Abstract
Learning-correlated changes in the excitability and photoresponses of Hermissenda's ocular type B photoreceptors are mediated by reductions in two distinct K+ currents, IA and IK-Ca. The suppression of these K+ currents has been linked to conditioning-produced activation of protein kinase C (PKC). The question of whether PKC accounts completely for the changes in excitability and K+ currents or whether other kinase(s) are involved has received little attention. In the present experiments, we asked whether protein tyrosine kinases (PTKs) might also contribute to conditioning-produced alterations in B cells. We found that the PTK inhibitors genistein and lavendustin A greatly reduced cumulative depolarization of type B cells, a short-term correlate of associative learning. This disruption occurred even when PKC activation had been either occluded by preexposure of type B cells to a phorbol ester or otherwise prevented by the pseudosubstrate inhibitor peptide PKC[19–31]. PTK inhibitors also increased the amplitude of the transient (IA) and delayed (IDelayed) components of voltage-dependent K+ current that have previously been shown to be selectively reduced by conditioning and to contribute to cumulative depolarization. Genistein partially prevented the reduction of IA and IDelayed due to in vitro conditioning and blocked the changes in their voltage dependencies. Ionophoresis of pervanadate ion, a potent inhibitor of protein tyrosine phosphatases, depolarized type B photoreceptors and occluded conditioning-produced cumulative depolarization. Pervanadate also suppressed IA and IDelayed, reduced their voltage dependence, and altered inactivation kinetics for IA, mimicking conditioning. Western blot analysis using a phosphotyrosine antibody indicated that conditioning increased the phosphotyrosine content of many proteins within the Hermissenda CNS. Collectively, our results suggest that in addition to PKC, one or more PTKs play an important role in conditioning-produced changes in type B cell excitability. PTKs and PKCs converge to effect reductions in B cell K+ currents during conditioning, apparently through distinct biophysical mechanisms.
INTRODUCTION
Repeated pairings of light and rotation (conditioning) result in persistent changes in the excitability of ocular type B photoreceptors of Hermissenda crassicornis (H.c.) (Crow and Alkon 1980; Farley 1987, 1988; Farley and Alkon 1982). These cells play important roles in the suppression of phototactic behavior by conditioning (Farley et al. 1983; Goh et al. 1985).
The calcium/phospholipid-dependent enzyme family, protein kinase C (PKC), plays a crucial role in learning-correlated changes in type B photoreceptor excitability. Exposure of B cells to phorbol esters or injections of exogenous PKC mimic many of the effects of conditioning on input resistance, light response, and voltage-dependent K+ currents (Farley and Auerbach 1986; Farley and Schuman 1991). PKC inhibitors block conditioning-produced changes in B cell excitability (Crow and Forrester 1993; Farley and Schuman 1991; Matzel et al. 1991) and also reverse reductions in K+ currents apparent during retention days (Farley and Schuman 1991).
In addition to their roles in cellular differentiation and growth (Pazin and Williams 1992; Sun and Tonks 1994), protein tyrosine kinases (PTKs) are increasingly implicated in the control of membrane potential and regulation of receptor (Hopfield et al. 1988; Moss et al. 1995; Narisawa-Saito et al. 1999; Valenzuela et al. 1995; Wallace 1995; Wang and Salter 1994; cf. Thornton et al. 2003) and ion channel activities (Boixel et al. 2000; Jonas and Kaczmarek 1996; Lev et al. 1995).
Several studies have suggested a direct (Grant et al. 1992; O'Dell et al. 1991) or modulatory (Terlau and Seifert 1989) involvement of PTKs in hippocampal long-term potentiation (LTP) (see also Kang and Schuman 1995; Patterson et al. 1992), as well as in cerebellar long-term depression (LTD) (Boxall et al. 1996), prominent cellular models of learning and memory in the vertebrate brain. At Aplysia synapses, a Trk-like presynaptic receptor (ApTrkI) has been reported to be involved in long-term facilitation (Ormond et al. 2004; Purcell et al. 2003). PTKs have also been implicated in several other learning and memory-related processes (Rattiner et al. 2004; Schafe et al. 1996; Yamada and Nabeshima 2003; Zhao et al. 2000).
The reports that PTKs are implicated in learning-related neural plasticity in several systems and that voltage-dependent K+ channels are modulated by PTKs in some cell types prompted us to examine the possible involvement of PTKs in conditioning-produced changes in H.c. type B photoreceptors. The results presented here indicate that the PTK inhibitors genistein and lavendustin A reduced in vitro conditioning-produced changes in type B photoreceptor excitability and increased the amplitude of two components of K+ current (transient [IA] and delayed [IDelayed]) that are reduced by conditioning. Pervanadate ion, a potent tyrosine phosphatase inhibitor, mimicked the effects of in vitro conditioning. Pervanadate depolarized B cells, suppressed K+ currents, and occluded the effects of in vitro conditioning. Behavioral conditioning was also accompanied by increases in the phosphotyrosine content of many proteins within the Hermissenda CNS. Collectively, our results suggest that PKC and PTK(s) both contribute to learning-produced alterations in type B cell K+ currents, with distinguishable biophysical effects.
METHODS
Animals
Adult Hermissenda were obtained from Sea Life Supply (Sand City, CA) and maintained as previously described (Farley 1988) in 30- or 40-gallon artificial sea water (ASW) aquaria, at 15°C. Each aquarium was illuminated on a 12-h light/dark cycle. The animals were fed with small pieces of Mytilus every other day.
Nervous system preparation
The isolated circumesophageal nervous system was dissected from the animal and prepared for recording as previously described (Farley and Alkon 1982, 1987) (Fig. 1 A). A nervous system was placed on a glass microscope slide, within an approximately 300- to 500-μL well of standard ASW, circumscribed by walls of Vaseline petroleum jelly. The nervous system was immobilized between two strips of Vaseline using stainless steel insect pins. In voltage-clamp experiments, type B photoreceptors were synaptically isolated by means of a razor lesion of the optic nerve, as previously described (Farley and Auerbach 1986).
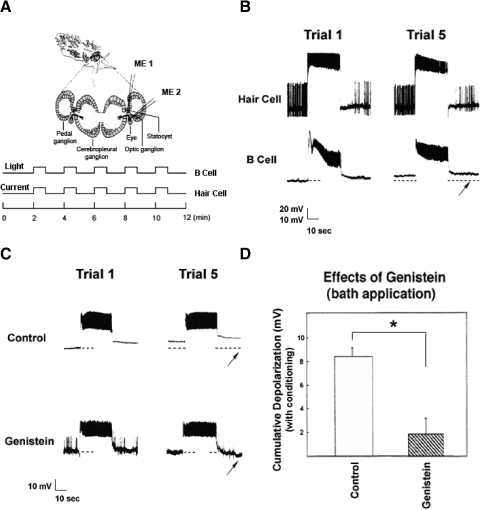
Fig. 1.
Bath application of the tyrosine kinase inhibitor genistein blocks in vitro conditioning-produced cumulative depolarization of type B photoreceptors. A: nervous system preparation and in vitro conditioning protocol. Simultaneous intracellular recordings from a type B photoreceptor (using microelectrode #2; ME2) and an ipsilateral caudal hair cell (ME1) were obtained. Following an initial 10 min of dark adaptation, 5 pairings of 30 s of light with 30 s of intracellular current stimulation of the hair cell were administered, at 2-min intervals. B: light stimulation of a type B photoreceptor (bottom trace) paired with depolarizing current stimulation of a caudal statocyst hair cell (top trace) results in a nearly 9-mV depolarization of the B cell. Hair cell stimulation elicited a high frequency train of action potentials, followed by a transient afterhyperpolarization (AHP) and reduction in spiking below the initial baseline level. Whole-field visual stimulation of the photoreceptor resulted in a sustained depolarizing generator potential and superimposed spiking in the type B cell. Dashed line underneath the type B photoreceptor record indicates the original baseline membrane potential of the B cell; the arrow indicates the 30-s time point following trial 5. This preparation was bathed in a 0.3% dimethyl sulfoxide (DMSO) artificial seawater (ASW). C: with 100 μM genistein in the bath, pairings of light and hair cell stimulation (not shown) resulted in a small hyperpolarization (∼1–2 mV) of this B cell, measured 30 s after trial 5 (arrow). The light response and spiking of the type B photoreceptor were not conspicuously affected by genistein, although genistein-treated cells generally showed a reduced long-lasting depolarization (LLD) response following light offset. D: summary data for B cells conditioned in the presence or the absence of bath-applied genistein. Depolarization was measured 2 min following the 5th conditioning trial. Cells exposed to genistein exhibited significantly less conditioning-produced cumulative depolarization than that of cells exposed to the DMSO bath solution. Error bars in this and all subsequent figures = ±SE.
Electrophysiology
Sharp-electrode intracellular recordings of type B photoreceptors and ipsilateral statocyst hair cells were obtained, at room temperature (20°C), as previously described (Farley 1987; Farley and Alkon 1987) (Fig. 1A). Cell impalement was facilitated by proteolytic digestion of encapsulating tissue. The exact conditions used varied as a function of the particular lot of protease (Type XXVII, cat. #4789; Sigma, St. Louis, MO). In general, the concentration ranged between 1 and 4 mg/mL of ASW. Exposure times ranged between 8 and 15 min at room temperature (20°C). Following incubation in protease, the nervous system was washed with a minimum of six volumes of 15°C ASW. Glass microelectrodes (cat. #6020; A-M Systems) filled with 3 M KCl (30–40 MΩ), connected to the head stages of high-impedance amplifiers (Axoclamp 2A, Axon Instruments, Foster City, CA), were used. Electrical stimulation of photoreceptors and hair cells was accomplished by injecting current through the recording electrode via a bridge circuit. Continuous records of electrical activity for each experiment were PCM-digitized using a Neuro Data Recorder (model #284; Neuro Data Instruments, New York, NY), stored on VCR (Panasonic #PV-1530) tapes, and displayed on a digital oscilloscope (model #5020A; Kikusui America, Torrance, CA) and a chart recorder (model #260; Gould Instrument Systems, Cleveland, OH). A 110-V light source was used for visual stimulation, at an intensity of about 300 μW·cm−2 when measured at the preparation site.
All type B photoreceptors for which current-clamp measurements are reported satisfied the following criteria: 1) resting membrane potential (Vm) more negative than −40 mV, 2) a steady-state light response (20–30 s after light onset) >10 mV, and 3) a resting input resistance (Rin) >20 MΩ. For hair cells, the criteria for acceptance were: 1) a Vm more negative than −20 mV, 2) the occurrence of spontaneous action potentials (APs) with amplitudes >40 mV, and 3) elicitation of trains of APs (5–15 Hz) by depolarizing current injections (0.5–1.5 nA). Cells that failed to satisfy these criteria were considered damaged and were excluded from further study. These criteria were essentially identical to those used in previous studies from this laboratory (Farley 1987; Farley and Alkon 1987; Farley and Schuman 1991; Huang and Farley 2001).
In vitro conditioning procedure
Previous research has shown that an in vitro conditioning protocol, in which light is paired with stimulation of statocyst hair cells, results in a pairing-specific cumulative depolarization of type B photoreceptors (Farley 1987; Farley and Alkon 1982, 1987; Farley and Schuman 1991; Grover et al. 1989). Individual hair cells in the caudal portion of the statocyst inhibit medial and intermediate type B photoreceptors (Alkon 1974; Farley and Alkon 1987) not only through direct, monosynaptic GABAergic synapses (Alkon et al. 1993), but also through inhibition of the S/E optic ganglion cell that (in the absence of hair cell inhibition) synaptically excites type B photoreceptors (Tabata and Alkon 1982). Following trains of APs in caudal hair cells (e.g., a pairing of light and caudal hair cell stimulation), the type B photoreceptors are excited via increased excitatory feedback from the disinhibited S/E optic ganglion cell, as well as through disinhibition from caudal hair cells, which show prolonged afterhyperpolarization (AHP) and cessation of spike activity (for detailed discussion, see Fig. 8 and associated text in Farley and Alkon 1987).
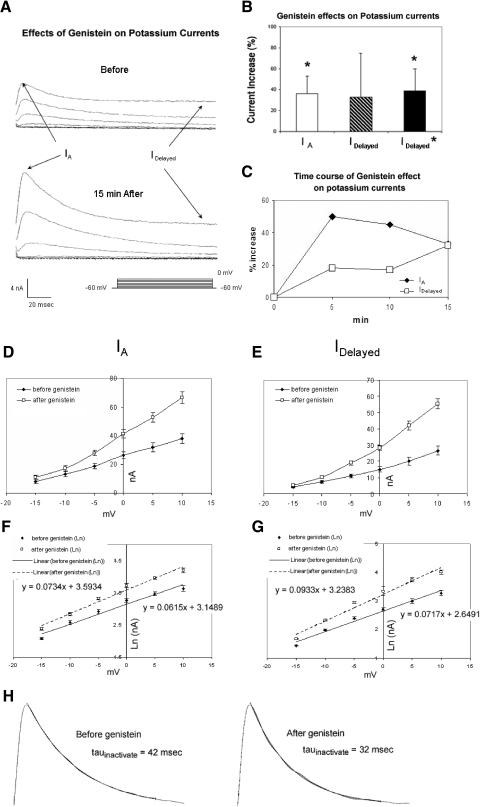
Fig. 8.
