Abstract
Network outputs elicited by a specific stimulus may differ radically depending on the momentary network state. One class of networks states—experience-dependent states—is known to operate in numerous networks, yet the fundamental question concerning the relative role that inputs and states play in determining the network outputs remains to be investigated in a behaviorally relevant manner. Because previous work indicated that in the isolated nervous system the motor outputs of the Aplysia feeding network are affected by experience-dependent states, we sought to establish the behavioral relevance of these outputs. We analyzed the phasing of firing of radula opening motoneurons (B44 and B48) relative to other previously characterized motoneurons. We found that the overall pattern of motoneuronal firing corresponds to the phasing of movements during feeding behavior, thus indicating a behavioral relevance of network outputs. Previous studies suggested that network inputs act to trigger a response rather than to shape its characteristics, with the latter function being fulfilled by network states. We show this is an oversimplification. In a rested state, different inputs elicited distinct responses, indicating that inputs not only trigger but also shape the responses. However, depending on the combination of inputs and states, responses were either dramatically altered by the network state or were indistinguishable from those observed in the rested state. We suggest that the relative contributions of inputs and states are dynamically regulated and, rather than being fixed, depend on the specifics of states and inputs.
INTRODUCTION
A multitasking motor network can generate differing outputs depending on which triggering stimuli or contextual stimuli impinge on the network (Esch et al. 2002; Friesen and Kristan 2007; Heinrich et al. 2001; Marder and Bucher 2007; Nolan and Hoy 1984; Salinas 2004; Susswein et al. 1978). When different inputs elicit different outputs there is an appearance of responses being controlled by stimuli that impinge on the network, although a network can generate different responses not only when it is activated by distinct stimuli. The same stimulus can elicit different network outputs depending on its recent experiences (Abraham and Willows 1971; De Nunzio et al. 2009; Friedman 2000; Gold and Shadlen 2000; Meiran and Marciano 2002; Nadim et al. 2008; Peña and Ramirez 2005; Proekt et al. 2004). In essence, recent experiences modify the state of the network and network states become an important factor in determining the characteristic network outputs.
Given that network states can assume an important role in shaping network outputs the question arises as to what is the role(s) of inputs versus states in determining network outputs. Here, to characterize contributions that inputs and states make to motor outputs we studied the feeding network of Aplysia in which different inputs can be used to trigger motor programs and the network states can be experimentally manipulated (Proekt and Weiss 2003; Proekt et al. 2004). Previous studies of state dependence (Proekt et al. 2004, 2007, 2008) suggested that network inputs may act solely as triggers that release state-determined sequences of motoneuronal firing. Our data suggest that is an oversimplification.
Aplysia generate a broad range of feeding-related behaviors that vary in their degree of ingestiveness versus egestiveness (Church and Lloyd 1994; Morton and Chiel 1993; Susswein et al. 1976, 1978; Weiss et al. 1982; Zhurov et al. 2005). The spectrum of motor programs that has been recorded in the isolated nervous system is believed to correspond to the spectrum of feeding behaviors (Lechner et al. 2000; Morgan et al. 2002; Morton and Chiel 1993; Nargeot et al. 1997). However, the work on state dependence of motor programs was limited. It classified motor programs on the basis of the relative activity phasing of radula closing motoneuron B8 and a series of protracting and retracting motoneurons, but did not include the set of radula opening motoneurons that have to be appropriately phased to produce functional motor outputs (Kupfermann 1974; Morton and Chiel 1993; Susswein et al. 1976; Weiss et al. 1986). The radula, the feeding apparatus, grasps seaweed by closing around it when the radula is fully protracted; the grasped seaweed is then pulled into the buccal cavity as the radula retracts. The radula then opens inside the mouth to release the food and protracts open for another bite. During egestion the radula does the opposite: it closes around an inedible object and protracts and opens to release what it has removed and then retracts into the buccal cavity open to repeat the process. Here, we characterized the firing of radula opening motoneurons B48 and B44 (Church and Lloyd 1994; Evans and Cropper 1998; Evans et al. 1996) in different states and found that state-dependent differences in the patterns of firing of these motoneurons are likely to be functionally meaningful and that the frequencies of firing of the motoneurons are likely to have behavioral manifestations. We found that, depending on the specific combinations of stimulated inputs and states, motor responses can be shaped exclusively by the network state, exclusively by the input, or by a combination of inputs and states. Remarkably, we also found that the degree to which inputs codetermine the outputs is state dependent.
METHODS
Experiments were performed on Aplysia californica obtained from Marinus (Long Beach, CA). Aplysia were maintained in circulating artificial sea water (ASW) made from Instant Ocean (Aquarium Systems, Mentor, OH), at 14–15°C. Animals weighing 150–250 g were anesthetized by injection of approximately half their weight of isotonic MgCl2 (337 mM).
Conventional intracellular and extracellular recording techniques were used. Intracellular electrodes were filled with an electrolyte, containing 2 M K-acetate and 100 mM KCl, and beveled to the resistance of 6–10 MΩ. Intracellular recordings were performed using either the Axoclamp 2B amplifier (Molecular Devices, Foster City, CA) or a Getting 5A amplifier (Getting Instruments, Iowa City, IA). Extracellular recordings were performed by applying suction electrodes constructed from polyethylene tubing on buccal nerves. Signals were amplified by a CyberAmp 380 (Molecular Devices) and digitized using a Digidata 1200 (Molecular Devices) data acquisition system.
The cerebral ganglion, buccal ganglia, and the attached buccal mass were dissected out with the cerebral–buccal connectives and buccal nerves intact since they contain the nerves of B48 and B44. The buccal mass was separated from the distal digestive system at the buccal–esophageal junction and a portion of the buccal musculature was cut away to reveal the radula. The cerebral and buccal ganglia were pinned and desheathed in a Sylgard dish, with the buccal mass pinned dorsal side down, exposing the radula. The dish was perfused with ASW (in mM: 460 NaCl, 10 KCl, 55 MgCl2, 11 CaCl2, and 10 HEPES buffer, pH 7.6) for 1 h. Motoneurons B48 and B44 were identified by the distinct radula opening movements produced during direct intracellular stimulation and the locations described previously (Church and Lloyd 1994; Evans and Cropper 1998; Evans et al. 1996, 1999; Horn et al. 2004; Jing and Weiss 2001; Orekhova et al. 2001; Zhurov et al. 2005).
After physiological identification of B48 and B44, the cerebral and buccal ganglia were then transferred to a recording chamber with a volume of about 1.5 ml of ASW. The preparations were continuously perfused with ASW at 0.3 ml/min and maintained at 14–17°C for 1 h before beginning experiments. Previously identified neurons cerebral–buccal interneuron 2 (CBI-2) and B8 were identified based on location, size, and electrophysiological and morphological characteristics (Church and Lloyd 1994; Rosen et al. 1991).
We elicited motor programs using two different types of inputs. They were elicited via stimulation of a command-like neuron, CBI-2, and through stimulation of the esophageal nerve (EN) (Chiel et al. 1988; Morton and Chiel 1993). Both of these inputs are important in feeding behavior and are capable of eliciting reliable motor programs in semiintact preparations (Due et al. 2004; Evans et al. 2003; Horn et al. 2004; Jing and Weiss 2002, 2005; Koh and Weiss 2007; Morgan et al. 2002; Proekt et al. 2004; Rosen et al. 1991; Sasaki et al. 2008; Zhurov et al. 2005). CBI-2 was stimulated at 8–9 Hz so that each current pulse was set to trigger a single action potential. CBI-2 stimulation was manually terminated after the end of the protraction phase, as determined by the termination of activity of the I2 nerve (I2N), which contains the nerve projections of known protraction phase neurons (Hurwitz et al. 1996, 2000). Two different CBI-2 stimulation paradigms were used. CBI-2 programs were elicited using either a short interstimulus interval (S-ISI) of 30 s, in which CBI-2 was stimulated to elicit a program 30 s after the end of retraction of the previous program, or a long interstimulus interval (L-ISI) in which we waited 3 min after the end of the previous program's retraction phase to elicit the next program.
To elicit programs via EN stimulation the EN was stimulated continuously using 3-ms pulses at 2 Hz for 2 min. The pulse amplitude was adjusted to elicit five successive cycles of motor programs. An S88 stimulator (Grass Medical Instruments, West Warwick, RI) was used to generate current pulses that were injected into the extracellular polyethylene suction electrode into which the esophageal nerve had been aspirated. Prior to all experiments, pretest stimulations of EN and CBI-2 programs were elicited to ensure that motor programs were generated. After a 15-min rest period experiments were initiated.
Classification of feeding motor programs in Aplysia
The Aplysia central pattern generator (CPG) can generate a variety of different types of feeding motor programs, although all programs share a similar pattern in which protraction is followed by retraction (Fig. 1). The feeding programs can be classified as ingestive, egestive, or intermediate (Fig. 1). The central difference among the three classes of behavior is the relative timing of the radula opening/closing versus the fixed activity of radula protraction/retraction. In ingestive behaviors, the radula opens during protraction and closes during retraction, whereas in egestive behaviors the radula closes during protraction and opens during retraction. Intermediate programs were defined by incomplete closure during either protraction or retraction.
Fig. 1.
Classification of feeding motor programs and B8 activity in different types of programs. A: examples of 3 types of motor programs: ingestive, egestive, and intermediate. Protraction (filled bar) was monitored by activity in the I2 nerve. Retraction (open bar) was monitored by activity in buccal nerve 2 (BN2) after protraction. In the ingestive program, the radula closer motoneuron B8 fired predominantly during the retraction phase; in the egestive program, B8 fired vigorously during the protraction phase; in the intermediate program, B8 was active during both protraction and retraction. B: plot of B8 activity during protraction vs. B8 activity during retraction illustrates cluster boundaries for ingestive and egestive motor programs. Intermediate programs are motor programs that are between clusters for ingestive and egestive programs. With repeated stimulation of CBI-2 or EN, B8 firing frequency increases in either the retraction phase or the protraction phase and motor programs migrate toward the ingestive or egestive cluster, respectively.
To classify programs we used the standard definitions of protraction and retraction phases (Nargeot et al. 1997; Sasaki et al. 2007). The protraction phase was determined by extracellular recordings of the I2 nerve. Since only protraction phase motor neurons send their axons to the I2 nerve, it was feasible to monitor the length of protraction with this nerve recording (Dembrow et al. 2004; Hurwitz et al. 1996; Jing and Weiss 2001, 2002). The retraction phase was monitored by the presence of large unit activity in buccal nerve 2 (BN2) as well as with intracellular recordings of B48, which has a prominent hyperpolarization during retraction (Morton and Chiel 1993; Nargeot et al. 1999a,b; Wu et al. 2007). The postretraction phase begins at the end of retraction. The activity of specific neurons defines its length (Borovikov et al. 2000; Cropper et al. 2004; Evans et al. 1996; Serrano et al. 2007). For the purpose of data analysis, activity of individual neurons was expressed in terms of the average firing frequency within a given phase of the program. This procedure smooths over individual variability between preparations. The method, however, is sufficiently sensitive to distinguish between different states. Also, these average frequencies were used in stimulation experiments and therefore movements were also expressed as averages.
