Abstract
Synaptic plasticity has been extensively studied in principal neurons of the neocortex, but less work has been done on GABAergic interneurons. Interneurons consist of multiple subtypes and their synaptic properties vary between subtypes. In the present study, we have examined long-term potentiation (LTP) of excitatory synapses on somatostatin (SS)-expressing interneurons in neocortex using transgenic mice that express enhanced green fluorescent protein in these interneurons. We found that a strong theta burst stimulation was required to induce LTP in SS interneurons. LTP was associated with a reduction in paired-pulse facilitation and was not blocked by an N-methyl-d-aspartate receptor (NMDAR) antagonist. LTP was not affected by chelating postsynaptic Ca2+ with BAPTA, a fast Ca2+ chelator, and blocking L-type voltage-dependent Ca2+ channels with nimodipine. Application of forskolin, an activator of adenylate cyclase that increases cyclic adenosine monophosphate (cAMP) concentration, enhanced synaptic transmission and occluded subsequent induction of LTP. Finally, we found that LTP was blocked by protein kinase A (PKA) inhibitors. Our results suggest that excitatory synapses on SS interneurons express a presynaptic form of LTP that is not dependent on NMDARs or postsynaptic Ca2+ rise but is dependent on the cAMP–PKA signaling pathway.
INTRODUCTION
GABAergic interneurons play a key role in controlling the excitability of glutamatergic principal cells and synchronization of neuronal networks (Buzsáki et al. 2004; Mott and Dingledine 2003). These interneurons receive numerous inputs from principal cells and their excitability may be regulated by the efficacy of excitatory synapses on them. Activity-dependent changes in synaptic strength have been well studied in principal cells of the neocortex. The presence of long-term potentiation (LTP) and depression (LTD) of synaptic transmission in principal cells is believed to be a basis for many brain functions, such as learning and memory (Bear 1996; Castro-Alamancos and Connors 1996; Kirkwood and Bear 1994; Rioult-Pedotti et al. 2000). In contrast, much less is known about synaptic plasticity in cortical interneurons.
Cortical GABAergic interneurons vary greatly in terms of their morphology, intrinsic properties, and molecular makeup (Cauli et al. 1997; Markram et al. 2004). The synaptic properties in interneurons also differ strikingly depending on their specific subtype (Markram et al. 1998; Reyes et al. 1998). A substantial number of studies have examined long-term synaptic changes including LTP and LTD in interneurons in hippocampus, amygdala, and neocortex (Bauer and LeDoux 2004; Kullmann and Lamsa 2007; Lu et al. 2007; Mahanty and Sah 1998; Sarihi et al. 2008). It appears that multiple forms of LTP exist, depending on the subtype and location of the interneuron, and Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), metabotropic glutamate receptors (mGluRs), and N-methyl-d-aspartate receptors (NMDARs) have all been reported to play essential roles in different forms of LTP (Bauer and LeDoux 2004; Lamsa et al. 2005, 2007; Lu et al. 2007; Mahanty and Sah 1998; Oren et al. 2009; Pelkey et al. 2005; Perez et al. 2001; Sarihi et al. 2008). Relative to the hippocampus, less work has been done in the neocortex. In mouse visual cortex, it was found that LTP was present in fast-spiking (FS) interneurons but not in non-FS interneurons. The induction of LTP required activation of mGluR5 and postsynaptic Ca2+ rise, but the expression was mediated presynaptically (Sarihi et al. 2008). In rat somatosensory cortex, repetitive correlated pre- and postsynaptic spiking induced LTP in low-threshold spiking (LTS) interneurons, but LTD in FS interneurons. LTP in this study required the activation of NMDARs and was also expressed presynaptically (Lu et al. 2007). These varied and sometimes conflicting results probably reflect the wide diversity of interneurons and their synaptic properties (Kullmann and Lamsa 2007). It may also imply that different stimulation patterns yield different forms of plasticity in different subtypes of interneurons.
Somatostatin (SS)-expressing interneurons are one major subtype of interneuron in the cortex. They are found in the hilus of dentate gyrus, stratium oriens of CA1 of the hippocampus, and all layers of the neocortex. Axons of these interneurons form synapses primarily on the distal dendrites of pyramidal cells (Di Cristo et al. 2004; Kawaguchi and Kubota 1993; Kubota and Kawaguchi 2000; Miles et al. 1996; Somogyi and Klausberger 2005). The excitatory synapses on neocortical SS interneurons show a low release probability and exhibit robust short-term facilitation with repetitive stimulation (Beierlein et al. 2003; Reyes et al. 1998). They have been shown to be particularly vulnerable to injury during prolonged seizures, suggesting a possible role in epilepsy (Cossart et al. 2001; Oliva Jr et al. 2002; Sloviter 1987). In the present study, we examined LTP of the excitatory synapses on SS interneurons in a line of transgenic mice that express enhanced green fluorescent protein (eGFP) in a subpopulation of SS interneurons (Oliva Jr et al. 2000). We found that monosynaptic LTP could be induced by a strong theta burst stimulation (TBS). Subsequent experiments showed that induction of LTP is not dependent on NMDAR activation or postsynaptic Ca2+ rise, but probably dependent on presynaptic activation of the protein kinase A (PKA) pathway.
