Abstract
Neurons of the subthalamic nucleus (STN) are very sensitive to applied currents, firing at 10–20/s during spontaneous activity, but increasing to peak firing rates of 200/s with applied currents <0.5 nA. They receive a powerful tonic excitatory input from neurons in the cerebral cortex, yet in vivo maintain an irregular firing rate only slightly higher than the autonomous firing rate seen in slices. Spike frequency adaptation acts to normalize background firing rate by removing slow trends in firing due to changes in average input. Subthalamic neurons have been previously described as showing little spike frequency adaptation, but this was based on tests using brief stimuli. We applied long-duration depolarizing current steps to STN neurons in slices and observed a very strong spike frequency adaptation with a time constant of 20 s and that recovered at a similar rate. This adaptation could return firing to near-baseline levels during prolonged current pulses that transiently drove the cells at high rates. The current responsible for adaptation was studied in voltage clamp during and after high-frequency driving of the cell and was determined to be a slowly accumulating K+ current. This current was independent of calcium or sodium entry and could be induced with long-duration voltage steps after blockade of action potentials. In addition to the adaptation current, driven firing produced slow inactivation of the persistent Na+ current, which also contributed to the reduced excitability of STN cells during and after driven firing.
INTRODUCTION
Subthalamic nucleus (STN) neurons are capable of repetitive firing over a wide range of frequencies, from about 1 to >200/s. This wide dynamic range of the cells is evident in their firing in vivo. In awake primates they exhibit an irregular background firing at 18–28/s (Bergman et al. 1994; Georgeopoulos et al. 1983; Isoda and Hikosaka 2008; Matsumura et al. 1992) and peak firing of >100/s transiently during movement-related responses (Isoda and Hikosaka 2008; Matsumura et al. 1992). In slices, STN cells spontaneously fire rhythmically at 5–20/s (Abbott et al. 1997; Beurrier et al. 1999; Bevan and Wilson 1999; Nakanishi et al. 1987). Autonomous firing is even faster in dissociated cells (Do and Bean 2003). When driven by intracellular depolarizing current pulses, STN cells in slices can maintain high-frequency firing for >1 s. At the onset of 1-s current pulses of sufficient amplitude, their firing rate increases rapidly over the first 10–200 ms (Hallworth et al. 2003; Wilson et al. 2004) and can be maintained at >200/s throughout the remainder of the stimulus. For such stimuli, a moderate spike frequency adaptation is usually seen toward the end of the train of high-frequency firing. Some adaptation occurs in isolation when the cell is driven to fire at rates slower than those that trigger the initial speedup (e.g., Wilson et al. 2004). The mechanisms responsible for these intrinsic changes in firing rate certainly influence the time course of firing in vivo and differentially affect background firing and the cells' responses to movement.
Spike frequency adaptation is usually attributed to the accumulation of an afterhyperpolarization (AHP) current, an outward current that is triggered in the wake of action potentials (Baldissera and Gustafsson 1971; Partridge and Stevens 1976). To be effective over a wide range of firing rates, individual action potentials should activate only a small proportion of the total available current; otherwise, the adaptation current will saturate, the firing rate will become unstable, and firing will fail in depolarization block (Baldissera et al. 1978; Wilson et al. 2004). If adaptation is to be able to develop slowly over time, the decay of the adaptation current contributed by single action potentials must also be slow. The slowly developing spike frequency adaptation observed in STN neurons during 1-s current pulses over firing rates ranging from 25 to 500/s suggests the presence of a long-lasting adaptation current that can act over the entire range of firing rates seen in these cells (Teagarden et al. 2008).
STN neurons also exhibit long pauses in their spontaneous activity following the offset of stimuli that drive firing to high rates (Beurrier et al. 2001; Bevan and Wilson 1999; Hallworth et al. 2003). These pauses also suggest the presence of a slowly decaying adaptation current, which accumulates during high-frequency firing. The pause following high-frequency firing has been attributed in part to the SK current generated by calcium influx during high-frequency firing (Hallworth et al. 2003) and to slow inactivation of the persistent Na+ current that is known to be responsible for maintenance of the background firing pattern (Beurrier et al. 2001).
A subset of STN neurons also exhibit plateau potentials and sustained high-frequency firing when depolarized from a sustained hyperpolarized state or on termination of a hyperpolarizing current pulse (Beurrier et al. 1999; Kass and Mintz 2006). The plateau potentials do not rely on persistent Na+ current, but are generated by calcium currents (Beurrier et al. 1999; Nakanishi et al. 1987; Otsuka et al. 2001). The termination of the plateau potential apparently relies on action potential firing because if firing does not occur (because of depolarization block or after tetrodotoxin [TTX]-induced block of Na+ channels), plateau potentials may assume arbitrary durations, thus making the cells multistable (Beurrier 1999; Kass and Mintz 2006). In this subset of neurons, the same mechanism responsible for spike frequency adaptation may also help to stabilize repetitive firing by terminating plateau potentials.
Teagarden et al. (2008) demonstrated the presence of a slowly decaying outward current that followed 1-s periods of 25–200 spike/s driven firing and increased linearly with firing rate over that range. The nature of the adaptation current or its relationship to spike frequency adaptation was not established, but it was shown not to be dependent on calcium entry because it was not reduced by blockade of calcium currents with cadmium.
In this report, we show that subthalamic neurons exhibit a profound spike frequency adaptation that develops over periods of 10–20 s, making the cells relatively insensitive to steady-state changes in the average level of excitatory input. Spike frequency adaptation in subthalamic cells is shown to arise primarily from the accumulation of a very slow potassium current that is triggered by action potentials and that decays with a time constant of tens of seconds. A smaller and more rapidly decaying component of the adaptation current arises from inactivation of persistent sodium current.