Radula closure was monitored by intracellular recordings of motor neuron B8. Programs were classified as ingestive if B8 firing activity occurred predominantly during retraction, whereas programs were classified as egestive if B8 firing activity occurred predominantly during protraction (Jing and Weiss 2001, 2002; Morgan et al. 2002; Proekt et al. 2004, 2007). The program was considered intermediate when B8 firing was similar during protraction and retraction and therefore could not produce either ingestion or egestion. Here we used a plot of B8 retraction versus B8 protraction phase activity along with the previous classification scheme to operationally assign the degree of ingestiveness or egestiveness (Morgan et al. 2002; Proekt et al. 2004, 2007) (Fig. 1B). The approximate boundaries of the ingestive and egestive clusters were shown as defined previously by the phasing of activity of B8. The intermediate programs were those that were outside the boundaries that define the ingestive and egestive programs. With repeated stimulation of CBI-2, programs migrate from intermediate (Fig. 1B, star) toward ingestive, as manifested by the increase in B8 firing frequency during retraction. In contrast, with repeated stimulation of EN, the programs migrate from intermediate toward the egestive cluster, as manifested by the increase in B8 firing frequency during protraction. For the purpose of data analysis we used average spike frequencies over the entire phase.
Characterization of radula movement
The buccal mass preparation was similar to that previously used by Evans and Cropper (1998) and Orekhova et al. (2001). The entire buccal mass was removed from the animal, together with the buccal ganglia, and anchored dorsal-side up in a Sylgard-lined dish by pinning the lip tissue. The esophageal nerves were also cut to allow mobility of the buccal ganglia, but other buccal nerves and the radula nerve remained intact. To gain access to the radula and for visualization of the entire radula surface, a part of the esophagus was removed from the area where it emerges from the buccal mass and its caudal surface was pinned to the Sylgard-lined platform. A suture from black silk Ethicon 6-0 was made as a mark on the edge of each half of the radula. The buccal ganglia were pinned to a Sylgard-lined platform and desheathed on the caudal surface for B44 or B48 experiments and on the rostral surface for B8 experiments. Before experimentation the preparation was perfused continuously with ASW at 0.3 ml/min and cooled to 14.5–16°C. Additionally, the buccal mass was perfused with ASW through the abdominal aorta. Movements were elicited by electrically stimulating both B44 simultaneously in 7-s blocks at 1, 2, 3, 4, 5, 6, 7, 8, and 15 Hz, separated by 1-min rest periods (for B44 experiments). For B48, movements were elicited by electrically stimulating both B48 motoneurons simultaneously in 15-s blocks at 2, 3, 4, 5, 6, 7, 8, and 15 Hz, separated by 1-min rest periods. B8 movements were elicited by electrically stimulating the four bilateral B8s simultaneously in 15-s blocks at 2, 4, 6, 8, 10, 12, and 20 Hz, separated by 1-min rest periods. Individual current pulses were typically modified to elicit a series of action potentials, 15–25 ms in duration. Movements of the radula were recorded using a digital video camcorder (Canon ZR45). Relevant parts of the video were captured using VirtualDub (GNU, Cambridge, MA) and data were analyzed using Screen Calipers (Iconico, New York, NY).
Statistics
Digitized electrophysiological recordings were plotted using CorelDRAW v. 13 (Ottawa, Ontario, Canada) and data were expressed as means ± SE. Each “n” represents an individual preparation. Two-group statistical comparisons were performed using t-test statistics. Data with more than two groups were first analyzed using appropriate forms of ANOVA. Subsequent comparisons were performed using Bonferroni's multiple-comparison tests. The significance level was set at P < 0.05 (***P < 0.001; **P < 0.01; *P < 0.05; n.s., P > 0.05). All statistical tests were performed using SigmaPlot (Systat Software, San Jose, CA).
RESULTS
In the isolated nervous system of Aplysia frequent repetitive stimulation of network inputs produces progressively better articulated fictive motor programs. This phenomenon, which we refer to as “buildup,” is a specific case of a broader class of phenomena referred to as repetition priming. In a rested preparation (defined operationally as 15 min of no stimulation) the first stimulation produces a poorly articulated program that is classified as an intermediate type program (Fig. 1) (Proekt et al. 2004; Zhurov et al. 2005). However, following repetitive CBI-2 prestimulation, stimulation of CBI-2, an integrator of mechanosensory and chemosensory inputs from the mouth (Rosen et al. 1991), elicits ingestive programs (Fig. 1). In contrast, following repetitive esophageal nerve (EN) prestimulation, stimulation of the EN, a mediator of mechanosensory information from the esophagus (Chiel et al. 1988), elicits egestive programs. Previous work indicated that buildup in Aplysia was a result of repetitive-stimulation–induced alterations of the state of the feeding network. Another state-dependent form of network plasticity that was observed as a result of repetitive stimulation of inputs was negative biasing that corresponds to a broader class of phenomena referred to as task switch cost (Monsell et al. 2003; Waszak et al. 2003; Wylie and Allport 2000), which consists of impaired articulation of responses elicited by stimulation of CBI-2 following a period of repetitive EN stimulation (Proekt et al. 2004, 2008). The existence of repetition priming and task switch costs suggested that CBI-2 and EN stimulations exert actions that operate on two different timescales. First, each input acts with fast dynamics to trigger a response. Second, each input acts to alter the network state, albeit in a different direction, with dynamics that develop on the timescale of minutes. These findings were consistent with the hypothesis that at any given moment CBI-2 and EN may release sequences of activity that reflect the momentary state of the network.
However, the ability to accept the behavioral relevance of the hypothesis described earlier was limited in that the characterization of the nature of specific motor programs was based on traditional criteria (Morgan et al. 2002; Morton and Chiel 1993)—i.e., it was based on the relationship between the firing patterns of motoneurons that implement the protraction–retraction and only the closing movements of the radula (Fig. 1A). Yet, in normal behavior radula opening plays a critical functional role. Specifically, in ingestion the radula opens during protraction and closes during retraction, whereas opposite phasing of radula opening/closing relative to protraction/retraction is observed in egestion. The two types of phasing ensure that during ingestion the radula can effectively bring food into the buccal cavity and that during egestion the radula can remove indigestible objects from the buccal cavity. Since both radula closing and opening activity must be appropriately phased with the protraction and retraction phases to produce functional programs, the radula motoneurons need to be included in such classification. It follows that when the phasing of activity of the radula closers changes with repetitive stimulation of inputs, the phasing of the radula opening motoneurons has to be appropriately adjusted to give rise to both repetition priming and task switch cost.
Here, we use as a point of departure the findings of previous studies on the radula opening motoneurons that showed that when different inputs were used to initiate motor programs that varied in their ingestiveness/egestiveness, either radula opener B44 or B48 fired preferentially (Church and Lloyd 1994; Evans and Cropper 1998; Evans et al. 1996). Although these studies did not quantify the degree of ingestiveness/egestiveness and could not distinguish between the contribution that states and inputs make to the outputs, the differences in B44 and B48 firing rates raised the possibility that motor outputs may reflect not only the state, but also the triggering input. To gain insights into this possibility we characterized activity patterns of B44 and B48 while experimentally manipulating the state of the network.
Motoneuronal activity in CBI-2– and EN-elicited programs
To characterize the firing patterns of radula openers B48 and B44 as ingestive and egestive motor programs (as defined by B8 activity patterns), we followed previously established procedures (Proekt et al. 2004; Sasaki et al. 2008; Wu et al. 2007), which showed that when CBI-2s or ENs are repeatedly elicited at short interprogram intervals, the network begins to generate stable, putatively ingestive or egestive programs, respectively. Based on previous studies that characterized the stability of CBI-2– and EN-elicited programs, we analyzed the tenth CBI-2– and the fifth EN-elicited programs. The ingestive versus egestive characteristics of motor programs were determined based on whether B8 fired at high frequency during retraction or protraction. Quantitative data derived from eight preparations (n = 8) in Fig. 2, A2 and B2 show that programs elicited by CBI-2 and EN were respectively ingestive and egestive.
Fig. 2.
Activity of radula openers in cerebral–buccal interneuron 2 (CBI-2) and esophageal nerve (EN)–elicited steady-state feeding motor programs. Programs consist of 2 fixed phases of activity, protraction, and retraction. Protraction (filled bar) was monitored by activity in the I2 nerve. Retraction (open bar) was monitored by activity in BN2 after protraction. Postretraction was monitored by activity in B48 (gray bar). A1: representative recording of a steady-state CBI-2–elicited program that, based on B8 activity, was classified as being ingestive. A2: grouped data show the firing frequencies of B48, B44, and B8 in each phase during an ingestive program. B1: representative recording of a steady-state EN-elicited program that, based on B8 activity, was classified as egestive. B2: grouped data show the firing frequencies of B48, B44, and B8 in each phase during an egestive program. Throughout the figures, all group data are shown as means ± SE and statistical significance is denoted (***P < 0.001; **P < 0.01; *P < 0.05; n.s., P > 0.05).
We observed a fundamental difference in the pattern of activity of B48 and B44 in CBI-2–elicited ingestive programs and EN-elicited egestive programs. These differences are illustrated in Fig. 2. Figure 2A1 illustrates results from a single preparation. Specifically, this figure shows that in CBI-2–elicited ingestive programs B48 fired at high frequency during protraction and was essentially silent during retraction. B48 also showed a burst of activity following the retraction phase (Church and Lloyd 1994). In contrast to B48, radula opener B44 was essentially silent in CBI-2–elicited ingestive programs. The differences between B48 and B44 firing during both the protraction and the postretraction phases were statistically significant (protraction: t = 18.93 P < 0.001, degree of freedom [df] = 7; postretraction: t = 12.80, P < 0.001, df = 7).
The pattern of B48 and B44 activity was radically different in EN-elicited egestive programs. Figure 2B1 illustrates results from a single experiment in which we monitored the firing of B48 and B44. Aggregate data are shown in Fig. 2B2. We found that in EN-elicited egestive programs B44 fired robustly during retraction but was silent during protraction, whereas B48 was essentially silent during both protraction and retraction but active during the postretraction period. The difference between B48 and B44 firing during retraction and postretraction was statistically significant (retraction: t = 15.88, P < 0.001; postretraction: t = 8.82, P < 0.001).
Taken together our data show that B48 and B44 are differentially recruited in CBI-2–elicited ingestive programs and EN-elicited egestive programs. Importantly, the phasing of the two neurons is distinct in the two types of programs. In CBI-2–elicited ingestive programs B48 is strongly active during the protraction phase, a phase in which radula closer motoneuron B8 shows low levels of activity. In contrast, in EN-elicited egestive programs, B44 shows a high level of activity during the retraction phase, a phase during which B8 shows low levels of activity. Thus activities of motoneurons that open and close the radula are out of phase with each other and, as such, are likely to promote out-of-phase radula opening and closing movements.
Experience-dependent B48 activity.
Studies of radula closer B8 indicated that repeated stimulation of CBI-2 and EN altered the state of the network and modified the firing of radula closer B8. In principle, there was no reason to assume B48 and/or B44 firing patterns are modified by recent experience and, even if they are modified, there was no unequivocal way to predict what such modification could engender. For instance, the two neurons could maintain a phase-constrained constant firing rate independent of recent history of stimulation and, as long as they fired out of phase with B8, the integrity of motor programs would be maintained. Alternatively, similar to B8, one or both radula openers could initially fire during both protraction and retraction and only with repeated stimulation assume a phase-delimited firing. We sought to determine what actually happens to B44 and B48 firing when CBI-2 or EN is repetitively stimulated.