Genistein increases K+ currents of type B photoreceptors. A, top traces: currents elicited by depolarization of a voltage-clamped type B photoreceptor to indicated membrane potentials (see bottom right), just prior to genistein exposure. Bottom traces: currents from the same cell recorded 15 min following exposure to genistein. Both the transient and sustained components of K+ current were increased. B: summary data depicting genistein's enhancement of the transient K+ current (IA), at 0 mV, for 5 cells measured 15 min after bath application. The sustained K+ current (IDelayed *) was also significantly enhanced after the results of one anomalous cell were omitted. Enhancement is expressed as a percentage of the original baseline (pregenistein) value. C: time course of genistein's enhancement of K+ currents. Genistein's effects were apparent within a few minutes of application, peaked at about 5 min for IA and slowly declined thereafter. IDelayed continued to increase over the next 10 min. D–G: summary I–V relationships for IA and IDelayed, before and after exposure of cells (n = 4) to genistein. Genistein increased the voltage dependence of both the A current (D, F) and IDelayed (E, G). In the absence of genistein, IA and IDelayed both showed e-fold increases in amplitude per about 14 mV (from −15 to +10 mV). Genistein enhancement of IA and IDelayed was accompanied by an average 19% increase in the voltage dependence of IA and a 30% increase in voltage dependence for IDelayed. These changes are most apparent in the slopes of semi-log (log–linear [log–lin]) plots in F and G (indicated by the linear regression lines and associated equations). H: genistein increases the rate of inactivation of IA. Examples shown are of single-exponential fits of current records of IA inactivation (at +10 mV), before (left) and 10 min after (right) genistein exposure. Genistein increased IA inactivation by roughly 26%. Current traces have been scaled to the same level.
Cumulative depolarization of a type B cell is accompanied by an increase in resting input resistance (Rin) and suppression of transient and delayed components of voltage-activated K+ currents (Farley 1987; Farley and Schuman 1991). In addition to being an extremely reliable outcome of associative conditioning, cumulative depolarization of type B cells has also been proposed to contribute to the production of persistent changes in B cell excitability (Alkon 1980; Farley and Alkon 1987), through voltage-dependent inactivation and calcium-mediated suppression of K+ currents (Farley and Alkon 1987; but see Matzel and Rogers 1993).
The in vitro conditioning protocol used here was essentially identical to that of earlier studies (Farley 1987; Farley and Alkon 1987; Farley and Schuman 1991) (Fig. 1A). After stable recordings from a type B photoreceptor and an ipsilateral caudal hair cell were obtained, the preparation was dark-adapted for 10 min. When it was possible, Rin of the B cell was measured during the final 2 min of the dark-adaptation period, from the current–voltage (I–V) plot obtained by injecting 200-ms current steps (spanning the range from −0.5 to +0.2 nA, in 0.1-nA increments) into the B cell through a balanced bridge circuit. However, in many experiments in which a drug or peptide was present in the electrode, the high resistance (>100 MΩ) and/or plugging of the electrode precluded accurate bridge balancing and measurement of Rin.
Following dark adaptation, associatively trained preparations were exposed to five successive 30-s conditioning trials at 2-min intervals (see Fig. 1A). Each trial consisted of 30 s of whole-field illumination of the preparation (∼300 μW·cm−2, at 510 nm) and 30 s of 0.5- to 1.5-nA depolarizing current stimulation of a caudal hair cell.
Two minutes following the fifth trial, the resting membrane potential of the type B cell was recorded. Previous results indicate that membrane potential changes only slightly (<1.0 mV) after this time (Farley and Alkon 1987). In the majority of experiments, no attempt was made to measure Rin after in vitro conditioning because of the bridge-balancing and electrode tip-clogging problems mentioned earlier.
Drug delivery
Genistein, a widely used inhibitor of protein tyrosine kinases (Akiyama et al. 1987), was applied in the bath, ≥30–40 min prior to the start of in vitro conditioning. In other experiments, genistein or lavendustin A was introduced into a type B cell by leakage from the tip of a low-resistance microelectrode (∼10–15 MΩ when filled with 3 M KCl), for about 30 min prior to the start of in vitro conditioning.
Before its use in in vitro conditioning experiments (e.g., Fig. 4), we first determined ionophoresis conditions for the synthetic peptide inhibitor of PKC, PKC[19–31] (House and Kemp 1988), which blocked reductions in B cell K+ currents caused by bath-applied phorbol 12-myristate, 13-acetate ester (PMA) application. These conditions, also used in subsequent in vitro conditioning experiments, were as follows. Following a 10-min dark-adaptation period, a 3-min exposure to +4.0-nA current pulses (200 ms, 1 Hz) was used to eject the peptide. An average somatic B cell volume of about 5.3 × 10−10 L was estimated from simple geometry, assuming the soma of the cell to be a simple sphere with a radius of 50 μm. Upper- and lower-limit transference numbers for the peptide of 0.0004 and 0.017 were empirically determined (see following text). Using the Faraday–Hittdorf law (Purves 1980), the cited transport numbers and a valence (z) of +12 for the peptide, we estimated the intracellular concentration to range between 150 and 600 nM, which exceeds the half-maximal inhibitory concentration (IC50) values for PKC[19–31] (147 ± 9 nM) that have been reported in enzymatic assays (House and Kemp 1988). Following ionophoresis, in vitro conditioning was then initiated.
Fig. 4.
Genistein blocked conditioning-produced cumulative depolarization of B cells that resisted reduction by the PKC pseudosubstrate inhibitor peptide PKC[19–31]. A, top trace: ionophoretic control preparation depolarized >10 mV after the 5th in vitro conditioning trial (middle trace). Ionophoresis of PKC[19–31] into the B cell resulted in conditioning producing only 3–4 mV of depolarization in this B cell (bottom trace). A type B photoreceptor injected with PKC[19–31] and exposed to bath-applied genistein depolarized by only 1.5 mV. B: summary data for B cells conditioned in the presence of PKC[19–31], the combination of genistein and PKC[19–31], or control vehicle solution. PKC[19–31]-injected B cells showed significantly less depolarization than that of ionophoresis controls. B cells conditioned in the presence of both PKC[19–31] and bath-applied genistein showed significantly less cumulative depolarization than that of the PKC[19–31]-alone–treated cells.
In experiments in which nervous systems were treated with a phorbol ester to activate PKC, a preparation was perfused with an ASW containing 10−7 M PMA (final solvent concentration of 0.01% acetone) for about 30 min prior to in vitro conditioning. In separate solvent control experiments, preparations were exposed to standard ASW to which acetone had been added to a final concentration of 0.01%.
In current-clamp experiments, pervanadate ion was ionophoresed into a type B photoreceptor from a single microelectrode filled with 250–300 μM pervanadate and 100 mM potassium acetate, using a constant current of −0.4 nA for 3 min. The electrodes used in these experiments typically had resistances of about 40 MΩ when filled with pervanadate/potassium acetate. Assuming an average somatic volume for a type B photoreceptor of 5.3 × 10−10 L and an empirically determined transference number of 0.03 for pervanadate (see following text), an intracellular concentration of about 44 μM was estimated. This value falls within the range of half-maximal activation values for PTKs by pervanadate in other cells: range of 15–100 μM (Heffetz et al. 1990; Inazu et al. 1990; Zick and Sagi-Eisenberg 1990). In voltage-clamp experiments, pervanadate was present in the current-passing electrode. Ionophoresis was accomplished by switching from voltage-clamp to current-clamp mode and passing appropriate current (−0.4 nA for 3 min) between the pervanadate-containing electrode and the bath ground. An equal but opposite current was passed through the second intracellular electrode (the voltage-recording electrode), to minimize changes in membrane potential during pervanadate injection. Following ionophoresis, control of membrane potential was again restored by switching back to voltage-clamp mode. In pervanadate-control experiments, the above-cited protocols were also used, although pervanadate was omitted from the electrode.
In two series of experiments we also introduced the calcium chelator ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) into B cells, to assess the effect of eliminating the calcium-activated K+ current (IK-Ca) on the resting Vm and Rin of synaptically isolated B cells. Microelectrodes used to deliver EGTA into cells contained 1 M EGTA (pH 7.0, Sigma) and 1 M KCl (45–70 MΩ). EGTA was allowed to leak into a type B photoreceptor from the microelectrode for 8–10 min followed by measurement of Vm and Rin. We previously used these methods to eliminate IK-Ca, as measured under voltage-clamp (Alkon et al. 1984; Farley 1988; Farley and Han 1997; Farley et al. 1990).
Determination of microelectrode ionophoresis transfer numbers
For ionophoresis microelectrodes containing the peptide PKC[19–31], we first measured the current required to produce a constant depolarization of 50 mV for a uniform set of electrodes (n = 4) in which the electrode solution was 3 M KCl and the external (bath ground) solution was standard ASW. We then made the same current measurements for these electrodes when they were filled with 2.16 × 10−7 M PKC[19–31] in distilled H2O and then when they were filled with KCl and pervanadate. In the latter condition, we confirmed that the mix of the two electrolytes together in the same microelectrode resulted in a measured resistance of the microelectrode that was less than that when it was filled with just 3 M KCl, or peptide, alone. We assumed in all three conditions that the current passed by these electrodes was carried by some (unknown) combination of cation efflux [K+ and PKC[19–31] peptide (+12)] from the microelectrode tip into the external bath and anion (chiefly Cl−) influx from the bath into microelectrode and, further, that the anion influx would not differ in the three situations. Thus any differences in current flow between the first two conditions (electrodes filled with 3 M KCl and PKC[19–31], respectively) would reflect the relative permeabilities/mobilities of the peptide versus K+, taking into account their charge and concentration differences. This allowed us to estimate a permeability/mobility coefficient for PKC[19–31], relative to K+. Under the assumption that all of the current passed when 3 M KCl electrodes were used was due to K+ efflux (i.e., anion influx was negligible) and that the transfer number for K+ in this situation was thus 1.0, the relative permeability/mobility coefficient for PKC[19–31] was used to calculate an upper-limit estimate of its transfer number for the situation in which both electrolytes were present in the microelectrode. To obtain a lower-limit estimate of the transfer number for PKC[19–31], we assumed that the transfer number for K+ was 0.5 (and that for Cl− was also 0.5).
For vanadate/pervanadate electrodes, we first measured the current required to produce a constant hyperpolarization of 100 mV for a uniform set of electrodes in which the electrode and bath (external) solution was 100 mM K+ acetate. We then made the same current measurements for these electrodes when they were filled with 250–300 μM vanadate/pervanadate dissolved in distilled water; the external (bath) solution here was again 100 mM K+ acetate. We assumed that the difference in measured currents for these two microelectrode solutions primarily reflected the relative mobility of the vanadate/pervanadate versus acetate anion, taking into account their charge and concentration differences. The contribution of influx of K+ cations into the pipette from the external bath was unknown in both cases; however, it would be expected to be equivalent. For the first condition (100 mM K+ acetate solution in electrode), we assumed a maximum transfer number for acetate of 0.50 and a minimum of 0.00. These relative permeability/mobility coefficients for acetate were then used to calculate upper- and lower-limit transfer-number estimates for acetate and vanadate/pervanadate for the situation in which both electrolytes were present in the microelectrode.
Obviously, the preceding calculated transfer numbers are only approximate at best and should probably not be taken too seriously, given the many difficulties associated with quantitative ionophoresis from microelectrodes (Purves 1980). Nevertheless, we felt that some estimates of the intracellular concentrations that were likely to have been achieved for PKC[19–31] and vanadate/pervanadate were preferable to none, if only to check on the plausibility that the effects of these compounds were consistent with those reported for them in test-tube biochemistry assays.
Voltage-clamp measurements and protocol
Behavioral training, in vitro conditioning, pharmacological stimulation of B cells by neurotransmitters (e.g., serotonin) implicated in conditioning, and PKC activation by phorbol esters have all been shown to reduce two kinetically and pharmacologically distinct somatic K+ currents in type B cells: a slow, calcium-activated K+ current (IK-Ca) (Farley 1988; Farley and Auerbach 1986; Farley and Schuman 1991; Farley and Wu 1989) and a rapidly inactivating, voltage-dependent “A-type” current (Alkon et al. 1982; Farley 1988; Farley and Auerbach 1986; Farley and Wu 1989). Because the calcium-activated K+ current is a major contributor to the composite, delayed outward current at potentials more positive than −30 mV (Alkon et al. 1984; Farley 1988), IDelayed (rather than IK-Ca per se) was measured in these experiments.
These K+ currents of B cells were studied as described previously (Farley 1988; Farley and Auerbach 1986; Farley and Schuman 1991), using standard two microelectrode voltage-clamp methods. An Axoclamp 2A amplifier (Axon Instruments) was used for voltage clamp.
