Abstract
Previous research has suggested distinct predictive and reactive control mechanisms for bimanual movements compared with unimanual motion. Recent studies have extended these findings by demonstrating that movement corrections during bimanual movements might differ depending on whether or not the task is shared between the arms. We hypothesized that corrective responses during shared bimanual tasks recruit bilateral rapid feedback mechanisms such as reflexes. We tested this hypothesis by perturbing one arm as subjects performed uni- and bimanual movements. Movements were made in a virtual-reality environment in which hand position was displayed as a cursor on a screen. During bimanual motion, we provided cursor feedback either independently for each arm (independent-cursor) or such that one cursor was placed at the average location between the arms (shared-cursor). On random trials, we applied a 40 N force pulse to the right arm 100 ms after movement onset. Our results show that while reflex responses were rapidly elicited in the perturbed arm, electromyographic activity remained close to baseline levels in the unperturbed arm during the independent-cursor trials. In contrast, when the cursor was shared between the arms, reflex responses were reduced in the perturbed arm and were rapidly elicited in the unperturbed arm. Our results thus suggest that when both arms contribute to achieving the task goal, reflex responses are bilaterally elicited in response to unilateral perturbations. These results agree with and extend recent suggestions that bimanual feedback control might be modified depending on task context.
INTRODUCTION
A large body of research has led to the notion that the control of bimanual limb movements might recruit unique neural mechanisms when compared with unimanual movements. Support for this idea has come from several lines of work including neural imaging studies (Jancke et al. 1998, 2000; Roland et al. 1980a,b; Sadato et al. 1997), neurophysiological recordings (Donchin et al. 1998; Tanji et al. 1987, 1988), and lesion studies (Brinkman 1984), as well as other behavioral studies comparing the two types of movements (Kelso et al. 1979a,b; Nozaki et al. 2006; Swinnen 2002; Theorin and Johansson 2007; Westenberg et al. 2004; Wiesendanger and Serrien 2004). Most behavioral studies have focused on explaining the spatiotemporal synchronization between the arms observed during rhythmic bimanual movements and have suggested that such “coordinated” behavior could be predicted from interactions among coupled oscillatory processes (Kelso et al. 1981; Yamanishi et al. 1980). However, the neural mechanisms that underlie this coordination remain poorly understood.
More recent studies have suggested that control of bimanual movements might be subject to modification depending on task conditions (Diedrichsen 2007; Domkin et al. 2002; Swinnen et al. 1988, 1991a,b, 1993; Walter et al. 1997). Swinnen and colleagues showed that while learning novel interlimb coordination patterns during rhythmic tasks, changes in control strategies were manifested as a “decoupling” or “dissociation” of preferred synchronous limb activation patterns (Swinnen et al. 1993). The modification of bimanual coordination strategies were thus reflected in the way movements were planned and executed while learning the novel task requirements. More recently, Diedrichsen (2007) demonstrated that reactive processes involved in correcting movement trajectories during bimanual motion could also be modified based on the goal of the task. This study showed that when both arms moved independent cursors to respective targets, velocity-dependent forces applied to one arm were corrected using only that arm. In contrast, when both arms shared movement of a single cursor to one target, the same unilateral perturbation resulted in bimanual corrections. These differences were attributed to changes in task-dependent “feedback control” strategies, although the specific circuits underlying these changes were not explored. Moreover the corrections observed in this study were relatively small and occurred during the late phases of movement (around 190 ms following perturbation onset, in kinematics), leaving open the question whether rapid feedback mechanisms such as reflexes could be associated with such bimanual corrective responses.
We now investigate whether reflex responses are elicited bilaterally during the performance of bimanual movements. Specifically, we ask whether a perturbation applied to one arm might elicit rapid reflex responses in the unperturbed arm. We expect that the emergence of bimanual reflexes would depend on the degree of coordination required between the arms. Subjects performed movements under three task conditions: unimanual movements in which only the right arm performed the required reaching movement, bimanual movements with each arm moving its respective cursor to separate targets (independent-cursor condition), and bimanual movements in which both arms shared the movement of one cursor to a target (shared-cursor condition). Occasionally and pseudo-randomly, force pulses were applied to the right arm to elicit reflex responses and the influence of movement condition on responses in the perturbed and unperturbed arm was examined.
METHODS
Participants
Nine healthy right-handed volunteers were recruited for the study. Handedness was determined using a 12-item version of the Edinburgh inventory (Oldfield 1971). All participants gave informed consent prior to participation. Informed consent had been approved by the Institutional Review Board of the Pennsylvania State University.
Experimental setup
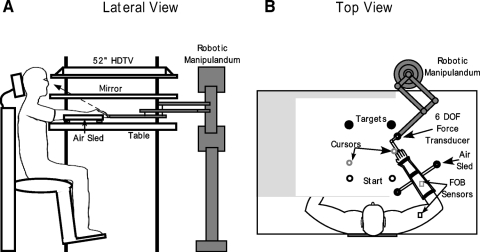
The experimental setup used for this study is shown in Fig. 1. Subjects were seated facing a table with their fists closed and hands supported over the table surface positioned just below shoulder height by two air-sled systems, one for each hand. Cursors representing the positions of the interphalangeal joint location of the left and right index fingers, start circles (1.2 cm diam) and targets (3.5 cm diam) were projected using a horizontally mounted 52-in flat screen TV (Sony) onto a mirror. The mirror reflected the visual display to give the illusion that the display was in the same horizontal plane as the fingertip. Calibration of the display assured that this projection was veridical. The right air sled was attached to a MIT IMT2 robot arm (Krebs et al. 1999) via an L-shaped metal bracket. This arrangement ensured that subjects did not hold on to the robot arm and thus haptic influences on our results were minimized. The robot arm was fitted with a 6 degree of freedom (dof) force transducer (ATI Industrial Automation) to measure interface forces. The asymmetrical effects of robot arm inertia were compensated using custom software. A mass was added to the left sled to match the compensated inertia of the robotic system. Position and orientation of the forearm and upper-arm segments were sampled using a Flock of Birds (Ascension Technology) electromagnetic 6-dof movement recording system. The positions of the interphalangeal joint, the lateral epicondyle of the humerus and the acromion, directly posterior to the acromio-clavicular joint for the left and right arms were recorded using a stylus that was rigidly attached to a 6-dof Flock of Birds (FOB) sensor. One 6-dof sensor was then attached to the upper arm segment by means of a plastic arm cuff, whereas the other sensor was attached to the air sled on which the forearm was rested. The sensors were positioned at approximately the center of each segment. As sensor data were received from the FOB, the 3-D position of the above-mentioned landmarks was computed using custom software, with the x-y plane parallel to the tabletop. We used our computed x-y coordinates of the interphalangeal joint to define the projected cursor position. Digital data were collected at 103 Hz using a Macintosh computer, which controlled the sensors through separate serial ports. Custom computer algorithms for experimental control and data analysis were written in REAL BASIC (REAL Software) and Igor Pro (Wavemetrics), respectively. Electromyographic (EMG) activity was recorded from the biceps brachii, long and lateral heads of the triceps, anterior deltoid, and posterior deltoid muscles of both arms. EMG was recorded using active stainless steel electrodes with a built-in reference electrode (B and L Engineering). EMG signals were digitized at 1 kHz using a Macintosh computer equipped with an A/D board (National Instruments, PCI-MIO-16XE-50).
Fig. 1.
Experimental setup. A: lateral view showing the arrangement of the experimental apparatus. Subjects sat facing a mirror onto which the start positions and targets were projected using a 52-in HDTV. Subjects rested their arms in an air-sled system placed on a glass tabletop. The air sled support was attached to a robotic manipulandum at its distal end. B: top view of the setup showing attachment of the robot arm to the air sled, attachment of the Flock of Birds (FOB) sensors on the subject's arm and a typical screen display. The robot arm was connected to the air-sled using an L-shaped bracket. Two FOB sensors were used to record subjects' movements per arm. One sensor was attached to the air sled while the other was attached to the subject's upper arm. The screen display consisted of filled circles representing the start positions and targets for each arm. In addition, a cursor representing the position of the left and right index fingertip was displayed on the screen. A trial was typically initiated by bringing the cursor into the start circle followed by the presentation of an audiovisual “go” signal. Note that subjects could not see their arms during the experiment because of the mirror placed above. The mirror has been “cut out” in this figure for display purposes.
Experimental task
The experimental session comprised of 220 rapid multi-joint movements. We did not ask subjects to move as fast as possible, but subjects were instructed to make “rapid movements” and to try and maintain the same speed throughout the entire session. All the movements were made without visual feedback of the subject's arms, but the on-screen cursor position representing the hand position was visible throughout the movement. The first 10 trials were considered practice. The remaining trials were then divided into three blocks of 70 trials each. During the first block, subjects performed unimanual movements with only their right hand. During the second and third blocks, subjects performed bimanual movements, during which cursor feedback was varied. In one such block of bimanual movements, each hand was assigned its own cursor that was to be moved to two separate targets (independent-cursor condition), whereas in the other bimanual movement block, both hands shared the movement of a single cursor to one target (shared-cursor condition). During the shared-cursor trials, the cursor was located at the average position between the two arms. The order of the task performed by the subjects during the second and third blocks, either the independent-cursor task or the shared-cursor task, was counterbalanced across subjects.