We first characterized the progression of firing patterns of B48 in motor programs elicited by CBI-2 stimulation. At 15 min before the repetitive stimulation of CBI-2, we tested the ability of CBI-2 to elicit motor programs. We then repeatedly stimulated CBI-2 at 9 Hz throughout the protraction phase using a short interprogram interval of 30 s (S-ISI). Ten consecutive motor programs were elicited. Figure 3 A illustrates the first and the tenth programs that CBI-2 elicited in the same preparation. Consistent with previous observations (Proekt et al. 2004, 2007) we found that, as defined by B8 activity, the first program was intermediate as B8 fired at 1.8 Hz during retraction and at 0.6 Hz during protraction (see Fig. 1). The tenth program was ingestive as B8 fired at 1.2 Hz during protraction and at 5.8 Hz during retraction (see Fig. 1). Note that in the initial program B48 fired at a low rate during protraction and was silent during retraction. In contrast, in the tenth ingestive program B48 fired at a high rate in protraction and again was silent in retraction. In addition, in the initial program the delay between the onset of CBI-2 stimulation and the first B48 action potential was longer than that in the tenth programs. Finally, compared with the first program the postretraction phase firing of B48 was increased during the tenth CBI-2–elicited program.
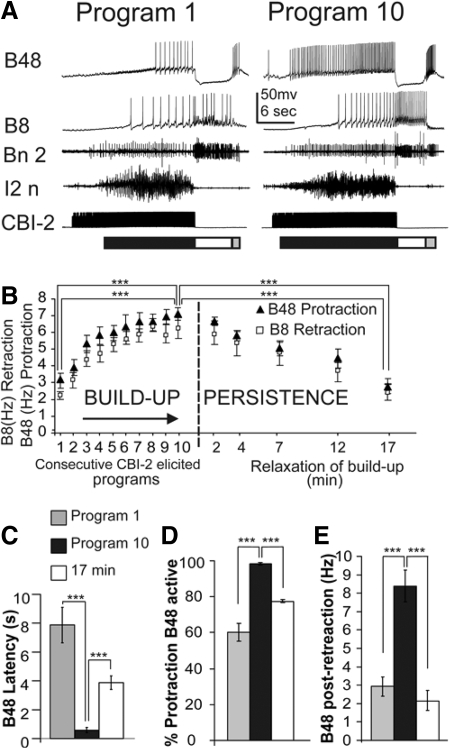
Fig. 3.
Activity of motoneurons B48 and B8 during repetitive CBI-2–stimulated motor programs. A: shown are the 1st and the 10th motor programs that were elicited by CBI-2 stimulation. CBI-2 was stimulated at 9 Hz for the duration of the protraction phase (18.6 ± 0.6 s). A 30-s rest was then implemented before the beginning of the next CBI-2 stimulation. Ten consecutive programs were elicited in this way and then a single program was elicited 2, 4, 7, 12, and 17 min later. B: group data of the effects of repeated CBI-2–elicited programs on B48 protraction firing frequency (black triangle) and B8 retraction firing frequency (open square). Repeated CBI-2 stimulation elicited progressively increasing B48 firing in protraction and B8 firing in retraction (buildup). CBI-2 programs elicited at 2, 4, 7, 12, and 17 min show a gradual decline in B48 firing during protraction and B8 firing during retraction (persistence). C: shift of the latency of B48's first action potential from the start of CBI-2 stimulation. Grouped data show that there is a significant difference between B48 latency in the 1st, 10th, and at the 17-min programs. D: grouped data show that there is a significant difference between the percentage of protraction phase with B48 activity in the 1st, 10th, and at 17-min programs. E: grouped data of B48 postretraction firing frequency show an increase from the 1st to the 10th motor programs and the return toward control values at 17 min.
Figure 3B illustrates the progression of B48 firing during protraction when ten CBI-2 programs were repeatedly elicited. As judged by the firing pattern of B8, these programs became progressively ingestive—i.e., the firing rate of B8 during retraction increased without a concomitant change of its firing rate during protraction. Importantly, increased ingestiveness of motor programs was accompanied by a gradual buildup of the firing frequency of B48 during protraction (Fig. 3B). To probe the persistence of the effects of repeated activation of motor programs following the tenth program, we increased the interprogram interval and elicited programs at 2, 4, 7, 12, and 17 min. B48 and B8 firing frequencies gradually relaxed toward the firing frequencies that were recorded during the first program. Therefore the effects of repeated stimulation on B48's firing frequency persisted beyond the stimulation of CBI-2.
To statistically analyze our data (n = 10) we performed a one-way repeated-measures ANOVA on the first program, the tenth program, and the program elicited 17 min after the tenth CBI-2–elicited program. B48 and B8 were analyzed separately. B8 was analyzed to verify that as a result of repeated stimulation programs became more ingestive, as defined by increased B8 firing frequency during retraction. As previously reported (Proekt et al. 2004, 2007) B8 activity during protraction did not significantly change as a result of repeated CBI-2 stimulation [ANOVA, F(2,18) = 0.529; NS]. However, we found significant overall differences in the firing frequencies of B8 during retraction and B48 during protraction [B8: F(2,18) = 23.97; P < 0.001; B48: F(2,18) = 107.8; P < 0.001; n = 10]. Individual comparisons (t-test with Bonferroni corrections) showed that B48 fired at significantly higher frequency in the tenth program compared with that in the first program (t = 14.07; P < 0.001, paired t-test) and compared with the program at 17 min (t = 10.66, P < 0.001, n = 10). During retraction B8 also fired at a significantly higher frequency in the tenth program compared with that in the first program (t = 6.16; P < 0.001, paired t-test) and the program at 17 min (t = 5.82, P < 0.001, n = 10).
Further analysis of B48 activity during protraction revealed that the latency from the onset of CBI-2 stimulation to the first action potential in B48 decreased from the first program to the tenth program (t = 8.3; P < 0.001) (Fig. 3C). Consistent with this observation we found that the percentage of protraction phase during which B48 is active also increases from the first to the tenth programs (t = 8.445; P < 0.001) (Fig. 3D). At 17 min, B48 latency and percentage of protraction phase during which B48 was active returned toward starting values (latency: tenth vs. 17-min program: t = 3.78, P < 0.01; percentage of protraction with B48 activity: tenth vs. 17-min program: t = 4.59, P < 0.01). Finally, we compared the firing frequency during the postretraction burst and found that the firing frequency of B48 was significantly increased in the tenth motor program (8.4 ± 0.53 Hz) compared with the first program (2.9 ± 0.52 Hz) and the 17-min test program (2.3 ± 0.61 Hz) (first vs. tenth: t = 8.75, P < 0.001; tenth vs. 17-min test: t = 8.04, P < 0.001) (Fig. 3E). Taken together, the data shown in Fig. 3 indicated that the output of the CPG, reflected in the activity of B48, depends on the history of the network.
We also examined the specificity of B48 firing in CBI-2– versus EN-elicited motor programs. Under steady-state conditions of EN stimulation B48 is essentially silent (Fig. 2) during protraction. We posit that this silence may be explained by the fact that, independently of recent history, EN stimulation does not activate B48. Alternatively, B48 could be activated by EN in preparations that had no recent EN stimulation, but this activation could decrement on repeated EN stimulation. To distinguish between these alternatives in rested preparations, we elicited a series of motor programs through EN stimulation. At 15 min prior to the repeated stimulation of EN we tested the ability of EN stimulation to elicit motor programs. Figure 4 A illustrates the first and fifth EN-elicited programs from a single preparation. Consistent with previous work, as defined by B8 activity, the first program was intermediate because B8 fired at a low frequency. The fifth program was egestive, given that B8 fired at a high frequency during protraction and not at all during retraction. In both the EN-elicited intermediate and egestive programs, B48 firing frequency during protraction was near zero. Although radula opener B48 was not active during protraction or retraction, B48 was active during the postretraction period of both intermediate and egestive programs.
Fig. 4.
Activity of motoneurons B48 and B8 during motor programs elicited by repetitive EN stimulation. A: shown are the 1st and the 5th motor programs that were elicited by EN stimulation. B: group data show a comparison of the 1st and the 5th programs of the effects of repeated EN stimulation on B48 protraction, B8 protraction, and B48 postretraction (rebound) activity. Repetitive EN stimulation elicited a progressive increase of B8 firing during protraction but no change in B48 firing.
Analyses of aggregate data (n = 5) from repeated EN-elicited programs (Fig. 4B) showed that B48 fired at a low frequency during protraction, irrespective of whether they were intermediate or egestive (first vs. fifth: t = 0.333; NS). Also, B48 firing frequency during postretraction did not change as the programs became more egestive (first vs. fifth: t = 1.43; NS). In comparison, B8 firing frequency during protraction was higher in egestive programs than that in intermediate ones (first vs. fifth: t = 3.325; P < 0.01). Importantly, although B48 firing frequency was low during EN-elicited programs, it did not change with repeated EN stimulation.
Experience-dependent B44 activity.
As discussed at the beginning of the preceding section for motoneuron B48, a variety of mechanisms could be responsible for selective firing of the second radula opening motoneuron, B44 (Fig. 2). B44 firing frequency might demonstrate a gradual decay during repeated CBI-2 programs or might be actively inhibited during all CBI-2 programs. It is also possible that B44 activity may not reflect recent experience and retain a constant firing frequency throughout repeated EN stimulation. We thus sought to characterize activity patterns of radula opener B44 in repeatedly elicited EN or CBI-2 programs. We elicited a series of five programs by stimulating EN. Figure 5 A illustrates the first program and fifth program from a single preparation. The first program was intermediate in terms of B8 activity because B8 fired at a low frequency. The fifth program was egestive because B8 fired at high frequency during protraction. In contrast, the firing frequency of B44 increased robustly during retraction from the first to the fifth programs.
Fig. 5.
Activity of motoneurons B44 and B8 during motor programs elicited by repetitive EN stimulation. A: shown are the 1st and the 5th motor programs that were elicited by EN stimulation. B: group data of the effects of repeated EN-elicited programs on B44 retraction frequency (black circle) and B8 protraction frequency (open square). Repetitive EN stimulation elicited progressively increasing B44 firing during retraction and B8 firing during protraction. Individual EN-elicited programs at 1, 3, and 6 min after repetitive stimulation shows the relaxation of buildup in which B44 firing during retraction and B8 firing during protraction returns to starting levels. C: grouped data show the latency of B44's first action potential from the start of retraction in the 1st (EN P-1), 5th (EN P-5), and 6-min EN-elicited programs. There is a significant decrease in B44 latency from the 1st to the 5th EN-elicited programs. D: grouped data show that there is a significant increase in the percentage of retraction phase with B44 activity when comparing 1st and 5th programs.
Figure 5B illustrates the progression of B8 and B44 firing. Aggregate data (n = 10) showed that, as defined by B8 firing pattern, over five programs of EN stimulation motor programs became progressively more egestive, thus reproducing the data of Proekt et al. (2004). Importantly, increased B8 activity-defined egestiveness was accompanied by a gradual buildup of the firing frequency of B44 during retraction. To probe the persistence of the effects of repeated activation of EN, following the fifth program, we elicited programs at 15 s and 1, 3, and 6 min. B44 and B8 firing frequencies relaxed toward the firing frequencies recorded in the first program.
