in each step after the termination of the step currents. B1: a 5-s bivoltage ramp from −70 to 0 mV was applied to a type 3 cell in lamina VIII. A small persistent inward current (PIC) with negative current slope was demonstrated on the rising phase of the voltage ramp. B2: the similar voltage ramp was applied to a type 3 EGFP+ neuron in lamina VII with 10 mM TEA and 5 mM 4-aminopyridine (4AP) applied to the recording solution. A persistent inward current (PIC) of ∼100 pA was demonstrated on the ascending phase of the ramp while a small PIC (∼50 pA) was also observed in the descending phase of the ramp. C1: the repetitive firing was elicited by a 2-s step current of 0.7 nA (bottom) in an EGFP+ neuron patched in target areas. C2: bath application of 12 μM FPL64176, an agonist of L-type calcium channels, generated a plateau potential with bistable firing. C3: the bistable firing was blocked by 20 μM nifidipine with FPL, the L-channel antagonist.
Persistent inward currents (PICs) were also tested in this study. The currents could be explored by a slow voltage ramp (Lee and Heckman 2001) as shown in Fig. 6B1, where a 5-s triangular voltage ramp from −70 to 0 mV was applied to a type 3 EGFP+ neuron in lamina VIII. A small PIC (∼50 pA) with negative current slope was demonstrated on the rising phase of the voltage ramp. A larger PIC could be recorded with blockade of potassium currents by TEA and 4-aminopyridine (4AP). An example is shown in Fig. 6B2, where a 10 mM TEA and 5 mM 4AP were administrated in the recording solution. A similar voltage ramp was applied to a type 3 EGFP+ neuron in lamina VII. A larger PIC (∼100 pA) was demonstrated on the ascending phase of the ramp while a small PIC (∼50 pA) was also observed in the descending phase of the ramp. While PIC could be enhanced by blockade of potassium currents, potentiator of PIC could also facilitate the induction of PIC in EGFP+ neurons, which was shown as an induction of plateau potential with bistable firing in current clamp recording in Fig. 6C. A repetitive firing was elicited by a 2-s step current of 0.7 nA (Fig. 6C1) in an EGFP+ neuron. Bath application of 12 μM FPL64176, a L-type calcium channel potentiator, generated a plateau potential with bistable firing outlasting the termination of the step current (Fig. 6C2). The plateau potential and bistable firing were blocked by 20 μM nifidipine (with FPL64176), the L-channel antagonist (Fig. 6C3). The facilitation of plateau potential was observed in four of five EGFP+ neurons recorded with FPL64176 in current-clamp protocol from P8–P15 cfos-EGFP mice. In a total of 67 neurons (55 EGFP+ and 12 EGFP−) recorded with voltage-clamp protocol from P4–P8 mice, PIC was demonstrated in 51% of the neurons (34/67), 56% of the EGFP+ neurons (31/55), and 25% of EGFP- neurons (3/12). The expression of PICs was not related to the lamina distribution of the neurons.
5-HT-induced membrane potential oscillations
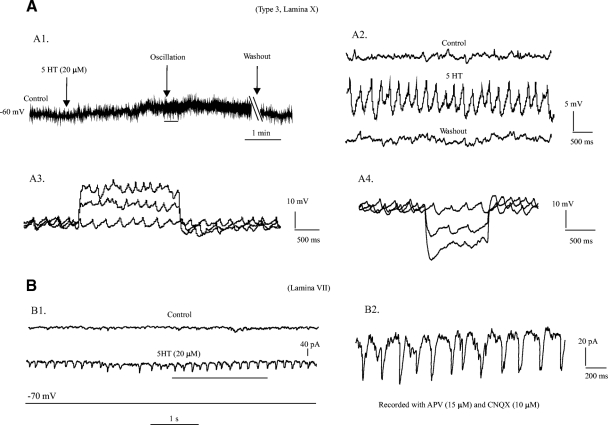
5-HT-induced membrane potential oscillations were observed in 7 of 20 cells tested. Similar observations were reported in recent studies in ascending commissural interneurons in the neonatal mouse (Carlin et al. 2006; Zhong et al. 2006a) and in previous studies in rat spinal interneurons in lamina VII (MacLean et al. 1995) and X (Hochman et al. 1994a,b). Figure 7 shows a voltage oscillation in a type 3 neuron in lamina X after bath application of 20 μM 5-HT. The 5-HT depolarized the membrane potential by ∼10 mV (Fig. 7A1) and induced membrane potential oscillations within ∼2 min of application. The peak-to-peak amplitude of these oscillations was ∼7 mV and frequency ∼4 Hz (Fig. 7A2). The oscillations were voltage dependent with a small increase in frequency (1–2 Hz) when the membrane potential was depolarized (Fig. 7A3) and a small decreased in the frequency (∼2 Hz) when the membrane potential was hyperpolarized (A4). The amplitude of the oscillations was relatively stable when the membrane potential was perturbed by the step currents. The oscillations stopped after 15 min washout (Fig. 7A, 1 and 2).
Fig. 7.
5-HT-induced membrane oscillations. A1: the membrane potential was maintained at about −60 mV in a cell of type 3 in lamina X. About 10 mV membrane depolarization was induced by bath application of 20 μM 5-HT. Membrane oscillation was observed 2 min after application of the 5-HT. The 5-HT-induced oscollation could be removed after 15 min washout. A2: the oscillation underlined by black bar in A1 was enlarged to show the details with control and washout. The peak-to-peak amplitude of the oscillation in this cell was ∼7 mV and frequency ∼4 Hz. A3: a 2 s depolarizing step current with step of 50 pA was injected into the same cell. The membrane oscillation remained with a small increase (1–2 Hz) in frequency of the oscillation. A4: a 1-s hyperpolarizing step current with step of −50 pA was injected into the same cell. Again, the membrane oscillation remained but the frequency decrease by ∼2 Hz. The last step evoked sag current and resulted in a membrane depolarization. But the oscillation still remained. B1: 5-HT-induced membrane (current) oscillation was observed in an EGPF neuron in lamina VII with voltage-clamp protocol. The recordings were made with membrane potential clamped at −70 mV and 2-amino-5-phosphonovaleric acid (APV, 15 μM) and 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX, 10 μM) applied to the solution. Top: control; middle: 5-HT-induced oscillation; bottom: clamped voltage. B2: the oscillation underlined by black bar in B1 was enlarged to show the details: peak-to-peak amplitude was ∼50 pA and frequency ∼6 Hz.