The electrodes used to measure membrane potential in voltage-clamp experiments were pulled from microcapillary glass and had resistances of 15–25 MΩ when filled with 1.5–2.0 M KCl. A lower-resistance electrode (∼10–15 MΩ when filled with 1.5 M KCl) was used for current passage. Series resistance under typical recording conditions was measured with a step current pulse and ranged from 50 to 100 kΩ. No series resistance compensation was used. For an average series resistance value of 75 kΩ the error in measured membrane potential, without compensation, for a 60-nA membrane current (which is among the largest we recorded) is 4.5 mV (Verror = ImRS). Quantitation and analysis of ionic currents from voltage-clamp experiments were accomplished using pClamp v. 5.5.1 and 6.0 program suites (Axon Instruments). Depolarizing pulses were administered 30 s apart, to prevent any cumulative inactivation of IA or IDelayed.
All voltage-clamp measurements were obtained from synaptically isolated type B photoreceptors that satisfied the following criteria. When impaled with a single microelectrode, the cell had an initial resting membrane potential more negative than −40 mV. Following impalement with the second (current-passing) electrode, the membrane potential of the cell was more negative than −25 mV and the membrane potential measured through both electrodes differed by no more than 2 mV. The holding current at −60 mV was no greater than −4.0 nA and changed by no more than 2 nA over the course of continuous constant recording conditions. Finally, illumination of the dark-adapted cell elicited rapid (peaking within 300 ms) inward currents >5 nA, when measured from a holding potential (Vh) of −60 mV. The preceding criteria were essentially identical to those of previous two-electrode voltage-clamp studies from this laboratory (Farley 1988; Farley and Auerbach 1986; Farley and Schuman 1991; Farley and Wu 1989; Huang and Farley 2001) and others (Alkon et al. 1984).
In the experiments that characterized changes in the K+ currents produced by associative training, voltage-clamp measurements were obtained after first carrying out paired or random in vitro conditioning, as described earlier. Intracellular recordings were not obtained from B cells during these in vitro conditioning experiments, to minimize damage to cells that result from multiple reimpalements following synaptic isolation and establishment of voltage clamp. Approximately 5–10 min following in vitro conditioning, the B cells were synaptically isolated and voltage-clamped as described earlier.
Drugs and solutions
Genistein, lavendustin A, sodium orthovanadate, and PKC[19–31] were purchased from LC Laboratories (Woburn, MA). PMA and dimethyl sulfoxide (DMSO) were purchased from Sigma. Stock solutions of kinase inhibitors and activators were made fresh, every day or two. The standard artificial sea water (ASW) contained (in mM): 430 Na+; 10 K+; 10 Ca2+; 50 Mg2+; 10 Tris; 570 Cl− (pH = 7.6–7.8).
When bath applied, genistein was dissolved in standard ASW at a final concentration of 100 μM and 0.3% DMSO. This concentration is <55% of the smallest IC50 value (185 μM) reported for inhibition of PKC by genistein (Akiyama et al. 1987; Onoda et al. 1989), as determined from in vitro kinase assays. The solvent control solution contained 0.3% DMSO in ASW (pH = 7.6–7.8). When allowed to leak directly into type B cells from a microelectrode, genistein and lavendustin A were each dissolved at a 1.0 mM concentration in the 1–3 M KCl solution (0.5% DMSO) used to fill the electrode (pH = 7.2). The solution present in the electrode during control experiments contained 0.5% DMSO in 1–3 M KCl (pH = 7.2).
Pseudosubstrate inhibitor of PKC, PKC[19–31] (1 mg), was dissolved in 3 ml of double-distilled H2O. This stock solution was diluted 1,000-fold in a filtered 3 M KCl solution, to a final concentration in the electrode of 2.16 × 10−7 M. Low-resistance microelectrodes were then filled with the solution and the peptide was ionophoresed as described earlier.
Stock solutions of PMA were made by dissolving the drug in a 0.01% acetone solution. A 0.01% solution of acetone in ASW was used in control experiments. Special care was taken to minimize exposure of the PMA solutions to light, which were kept at 4°C.
Pervanadate solution was prepared by mixing vanadate with H2O2 (10−3 M) and then letting it stand for 15 min at room temperature. Catalase (200 μg/ml) was then added to this mixture to quench any extra H2O2. This peroxidized form of vanadate is stable for about 2 h (Fantus et al. 1989) and all of our solutions were used within 90 min. The concentration of pervanadate is denoted by vanadate concentration added to the mixture (Fantus et al. 1989).
The mixed electrode buffer containing vanadate and potassium acetate was prepared daily from concentrated stocks. Vanadate ion was dissolved at a 10 mM concentration in 100 mM potassium acetate at pH = 7.2. At this pH, vanadate exists in equilibrium as roughly 30% monomer, 10% dimer, 50% tetramer, and 10% other species (e.g., decavanadate) (Petterson et al. 1983), but over the course of several hours will degrade to primarily polymeric species. Because most previous studies using vanadate to stimulate PTK-mediated phosphorylation have tried to avoid the use of polymeric species, and some polymeric species of vanadate can perturb other signal-transduction pathways [e.g., decavanadate is reportedly a potent antagonist at the inositol-trisphosphate (IP3) receptor (Fohr et al. 1989)], vanadate solutions were made fresh every 1–2 h.
Vanadate and pervanadate ionophoresis were accomplished by application of a constant −0.4-nA current to the intracellular electrode for 3 min. The drug concentration was about 250 μM. Prior to and after controlled ionophoresis, a retaining current of +0.05 nA was applied. Application of the Faraday–Hittdorf law under these conditions, with an empirically determined transport number of n ≅ 0.03, yielded a predicted net efflux of about 3.7 μmol of vanadate for an approximate intracellular concentration of 44 μM.
Immunoblots
MATERIALS.
Mouse monoclonal antiphosphotyrosine antibody 4G10 was obtained from Upstate Biotechnology (Lake Placid, NY) and used according to the supplier's instructions.
IMMUNOBLOTTING.
After training, Hermissenda nervous systems were dissected from the animals and stored at −70°C until processed. Nervous system tissues were disrupted by sonication (two × 15-s pulse) in radioimmunoprecipitation assay (RIPA) buffer containing 1× phosphate-buffered saline (PBS), 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and supplemented with 0.1 mg/ml phenylmethylsulfonyl fluoride, 3% aprotinin, and 1 mM sodium orthovanadate. Protein concentrations in the lysates were determined by the use of Bradford protein assays. Cell lysates (40 μg/lane) were separated on 10% sodium dodecyl sulfate–polyacrylamide gels (SDS–PAGE) and electrophoretically transferred to a polyvinylidene fluoride membrane. The membrane was blocked by incubating in 3% Carnation instant milk in PBS for 1 h. Subsequently, the membrane was incubated in PBS/milk in the presence of 4G10 antibody overnight with agitation at 4°C. After two washes with water, the membrane was incubated with a secondary horseradish peroxidase–conjugated goat anti-mouse IgG antibody for 1 h. Immunoreactive bands were developed by an enhanced chemiluminescence substrate (ECL kit; PerkinElmer, Waltham, MA) and visualized by exposure to film.
BEHAVIORAL TRAINING FOR PREPARATIONS USED IN IMMUNOBLOTS.
Standard behavioral conditioning apparatus and methods were used (Farley 1988). In brief, intact animals received three successive training days. During each session, associatively trained animals were exposed to 50 simultaneous and overlapping pairings of light (∼300 μW·cm−2, at 510 nm) and turntable rotation (∼100 rpm, 2.1 × g centrifugal force), delivered at an average intertrial interval (ITI) of 2 min (range of 1.0–3.5 min). Random control animals also received 50 presentations each of light and rotation, but these were delivered randomly and independently of one another at an average interstimulus interval (ISI) of 2 min. For both paired and random animals, each of the light and rotation presentations was 30 s in duration.
Statistical analysis
Differences in cumulative depolarization, light response magnitudes, Vm or Rin, and ionic current amplitudes produced by two different treatment conditions were assessed using appropriate Student's t-tests or ANOVAs and Tukey's honestly significant difference (HSD) multiple comparisons for three or more treatments and reported as significant if P < 0.05.
Comparisons of the voltage dependencies of IA and IDelayed were assessed by linear regression analyses of their I–V relationships, and appropriate Student's t-tests conducted on the slope coefficients of the resulting regression equations. These analyses could be of two types: 1) between-group comparisons of the slope coefficients for different cells exposed to different drugs (e.g., presence vs. absence of genistein in the bath) and/or different behavioral treatments (e.g., paired vs. random training); and 2) within-group comparisons of the slope coefficients for the same cells, before and after exposure to a drug (e.g., genistein). In the former comparisons, independent-sample Student's t-tests were used; in the latter, correlated-sample t-tests were used to assess statistical significance. Two-tailed tests were used and the results were reported as significant if P < 0.05.
Inactivation time constants for IA (tauinactivate) were fit by single-exponential functions to the 100 ms of data following the peak current. The goodness-of-fit values (R2) were >0.98 in all cases.
RESULTS
Tyrosine kinase inhibitors block in vitro conditioning-produced cumulative depolarization
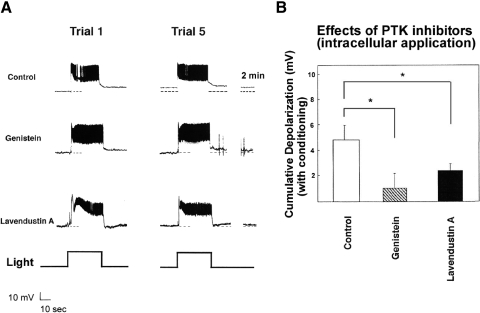
In control experiments (0.3% DMSO ASW bath solution), preparations exposed to in vitro conditioning depolarized by an average (mean ± SE) of 8.4 ± 0.7 mV (n = 8), measured 2 min after the fifth conditioning trial (Fig. 1, B–D). An average depolarization of only 1.9 ± 1.3 mV developed in type B cells conditioned in the presence of 100 μM bath-applied concentration of genistein (n = 10), which was significantly less than that of the DMSO controls [t(16) = 4.29, P < 0.001] (Fig. 1, C and D). These results suggest that a genistein-sensitive kinase is involved in the learning-produced changes in B cells, although the method of drug delivery used did not allow us to conclude that the kinase(s) affected by genistein were located in type B cells.
We next asked whether tyrosine kinase inhibitors would disrupt learning-related excitability changes in type B photoreceptors if their effects were largely confined to these cells. We therefore conducted in vitro conditioning in standard ASW after a PTK inhibitor had been introduced into a B cell by passive diffusion from a low-resistance recording electrode. Genistein-loaded cells showed only 1.0 ± 1.2 mV depolarization (n = 9) (Fig. 2, A and B). Because the possibility exists that membrane-permeable genistein might diffuse out of the B cell and affect PTK activity in cells presynaptic to type B photoreceptors, we repeated these experiments with ionophoresis of a second, structurally unrelated, less-permeable PTK inhibitor, lavendustin A. A similar reduction of cumulative depolarization was observed with this compound: an average depolarization of only 2.4 ± 0.6 mV (n = 11) (Fig. 2, A and B). Control cells showed an average depolarization of 4.8 ± 1.2 mV (n = 6) (Fig. 2, A and B). A one-way ANOVA revealed a significant main effect of treatment [F(2,22) = 6.48, P < 0.01]. Tukey HSD tests revealed significant differences between the genistein-treated and control cells (P < 0.01), as well as between lavendustin A–treated cells and controls (P < 0.05). The genistein- and lavendustin A–treated cells did not differ significantly from one another. These results and those for bath-applied genistein suggest that PTK activity within type B photoreceptors is involved in conditioning-produced excitability changes.
Fig. 2.
Introduction of genistein or lavendustin A into a type B photoreceptor blocks in vitro conditioning-produced cumulative depolarization. A, top trace: 5 pairings of light and hair cell stimulation, in a preparation in which a solvent-control solution (0.3% DMSO) had been introduced into the type B photoreceptor, produced about 7-mV depolarization of the B cell after the 5th conditioning trial. In this and subsequent figures, the discontinuous trace at far right begins 2 min after offset of 5th conditioning trial (middle trace). Intracellular application of genistein into the type B photoreceptor cell substantially reduced cumulative depolarization. Following conditioning, this B cell depolarized only 2 mV (bottom trace). Intracellular application of lavendustin A into the type B photoreceptor greatly reduced cumulative depolarization. The B cell depicted here depolarized by only about 1.5 mV. In general, the light response and spiking of type B photoreceptors were not conspicuously affected by genistein or lavendustin A. However, B cells exposed to these protein tyrosine kinase (PTK) inhibitors showed substantially reduced LLD responses following light offset. Often (7 of 11 experiments), as in the cell shown here, lavendustin A–exposed B cells showed an AHP response following light offset. B: summary data for B cells conditioned after DMSO, genistein, or lavendustin A had been applied to B cells from low-resistance microelectrodes. Depolarization was measured 2 min following the 5th conditioning trial. Both genistein and lavendustin A exposure resulted in significantly less conditioning-produced cumulative depolarization than that of the DMSO solvent-control condition.