To analyze aggregate data from repeated EN-elicited programs we performed a one-way repeated-measures ANOVA on the first program, fifth program, and the program elicited 6 min after the fifth EN-elicited program. B44 and B8 were analyzed separately. As previously reported, B8 activity during retraction did not change with repeated EN-elicited programs and statistical analysis showed that there was no difference between the first, fifth, or 6-min program [ANOVA, F(2,18) = 3.287; NS]. We found significant overall differences in the firing frequencies of B44 during retraction and B8 during protraction in the first, fifth, and 6-min programs [ANOVA, B44: F(2,18) = 23.761; P < 0.001; B8, F(2,18) = 34.612; P < 0.001]. Individual comparison (t-test with Bonferroni corrections) showed that B44 fired at a significantly higher frequency in the fifth program compared with that in both the first program (t = 5.661, P < 0.001) and the program elicited at 6 min (t = 6.237, P < 0.001). We also confirmed that, as defined by B8 firing patterns, the fifth program was fully egestive. Note that compared with the first program there was a significant increase in B8 firing during the fifth program (t = 8.192, P < 0.001). Thus as programs progress toward fully egestive behavior, B44 activity increased, suggesting that B44 firing reflects changes in the state of the network.
Further analyses of B44 activity during the retraction phase revealed overall differences in the latency from onset of the retraction phase to the first action potential in B44 between the first, fifth, and 6-min programs [ANOVA, F(2,18) = 34.381; P < 0.001]. Importantly, the latency significantly decreased from the first to the fifth programs (t = 6.23, P < 0.001) (Fig. 5C). Consistent with this observation we also found that there were differences in the percentage of retraction phase occupied by B44 activity between the three groups [ANOVA, F(2,18) = 46.22; P < 0.001] (Fig. 5D). There was a significant increase in the percentage of retraction phase occupied by B44 activity in the fifth program (first vs. fifth program: t = 9.321, P < 0.001). This indicated that there was not only an increase in the firing frequency but also an increase in the duration of time during which B44 was active. Together, Fig. 5 revealed that when EN was repeatedly stimulated and motor programs became more egestive (as defined by B8 firing) B44 firing frequency progressively increased during the retraction phase.
We also examined the specificity of B44 firing in EN- versus CBI-2–elicited motor programs. Under steady-state conditions of CBI-2 stimulation, B44 for all practical purposes remains silent (Fig. 2). This silence could be explained by the fact that, independent of recent history, CBI-2 stimulation does not activate B44. At least in principle, B44 could be activated by CBI-2 in preparations that had no recent history of CBI-2 stimulation, although this activation could decrement on repeated CBI-2 stimulation. To distinguish between these alternatives, in rested preparations, we elicited a series of motor programs through CBI-2 stimulation. We compared the firing frequencies of B44 in the first and the tenth motor programs (see Fig. 6 A for representative recordings from a single preparation and Fig. 6B for aggregate data). Figure 6A illustrates B44 and B8 activity in the first and tenth programs in a series of ten CBI-2–elicited programs. Consistent with previous work, B8 firing frequency during retraction increased from the first program to the tenth program, producing a fully ingestive program. Although the programs were progressively becoming more ingestive (as defined by B8 activity), in both the first and the tenth CBI-2–elicited programs, B44 showed a similar low level of activity during retraction.
Fig. 6.
B44 activity in motor programs elicited by repeated CBI-2 stimulation. A: shown are the 1st and the 10th programs elicited by repeated CBI-2 stimulation. B: grouped data show the firing frequencies of B8 and B44 in the 1st, 10th, and 12-min CBI-2–elicited programs. Repeated CBI-2 stimulation elicited progressively increasing B8 firing during retraction and no change in B44 activity. C: grouped data show that there is no significant difference in B44 latency in the 1st, 10th, and 12-min programs. D: grouped data show that there is no significant difference in the percentage of retraction phase occupied by B44 activity when comparing the 1st, 10th, and 12-min programs.
Aggregate data (n = 5) revealed that over the ten programs elicited by CBI-2 stimulation, motor programs became progressively more ingestive—i.e., the firing rate of B8 during retraction increased without a concomitant change of its firing rate during protraction (Fig. 6B). Importantly, increased ingestiveness of motor programs was not accompanied by a change in the firing frequency in B44. B44's firing frequency did not change significantly as programs became more ingestive or after a 12-min recovery [F(2,18) = 0.045; NS]. We also compared the delay from the onset of retraction to the first action potential in B44 and found no differences between the first program, tenth program, or 12 min after the tenth program (Fig. 6C) [F(2,18) = 0.141; NS]. Similarly, we found no change in the percentage of time during the retraction phase in which B44 was active (Fig. 6D) [F(2,18) = 1.484; NS]. Thus B44 activity was not affected when repeated CBI-2 stimulation altered B8 activity.
Bias of B48 and B44 activity.
The experiments presented earlier showed that repetitive stimulation of CBI-2 increased the firing of B48 during protraction and that EN stimulation increased B44 firing during retraction. Previous studies of radula closing motoneuron B8 suggested that its activity and phasing may reflect a momentary state of the feeding CPG (Proekt et al. 2004, 2007). In these studies fully ingestive CBI-2 programs were first established by repetitively stimulating ten consecutive CBI-2 programs using a 30-s S-ISI. Stimulation of CBI-2 was stopped for 5 min during which the EN was stimulated. At the end of 5 min, stimulation was switched back to CBI-2. Following repetitive EN stimulation, CBI-2 stimulation elicited egestive rather than ingestive motor programs. Since different inputs were used to modify the CPG state and to probe its state, these data suggested that an alteration of the state of the network rather than an alteration of network inputs was critical for changes in B8 firing. However, in these studies the effects of not firing CBI-2 during the period of EN stimulation were not separated from the effects of EN stimulation. To rectify this problem, in our study we compared CBI-2 programs elicited following a 5-min period of no stimulation to programs elicited by CBI-2 stimulation following a 5-min period of EN stimulation. Using this modified paradigm we further investigated the putatively state-dependent regulation of activity patterns of B8, B48, and B44.
Figure 7 A is a representative recording of B48 and B8 from a single preparation in which this experimental paradigm was used. First, a fully ingestive state in which B48 had a high firing frequency during protraction and B8 had a high firing frequency during retraction was established by repeated stimulation of CBI-2 (Fig. 7A). At 15 s following the end of retraction we initiated a stimulation of EN. The 15-s delay was used to avoid stimulation during the postretraction phase. At 15 s CBI-2–elicited programs remain maximally ingestive (Fig. 3B). This ensured that we were probing persistent rather than transient effects of network activation (Proekt et al. 2008). EN was stimulated for 5 min to produce a fully egestive program, in which B48 activity was absent and B8 fired robustly during protraction. For the same reason as described earlier, a CBI-2 program was elicited 15 s after the last EN program ended. Consistent with previous reports, when we switched the stimulation back to CBI-2, the program did not return to an ingestive program as defined by the B8 activity pattern. Specifically, B8 fired at high frequency during protraction instead of firing at high frequency during retraction. Importantly, despite receiving a CBI-2 input, B48 fired at negligibly low frequencies that were comparable to those with which it fired in egestive motor programs elicited by EN. Control experiments showed that the egestive character of CBI-2 programs elicited after EN stimulation was not a result of a 5-min break in stimulation of CBI-2 (Fig. 7B). When a 5-min break in CBI-2 stimulation was not accompanied by EN stimulation, the B8 activity-defined ingestiveness (higher firing rate during the retraction phase than that during the protraction phase) of motor programs was still evident, although it appeared to be reduced compared with activity observed in the tenth CBI-2 program. Changes in the firing of B8 and B48 that were observed following EN stimulation were not permanent because the firing of these neurons relaxed toward the pre-EN stimulation levels when CBI-2 was repeatedly stimulated.
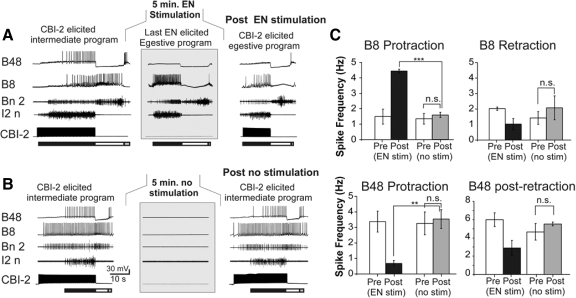
Fig. 7.
Activity patterns of B48 and B8 reflect the network state. A: a representative recording of the 10th CBI-2–elicited program (P-10), the last EN-elicited program, and the CBI-2 program elicited by CBI-2 15 s after EN stimulation are shown. B: a representative recording of the 10th CBI-2–elicited program (P-10), no stimulation, and the program elicited by CBI-2 after no stimulation. C: group data for B8 firing frequencies in the 1st and 10th CBI-2 programs (P-1, P-10) and CBI-2 programs following EN stimulation (black square) and following no stimulation (open square). Following EN stimulation B8 retraction firing frequency is significantly reduced compared with CBI-2 programs following no stimulation. B8 protraction firing frequency is significantly increased following EN stimulation compared with that following no stimulation. D: group data for B48 firing frequencies in the 1st (P-1) and 10th (P-10) CBI-2 programs and CBI-2 programs following EN stimulation (black triangle) and following no stimulation (open triangle). The gray bar indicates 5 min of either EN stimulation or 5 min of no stimulation. Following EN stimulation B48 firing frequency was significantly reduced compared with that of CBI-2 programs following no stimulation.
Previous work demonstrated a switch in the pattern of B8 activity during CBI-2 programs following EN stimulation, but in this work the contributions of EN stimulation versus time-dependent processes were not separated. Here, we used a two-way repeated-measures ANOVA to separate the effect of a time-dependent waning of B8 retraction activity from the EN-dependent decrement of B8 retraction activity. The two variables we analyzed were 1) B8 retraction activity in EN stimulation (n = 5) versus no-EN stimulation (n = 5) treatments and 2) B8 retraction activity during the tenth CBI-2 program versus the CBI-2 program that followed the EN/no EN stimulation period. We found a significant interaction between the two variables [F(1,8) = 18.65; P < 0.01]. Individual comparisons showed that in the tenth cycle of CBI-2 activity the two treatment groups did not differ significantly in terms of B8 retraction activity (t = 1.04, NS). However, B8 retraction activity following either EN stimulation or no-EN stimulation decreased relative to the tenth program (for the EN stimulation: t = 9.43, df = 4, P < 0.001; for the no-EN stimulation: t = 4.55, df = 4, P < 0.05) (Fig. 7C1). Importantly, B8 retraction activity following EN stimulation was significantly lower than that following no-EN stimulation (t = 3.01, df = 4, P < 0.05). Thus EN stimulation produced a larger decrement of B8 activity during retraction than that due to the time-dependent waning of B8 firing.