All subjects started from an initial configuration such that the starting shoulder and internal elbow angles for each arm were 25 and 75°, respectively. Start positions were displayed as circles, 1.2 cm in diameter, for each arm based on this initial configuration. Due to our requirement that subjects start from the same initial joint configuration, the exact location of the start circles varied slightly across subjects. All trials were composed of movements from this start position to a target located 13 cm along a straight line in the +y direction of the workspace, i.e., further away from the subject.
Achievement of this target thus required flexion of the shoulder along with elbow extension. When performing bimanual movements (either independent or shared cursor), starting arm configuration was required to be symmetric such that both arms maintained starting shoulder and internal elbow angles at 25 and 75°, respectively. During the independent-cursor bimanual trials, two start circles and targets, one for each arm, were displayed. Across all subjects, these start circles were located 28 ± 1 (SE) cm apart for this condition. In the shared-cursor trials, one start circle and one target (located at the average distance between the start circles and targets for the independent-cursor condition) were displayed.
In general, to initiate a trial, subjects brought the cursor for each hand into its starting location and waited for an audiovisual “go” signal. This signal occurred 500 ms after the cursor was in the start circle and comprised a beeping sound and graying of the target, which indicated to the subjects that they must initiate the desired movement. In the independent-cursor condition, the go signal did not occur until the cursors for both arms were in the respective start circle. During the shared-cursor condition, as mentioned earlier, only one cursor was displayed at the average location of the two hands. During these trials, it was plausible that subjects started from asymmetrical arm configurations and yet could place the cursor in the desired start position. To avoid this scenario and to maintain symmetry of the starting arm configuration, as subjects brought the shared cursor toward the start circle to initiate a trial, if the subjects were within a radius of 3 cm from the center of the start circle, we switched the display to show two “start” positions and two cursors. This served to guide the subjects toward the desired starting locations for the two arms. As soon as subjects “touched” these two start circles with the two cursors, we switched the display back to a single shared cursor and common start circle. The go signal was given 500 ms later. The single cursor feedback was maintained until after the end of the trial, up to the point at which the subject returned back to initiate a new trial and got within 3 cm of the start position with the shared-cursor. The screen was then switched to briefly display two start positions and two cursors, and the process was repeated as described in the preceding text.
On random trials, 100 ms after movement initiation, we applied a mechanical perturbation to the right arm using the robotic manipulandum. In this case, movement initiation was defined as the point at which the subject first breached the start circle boundary. The perturbation, a 40 N force lasting 50 ms and was applied in the −y direction of the workspace (away form the robot body, toward the subject). Only 6 such perturbation trials were used in each block, thus yielding 18 perturbation trials in all for the entire session. Thus majority of trials (∼92%) in our case were baseline trials, on which no perturbation was applied. We restricted the number of perturbation trials per block to six to prevent subjects from anticipating when a perturbation would occur and to ensure consistent baseline performance. Regardless of whether the perturbation occurred or not, subjects were instructed to reach the target on screen in all cases. Points were awarded based on the accuracy of these trials. Final position errors of <1 cm were awarded 10 points, errors between 1 and 2 cm were awarded 3 points, and errors between 2 and 3 cm were given 1 point. No points were awarded for position errors larger than 3 cm. Points were displayed on the screen following each trial.
Kinematic data analysis
Elbow and shoulder angles for each arm were calculated from the two-dimensional (2-D) positions of the finger, elbow, and shoulder. All kinematic data were filtered at 8 Hz using a third-order dual-pass Butterworth filter, and angular data were differentiated to yield velocity and acceleration values. The first 10 trials were considered practice trials and were not considered for analysis. Trials on which the subject failed to make a corrective response to the perturbation were also excluded from analysis. On average, one perturbation trial per subject was excluded. As described earlier, movement onset was defined as the point at which subjects first breached the start circle. The small diameter of the start circle ensured that this time of movement initiation was fairly consistent across trials. Movement termination was defined as the first minimum (<3% maximum tangential velocity) following peak tangential finger velocity.
Kinetic data analysis
We used a planar two-segment rigid body model to calculate joint torques at the elbow and shoulder. Each arm was modeled as two interconnected rigid links with frictionless joints at the shoulder and elbow. For the right arm, we took into account the interface forces between the robot manipulandum and the distal end of the forearm during the calculation of joint torques. For the left arm, we included the effects of the added mass into our equations of motion. Limb segment inertias, centers of mass, and mass were computed from the regression equations using the subjects' body mass and segment lengths for each arm (Winter 1990).
EMG data analysis
EMG was recorded from 500 ms prior to 2 s after movement initiation. For processing, EMG signals were normalized, full-wave rectified and subsequently low-pass filtered at 400 Hz using a third-order dual-pass Butterworth filter. Temporal resolution of the EMG data were maintained at 1 kHz. Normalization was done to percent of maximum of EMG for each muscle within subjects. Maximum EMG was obtained by asking subjects to perform maximum isometric voluntary contractions during isolated flexor and extensor direction movements at the shoulder and elbow. Two such trials for each direction were obtained and the maximum value during these trials was found using a custom computer algorithm.
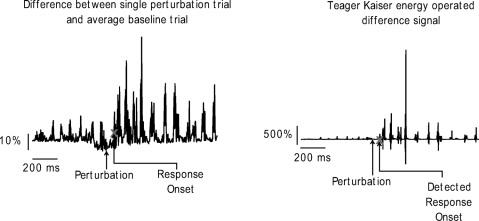
To determine when the EMG responses to perturbations deviated from baseline EMG, we used the following method: for the muscle of interest, we first obtained a difference between the response on each perturbation trial and an average of all baseline trials. This difference profile was then subjected to a Teager Kaiser energy operation (TKEO) to reduce background noise and amplify changes in the EMG signal. This process vastly improves the EMG signal to noise ratio, enabling a more robust and accurate detection of response onset (Li et al. 2007). Once the TKEO was complete, we applied a thresholding algorithm on the transformed EMG signal to determine the time when the response occurred. To do this, we first found the mean ± SD of the first 300 ms of the TKEO transformed EMG difference signal. We then found the point in time where the average magnitude of three consecutive data points in this TKEO transformed signal exceeded 8 SD from this mean. This point was taken as the point where the response occurred and the time was recorded. Thus detection of the onset of the response to the perturbation was done in the Teager Kaiser domain.
Figure 2 shows an example of this kind of operation. On the left is a profile obtained as a difference between the EMG response of the posterior deltoid muscle on a perturbation trial and the average baseline EMG response in the same muscle. The plot on the right shows the same difference signal following the TKEO transformation. As can be seen, the signal to noise ratio in the TKEO transformed waveform is drastically improved. The gray X on the profiles shows the onset time identified by our thresholding algorithm after the TKEO process.
Fig. 2.
Example of the Teager Kaiser Energy operation on the EMG signal. Left: the difference between a perturbation response and the average baseline response for the anterior deltoid muscle. Right: the same difference signal after the Teager Kaiser energy operation. ×, the detected reflex onset latency.
We observed reflex responses in the triceps and posterior deltoid muscles ∼50 ms following the onset of the perturbation (see results). We therefore quantified the EMG impulse for each recorded muscle of each arm from 50 to 110 ms after the perturbation. This duration is consistent with previously reported values for the medium- and long-latency components of a reflex response to muscle stretch (Mutha et al. 2008; Perreault et al. 2008; Pruszynski et al. 2008), typically called “M2–M3” (Latash 1996). Selecting this interval also allowed us to maintain consistency across the different muscles for our statistical analysis. For the reflex interval from 50 to 110 ms, we calculated the change in EMG impulse on perturbation trials, relative to the baseline EMG impulse. To do this, we first calculated the difference between the perturbation and baseline EMG impulses. We divided this difference by the baseline EMG impulse and multiplied the result by 100 to yield a percentage value. This was done for the uni- and bimanual movement conditions for the right arm and only the bimanual movement conditions for the left arm. This percent change value was used as the dependent measure for our statistical analysis.
Statistical analysis
We first wanted to determine whether EMG activity following a perturbation changed significantly relative to baseline levels during the reflex interval. For each arm, we therefore evaluated whether the mean percent change in reflex EMG impulse on perturbation trials from a particular movement condition (unimanual, bimanual independent cursor, or bimanual shared cursor) significantly deviated from zero. In this case, zero represents the percent “change” in baseline EMG during the reflex interval, calculated as described in the earlier section.
Second, we wanted to compare changes in EMG during the reflex interval across movement conditions. To determine differences between the shared- and independent-cursor conditions, statistical analysis was performed on our percent change measures. If these values tended to deviate from normality, we transformed them using appropriate Box-Cox transformations. The data were then subjected to a two-way ANOVA, separately for each muscle. Arm [perturbed (right) or unperturbed (left)] and cursor-condition (independent or shared cursor) were our independent factors. Subjects were treated as a random factor for this analysis. Statistical significance levels were set to 0.05. Post hoc comparisons were performed using Tukey's HSD tests when warranted by significant main effects.