Using voltage clamp, 5-HT induced membrane current oscillations were observed in another EGFP+ neuron in lamina VII (Fig. 7B1). The neuron was recorded with membrane potential clamped at −70 mV and 2-amino-5-phosphonovaleric acid (APV, 15 μM) and 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX, 10 μM) applied in the bath (Fig. 7B1). Bath application of 5-HT (20 μM) induced the current oscillations with peak-to-peak amplitude of ∼50 pA and frequency of ∼6 Hz (Fig. 7B2). Of the seven neurons in which 5-HT induced oscillations, two were located in lamina VII, three in lamina VIII, and two in laminar X. Five of the neurons were classified as type 3, one as type 2, and one was not tested in current clamp. The mean value of peak-to-peak amplitude for the six cells recorded with current clamp was 8.6 mV (from 7 to 10 mV), and the mean frequency was 8.7 Hz (from 4 to 16.4 Hz). The 5-HT-induced membrane potential oscillations appeared to be related to the neuron types (2 and 3) but not laminar distribution (n = 7).
5-HT modulation of cell membrane properties
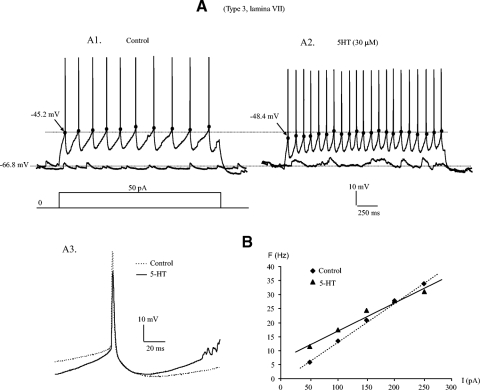
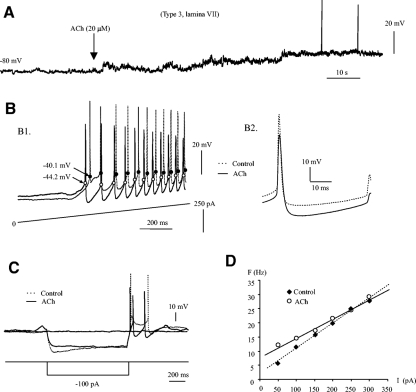
As 5-HT is critical for locomotor activity. Its effect on EGFP+ neurons are investigated in this study. Our study shows that 5-HT modulated membrane properties of EGFP+ neurons and increased neuronal excitability. A typical example is shown in Fig. 8. Repetitive firing was evoked by step currents injected into a type 3 neuron in lamina VII (Fig. 8A1). Bath application of 30 μM 5-HT generated 11 additional spikes with no change in Em (Fig. 8A2). This increased excitability by 5-HT was accompanied by hyperpolarization of Vth by ∼2.8 mV (Fig. 8A3), reduction of AHP by ∼1.6 mV (A3), and increase of Rin by ∼7.5 MΩ. The slope of F-I relation was reduced by 38.1 Hz/nA without shift of the F-I curve (Fig. 8B). These data suggest that neuronal excitability is modestly increased by 5-HT especially in the lower range of injected current (<200 pA).
Fig. 8.
5-HT modulation of membrane properties of EGFP+ neuron. A1: a repetitive firing (top) was evoked in a type 3 cell in lamina VII by a 2-s step current of 50 pA (bottom). The voltage threshold (marked by back dots) for the first spike was −45.2 mV. A2: bath application of 30 μM 5-HT resulted in a hyperpolarization of Vth in all spikes and an elicitation of 11 more spikes with the same step current. The Vth was hyperpolarized by 3.2 mV in the 1st spike while the resting membrane potential was almost unchanged. A3: the 1st 5 spikes from A, 1 and 2, were averaged respectively and overlapped on each other (- - -, control; —, 5-HT). It shows that 5-HT lowered the Vth by ∼2.8 mV and reduce the AHP by ∼1.6 mV. B: the F-I relation was plotted for steady-state firing (- - -, control; —, 5-HT). The F-I slope was reduced by 38.1 Hz/nA without shift of the F-I curve.
The effects of 5-HT on 14 neurons (11 EGFP+ and 3 EGFP−) are summarized in Table 2. Because 5-HT-induced changes in the 11 EGFP+ neurons are generally agreed with those in all 14 neurons (see Table 2), the data from the 14 neurons are used in the following presentation. Of the 14 neurons, 5 were located in lamina VII, 4 in lamina VIII, and 5 in lamina X. Three were type 1, two were type 2, and nine were type 3. The significant changes induced by 5-HT in the membrane properties included an increase in Rin (64.3 ± 90 MΩ, 18.5%), hyperpolarization of Vth (5.1 ± 6 mV), increase in AP width (0.6 ± 1 ms) and reduction in AHP depth (1.9 ± 3 mV). Reduction in membrane potential (−1.3 ± 7 mV), rheobase (−3.6 ± 31 pA), AP height (−1.7 ± 11 mV), and AHP half decay (−8.7 ± 31 ms) were also observed in these neurons but these changes were not significant.
Table 2.
5-HT-induced changes in cell membrane properties
| Changes in Membrane Properties |
||||||
|---|---|---|---|---|---|---|
| Control |
Mean |
|||||
| Total | EGFP+ | Total | EGFP+ | ↑Em Total | ↓Em Total | |
| n | 14 | 11 | 14 | 11 | 7 | 7 |
| Em, mV | −72.3 ± 8 | −72.9 ± 8 | −1.3 ± 7 | −0.8 ± 5 | 4.5 ± 3* | −7.1 ± 3* |
| Ith, pA | 107.1 ± 61 | 118.2 ± 64 | −3.6 ± 31 | −4.5 ± 35 | −7.1 ± 34 | 0.0 ± 29 |
| Rin, MΩ | 347.8 ± 111 | 342.5 ± 123 | 64.3 ± 90* | 71.5 ± 99* | 70.6 ± 54* | 58.0 ± 121 |
| Vth, mV | −39.6 ± 10 | −39.7 ± 11 | −5.1 ± 6* | −3.4 ± 4† | −2.4 ± 4† | −8.3 ± 6† |
| AP height, mV | 43.5 ± 13 | 39.2 ± 11 | −1.7 ± 11 | −3.4 ± 11 | −2.5 ± 13 | −0.7 ± 9 |
| AP width, ms | 3.8 ± 1 | 3.9 ± 1 | 0.6 ± 1† | 0.6 ± 1† | 0.4 ± 1 | 0.7 ± 1† |
| AHP depth, mV | 16.4 ± 8 | 15.8 ± 8 | −1.9 ± 3† | −1.7 ± 3† | −1.5 ± 4 | −2.4 ± 2† |
| AHP 1/2 decay, ms | 52.1 ± 43 | 46.3 ± 27 | −8.7 ± 31 | −5.6 ± 21 | −17.9 ± 39 | 2 ± 13 |
| Slope SS, Hz/nA | 88.3 ± 37 | 88.1 ± 39 | −11.1 ± 37 | −20.6 ± 27 | −19.2 ± 29 | −3.1 ± 47 |
↑, depolarization (≥2 mV in average); ↓, hyperpolarization (≤−2 mV in average);
, significant difference with P < 0.05 and
, P < 0.005 performed by paired two-sample t-test.