METHODS
Slice preparation
Breeding pairs of homozygous, transgenic mice [FVB-Tg(GadGFP) 45704Swn/J] were purchased from The Jackson Laboratory (Bar Harbor, ME). Their offspring were used for the experiments. Brain slices were obtained from mice aged postnatal day 15 (P15) to P42 (median age = P21). Mice were anesthetized by inhalation of isoflurane, decapitated, and the brain was rapidly removed. Coronal, 400-μm-thick sensorimotor cortical slices were cut using a vibrating microtome (Leica Microsystems, Wetzlar, Germany) in ice-cold artificial cerebrospinal fluid (ACSF; see following text) containing 1 mM Ca2+ and 6 mM Mg2+. Slices were incubated on cell-culture inserts (8-μm pore diameter) covered by a thin layer of ACSF (same as cutting solution) and surrounded by a humidified 95% O2-5% CO2 atmosphere at room temperature (22°C). After incubation for ≥1 h, slices were transferred to a submerged recording chamber with continuous flow (2–3 ml/min) of ACSF that contained (in mM) 124 NaCl, 26 NaHCO2, 1.25 NaH2PO4, 2.5 KCl, 2 CaCl2, 2 MgCl2, 10 d-glucose, and 0.5 picrotoxin gassed with 95% O2-5% CO2 (giving pH 7.4). All experiments were carried out at room temperature.
Electrophysiology
Whole cell recordings were made from eGFP-expressing interneurons in layers II to IV of the sensorimotor cortex. Each eGFP interneuron was first identified using fluorescence microscopy and was then patched using infrared differential interference contrast (IR-DIC) videomicroscopy (Axioskop-FS; Carl Zeiss, Jena, Germany) using a ×40, 0.8-W water-immersion objective. In some experiments, recordings were made from layer III/V pyramidal cells using IR-DIC microscopy. Patch electrodes had a resistance of 3–5 MΩ when filled with an internal solution containing (in mM): 120 K-gluconate, 8 NaCl, 10 HEPES, 2 MgATP, 0.3 Na3GTP, and 0.2 EGTA (pH 7.3 with KOH; osmolarity = 290–300 mOsm). After establishing whole cell recordings, the spiking pattern was tested in current-clamp mode by injection of suprathreshold current pulses (200–300 pA, 300 ms). Short-term plasticity of evoked excitatory postsynaptic currents (EPSCs) was examined using a five-pulse (20 Hz) train stimulation. In LTP experiments, neurons were held at −70 mV in current-clamp mode by injecting continuous current or voltage-clamped at −68 mV using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA). GABAergic activity was blocked by adding the γ-aminobutyric acid type A (GABAA) receptor blocker, picrotoxin (50 μM), to the bath solution. Data were acquired using Clampex 10 software (Axon Instruments) and digitized at 10 kHz. Off-line analysis was performed with Clampfit 10 (Axon Instruments) and Origin 7.5 (OriginLab, Northampton, MA).
To evoke monosynaptic excitatory postsynaptic potentials (EPSPs) or EPSCs, a glass electrode (3–5 MΩ) filled with ACSF was placed 30–50 μm away from a thick dendritic shaft of the recorded neuron and the position was carefully adjusted so as not to evoke polysynaptic responses. Monosynaptic potentials and currents were identified by their constant latency and a single peak. Paired-pulse stimulation (interstimulus interval = 50 ms) was used in LTP experiments as a test stimulus. For baseline recordings, the amplitude of the first EPSP or EPSC was set to 5–10 mV or 50–100 pA, respectively, by adjusting the strength of stimulation. LTP was attempted only in inputs where doubling the amplitude of baseline EPSP or EPSC by increasing the strength of stimulation did not induce polysynaptic activities. Series resistance was 14–25 MΩ and was monitored continuously by applying a hyperpolarizing pulse before each stimulus. The recordings were discarded if a change of series resistance >10% occurred.
TBS was used to induce LTP. One episode of TBS contained 20 bursts at 5 Hz, each burst containing five pulses at 100 Hz. LTP was induced by 6 to 10 episodes (total pulses = 600 to 1,000) with a 10-s interval between each episode. Test stimulation with paired pulses at 20 Hz was given at 0.1 Hz and LTP was induced after a 5- to 10-min stable baseline recording. The peak amplitudes of synaptic responses were measured and an average of 5-min recordings prior to TBS was set at 100%. Paired-pulse facilitation (PPF) was calculated as a ratio of the amplitude of the second response to that of the first response based on an average of six consecutive sweeps.
Data analysis and drugs
The Student's t-test was used for statistical comparison before and after LTP and drug treatments. All summary data are expressed as means ± SE. Statistical significance was defined as P < 0.05. All drugs used were purchased from Sigma (St. Louis, MO). Forskolin, nimodipine, H-89, and K-5720 were first dissolved in 100% DMSO as stock solution and then applied in the bath solution at desired concentrations. The final concentration of DMSO in the bath solution was 0.05%.
Immunohistochemistry
Animals were deeply anesthetized with isoflurane inhalation and then perfused transcardially with 4% fresh paraformaldehyde in 0.02 M phosphate buffer solution (PBS, pH 7.4). Brains were removed and left overnight in the same fixative at 4°C and then cryoprotected in 30% sucrose in PBS. Brains were then rapidly frozen by immersion in 2-methylbutane on dry ice and cryostat sections (30 μm) were cut. The sections were then incubated with mouse antiserum to parvalbumin (PV, monoclonal anti-PV clone PARV-19; Sigma) at 1:5,000 and SS (SOM-018; Sigma) at 1:1,000 in PBS plus 0.5% Triton X-100 and 1% BSA. After an overnight incubation at 4°C, sections were washed with PBS and incubated with the goat anti-mouse IgG (H + L) conjugated with Alexa Fluor 594 fluorescent dyes (Invitrogen, Carlsbad, CA) at 1:500 for 2 h at room temperature. Sections were then washed in PBS plus 0.5% Triton X-100, mounted on slides, and coverslipped. Immunofluorescence was examined with a confocal microscope and fluorescent photomicrographs were taken.