METHODS
Experiments were performed following National Institutes of Health Guidelines and were approved by the UTSA institutional care committee. Sprague–Dawley rats aged 17–25 days were anesthetized with a combination of ketamine (160 mg/kg) and xylazine (24 mg/kg) and perfused intracardially with ice-cold artificial cerebrospinal fluid (ACSF) modified as described by Aghajanian and Rasmussen (1989). The brains were removed and sectioned in the parasagittal plane at 300 μm using a vibrating microtome. Slices including the subthalamic nucleus were stored at room temperature in ACSF containing (in mM) 125 NaCl, 3.0 KCl, 1.25 NaH2PO4, 2 CaCl2, 1.5 MgCl, 26 NaHCO3, and 10 glucose, bubbled with 95% O2-5% CO2. Slices were visualized using a Zeiss Axioskop microscope equipped with infrared differential interference contrast optics, maintained at 32–35°C, and submerged and superfused with ACSF. Whole cell and perforated-patch recordings were obtained using glass pipettes with tip diameters of 1–3 μM and tip resistances of 2–7 MΩ, pulled using a horizontal puller (P-97, Sutter Instrument, Novato CA). For perforated-patch recordings, electrodes were filled with a solution containing (in mM) 140.5 KMeSO4, 7.5 NaCl, 10 HEPES buffer, and 0.01 phosphocreatine, to which had been added 100 μg/ml gramidicin-D. Gramicidin-D stock solutions containing 10 mg/ml gramicidin in DMSO were prepared daily; 5 μl of this solution was added to a 500-μl aliquot of electrode filling solution and mixed by sonication within 20 min of use. Perforated-patch electrodes were front-filled with gramicidin-free solution and then back-filled with the solution containing gramicidin. For whole cell recordings, electrodes were filled with a solution containing (in mM) 140.5 KMeSO4, 7.5 NaCl, 10 HEPES buffer, 0.01 phosphocreatine, 2 Mg-ATP, and 0.2 Na-GTP. In some experiments, BAPTA [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra-potassium salt] was added to the electrode solutions at 2 or 5 mM. In these cases the concentration of KMeSO4 was adjusted to keep K+ concentration constant, assuming complete dissociation of the BAPTA salt.
Recordings were made using an Axoclamp 200B amplifier, filtered at 5 or 10 kHz, and digitized at 10 or 20 kHz, using a DigiData 1332A (Molecular Devices, Palo Alto, CA) or a HEKA ITC-18 digitizer. Data were analyzed using custom routines programmed using Mathematica (Wolfram Research, Champaign, IL). Means are presented ± SDs except where otherwise noted.
RESULTS
Slow spike frequency adaptation
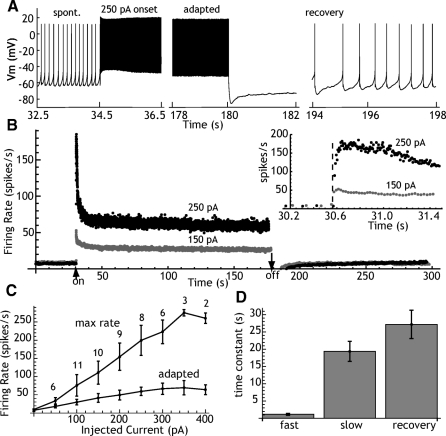
Slow adaptation was studied in 11 spontaneously active STN neurons recorded using the perforated-patch method. All of the cells fired in a rhythmic single spike pattern in the absence of applied currents. Spontaneous firing rates ranged from 3.6 to 14.6/s, with an average of 7.6 ± 3.4/s. The perforated-patch method was preferred for these experiments to avoid the effects of dialysis, which often causes gradual changes in firing rate over time. Spontaneous firing was interrupted by 2- to 3-min applications of constant current ranging from 50 to 400 pA, selected to produce initial firing at 20 to 250/s. Instantaneous firing rate was monitored over the entire period of driven firing and for a period of ≥2 min following the offset of depolarizing current. When the firing rate returned to normal, the experiment was repeated with a different level of constant current. The stability of perforated-patch recordings enabled us to apply more than one current pulse to each cell, although we did not cover the entire range in every cell. An example showing samples of the spike train for one level of injected current is shown in Fig. 1 A. Examples showing the effects on instantaneous rate are shown in Fig. 1B. As reported previously, the firing rate increased for the first 20–100 ms and then showed a small decline over the first second of driven firing. Figure 1B shows that over the course of the next 20–30 s, a much more profound adaptation gradually developed and reduced the firing rate to 25–50% of its initial peak. Stable adapted firing rates were maintained for the remainder of the period. At higher current levels, some cells failed to sustain firing and entered depolarization block (not shown). When this occurred, it was always in the first few seconds of driven firing, before adaptation was complete. Cells that established a steady adapted firing rate continued to fire for the entire period of the current application. The degree of adaptation increased at high firing rates, with adapted rates being 54% of peak at 50 pA and 24% at 400 pA. Sustained adapted firing rates were possible at rates ≤70 spikes/s.
Fig. 1.