5-HT had a paradoxical effect on membrane potential. While 5-HT depolarized the membrane potential in 50% of the recorded neurons (n = 14), another 50% of the neurons displayed a hyperpolarization of membrane potential by 5-HT. We separated these neurons into two groups based on their membrane potential response to 5-HT and summarized the effects of 5-HT on their membrane properties in Table 2. The results show that although 5-HT induced a strong and opposite effect on membrane potential, changes in other membrane properties in these two groups of cells were generally the same (only small, nonsignificant difference in AHP decay). These results suggest that the variable effects of 5-HT on membrane potential may not result from the changes in other membrane properties measured in this study. The mechanism underlying this paradox remains unknown. While the major effect of 5-HT on membrane properties (mean in Table 2) were observed in most of the recorded neurons, a small portion of the neurons (<35%) showed no change or opposite changes in Rin, Vth, AP, and AHP depth and width, and 64% of the neurons showed no change (or changes <50 pA) in Ith. Note that the measurement of Ith in this study might not reflect the real changes in some cells because the step current injected into cells for determination of the Ith was increased by 50 pA at each step, therefore any changes in Ith <50 pA would not be detected.
Data from 8 (all type 3) of the 14 cells were used to calculate the F-I relations. Five of the eight cells showed a significant reduction in the F-I slope, accompanied by a left (n = 1) or up shift (n = 4) of the F-I curves. Two of the eight cells showed an increased in F-I slope with nonshift (n = 1) or right shift (n = 1) of the F-I curves. A decrease in neuronal output was observed in one cell that showed a down shift of the F-I curve with unchanged slope. These data demonstrate that 5-HT generally increased the neuronal excitability and/or enhanced the output of EGFP+ neurons although the effects were variable.
ACh modulation of cell membrane properties
Modulation of EGFP+ neurons by ACh was also investigated (n = 19). A typical example is shown in Fig. 9, which demonstrates recordings from a type 3 neuron in lamina VII. Bath application of ACh (20 μM) depolarized the membrane potential by ∼17 mV with increased background noise and spontaneous firing (Fig. 9A). ACh also led to a hyperpolarization of Vth by ∼4.1 mV (Fig. 9B), increase of the AHP by ∼3.5 mV (B), reduction of Rin by ∼55 MΩ (C); and lowering of the F-I slope by ∼35.6 Hz/nA (D). Note that the Ih was also blocked by ACh in this neuron (Fig. 9C). The change in the F-I relation suggests that the excitability of the cell was increased by ACh in the lower range of injected current ≤250 pA but reduced in the higher range over 250 pA. This “current-dependent” modulation of neuronal excitability might result from a nonlinear balance of excitatory (slow depolarization of Em and lowering of Vth) and inhibitory (increase of AHP and reduction of Rin and Ih) forces driven by ACh.
Fig. 9.
ACh modulation of membrane properties of EGFP+ neuron. A: recordings were made from a type 3 cell in lamina VII. A: 20 μM ACh was applied to the bath solution (↓), and the membrane potential was depolarized by ∼17 mV after 1-min application of the drug. Spontaneous firing occurs occasionally during the depolarization of the membrane potential. B1: the hyperpolarization of Vth and increase of afterhyperpolarization (AHP) were shown in the repetitive firings evoked by a 2-s ramp current starting from 0 pA. Spike trains from control (- - -) and ACh (—) were overlapped with unchanged baseline for resting membrane potentials. Voltage thresholds were marked by dots on the spikes (black for control and open for ACh). B2: the 1st 5 spikes from both control and ACh conditions were averaged, respectively, and overlapped on each other (- - -, control; —, Ach) to show the changes in Vth, AHP and action potential (AP) in this neuron. C: a hyperpolarizing current (1 s, −100 pA) was injected into the same cell before and after the ACh application. Membrane potential deflections from the control (- - -) and ACh (—) conditions were overlapped and aligned to the resting membrane potentials. Spikes were elicited from postinhibitory rebound in both conditions. ACh hyperpolarized the Vth by ∼4.1 mV, reduced input resistance by ∼55 MΩ and increased the AHP by ∼3.5 mV in this neuron. D: the F-I curves were plotted for steady-state spikes. ACh reduced the F-I slope by ∼35.6 Hz/nA. The excitability of the cell was increased in the lower range of injected current (≤250 pA) but slightly reduced in the higher range over 250 pA.
The effects of ACh on neuronal membrane properties from 19 neurons (15 EGFP+ and 4 EGFP−) are summarized in Table 3. The table is organized in the same format as Table 2 for similar observation of changes in membrane potential with 5-HT and ACh. Because the Ach-induced changes in the 15 EGFP+ neurons are not significantly different from those in all 19 neurons (see Table 3), the data from the 19 neurons are used in the following presentation. Of 19 recorded cells, 4 were located in lamina VII (1 type 1 and 3 type 3), 4 in lamina VIII (1 type 1, 1 type 2, and 2 type 3), and 11 in lamina X (1 type 1, 5 type 2, and 5 type 3). Although >50% of the cells were distributed in lamina X and the majority of the cells were type 3, the ACh-induced changes in membrane properties were not significantly related to either laminar distribution or cell type.
Table 3.