RESULTS
As illustrated in Fig. 1A, most eGFP interneurons were located in layers II–IV. These neurons express SS, but not PV, as illustrated in Fig. 1, B and C. We also noticed that not all SS-positive neurons have eGFP, consistent with previous reports that eGFP neurons represent only a subpopulation of SS interneurons (Oliva Jr et al. 2000). All eGFP cells recorded in the present study demonstrated a similar spiking pattern to suprathreshold current pulses (300 pA, 300 ms, Fig. 1D). In addition, short-term plasticity (STP) of EPSCs evoked by five-pulse train stimulation displayed a robust facilitation (Fig. 1E) in all cells recorded. The percentages of amplitude of the second and fifth EPSCs relative to that of the first EPSC were 206 ± 14, 314 ± 26, 363 ± 34, and 427 ± 36%, respectively (n = 26). We also noticed that STP evoked from different inputs in individual eGFP interneurons had a similar pattern, implying that the release probability of the excitatory synapses on a single interneuron is similar. Consistent with previous reports (Goldberg et al. 2003), NMDAR-mediated current was present in eGFP interneurons, as illustrated in Fig. 1F. NMDAR components of the evoked response were isolated by holding neurons at −30 mV and pharmacologically blocking both AMPA- and GABAA-mediated currents. Application of d-2-amino-5-phosphonopentanoic acid (AP5, 50 μM), an NMDAR antagonist, blocked the NMDAR-mediated components.
Fig. 1.
Distribution of eGFP-expressing interneurons in the neocortex and their intrinsic and synaptic properties. A: a photomicrograph showing the distribution of fluorescent cells in the neocortex (a coronal section) taken with a fluorescent microscope. B and C: immunostaining for SS and PV, respectively. Pictures were taken with a confocal microscope. All eGFP-expressing neurons colabeled with SS antibody but no eGFP-expressing neurons were stained with PV antibody. D: a representative current-clamp recording showing the typical firing pattern to suprathreshold current injection (300 pA) with frequency adaptation. E: an averaged trace showing short-term facilitation of EPSCs evoked by 5-pulse train stimulation (20 Hz). F: NMDAR-mediated EPSCs recorded at a holding potential of −35 mV. AMPA and GABAA components were blocked by NBQX (10 μM) and picrotoxin (50 μM), respectively. AP5 (50 μM) blocked the NMDAR-mediated EPSCs (arrow). eGFP, enhanced green fluorescent protein; SS, somatostatin; PV, parvalbumin; EPSC, excitatory postsynaptic current; NMDAR, N-methyl-d-aspartate receptor; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GABAA, γ-aminobutyric acid type A; NBQX, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione; AP5, d-2-amino-5-phosphonopentanoic acid.
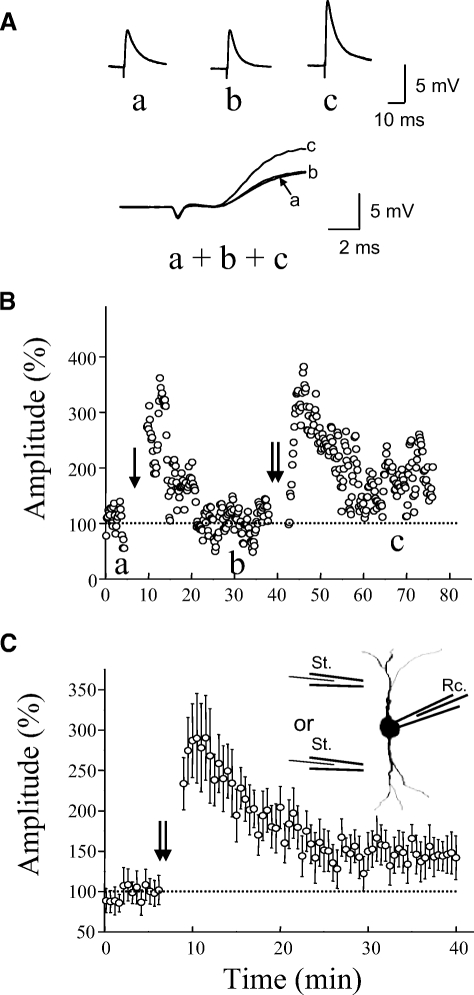
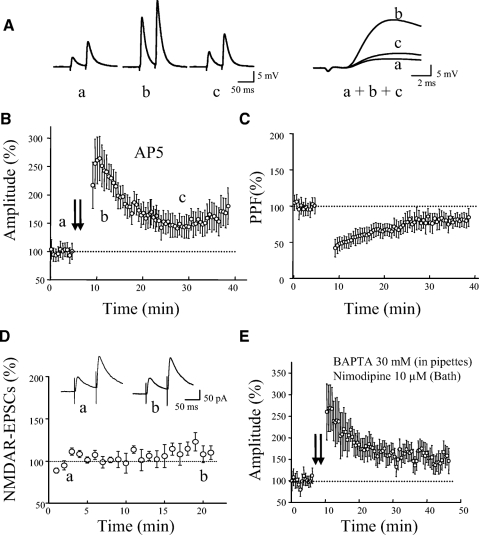
In initial attempts, we used a TBS containing two to three episodes, similar to the protocols that have been commonly used to induce LTP in pyramidal neurons of neocortex (Castro-Alamancos and Connors 1996). We found, however, that this protocol produced a short-term potentiation lasting <30 min in all eGFP interneurons tested ( n = 9). We also tried a tetanic stimulation of 100 Hz for 1 s given three times and found it failed to induce LTP in all tested cells (n = 6). We then tried more episodes and found that a TBS with 6 to 10 episodes could regularly induce LTP (Fig. 2, B and C, 144 ± 13% of control at 30 min after TBS, n = 9, P < 0.01). We found that LTP was not affected by blocking NMDARs with AP5 (50 μM). It was 151 ± 22% of control around 30 min after TBS in the presence of AP5 (Fig. 3, A and B, n = 12). LTP was associated with a reduction in PPF (Fig. 3C). PPF was 78 ± 10% of the baseline level at 30 min after TBS (n = 12, P < 0.05). Since LTP was not affected by AP5, the remaining experiments were all performed in the presence of AP5. The presence of AP5 may reduce the incidence of polysynaptic activity (Sutor and Hablitz 1989).