Spike frequency adaptation in subthalamic nucleus (STN) neurons. A: an example showing the spontaneous firing, transient response to a current pulse (first 2 s of driven firing), adapted firing pattern (last 2 s of driven firing), and recovery of spontaneous firing after a 250-pA current pulse. B: instantaneous firing rate of the same cell shown in A, for current pulses of 150 and 250 pA. Currents were initiated after 30 s of spontaneous firing and continued for about 140 s. Arrows indicate current onset and offset. Spontaneous firing is lost for 10–20 s after offset of the current. The inset shows the transient response at higher resolution. Individual points indicate frequencies calculated from single interspike intervals. The dashed line indicates the onset of the current pulse. Note that firing increases for the first 50–100 ms of firing and that firing is not much affected by adaptation for the first 500 ms or longer. C: the relationship between firing rate and injected current, for the maximum rate during the transient (max rate) and the rate in the last second of a 120-s current pulse (adapted). Error bars are SEs and the numbers by each point are the number of cells examined at each current level. D: the fast and slow time constants of adaptation obtained by fitting a sum of exponentials to the time course of instantaneous firing rate and the single time constant from the late portion of the recovery.
After termination of the depolarizing current, neurons spontaneously hyperpolarized and ongoing firing was lost for several seconds. Firing resumed gradually and returned to baseline levels over the course of 30–60 s. During the silent period following the offset of current, repetitive firing could be elicited by 2-s 20- to 100-pA current pulses and action potentials evoked in that way were normal in size and duration (not shown).
The rate of adaptation was measured by fitting exponential decays to the instantaneous rate versus time plots. Single-exponential curves did not adequately fit the early part of the adaptation process, but the data were fit well using a sum of two exponential processes. There was no correlation between the level of injected current and either the fast (r = −0.076 for all traces used in the frequency–current curves in Fig. 1C) or the slow adaptation time constants (r = 0.014). To equalize the contribution of each cell to the mean, the average time constants were calculated for the largest current applied to each cell. For the 11 cells in this sample, the time constant of the fast component averaged 1.2 ± 0.9 s and the slow component was 19.4 ± 9.7 s. Only the late part of the recovery curve was available for spike rate analysis and it was adequately fit with a single-exponential function with a time constant of 27.1 ± 13.6 s.
Adaptation current
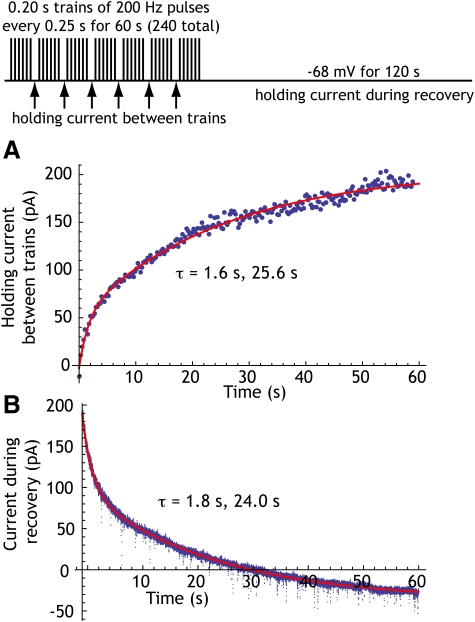
To measure the adaptation current, we used an elaboration of the method used by Partridge and Stevens (1976) and Teagarden et al. (2008). The cells were recorded using the whole cell method and action potentials were triggered from a holding potential of −68 mV by brief (2-ms) voltage pulses (see Fig. 2). The pulses occurred at 200/s and were presented in trains with 0.2-s duration, given every 0.25 s for 60 s. Although these stimulus pulses were applied in voltage-clamp recording, voltage control was not maintained during these brief pulses and they triggered action potentials that were detected as action currents. No measurements could be taken during the pulse trains beyond determining whether action currents were reliably elicited on each pulse. The 0.2-s trains of pulses were separated by 50-ms holding periods at −68 mV. The holding current during the last 10 ms of these 50-ms periods was averaged as an estimate of accumulating adaptation current. These measurements were made four times/s over the entire 60-s period of 200/s driven firing. Following the high-frequency pulses, the cell was held at −68 mV for 60 s to follow the time course of decay of the adaptation current. Measurements were made in 13 cells, of which 9 were recorded using electrodes containing 2 or 5 mM BAPTA, and 4 contained no calcium buffer. In agreement with the report by Teagarden et al. (2008) the presence of BAPTA had no influence on the size or time course of the adaptation current and these data were pooled for purposes of the following measurements. In all cases, action currents were evident on every pulse in the trains throughout the entire 60-s stimulation period. In the measuring periods interleaved with stimulation, a gradually increasing outward current was detected in all cells. The maximum current measured at the end of 60 s of stimulation ranged from 117 to 400 pA and averaged 193.6 ± 77.7 pA. The time course of development for the adaptation curve was measured by fitting an exponential curve to the current, as shown in Fig. 2A. The early phase of development of adaptation current could not be adequately fit by a single exponential, so a sum of exponentials was used to fit the curves. In all 13 cells, the time constants of the development of adaptation current closely matched the time constant of spike frequency adaptation; the early time constant averaged 1.2 ± 1.1 s and the late component averaged 23.5 ± 12.5 s. The decay of the adaptation current was obtained from the decay of holding current sampled at 20 kHz and downsampled to 2 kHz, as shown in Fig. 2B. It was also fit with a sum of two exponentials and these were similar to the values obtained for the accumulation of adaptation current. The fast time constant of decay averaged 1.9 ± 1.7 s and the slow time constant averaged 20.1 ± 5.3 s.
Fig. 2.