ACh-induced changes in cell membrane properties
| Changes in Membrane Properties |
||||||
|---|---|---|---|---|---|---|
| Control |
Mean |
|||||
| Total | EGFP+ | Total | EGFP+ | ↑Em Total | ↓Em Total | |
| n | 19 | 15 | 19 | 15 | 10 | 6 |
| Em, mV | −68.7 ± 7 | −69.3 ± 7 | 3.7 ± 9† | 3.1 ± 9† | 10.4 ± 7* | −5.7 ± 5† |
| Ith, pA | 73.7 ± 31 | 66.7 ± 24 | 2.6 ± 48 | 10.0 ± 48 | −10.0 ± 39 | 25 ± 69 |
| Rin, MΩ | 426.7 ± 241 | 446.7 ± 242 | −56.4 ± 78* | −69.6 ± 78† | −60.9 ± 92† | −37.2 ± 68 |
| Vth, mV | −40.7 ± 10 | −41.8 ± 8 | −3.9 ± 4* | −3.3 ± 3* | −3.4 ± 4† | −5.6 ± 3* |
| AP height, mV | 50.4 ± 13 | 50.3 ± 12 | −5.5 ± 12† | −5.6 ± 12† | −6.6 ± 16 | −2.8 ± 10 |
| AP width, ms | 3.3 ± 1 | 3.1 ± 1 | 0.3 ± 1† | 0.3 ± 0.7† | 0.4 ± 1 | 0.2 ± 0.3 |
| AHP depth, mV | 14.1 ± 4 | 14.0 ± 5 | 1.1 ± 3† | 0.8 ± 3† | 1.4 ± 4 | 0.5 ± 2 |
| AHP 1/2 decay, ms | 51.6 ± 30 | 50.7 ± 30 | 3.8 ± 15 | 3.0 ± 15 | 0.4 ± 17 | 5.9 ± 15 |
| Slope SS, Hz/nA | 94.8 ± 30 | 99.1 ± 29 | 4.8 ± 52 | 12.2 ± 52 | 12.5 ± 59 | −2.4 ± 5 |
↑, depolarization (≥2 mV in average); ↓, hyperpolarization (≤−2 mV in average);
, significant difference with P < 0.05 and
, P < 0.005 performed by paired two-sample t-test.
The significant changes induced by ACh include a depolarization of Em (3.7 ± 9 mV), reduction of Rin (−56.4 ± 78 MΩ, 13.2%), hyperpolarization of Vth (−3.9 ± 4 mV), reduction of AP amplitude (−5.5 ± 12 mV) with an increase in AP width (0.3 ± 0.7 ms), and an increase of AHP depth (1.1 ± 3 mV) with an increase in AHP half-decay time (3.8 ± 15 ms). Similar to the case of 5-HT, ACh also induced paradoxical changes in membrane potential. Of the 19 cells, 10 cells (52%) showed depolarization of Em (mean = 10.4 ± 7 mV), 6 cells (31%) displayed hyperpolarization of Em (mean = −5.7 ± 5 mV), and 3 cells (15%) did not respond to ACh in Em. Both changes (depolarization and hyperpolarization) were statistically significant. Except for the differences in rheobase and slope of the F-I relation, all other changes in membrane properties in these two groups of neurons were very similar (see Table 3), suggesting that the mechanism underlying the changes in membrane potential by ACh may not result from the changes in other properties shown in the Table 3. Instead the changes in membrane potential might cause the changes in Ith and F-I slope. The reduction of rheobase and increase of F-I slope could be directly induced by the depolarization of Em in the group of depolarizing neurons, whereas the hyperpolarization of Em could lead to the opposite effects on rheobase and F-I slope in the group of hyperpolarizing neurons. Similar to the experiments of 5-HT, a small portion of the neurons (<35%) displayed no change or opposite changes in Rin, Vth, AP, and AHP properties, and a large number of the cells (68%) showed no change (or changes <50 pA) in rheobase.
The F-I relations were calculated in 11 of the 19 neurons (8 type 3 and 3 type 2). Of the 11 neurons, 64% of the cells (7/11) showed a significant reduction of the F-I slope with left (n = 3), up (n = 3), or down shift (n = 1) of the F-I curves. 36% (4/11) of the cells showed an increase in the F-I slope with nonshift (n = 1), down (n = 2), or up shift (n = 1) of the F-I curve. These changes in F-I relation demonstrate the ACh modulation of neuronal excitability and output through a balance of excitatory and inhibitory forces driven by ACh in the EGFP+ neurons.
Combined effects of 5-HT and ACh on EGFP+ neurons
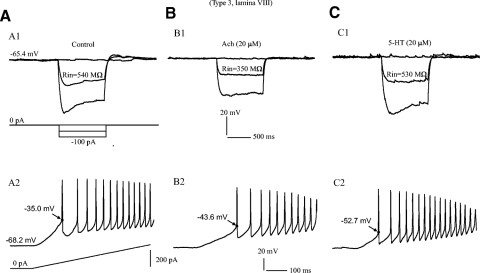
The combined effects of 5-HT and ACh on cell membrane properties were investigated in this study to examine the relative mechanisms mediating the modulation of membrane properties by the two agents. Figure 10 shows modulation of neuronal properties by these agents with recordings made from a type 3 neuron in lamina VIII. In control, the Rin was ∼540 MΩ (Fig. 10A1) and Vth was −35.0 mV (A2). Bath application of 20 μM ACh reduced the Rin by ∼190 MΩ (Fig. 10B1) and hyperpolarized the Vth by 8.6 mV (B2). Subsequent application of 20 μM 5-HT increased the Rin by ∼180 MΩ from 350 MΩ measured in ACh condition (Fig. 10C1) and further hyperpolarized the Vth by 9.1 mV from −43.6 mV measured in ACh condition (C2). 5-HT also induced excitatory postsynaptic potentials (EPSPs) in this cell (Fig. 10C1). This figure also provides evidence for a reduction of Ih by ACh (Fig. 10B1). Interestingly, this reduction is reversed by subsequent application of 5-HT (Fig. 10C1). A similar reduction (or blockade) of Ih by ACh is also shown in Fig. 9C. These results demonstrate that the effects of 5-HT and ACh on EGFP+ neurons can be additive (e.g., Vth) or subtractive (e.g., Rin and Ih), depending on the specific membrane properties they modulate.
Fig. 10.
Combined effects of ACh and 5-HT on EGFP+ neurons. Recordings were made from a type 3 cell in lamina VIII in control (A) and conditions of ACh (B) and 5-HT (C). The current injections in A–C were the same as those shown in A. A: control recordings. A1: hyperpolarizing step current (1 s with 50 pA for each step, bottom traces) was injected into the cell. Voltage deflection (top traces) produced by the −50-pA step current was used to calculate Rin (540 MΩ). A2: repetitive firing (top trace) was evoked in the same cell by a 2-s ramp current (bottom trace). The Vth was measured from the 1st spike (−35.0 mV). B: recordings with bath application of 20 μM ACh. B1: the Rin (358 MΩ) was reduced by 182 MΩ and Ih was diminished. B2: in the same condition of ACh the Vth (−43.6 mV) of the 1st spike was hyperpolarized by 8.6 mV. C: recordings with bath application of 5-TH (20 μM) subsequently. C1: 5-HT increased the Rin (530 MΩ) by 172 MΩ from that measured in the condition with ACh. C2: Vth (−52.7 mV) was further hyperpolarized by 9.1 mV by 5-HT.