Fig. 2.
LTP induced in excitatory synapses on eGFP-expessing interneurons by TBS. A: the top traces (a, b, and c) were from a representative cell taken at times marked by letters in B. Bottom traces show superimposed responses with an expanded timescale. Each trace was an average of 10 consecutive responses. B: a representative cell showing STP and LTP of EPSPs induced by 4 episodes (single arrow) and 10 episodes (double arrows) of TBS, respectively. C: summary data showing LTP induced by TBS without blocking NMDARs (n = 9). Double arrows in C and the following figures indicate TBS of 6 to 10 episodes. Inset: a schematic diagram showing the arrangement of the recording electrode and stimulation electrodes. LTP, long-term potentiation; TBS, theta burst stimulation; STP, short-term plasticity; EPSP, excitatory postsynaptic potential.
Fig. 3.
LTP was independent of NMDARs and postsynaptic Ca2+ rise, but associated with a reduction in paired-pulse facilitation (PPF). A: EPSP traces induced by paired-pulse stimulation in current-clamp mode. The traces (a, b, and c) were taken from a representative experiment at times indicated by letters in B. Superimposed and expanded traces on the right (a, b, and c) demonstrate the initial portions of the first EPSPs. Each trace was an average of 10 consecutive responses. B: summary data showing LTP (n = 12) in the presence of AP5 (50 μM). C: PPF was reduced in association with LTP in B. D: NMDAR-mediated EPSCs recorded immediately after the establishment of whole cell recording. The cells were held at +50 mV in the presence of NBQX (10 μm) and picrotoxin (100 μm), which blocked AMPA and GABAA receptors, respectively. Inset: traces from an experiment taken at time indicated by letters. E: summary data showing LTP induced with recording pipette loaded with internal solution containing 30 mM BAPTA, a fast calcium chelator, and with a bath solution containing 10 μM nimodipine, an L-type calcium channel blocker (n = 7). AP5 (50 μM) was present in all experiments. BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid.
It has been reported in hippocampus that NMDAR-mediated currents run down quickly in conventional whole cell recording (Lamsa et al. 2005), which could explain the lack of NMDAR-dependent LTP in interneurons. To test whether the NMDAR component has a similar rundown feature in eGFP interneurons in cortex, NMDAR components were evoked at a holding potential of +50 mV with blockade of AMPA receptors with 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX, 10 μM) and GABAA receptors with picrotoxin (100 μM). The recordings of NMDAR components were started immediately after the establishment of whole cell recording. As shown in Fig. 3D, no significant reduction was noticed in the amplitude of NMDAR components during the first 20 min of the recording (n = 8, P = 0.9).
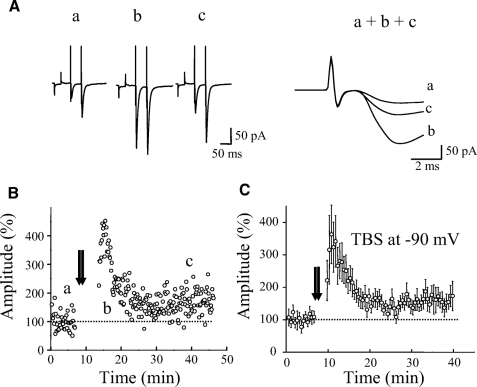
We next tested whether an elevation in postsynaptic Ca2+ is required for LTP. In these experiments, patch pipettes were filled with an internal solution containing 30 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), a fast Ca2+ chelator, and, at the same time, nimodipine (10 μM), an L-type voltage-dependent Ca2+ channel blocker, was added to the bath solution. TBS was given 30 to 60 min after recording started. Under these conditions (Fig. 3E), LTP was not affected (157 ± 24% at 30 min after TBS, n = 7, P < 0.001). A 30 mM concentration of BAPTA was reported to be sufficient to block Ca2+ rise either from external (NMDARs or mGluRs) or internal sources in previous studies (Alle et al. 2001; Sarihi et al. 2008; Yeckel et al. 1999). We also attempted to induce LTP while the membrane potential was held at −90 mV during TBS in a voltage-clamp mode. As shown in Fig. 4, LTP could still be induced (160 ± 24% at 30 min after TBS, n = 6, P < 0.01).
Fig. 4.