The difference in depolarization observed for control cells where DMSO was present in the bath (∼8.5 mV; from Fig. 1D) versus that observed when DMSO was present in the recording electrode (∼4.8 mV; Fig. 2B) was also significant [t(12) = 3.03, P < 0.01], suggesting that application of DMSO into B cells (and/or the use of low-resistance electrodes) also interfered somewhat with normal in vitro conditioning-produced changes in B cells.
Two additional effects of genistein and lavendustin A on type B photoreceptor excitability were conspicuous. First, B cells exposed to these inhibitors had more negative resting membrane potentials (−52.4 ± 1.6 mV, n = 27) than those of controls (−47.7 ± 1.5 mV, n = 15) [t(40) = 2.03, P < 0.05]. These data suggest that basal PTK activity plays a role in determining the resting membrane potential of B cells. In addition, type B cells exposed to PTK inhibitors showed a reduced long-lasting depolarization (LLD) response following light offset. In many (7 of 11) experiments with lavendustin A, for example, type B cells showed a transient AHP following light offset (e.g., Fig. 2A, bottom trace). Comparison of the half-maximal decay time constants (for the first light response) for cells exposed to PTK inhibitors (60.8 ± 5.5 s) versus controls (90.0 ± 1.0 s) indicated the former to be significantly smaller than the latter [t(40) = 4.12, P < 0.005].
PTK inhibitors block cumulative depolarization despite PMA treatment
In addition to their potent inhibition of tyrosine kinases at concentrations <20 μM (IC50 values = 2.6–18 μM; Akiyama et al. 1987; O'Dell et al. 1991), genistein and lavendustin A also inhibit several mammalian serine/threonine kinases at higher concentrations. For genistein, IC50 values >185 μM have been reported for inhibition of PKA, CAM kinase II, and PKC (Akiyama et al. 1987; O'Dell et al. 1991). IC50 values of lavendustin A for these same kinases are >100 μM (O'Dell et al. 1991; Onoda et al. 1989). The intracellular concentrations of genistein and lavendustin A were unknown in the experiments in which these compounds were introduced into B cells by passive diffusion from a microelectrode, but conceivably could have reached several hundred micromoles. Therefore it is possible that the PTK inhibitor effects were mediated in part by inhibition of a serine/threonine kinase, perhaps even PKC.
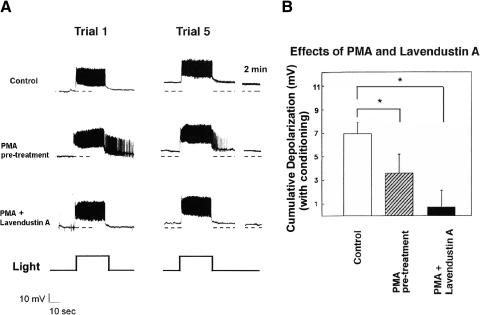
Consequently, we reassessed the effects of PTK inhibitors using conditions that greatly reduced the possibility that their effects could be solely attributed to PKC inhibition. In addition to addressing the issue of the specificity of the PTK inhibitors, these experiments also potentially address the issue of whether conditioning-relevant PTK- and PKC-signal transduction cascades interact, or are independent, in the case of type B photoreceptors. We first used an occlusion strategy in which we examined the effects of exposure to PMA (10−7 M) on conditioning-produced B cell cumulative depolarization. In these experiments, PMA was present in the bath for 30 min prior to (and throughout) in vitro conditioning. Given PKC's previously demonstrated role in conditioning, our expectation was that pretreatment of nervous systems with PMA would partially occlude in vitro conditioning-produced changes in B cells. We then asked whether any residual conditioning-produced depolarization could be blocked by a PTK inhibitor. If genistein's and lavendustin A's reductions of cumulative depolarization in earlier experiments were due to PTK (rather than PKC) inhibition—and the effects of PTKs and PKC are at least partially independent of one another in B cells—then reductions of cumulative depolarization in PMA-treated cells by PTK inhibitors would also be expected. Any residual depolarization would be expected to be blocked by lavendustin A. This is precisely the pattern that we observed.
PMA-exposed preparations that were conditioned in vitro depolarized by 3.5 ± 1.7 mV (n = 5) (Fig. 3, A and B). Solvent-control cells showed a mean depolarization of 7.0 ± 1.0 mV (n = 10) (Fig. 3, A and B). PMA-treated preparations in which lavendustin A had been allowed to leak into a type B cell showed minimal cumulative depolarization following in vitro conditioning [0.7 ± 1.4 mV (n = 5)] (Fig. 3, A and B). A one-way ANOVA revealed a significant main effect of treatment [F(2,17) = 10.10, P < 0.005]. Tukey HSD tests revealed a significant difference between the PMA-treated and control cells (P < 0.05), consistent with PKC's activation by PMA having produced a partial occlusion of conditioning-produced changes in type B cells. The difference between PMA and lavendustin A–treated cells and controls was also highly significant (P < 0.01). Although the PMA and lavendustin A–treated cells showed less cumulative depolarization than PMA-alone–treated cells, the difference was not significant with the HSD test. However, a less stringent t-test did indicate a significant difference [t(8) = 2.17, P < 0.05].
Fig. 3.
Phorbol 12-myristate, 13-acetate ester (PMA) exposure partially occludes conditioning-produced cumulative depolarization of type B photoreceptors and lavendustin A blocks the remainder of cumulative depolarization. A, top trace: control-condition B cell (10−7 M acetone solution) showed about 9-mV depolarization following in vitro conditioning (middle trace). Pretreatment of B cell with the protein kinase C (PKC)-activator PMA (10−7 M) reduced cumulative depolarization due to in vitro conditioning. This PMA-exposed B cell showed only about 5 mV of depolarization 2 min following the 5th conditioning trial (bottom trace). Introduction of lavendustin A into a PMA-exposed B cell eliminated PMA-resistant cumulative depolarization of the B cell. This cell showed only about 1.5 mV of depolarization following conditioning. PMA-exposed type B photoreceptors also showed greater steady-state light responses, compared with those of solvent-control cells. B: summary data for B cells conditioned in the presence of PMA, the combination of lavendustin A and PMA, or acetone control. PMA-exposed B cells showed significantly less depolarization than that of controls. B cells conditioned in the presence of both PMA and lavendustin A showed significantly less depolarization than that of PMA-exposed cells.
In addition, the magnitude of the dark-adapted type B photoreceptor's steady-state light response during the first conditioning trial (see Trial 1 light responses in Fig. 3) was also found to be greater for PMA-exposed (15.0 ± 2.1 mV, n = 5) versus control preparations (10.9 ± 1.1 mV, n = 10) [t(13) = 2.31, P < 0.05]. Similar results were obtained for Rin: 41.7 ± 4.2 MΩ for PMA-treated B cells versus 25.3 ± 3.1 MΩ for controls [t(13) = 2.27, P < 0.05]. These data are consistent with those reported previously (Crow et al. 1991; Farley and Auerbach 1986; Farley and Schuman 1991). They provide further evidence for PMA having induced conditioning-associated changes in B cells, since two of the primary persistent effects of behavioral conditioning on B cell excitability are enhanced steady-state light responses and resting input resistances of the dark-adapted B cell (Farley 1987, 1988; Farley and Alkon 1982). There was no significant difference in the steady-state light response (during the first conditioning trial) of cells exposed to the combination of PMA and lavendustin A (13.8 ± 2.4 mV) (n = 5) versus B cells exposed to PMA alone (15.0 ± 2.1 mV) [t(9) = 1.38, P > 0.05].
Taken together, these results suggest that conditioning-produced changes in type B photoreceptors were blocked by PTK inhibitors, even when a possible contribution of PKC to these changes had been minimized. However, other explanations are possible. Perhaps PMA exposure resulted in only a partial activation (and hence partial occlusion) of conditioning-produced changes in type B cells. The further attenuation of cumulative depolarization by lavendustin A might then be interpreted as being attributed to its inhibition of residual PKC that had not been activated by PMA (e.g., “atypical” PKC isoforms), but that was nevertheless activated by conditioning stimulation.
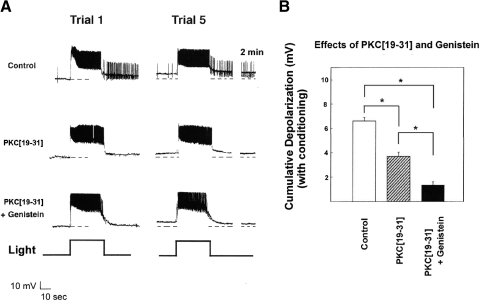
Genistein blocks cumulative depolarization despite injection of PKC[19–31]
We next attempted to minimize PKC activity by introducing into B cells the potent and specific PKC pseudosubstrate inhibitor, PKC[19–31] (House and Kemp 1988). In separate experiments, we first determined that the PKC[19–31] ionophoresis conditions used here were effective in blocking PMA-produced reductions in the transient and delayed components of K+ current, when measured under voltage clamp (e.g., Farley and Auerbach 1986; n = 3; not shown).
Cells injected with PKC[19–31] were depolarized by an average of 3.9 ± 0.3 mV (n = 5) by in vitro conditioning, which was clearly less than the 6.6 ± 0.3-mV depolarization shown by ionophoretically injected control cells (n = 4) (Fig. 4, A and B). In contrast, genistein-treated cells injected with PKC[19–31] depolarized by only 1.4 ± 0.2 mV (n = 4) (Fig. 4, A and B) following in vitro conditioning, which was also clearly less than that shown by cells merely injected with PKC[19–31]. A one-way ANOVA indicated a significant main effect of treatment [F(2,11) = 59.9, P < 0.0001] and HSD tests revealed that all three treatment conditions differed from each other (all values of P < 0.01). Ionophoresis of PKC[19–31] had no consistent effect on type B cell membrane potential or light response.
Genistein fails to block the effects of PMA on B cell excitability
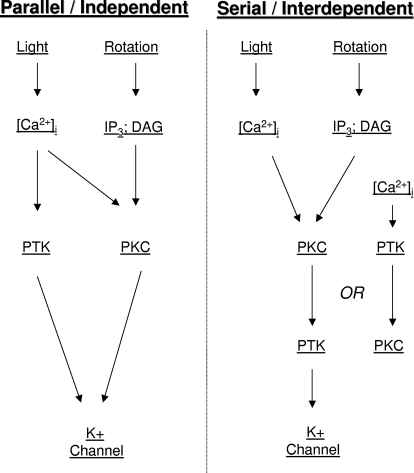
Genistein and lavendustin A were both found to block in vitro conditioning-produced depolarization of B cells that survived exposure to PKC inhibitors and activators, suggesting that their primary effects were through perturbation of PTK-signaling pathways, rather than through interactions with PKC. However, in the absence of biochemical data demonstrating complete inhibition of PKC in B cells by PKC[19–31], or complete activation by PMA, the possibility remains that genistein and lavendustin A might be affecting B cell excitability changes through effects on other protein kinases, including residual PKC activity. Such effects might occur because the inhibitors directly blocked Hermissenda PKC activity or indirectly through their interference with PTK modulation of PKC activity (i.e., the two signal-transduction cascades interact).
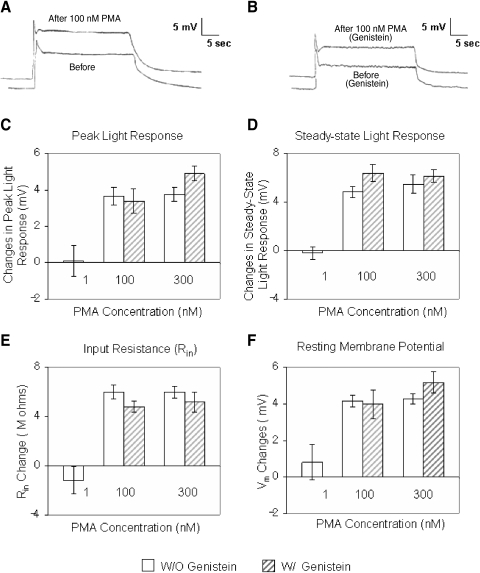
To further address these possibilities, we tested whether genistein blocked any membrane excitability changes in B cells produced by the phorbol ester PMA. Synaptically isolated type B cells from untrained specimens were exposed to a final bath concentration of 1, 100, or 300 nM PMA, in either the presence or the absence of 100 μM bath-applied genistein. Because the concentration of 1 nM PMA failed to reliably affect B cells, we tested genistein with only the 100- and 300-nM concentrations of PMA. Membrane-excitability changes were followed for 20–25 min following PMA exposure, by monitoring Vm, Rin, and peak- and steady-state components of 30-s light-induced generator potentials (light ISI of 2 min).