Eleven cells were investigated for combined modulation of cell membrane properties by 5-HT and ACh. Six cells were recorded with application of ACh followed by 5-HT. ACh reduced the Rin by −63.5 ± 134 MΩ, and subsequent application of 5-HT reversed this reduction of Rin and led to an increase in Rin by 84.3 ± 147 MΩ. Thus prior application of ACh did not reduce the ability of 5-HT to increase Rin, consistent with the notion that these two agents may alter Rin via different mechanisms. ACh hyperpolarized the Vth by −3.4 ± 4 mV, whereas 5-HT further lowered it by an additional −4.3 ± 5 mV. Thus prior application of ACh did not diminish the ability of 5-HT to hyperpolarize voltage threshold, suggesting that the two agents may act by different mechanisms to achieve the same effect on Vth. Similarly, ACh increased AHP depth (0.1 ± 2 mV) and width (1.1 ± 20 ms), whereas 5-HT reduced this (−1.9 ± 3 mV; −6.7 ± 10 ms). In another five cells, the inverse sequence of drug application was used. 5-HT increased the Rin (24.0 ± 83 MΩ), hyperpolarized Vth (−7.0 ± 5 mV), and reduced AHP (−2.0 ± 1 mV), whereas ACh applied subsequent to 5-HT reduced Rin (−52.4 ± 41 MΩ), further hyperpolarized the Vth (−5.6 ± 8 mV), and increased AHP (2.2 ± 2 mV).
In general, the effects of 5-HT after ACh or the effects of ACh after 5-HT were consistent with those of 5-HT and ACh alone on the EGFP+ neurons as listed in Tables 2 and 3. Thus neither agent alters the modulatory properties of the other on EGFP+ neurons, consistent with the suggestion that their actions on membrane properties of these cells are exerted by separate mechanisms. The additive effects of 5-HT and ACh on Vth would increase neuronal excitability, whereas the subtractive effects of 5-HT and ACh on Rin and AHP would either increase or decrease the cell excitability depending on the balance of the forces driven by the two agents.
Changes in membrane potential with modulation of intrinsic membrane properties
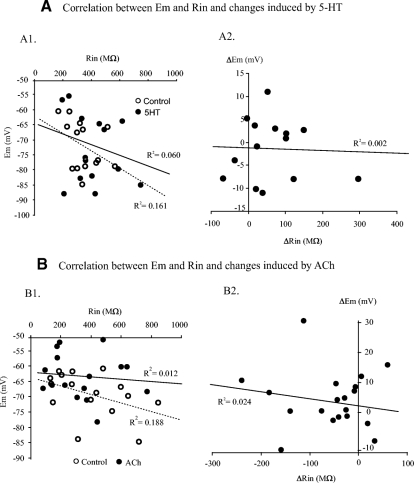
Both 5-HT and ACh induced variable effects on membrane potential. Because Em and Rin were both shown to be modulated by 5-HT (Table 2) and ACh (Table 3), we would expect that the changes induced by 5-HT and ACh in Em could result from the changes in Rin. Figure 11 shows the correlations between Em and Rin and the changes induced by 5-HT (n = 14) and ACh (n = 19). No significant correlation was found between Em and Rin (Fig. 11, A1 and B1) before or after administration of 5-HT (correlation coefficient R = 0.24 for control, 0.40 for 5-HT) or ACh (R = 0.11 for control, 0.43 for ACh). These results suggested that the resting membrane potential was not regulated simply by the passive membrane properties such as the size of EGFP+ neurons. Also, the ΔEm was not correlated to the ΔRin in either 5-HT (R = 0.05, Fig. 11A2) or ACh (R = 0.15, Fig. 11B2) condition. Furthermore, the changes in Em and Rin were neither correlated to the laminar distributions of the neurons nor to the types of the neurons (not shown). These results suggest that modulation of some unknown active (rather than passive) membrane properties by 5-HT and ACh result in the observed changes in membrane potentials in EGFP+ neurons.
Fig. 11.
Correlation analysis between membrane potential and input resistant. A: correlations between Em and Rin in both control (○ - -) and 5-HT (● –) conditions are established from 14 cells in A1. Changes induced by 5-HT in Em and Rin in the same group of neurons are plotted in A2. B: similar to A, the Em is correlated with Rin in both control (○ - -) and ACh (● –) conditions from the recordings of 19 cells in B1. Changes induced by ACh in Em and Rin are shown in B2. None of the preceding relations is significantly correlated, suggesting that rather than passive membrane properties, the 5-HT and ACh induced changes in Em and Rin may mainly result from modulation of active membrane properties of the EGFP+ neurons.
DISCUSSION
In this study, we demonstrate a new method by which neurons active in locomotion can be identified and their intrinsic properties studied in the mouse spinal cord. Identification of spinal interneurons involved in locomotion is a necessary step to understand how the spinal cord produces rhythm and pattern. As shown in other systems, such as the stomatogastric ganglion (Marder and Bucher 2001), it is necessary to understand neuronal connections, their intrinsic properties, and the modulation of these properties to understand rhythm generation. In the adult cat, interneurons can be identified by their location and synaptology (Jankowska 2008). However, the intrinsic and modulatable properties of these neurons have not been studied. In the mouse, interneurons can be identified through labeling with GFP driven by, for example, transcription factors expressed during development (e.g., Wilson et al. 2005; Zhang et al. 2008), or through retrograde labeling with dyes (Carlin et al. 2006; Eide et al. 1999; Stokke et al. 2002). With these techniques, the intrinsic membrane properties of the identified neurons can be studied directly in either in vitro or in vivo preparations. The present study demonstrates a method of studying spinal interneurons that are functionally activated by locomotor activities and visualized by EGFP expression driven by the promoter for the immediate early gene, c-fos. The membrane properties of the EGFP+ neurons and their modulatory properties by neurotransmitters 5-HT and ACh are studied in detail in this study. A conceptually similar circadian clock gene promoter-driven d2EGFP transgenic reporter mouse line has been used to target and study functionally activated biological clock neurons and circadian modulation of locomotor behavior (Ciarleglio et al. 2009; Kuhlman et al. 2003; Quintero et al. 2003).