LTP did not require postsynaptic depolarization. A: EPSC traces induced by paired-pulse stimulation in voltage-clamp mode from a representative experiment in B. Single traces (a, b, and c, left column) were taken at time indicated by the letters in B. Right column shows superimposed and expanded initial portion of traces (a, b, and c). Each trace was an average of 10 consecutive responses. B: a representative experiment of LTP induced in voltage-clamp configuration at a holding potential of −90 mV during TBS. C: summary data showing that LTP was induced at −90 mV (n = 6).
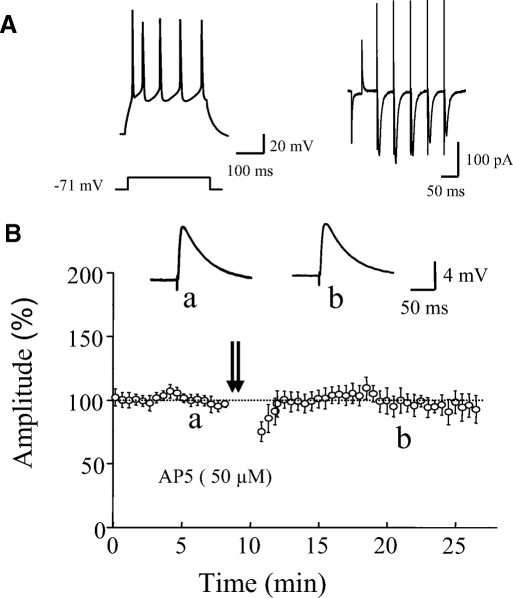
NMDAR-independent LTP has been reported in pyramidal cells of the neocortex (Aroniadou and Teyler 1992). So it is possible that LTP in our study might be caused by a passive propagation of LTP in nearby pyramidal cells, as has been reported in hippocampus (Maccaferri and McBain 1996). We tested whether the TBS protocol that we used in this study could induce NMDAR-independent LTP in layer II/IV pyramidal cells. As shown in Fig. 5, this TBS protocol did not induce LTP in pyramidal cells in the presence of AP5 in bath solution (97 ± 8% of control 15 min after TBS, n = 6, P = 0.62).
Fig. 5.
TBS did not induce NMDAR-independent LTP in pyramidal cells. A: the typical spiking pattern of a representative pyramidal cell to a current pulse (left) and short-term plasticity of EPSCs evoked by a 5-pulse train stimulation at 20 Hz (right). B: summary data showing that the same TBS protocol that induced LTP in eGFP-expressing interneurons did not induce LTP in pyramidal cells in the presence of AP5 (50 μM, n = 6). Insets: averaged traces of EPSP from a cell taken at time indicated by letters.
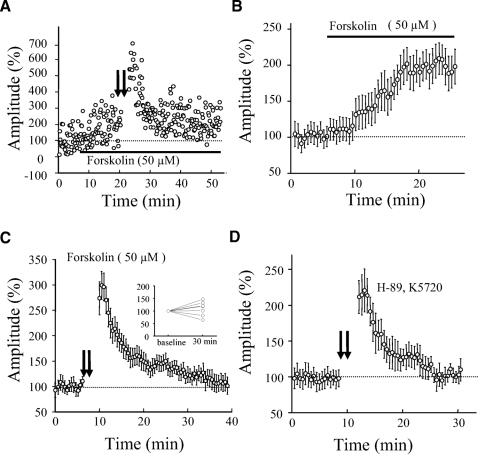
Presynaptic LTP has been shown in hippocampal mossy fiber synapses, cerebellar parallel fiber synapses, and corticothalamic synapses and, in these synapses, presynaptic cyclic adenosine monophosphate (cAMP) signaling pathway is required (Castro-Alamancos and Calcagnotto 1999; Linden and Ahn 1999; Salin et al. 1996; Weisskopf et al. 1994). We tried to determine whether LTP in SS interneurons might also be mediated by this signaling pathway. We first tested whether the application of forskolin (an activator of adenylate cyclase) was able to enhance synaptic transmission. Forskolin has been shown to enhance synaptic transmission by increasing presynaptic release probability (Chavez-Noriega and Stevens 1994). As illustrated in Fig. 6, A and B, 50 μM forskolin enhanced synaptic responses to 200 ± 23% of control and LTP could not be subsequently induced (107 ± 18%, 30 min after TBS, n = 7, P > 0.1, Fig. 6, A and C). We then tested whether PKA activity was required for LTP induction. We incubated slices with H-89 (10 μM) alone or a combination of H-89 (5 μM) and K5720 (3 μM), two PKA inhibitors, and LTP was attempted after ≥2 h of incubation. As illustrated in Fig. 6D, we were not able to induce LTP (97 ± 13% of control 20 min after TBS, n = 7, P = 0.2). This result indicated that PKA activity may be required for LTP induction in eGFP interneurons.
Fig. 6.
Induction of LTP by TBS in eGFP-expressing neurons required protein kinase A (PKA) activity. A: an experiment showing that bath application of the adenylate cyclase activator forskolin (50 μM) enhanced synaptic transmission, which occluded subsequent induction of LTP by TBS. Bath application of forskolin is indicated by a bar. B: summary data showing the enhancement of synaptic transmission by bath application of forskolin (50 μM, n = 7). C: summary data showing that LTP was not induced after synaptic transmission was enhanced by forskolin (n = 7). Inset: showing the percentage change in amplitude 30 min after TBS for each cell. D: summary data showing that LTP was blocked by PKA inhibitor, H-89 (10 μM, n = 4), alone or a combination of H-89 (5 μM) with another PKA inhibitor, K5720 (3 μM, n = 3). Since no clear difference was seen between H-89 alone and H-89 + K5720, these data were pooled. Slices were incubated with PKA inhibitors at least for 2 h before recording. AP5 (50 μM) was present in all experiments.