An example showing the growth and decay of the adaptation current during and after driven firing, respectively. The experimental protocol is shown at the top. Firing was driven by trains of 2-ms voltage pulses to 0 V from a holding voltage of −68 mV. During each pulse, voltage control was lost and the cell fired an incompletely clamped action potential. During 50-ms pauses in the trains, the holding current was measured at −68 mV. These occurred 4 times/s. A: the growth of current measured in pauses interleaved between trains of driven firing. Each point represents a measurement made during a single 50-ms pause. The solid red line shows the fit with a sum of exponentials with time constants as indicated. B: the decay of adaptation current after offset of driven firing in the same cell. A 2-exponential fit to current decay is shown as a solid line and the time constants are as given. In a sample of 13 cells studied, the adaptation current at the end of 60 s of 200-Hz firing (with 50-ms pauses every 0.2 s) averaged 193.6 ± 77.7 pA, the time constants of accumulation of current were 1.2 ± 1.1 and 23.5 ± 12.5 s, and the time constants of decay were 1.9 ± 1.7 and 20.1 ± 5.3 s.
Ionic composition of the adaptation current
To determine the species of ions responsible for the adaptation current, we initially performed a series of experiments like those shown in Fig. 2, but varied the holding potential during the decay period to attempt to reverse the adaptation current. This required holding the cells at very negative potentials for >1 min at each holding potential and we were unable to hold cells reliably below −90 mV for such long times without damaging the cells. In a sample of 14 cells measured in 2.5 mM extracellular K+, we obtained an extrapolated reversal potential based on only three to four measurements per cell. The measured reversal potential was −105 ± 20 mV, which was close to the calculated equilibrium potential for potassium (−106 mV). This took too long to combine with changes in K+ concentration, so we measured the reversal potential of the current using ramps.
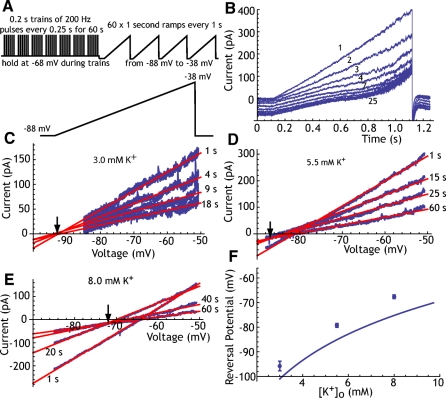
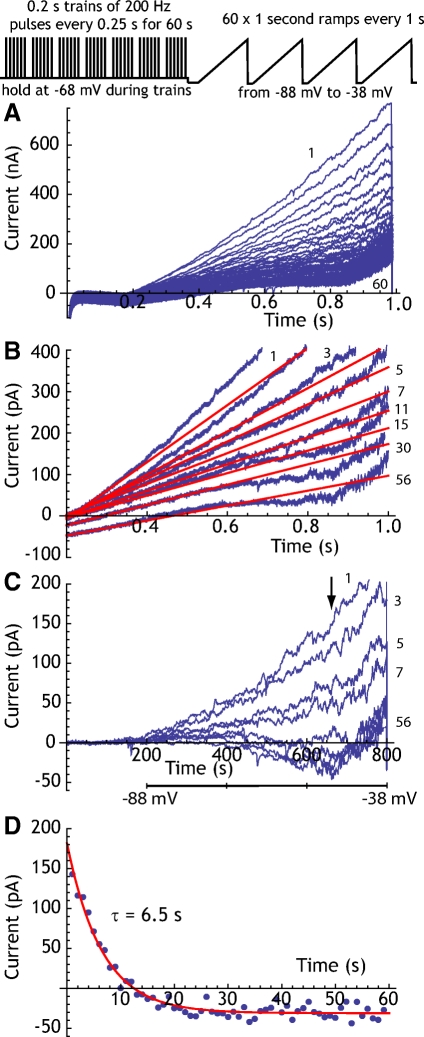
We applied trains of 200/s pulses in voltage clamp for 60 s as before, but during the recovery period we applied a series of voltage ramps from −88 to −38 mV applied every 1 s, as shown in Fig. 3, A–F. These experiments were conducted in eight neurons using whole cell recording and the K+ concentration of the extracellular solution was varied to manipulate the K+ equilibrium potential. In 3.0 mM extracellular K+ (with a calculated EK of −101 mV), the adaptation current was not reversed at −88 mV, as indicated by the inward shift of holding current at the most hyperpolarized level in the ramp during the decay of the adaptation current. We estimated the reversal potential of the adaptation current by fitting a straight line to the early linear part of the current response to the ramp voltage (between −88 and −60 mV) and extrapolating those lines to their intersection point (Fig. 3C). Increasing the extracellular K+ concentration to 5.5 mM shifted the reversal potential in the positive direction, into the range of our ramp (Fig. 3D). After this treatment, however, it was evident that the current in response to ramps early in the recovery period (during the first 1–5 s) reversed at a more positive potential than that of the remaining ramps. This can be seen in Fig. 3D, in which the ramp taken 1 s after termination of the pulses intersects the later ramp currents (15–60 s) at a point more positive than their common intersection. This suggested that there may be a different ionic composition of the adaptation current early in the decay period compared with later. Separating the ramps into early and late components showed that beginning 5–10 s after the stimulus trains the reversal potential was constant, but during the first few seconds of decay of adaptation current, there was no common intersection. Therefore we calculated the reversal potential of the longer-lasting component of the adaptation current, using the currents evoked by ramps from 10 to 60 s after termination of the stimulation. This procedure was repeated using 8 mM K+ extracellular solution in the same cells (Fig. 3E).
Fig. 3.