Expression of c-fos-EGFP in spinal interneurons
In addition to locomotor task, c-fos expression can be also induced by many other factors (Coggeshall 2005) such as cell death and surgical procedures in spinal neurons. To reduce these effects on our study, we have set several criteria to induce c-fos expression and to choose the EGFP+ neurons in this study: 1) the locomotor task (swimming) was induced in animals for 60–90 min. This long-duration, persistent, and nonstopping locomotor activity would allow spinal neurons driven by the locomotion to have enough time and intensity to express c-fos (Coggeshall 2005). In fact, our results confirmed the validity of this protocol for induction of the c-fos-EGFP expression in spinal interneurons (Fig. 3). 2) Apoptosis is known to be associated with c-fos expression (Smeyne et al. 1993). In spinal interneurons, the death of cells due to apoptosis reached the peak at P0 and decreased rapidly by ∼80% after P4 (Lowrie and Lawson 2000). For this reason, the animals we chose for patch-clamp experiments were all at (16%, 7/44) or older (84%, 37/44) than P4. This selection of animals could reduce the effect of apoptosis on cfos-EGFP expression. 3) Although surgical procedures such as dissection and slicing process could induce cfos expression, neurons expressing c-fos-EGFP by this way are usually unhealthy. This can be recognized by the shrinking cell body, visualized nuclear, rough surface, and ultra brightness of the EGFP expression. These cells were excluded for patch-clamp recording. In this study, only healthy neurons that were usually shown as medium (or sometimes weak) brightness of EGFP expression in target areas (lamina VII, VIII, and X) were chosen for patch-clamp study. Although the number of EGFP+ neurons induced by nonlocomotor activity could be largely reduced by these criteria, we could not absolutely rule out the c-fos-EGFP expression triggered by some unwanted factors. The EGFP+ neurons that can be always seen in dorsal horn in both control and locomotor slices are such an example. As shown in Fig. 3, however, the pronounced increase in EGFP expression in ventral horn after swimming suggested that the locomotor activity indeed induced EGFP expression in spinal interneurons. These neurons are the target of the present study.
Differences among the firing patterns, lamina distributions, and membrane properties
A significant difference in Rin and Cm was shown to be correlated with neuronal laminar distribution but not type. This suggests differences in size and/or morphology of the activated neurons in the target areas. The active properties (Vth, AP, AHP, and F-I properties, etc), however, were not significantly related to the laminar distribution. Therefore the electrophysiological or functional differences in these neurons with respect to their role in locomotion is not clear.
Overlapping of the EGFP+ neurons with some identified locomotor neurons
The functional role of the EGFP+ neurons in locomotion is unknown. It is not possible to classify the EGFP+ neurons into any known type of locomotor interneuron given differences in the types of studies. However, the EGFP+ neurons and ascending commissural interneurons previously studied (Carlin et al. 2006; Zhong et al. 2006a,b) are modulated in a similar fashion by the neurotransmitters ACh and 5-HT. These similarities include the depolarization of membrane potential and reduction of input resistance by ACh, and the depolarization of membrane potential, reduction of AHP, hyperpolarization of Vth, reduction of rheobase, and increase of input resistance and action potential width by 5-HT. In addition, the target areas (laminar VII, VIII, and X) for patching the EGFP+ neurons are overlapped with the areas where the ascending commissural interneurons locate (Carlin et al. 2006; Eide et al. 1999). It is therefore possible that a proportion of the EGFP+ neurons studied here are ascending commissural interneurons.
Membrane currents in EGFP+ neurons
Two important ionic currents were found in EGFP+ neurons in the present study: hyperpolarization-activated inward currents and persistent inward currents. Ih has been shown to be involved in the production of bursting during various forms of rhythmic activity (Bertrand and Cazalets 1998; Butt et al. 2002; Kiehn et al. 2000; Thoby-Brisson et al. 2000) while PICs have been also shown to play an important role in rhythm generation in respiration (Del Negro et al. 2002, 2005) and locomotion (Tazerart et al. 2008; Zhong et al. 2007). The expression of Ih and PICs in EGFP+ neurons supports the importance of these two currents in the generation of locomotion. However, further studies are required to characterize Ih and PICs in EGFP+ neurons.
5-HT-induced membrane oscillations
5-HT-induced membrane oscillation was observed in EGFP+ neurons. Similar observations have been reported in recent studies in ascending commissural interneurons in neonatal mice (Carlin et al. 2006; Zhong et al. 2006a), where the 5-HT-induced membrane oscillations were shown to be either voltage dependent (Carlin et al. 2006) or independent (Zhong et al. 2006a) and could be blocked by TTX or the gap junction blocker carbenoxolone (Zhong et al. 2006a), suggesting that multiple mechanisms could be responsible for the oscillations. In this study, we demonstrated that in some cells, the frequency of the oscillations was voltage dependent (Fig. 7A) and could be persisted with APV and CNQX in the recording solution (B). These results indicate that intrinsic membrane properties play a role in generating the 5-HT-mediated membrane potential oscillations in some EGFP+ neurons, which may therefore have a role in rhythm generation. Membrane oscillation requires a coupling of inward and outward currents with precise occurrence of the currents in time. As suggested by many previous studies (e.g., Marder and Bucher 2001; Wilson et al. 2005), Ih, T-type calcium currents, and PICs (calcium and/or sodium components) are all candidates for induction of membrane oscillations in locomotor neurons. Enhancement of Ih and PICs by 5-HT have been reported in our recent studies (Dai and Jordan 2006; Dai et al. 2005a). Therefore the action of 5-HT on Ih and PICs could facilitate the membrane oscillation in EGFP+ neurons observed in this study. Further study is required to investigate this issue.
Aminergic effects on EGFP+ neurons
In this study, we demonstrate the modulation of membrane properties by 5-HT and ACh in EGFP+ neurons. Although both agents increase the neuronal excitability, their effects on membrane properties are not exactly the same. Both agents induced paradoxical effects on membrane potential and hyperpolarized Vth. On the other side, however, 5-HT increased Rin and reduced AHP, whereas ACh reduced Rin and increased AHP. The variable effects of 5-HT and ACh on EGFP+ neurons could result in different mechanisms to regulate the neuronal excitability and output. 5-HT-induced changes in F-I slope (n = 7) tended to be correlated to changes in Rin (absolute value of correlation coefficient R = 0.6), AHP depth (R = 0.6), and Vth (R = 0.8), whereas ACh-induced changes in F-I slope (n = 11) were coupled relatively strongly to changes in Rin (R = 0.7) and AP height and width (R = 0.5) but weakly to AHP duration (R = 0.3) and Vth (R = 0.2). These differences in correlation reflected different levels of balance in modulatory forces driven by different mechanisms.