DISCUSSION
In the present study, we have examined LTP of excitatory synapses on a subpopulation of SS-expressing interneurons in the necortex of the transgenic mice (Oliva Jr et al. 2000). The excitatory synapses on these interneurons have a low release probability and exhibit robust short-term facilitation. LTP could be induced in these synapses with a vigorous TBS protocol using a total of 600 to 1,000 stimuli. It was not affected by the NMDAR antagonist, AP5, or postsynaptic loading of 30 mM BAPTA. It was associated with a reduction in PPF. These results indicate that this form of LTP does not require NMDARs or postsynaptic Ca2+ rise and has a presynaptic locus. Finally, we found LTP was blocked by PKA inhibitors, implying the involvement of the PKA signaling pathway.
Previous studies have shown that mechanistically different forms of LTP exist in excitatory synapses on interneurons, depending on the specific subtype of interneuron and its location (Kullmann and Lamsa 2007). For instance, NMDAR-independent LTP has been reported in interneurons in stratum oriens of the hippocampal CA1 region. The induction of LTP in these interneurons required a postsynaptic Ca2+ rise through Ca2+-permeable AMPA receptors and LTP was expressed presynaptically (Lamsa et al. 2007; Oren et al. 2009; Perez et al. 2001). However, in interneurons located in stratum radiatum, LTP could not be induced with conventional whole cell recording with tetanic stimulation or paring protocols, but could be induced with the perforated-patch recording method, which prevents the cytoplasm from being diluted by the recording solution. This LTP was NMDAR dependent and was expressed postsynaptically (Lamsa et al. 2005, 2007). Different forms of LTP have also been reported in interneurons in neocortex. NMDAR-independent, presynaptic LTP was induced by TBS in FS interneurons, but not non-FS interneurons in mouse visual cortex (Sarihi et al. 2008). In rat somatosensory cortex, repetitive correlated pre- and postsynaptic spiking induced LTP in LTS interneurons, but LTD in FS interneurons. The induction of LTP required the activation of NMDARs, but expression had a presynaptic mechanism (Lu et al. 2007).
The present results may reveal a new form of LTP in cortical SS interneurons. The induction of LTP required a strong TSB stimulation protocol, was NMDAR independent, did not require a postsynaptic Ca2+ rise, and was expressed presynaptically. Several previous studies have shown that 30 mM BAPTA is sufficient to prevent the induction of LTP, which requires a postsynaptic Ca2+ rise either from external or internal sources (Alle et al. 2001; Sarihi et al. 2008; Yeckel et al. 1999). The induction of LTP in our study was not affected when TBS was given 30 to 60 min after the onset of recording with an internal solution containing 30 mM BAPTA. Although it is unlikely, we cannot completely rule out the possibility that residual Ca2+ was present in concentrations sufficient to induce this form of LTP. Previous studies showed that a Ca2+ influx induced by synaptic responses occurs mainly through NMDARs in cortical SS interneurons (Goldberg et al. 2003; Kaiser et al. 2004). Our results also show synaptically induced NMDAR-mediated EPSCs in SS interneurons. However, it seems that Ca2+ influx through the opening of NMDARs by TSB did not trigger the biochemical cascades required for NMDAR-dependent LTP. This may be due to the fact that interneurons lack a key molecule—Ca2+/calmodulin-dependent protein kinase II—that is required for induction of NMDAR-dependent LTP (Chen et al. 2001; Malinow et al. 1989; Otmakhov et al. 1997; Sik et al. 1998). It has been reported that NMDAR-mediated components of synaptic responses in hippocampal interneurons decay rapidly during conventional whole cell recordings, which may eliminate or dampen postsynaptic Ca2+ influx (Lamsa et al. 2005), although this was not the case in our study. We examined the NMDAR component of EPSCs in cortical SS interneurons with conventional whole cell recording and found it did not show a reduction during at least the first 20 min of recording. Previous work demonstrated that pairing presynaptic and postsynaptic spiking induced NMDAR-dependent LTP in excitatory synapses in LTS interneurons in rat somatosensory cortex (Lu et al. 2007). LTS interneurons show similar intrinsic and synaptic properties with SS interneurons and they have been reported to express SS (Beierlein et al. 2003; Kubota and Kawaguchi 2000). The discrepancy between our results and this study may be due to species differences, the effect of different protocols for inducing LTP, or it is possible that the SS interneurons that express eGFP in this transgenic mouse are not LTS cells.