The reversal potential of the adaptation current. A: the voltage-clamp protocol used to measure the reversal potential. B: example currents evoked in one neuron by voltage ramps during recovery from adaptation at 3.0 mM K+. The numbers by traces indicate the ramp number (seconds since offset of driven firing). Note that at this K+ concentration the adaptation current is outward at all voltages tested (the traces do not cross). C: determining the reversal potential for the current traces in B. Red lines are the linear fit to the current over the voltage range between −88 and −60 for each ramp. Ramps are numbered by time since offset of driven firing. For clarity, only 4 ramps are shown, although this process was done for all ramps. The reversal potential is measured as the voltage at which the lines converge (arrow). D: as in C, except at 5.5 mM extracellular K+. For ramps given after the first 10 s, the crossing point is stable. For earlier ramps, there is no shared point of crossing, indicating that the reversal potential is changing over time. E: as in B and C, but at 8.0 mM extracellular K+. Again, a stable reversal point can be measured for late parts of the adaptation current decay. F: reversal potentials for a sample of 9 cells measured at 3.0, 5.5, and 8.0 mM extracellular K+. Error bars are SEs. The solid line is the K+ equilibrium potential calculated using 140 mM as the intracellular concentration of K+ (the concentration in the recording electrode).
The reversal potentials calculated in this way were plotted against the extracellular K+ concentration and compared with the expected K+ equilibrium potentials calculated using the electrode solution K+ concentration as the intracellular K+. The results of this measurement for eight neurons are shown in Fig. 3F. The reversal potentials were 8–10 mV more positive than the K+ equilibrium potential and tracked K+ concentration closely, indicating that the long-lasting phase of the adaptation current is mainly carried by K+ ions, although there may be a small contribution from an ion with a more positive equilibrium potential. For example, if there were a Na+ component of the late component current measured here, it would be <5% of the total current. At earlier times during the recovery from adaptation, on the other hand, there may be a large current contribution by ions with a more positive equilibrium potential. Because the reversal potential of the adaptation current is changing rapidly during this the first 1–5 s, our method does not allow a good estimate of its ionic composition at that time.
What triggers the adaptation current?
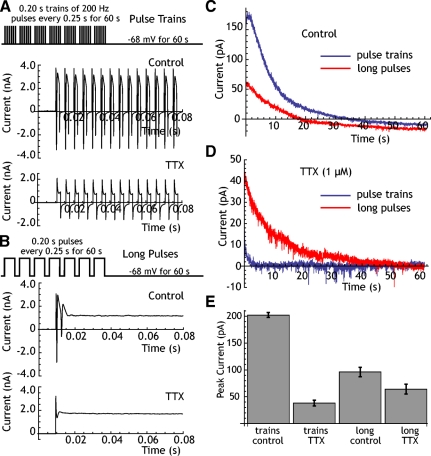
The adaptation current triggered by trains of 200/s pulses as shown in Fig. 4 was blocked by TTX, indicating that it was the series of action potentials triggered by the pulses, and not the voltage-clamped waveform, that was responsible for activating the current. However, trains of 2-ms pulses delivered in voltage clamp were probably not very effective at depolarizing the neurons in our sample because of uncompensated cell capacitance. To determine whether action potentials and Na+ entry were necessary to trigger the adaptation current or whether the adaptation current depended only on depolarization, we attempted to evoke it without action potentials using 0.2-s depolarizing voltage steps to 0 mV, presented every 0.25 s for 60 s. Although poorly controlled action potentials occurred at the onset of these pulses, inactivation limited sodium current during most of each pulse (Fig. 4B). After the offset of this train of voltage pulses, we observed an adaptation current that decayed at the same rate as that seen after trains of action potentials (Fig. 4C). Although the adaptation current triggered by brief depolarizing pulses was mostly gone after treatment with TTX, the current evoked by long pulses (which are more effective at depolarizing the cell) were mostly intact (Fig. 4D). We then measured the amplitude and decay time constants of this adaptation current after application of TTX in five neurons (Fig. 4E). TTX did not block the adaptation current when it was triggered by long pulses (control: 96 ± 44 pA; TTX: 64 ± 45 pA, t = 2.36, df = 4, P > 0.05). The time course of the adaptation current was also not changed by TTX under these conditions, being fit with a faster and a slower component (control τdecay = 1.9 and 13.8 s; TTX: τdecay = 3.0 and 20.1 s). This result indicates that the adaptation current measured at −68 mV does not require entry of Na+ into the cell during action potentials, but is triggered by depolarization. As expected from previous reports (Beurrier et al. 1999; Bevan and Wilson 1999), there was no detectible TTX-sensitive current at the holding potential of −68 mV (−4 ± 18.4 pA).
Fig. 4.
The adaptation current is triggered by depolarization during action potentials. A: action potential train stimulation protocol and fast sweep traces showing currents evoked in control solution and after application of tetrodotoxin (TTX). Action currents are not triggered after TTX. B: pulse stimulation protocol, in which each train of stimulation pulses is replaced by a single long depolarizing pulse. In control solution, only one full action current is triggered at the beginning of the pulse. After TTX, this action current is blocked. C: comparison of the decay of adaptation current seen during the recovery period using trains and long pulses. Long pulses are less effective. D: after TTX blockade of action potentials, trains evoke almost no adaptation current, whereas long pulses are nearly as effective as they were under control conditions. E: comparison of currents evoked in a sample of 5 neurons by trains and by long pulses before and after TTX.
Inactivation of persistent Na+ current
The change from a linear to a concave ramp response seen in Fig. 3B during recovery from adaptation suggests that the adaptation current may not be solely attributable to an accumulating K+ current. In addition to the increase in the voltage-independent membrane conductance that dominated the response at the most hyperpolarized membrane potentials, there was also a change in the voltage-dependent component of the response to voltage ramps during recovery from adaptation. It was previously established that STN neurons have a prominent persistent Na+ current that activates positive to −65 mV and is primarily responsible for the curvature in the current response to a ramp voltage (Beurrier et al. 1999; Bevan and Wilson 1999; Do and Bean 2003). This current generates the negative slope conductance seen in the STN neurons' steady-state current–voltage (I–V) curve at rest and drives spontaneous firing. Slow inactivation of this current has been shown to contribute to the silent period of STN neurons after high-frequency firing (Beurrier et al. 2001). Because the persistent Na+ current is normally a contributor to the resting current above −65 mV, its inactivation would appear as an outward component of the holding current at these voltages.