Figure 5 A depicts a typical light response of a B cell following 15 min of exposure to 100 nM bath-applied PMA (no genistein). Following PMA exposure, the B cell had depolarized by about 3 mV and Rin had increased by about 24% [main effect of PMA concentration: F(2,25) = 36.6 and 64.3 for Vm and Rin, respectively]. The peak- and steady-state components of the light response were also increased, by 24 and 42%, respectively (Fig. 5A). Similar results were observed in four other cells (Fig. 5, C–F) [PMA main effect: F(2,25) = 44.2 and 92.8, for the peak and steady-state light response, respectively].
Fig. 5.
Genistein fails to affect PMA-induced excitability changes in B cells. A: type B cell light response before and about 15 min after exposure to 100 nM PMA. All components of the light response were enhanced by PMA. In addition, the cell had depolarized by 3 mV, relative to baseline. The 2 traces have been offset slightly along the time axis (∼1 s). B: type B cell light response before and about 15 min after exposure to 100 nM PMA, in the presence of 100 μM genistein. All components of the light response were enhanced by PMA. In addition, the cell had depolarized by 4 mV, relative to baseline. The 2 traces have been offset slightly along the time axis (∼0.5 s). C–F: summary data for changes in the light responses and excitability of B cells exposed to different concentrations of PMA (1, 100, or 300 nM), in either the presence or the absence of 100 μM bath-applied genistein. Because 1 nM PMA failed to affect the B cells, the combination of genistein and PMA was not assessed for this concentration. For all measures, the 100- and 300-nM concentrations of PMA produced approximately equivalent changes in light responses and excitability, regardless of whether cells had been preexposed to genistein. There were no significant differences in the magnitude of the changes for 100- vs. 300-nM PMA concentrations for any measure; n = 5 in all conditions, except for the 1-nM PMA concentration condition where n = 3. C and D: summary data for changes in the peak- and steady-state components of the light responses of B cells exposed to different concentrations of PMA (1, 100, or 300 nM), in either the presence or the absence of 100 μM bath-applied genistein. The 100- and 300-nM concentrations of PMA enhanced both peak- and steady-state components of the light response, regardless of whether cells had been preexposed to genistein. E: summary data for changes in input resistance (Rin) of B cells exposed to different concentrations of PMA (1, 100, or 300 nM), in either the presence or the absence of 100 μM bath-applied genistein. The 100- and 300-nM concentrations of PMA produced comparable increases in Rin, regardless of whether cells had been preexposed to genistein. F: summary data for changes in membrane voltage (Vm) of B cells exposed to different concentrations of PMA (1, 100, or 300 nM), in either the presence or the absence of 100 μM bath-applied genistein. The 100- and 300-nM concentrations of PMA produced comparable depolarization of Vm, regardless of whether cells had been preexposed to genistein.
In contrast, the 1-nM concentration had negligible effects (n = 3) on all indices of membrane excitability examined (Fig. 5, C–F). Conversely, the 300-nM concentration of PMA had robust effects very similar to those of 100 nM. On average, B cells (n = 5) depolarized by about 4 mV (Fig. 5F) and Rin increased by roughly 29% (Fig. 5E). The peak- and steady-state components of the light response were also increased by 27 and 40%, respectively (Fig. 5, C and D). Although the effects of 300 nM PMA were slightly greater than the effects of 100 nM PMA, for all measures of light response (peak and steady-state) and membrane excitability (Vm and Rin) examined, the differences in no case approached statistical significance (Fig. 5, C–F), as assessed by post hoc HSD tests. Thus the effects of 100 nM PMA appeared to have been close to saturating.
Exposure of B cells to genistein failed to appreciably alter their responses to PMA. Genistein-treated cells stimulated with 100 nM PMA (n = 5) depolarized by 4 mV (Fig. 5, B and E) and showed increases in Rin of 29% (Fig. 5F). The peak and steady-state components of the light response were also increased by 27 and 39%, respectively (Fig. 5, C and D). Similarly, in response to 300 nM PMA, genistein-exposed cells depolarized by 4.7 mV (Fig. 5E) and Rin increased by about 26% (Fig. 5F). The peak and steady-state components of the light response were increased by 28 and 43%, respectively (Fig. 5, B–D). In summary, genistein failed to interfere with PMA-produced increases in light responses [main effect of genistein: F(1,25) = 0.46 and 4.07, P > 0.05 for peak and steady-state, respectively; interaction of genistein × PMA: F(2,25) = 1.31 and 1.04, P > 0.05 for peak and steady-state, respectively] and excitability of B cells [main effect of genistein: F(1,25) = 0.001 and 0.30, P > 0.05 for peak and steady-state, respectively; interaction of genistein × PMA: F(2,25) = 1.23 and 2.20, P > 0.05 for peak and steady-state, respectively].
Changes in IA and IDelayed with in vitro conditioning and genistein's block of altered voltage dependence
Conditioning-produced cumulative depolarization of type B photoreceptors is accompanied by, and in part dependent on, suppression of the K+ currents IA and IDelayed (Farley 1987, 1988; Farley and Schuman 1991). In vitro conditioning also reduces the voltage dependence of IA and IDelayed (Farley and Schuman 1991). In contrast, although phorbol esters reduce B cell K+ current amplitudes (Farley and Auerbach 1986; see Fig. 9 of the current study) and partially occlude the effects of in vitro conditioning, they fail to alter the voltage dependence of the K+ currents. Similarly, inhibitors of serine/threonine (S/T) protein phosphatases 1 and 2A (PP1/PP2A) reduce B cell K+-current amplitudes and occlude in vitro conditioning (Huang and Farley 2001). These effects of phosphatase inhibitors are blocked by the general S/T kinase inhibitor H-7 (Huang and Farley 2001), suggesting that constitutive S/T kinase activity regulates the A current. However, PP1/PP2A inhibitors failed to affect IA voltage dependence (Huang and Farley 2001). Thus conditioning-related changes in S/T kinase activity do not appear to account for the changes in voltage dependence of IA and IDelayed that are produced by both behavioral-training and in vitro conditioning. The above-cited considerations suggest that these alterations in K+ I–V dependencies may be due to conditioning-produced PTK activity.
Fig. 9.
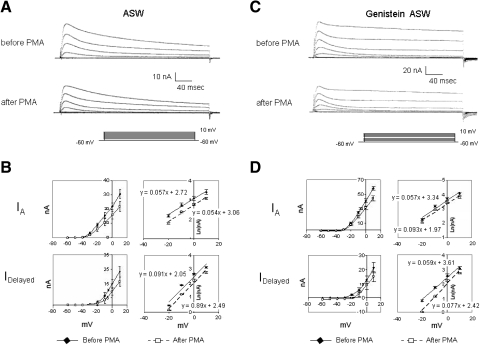
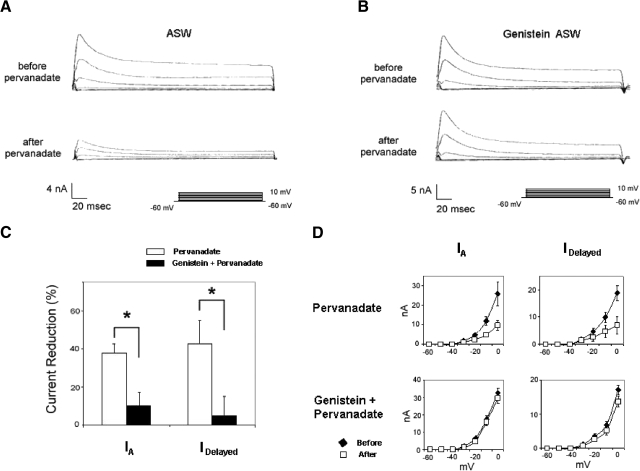
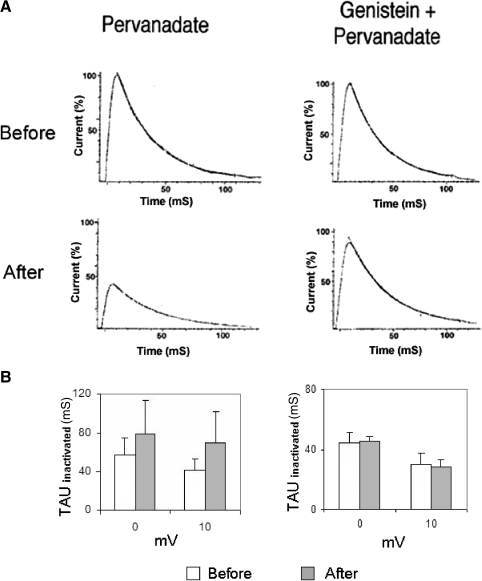
Genistein fails to attenuate PMA suppression of K+ currents. A: control preparation exposed to 100 nM PMA showed reductions in IA and IDelayed measured about 20 min following PMA exposure (top traces: currents before PMA; bottom traces: currents ∼20 min after PMA exposure). B: summary data depicting PMA's suppression of K+ currents, for 4 cells, measured 20 min after bath application of PMA. Both the transient (IA) and sustained (IDelayed) components of K+ current were decreased. The voltage dependence of IA and IDelayed was unaffected by PMA. C: genistein-treated preparation exposed to 100 nM PMA also showed reductions in IA and IDelayed measured about 20 min following PMA exposure (top traces: currents before PMA; bottom traces: currents about 20 min after PMA exposure). D: summary data depicting PMA's suppression of K+ currents for cells treated first with genistein, measured 20 min after bath application of PMA. For genistein-treated preparations, the reductions of IA and IDelayed by PMA were comparable to those observed in cells that had not been exposed to genistein (compare with B). Prior to PMA exposure, however, the K+ currents were larger for the genistein-treated cells (compare with A). The voltage dependence of IA and IDelayed was not significantly affected by PMA in the presence of genistein.
To test this hypothesis directly and to further explore the role of PTK activity during conditioning, we compared the amplitude and voltage dependence of IA and IDelayed in B cells following in vitro conditioning for preparations conditioned in the presence or the absence of bath-applied genistein (100 μM). The same in vitro conditioning protocols used in earlier experiments of this series (Fig. 1A) were used here as well.
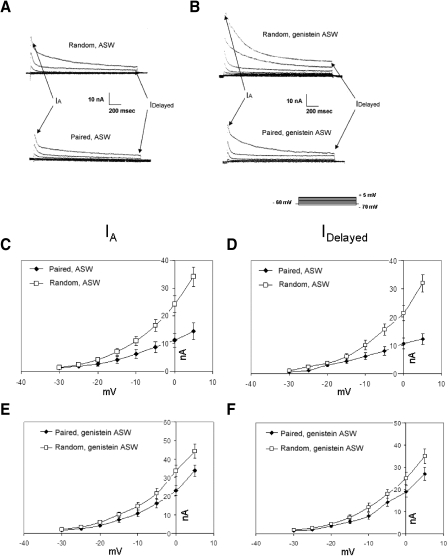
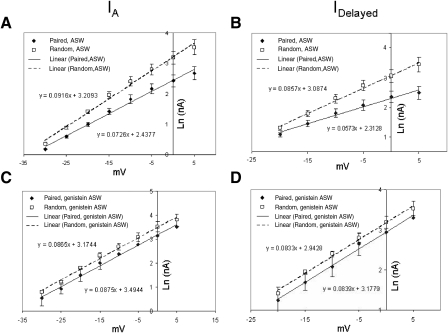
Type B cells from preparations exposed to pairings of light and statocyst hair cell stimulation showed reduced amplitudes of IA and IDelayed, compared with preparations exposed to random presentations of these stimuli. In the absence of genistein, and when averaged across the range of −30 to +5 mV, the amplitudes of IA (Fig. 6, A and C) and IDelayed (Fig. 6, A and D) for paired cells were roughly 60 and 65%, respectively, of those of random control cells. These reductions in the different components of K+ current were smaller when preparations were conditioned in the presence of genistein. Peak amplitudes of IA for paired cells were roughly 74% of random control values (Fig. 6E). IDelayed amplitudes for paired cells were about 81% of random control values (Fig. 6F). Thus genistein partially prevented the conditioning-specific reductions in IA and IDelayed. Consistent with the following experiments (Fig. 8), the K+ currents of genistein-exposed cells were larger than those of comparable nonexposed cells (Fig. 6, A, C, and D vs. B, E, and F).
Fig. 6.