TBS is commonly used to induce LTP in cortex. It mimics natural bursts of spikes in cortex and hippocampus that are believed to play an important role in synaptic plasticity and information processing (Degenetais et al. 2002; Lisman 1997). Compared with cortical pyramidal cells, more episodes of TBS were required to induce LTP in cortical SS interneurons. In cortical pyramidal cells, LTP was induced by TBS with a total of 80 to 300 pulses (Castro-Alamancos and Connors 1996; Kirkwood and Bear 1994). However, LTP in cortical SS interneurons requires a stronger TBS protocol, with a total of 600–1,000 pulses. The reason for this difference is not known, although it could be due to distinct properties of excitatory synapses on interneurons as opposed to pyramidal cells. We presume that this form of LTP may occur naturally when excitatory neurons are extensively activated or in a state of hyperexcitability, such as during a seizure. A previous study in mouse visual cortex reported that LTP was noticed in FS interneurons, but not in regular-spiking interneurons that may express SS (Sarihi et al. 2008). The reason for not finding LTP in SS interneurons in their study may be because the TBS protocol that they used was not vigorous enough to induce LTP. Our results also indicated that the magnitude of LTP varied significantly among cells, which could be due to many factors. A recent study showed that eGFP cells in transgenic mice could be further divided into four subgroups according to their intrinsic properties and EPSC dynamics (Halabisky et al. 2006). It is possible that synapses in different subgroups may display different types of long-term plasticity. Localization of a cell and age of the animals may also be contributing factors. It is not clear whether these differences could explain the discrepancies between our results and those of previous works in the neocortex (Lu et al. 2007; Sarihi et al. 2006). Further systematic studies are required to answer these questions. Our results support the notion that different stimulation paradigms may be required to induce long-term synaptic changes in characteristically different synapses. This concept has also been supported by previous studies in hippocampus. For instance, tetanic stimulation in CA1 and CA3 induced LTP in pyramidal cells, but interneurons have shown either LTP, LTD, or no change (Laezza et al. 1999; Lamsa et al. 2007; Maccaferri and McBain 1996; Maccaferri et al. 1998; McMahon and Kauer 1997). Christie et al. (2000) reported that 200 Hz, but not 100 Hz, tetanic stimulation produced LTP in interneurons located in the stratum radiatum of CA1. Given the huge diversity of synapses on different subtypes of interneurons, it is not surprising that diverse findings have been derived from different studies.
Could LTP in neocortical SS interneurons have resulted from a passive propagation of LTP in principal cells? This form of LTP has been reported in hippocampal interneurons (Maccaferri and McBain 1996). We think this is not the case in the current study. First, we carefully identified monosynaptic responses by adjusting stimulation position and intensity. Second, LTP in cortical pyramidal cells is largely NMDAR dependent (Castro-Alamancos and Connors 1996; Kirkwood and Bear 1994); however, LTP in cortical SS interneurons in our study was NMDAR independent. Third, we found that prolonged TBS that induced LTP in SS interneurons did not induce LTP in pyramidal cells in the presence of AP5; i.e., no NMDAR-independent LTP was seen.
LTP in neocortical SS interneurons seems to share many common features with presynaptic LTP in area CA3 of the hippocampus, in cerebellar parallel fiber synapses, and corticothalamic synapses (Castro-Alamancos and Calcagnotto 1999; Linden and Ahn 1999; Salin et al. 1996; Zalutsky and Nicoll 1990). LTP in these areas does not require postsynaptic Ca2+ elevation, but it is dependent on the presynaptic cAMP–PKA signaling pathway (Mellor and Nicoll 2001; Weisskopf et al. 1994; but see Yeckel et al. 1999). It has been reported that cerebellar granule cells, hippocampal dentate granule cells, and neocortical pyramidal cells—which give rise to mossy fibers, parallel fibers, and corticothalamic fibers, respectively—contain abundant mRNA for adenylyl cyclases (Xia et al. 1991). Therefore one might predict that the presynaptic terminals from pyramidal cells on SS interneurons may also contain high levels of adenylyl cyclases; however, this information is currently not known. The induction of mossy fiber LTP requires extracellular calcium, indicating that calcium influx into the presynaptic terminal during tetanic stimulation is essential (Mellor and Nicoll 2001). It remains to be determined whether the same conditions apply to LTP of neocortical SS interneurons. The downstream pathway of PKA activation involved in SS interneuron LTP also remains to be determined.
In contrast to pyramidal cells that are relatively stereotypic, cortical interneurons are very heterogeneous, even though their number is about one fourth that of excitatory neurons (Markram et al. 2004). The properties of excitatory synapses on each type are strikingly different, indicating that they may play different roles in neuronal networks (Beierlein et al. 2003; Koester and Johnston 2005; Reyes et al. 1998). The unique property of robust short-term facilitation endows SS interneurons with a great ability to sense repetitive impulses from afferent fibers. Presumably, the occurrence of LTP in these interneurons would enhance this ability and provide more powerful and prompt inhibition to pyramidal cells in response to prior periods of increased excitatory activity.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-35651 to S. N. Roper, the Partnership for Pediatric Epilepsy Research, and the Densch Foundation.