Finally, we also wished to compare whether the timing of the EMG response to the perturbation would be different across conditions. We therefore performed separate t-test for specific comparisons of perturbation related changes in reflex latency under the various task conditions for either arm.
RESULTS
Baseline performance
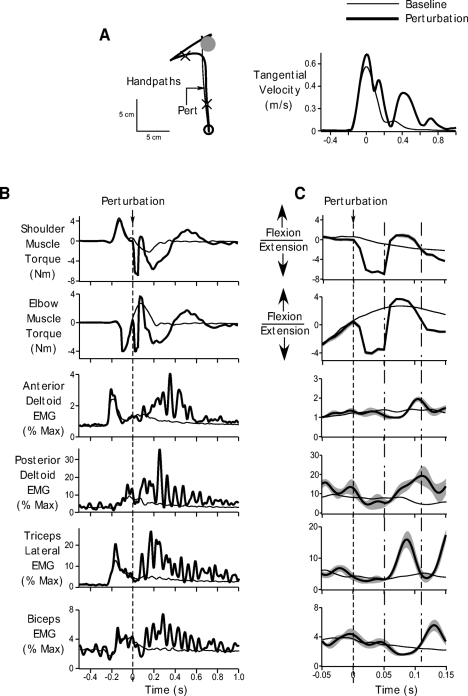
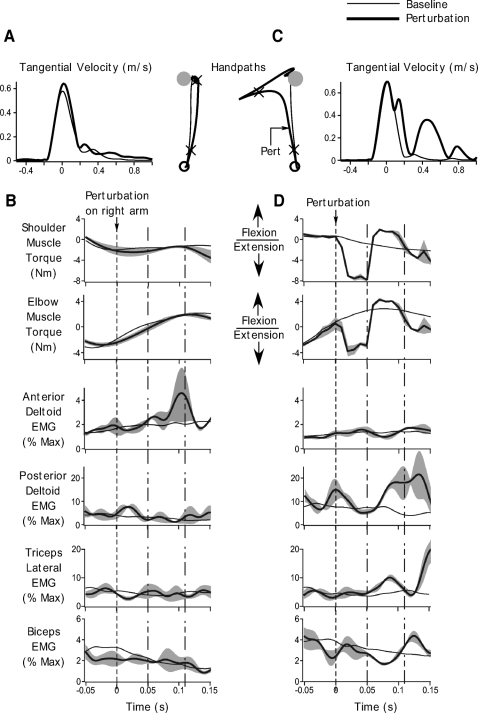
Majority (∼92%) of our trials comprised baseline movements during which subjects simply reached from a start position to the displayed target on receiving the “go” signal. The thin black lines in Fig. 3 show right arm performance under baseline conditions for a representative subject. Figure 3A shows the handpath and tangential velocity profiles, while Figure 3B shows EMG and muscle torques associated with these baseline movements. All data are shown from 500 ms prior to 1 s after the point at which we applied the mechanical perturbation to the limb. EMG data are shown for the anterior deltoid, posterior deltoid, lateral head of the triceps, and biceps muscles. Although we recorded from the long head of the triceps, we did not get consistent responses from this muscle and the signal tended to be contaminated with noise. We therefore chose not to show the data from this muscle. Figure 3C shows the same data as in B on an expanded time scale, from 50 ms prior to 150 ms after the perturbation. We must also point out that for a clearer presentation of our data, our EMG data are shown in a more filtered form (30-Hz low- pass). However, this is being done for display purposes only. All our analyses have been performed on the original EMG signal filtered at 400 Hz to include all essential components of the EMG signal and maintain consistency with other studies in the literature.
Fig. 3.
Performance during baseline and unimanual perturbation trials. A: ensemble averaged handpaths (left) and tangential hand velocity profiles (right) during baseline movements (thin black) and unimanual perturbation trials (thick black). Arrow on the handpath profile shows the time of perturbation. The cross hairs represent the time from 50 ms prior to 150 ms after the perturbation. B: torque and electromyographic (EMG) data associated with baseline (thin black) and perturbation trials (thick black). Ensemble averaged shoulder and elbow muscle torque, and anterior deltoid, posterior deltoid, triceps lateral and biceps EMG are shown. Data are shown from 500 ms prior to 1 s after the perturbation. Dotted line indicates perturbation onset. C: same data as in B, but time axis is restricted from 50 ms before to 150 ms after the perturbation to better demonstrate the effects of the perturbation and the associated muscle responses. Gray bands represent standard error of the ensemble averages. Dotted line represents perturbation onset. The time between the dotted-and-dashed lines represents the reflex interval from 50 to 110 ms after the perturbation. All data are for a representative subject.
As can be seen from Fig. 3 (thin black lines), baseline movements were associated with typical kinematic, torque, and EMG profiles. The handpaths were fairly straight and smooth (Morasso 1981). These handpaths were associated with typical bell- shaped velocity profiles (Fig. 3A, right, thin black lines). The EMG profiles (Fig. 3B, thin black lines) showed an initial burst of activation in the anterior deltoid and triceps muscles. These muscle activation patterns caused an initial flexor torque at the shoulder and extensor torque at the elbow to propel the arm forward toward the target. The movement was decelerated by activation of the antagonist muscles following the first agonist bursts. At about the time of the end of the early burst of the anterior deltoid, the posterior deltoid muscle became active, which along with biceps activation led to slight extensor torques at the shoulder and flexor torques at the elbow to decelerate the motion of the arm. A small degree of coactivation of these muscles later in the movement was also seen that was likely associated with stopping the movement on the baseline target. These patterns of EMG activation and the ensuing kinematic movement pattern were consistent across all subjects. Baseline performance in the left arm was not very different. The kinematic, torque, and EMG patterns were similar to those observed in the right arm.
Unimanual perturbation responses
The thick black profiles in Fig. 2, overlaid on top of the baseline trials show the average response obtained when a mechanical perturbation was applied to the right arm 100 ms after movement onset. The vertical dotted line shows the time of perturbation onset, which, as can be seen from Fig. 3B, was after the first burst in the anterior deltoid and triceps muscles. Prior to the perturbation, as can be seen from Fig. 3, the kinematic, torque and EMG profiles in the baseline and perturbation conditions were similar. The overlap between these responses prior to the perturbation suggests that subjects did not anticipate the perturbation.
The perturbation comprised a 40 N force pulse in the −y direction of the workspace, applied 100 ms after movement onset. At the time of perturbation application, the shoulder and elbow were undergoing active flexion and extension, respectively. The most direct effect of the perturbation was at the elbow; the shoulder could continue moving. The force pulse produced an external flexor torque at the elbow joint, thus accelerating the elbow into flexion. The intersegmental effect of this elbow acceleration on shoulder torque depends on the cosine of the elbow angle (Bagesteiro and Sainburg 2002). Accordingly, if elbow acceleration is flexorward and the internal elbow angle is <90°, the resulting intersegmental torque at the shoulder is flexor. In our study, subjects started with an initial internal elbow angle of 75°. At the time of perturbation, application (100 ms after movement onset), internal elbow angle changed on average by 8 ± 1° (mean ± SE) across all our subjects, indicating that the internal elbow angle was still <90° at the time the perturbation was applied. These two factors, the flexor elbow acceleration and the acute (<90°) internal elbow angle resulted in a flexor interaction torque at the shoulder joint. This flexor interaction torque, coupled with the ongoing shoulder motion, resulted in further flexion of the shoulder. The lack of elbow extension combined with shoulder flexion caused a leftward medial deviation of the handpath under the perturbation trials, as can be seen from the handpath profile of Fig. 3A (thick black trace). Across all subjects, the perturbed handpaths deviated from the baseline movement paths starting at 61 ± 9 ms after the perturbation. This delay between the application of the perturbation and the deviation of the trajectory was related to both the inertial and viscoelastic properties of the moving limb. The fact that this delay did not occur in the torque response (see next paragraph and also see torque profiles in Fig. 3C, thick black lines) emphasizes the role of limb dynamics in producing this lag on the handpath kinematics. Hand velocity also diverged from its typical bell-shaped profile during baseline trials (Fig. 3A, right, thick black line). We observed changes in hand velocity at 68 ± 4 ms after perturbation onset. Thus the perturbations produced large and significant changes in the subjects' movement patterns.