Similar analyses were performed for B8 protraction activity following EN stimulation. We used a two-way repeated-measures ANOVA to separate the effect of time on B8 protraction activity from the EN-dependent increase in B8 protraction activity. Again, the two variables we analyzed were B8 protraction activity during EN stimulation versus no-EN stimulation, and B8 protraction activity during the tenth CBI-2 program versus the CBI-2 program that followed the EN/no-EN stimulation (Fig. 7C2). We found a significant interaction between the two variables [F(1,8) = 16.11; P < 0.01]. Individual comparisons of B8 firing during protraction in the tenth CBI-2 programs (i.e., before EN stimulation/no stimulation treatments) showed that there was no significant difference (t = 1.53, NS). However, following EN/no-EN stimulation periods there was a significant difference between B8 protraction phase firing in the EN stimulation versus no-EN stimulation treatments (t = 13.26, P < 0.001). Importantly, individual comparisons revealed that there was a significantly higher B8 protraction-phase activity following EN stimulation than that following no-EN stimulation (t = 9.34, df = 4, P < 0.001). This indicated that EN stimulation contributed to the increase in the B8 protraction activity. Taken together, changes in the protraction and retraction phase activity of B8 indicated that CBI-2–elicited programs became more egestive.
We examined this phenomenon further by probing the activity of the radula opener B48. To separate the effect of a time-dependent waning of B48 protraction activity from EN-dependent decrement of B48 protraction activity we used a two-way repeated-measures ANOVA. Again, the two variables we analyzed were EN stimulation versus no EN stimulation and the tenth CBI-2 program versus the program that followed the EN/no-EN stimulation period. We found that there was a significant interaction between the two variables [F(1,8) = 8.48; P < 0.05] (Fig. 7D1). Individual comparisons with Bonferroni corrections showed that during protraction, B48 activity in the two groups that were to receive either EN stimulation or no stimulation treatments did not differ from each other in the tenth CBI-2–elicited program (t = 0.64, df = 4, NS). Following both the EN stimulation or no EN stimulation treatment B48 activity showed a significant decrement compared with that recorded in the tenth CBI-2 program (for the EN-stimulated group: t = 10.26, df = 4, P < 0.001; for the no-EN stimulation group: t = 3.62, df = 4, P < 0.05). Importantly, the firing frequency of B48 in the CBI-2 program following EN stimulation was significantly lower compared with that of the no-EN stimulation treatment group (t = 3.52, df = 4, P < 0.05). Thus EN stimulation contributed to the decrement of B48 protraction firing.
Similar analyses of B48 postretraction activity (Fig. 7D2) showed that there was a significant decrease in activity following both EN stimulation and no-EN stimulation treatments compared with the tenth CBI-2 program (for the EN-stimulated group: t = 5.56, df = 4, P < 0.05; for the no-EN stimulation group: t = 4.34.62, df = 4, P < 0.05). Interestingly, the firing frequency of B48 postretraction activity following EN or no-EN stimulation period was not significantly different in the EN-stimulated group (t = 1.3 df = 4, NS), indicating that EN stimulation did not further contribute to the decrement in activity.
We examined the state-dependent regulation of B44 activity using a similar paradigm in which we stimulated CBI-2 following 5 min of EN stimulation. In previous experiments we showed that B44 firing frequency increased during repeated EN stimulation and appeared not to change as a result of repeated CBI-2 stimulation. Therefore we examined what contribution a CBI-2 stimulatory input would have on the activity of B44 given a fully egestive state. An EN stimulation control was not necessary since B44 firing frequency did not change with repeated CBI-2 stimulation; therefore any alterations in B44 activity would be attributed to the 5-min EN stimulation.
Figure 8 A is a representative recording of B44 and B8 from a single preparation. Similar to the paradigm previously described by eliciting a series of ten CBI-2 programs, we established fully ingestive programs in which B8 fired robustly during retraction and B44 fired weakly during retraction. After 5 min of EN stimulation, in which B44 firing frequency increased during retraction and B8 firing frequency increased during protraction and decreased during retraction, we stimulated CBI-2. Consistent with previous experiments, B8 activity was high during protraction and low during retraction, thus indicating that the motor programs were egestive when CBI-2 programs were elicited following EN stimulation (n = 5). B44 activity was also significantly higher during CBI-2 programs following EN stimulation compared with the tenth CBI-2 program (t = 6.03, P < 0.001) (Fig. 8B). With repeated stimulation of CBI-2 these alterations of B8 and B44 activity relaxed toward the levels recorded before EN stimulation.
Fig. 8.
Activity patterns of B44 and B8 reflect the network state. A: shown are representative recordings of the 10th CBI-2–elicited ingestive program (program 10), the last egestive EN-elicited program, and the egestive program elicited by CBI-2 15 s after EN stimulation. B: group data for B44 (open circle) and B8 (black square) firing frequencies during retraction in the 10th CBI-2 control program, last EN program, and during CBI-2 programs following EN stimulation. Following EN stimulation B8 retraction firing frequency is significantly reduced and B44 firing frequency is significantly increased. With repeated CBI-2 stimulation every minute, B8 firing frequency and B48 firing frequency gradually return to CBI-2 control levels. C: grouped data show that there is no significant difference in B44 latency in the EN-elicited program compared with the CBI-2 program elicited following EN stimulation.
In addition, there were significant differences in the latency from the onset of the retraction phase to the first action potential in B44 recorded in the tenth CBI-2 program, the last EN program, and the post-EN CBI-2 program [ANOVA, F(2,14) = 23.74; P < 0.001] (Fig. 8C). Compared with the tenth CBI-2 program, the latency was significantly shorter in the CBI-2 program elicited following EN stimulation (t = 6.11, P < 0.001). Importantly, B44's latency during EN programs was not significantly different from the CBI-2 program elicited following EN stimulation (t = 0.22, P = NS).
Intermediate program bias.
To further characterize the contribution that stimulation might have on subsequent outputs of the network we used a modified biasing paradigm. We considered the possibility that the initial buildup of ingestive programs might be necessary for the ability of EN stimulation to alter subsequent CBI-2 programs from ingestive programs into egestive ones. Rather than producing a buildup of ingestiveness through a frequent CBI-2 stimulation (30-s S-ISI) we stimulated CBI-2 infrequently (3-min L-ISI). Under these conditions no buildup of ingestiveness occurs and motor programs remain intermediate, as defined by the low firing frequency of B8 during both protraction and retraction phases. After a series of CBI-2–elicited intermediate programs, a 5-min stimulation of EN or 5-min break was implemented. Following either the stimulation or the break in stimulation a CBI-2 program was elicited.
Figure 9 A illustrates results from a single preparation in which we examined B48 and B8 activity during CBI-2 programs before and after EN stimulation. The initial CBI-2–elicited program was intermediate, as defined by the low frequency of B8 during both protraction and retraction. EN was then stimulated for 5 min. At 15 s after the EN stimulation the CBI-2–elicited program that resulted was egestive, as defined by the high frequency of B8 firing during protraction and the absence of B48 firing. The control experiment in which the 5 min of EN stimulation was replaced with a 5-min break (Fig. 9B) showed that the intermediate programs elicited by CBI-2 were the same before and after the 5-min break in stimulation (B48: t = 0.30, NS; B8 protraction: t = −0.61, NS; B8 retraction: t = 1.739, NS; B48 postretraction, t = −0. 67, NS).
Fig. 9.
Alterations in B48 and B8 activity patterns in CBI-2 programs following EN stimulation are not dependent on a preceding CBI-2 buildup. CBI-2 was stimulated every 3 min for the duration of the protraction phase to produce a series of intermediate programs. Following this either EN was stimulated for 5 min or a 5-min break was implemented, then CBI-2 was stimulated again every minute. A: shown are data for a control intermediate CBI-2–elicited program, last egestive EN-elicited program, and the egestive program elicited by CBI-2 15 s after EN stimulation. Following EN stimulation B48 protraction firing frequency during CBI-2 programs is significantly reduced. B: shown are data for a control intermediate CBI-2–elicited program, no stimulation, and the program elicited by CBI-2 15 s after no stimulation. C: group data for B8 and B48 firing frequencies during control CBI-2 intermediate programs (white), CBI-2 programs post-EN stimulation (black), and CBI-2 programs after no stimulation (gray).
Analyses of aggregate data (n = 5) showed that following EN stimulation the CBI-2 program elicited was egestive, as defined by the high frequency of B8 during protraction and the absence of B48 activity (Fig. 9C). Importantly, despite starting from an intermediate program, the CBI-2 program elicited following EN stimulation was egestive. In addition, the activity of B48 was significantly lower and B8 protraction-phase activity was significantly higher following EN stimulation than that following a 5-min break in stimulation, even when the initial fully ingestive control was not established (post-EN vs. postbreak; B48: t = 6.45, P < 0.01; B8 protraction: t = 13.26, P < 0.001). Taken together this suggests that the degree of egestiveness established by EN stimulation and its ability to alter the activity of B48 and B8 following CBI-2–elicited programs were not dependent on having established fully ingestive programs via a frequent stimulation of CBI-2.
Using the same paradigm, we also examined B44 activity. Figure 10 A illustrates results from a single preparation in which we examined B44 and B8 activity during post-EN CBI-2 programs, following a series of CBI-2–elicited intermediate programs. The initial CBI-2–elicited program was intermediate. Following the intermediate programs, the EN was stimulated for 5 min, increasing B44 firing frequency during retraction. By 15 s following the EN stimulation, the CBI-2 program elicited remained in an egestive conformation, as defined by the high frequency of B8 activity during protraction and high frequency of B44 during retraction. Control experiments showed that the egestive character of CBI-2 programs elicited following EN stimulation could not be accounted for by a 5-min break in stimulation of CBI-2. Figure 10B illustrates B44 and B8 activity from a single preparation during CBI-2–elicited intermediate programs before and after a break in stimulation. Similar to what we observed previously, the programs (n = 5) remained intermediate, as defined by B8 activity before and after the 5-min break (B44: t = 0.02, NS; B8 protraction: t = −0.04, NS; B8 retraction: t = −0.68, NS).
Fig. 10.
Alterations in B44 and B8 activity patterns during CBI-2 programs following EN stimulation are not dependent on a preceding CBI-2 buildup. CBI-2 was stimulated for the duration of the protraction phase to a series of intermediate programs, then either EN was stimulated for 5 min or a 5-min break was implemented, then CBI-2 was stimulated again every minute. A: shown are data for a control intermediate CBI-2–elicited program, last egestive EN-elicited program, and the egestive program elicited by CBI-2 15 s after EN stimulation. B: shown are data for a control intermediate CBI-2–elicited program, no stimulation, and the program elicited by CBI-2 15 s after no stimulation. C: group data (n = 5) for B8 and B44 firing frequencies during control CBI-2 intermediate programs (white), post-EN stimulation (black), and after no stimulation (gray).
In contrast, the B44 retraction activity and B8 protraction activity were significantly higher following EN stimulation than those following a 5-min break (n = 5). This was found to be true even when the initial fully ingestive programs were not established (post-EN vs. postbreak; B44: t = 5.41, P < 0.05; B8 protraction: t = 47.21, P < 0.001) (Fig. 10C). Thus independent of whether before EN stimulation the CBI-2 programs were ingestive or intermediate, programs elicited by CBI-2 following repetitive EN stimulation were egestive.
State as a determinant of motor output.