In vitro conditioning reduces B cell K+-current amplitudes; reductions are smaller in the presence of genistein. A, top traces: family of transient (IA) and delayed (IDelayed) components of K+ current in a type B photoreceptor from a random control preparation, measured about 10 min following the conclusion of random in vitro conditioning. Currents elicited in standard ASW by depolarization to indicated membrane potentials (bottom right inset, B). Bottom traces: currents recorded from a B cell of an associatively trained preparation, about 10 min following the conclusion of paired in vitro conditioning. These preparations were conditioned in standard ASW, without genistein. K+ currents were smaller in the B cell exposed to paired conditioning. B, top traces: family of transient (IA) and delayed (IDelayed) components of K+ current in a type B photoreceptor from a random control preparation, measured about 10 min following the conclusion of random in vitro conditioning. Currents elicited by depolarization to indicated membrane potentials (bottom right inset). Bottom traces: currents recorded from a B cell of an associatively trained preparation, about 10 min following the conclusion of paired in vitro conditioning. These preparations were conditioned in ASW with genistein (100 μM). K+ currents were smaller in the B cell exposed to paired conditioning, although the reductions were not as great as those for the B cell exposed to pairings in the absence of genistein (bottom traces, A). Currents were recorded in ASW with genistein present. C and D: summary current–voltage (I–V) relations for IA (C) and IDelayed (D) components of K+ currents in B cells, from preparations exposed to paired (n = 6) or random (n = 4) in vitro conditioning, in the absence of genistein. Pairings of light and hair cell stimulation decreased both components of K+ current. E and F: summary I–V relations for IA (E) and IDelayed (F) components of K+ currents in B cells, from preparations exposed to paired (n = 5) or random (n = 5) in vitro conditioning, in the presence of genistein. Pairings of light and hair cell stimulation decreased both components of K+ current, but the reductions were smaller than those when genistein was absent. Compare with C and D.
When B cells were conditioned in the absence of genistein, the voltage dependencies of the K+ current I–V relations were clearly affected by pairings (Figs. 6, C and D and 7, A and B). IA from random control cells showed e-fold increases per 10.91 ± 2.8 mV, whereas IA in cells from animals exposed to paired in vitro conditioning showed e-fold increases per 14.80 ± 3.4 mV (Fig. 6C), a significant difference of about 36% [t(8) = 3.89, P < 0.01]. Similarly, IDelayed from random control cells showed e-fold increases per 11.73 ± 2.5 mV, whereas IDelayed in cells from animals exposed to pairings showed e-fold increases per 16.60 ± 3.6 mV (Fig. 6D), a significant difference of about 42% [t(8) = 4.87, P < 0.001]. Semilogarithmic (log–linear [log–lin]) plots (Fig. 7, A and B) and regression analysis of the I–V data further confirmed that the voltage dependencies were altered by in vitro conditioning: the slopes of the log–lin regression lines for the voltage dependencies of IA and IDelayed were significantly shallower for cells from paired versus random in vitro conditioning [t(8) = 3.16 and 4.72, P < 0.01 and 0.001, respectively].
Fig. 7.
In vitro conditioning effects on B cell K+-current voltage dependencies in the absence (A and B) vs. the presence (C and D) of genistein (100 μM). A and B: semilogarithmic plots of IA (A) and IDelayed (B) amplitudes vs. voltage following paired or random control in vitro conditioning in standard ASW (no genistein), replotted from Fig. 6, C and D. Paired conditioning reduced the slopes of the I–V relation, in addition to reducing the maximum amplitudes. C and D: semilogarithmic plots of IA (C) and IDelayed (D) amplitudes vs. voltage following paired or random control in vitro conditioning in the presence of genistein, replotted from Fig. 6, E and F. Paired conditioning failed to affect the slopes of the I–V relations and, although it did reduce the amplitudes of both components of the K+ current, the reductions were smaller than those when genistein was absent (compare with A and B).
When B cells were conditioned in genistein, however, the voltage dependencies of the K+ currents were not significantly affected (Fig. 6, E and F). IA from random control cells showed e-fold increases per 11.60 ± 2.9 mV, whereas IA of cells from paired preparations showed e-fold increases per 11.43 ± 2.7 mV. IDelayed from random control cells showed e-fold increases per 12.00 ± 2.3 mV, whereas IDelayed of cells from paired preparations showed e-fold increases per 11.85 ± 2.6 mV. The log–lin plots (Fig. 7, C and D) and regression analysis of the I–V data indicated that the slopes of the regression lines for the voltage dependencies of IA and IDelayed were not significantly different for cells from paired versus random in vitro conditioning, in the presence of genistein (Fig. 7, C and D).
In summary, although both PTK- and PKC-signaling pathways contribute to the conditioning-produced reductions in IA and IDelayed K+ currents, the PTK-signaling pathway(s) appears to be specifically responsible for the reductions in their voltage dependence.
It seems very unlikely that the paired versus random differences in amplitude and/or voltage dependence for IA and IDelayed (Figs. 6 and 7) could be due to sampling variability—i.e., random differences between the two different sets of B cells exposed to paired versus control training stimulation. The same qualitative conditioning-produced differences in amplitude for both K+ currents (smaller for paired than for random) have previously been observed in three separate and independent studies (Alkon et al. 1985; Farley 1988; Farley and Schuman 1991). Another early study by Alkon et al. (1982) looked primarily at IA and also found smaller A currents for paired versus control cells. Similarly, the same qualitative changes in voltage dependence observed here were also observed in two previous studies where it was looked at, albeit less thoroughly (Farley 1988; Farley and Schuman 1991).
It remains possible, although unlikely, that the paired versus random differences in K+-current amplitudes could be due to systematic differences in cell size (e.g., perhaps conditioning and PTK activation lead to rapid reductions in B cell size/volume). Technical considerations prevented us from normalizing our current measurements to cell size. Our use of two microelectrodes to effect voltage clamp precluded accurate measurements of cell capacitance, since the series resistance and capacitance of the electrodes swamps the contribution of the cell. However, our previous attempts to look for conditioning-associated changes in capacitance on the basis of single-electrode/current-clamp measurements of time constants for charging curves have failed to detect any (Farley, unpublished observations). It is also relevant that not all currents in B cells are reduced by conditioning, as might be expected if conditioning simply reduced cell size/membrane area but channel densities were unaltered. Measurements of the light-activated currents in B cells on retention days have found no significant reductions due to pairings (Alkon et al. 1985; Farley 1988). Similarly, the voltage-dependent Ca2+ current (ICa) has been variously reported to be increased (Farley 1988) or unchanged by pairings (Collin et al. 1998) 1–2 days post training. Several imaging studies of paired versus control B cells have also failed to note any obvious differences in cell (soma) size due to conditioning history (Ito et al. 1994; Kawai et al. 2002). Although rapid changes in morphology of type B photoreceptor somas have been reported for phorbol esters (Lederhendler et al. 1990), the changes involved outgrowth from the cell surface (“blebbing”) that the authors interpreted as increasing the cell surface area. In summary, although the issue has not been thoroughly or definitively examined yet, there is no evidence to support the conjecture that pairings reduce K+ currents via reductions in somatic volumes/cell size. Moreover, it is not obvious how reductions in cell size/volume could explain the altered voltage dependence of IA and IDelayed.
Although both IA and IDelayed (more specifically, IK-Ca) are suppressed both by in vitro and by behavioral-conditioning protocols (Farley 1988; Farley and Schuman 1991), whether these currents contribute appreciably to the resting Vm (and thus cumulative depolarization) and Rin of B cells is somewhat unclear. To address this issue, we conducted an additional series of experiments using (synaptically isolated) B cells from untrained animals in which we assessed the effects on Vm and Rin of eliminating IA and IK-Ca, each alone and then both together. We first examined the effect of 2 mM 4-aminopyridine (4-AP) on Vm and Rin for two sets of B cells in which each cell was used as its own control (n = 4). For the first set, cells were maintained at their normal resting potential; no extrinsic current injection was applied. Vm in the absence of 4-AP was −48.9 ± 1.3 mV; about 5 min after 4-AP bath addition, Vm was −46.7 ± 0.9 mV. 4-AP depolarized B cells by about 2.2 mV [t(3) = 6.18, P < 0.01]. It also increased resting Rin by roughly 23% in these same cells. These results agree well with those of a previous study (Farley 1988, where it was observed that Vm for random control cells, in the absence of 4-AP, was −50.3 ± 2.2 mV (n = 7), whereas for a different group of random control cells, in the presence of 2 mM 4-AP, Vm was −47.6 ± 2.0 mV (n = 7). In this earlier study, 4-AP depolarized B cells by about 2.7 mV. For a second set of B cells in the current study, cells were current-clamped to a hyperpolarized membrane potential (−70 mV) at which the A current is largely deinactivated but not activated. At −70 mV IA would not be expected to contribute to Vm and Rin of B cells. At these hyperpolarized conditions, 4-AP addition had no consistent effect on Vm (−69 0.6 ± 1.7 mV) and a greatly reduced effect on Rin (increase of 9 ± 5% change). The small increase in Rin observed may be attributable to an effect of 4-AP on “leakage” and/or inward-rectifier type K+ channels. These observations suggest that appreciable standing A current is present at the resting membrane potential of B cells such that blocking it with 4-AP produces clearly detectable depolarization and increases in Rin.
To assess the contribution of IK-Ca to Vm and Rin of B cells, we compared their values in cells into which EGTA had been allowed to leak (to preclude activation of IK-Ca) versus cells in which 1 M KCl was allowed to leak into them. Cells injected with EGTA had less negative membrane potentials (−44.7 ± 1.3 mV, n = 6) than those of cells not injected with EGTA (−48.9 ± 1.6, n = 3): about 4.2-mV difference [t(7) = 1.95, P < 0.05, one-tailed]. The former cells also showed slow depolarizations of 3–4 mV during the EGTA leakage period. Rin values were also greater for the EGTA-injected cells (42.3 ± 3.2 vs. 35.2 ± 2.8 MΩ). These observations indicate that a standing IK-Ca contributes to the resting Vm and Rin of type B photoreceptors and, further, that elimination of this current results in depolarization and increases in Rin.
For four of the EGTA-injected cells, we subsequently added 2 mM 4-AP to the bath and observed a net depolarization (due to the combination of EGTA and 4-AP) of 5.6 ± 1.8 mV [t(3) = 7.22, P < 0.01], as well as a further increase in Rin (50.2 ± 3.4 MΩ). These effects are approximately those expected from the summation of the independent effects of EGTA and 4-AP, indicating that the combined suppression of IA and IK-Ca can lead to a clear depolarization and increase in Rin of B cells.
Genistein increases K+ currents in B cells, alters their voltage dependence, and speeds A-current inactivation
Bath application of 100 μM genistein to voltage-clamped B cells from untrained animals consistently and significantly increased the peak of the transient current (IA) by an average of 36 ± 17% (n = 5) (Fig. 8, A and B), when measured 15 min following application [t(4) = 8.01, P < 0.002]. The increase was first apparent within 2–3 min following genistein application, peaked after about 5 min, and declined slowly over the next 10 min (Fig. 8C). Increases in the delayed component of K+ current were also observed (Fig. 8A), although the effects were more variable (Fig. 8B). On average, for all cells examined in this replication, the delayed component increased by 33 ± 42% (n = 5) 15 min after application. This increase was not statistically significant [t(4) = 1.62, P > 0.18], stemming in large part from the results of one atypical cell that showed a 28% decrease in amplitude. The other four cells showed an average increase of 39 ± 21%, an increase that was statistically significant [t(3) = 3.70, P < 0.05]. Bath addition of acetone to 0.01% in control experiments failed to affect either the peak amplitudes of the transient or the maximum amplitude of the delayed components (n = 2; data not shown).
The increases in IA and IDelayed by genistein were accompanied by changes in the voltage dependencies of the currents. In these analyses, results for the one anomalous cell (see preceding text) that showed a decrease in IDelayed were omitted. In the absence of genistein, IA and IDelayed both showed e-fold increases in amplitude per approximately 14–16 mV (over the range of −15 to +10 mV; Fig. 8, D and E). Semilogarithmic (log–lin) plots (Fig. 8, F and G) and regression analysis of the I–V data indicated that genistein enhancement of IA and IDelayed was accompanied by an average 18% increase in the voltage dependence of IA (from 16.7 ± 2 to 13.6 ± 3.2 mV, n = 5) and a 22% increase in voltage dependence for IDelayed (from 13.9 ± 1.2 to 10.8 ± 3.3 mV, n = 4). The slopes of the log–lin regression lines for IA (Fig. 8F) and IDelayed (Fig. 8G) were significantly steeper in the presence versus the absence of genistein [t(4,3) = 2.56 and 2.73, respectively; P < 0.05 for both]. In acetone control experiments, a small decrease of 4 ± 8.2% was observed for IA and a slight increase of 5.5 ± 4.0% was measured for IDelayed over the same time period in cells (n = 2) not exposed to genistein (not shown).
The rate of inactivation for IA, tauinactivate, was increased by genistein (Fig. 8H). Single-exponential fits of the decline in the peak outward current during the following 100 ms yielded the following average tauinactivate values at −10, 0, and +10 mV, prior to genistein: 79.2 ± 21.2, 58.1 ± 17.8, and 47.4 ± 14.3 ms. Genistein decreased tauinactivate by an average of roughly 31%: 51.1 ± 11.2, 41.2 ± 7.5, and 33.5 ± 7.9 ms, at −10, 0, and +10 mV, respectively.