The passive extensor resistance to the imposed flexor torque at the elbow and shoulder can be seen as a sharp transition in the shoulder and elbow torque profiles of Fig. 3, B and C (thick black lines). This increase in extensor torque occurred almost instantaneously following the perturbation. The average latency of the increase in shoulder and elbow torques after perturbation onset was 12 ± 2 and 10 ± 0.008 ms, respectively. As can be seen from the EMG profiles in Fig. 3C (thick black lines), the applied external torques at the shoulder and elbow were resisted actively by rapid activation of the posterior deltoid and triceps muscles. The increase in posterior deltoid activity was observed on average at a latency of 48 ± 8 ms following the perturbation, whereas the increase in triceps activity occurred slightly earlier, on average 46 ± 3 ms after perturbation onset. Given these latencies and consistent with previous reports (Mutha et al. 2008; Perreault et al. 2008; Pruszynski et al. 2008), we integrated the EMG response starting from 50 to 110 ms after the perturbation (time between the dotted-and-dashed lines in Fig. 3C). On comparison with baseline levels, we observed a significant increase in triceps EMG during this interval (t = 4.1646, P = 0.0031). Across all subjects, triceps activity increased by and average of 75 ± 18%. In addition, posterior deltoid muscle activity in the reflex interval increased by 98 ± 26%, and this increase was also significant compared with baseline posterior deltoid activity during the same interval (t = 3.742, P = 0.0057). Biceps activity, however, remained close to baseline levels (t = −1.3395, P = 0.2172). Similarly, anterior deltoid EMG was similar to baseline activity for this subject although this was not consistent across all subjects. Some subjects tended to show an increase in the anterior deltoid response starting ∼80 ms after the perturbation, which could be related to removal of the force pulse. Nevertheless, our comparison revealed that across all subjects, anterior deltoid activity following the perturbation was not significantly different from baseline levels during the reflex interval (t = 1.8475, P = 0.1019). Thus the largest and most consistent effect of the perturbation was to elicit a rapid increase in the activity of the posterior deltoid and triceps muscles.
Perturbation responses during bimanual independent-cursor movements
After performance of the unimanual block of trials, subjects performed two blocks of bimanual movements. During one of these blocks, which could be performed immediately following the unimanual block or after the other block of bimanual movements, subjects were required to move two cursors, one for each hand, from separate start positions to separate targets on the screen. During this independent-cursor condition, we randomly applied the same perturbations to the right arm as during the previous movement block, 100 ms after movement onset.
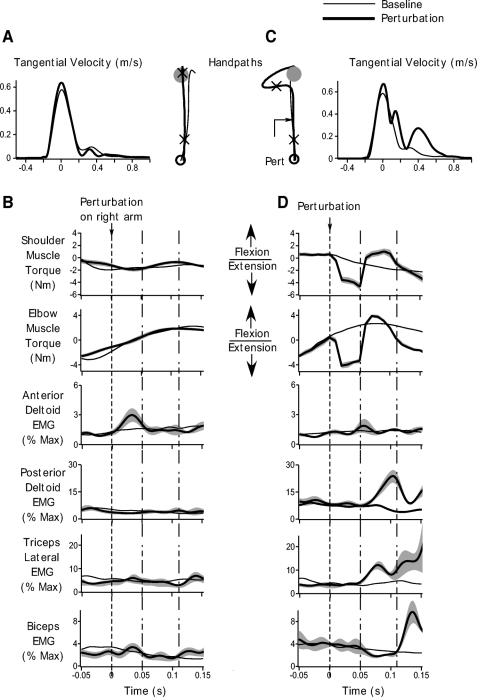
The thick black lines in Fig. 4 show the average responses obtained on perturbation trials in the independent-cursor condition for the left (A and B) and right (C and D) arms. Baseline trials are shown as thin black lines. Results are shown for the same representative subject as in Fig. 3. This data are restricted to 50 ms prior to 150 ms following the perturbation. Overall the pattern of responses to perturbations obtained in the right arm was very similar to the unimanual perturbation conditions. The flexor external torques at the shoulder and elbow led to a large medial deviation in the handpath and caused a substantial change in hand velocity (Fig. 4C, thick black line). These torques elicited rapid reflex responses in the posterior deltoid and triceps muscles. A significant increase in posterior deltoid reflex activity relative to its baseline levels (t = 3.5417, P = 0.0076) occurred on average at 48 ± 9 ms following the perturbation (Fig. 4D, posterior deltoid response, thick black line). Posterior deltoid activity increased by 84 ± 23% relative to baseline EMG levels during the reflex interval (time between the dotted-and-dashed lines in Fig. 4D) for this independent-cursor condition. We also observed a significant increase in triceps activity relative to baseline EMG levels following the perturbation (t = 5.7472, P = 0.0004; Fig. 4D, triceps response, thick black line). This increase occurred at a latency of 45 ± 6 ms after the force pulse was applied. Across all subjects, for the triceps, we observed an increase of 89 ± 15% from baseline levels during our reflex interval. There was no consistent change in EMG levels of the anterior deltoid (t = 2.2595, P = 0.0538) and biceps (t = −1.7496, P = 0.1183) muscles during the reflex interval for the independent-cursor condition for this subject.
Fig. 4.
Performance during baseline and perturbation trials in the bimanual independent-cursor conditions. A: ensemble averaged left arm handpaths and tangential velocity profiles during baseline trials (thin black) and during trials on which a perturbation was applied to the right arm (thick black). Cross hairs on the handpath profile represent the time from 50 ms prior to 150 ms after the perturbation. B: torque and EMG data for the left arm during baseline trials (thin black) and trials on which a perturbation was applied to the right arm (thick black). Ensemble averaged shoulder and elbow muscle torque, and anterior deltoid, posterior deltoid, triceps, and biceps EMG are shown. Gray bands represent SE. Data are shown from 50 ms prior to 150 ms after the perturbation. Dotted line indicates when the perturbation was applied to the right arm. The time between the dotted-and-dashed lines represents reflex interval from 50 to 110 ms after the perturbation. C: ensemble averaged right arm handpaths and tangential velocity profiles during baseline (thin black) perturbation trials (thick black). Cross hairs on the handpath profile represent the time from 50 ms prior to 150 ms after the perturbation. D: torque and EMG data for the right arm during baseline (thin black) and perturbation trials (thick black). Ensemble averaged shoulder and elbow muscle torque, and anterior deltoid, posterior deltoid, triceps, and biceps EMG are shown. Gray bands represent SE. Data are shown from 50 ms prior to 150 ms after the perturbation. Dotted line indicates perturbation onset. The time between the dotted-and-dashed lines represents reflex interval from 50 to 110 ms after the perturbation. All data are for the same representative subject as in Fig. 3.
Interestingly, the reflex responses in the posterior deltoid and triceps muscles were similar in timing and magnitude in the independent-cursor and unimanual conditions. We observed no significant difference between the latency of response onset between the independent-cursor and the unimanual condition for either the triceps (t = −0.1840, P = 0.8585), or the posterior deltoid muscle (t = −0.0247, P = 0.9808). The magnitude of increase in the EMG activity of these muscles was also similar for the two conditions (triceps: t = −0.9719, P = 0.3562; posterior deltoid: t = 0.8802, P = 0.4044).
Importantly, the left arm, which was not perturbed in this independent-cursor condition, showed no appreciable change from baseline movement patterns. As can be seen from Fig. 4A, the velocity profiles for the perturbation and baseline trials closely overlapped (compare thick and thin black lines). Our primary interest, however, was in examining whether muscle responses would change in this unperturbed arm following the perturbation to the other arm especially during the reflex interval (time between the dotted-and-dashed lines in Fig. 4B). As can been from Fig. 4B, during the reflex epoch, EMG activity did not change significantly from baseline levels (compare thick and thin black lines). We did not find any significant differences in the EMG response between the baseline and perturbation trials during the reflex interval. This result was consistent across all muscles and all subjects as revealed by our statistical analysis (posterior deltoid: t = 0.1630, P = 0.8746; anterior deltoid: t = −1.7813, P = 0.1127; triceps: t = −0.0163, P = 0.9874; biceps: t = 0.4910, P = 0.6366).
Thus in the independent-cursor condition, the perturbation caused large reflex changes in the EMG response of the perturbed right arm. More importantly, however, we did not observe any significant changes in the EMG response for the unperturbed left arm during the reflex period.
Perturbation responses during bimanual shared-cursor movements
In the bimanual shared-cursor condition, subjects moved a single cursor located at the average distance between the two arms to a single target. Thus both arms shared the task of bringing the cursor to the target. This block of movements could be performed either directly after the unimanual block or following the independent-cursor movement block. Again occasional perturbations of the same magnitude and duration as the unimanual and the bimanual independent-cursor blocks were applied to the right arm to elicit reflex responses in that arm.
Reflex responses are elicited bilaterally in the shared-cursor task
The thick black lines of Fig. 5 show the average responses observed in the right as well as the left arm during the shared-cursor perturbation trials for our representative subject. Note that subjects only saw one start circle and one target on the screen. For comparison with the independent-cursor condition, we plot the motion of the arms as if they were moving to their respective targets. Figure 5, A and C, shows the handpaths and velocity profiles, while B and D show the torque and EMG profiles for the left and right arm respectively. Data display for Fig. 5, B and D, is restricted to 50 ms prior to 150 ms after the perturbation.
Fig. 5.