Repetitive EN stimulation establishes a network state in which stimulation of either CBI-2 or EN elicits a motor program that appears to be egestive (see Figs. 7–10). This suggests that after the EN stimulation the critical determinant of network output may be its state, rather than its input. If this were the case, the firing frequencies of B48, B44, and B8 in the last EN motor program and the following CBI-2–elicited programs would be expected to share the same characteristics. To quantify this we compared firing frequencies of motoneurons during the last EN-elicited program and CBI-2 program that were elicited following a period of repetitive EN stimulation (data from Figs. 7 and 8). Following EN stimulation there were no significant differences in the firing frequencies of B48, B44, and B8, irrespective of whether the egestive program was elicited by EN stimulation or CBI-2 stimulation (B48: t = 0.633, NS; B44: t = 0.59, NS; B8 protraction: t = 0.24, NS; B8 retraction: t = 0.75, NS) (Fig. 11 A). Similarly, in the intermediate bias paradigms (Figs. 9 and 10), we found that the firing frequencies of B48, B44, and B8 were not significantly different during the last EN-elicited program and the following CBI-2–elicited program (B48: t = 0.199, NS; B44: t = 0.728, NS; B8 protraction: t = 0.125, NS; B8 retraction: t = 0.851, NS) (Fig. 11B). This suggested that after repetitive EN stimulation, network states, rather than network inputs, were in control of firing of B48, B44, and B8.
Fig. 11.
Similar activity patterns of B8, B48, and B44 during EN- and CBI-2–elicited programs following repetitive EN stimulation illustrate state dependence. A: shown are the group data (from Figs. 7A and 8A) of B8, B48, and B44 activity during the last EN-elicited program, preceded by CBI-2 buildup (gray), and during the first CBI-2 program elicited following EN stimulation (black). B: shown are the group data (from Figs. 9A and 10A) of B8, B48, and B44 activity during the last EN-elicited program, preceded by intermediate programs (gray), and during the first CBI-2 program elicited following EN stimulation (black). There are no significant differences in firing frequencies of B8, B48, and B44 in programs elicited by CBI-2 or EN following EN stimulation.
Input as a determinant of motor output.
To further probe the generality of the hypothesis that network states are the sole determinant of network outputs we compared responses elicited by CBI-2 and EN in a rested state. Analyses of these responses showed that, although the preparations were in the same rested state, responses elicited by CBI-2 and EN were different (Fig. 12 A). Both B48 and B44 had significantly different firing frequencies during motor programs, depending on the eliciting input. B48 exhibited a significantly higher firing frequency during the CBI-2–elicited program, whereas B44 exhibited a significantly higher firing frequency during the EN-elicited program (B48: t = 4.38, P < 0.01; B44: t = 4.08, P < 0.001). In addition, B8 exhibited a significantly higher firing frequency in the retraction phase during CBI-2–elicited programs (B8 retraction: t = 4.44, P < 0.002). The fact that stimulation of two inputs elicited different responses although the network was in the same state (rested) suggested that, at least in a rested state, inputs can make a state-independent contribution to network outputs.
Fig. 12.
Network states and activity patterns of B8, B48, and B44. A: in a rested state activity patterns of neurons B8, B48, and B44 depend on the input [CBI-2 (black) vs. EN (gray)] used to activate the program. B: there are no differences between activity patterns of B8, B44, and B48 in EN programs that were elicited in a rested state (gray) and following repeated stimulation of CBI-2 (black).
Data presented so far may suggest that in a rested state network inputs determine network outputs, whereas in states established by repetitive stimulation, network states are the determinant of outputs. However, this supposition was based on states established by a repeated EN stimulation. If the distinctive role of inputs and states can indeed be reduced to a difference between rested and stimulation-induced states, one would expect that following a period of repetitive stimulation of CBI-2, when CBI-2 programs are ingestive, the EN-elicited program should be ingestive as well. Analysis of the first responses elicited following EN stimulation (from experiments illustrated in Figs. 7 and 8) showed that this was not the case. Rather than being ingestive, EN programs elicited following a period of CBI-2 stimulation appeared to be intermediate. A quantitative analysis of the firing of neurons B8, B44, and B48 showed that this was the case. Importantly, comparisons of the activity patterns of B8, B44, and B48 (Fig. 12B) recorded during protraction and retraction phases in EN-elicited programs following CBI-2 stimulation were indistinguishable from the intermediate programs that were elicited in rested preparations (B48: t = 0.38, NS; B44: t = 0.74, NS; B8 protraction: t = 1.30, NS; B8 retraction: t = 3.50, NS) (n = 5). Taken together our data are not consistent with the simple view of inputs acting solely as response triggers and states acting as response shapers.
Radula movements elicited by motoneuronal activity.
Because the studies presented so far were performed in the isolated nervous system, changes in activity of motoneurons inform us about events that take place only when fictive feeding motor programs are generated. The fundamental question that needed to be answered was whether the changes in firing frequencies recorded in an isolated CNS have behavioral consequences. To answer this question we stimulated B48, B44, and B8 with frequencies that spanned the range of frequencies with which these neurons fired in the isolated nervous system while monitoring the size of radula opening and closing movements. Single B48 and B44 neurons are present in each hemiganglion. A bilateral stimulation was used for each of the motoneurons. Since each hemiganglion contains two of the B8 neurons, all four of the B8 neurons were stimulated.
Figure 13 illustrates the relationships between the size of radula opening and closing movements and the frequencies of firing of neurons B48 (Fig. 13A; n = 9), B44 (Fig. 13B; n = 8), and B8 (Fig. 13C; n = 6). Stimulation of all three neurons produced a movement that increased in size as a function of stimulation frequencies. Importantly, when B48 was stimulated at 3 Hz, a frequency similar to which B48 fires in a rested preparation, the opening movement was 0.4 mm. When B48 was stimulated at 7 Hz, a frequency at which it fires in the tenth program of CBI-2–elicited programs, the opening movement was 1.11 mm. This represents a 178% increase in the size of movement. Similarly when B44 was stimulated at 2 Hz, a frequency similar to which B44 fires in CBI-2 programs, the opening movement was 0.04 mm. When B44 was stimulated at 7 Hz, the frequency at which it fires during the fifth EN-elicited programs, the opening movement was 1.14 mm. This represents a 2,758% increase in the size of movement. Finally, when B8 was stimulated at 2 Hz, a frequency similar to that with which B8 fires in rested preparations, there was no detectable movement. In fully ingestive and egestive programs B8 firing frequency was in the range of 6 to 8 Hz. B8 simulation at these frequencies produced 0.7- and 1.2-mm radula closing movements, respectively. From a functional perspective it is interesting to note that when B8 was stimulated at 6 Hz, the radula of 67% of the animals showed a bilateral contact of the leading edges of the radula, indicating that a full closure occurred. When B8 was stimulated at 8 Hz, full radula closure occurred in 100% of the animals. In summary, motoneuronal stimulation in the range of frequencies in which B44, B48, and B8 fire in fictive motor programs showed that experience-dependent alterations of the firing of these motoneurons are reflected in the size of movements that activity of these motoneurons would produce.
Fig. 13.
Plots of radula opening and closing movements vs. the firing frequencies of the motoneurons B48 (A), B44 (B), and B8 (C). Increases in the frequency of bilateral stimulation of B48 and B44 increase the degree of radula opening. As B8 stimulation frequency is increased, the degree of radula closing increases until there is complete closure of the leading edge of the radula halves. In all, 100% of the animals showed full contact of the leading edge at 8 Hz.
DISCUSSION
Experience-dependent states that emerge following a period of repetitive stimulation constitute one of the major determinants of the type and quality of behaviors that organisms, including humans (Allport et al. 1994; Blitz and Ramirez 2002; Blitz et al. 2008; Leopold et al. 2001; Meiran and Marciano 2002; Meiran et al. 2000; Popescu and Frost 2002; Sharma et al. 2003), generate at any given time (Abraham and Willows 1971; Brown et al. 1996; Gold and Shadlen 2000, 2003; Prut and Fetz 1999; Susswein et al. 1978; Weiss et al. 1982). Despite the widespread influence of experience-dependent states, there is a limited understanding of the relative contribution that states and stimuli make to response specification, partly because of a dearth of widely accepted model systems. Previous work that investigated the effects of network states established by a repetitive stimulation of inputs to the feeding CPG of Aplysia suggested that this network may be a valid model system for gaining insights into the relative contribution that inputs and network states make to the characteristics of responses that the network generates (Proekt and Weiss 2003; Proekt et al. 2004).
Motor program characteristics and network states
To be effective, feeding responses have to coordinate two sets of radula movements: protraction/retraction and opening/closing (Kupfermann 1974; Morton and Chiel 1993). In both ingestion and egestion, protraction always precedes retraction, whereas the phasing of opening and closing movements differs. In ingestion, opening occurs during protraction and closing occurs during retraction. In egestion, closing occurs during protraction and opening during retraction. Traditional classification of feeding motor programs as ingestive, egestive, or intermediate is largely based on “topography” of motor programs, specifically on the relative phasing of B8 activity in protraction and retraction phases (Jing and Weiss 2005; Zhurov et al. 2005). Previous work that investigated state dependence of motoneuronal firing in motor programs was consistent with the idea that state-induced alterations may be functionally meaningful. This work showed that in a rested state CBI-2 and EN stimulation resulted in B8 firing both in the protraction phase and in the retraction phase of programs, suggesting that those programs were neither ingestive nor egestive (Proekt et al. 2004). However, repetitive stimulation of CBI-2 increased the firing of radula closing motoneuron B8 in retraction, whereas repetitive stimulation of EN resulted in an increased firing of B8 during protraction. Based on the correspondence to B8 firing patterns in feeding animals, these programs could be classified as ingestive and egestive (Jing and Weiss 2001, 2002; Morgan et al. 2002). However, the phasing of radula closing motoneurons on its own is not sufficient for deciding whether the CPG output is functional. Functionality requires that in addition to radula closing motoneurons, the firing of opening motoneurons must be appropriately phased. What makes this requirement essential is that the phasing of B8 firing changes during repetitive stimulation of CPG inputs. Thus if the programs are to be functional, the firing characteristics of radula opening motoneurons would need to change as well. This indeed happened. As a result of repetitive stimulation of CBI-2, opening motoneuron B48 increased its firing rate during protraction, whereas as a result of EN stimulation B44 increased its firing rate during retraction. Thus activity of opening motoneurons was appropriately phased to produce functional ingestive and egestive movements.
Behavioral implications
It has been pointed out that, on its own, phasing may not suffice to establish a behavioral relevance of motor programs (Marder and Calabrese 1996). For instance, neutral motor programs that are properly phased but result in no movements have been reported (Croll et al. 1985a,b; McClellan 1982; Morton and Chiel 1993). It was thus important to confer a behavioral meaning to the state-dependent differences in firing frequencies of motoneurons. Stimulation of radula opening and closing motoneurons in the range of frequencies recorded during repetitive stimulation of CBI-2 and EN showed that at frequencies recorded in rested preparations there were no significant radula movements. However, when the frequencies of motoneuronal firing increased, the magnitude of movements increased accordingly. At the behavioral level this may correspond to a transition from poorly articulated intermediate type responses toward clearly articulated ingestive or egestive type responses (Horn et al. 2004; Orekhova et al. 2001; Proekt et al. 2008; Susswein et al. 1978).