Genistein fails to block the suppression of K+ currents by PMA
As a further check on the possibility that genistein's effects on B cell excitability and K+ currents might be mediated through a PKC-dependent pathway, we tested the ability of 100 μM bath-applied genistein to block the effects of PMA (100 nM) on somatic K+ currents (n = 4). Figure 9A depicts representative suppression of IA and IDelayed in standard ASW (no genistein) observed about 20 min following PMA exposure. Peak A currents were reduced by about 30% (Fig. 9B, top). IDelayed was decreased by about 35% (Fig. 9B, bottom). The voltage dependence of either IA (Fig. 9B, top right) or IDelayed (Fig. 9B, bottom right) was not affected by PMA. The slopes of the log–lin regression lines for IA and IDelayed were not significantly different before and after PMA exposure [t(3) = 0.82 and 0.96, respectively].
PMA suppression of K+ currents was relatively unaffected by prior exposure (30 min) of cells to genistein. At 20 min after addition of PMA, peak A currents were reduced by about 29% (Fig. 9, C and D, top). IDelayed was decreased by about 30% (Fig. 9, C and D, bottom). The voltage dependence of either IA (Fig. 9D, top right) or IDelayed (Fig. 9D, bottom right) was not affected by PMA. The slopes of the log–lin regression lines for IA and IDelayed were not significantly different before and after PMA exposure [t(3) = 0.21 and 1.88, respectively]. In addition to further showing that genistein's effects on B cell K+ currents are independent of possible PKC inhibition, these results also imply that the PTK(s) that are activated during in vitro conditioning are unlikely to lie downstream of PKC activation; otherwise, genistein would have been expected to have blunted the effects of PMA.
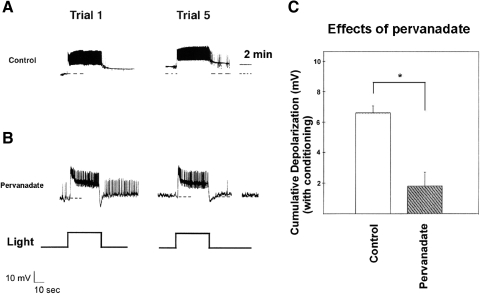
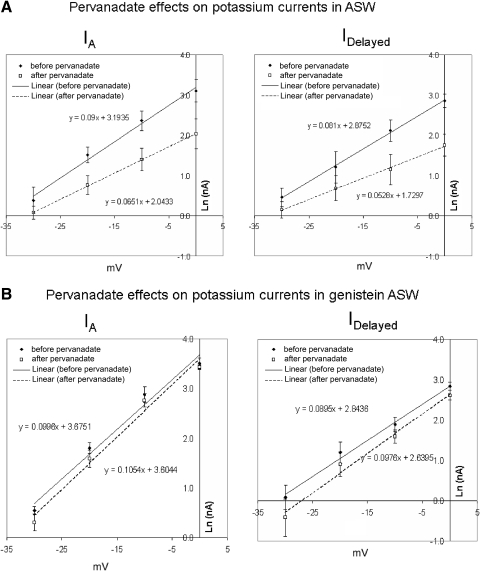
Pervanadate depolarizes type B cells and partially occludes in vitro conditioning
Vanadate and pervanadate ion are widely used to increase phosphotyrosine levels in many cell types (Brown and Gordon 1984; Inazu et al. 1990; Koshio et al. 1988), through their inhibition of protein tyrosine phosphatases (Bernier et al. 1988; Heffetz et al. 1990; Swarup et al. 1982). Therefore we tested their ability to affect type B photoreceptor excitability, photoresponses, and cumulative depolarization due to in vitro conditioning.
Ionophoresis of pervanadate ion from electrodes containing 1–100 mM of the drug depolarized type B photoreceptors by >20 mV (n = 4) and drastically reduced their light response (not shown). When a lower concentration (250–300 μM) was used in the electrode, a more modest depolarization of 5–10 mV resulted (n = 7) and the B cell retained its light response. Type B photoreceptors injected with pervanadate ion (250–300 μM concentration in electrode) and exposed to in vitro conditioning (n = 7) showed greatly reduced cumulative depolarization: 1.7 ± 1.0 mV (Fig. 10, B and C). This change was significantly less than the 6.6 ± 0.3-mV depolarization shown by ionophoretically injected control cells (n = 4) [t(9) = 3.78, P < 0.005] (Fig. 10, A and C). As already noted, the resting membrane potential of type B cells exposed to pervanadate was less negative than that of controls [−33.4 ± 2.7 vs. −50.0 ± 3.6 mV, t(9) = 4.17, P < 0.005]. In addition, type B photoreceptors exposed to pervanadate showed a significantly greater steady-state depolarizing response to light, compared with that of controls [first light response: 14.8 ± 1.3 mV (n = 7) vs. 9.8 ± 1.4 mV (n = 4), t(9) = 2.97, P < 0.05]. Many pervanadate-injected cells also showed AHP responses following light offset (Fig. 10B). Results with vanadate injection (n = 3; not shown) were essentially identical to those of pervanadate.
Fig. 10.
Pervanadate occludes in vitro conditioning-produced cumulative depolarization. A: control preparation exposed to in vitro conditioning depolarized by 9 mV. B: ionophoretic injection of pervanadate into type B cell resulted in the cell showing only 1 mV of depolarization, 2 min following conditioning (discontinuous trace at right). C: summary data of pervanadate or acetate ion (control) ionophoretic effects on cumulative depolarization of B cells. Cells injected with pervanadate showed significantly less conditioning-produced depolarization than that of control cells injected with acetate.
Pervanadate suppresses K+ currents in B cells
We next determined whether the same K+ currents (IA and IDelayed) that were suppressed by in vitro and behavioral conditioning were also modulated by pervanadate ion and, by implication, PTK activity. Ionophoresis of pervanadate ion into a B cell reduced IA by an average of 36 ± 7.3% (n = 7) (Fig. 11, A and C). These effects were apparent within 3–5 min following ionophoresis and plateaued at about 10 min. Similarly, pervanadate reduced IDelayed by an average of 43 ± 11.2% (n = 7) (Fig. 11, A and C). Bath-applied genistein (100 μM) prevented the pervanadate reductions of IA [9 ± 6.4%, n = 3; comparison with pervanadate alone, t(8) = 2.39, P < 0.05] and IDelayed [4 ± 9.5%, n = 3; comparison with pervanadate alone, t(8) = 2.29, P < 0.05] (Fig. 11, B and C).
Fig. 11.
Pervanadate ionophoresis suppresses K+ currents in type B photoreceptors. A, top traces: family of transient (IA) and delayed (IDelayed) components of K+ current in a type B photoreceptor, elicited by depolarization to indicated membrane potentials (bottom), before ionophoresis of pervanadate ion. Bottom traces: currents recorded from the same cell 10 min after ionophoresis of pervanadate ion. Pervanadate suppressed both IA and IDelayed at all potentials tested. B: pervanadate's inhibition of K+ currents was blocked by 100 μM genistein. K+ currents before (top traces) and 10 min after (bottom) pervanadate ionophoresis in the presence of genistein. C: summary data for suppression of K+ currents resulting from pervanadate ionophoresis (n = 7) or pervanadate ionophoresis in the presence of genistein (n = 3). Pervanadate ionophoresis suppressed the K+ currents, but not if genistein was present in the bath. Currents measured at 0 mV. D: summary data depicting the effects of pervanadate (n = 7) or the combination of pervanadate and genistein (n = 3) on K+ currents, at all voltages, measured about 10–12 min after ionophoresis of pervanadate. Both the transient (IA) and sustained (IDelayed) components of K+ current were decreased by pervanadate (top), although genistein blocked the suppressive effects of pervanadate on both components of K+ current (bottom).
The reductions in IA and IDelayed by pervanadate were accompanied by clear changes in the voltage dependencies of the currents (Figs. 11D and 12A). Pervanadate suppression of IA and IDelayed was accompanied by an average 38% decrease in the voltage dependence of IA (from 11.1 ± 3 to 15.4 ± 4.2 mV, n = 7) and a 53% decrease in voltage dependence for IDelayed (from 12.3 ± 1.2 to 18.2 ± 4.3 mV, n = 7) (Figs. 11D, top and 12A). The slopes of the log–lin regression lines for IA and IDelayed were significantly reduced by pervanadate (Fig. 12 A) [t(6) = 3.47 and 9.89, P < 0.01 and 0.001, respectively]. In control experiments (n = 3), slight increases of 1 ± 11.3 and 9.5 ± 7.0% were measured for the voltage dependencies of IA and IDelayed over the same time period in cells that had not been exposed to pervanadate (not shown). Genistein blocked the pervanadate changes in voltage dependence for IA (before: 10.0 ± 4.3 mV, after: 9.5 ± 5.3 mV; average increase of ∼5%) and IDelayed (before: 11.2 ± 4.9 mV, after: 10.3 ± 4.5 mV, average increase of ∼9%) (Figs. 11D, bottom and 12B). In the presence of genistein, the slopes of the log-lin regression lines for IA and IDelayed were not significantly affected by pervanadate (Fig. 12B) [t(6) < 1.0, for both]. The rate of inactivation for IA (tauinactivate) was slowed by pervanadate (Fig. 13), a change opposite that produced by genistein (Fig. 8H). The pervanadate increase in tauinactivate cannot readily explain the reduction in the peak current. Single-exponential fits of the decline in the peak outward current during the following 100 ms yielded the following average tauinactivate values at −10, 0, and +10 mV: 87.2 ± 20.9, 57.5 ± 6.3, and 41.4 ± 4.4 ms (Fig. 13, A and B). Pervanadate slowed inactivation, increasing tauinactivate by an average of roughly 79%: 202.2 ± 39.8, 79.5 ± 12.7, and 70.1 ± 12.1 ms at −10, 0, and +10 mV, respectively (Fig. 13, A and B). Genistein blocked the pervanadate-produced slowing of inactivation (Fig. 13, A and B). Prior to the addition of pervanadate, IA currents from genistein-exposed cells had tauinactivate values of: 59.6 ± 10.3, 44.2 ± 6.9, and 29.3 ± 7.7 ms (−10, 0, +10 mV, respectively). Following pervanadate exposure, IA currents from these genistein-treated cells had tauinactivate values of: 79.8 ± 24.3, 45.6 ± 2.9, and 28.4 ± 4.1 ms, for an average increase in tauinactivate of only 11% (Fig. 13B).
Fig. 12.
Pervanadate ionophoresis reduces the voltage dependence of IA and IDelayed. A: summary log–lin I–V plots for IA (left) and IDelayed (right), before and after pervanadate ionophoresis. Pervanadate reduced the voltage dependence of both currents, as indicated by the reduced slope of the linear regression line and associated equation's coefficient for the I–V relation. B: genistein prevented the pervanadate-induced changes in voltage dependence of both K+ currents.
Fig. 13.
Pervanadate slowed the inactivation kinetics of IA. A: examples of single-exponential fits of current records of IA inactivation (at 0 mV), before (top) and 10 min after (bottom) pervanadate treatment. Currents suppressed by pervanadate (bottom) have been scaled relative to the preionophoresis records. Pervanadate slowed IA inactivation by roughly 38% (left). Genistein prevented the changes in IA inactivation rate (right). B: summary data for effects of pervanadate on tauinactivate, in the absence (left) or the presence (right) of genistein. Averages given are for 5 (pervanadate) and 3 (pervanadate/genistein) cells, respectively. Data were obtained about 10 min after pervanadate ionophoresis at 0 and +10 mV.
Behavioral conditioning results in pairing-specific increases in protein tyrosine phosphorylation in the CNS
It is unclear whether the PTK inhibitors' abrogation of conditioning-induced changes in type B cells' excitability occurs because the inhibitors block a PTK that is specifically activated by conditioning stimulation or whether the inhibitors render the B cell K+ conductance systems insensitive to modulation by non-PTK signaling pathways that are subsequently activated by conditioning. For example, perhaps dephosphorylation of tyrosine residues from the A-type and Ca2+-activated K+ channels or associated proteins (a possible consequence of prolonged exposure of B cells to genistein or lavendustin A) renders these channels unresponsive to PKC-mediated phosphorylation events. In this scenario, constitutive PTK activity might be part of the biochemical background that is critical for learning-related plasticity, but would not be stimulated by conditioning.
To address this issue, we asked whether behavioral conditioning of H.c. is accompanied by increased PTK activity, as assessed by increased phosphotyrosine levels in Western blots of proteins from the entire nervous system. In these experiments, conducted as two separate and independent replications, intact animals were exposed to one of three treatment conditions (n = 5 per condition, per replication): 1) pairings of light and rotation, 2) random presentations of light and rotation, and 3) no training stimulation (untrained control). At 24 h following treatment, animals were killed, their nervous systems removed, and changes in tyrosine phosphorylation were examined by SDS–PAGE and immunoblotting of proteins with the mouse monoclonal antiphosphotyrosine antibody 4G10 (see methods).
Phosphate incorporation into tyrosine residues of many proteins was increased by paired conditioning, compared with that of either random or untrained controls (Fig. 14). The Bradford assay results, as well as the immunoblot results for actin, indicated approximately equivalent protein concentrations for samples from the three treatment conditions. The increased phosphotyrosine levels were evidently conditioning specific, since no conspicuous differences were observed between random and untrained control animals. Similar results were obtained in both replications of the experiment for proteins from the entire CNS. Little can be said at present as to the possible identities of the proteins whose phosphotyrosine content was increased by conditioning. However, the mass of those falling within the 34.7- to 51.8-kDa range is not inconsistent with that of voltage-dependent K+ channel alpha subunits.