Performance during baseline and perturbation trials in the bimanual shared-cursor conditions. A: ensemble averaged left arm handpaths and tangential velocity profiles during baseline trials (thin black) and during trials on which a perturbation was applied to the right arm (thick black). Cross hairs on the handpath profile represent the time from 50 ms prior to 150 ms after the perturbation. B: torque and EMG data for the left arm during baseline trials (thin black) and trials on which a perturbation was applied to the right arm (thick black). Ensemble averaged shoulder and elbow muscle torque, and anterior deltoid, posterior deltoid, triceps and biceps EMG are shown. Gray bands represent SE. Data are shown from 50 ms prior to 150 ms after the perturbation. Dotted line indicates when the perturbation was applied to the right arm. The time between the dotted-and-dashed lines represents reflex interval from 50 to110 ms after the perturbation. C: ensemble averaged right arm handpaths and tangential velocity profiles during baseline (thin black) perturbation trials (thick black). Cross hairs on the handpath profile represent the time from 50 ms prior to 150 ms after the perturbation. D: torque and EMG data for the right arm during baseline (thin black) and perturbation trials (thick black). Ensemble averaged shoulder and elbow muscle torque, and anterior deltoid, posterior deltoid, triceps, and biceps EMG are shown. Gray bands represent SE. Data are shown from 50 ms prior to 150 ms after the perturbation. Dotted line indicates perturbation onset. The time between the dotted-and-dashed lines represents the reflex interval from 50 to 110 ms after the perturbation. All data are for the same representative subject as in Figs. 3 and 4.
Consistent with the uni- and bimanual independent-cursor movements, our data show that reflexes were rapidly elicited in the right arm following the perturbation. An early increase in posterior deltoid and triceps activity relative to baseline levels was observed (Fig. 5D, thick black lines). Compared with baseline levels, posterior deltoid activity increased significantly (t = 4.4803, P = 0.0021) at a latency of 49 ± 7 ms following the perturbation. On average, this increase was 77 ± 17% larger than baseline activity during the same interval. Further, the latency of response onset in the posterior deltoid muscle was not significantly different from that in the unimanual (t = −0.0308, P = 0.4880) or bimanual independent-cursor (t = −0.0555, P = 0.4785) conditions. Triceps activity also increased, beginning 46 ± 7 ms after perturbation onset (Fig. 5D, triceps response, thick black lines). Relative to baseline levels, the change in triceps activity was significant during the reflex interval (t = 6.4216, P = 0.0002) and measured 46 ± 7% on average across all subjects. Again the latency of the increase in triceps activity was not significantly different from that of unimanual (t = 0.0476, P = 0.4815) or independent-cursor trials (t = −0.0803, P = 0.4689).
Muscle activity in the anterior deltoid and biceps muscles of the perturbed arm, however, did not deviate significantly from their respective baseline levels during the shared-cursor perturbation trials. These effects can be seen for our representative subject in Fig. 4D by comparing the thick and thin black lines on the anterior deltoid and biceps EMG plots. The consistency of this result across all our subjects was revealed by our statistical analysis, which showed no significant differences between baseline and perturbation EMG responses in these muscles (anterior deltoid: t = 1.9763, P = 0.0835; biceps: t = −0.3392, P = 0.7432). Thus our overall pattern of responses in the perturbed arm in the shared-cursor condition was similar to that in the uni- and bimanual independent- cursor conditions.
Most interestingly, however, during this shared-cursor condition, the unperturbed left arm showed strong activation in the anterior deltoid muscle following the perturbation to the right arm (Fig. 5B, anterior deltoid EMG, thick black line). Across all subjects, EMG activity increased by 35 ±13% relative to baseline anterior deltoid activity in the unperturbed arm. This increase was significant (t = 2.6851, P = 0.0277) and occurred at a latency of 54 ± 7 ms. Thus in the shared-cursor conditions, a perturbation applied to only the right arm led to strong reflex responses in both arms. When we compared the latency of this increase in anterior deltoid activity of the unperturbed left arm to the onset of reflex activity in the perturbed right arm, our results showed no significant differences between the onset times of the unperturbed anterior deltoid response and perturbed posterior deltoid activity (t = −0.4564, P = 0.3301). Moreover even though there was a difference of almost 10 ms between the latency of onset of the triceps response in the perturbed arm and the anterior deltoid arm in the unperturbed arm, this difference did not reach statistical significance in our data (t = −1.4171, P = 0.0970). As can be seen by comparing the thick and thin black traces in Fig. 4B, responses in other muscles of the unperturbed left arm did not deviate significantly from their baseline levels, a result consistent on average across all our subjects (posterior deltoid: t = 1.7352, P = 0.1209; triceps: t = 0.5283, P = 0.6116; biceps: t = −0.5975, P = 0.5667).
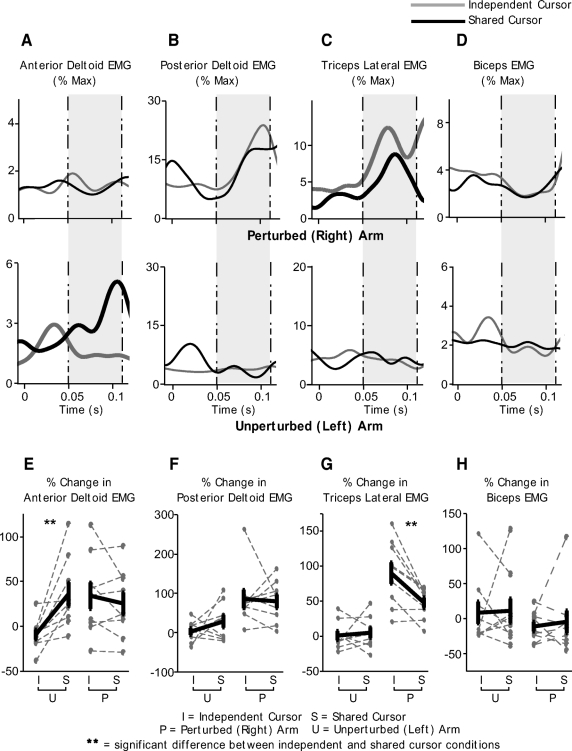
Next we directly compared whether these reflex responses obtained during the shared-cursor condition were different from those in the independent-cursor condition for the two arms. Figure 6 shows this comparison with A–D showing EMG traces for our representative subject and E–H showing the change in EMG between the shared- and independent-cursor conditions for each of our subjects (gray dotted lines) along with the mean change in EMG across all subjects highlighted in thick black. Statistical comparison between the independent- and shared-cursor conditions was done using a two-way (arm × cursor-condition) ANOVA. As can be seen from Fig. 6D, the biceps response for our representative subject was not different between the two cursor conditions during the reflex interval (shaded gray area) for either the perturbed or the unperturbed arm. This result was consistent across all subjects (Fig. 6H) and confirmed by our ANOVA. For the biceps, we observed no significant effects for either arm [F(1,8) = 2.4290, P = 0.1322] or cursor condition [F(1,8) = 0.0312, P = 0.8613]. No significant hand × cursor-condition interaction [F(1,8) = 0.0186, P = 0.8927] was observed for this muscle either, suggesting that biceps activity was not significantly different across the shared- and independent-cursor conditions for either the left or the right arm. For the posterior deltoid muscle, only a significant main effect for hand [F(1,8 = 19.5697, P = 0.0002] was observed. As can be seen from Fig. 6, B and F, a large change in EMG was seen for the perturbed right but not the unperturbed left arm, consistent with the effects of the applied perturbation. However, no significant effect of cursor-condition [F(1,8) = 0.8633, P = 0.3621] was observed, suggesting that for either arm, the change in EMG between the shared- and independent-cursor conditions was not significantly different. No interaction effects [F(1,8) = 1.3458, P = 0.2574] were observed for this muscle either.
Fig. 6.
Comparison of responses in the independent- and shared-cursor conditions. A–D: ensemble averaged EMG responses in the independent-cursor (gray) and shared- cursor (black) conditions for the same representative subject as in Figs. 3–5 during trials on which a perturbation is applied to the right arm. Anterior deltoid (A), posterior deltoid (B), triceps lateral (C), and biceps (D) responses are shown. Top: data for the perturbed (right) arm; bottom: data for the unperturbed (left) arm. Time 0 indicates when the perturbation was applied to the right arm. The time between the dotted-and-dashed lines (shaded gray area) represents the reflex interval from 50 to 110 ms after the perturbation. E–H: percent change in EMG response in the independent- and shared-cursor conditions for the perturbed (right) and unperturbed (left) arms. Data for anterior deltoid (E), posterior deltoid (F), triceps lateral (G), and biceps (H) are shown. Dotted gray lines show data for each individual subject while thick black lines represent the mean ± SE across all our subjects.
More interestingly, our ANOVA showed a significant interaction between arm and cursor-condition for the change in reflex EMG in the triceps muscle [F(1,8) = 7.6796, P = 0.0106]. Post hoc statistical analysis revealed that while in the left, unperturbed arm, triceps response was not different (P > 0.05, Tukey's HSD test) between the independent- and shared-cursor conditions, the perturbed (right) arm response was significantly smaller during the shared-cursor condition relative to the independent-cursor trials (P < 0.05, Tukey's HSD test). These effects are shown in bold in Fig. 6C, which illustrate the mean triceps response in the shared-cursor (black) and the independent cursor condition (gray) for our representative subject. The consistency of this result across all subjects is demonstrated in Fig. 6G. As can be seen from this figure, there was no significant change in the triceps EMG response in the unperturbed left arm in the independent versus the shared cursor conditions. In contrast, the right arm triceps EMG response was considerable smaller during the shared-cursor conditions compared with the independent- cursor response. Thus the shared-cursor condition led to a significant reduction in the triceps response of the perturbed arm.