State-dependent and functional characteristics of motoneurons
Previous studies in invertebrates (Briggman and Kristan Jr 2006; Church and Lloyd 1994; Combes et al. 1999; Saideman et al. 2007) and vertebrates (Bizzi et al. 2002, 2008; D'Avella and Bizzi 2005; Li et al. 2007; Stein 2005) suggested that distinct but related behaviors are implemented by use of different combinations of motoneurons: some motoneurons that are shared by different behaviors and other motoneurons that are behavior specific. Within this framework, shared components of different behaviors would be implemented by shared motoneurons and unique components would be implemented by behavior-specific motoneurons. However, since experiments on which this classification was based used different stimuli to elicit different responses, one could have concluded that specific neurons, rather than being response specific, were actually specific with respect to the stimulus. Essentially, in these experiments, stimuli and responses were inextricably linked and could not be dissociated from each other. The paradigms in the present study allowed us to examine and dissociate the stimulus versus response sharing/specificity characteristics of individual motoneurons.
In repetition priming, we found that in a rested state CBI-2 stimulation preferentially activated B48, whereas B44 was essentially silent. The opposite pattern held for EN so that its stimulation preferentially activated B44 and B48 was silent. With repetitive stimulation of CBI-2 there was an increase of B48 firing, whereas with repetitive stimulation of EN the firing of B44 was increased. Since stimulated inputs were kept constant, the increased firing rates of B48 and B44 did not reflect the inputs, but the type of motor program that could be assessed based on B8 activity. The conclusion that B44 and B48 activity depends on the type of motor program, rather than on input, was further reinforced by results obtained in task switch experiments in which the test input was altered. Specifically, following EN stimulation, CBI-2 stimulation elicited egestive rather than ingestive or intermediate type programs as defined by B8 activity. Activity of neurons B48 and B44 was altered to reflect the motor program type rather than the input used to elicit the program. Notably, B8 behaved as a shared neuron as it fired, albeit at different rates and in different phases, in different types of programs that were elicited by either CBI-2 or EN. In summary, specific neurons are indeed response rather than stimulus specific. Importantly, our data expand to state-dependent regulation the principle that different motor programs can be generated through the use of various combinations of specific and shared neurons.
Motor programs: contribution of inputs and states
When the same stimulus elicits different network outputs depending on the network's recent experiences, inputs may appear to act solely as triggers that release state-determined sequences of motoneuronal firing. If inputs were to act only as response initiators and states as determinants of response characteristics, then in the same state, responses elicited by CBI-2 and EN should be the same. This was the case in the task switch paradigm because following a period of EN stimulation, responses elicited by both CBI-2 and EN were egestive and were indistinguishable from each other (Fig. 11). Here, an egestive state, established by repetitive stimulation of EN, determined the characteristics of the following motor program, regardless of the triggering input. This illustrates the critical contribution that states may have in determining the characteristics of network outputs. If states act solely as a determinant of response characteristics, a shared state should produce the same motor response regardless of the input, whereas two different states should produce different motor responses to the same network input. However, we found this was not always the case.
We found that in a rested state EN and CBI-2 elicited different responses (Fig. 12A), whereas EN programs elicited in different states shared the same response characteristics (Fig. 12B). In the rested state, CBI-2 stimulation preferentially activated B48 and EN stimulation preferentially activated B44, thus demonstrating that in a rested network state, the input is the determining factor in the characteristics of network outputs. Previous work (Proekt et al. 2004) indicated that B8 fired in both protraction and retraction phases in programs elicited in a rested preparation, but this work did not report a quantitative comparison of B8 firing. When we did so, we found that in response to CBI-2 stimulation there was no phase preference in B8 firing. In contrast to CBI-2, when EN was stimulated in rested preparations, B8 fired more during protraction than it did during retraction. Thus when seen from the perspective of B8 firing, the role of inputs appears not to be limited to initiating responses, but also includes a response shaping function. Notably, this response-shaping function is not constant because following a repetitive stimulation of CBI-2 or EN, the frequency of B48 and B44 changes, and in the case of B8 the phasing of firing changes as well.
Differences in contributions made by input and network states could not be reduced to a general difference between rested states and repeated stimulation-induced states. When “ingestive” network states were induced by repeated stimulation of CBI-2, these states did not affect the characteristics of EN-elicited responses. Specifically, EN responses elicited following a period of CBI-2 stimulation were indistinguishable as measured by the activity of B8, B48, and B44 during protraction and retraction phases from those that EN stimulation elicited in a rested preparation. These findings are not in agreement with the hypothesis that network states, rather than stimuli, are the ultimate determinant of response characteristics. In its general form this hypothesis would have predicted that following CBI-2 stimulation, not only CBI-2 but also EN stimulation should elicit ingestive responses.
In this study, our experiments focused on network states that were induced by stimulation of program-initiating inputs, specifically CBI-2 and EN. It is important to note, however, that response-initiating inputs are not the sole inputs that can affect the network state. Even when Aplysia are not feeding—i.e., they remain in a rested state—the feeding CPG may receive a number of modulatory inputs that can both up- and down-modulate the outputs of the CPG (Jing et al. 2007, 2008; Proekt and Weiss 2003; Rosen et al. 1989; Weiss et al. 1978). Some of these inputs may serve as a bridge between the feeding CPG and other parts of the nervous system that control feeding-relevant responses (Koch et al. 1984; Romanova et al. 2007). Interestingly, the state induced by stimulation of one of these inputs, the MCC (metacerebral cell) neuron, appears to act on the same targets and use the same mechanisms that CBI-2 uses to generate an “ingestive state” (Proekt and Weiss 2003). The fact that in freely moving animals, a number of modulatory inputs are likely to determine the momentary state of the CPG poses a serious experimental challenge to investigations of the relative contribution that network inputs and states make to the feeding behavior. An important advantage of the preparation we used is that other modulatory inputs are not active, making it possible to isolate the specific contributions of network states that are induced by repetitive stimulation of EN and CBI-2.
Summary
The advantageous features of the Aplysia nervous system allowed us to probe the contributions that inputs and states make to network output. The point of departure for this study was the idea that actions of states determine the nature of the response that networks generate. A corollary of this assumption was that in the same state different inputs should trigger the same response. Surprisingly, we found that although this was the case for the state induced by repetitive stimulation of EN this was not the case for a CBI-2–induced network state. The scope of actions of these two stimulation-induced states was different, thus indicating that one cannot assume that the effects of one state should have a similar scope of actions as that described for another state. A significant corollary of this conclusion is that the same holds true for the role that stimuli play in determining network responses. Even though the contributions that inputs and states make to network outputs depend on the specifics of input and states, when seen from a broader perspective our data suggest that dynamically regulated actions of inputs and states determine the characteristics of behaviorally relevant motor programs that are generated by the feeding CPG of Aplysia. Given that repetition priming and task-switch cost are detected in a variety of behaviors, it may be attractive to hypothesize that in those other behaviors state and stimuli make similar contributions as those described earlier.
GRANTS
This work was supported by National Institutes of Health Grants NS-066587 and MH-035564. Some of the Aplysia were provided by the National Resource for Aplysia of the University of Miami under National Institutes of Health National Center for Research Resources Grant RR-0294.
ACKNOWLEDGMENTS
We thank M. R. Due for valuable comments on this manuscript.
REFERENCES
- Abraham FD, Willows AOD. Plasticity of a fixed action pattern in the sea slug Tritonia diomedia. Comm Behav Biol 6: 271–280, 1971 [Google Scholar]
- Allport DA, Styles EA, Hsieh S. Shifting intentional set: exploring the dynamic control of tasks. In: Attention and Performance XV: Conscious and Nonconscious Information Processing, edited by Ulmità C. Cambridge, MA: MIT Press, 1994, p. 421–452 [Google Scholar]
- Bizzi E, Cheung VCK, D'Avella A, Saltiel P, Tresch M. Combining modules for movement. Brain Res Rev 57: 125–133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E, D'Avella A, Saltiel P, Tresch M. Modular organization of spinal motor systems. Neuroscientist 8: 437–442, 2002 [DOI] [PubMed] [Google Scholar]
- Blitz DM, Ramirez J-M. Long-term modulation of respiratory network activity following anoxia in vitro. J Neurophysiol 87: 2964–2971, 2002 [DOI] [PubMed] [Google Scholar]
- Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol 211: 1000–1011, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikov D, Evans CG, Jing J, Rosen SC, Cropper EC. A proprioceptive role for an exteroceptive mechanoafferent neuron in Aplysia. J Neurosci 20: 1990–2002, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Kristan WB., Jr Imaging dedicated and multifunctional neural circuits generating distinct behaviors. J Neurosci 26: 10925–10933, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Frost WN, Getting PA. Habituation and iterative enhancement of multiple components of the Tritonia swim response. Behav Neurosci 110: 478–485, 1996 [DOI] [PubMed] [Google Scholar]
- Chiel H, Kupfermann I, Weiss K. An identified histaminergic neuron can modulate the outputs of buccal–cerebral interneurons in Aplysia via presynaptic inhibition. J Neurosci 8: 49–63, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church PJ, Lloyd PE. Activity of multiple identified motor neurons recorded intracellularly during evoked feedinglike motor programs in Aplysia. J Neurophysiol 72: 1794–1809, 1994 [DOI] [PubMed] [Google Scholar]
- Combes D, Meyrand P, Simmers J. Dynamic restructuring of a rhythmic motor program by a single mechanoreceptor neuron in lobster. J Neurosci 19: 3620–3628, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll R, Davis W, Kovac M. Neural mechanisms of motor program switching in the mollusc Pleurobranchaea. I. Central motor programs underlying ingestion, egestion, and the “neutral” rhythm(s). J Neurosci 5: 48–55, 1985a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll R, Kovac M, Davis W. Neural mechanisms of motor program switching in the mollusc Pleurobranchaea. II. Role of the ventral white cell, anterior ventral, and B3 buccal neurons. J Neurosci 5: 56–63, 1985b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper EC, Evans CG, Hurwitz I, Jing J, Proekt A, Romero A, Rosen SC. Feeding neural networks in the mollusc Aplysia. Neurosignals 13: 70–86, 2004 [DOI] [PubMed] [Google Scholar]