To measure the contribution of inactivation of persistent Na+ current to the adaptation current in STN neurons, we again used the changes in current evoked by ramp voltages during recovery from adaptation. The results are shown in Fig. 5. A series of currents evoked by 60 ramp voltages applied during the decay of the adaptation current are shown in Fig. 5A. The increase in K+ conductance measured in Fig. 3 produced a large current throughout the ramp. This component of the response was estimated by fitting a line to the current during the first 100 ms of the ramp, corresponding to voltages between −88 and −82 mV. In this part of the ramp the I–V relation was linear, as shown in Fig. 5B, and this corresponds to the component whose reversal potential was measured in the previous section. The conductance was estimated from the −88- to −82-mV part of the ramp and the predicted current was subtracted from the remainder of the response to the ramp to yield currents not accounted for by the adaptation current already described. This method allows an estimate of the contribution of the voltage-independent K+ conductance adaptation current, although it also contains all voltage-insensitive currents not affected by adaptation (e.g., Fig. 5C). This residual current contained the voltage-sensitive slowly or noninactivating currents activated between −80 and −40 mV. It included the persistent Na+ current, whose contribution grew rapidly over the first 10 s of recovery from inactivation and that dominates the current after recovery from adaptation. It also included a voltage-dependent outward current that activated rapidly at voltages more positive than −50 mV. To estimate the recovery of the persistent Na+ current, an average current was calculated for a 50-ms period centered on 650 ms after the start of each ramp (corresponding to about −48 mV on the ramp). Recovery of the persistent Na+ current after termination of the stimulus trains was measured as an inward trend in this current. Its time course is shown in Fig. 5D. For 10 cells measured in this way, the time constant of the recovery of the persistent Na+ current averaged 5.0 ± 2.1 s.
Fig. 5.
Measurement of the recovery of inactivation of the persistent Na+ current. A: the experimental protocol used to measure persistent Na+ current. As for the experiments in Fig. 3, currents evoked by ramp-voltage changes were measured every 1 s during the decay of adaptation current after induction by trains of driven firing. Examples showing the sequence of ramp currents evoked in one neuron. The first and last traces are labeled with numbers indicating seconds since offset of the trains of driven firing. B: some selected responses to voltage ramps from A are shown at higher resolution. Solid red lines are the estimate of the voltage-insensitive current, obtained by fitting to the linear part of the current trace (between −88 and −82 mV). C: residual currents, obtained by subtracting the linear fit from the ramp currents. The ramps are the same ones shown in B. The influence of inactivation of persistent sodium current was measured as the change in current over traces at the time indicated by the arrow. The voltage scale corresponding to the ramp timescale is shown below the abscissa. D: the removal of inactivation as measured in C, for the example cell shown in A–D. A fit to a single decay time constant is shown. For the sample of 10 cells, the time constant averaged 5.0 ± 2.1 s.
DISCUSSION
Multiple mechanisms of spike frequency adaptation
STN neurons characteristically have brief action potentials and it has often been noted that they are capable of firing at relatively high rates for periods of ≥1 s with minimal spike frequency adaptation or even reverse spike frequency adaptation (Wilson et al. 2004). The absence of a strong spike frequency adaptation over these timescales is also associated with the absence of any accumulation of the medium-duration afterhyperpolarization (mAHP) current during repetitive firing (Teagarden et al. 2008). Teagarden et al. (2008) observed a small and slow but accumulating AHP current in STN neurons driven to fire at high rates for ≥1 s. They showed that this current was not a calcium-dependent potassium current and, although relatively small (<50 pA) after 1 s of driven firing, it decayed very slowly and so might be capable of accumulating to much higher levels with prolonged stimulation. Another indication that STN cells may have an accumulating slow AHP current is evident from observations of a cessation of their spontaneous firing following brief episodes of high-frequency driven firing (Beurrier et al. 2001; Bevan and Wilson 1999; Hallworth et al. 2003). Beurrier et al. (2001) showed that this pause, induced by direct stimulation from extracellular electrodes, was not abolished by blockade of calcium channels and Hallworth et al. (2003) showed that a similar pause that followed firing driven by intracellular current injection was also not abolished (although it was reduced) by blockade of calcium-dependent potassium channels responsible for the mAHP. Beurrier et al. (2001) noted that excitability of the cells was greatly reduced during the pause in firing and that the persistent sodium current was significantly suppressed during the silent period; they suggested that slow inactivation of the persistent sodium current might be the principal cause of the suppression of spontaneous activity. The slow inactivation of persistent sodium current was studied in detail by Do and Bean (2003). They showed that not only the persistent but also the transient and resurgent components of the sodium current in STN neurons do undergo slow inactivation after high-frequency activity. However, the time course of recovery of the sodium currents from inactivation did not match the time course of recovery of spontaneous firing reported by Beurrier et al. (2001). Slow inactivation of persistent sodium current had a time constant near 5 s (Do and Bean 2003), whereas spontaneous activity could be suppressed by high-frequency firing for >1 min.