REFERENCES
- Alle H, Jonas P, Geiger JR. PTP and LTP at a hippocampal mossy fiber–interneuron synapse. Proc Natl Acad Sci USA 98: 14708–14713, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou VA, Teyler TJ. Induction of NMDA receptor-independent long-term potentiation (LTP) in visual cortex of adult rats. Brain Res 584: 169–173, 1992 [DOI] [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE. Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala. J Neurosci 24: 9507–9512, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci USA 93: 13453–13459, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90: 2987–3000, 2003 [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Geisler C, Henze DA, Wang XJ. Interneuron diversity series: circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci 27: 186–193, 2004 [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Calcagnotto ME. Pre-synaptic long-term potentiation in corticothalamic synapses. J Neurosci 19: 9090–9097, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Short-term synaptic enhancement and long-term potentiation in neocortex. Proc Natl Acad Sci USA 93: 1335–1339, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci 17: 3894–3906, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Stevens CF. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J Neurosci 14: 310–317, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HX, Otmakhov N, Strack S, Colbran RJ, Lisman JE. Is persistent activity of calcium/calmodulin-dependent kinase required for the maintenance of LTP? J Neurophysiol 85: 1368–1376, 2001 [DOI] [PubMed] [Google Scholar]
- Christie BR, Franks KM, Seamans JK, Saga K, Sejnowski TJ. Synaptic plasticity in morphologically identified CA1 stratum radiatum interneurons and giant projection cells. Hippocampus 10: 673–683, 2000 [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci 4: 52–62, 2001 [DOI] [PubMed] [Google Scholar]
- Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Electrophysiological properties of pyramidal neurons in the rat prefrontal cortex: an in vivo intracellular recording study. Cereb Cortex 12: 1–16, 2002 [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E, Svoboda K, Huang ZJ. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci 7: 1184–1186, 2004 [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R, Tamas G. Ca2+ imaging of mouse neocortical interneurone dendrites: contribution of Ca2+-permeable AMPA and NMDA receptors to subthreshold Ca2+dynamics. J Physiol 551: 67–78, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabisky B, Shen F, Huguenard JR, Prince DA. Electrophysiological classification of somatostatin-positive interneurons in mouse sensorimotor cortex. J Neurophysiol 96: 834–845, 2006 [DOI] [PubMed] [Google Scholar]
- Kaiser KM, Lübke J, Zilberter Y, Sakmann B. Postsynaptic calcium influx at single synaptic contacts between pyramidal neurons and bitufted interneurons in layer 2/3 of rat neocortex is enhanced by backpropagating action potentials. J Neurosci 24: 1319–1329, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol 70: 387–396, 1993 [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci 14: 1634–1645, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science 308: 863–866, 2005 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci 20: 375–386, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci 8: 687–699, 2007 [DOI] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: joint requirement for pre- and post-synaptic events. Science 285: 1411–1414, 1999 [DOI] [PubMed] [Google Scholar]
- Lamsa K, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci 8: 916–924, 2005 [DOI] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science 315: 1262–1266, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ, Ahn S. Activation of pre-synaptic cAMP-dependent protein kinase is required for induction of cerebellar long-term potentiation. J Neurosci 19: 10221–10227, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci 20: 38–43, 1997 [DOI] [PubMed] [Google Scholar]
- Lu JT, Li CY, Zhao JP, Poo MM, Zhang XH. Spike-timing-dependent plasticity of neocortical excitatory synapses on inhibitory interneurons depends on target cell type. J Neurosci 27: 9711–9720, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci 16: 5334–5343, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Toth K, McBain CJ. Target-specific expression of pre-synaptic mossy fiber plasticity. Science 279: 1368–1370, 1998 [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature 394: 683–687, 1998 [DOI] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of post-synaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245: 862–866, 1989 [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004 [DOI] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci USA 95: 5323–5328, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron 18: 295–305, 1997 [DOI] [PubMed] [Google Scholar]
- Mellor J, Nicoll RA. Hippocampal mossy fiber LTP is independent of post-synaptic calcium. Nat Neurosci 4: 125–126, 2001 [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16: 815–823, 1996 [DOI] [PubMed] [Google Scholar]
- Mott DD, Dingledine R. Interneuron diversity series: interneuron research—challenges and strategies. Trends Neurosci 26: 484–488, 2003 [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20: 3354–3368, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva AA, Jr, Lam TT, Swann JW. Distally directed dendrotoxicity induced by kainic acid in hippocampal interneurons of green fluorescent protein-expressing transgenic mice. J Neurosci 22: 8052–8062, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren I, Nissen W, Kullmann DM, Somogyi P, Lamsa KP. Role of ionotropic glutamate receptors in long-term potentiation in rat hippocampal CA1 oriens-lacunosum moleculare interneurons. J Neurosci 29: 939–950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N, Griffith LC, Lisman JE. Post-synaptic inhibitors of calcium/calmodulin-dependent protein kinase type II block induction but not maintenance of pairing-induced long-term potentiation. J Neurosci 17: 5357–5365, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron 46: 89–102, 2005 [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille JC. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci USA 98: 9401–9406, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci 1: 279–285, 1998 [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science 290: 533–536, 2000 [DOI] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a pre-synaptic form of LTP at cerebellar parallel fiber synapses. Neuron 16: 797–803, 1996 [DOI] [PubMed] [Google Scholar]
- Sarihi A, Jiang B, Komaki A, Sohya K, Yanagawa Y, Tsumoto T. Metabotropic glutamate receptor type 5-dependent long-term potentiation of excitatory synapses on fast-spiking GABAergic neurons in mouse visual cortex. J Neurosci 28: 1224–1235, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Hajos N, Gulacsi A, Mody I, Freund TF. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc Natl Acad Sci USA 95: 3245–3250, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science 235: 73–76, 1987 [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562: 9–26, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor B, Hablitz JJ. Long-term potentiation in frontal cortex: role of NMDA-modulated polysynaptic excitatory pathways. Neurosci Lett 97: 111–117, 1989 [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science 265: 1878–1882, 1994 [DOI] [PubMed] [Google Scholar]
- Xia ZG, Refsdal CD, Merchant KM, Dorsa DM, Storm DR. Distribution of mRNA for the calmodulin-sensitive adenylate cyclase in rat brain: expression in areas associated with learning and memory. Neuron 6: 431–443, 1991 [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neuronos use a common post-synaptic mechanism. Nat Neurosci 2: 625–633, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science 248: 1619–1624, 1990 [DOI] [PubMed] [Google Scholar]