The most striking result was obtained for the anterior deltoid muscle. Our ANOVA again showed a significant arm × cursor-condition interaction effect [F(1,8) = 9.4171, P = 0.0053]. Post hoc analysis showed that while the EMG response in the anterior deltoid was not different between the shared- and independent-cursor conditions for the perturbed right arm (P > 0.05, Tukey's HSD test), the unperturbed left arm showed a significant increase in EMG activity during the reflex interval in the shared-cursor trials compared with its activity in the independent-cursor conditions (P < 0.05, Tukey's HSD test). These results are demonstrated for our representative subject in Fig. 6A (compare black and gray lines) and the robustness of this result across all our subjects is shown in Fig. 6E. As this figure shows, the right arm anterior deltoid EMG was not different across the two cursor conditions, but the left arm showed an increased anterior deltoid response in the shared cursor-condition. Thus in the shared-cursor condition, strong reflex responses were elicited in the unperturbed left arm when the right arm was perturbed.
Kinematic consequences of reflex responses in the unperturbed arm
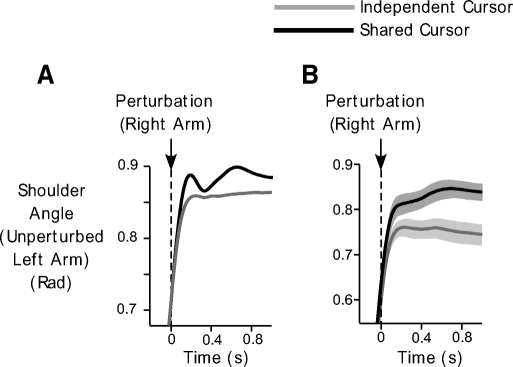
To assess what these changes in the left anterior deltoid EMG during the shared-cursor conditions might mean functionally, we examined whether anterior deltoid activation led to changes in shoulder angle in the unperturbed arm. Figure 7 shows the changes in shoulder angle for the unperturbed left arm in the independent-cursor (gray) and shared-cursor (black) conditions. Figure 7A depicts data for our representative subject, whereas B shows the mean shoulder angle across all our subjects. As can be seen from this figure, depending on the bimanual movement condition, distinct responses were obtained following the perturbation to the right arm. Increased shoulder flexion was observed during the shared-cursor trials, consistent with the reflex activation of the left anterior deltoid muscle. Our analysis showed that this increase in shoulder flexion occurred on average at 112 ± 10 ms after the perturbation to the right arm. This shoulder motion was in the direction that would assist in bringing the cursor back toward the target. Shoulder motion ultimately led to changes in tangential hand velocity during the shared cursor trials. Across all subjects, we observed changes in left hand velocity at an average latency of 130 ± 11 ms following the reflex response in the left arm. This was in contrast to the independent-cursor conditions, in which left hand velocity remained close to baseline levels (Fig. 5A). Thus reflex changes in the unperturbed left arm led to changes in its kinematics in manner that aided in the achievement of the task goal.
Fig. 7.
Kinematic consequences of EMG changes in the left arm. A and B: mean shoulder angle of the unperturbed left arm during the shared-cursor (black) and independent-cursor (gray) conditions, during trials on which a perturbation is applied to the right arm. Data from our representative subject (A) and across all subjects (B) are shown. In B, the gray bands represent SE.
In summary, our results demonstrate that significant responses can be elicited at reflex latencies in an unperturbed limb during a bimanual task when both arms contribute to the achievement of the task goal.
DISCUSSION
In this study, we investigated whether shared bilateral tasks might recruit bilateral reflexes in response to unilateral arm perturbations. Subjects made bimanual arm movements either to two separate targets when cursor feedback about each hand was provided separately, or to a single target when the cursor was presented at the average location of the two hands. Occasional perturbations applied to the right arm during these movements elicited strong reflex responses in the triceps and posterior deltoid muscles. We observed significant modulation of reflex responses to perturbations depending on task context. Reflex responses in the triceps muscle of the right arm were reduced during the shared-cursor condition. More strikingly however, the anterior deltoid muscle in the unperturbed left arm showed a strong increase in EMG activity at reflex latencies during this condition. This increase caused changes in left arm kinematics that contributed to bringing the cursor to the target. Our results thus indicate strong modulation of reflex responses across effectors, but under conditions in which the task goal is shared between the arms.
Cross-effector reflexes during movement
Sherrington's work during the early 20th century systematically demonstrated the existence of cross-effector reflexes in the lower limbs (Sherrington 1910). Both modulation and even reversal of this “crossed-extension” reflex have been documented over several studies in animals (Brown 1911; Brown and Sherrington 1912; Hinsey et al. 1930; Sherrington and Sowton 1911) and in humans (Grimby 1963; Hagbarth 1960; Kugelberg et al. 1960; Shahani and Young 1971). Although these studies suggested that this reflex modulation was important for maintenance of a stable posture, more recent studies have suggested that these reflex circuits might be part of a larger more complex network also involved in locomotion (Rossignol and Dubuc 1994; Sandrini et al. 2005; Zehr and Stein 1999). Thus in the lower limbs, not only are cross-effector bilateral reflexes present, but they contribute to the functionality of locomotion.
In the upper limbs, however, the existence and function of bilateral reflex responses during the course of an ongoing movement has not been convincingly demonstrated. Marsden et al. (1981) showed that a perturbation applied to one arm produced a response in the contralateral elbow muscle when the contralateral arm held a table to prevent the body from moving forward. This extensor response was reversed when the contralateral arm held a filled cup rather than the table. However, this task emphasized maintenance of a stable posture rather than performance of an actual movement. Moreover, while this study demonstrated reflex responses in the unperturbed arm, the response was based on its task context (holding a table or holding a cup) rather than how much that arm contributed to achievement of the overall task goal. Other studies, particularly those that have demonstrated bilateral modulation of grip force in response to object perturbations, have proposed that such bilateral effects might be mediated at least partly through cross-effector reflex mechanisms (Bracewell et al. 2003; White et al. 2008). However, most of these studies also examined grip force responses during positional rather than movement tasks. Moreover, perturbations occurred on most trials and it is unclear how much of the response was a consequence of reflex action rather than the early release of a stored voluntary response or a “triggered reaction” (see Hasan 2005 for a review).
Our study shows the emergence of bilateral reflexes during the course of movement. In our case, the perturbation was unpredictable and occurred after movement onset. Under these conditions, it is remarkable that we observed responses at reflex latencies in the unperturbed arm when both arms contributed equally to cursor movement. However, the lack of a change in response in the unperturbed arm during the independent-cursor condition underscores the importance of having the task shared between the two arms in order for such reflexes to be elicited.
Mechanisms underlying bimanual reflexes in the upper limbs
Studies investigating the modulation of grip force to sudden perturbations of an object held between the two hands have demonstrated that object perturbations resulted not only in a rapid (∼60–70 ms) increase in grip force in the perturbed hand but also led to a grip force increase in the unperturbed hand of the contralateral arm (Bracewell et al. 2003; Ohki and Johansson 1999; White et al. 2008). Importantly, Ohki and Johansson (1999) reported that responses in the unperturbed hand were slightly delayed compared with the onset of the response in the perturbed hand by ∼15 ms. Based on these results, Johansson and colleagues speculated that these bilateral effects might be dependent on crossing over of sensory information from the perturbed side to the unperturbed side at multiple levels of the nervous system, most likely due to transcallosal interactions between the sensorimotor cortices.
Our results show that proximal muscles at the shoulder can also be rapidly incorporated into reflex mechanisms depending on task goals. However, the relatively short latency of the bilateral reactions observed in our study as well as the lack of significant differences between the onsets of the responses between the arms leaves open the question whether the reactions observed in our case might utilize mechanisms other than direct cortical or transcallosal transfer of information. In contrast to distal hand muscles, which are more likely to be controlled by contralateral corticospinal tracts (therefore requiring calossal transfer of information to produce a response in the unperturbed side), it is possible that the bilateral reflex responses observed in our study are mediated by bilateral descending pathways that originate in one hemisphere. Proximal muscles of the trunk and limb girdle are controlled through bilateral projections from unilateral cortical areas (Brinkman and Kuypers 1972; Kuypers 1964; Kuypers and Brinkman 1970), and it is possible that these bilateral pathways might be recruited for the manifestation of bilateral reflex responses. However, we did observe a delay of nearly 10 ms between the onset of the triceps response in the perturbed arm and the anterior deltoid response in the unperturbed arm. Although not statistically significant, it remains possible that this delay was associated with transcalossal transfer of information from the perturbed to the unperturbed side leading to a slightly delayed response in the unperturbed arm, consistent with the suggestion of Ohki and Johansson (1999). It also remains plausible that transcalossal communication is required for setting up the bimanual responses through feedfoward or preparatory means. Thus transcollosal circuits might provide descending signals that ultimately disinhibit a response on the unperturbed side. It is also likely, given the short latency of the response following the perturbation in both arms, that these responses might be mediated, at least partly, at segmental or subcortical levels (White et al. 2008). Although our current results cannot distinguish between these alternatives, further experimentation that specifically targets certain pathways can shed more light on the circuitry involved.