Conclusion
Using cfos-EGFP transgenic mice, we demonstrate the ability to study interneurons activated by a locomotor task. The capacity to select neurons active during locomotion adds a new method in which interneurons can be studied and may be particularly powerful when used in combination with other techniques such as retrograde labeling technique for study of commissural interneurons specifically activated by locomotion. Characterization of EGFP+ neurons located in lamina VII, VIII, and X is important to provide insight into the cellular basis for generation of locomotion. The membrane properties of these neurons and the manner in which they are modulated by 5-HT and ACh suggest that the EGFP+ neurons could play a role in generating or mediating locomotion.
GRANTS
This work is supported by National Institutes of Health Grant 391313520 and the Canadian Institute of Health Research to L. M. Jordan.
ACKNOWLEDGMENTS
We thank J. McVagh, C. Gibbs, G. Detillieux, M. Ellis, J. Rutherford, and M. Setterbom for technical support.
REFERENCES
- Beato M, Nistri A. Serotonin-induced inhibition of locomotor rhythm ofthe rat isolated spinal cord is mediated by the 5-HT1 receptor class. Proc R Soc Lond B Biol Sci 265: 2073–2080, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand S, Cazalets JR. Postinhibitory rebound during locomotor-like activity in neonatal rat motoneurons in vitro. J Neurophysiol 79: 342–351 1998 [DOI] [PubMed] [Google Scholar]
- Bracci E, Beato M, Nistri A. Extracellular K+ induces locomotor-like patterns in the rat spinal cord in vitro: comparison with NMDA or 5-HT induced activity. J Neurophysiol 79: 2643–2652, 1998 [DOI] [PubMed] [Google Scholar]
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B Biol Sci 84: 308–319, 1911 [Google Scholar]
- Brownstone RM, Dimauro M, Li Z, McMahon DG, Jordan LM. Whole cell patch clamp recordings from locomotor activity-labelled spinal cord neurons in Cfos-EGFP mice. Soc. Neurosci Abstr 65.8, 2002 [Google Scholar]
- Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk S. On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Exp Brain Res 90: 441–445, 1992 [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurons in rhythmogenesis. Brain Res Rev 57: 64–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Harris-Warrick RM, Kiehn O. Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci 22: 9961–9971, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Lundfald L, Kiehn O. EphA4 defines a class of excitatory locomotor-related interneurons. Proc Natl Acad Sci USA 102: 14098–14103, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin KP, Dai Y, Jordan LM. Cholinergic and serotonergic excitation of ascending commissural neurons in the thoraco-lumbar spinal cord of the neonatal mouse. J Neurophysiol 95: 1278–1284, 2006 [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for instability. Eur J Neurosci 12: 1635–1646, 2000 [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci 15: 4943–4951, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets J-R, Borde M, Clarac F. The synaptic drive from the spinal locomotor network to motoneurons in the newborn rat. J Neurosci 16: 298–306, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol 455: 187–204, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio CM, Gamble KL, Axley JC, Strauss BR, Cohen JY, Colwell CS, McMahon DG. Population encoding by circadian clock neurons organizes circadian behavior. J Neurosci 29: 1670–1676, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cina C, Hochman S. Diffuse distribution of sulforhodamine-labeled neurons during serotonin-evoked locomotion in the neonatal rat thoracolumbar spinal cord. J Comp Neurol 423: 590–602, 2000 [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. Fos, nociception and the dorsal horn. Prog Neurobiol 77: 299–352, 2005 [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett 171: 147–150, 1994 [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol 77: 247–259, 1997 [DOI] [PubMed] [Google Scholar]
- Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O, Sharma K. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron 60: 70–83, 2008 [DOI] [PubMed] [Google Scholar]
- Dai Y, Jordan LM. Characterization of persistent inward currents (PICs) in locomotor activity-related neurons of Cfos-EGFP mice. Soc Neurosci Abstr 130.6, 2006 [Google Scholar]
- Dai Y, Jordan LM, Brownstone RM. Characterization of electrophysiological and pharmacological properties of locomotor activity related neurons in cfos-egfp mice. Soc Neurosci Abstr 882.14, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Jordan LM, Brownstone RM. Characterization of hyperpolarization-activated inward current in locomotor activity related neurons in Cfos-EGFP mice. Soc Neurosci Abstr 377.3, 2005a [Google Scholar]
- Dai X, Noga BR, Douglas JR, Jordan LM. Localization of spinal neurons activated during locomotion using the c-fos immunohistochemical method. J Neurophysiol 93: 3442–3452, 2005b [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ JR, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Botzinger complex inspiratory neurons in vitro. J Neurophysiol 88: 2242–2250, 2002 [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci 25: 446–453, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide AL, Glover J, Kjaerulff O, Kiehn O. Characterization of commissural interneurons in the lumbar region of the neonatal rat spinal cord. J Comp Neurol 403: 332–345, 1999 [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol 557: 355–361, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, McCrea D, Dai Y, Jones K, Jordan L. Motoneuron frequency/current relationships during fictive locomotion in the cat. Soc Neurosci Abstr 652: 15, 1998 [Google Scholar]
- Fields RD, Nelson PG. Resonant activation of calcium signal transduction in neurons. J Neurobiol 25: 281–293, 1994 [DOI] [PubMed] [Google Scholar]
- Gilman MZ, Wilson RN, Weinberg RA. Multiple protein-binding sites in the 5′-flanking region regulate c-fos expression. Mol Cell Biol 6: 4305–4316, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440: 215–219, 2006 [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods and fish. In: Handbook of Physiology. The Nervous System. Motor Control Bethesda: Am. Physiol. Soc., 1981, sect. 1, vol. II, p. 1179–1236 [Google Scholar]
- Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol 93: 1439–1449, 2005 [DOI] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Machacek DW, Shay BL. 5-HT receptorsand the neuromodualatory control of spinal cord function. In: Motor Neurobiology of the Spinal Cord, edited by Cope TC. New York: CRC, 2001, p. 48–87 [Google Scholar]
- Hochman S, Garraway SM, Pockett S. Membrane properties of deep dorsal horn neurons from neonatal rat spinal cord in vitro. Brain Res 767, 214–219, 1997 [DOI] [PubMed] [Google Scholar]
- Hochman S, Jordan L, MacDonald J. N-methyl-aspartate receptor-mediated voltage oscillations in neurons surrounding the central canal in slices of rat spinal cord. J Neurophysiol 72: 565–577, 1994a [DOI] [PubMed] [Google Scholar]