We have confirmed the results of Beurrier et al. (2001) using intracellular current and extended it to show that the same mechanism responsible for the pause in spontaneous firing can produce a powerful spike frequency adaptation over the same slow time course. Using firing driven by voltage-clamped pulses we measured an adaptation current responsible for both the spike frequency adaptation and the reduced excitability and pause in spontaneous firing that follows high-frequency firing in STN neurons. The adaptation current was insensitive to buffering of intracellular calcium with high concentrations of BAPTA, confirming that it does not arise from a calcium-dependent potassium channel. The adaptation current consisted primarily of a slowly accumulating potassium current, which was especially dominant at the longest timescale. Inactivation of persistent sodium current was seen as previously reported by Beurrier et al. (2001). But in agreement with Do and Bean (2003), it decayed with a time constant of about 5 s and so was not responsible for the slowest component of either the spike frequency adaptation or the reduction in excitability following high-frequency stimulation. Over the range of voltages visited by the membrane potential in the interspike interval (ISI), the persistent sodium current is relatively small. Its complete inactivation would effectively produce an outward current of about 50 pA, whereas the slow potassium current averaged about 200 pA at −68 mV after 60 s of stimulation.
What is the K+ adaptation current?
The properties of this current do not resemble those of channels usually associated with spike frequency adaptation. The best known examples of spike frequency adaptation develop much more rapidly and also decay much faster than the adaptation studied here. The time constant of accumulation and decay of the adaptation current suggests that whatever mechanism is responsible is very slowly decaying. Our observations suggest that this current may have both a faster and a slower component. Accumulation and decay curves of the adaptation current required description by a sum of two time constants, one near 1 s and the other near 20 s. The faster process is probably responsible for the tail currents measured by Teagarden et al. (2008) after 1-s periods of driven firing. Neither of these currents are evident after single action potentials or even brief trains of action potentials. Generally, accumulation of an exponentially decaying spike aftercurrent will grow according to IAHP = ΔI/[−1 + e−1/(fτ)], in which ΔI is the increment of current generated by a single action potential, τ is the time constant of decay of the current after an action potential, and f is the firing rate. Using an average value of 200 pA at a firing rate of 200/s and a time constant of −20 s (for the slowest component of the current), our results suggest that each action potential generates about 0.05 pA of adaptation current on average. Although this is a vanishingly small effect for a single action potential, this current can become large enough to dominate the cell's current balance after 60 s of high-frequency firing.
Implications for STN cell function
Spike frequency adaptation serves partly to counteract changes in firing rate caused by slow changes in synaptic input. It acts as a high-pass filter on synaptic inputs, counteracting changes that are as slow as or slower than the time required for accumulation of the adaptation current. Adaptation caused by relatively large, fast AHP currents following single action potentials can counteract temporal correlation in the input and even generate negative correlations in successive ISIs (e.g., Wang 1998). For extremely slow adaptation currents requiring many action potentials over a period of seconds, the response to brief changes in input may be relatively preserved, whereas the cell is rendered insensitive to slow changes in input levels. At high firing rates it preserves the response of the cell to transient inhibition or disfacilitation, while dampening but not blocking the effects of transient excitation or disinhibition.
STN neurons are autonomously active neurons that fire at rates of 5–30 spikes/s when isolated from synaptic inputs (Abbott et al. 1997; Beurrier et al. 1999; Bevan and Wilson 1999; Hallworth et al. 2003; Nakanishi et al. 1987; Wigmore and Lacy 2000). The autonomous firing seen in slices or in dissociated cells (Do and Bean 2003) is not much different from the range of rates seen in intact animals, when the cells are not responding to a specific stimulus (Georgeopoulos et al. 1983; Isoda and Hikosaka 2008; Matsumura et al. 1992; Wichmann et al. 1994) or the rate seen in intact anesthetized animals (Fujimoto and Kita 1993; Hollerman and Grace 1992; Ryan and Clark 1992). This is so despite the fact that STN neurons receive an enormous number of synapses made by neurons that are tonically active in these same preparations. For example, each STN neuron in rats receives about 900 synapses from GABAergic cells of the external segment globus pallidus (GPe; Baufreton et al. 2009), each of which is firing tonically at rates of 40–120/s in resting animals in vivo (e.g., Gardiner and Kitai 1992). A large excitatory input arises from a large region of the cerebral cortex (Kitai and Deniau 1981). Cortical projection cells in these regions are also tonically active in vivo, although they individually fire at a much lower rate (e.g., Stern et al. 1997). Antagonists of glutamatergic transmission block the response to cortical stimulation in vivo, but they have no substantial effect on the background firing rate (Rouzaire-Dubois and Scarnati 1987). In intact animals, destruction of the GPe, which should release the STN from tonic inhibition, produces almost no change in the mean firing rate of STN cells (Fujimoto and Kita 1993). The effectiveness of the slow spike frequency adaptation current studied here in equalizing mean rate over a wide range of input currents is demonstrated in Fig. 1C, in which the mean rate of firing after adaptation can be seen to remain mostly within the range of resting firing rates seen in vivo, despite the passage of very large depolarizing currents.
This mechanism may be responsible for a perplexing feature of STN neuron firing in Parkinson's disease and in animal models of the disease. It was widely expected that firing rates in the STN would be greatly increased in the disease because of a reduced inhibition from the GPe. Measurements of firing rate in STN neurons in parkinsonian animals (e.g., Bergman et al. 1994; Hollerman and Grace 1992; Wilson et al. 2006) have failed to show large expected changes in rate, although small increases in rate are common. We suggest that tonic changes in the overall level of input may produce smaller than expected changes in mean rate in the STN because of the adaptation mechanism studied here. During steady adaptation, transient high-frequency firing in response to rapid changes in input is still possible, but requires stronger stimuli because of the presence of the constant outward adaptation current. Because the slow adaptation current is a high-voltage–activated potassium current, it is effectively controlled by the firing rate of the neurons and so will increase or decrease in response to anything that changes firing rate. Its activation is independent of whether the change in rate arises because of increases or decreases in excitation or inhibition and whether the inhibition is primarily hyperpolarizing or shunting in nature.