- D'Avella A, Bizzi E. Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci USA 102: 3076–3081, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembrow NC, Jing J, Brezina V, Weiss KR. A specific synaptic pathway activates a conditional plateau potential underlying protraction phase in the Aplysia feeding central pattern generator. J Neurosci 24: 5230–5238, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nunzio A, Zanetti C, Schieppati M. Post-effect of forward and backward locomotion on body orientation in space during quiet stance. Eur J Appl Physiol 105: 297–307, 2009 [DOI] [PubMed] [Google Scholar]
- Due MR, Jing J, Weiss KR. Dopaminergic contributions to modulatory functions of a dual-transmitter interneuron in Aplysia. Neurosci Lett 358: 53–57, 2004 [DOI] [PubMed] [Google Scholar]
- Esch T, Mesce KA, Kristan WB. Evidence for sequential decision making in the medicinal leech. J Neurosci 22: 11045–11054, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Cropper EC. Proprioceptive input to feeding motor programs in Aplysia. J Neurosci 18: 8016–8031, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Jing J, Proekt A, Rosen SC, Cropper EC. Frequency-dependent regulation of afferent transmission in the feeding circuitry of Aplysia. J Neurophysiol 90: 3967–3977, 2003 [DOI] [PubMed] [Google Scholar]
- Evans CG, Rosen S, Kupfermann I, Weiss KR, Cropper EC. Characterization of a radula opener neuromuscular system in Aplysia. J Neurophysiol 76: 1267–1281, 1996 [DOI] [PubMed] [Google Scholar]
- Evans CG, Vilim FS, Harish O, Kupfermann I, Weiss KR, Cropper EC. Modulation of radula opener muscles in Aplysia. J Neurophysiol 82: 1339–1351, 1999 [DOI] [PubMed] [Google Scholar]
- Friedman D. Event-related brain potential investigations of memory and aging. Biol Psychol 54: 175–206, 2000 [DOI] [PubMed] [Google Scholar]
- Friesen WO, Kristan WB. Leech locomotion: swimming, crawling, and decisions. Curr Opin Neurobiol 17: 704–711, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. Representation of a perceptual decision in developing oculomotor commands. Nature 404: 390–394, 2000 [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The influence of behavioral context on the representation of a perceptual decision in developing oculomotor commands. J Neurosci 23: 632–651, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R, Wenzel B, Elsner N. Pharmacological brain stimulation releases elaborate stridulatory behaviour in gomphocerine grasshoppers: conclusions for the organization of the central nervous system. J Comp Physiol A Sens Neural Behav Physiol 187: 155–169, 2001 [DOI] [PubMed] [Google Scholar]
- Horn CC, Zhurov Y, Orekhova IV, Proekt A, Kupfermann I, Weiss KR, Brezina V. Cycle-to-cycle variability of neuromuscular activity in Aplysia feeding behavior. J Neurophysiol 92: 157–180, 2004 [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Cropper EC, Vilim FS, Alexeeva V, Susswein AJ, Kupfermann I, Weiss KR. Serotonergic and peptidergic modulation of the buccal mass protractor muscle (I2) in Aplysia. J Neurophysiol 84: 2810–2820, 2000 [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Neustadter D, Morton DW, Chiel HJ, Susswein AJ. Activity patterns of the B31/B32 pattern initiators innervating the I2 muscle of the buccal mass during normal feeding movements in Aplysia californica. J Neurophysiol 75: 1309–1326, 1996 [DOI] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Cropper EC, Weiss KR. Neural analog of arousal: persistent conditional activation of a feeding modulator by serotonergic initiators of locomotion. J Neurosci 28: 12349–12361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Zhurov Y, Kupfermann I, Sweedler JV, Weiss KR. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. J Neurosci 27: 3490–3502, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Neural mechanisms of motor program switching in Aplysia. J Neurosci 21: 7349–7362, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Interneuronal basis of the generation of related but distinct motor programs in Aplysia: implications for current neuronal models of vertebrate intralimb coordination. J Neurosci 22: 6228–6238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Generation of variants of a motor act in a modular and hierarchical motor network. Curr Biol 15: 1712–1721, 2005 [DOI] [PubMed] [Google Scholar]
- Koch UT, Koester J, Weiss KR. Neuronal mediation of cardiovascular effects of food arousal in Aplysia. J Neurophysiol 51: 126–135, 1984 [DOI] [PubMed] [Google Scholar]
- Koh H-Y, Weiss KR. Activity-dependent peptidergic modulation of the plateau-generating neuron B64 in the feeding network of Aplysia. J Neurophysiol 97: 1862–1867, 2007 [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol 10: 1–26, 1974 [DOI] [PubMed] [Google Scholar]
- Lechner HA, Baxter DA, Byrne JH. Classical conditioning of feeding in Aplysia: I. Behavioral analysis. J Neurosci 20: 3369–3376, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, O'Toole AJ, Vetter T, Blanz V. Prototype-referenced shape encoding revealed by high-level aftereffects. Nat Neurosci 4: 89–94, 2001 [DOI] [PubMed] [Google Scholar]
- Li W-C, Sautois B, Roberts A, Soffe SR. Reconfiguration of a vertebrate motor network: specific neuron recruitment and context-dependent synaptic plasticity. J Neurosci 27: 12267–12276, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69: 291–316, 2007 [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev 76: 687–717, 1996 [DOI] [PubMed] [Google Scholar]
- McClellan AD. Movements and motor patterns of the buccal mass of Pleurobranchaea during feeding, regurgitation and rejection. J Exp Biol 98: 195–211, 1982 [Google Scholar]
- Meiran N, Chorev Z, Sapir A. Component processes in task switching. Cogn Psychol 41: 211–253, 2000 [DOI] [PubMed] [Google Scholar]
- Meiran N, Marciano H. Limitations in advance task preparation: switching the relevant stimulus dimension in speeded same-different comparisons. Mem Cogn 30: 540–550, 2002 [DOI] [PubMed] [Google Scholar]
- Monsell S, Sumner P, Waters H. Task-set reconfiguration with predictable and unpredictable task switches. Mem Cogn 31: 327–342, 2003 [DOI] [PubMed] [Google Scholar]
- Morgan PT, Jing J, Vilim FS, Weiss KR. Interneuronal and peptidergic control of motor pattern switching in Aplysia. J Neurophysiol 87: 49–61, 2002 [DOI] [PubMed] [Google Scholar]
- Morton D, Chiel HJ. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol A Sens Neural Behav Physiol 173: 519–536, 1993 [DOI] [PubMed] [Google Scholar]
- Nadim F, Brezina V, Destexhe A, Linster C. State dependence of network output: modeling and experiments. J Neurosci 28: 11806–11813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Byrne JH. Contingent-dependent enhancement of rhythmic motor patterns: an in vitro analog of operant conditioning. J Neurosci 17: 8093–8105, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Byrne JH. In vitro analog of operant conditioning in Aplysia. I. Contingent reinforcement modifies the functional dynamics of an identified neuron. J Neurosci 19: 2247–2260, 1999a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Byrne JH. In vitro analog of operant conditioning in Aplysia. II. Modifications of the functional dynamics of an identified neuron contribute to motor pattern selection. J Neurosci 19: 2261–2272, 1999b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TG, Hoy RR. Initiation of behavior by single neurons: the role of behavioral context. Science 226: 992–994, 1984 [DOI] [PubMed] [Google Scholar]
- Orekhova IV, Jing J, Brezina V, DiCaprio RA, Weiss KR, Cropper EC. Sonometric measurements of motor-neuron-evoked movements of an internal feeding structure (the radula) in Aplysia. J Neurophysiol 86: 1057–1061, 2001 [DOI] [PubMed] [Google Scholar]
- Peña F, Ramirez J-M. Hypoxia-induced changes in neuronal network properties. Mol Neurobiol 32: 251–283, 2005 [DOI] [PubMed] [Google Scholar]
- Popescu IR, Frost WN. Highly dissimilar behaviors mediated by a multifunctional network in the marine mollusk Tritonia diomedea. J Neurosci 22: 1985–1993, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Brezina V, Weiss KR. Dynamical basis of intentions and expectations in a simple neuronal network. Proc Natl Acad Sci USA 101: 9447–9452, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Jing J, Weiss KR. Multiple contributions of an input-representing neuron to the dynamics of the Aplysia feeding network. J Neurophysiol 97: 3046–3056, 2007 [DOI] [PubMed] [Google Scholar]
- Proekt A, Weiss KR. Convergent mechanisms mediate preparatory states and repetition priming in the feeding network of Aplysia. J Neurosci 23: 4029–4033, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Wong J, Zhurov Y, Kozlova N, Weiss KR, Brezina V. Predicting adaptive behavior in the environment from central nervous system dynamics. PLoS ONE 3: e3678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut Y, Fetz E. Primate spinal interneurons show premovement instructed delay activity. Nature 401: 590–594, 1999 [DOI] [PubMed] [Google Scholar]
- Romanova EV, McKay N, Weiss KR, Sweedler JV, Koester J. Autonomic control network active in Aplysia during locomotion includes neurons that express splice variants of R15-neuropeptides. J Neurophysiol 97: 481–491, 2007 [DOI] [PubMed] [Google Scholar]
- Rosen S, Teyke T, Miller M, Weiss K, Kupfermann I. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J Neurosci 11: 3630–3655, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S, Weiss K, Goldstein R, Kupfermann I. The role of a modulatory neuron in feeding and satiation in Aplysia: effects of lesioning of the serotonergic metacerebral cells. J Neurosci 9: 1562–1578, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saideman SR, Blitz DM, Nusbaum MP. Convergent motor patterns from divergent circuits. J Neurosci 27: 6664–6674, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E. Context-dependent selection of visuomotor maps (Abstract). BMC Neurosci 5: 47, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Due MR, Jing J, Weiss KR. Feeding CPG in Aplysia directly controls two distinct outputs of a compartmentalized interneuron that functions as a CPG element. J Neurophysiol 98: 3796–3801, 2007 [DOI] [PubMed] [Google Scholar]
- Sasaki K, Jing J, Due MR, Weiss KR. An input-representing interneuron regulates spike timing and thereby phase switching in a motor network. J Neurosci 28: 1916–1928, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano GE, Martinez-Rubio C, Miller MW. Endogenous motor neuron properties contribute to a program-specific phase of activity in the multifunctional feeding central pattern generator of Aplysia. J Neurophysiol 98: 29–42, 2007 [DOI] [PubMed] [Google Scholar]
- Sharma J, Dragoi V, Tenenbaum JB, Miller EK, Sur M. V1 neurons signal acquisition of an internal representation of stimulus location. Science 300: 1758–1763, 2003 [DOI] [PubMed] [Google Scholar]
- Stein PSG. Neuronal control of turtle hindlimb motor rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 213–229, 2005 [DOI] [PubMed] [Google Scholar]
- Susswein AJ, Kupfermann I, Weiss KR. The stimulus control of biting in Aplysia. J Comp Physiol A Sens Neural Behav Physiol 108: 75–96, 1976 [Google Scholar]
- Susswein AJ, Weiss KR, Kupfermann I. The effects of food arousal on the latency of biting in Aplysia. J Comp Physiol 123: 31–41, 1978 [Google Scholar]
- Waszak F, Hommel B, Allport A. Task-switching and long-term priming: role of episodic stimulus-task bindings in task-shift costs. Cogn Psychol 46: 361–413, 2003 [DOI] [PubMed] [Google Scholar]
- Weiss K, Chiel H, Koch U, Kupfermann I. Activity of an identified histaminergic neuron, and its possible role in arousal of feeding behavior in semi-intact Aplysia. J Neurosci 6: 2403–2415, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss KR, Cohen JL, Kupfermann I. Modulatory control of buccal musculature by a serotonergic neuron (metacerebral cell) in Aplysia. J Neurophysiol 41: 181–203, 1978 [DOI] [PubMed] [Google Scholar]
- Weiss KR, Koch UT, Koester J, Rosen SC, Kupfermann I. The role of arousal in modulating feeding behavior of Aplysia: neural and behavioral studies. In: The Neural Basis of Feeding and Reward, edited by Hoebel BG, Novin D. Brunswick, ME: Haer Institute for Electrophysiological Research, 1982, p. 25–57 [Google Scholar]
- Wu J-s, Due MR, Sasaki K, Proekt A, Jing J, Weiss KR. State dependence of spike timing and neuronal function in a motor pattern generating network. J Neurosci 27: 10818–10831, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie G, Allport A. Task switching and the measurement of switch costs. Psychol Res 63: 212–233, 2000 [DOI] [PubMed] [Google Scholar]
- Zhurov Y, Proekt A, Weiss KR, Brezina V. Changes of internal state are expressed in coherent shifts of neuromuscular activity in Aplysia feeding behavior. J Neurosci 25: 1268–1280, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]