It is also possible that higher-level cognitive processes such as attention might influence sensorimotor cortical and/or spinal circuits that are ultimately responsible for the differential responses in the shared versus independent-cursor tasks as observed here. Recent studies have demonstrated that variations in the allocation of attentional resources to one arm significantly influence the strength of interlimb coupling (de Poel et al. 2007, 2008; Riek et al. 2003). It is plausible that in our study, attentional resources to the two arms could be differently distributed during the independent-cursor compared with the shared-cursor movements. In particular, a division of attention could have occurred in the independent-cursor condition, while this might be less likely in the shared-cursor task. These differences in the way attentional resources are allocated depending on task context could influence interlimb coupling strength, potentially affecting the reflex responses observed in the two cases. However, the specific interactions between these higher-level processes and sensorimotor cortical/spinal neuronal circuitry that ultimately produces the desired motor output remain incompletely understood. Future experiments could delineate these interactions.
Functional significance of bimanual reflexes
Several studies have examined modulation of reflex responses within a limb. These experiments have suggested that task-dependent reflex modulation provides the CNS the flexibility to regulate limb impedance to perturbations arising from the environment during the maintenance of a stable posture (Colebatch and McCloskey 1987; Colebatch et al. 1979; Doemges and Rack 1992a,b; Hammond 1955, 1956; Marsden et al. 1983; Perreault et al. 2006; Pruszynski et al. 2008; Rothwell et al. 1980) as well as during the course of an going movement (Kimura et al. 2006; Mutha et al. 2008). More recently, studies examining reflex responses to more complex perturbations that affect multiple joints within a limb have expanded the scope of such reflex modulation. These studies have suggested that multijoint reflex organization is useful in compensating perturbations in a manner that accounts for multijoint limb mechanics (Kurtzer et al. 2008; Nichols 1994; Nichols et al. 1999; Perreault et al. 2008). For example, Kurtzer et al. (2008) recently demonstrated that long-latency reflexes elicited at the shoulder were sensitive to both shoulder and elbow motion and compensated for the underlying multijoint torque pattern rather than local muscle stretch. Similar results had previously also been obtained by Lacquaniti and colleagues (Lacquaniti and Soechting 1986; Soechting and Lacquaniti 1988). Perreault et al. (2008) also recently demonstrated similar reflex coordination patterns in a task where multijoint perturbations were applied to the limb, although these reflex patterns did not change based on task condition. These authors showed that while the same muscles were active when subjects interacted with either a stiff or compliant environment in their task, the magnitude of reflex activation was quite different, pointing to the flexibility of these patterns during multijoint tasks. The general view emerging from all these studies has been that this multijoint organization of reflex circuitry provides the flexibility of assembling reflex coordination patterns based on task demands.
Our results support and extend the task-specific reflex modulation hypothesis described in the preceding text to include reflex responses elicited not just at multiple joints within a limb but also across limbs. It is plausible that similar to a within-limb multijoint reflex organization, between-limb reflex responses could be flexibly assembled based on task demands especially when the task is shared between the limbs. The near simultaneous responses in the left anterior deltoid and right posterior deltoid muscles, along with a decrease in right triceps activity at reflex latency observed during the shared-cursor condition in our study lend support to this idea. These changes can result in functionally relevant changes in arm kinematics so that the task of bringing the cursor to the target can be successfully completed.
Several everyday activities involve the coordination of activities of both hands to achieve the task goal. Manipulation of an object held between the two hands, for example, requires that the action of both hands be coordinated in a way that prevents the object from slipping. Perturbations arising during such instances, either at the object or at either arm, can adversely affect task performance. In such cases, rapid reflex responses elicited bilaterally might used to compensate the perturbations so that stability of the object being manipulated can be maintained. Thus bimanual reflexes might provide the CNS a rapid response mechanism to counteract the effects of the perturbations without compromising task performance or stability.
Task-dependent feedback control during bimanual movements
In this study, we also applied perturbations to the right arm when the left arm was stationary. An assessment of the reflex responses elicited during these unimanual movements suggests that they were similar to the responses obtained in the bimanual independent-cursor condition. These responses differed neither in the timing nor the amplitude of the response. Thus reflex mechanisms that resist perturbations might be utilized in a similar manner in these two task conditions. This suggests that similar to unimanual conditions, afferent information might be processed fairly locally during bimanual movements in which each arm is required to achieve its own goal. Sensorimotor feedback loops might be modified to act within an effector under such conditions. Such control could be achieved by maintaining separate control of the two limbs. This seems appropriate because a response in the unperturbed arm does not contribute to the achievement of the goal of the perturbed arm. In contrast, feedback loops might be set to act more “globally” in the shared-cursor condition, during which the unperturbed arm can contribute to achievement of the task goal. Such control could be implemented through a single higher-level controller that synchronizes the control of each limb. Thus the contrasting reflex responses observed in the independent- and shared-cursor conditions in our study demonstrates differential modulation of feedback circuitry based on bimanual task demands.
Support for this idea of task-dependent feedback control during bimanual movements comes from a recent study (Diedrichsen 2007). This study showed that velocity-dependent unilateral perturbations were corrected bilaterally if the task was shared between the arms. Although the corrective responses obtained during the bimanual conditions occurred late into the movement and were quite subtle, it nevertheless reflected changes in the feedback responses to perturbations based on task demands. Simulations using an optimal feedback control model (Todorov and Jordan 2002) showed the optimal control policy in the independent-cursor condition was such that only the perturbed arm responded to make a correction. In contrast, in the shared-cursor condition, both arms reacted to respond to a unimanual perturbation, minimizing the overall motor commands necessary.
Our current results extend Diedrichsen's (2007) suggestions that feedback circuits are modulated during bimanual movements based on task demands. We show that these circuits include our most rapid feedback mechanisms, i.e., reflexes. Their modulation appears to be optimal with respect to achievement of the current goal of the task.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant R01 HD-39311.
ACKNOWLEDGMENTS
We thank A. Przybyla for help with data collection and also for insight on some aspects of this research.