Because the slow adaptation current is effectively a constant hyperpolarizing current at high rates, it helps to repolarize the cell membrane potential and prevent the accumulation of sodium channel inactivation during high-frequency firing. This will work to prevent depolarization block when cells are driven at high rates. It contributed to the maintenance of high-frequency following of the incompletely clamped action potentials during our pulse-train stimulation. In the same way, it should contribute to maintenance of high-frequency following of STN neurons during deep brain stimulation. It will also reduce the sensitivity of STN neurons to inputs from the cortex and globus pallidus, which may carry pathologically synchronous patterns of activity.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-47085 to H. Kita and C. J. Wilson and National Science Foundation Undergraduate Mathematics and Biology Scholar Grant EF-0634588 to D. Barraza.
REFERENCES
- Abbott A, Wigmore MA, Lacey MG. Excitation of rat subthalamic nucleus neurones in vitro by activation of a group I metabotropic glutamate receptor. Brain Res 766: 162–167, 1997 [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult brain slices. Synapse 3: 331–338, 1989 [DOI] [PubMed] [Google Scholar]
- Baldissera F, Gustafsson B. Regulation of repetitive firing in motoneurons by the afterhyperpolarization conductance. Brain Res 30: 431–434, 1971 [DOI] [PubMed] [Google Scholar]
- Baldissera F, Gustafsson B, Parmiggiani F. Saturating summation of the afterhyperpolarization conductance in spinal motoneurones: a mechanism for “secondary range” repetitive firing. Brain Res 146: 69–82, 1978 [DOI] [PubMed] [Google Scholar]
- Baufreton J, Kirkham E, Atherton JF, Menard A, Magill PJ, Bolam JP, Bevan MD. Sparse but selective and potent synaptic transmission from the globus pallidus to the subthalamic nucleus. J Neurophysiol 102: 532–545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of Parkinsonism. J Neurophysiol 72: 507–520, 1994 [DOI] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85: 1351–1356, 2001 [DOI] [PubMed] [Google Scholar]
- Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J Neurosci 19: 599–609, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Wilson CJ. Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons. J Neurosci 19: 7617–7628, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MTH, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron 39: 109–120, 2003 [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kita H. Response characteristics of subthalamic neurons to the stimulation of the sensorimotor cortex in the rat. Brain Res 609: 185–192, 1993 [DOI] [PubMed] [Google Scholar]
- Gardiner TW, Kitai ST. Single-unit activity in the globus pallidus and neostriatum of the rat during performance of a trained head movement. Exp Brain Res 88: 517–530, 1992 [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, DeLong MR, Crutcher MD. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci 3: 1586–1598, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallworth NE, Wilson CJ, Bevan MD. Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus neurons in vitro. J Neurosci 23: 7525–7542, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Grace AA. Subthalamic nucleus cell firing in the 6-OHDA-treated rat: basal activity and response to haloperidol. Brain Res 590: 291–299, 1992 [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci 28: 7218–7209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass JI, Mintz IM. Silent plateau potentials, rhythmic bursts, and pacemaker firing: three patterns of activity that coexist in quadristable subthalamic neurons. Proc Natl Acad Sci USA 103: 183–188, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai ST, Deniau JM. Cortical inputs to the subthalamus. Brain Res 214: 411–415, 1981 [DOI] [PubMed] [Google Scholar]
- Matsumura M, Kojima J, Gardiner TW, Hikosaka O. Visual and oculomotor functions of monkey subthalamic nucleus. J Neurophysiol 67: 1615–1632, 1992 [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Electrical membrane properties of rat subthalamic nucleus neurons in an in vitro slice preparation. Brain Res 437: 35–44, 1987 [DOI] [PubMed] [Google Scholar]
- Otsuka T, Murakami F, Song W-J. Excitatory postsynaptic potentials trigger a plateau potential in rat subthalamic neurons at hyperpolarized states. J Neurophysiol 86: 1816–1825, 2001 [DOI] [PubMed] [Google Scholar]
- Partridge LD, Stevens CF. A mechanism for spike frequency adaptation. J Physiol 256: 315–332, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzaire-Dubois B, Scarnati E. Pharmacological study of the cortical-induced excitation of subthalamic nucleus neurons in the rat: evidence for amino acids as putative neurotransmitters. Neuroscience 21: 429–440, 1987 [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Sanders DJ, Clark KB. Auto- and cross-correlation analysis of subthalamic nucleus neuronal activity in neostriatal- and globus pallidal-lesioned rats. Brain Res 583: 253–261, 1992 [DOI] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol 77: 1697–1715, 1997 [DOI] [PubMed] [Google Scholar]
- Teagarden M, Atherton JF, Bevan MD, Wilson CJ. Accumulation of cytoplasmic calcium, but not apamin-sensitive afterhyperpolarization current, during high frequency firing in rat subthalamic nucleus cells. J Physiol 586: 817–833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-J. Calcium coding and adaptive temporal computation in cortical pyramidal cells. J Neurophysiol 79: 1549–1566, 1998 [DOI] [PubMed] [Google Scholar]
- Wilson CL, Cash D, Galley K, Chapman MG, Lacey MG, Stanford IM. Subthalamic nucleus neurones in slices from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mice show irregular, dopamine-reversible firing pattern changes, but without synchronous activity. Neuroscience 143: 565–572, 2006 [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Weyrick A, Terman D, Hallworth NE, Bevan MD. A model of reverse spike frequency adaptation and repetitive firing of subthalamic nucleus neurons. J Neurophysiol 91: 1963–1980, 2004 [DOI] [PubMed] [Google Scholar]