- Hochman S, Jordan LM, Schmidt BJ. TTX-resistant NMDA receptor-mediated voltage oscillations in mammalian lumbar motoneurons. J Neurophysiol 72: 2559–2562, 1994b [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev 57: 46–55, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816: 493–499, 1999 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol 70: 347–361, 2003 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Johnson BR, Raastad M. Plateau properties in mammalian spinal interneurons during transmitter-induced locomotor activity. Neuroscience 75: 263–273, 1996 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol 75: 1472–1482, 1996 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann NY Acad Sci 860: 110–129, 1998 [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Barajon I, Kiehn O. Sulphorhodamine-labeled cells in the neonatal rat spinal cord following chemically induced locomotor activity in vitro. J Physiol 478: 265–273, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O, Tresch MC, Harris-Warrick RM. Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord. Brain Res Bull 53: 649–659, 2000 [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol 77: 1155–1170, 1997 [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci 23: 1441–1450, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydstrom A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science 299: 1889–1892, 2003 [DOI] [PubMed] [Google Scholar]
- Kurihara T, Suzuki H, Yanagisawa M, Yoshioka K. Muscarinic excitatory and inhibitory mechanisms involved in afferent fiber-evoked depolarization of motoneurons in the neonatal rat spinal cord. Br J Pharmacol 110: 61–70, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry ES, Guertin PA. Differential effects of 5-HT1 and 5-HT2 receptor agonists on hindlimb movements in paraplegic mice. Prog Neuropsychopharmacol Biol Psychiatry 28: 1053–1060 2004 [DOI] [PubMed] [Google Scholar]
- Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB, Guertin PA. Contribution of spinal 5-HT1A and 5-HT7 receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. Eur J Neurosci 24: 535–546, 2006 [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 42: 375–386, 2004 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. J Neurophysiol 85: 472–475, 2001 [DOI] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol 94: 1392–1404, 2005 [DOI] [PubMed] [Google Scholar]
- Lowrie MB, Lawson SJ. Cell death of spinal interneurons. Prog Neurobiol 61: 543–555, 2000 [DOI] [PubMed] [Google Scholar]
- Maclean JN, Hochman S, Magnuson DSK. Lamina VII neurons are rhythmically active during locomotor-like activity in the neonatal rat spinal cord. Neurosci Lett 197: 9–12, 1995 [DOI] [PubMed] [Google Scholar]
- Madriaga MA, McPhee LC, Chersa T, Christie KJ, Whelan PJ. The modulation of locomotor activity by multiple 5-HT and dopaminergic receptor subtypes in the neonatal mouse spinal cord. J Neurophysiol 92: 1566–1576, 2004 [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986–996, 2001 [DOI] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci 104: 2448–2453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Nishimaru H, Kudo N. Basis of changes in left-right coordination of rhythmic motor activity during development in the rat spinal cord. J Neurosci 22: 10388–10398, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H, Kudo N. Formation of the central pattern generator for locomotion in the rat and mouse. Brain Res Bull 53: 661–669, 2000 [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Takizawa H, Kudo N. 5-Hydroxytryptamine-induced locomotor rhythm in the neonatal mouse spinal cord in vitro. Neurosci Lett 280: 187–190, 2000 [DOI] [PubMed] [Google Scholar]
- Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. Cell culture. Progenitor cells from human brain after death. Nature 411: 42–43, 2001 [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol 539: 817–836, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero JE, Kuhlman SJ, McMahon DG. The biological clock nucleus: a multiphasic oscillator network regulated by light. J Neurosci 23: 8070–8076, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling K, Luk D, Morgan JI, Curran T. Regulation of a fos-lacZ fusion gene: a paradigm for quantitative analysis of stimulustranscription coupling. Proc Natl Acad Sci USA 88: 5665–5669, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Vendrell M, Hayward M, Baker SJ, Miao GG, Schilling K, Robertson LM, Curran T, Morgan JI. Continuous c-fos expression precedes programmed cell death in vivo. Nature 363: 166–169. 1993 [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro studies of a mammalian respiration and locomotion. Novel approaches to the study of motor systems. Satellite Symp XXX Int. Cong. Physiol. Sci, Banff, Canada 55–56, 1986 [Google Scholar]
- Smith JC, Feldman JL. In vitro brain stem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods 21: 321–333, 1987 [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL, Schmidt BJ. Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. FASEB J 2: 2283–2288, 1988 [DOI] [PubMed] [Google Scholar]
- Stokke MF, Nissen UV, Glover JC, Kiehn O. Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat. J Comp Neurol 446: 349–359, 2002 [DOI] [PubMed] [Google Scholar]
- Szucs P, Odeh F, Szokol K, Antal M. Neurons with distinctive firing patterns, morphology and distribution in laminae V-VII of the neonatal rat lumbar spinal cord. Eur J Neurosci 17: 537–544, 2003 [DOI] [PubMed] [Google Scholar]
- Tazerart S, Vinay L, Brocard F. The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. J Neurosci 28: 8577–8589, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss RD, Heckman CJ. Systematic variation in effects of serotonin and norepinephrine on repetitive firing properties of ventral horn neurons. Neuroscience 134: 803–815, 2005 [DOI] [PubMed] [Google Scholar]
- Theiss RD, Kuo JJ, Heckman CJ. Persistent inward currents in rat ventral horn neurons. J Physiol 580: 507–522, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Telgkamp P, Ramirez JM. The role of the hyperpolarization-activated current in modulating rhythmic activity in the isolated respiratory network of mice. J Neurosci 20: 2994–3005, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Coding of locomotor phase in populations of neurons in rostral and caudal segments of the neonatal rat lumbar spinal cord. J Neurophysiol 82: 3563–3574, 1999 [DOI] [PubMed] [Google Scholar]
- Ung RV, Landry ES, Rouleau P, Lapointe NP, Rouillard C, Guertin PA. Role of spinal 5-HT2 receptor subtypes in quipazine-induced hindlimb movements after a low-thoracic spinal cord transection. Eur J Neurosci 28: 2231–2242, 2008 [DOI] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci 25: 5710–5719, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60: 84–96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Díaz-Ríos M, Harris-Warrick RM. Intrinsic and functional differences among commissural interneurons during fictive locomotion and serotonergic modulation in the neonatal mouse. J Neurosci 26: 6509–6517, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Masino MA, Harris-Warrick RM. Persistent sodium currents participate in fictive locomotion generation in neonatal mouse spinal cord. J Neurosci 27: 4507–4518, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Diaz-Rios ME, Harris-Warrick RM. Serotonin modulates the properties of ascending commissural interneurons in the neonatal mouse spinal cord. J Neurophysiol 95: 1545–1555, 2006a [DOI] [PubMed] [Google Scholar]
- Zieglgansberger W, Reiter C. A cholinergic mechanism in the spinal cord of cats. Neuropharmacology 13: 519–527, 1974 [DOI] [PubMed] [Google Scholar]