REFERENCES
- Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol 88: 2408–2421, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracewell RM, Wing AM, Soper HM, Clark KG. Predictive and reactive coordination of grip and load forces in bimanual lifting in man. Eur J Neurosci 18: 2396–2402, 2003 [DOI] [PubMed] [Google Scholar]
- Brinkman C. Supplementary motor area of the monkey's cerebral cortex: short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci 4: 918–929, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman J, Kuypers HG. Splitbrain monkeys: cerebral control of ipsilateral and contralateral arm, hand, and finger movements. Science 176: 536–539, 1972 [DOI] [PubMed] [Google Scholar]
- Brown TG. Studies in the phyiology of the nervous system. VIII. Neural balance and reflex reversal, with a note on progression in the decrebrate guinea pig. Q J Exp Physiol 4: 273–288, 1911 [Google Scholar]
- Brown TG, Sherrington CS. The rule of reflex response in the limb reflexes of the mammal and its exceptions. J Physiol 44: 125–130, 1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC, McCloskey DI, Potter EK. Subject instruction and long latency reflex responses to muscle stretch. J Physiol 292: 527–534, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, McCloskey DI. Maintenance of constant arm position or force: reflex and volitional components in man. J Physiol 386: 247–261, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Poel HJ, Peper CL, Beek PJ. Handedness-related asymmetry in coupling strength in bimanual coordination: furthering theory and evidence. Acta Psychol 124: 209–237, 2007 [DOI] [PubMed] [Google Scholar]
- de Poel HJ, Peper CL, Beek PJ. Laterally focused attention modulates asymmetric coupling in rhythmic interlimb coordination. Psychol Res 72: 123–137, 2008 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. Optimal task-dependent changes of bimanual feedback control and adaptation. Curr Biol 17: 1675–1679, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doemges F, Rack PM. Changes in the stretch reflex of the human first dorsal interosseous muscle during different tasks. J Physiol 447: 563–573, 1992a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doemges F, Rack PM. Task-dependent changes in the response of human wrist joints to mechanical disturbance. J Physiol 447: 575–585, 1992b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domkin D, Laczko J, Jaric S, Johansson H, Latash ML. Structure of joint variability in bimanual pointing tasks. Exp Brain Res Exp Hirnfsch 143: 11–23, 2002 [DOI] [PubMed] [Google Scholar]
- Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E. Primary motor cortex is involved in bimanual coordination. Nature 395: 274–278, 1998 [DOI] [PubMed] [Google Scholar]
- Grimby L. Normal plantar response: integration of flexor and extensor reflex components. J Neurol Neurosurg Psychiatry 26: 39–50, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth KE. Spinal withdrawal reflexes in the human lower limbs. J Neurol Neurosurg Psychiatry 23: 222–227, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond PH. Involuntary activity in biceps following the sudden application of velocity to the abducted forearm. J Physiol 127: 23–25P, 1955 [PMC free article] [PubMed] [Google Scholar]
- Hammond PH. The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. J Physiol 132: 17–18P, 1956 [PubMed] [Google Scholar]
- Hasan Z. The human motor control system's response to mechanical perturbation: should it, can it, and does it ensure stability?. J Mot Behav 37: 484–493, 2005 [DOI] [PubMed] [Google Scholar]
- Hinsey JC, Ranson SW, Doles EA. Reversal in the crossed extension reflex in decrebrate, decapitate and spinal cats. Am J Physiol 95: 573–583, 1930 [Google Scholar]
- Jancke L, Peters M, Himmelbach M, Nosselt T, Shah J, Steinmetz H. fMRI study of bimanual coordination. Neuropsychologia 38: 164–174, 2000 [DOI] [PubMed] [Google Scholar]
- Jancke L, Peters M, Schlaug G, Posse S, Steinmetz H, Muller-Gartner H. Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Brain Res Cogn Brain Res 6: 279–284, 1998 [DOI] [PubMed] [Google Scholar]
- Kelso JA, Holt KG, Rubin P, Kugler PN. Patterns of human interlimb coordination emerge from the properties of non-linear, limit cycle oscillatory processes: theory and data. J Mot Behav 13: 226–261, 1981 [PubMed] [Google Scholar]
- Kelso JA, Southard DL, Goodman D. On the coordination of two-handed movements. J Exp Psychol Hum Percept Perform 5: 229–238, 1979a [DOI] [PubMed] [Google Scholar]
- Kelso JA, Southard DL, Goodman D. On the nature of human interlimb coordination. Science 203: 1029–1031, 1979b [DOI] [PubMed] [Google Scholar]
- Kimura T, Haggard P, Gomi H. Transcranial magnetic stimulation over sensorimotor cortex disrupts anticipatory reflex gain modulation for skilled action. J Neurosci 26: 9272–9281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HI, Hogan N, Volpe BT, Aisen ML, Edelstein L, Diels C. Overview of clinical trials with MIT-MANUS: a robot-aided neuro-rehabilitation facility. Technol Health Care 7: 419–423, 1999 [PubMed] [Google Scholar]
- Kugelberg E, Eklund K, Grimby L. An electromyographic study of the nociceptive reflexes of the lower limb. Mechanism of the plantar responses. Brain 83: 394–410, 1960 [DOI] [PubMed] [Google Scholar]
- Kurtzer IL, Pruszynski JA, Scott SH. Long-latency reflexes of the human arm reflect an internal model of limb dynamics. Curr Biol 18: 449–453, 2008 [DOI] [PubMed] [Google Scholar]
- Kuypers HG. The descending pathways to the spinal cord, their anatomy and function. Prog Brain Res 11: 178–202, 1964 [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Brinkman J. Precentral projections to different parts of the spinal intermediate zone in therhesus monkey. Brain Res 24: 29–48, 1970 [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Soechting JF. EMG responses to load perturbations of the upper limb: effect of dynamic coupling between shoulder and elbow motion. Exp Brain Res Exp Hirnforsc 61: 482–496, 1986 [DOI] [PubMed] [Google Scholar]
- Latash ML. Neurophysiological Basis of Movement. Urbana, IL: Human Kinetics, 1996 [Google Scholar]
- Li X, Zhou P, Aruin AS. Teager-Kaiser energy operation of surface EMG improves muscle activity onset detection. Ann Biomed Eng 35: 1532–1538, 2007 [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Human postural responses. Brain 104: 513–534, 1981 [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Rapid postural reactions to mechanical displacement of the hand in man. Adv Neurol 39: 645–659, 1983 [PubMed] [Google Scholar]
- Morasso P. Spatial control of arm movements. Exp Brain Res Exp Hirnforsch 42: 223–227, 1981 [DOI] [PubMed] [Google Scholar]
- Mutha PK, Boulinguez P, Sainburg RL. Visual modulation of proprioceptive reflexes during movement. Brain Res 1246: 54–69, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR. A biomechanical perspective on spinal mechanisms of coordinated muscular action: an architecture principle. Acta Anat 151: 1–13, 1994 [DOI] [PubMed] [Google Scholar]
- Nichols TR, Cope TC, Abelew TA. Rapid spinal mechanisms of motor coordination. Exercise Sport Sci Rev 27: 255–284, 1999 [PubMed] [Google Scholar]
- Nozaki D, Kurtzer I, Scott SH. Limited transfer of learning between unimanual and bimanual skills within the same limb. Nat Neurosci 9: 1364–1366, 2006 [DOI] [PubMed] [Google Scholar]
- Ohki Y, Johansson RS. Sensorimotor interactions between pairs of fingers in bimanual and unimanual manipulative tasks. Exp Brain Res Exp Hirnforsch 127: 43–53, 1999 [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971 [DOI] [PubMed] [Google Scholar]
- Perreault EJ, Chen K, Lewis GN. Regulation of multijoint stretch reflexes during interactions with stiff and compliant environments. Conf Proc IEEE Eng Med Biol Soc 1: 300–302, 2006 [DOI] [PubMed] [Google Scholar]
- Perreault EJ, Chen K, Trumbower RD, Lewis G. Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination. J Neurophysiol 99: 2101–2113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol 100: 224–238, 2008 [DOI] [PubMed] [Google Scholar]
- Riek S, Tresilian JR, Mon-Williams M, Coppard VL, Carson RG. Bimanual aiming and overt attention: one law for two hands. Exp Brain Res Exp Hirnforsch 153: 59–75, 2003 [DOI] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol 43: 118–136, 1980a [DOI] [PubMed] [Google Scholar]
- Roland PE, Skinhoj E, Lassen NA, Larsen B. Different cortical areas in man in organization of voluntary movements in extrapersonal space. J Neurophysiol 43: 137–150, 1980b [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R. Spinal pattern generation. Curr Opin Neurobiol 4: 894–902, 1994 [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature 286: 496–498, 1980 [DOI] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y. Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci 17: 9667–9674, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol 77: 353–395, 2005 [DOI] [PubMed] [Google Scholar]
- Shahani BT, Young RR. Human flexor reflexes. J Neurol Neurosurg Psychiatry 34: 616–627, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol 40: 28–121, 1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS, Sowton SC. Chloroform and reversal of reflex effect. J Physiol 42: 383–388, 1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechting JF, Lacquaniti F. Quantitative evaluation of the electromyographic responses to multidirectional load perturbations of the human arm. J Neurophysiol 59: 1296–1313, 1988 [DOI] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: from behavioural principles to neural- network interactions. Nat Rev 3: 348–359, 2002 [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Beirinckx MB, Meugens PF, Walter CB. Dissociating the structural and metrical specifications of bimanual movement. J Mot Behav 23: 263–279, 1991a [DOI] [PubMed] [Google Scholar]
- Swinnen S, Walter CB, Shapiro DC. The coordination of limb movements with different kinematic patterns. Brain Cogn 8: 326–347, 1988 [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Walter CB, Lee TD, Serrien DJ. Acquiring bimanual skills: contrasting forms of information feedback for interlimb decoupling. J Exp Psychol Learn Mem Cogn 19: 1328–1344, 1993 [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Young DE, Walter CB, Serrien DJ. Control of asymmetrical bimanual movements. Exp Brain Res Exp Hirnforsch 85: 163–173, 1991b [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Relation of neurons in the nonprimary motor cortex to bilateral hand movement. Nature 327: 618–620, 1987 [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol 60: 325–343, 1988 [DOI] [PubMed] [Google Scholar]
- Theorin A, Johansson RS. Zones of bimanual and unimanual preference within human primary sensorimotor cortex during object manipulation. NeuroImage 36, Suppl 2: T2–T15, 2007 [DOI] [PubMed] [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci 5: 1226–1235, 2002 [DOI] [PubMed] [Google Scholar]
- Walter CB, Swinnen SP, Corcos DM, Pollatou E, Pan HY. Coping with systematic bias during bilateral movement. Psychol Res 60: 202–213, 1997 [DOI] [PubMed] [Google Scholar]
- Westenberg Y, Smits-Engelsman BC, Duysens J. Development of unimanual versus bimanual task performance in an isometric task. Hum Move Sci 23: 461–474, 2004 [DOI] [PubMed] [Google Scholar]
- White O, Dowling N, Bracewell RM, Diedrichsen J. Hand interactions in rapid grip force adjustments are independent of object dynamics. J Neurophysiol 100: 2738–2745, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesendanger M, Serrien DJ. The quest to understand bimanual coordination. Prog Brain Res 143: 491–505, 2004 [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. New York: Wiley, 1990 [Google Scholar]
- Yamanishi J, Kawato M, Suzuki R. Two coupled oscillators as a model for the coordinated finger tapping by both hands. Biol Cybern 37: 219–225, 1980 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 58: 185–205, 1999 [DOI] [PubMed] [Google Scholar]