Fig. 14.
Associative training increases phosphotyrosine levels in proteins from the Hermissenda CNS, as detected by Western blotting (see Immunoblots in methods for details). Intact animals were exposed to either standard associative training involving light and rotation (paired condition), random presentations of light and rotation, or no training (home cage). The nervous systems (n = 5 in all conditions) were then removed; proteins were extracted and subjected to SDS–PAGE, transferred onto polyvinylidene fluoride membranes, and probed with native 4G10 antiphosphotyrosine (top) or anti-actin (bottom). Paired training increased phosphotyrosine levels in high molecular weight (∼70 to 120 kDa) proteins. No differences were observed in actin-staining intensities. Highly similar results observed in a second replicate.
DISCUSSION
PTK inhibitors block cumulative depolarization and increase K+ currents
Our results implicate PTK activity in short-term, conditioning-produced excitability changes in Hermissenda type B photoreceptors. Exposure of B cells to genistein and lavendustin A substantially reduced conditioning-produced cumulative depolarization and increased Rin of type B photoreceptors. Type B cells exposed to these inhibitors also had more negative membrane potentials and larger K+ currents than those of control cells. These latter effects of the PTK inhibitors make it somewhat difficult to decisively attribute their block of conditioning effects to specific disruption of a PTK that is activated by paired conditioning, as opposed to producing an independent increase in the K+ currents that masks or opposes the conditioning-produced decreases. A specific disruption of a conditioning-activated PTK was suggested by the findings that the PTK inhibitors blocked the reductions in the voltage dependencies of IA and IDelayed. Collectively, our results suggest that the activity of B cell K+ channels is regulated by constitutive, as well as conditioning-stimulated, tyrosine phosphorylation/dephosphorylation cycles.
It is possible that the effects of genistein and lavendustin A on B cells might be partially mediated via inhibition of serine/threonine kinases, perhaps even PKC. However, several of our findings indicate that this cannot be the primary mechanism by which these compounds blocked conditioning-produced cumulative depolarization and reduction in K+ currents. First, the bath-applied concentration of genistein was about 50% of the reported IC50 values of genistein for PKC. Yet genistein reduced cumulative depolarization by ≳80%. Second, genistein was more effective in blocking cumulative depolarization (∼80%) than either PMA or PKC[19–31] (∼45–50%), which are more potent and selective disruptors of PKC signal-transduction pathways. Third, we also observed a block of cumulative depolarization by genistein and lavendustin A under circumstances in which PKC's role in conditioning would be expected to be minimal. PMA or PKC[19–31] reduced cumulative depolarization by roughly 50%. The remaining depolarization was prevented by lavendustin A in the case of PMA and by genistein in the case of PKC[19–31]. Fourth, for B cells from untrained animals, genistein failed to block the PMA-mediated effects on light responses, Rin, or K+ currents.
In addition to demonstrating a reduction of cumulative depolarization by a PTK inhibitor that is at least partially independent of any direct inhibition of PKC, these results also argue against the possibility that the effects of the PTK inhibitors were due to inhibition of other enzymes [such as phosphoinositide kinase (Onoda et al. 1989) or phospholipase C (Meisenhelder et al. 1989; Wahl et al. 1988)] that modulate PKC activity indirectly, through effects on inositol-trisphosphate (IP3) and diacylglycerol (DAG) levels or other components of calcium signal-transduction pathways.
Pervanadate mimics associative conditioning
Pervanadate depolarized B cells, enhanced the light response, reduced the same K+ currents that are suppressed by conditioning, and occluded in vitro conditioning-produced depolarization of B cells. These results are consistent with the hypothesis that pervanadate resulted in tyrosine-phosphorylation–mediated suppression of K+ channels, mimicking that which occurs during conditioning.
Although activation of both PKC- and PTK-dependent signal-transduction pathways in B cells reduces IA and IDelayed, the reductions were associated with distinct biophysical changes. Phorbol ester stimulation of B cells has little effect on the voltage dependence of IA (Farley and Auerbach 1986; Farley and Schuman 1991), shifting the I–V curve about 12–15 mV toward more depolarized potentials without affecting the slope of the relation, and also fails to affect inactivation kinetics (J. Farley, H. Huang, and I. Jin, unpublished observations). However, in addition to reducing the amplitudes of IDelayed and IK-Ca, in vitro conditioning also diminishes their apparent voltage sensitivity (Farley and Auerbach 1986; Farley and Schuman 1991). Similarly, calyculin and inhibitor-2, inhibitors of protein phosphatase 1 (PP1), duplicate the effects of PKC activators (Huang and Farley 2001). In contrast to these presumptive perturbations of serine/threonine kinase phosphorylation, pervanadate stimulation of B cells decreased the voltage sensitivity of IA and IDelayed by >35% and slowed the rate of A-current inactivation. Genistein had the opposite effects. Thus activation of PKC (a serine/threonine kinase) or inhibition of serine/threonine phosphatases both produce similar changes in B cell K+ currents that are distinguishable from those produced by PTK activation/inhibition.
Other important unresolved questions, for both behavioral conditioning and PKC- and PTK activation, concerns the effects of all of these manipulations on the voltage-dependent Ca2+ currents present in B cells (Alkon et al. 1984; Farley 1988; Yamoah and Crow 1996). However, because both phorbol esters (Farley and Auerbach 1986) and behavioral conditioning (Farley 1988) either increase the high-threshold noninactivating Ca2+ current (ICa, or sHVA; Yamoah and Crow 1996) in B cells, or do not change it for 1–2 days post training (Collin et al. 1988), conditioning-produced reductions in IK-Ca cannot readily be explained by reductions in ICa.
Our findings that type B cell K+ currents are regulated by inhibitors and activators of tyrosine phosphorylation add to a growing list of reports that tyrosine phosphorylation affects the activity of voltage-gated ion channels (Adams et al. 2000; Holmes et al. 1996; Huang et al. 1993; Jonas et al. 1996; Lev et al. 1995; Missan et al. 2006; Prevarskaya et al. 1995; Schrader et al. 2006; Tiran et al. 2003).
Contribution of reductions in IA and IK-Ca to resting potential and input resistance of B cells
Our results indicate that the suppression of IA and IK-Ca by conditioning is likely to contribute to the cumulative depolarization and increase in Rin of B cells. At the normal resting potential, elimination of IA by 2 mM 4-AP depolarized B cells by several millivolts and increased Rin. However, at −70 mV, at which there is essentially no activation of IA, 2 mM 4-AP had no effect on membrane potential and a greatly reduced effect on Rin. Several considerations further suggest that the effects of 2 mM 4-AP on B cells are largely restricted to A-type K+ channels, although some small contribution of voltage-independent (leak and inward-rectifier) channels cannot be excluded. Previous voltage-clamp studies have found that 4-AP concentrations <5 mM specifically block the transient A-type K+ current in B cells elicited by step depolarizations, with IDelayed being relatively unaffected (see Alkon et al. 1982; Farley and Wu 1989). In addition, whereas 2 mM 4-AP produced about 2–3 mV of depolarization at the resting Vm of B cells, high concentrations of tetraethylammonium and Ba2+ (which block a variety of voltage-dependent and leak K+ channels in B cells) produce much larger depolarizations of B cells, but have little effect on IA (e.g., Farley 1988; Farley and Wu 1989).
Similarly, our findings that EGTA leakage caused B cells to depolarize and Rin to increase are consistent with the presence of a standing IK-Ca in these cells. These findings are in agreement with the results of earlier voltage-clamp studies (Alkon et al. 1984; Figs. 1 and 2; Farley 1988; Fig. 7), where it was observed that in addition to eliminating IK-Ca elicited by depolarizing command steps to potentials equal to or more positive than −40 mV, EGTA injection into B cells also decreased the magnitude of the holding currents over the range of −60 to −40 mV. It is also relevant that our recent fura-2 ratiometric measurements of somatic intracellular calcium ion concentration ([Ca2+]i) in type B photoreceptors indicate that the basal bulk cytosolic concentrations in dark-adapted cells can be relatively high (e.g., ∼170 nM) (Farley, Cavallo, Hallahan, Johnson, unpublished observations). This level may be sufficient to support appreciable BK channel activity at resting membrane potentials in B cells (∼ −50 mV), as is the case for BK channels at negative potentials (≳ −70 mV) in several other cell types (e.g., Heppner et al. 1997; Kang et al. 1996; Wann and Richards 1994).
It is worth noting that BK channel properties are extremely diverse in different preparations, as a result of the production of splice variants and expression of different β-subunits (Orio et al. 2002). In most excitable (e.g., neuronal) cells, BK channel activation at negative resting potentials (≳ −70 mV) is negligible, due to both the hyperpolarized membrane potential and the low levels of [Ca2+]i. However, BK channels in some cells (e.g., smooth muscle) contain an auxiliary β-subunit that enhances their Ca2+ sensitivity (McManus et al. 1995; Valverde et al. 1999). This promotes gating at the resting potential. A similar mechanism may be at work in type B photoreceptors.
Current understanding of the dual activation of BK channels by voltage and Ca2+ envisions these as operating in parallel, with the channel voltage sensors and calcium-binding sites coupling to the activation gate independently, except for a weak interaction between the two mechanisms (see Cui et al. 2009; Latorre and Brauchi 2006; Magleby 2003). Both voltage and Ca2+ can activate the channel independently to increase the probability of channel opening (PO). However, neither depolarization nor Ca2+ binding is obligatory for BK channel activation. Although it enhances PO, voltage-sensor activation is not required for channel opening. At negative voltages (< −20 mV), where the voltage sensor of the BK channel is mostly at the resting state and the probability of channel opening due to voltage-sensor movement is small (PO <10−4), residual channel opening can be detected (Horrigan and Aldrich 2002). Elevating [Ca2+]i will increase PO and shift the G–V relation to more negative voltages.
The combination of 4-AP and EGTA, which should have resulted in nearly complete elimination of both IA and IK-Ca, produced about 6 mV of membrane depolarization at the resting Vm in B cells. This represents roughly 67–75% of the depolarization commonly produced by in vitro conditioning. Since these two currents are reduced by about 40% shortly after in vitro conditioning, it seems clear that additional currents are also reduced by in vitro conditioning and contribute to cumulative depolarization. Voltage-independent (leak) and/or inward-rectifier K+ currents are potential candidates for these additional targets of training.
Interaction or independence between PKC and PTKs in learning-produced changes in B cells?
Our results point to a joint involvement of PKC and one or more PTKs in the production of associative training-related changes in type B cell excitability. Two general classes of models for PKC and PTK interaction can be distinguished from one another (Fig. 15). In the first, PKC and PTK signal-transduction pathways might both be activated in parallel and independently by associative training, with little cross talk between the two prior to their convergence on common effector molecules.
Fig. 15.
Models for PTK and PKC interactions in the production of learning-produced changes in Hermissenda type B cell excitability. In the parallel/independent model (left), light leads to increases in intracellular calcium within B cells, whereas rotation leads to neurotransmitter (e.g., serotonin [5-HT] and γ-aminobutyric acid) stimulation of G-protein–triggered signal-transduction pathways, including those that result in membrane phospholipid hydrolysis and the production of IP3 and diacylglycerol (DAG). In this model, pairing specificity arises from the requirements for kinase activation (temporally contiguous increase in both intracellular calcium ion concentration, [Ca2+]i, and DAG are necessary for PKC activation) and the differential ability of each sensory stimulus to stimulate [Ca2+]i and DAG increases. Although IP3 would be produced by rotational stimulation and thereby lead to [Ca2+]i increases (in addition to DAG), we suggest that the [Ca2+]i increases produced are normally below the threshold needed for PKC activation. Thus repeated exposures to rotational stimulation in the absence of paired light presentations would be insufficient to induce PKC-mediated changes in B cell excitability. Similarly, in the case of PTK activation, we assume that for a calcium-dependent, nonreceptor-type PTK (e.g., PYK2), light-induced [Ca2+]i increases may be insufficient to activate the kinase unless rotation also adds to [Ca2+]i increases. Alternatively, the PTK(s) activated during conditioning may be receptor-type PTKs, dependent on both rotation-mediated stimulation and light-induced [Ca2+]i increases within B cells for their activation. In a second class of models (serial/interdependent, right), PTKs and PKCs interact and affect one another (see discussion). One possible interaction between PTKs and PKC in type B photoreceptors might involve PTK participation in activation of PLC-γ and subsequent activation of PKC by DAG and IP3. Our data are most consistent with the parallel/independent model (left).
In a second class of models, PTK(s) and PKC might interact with one another in their regulation of B cell excitability. Activation of PKC might phosphorylate a PTK, or another protein involved in PTK activation, that might in turn reduce K+-channel activity. Alternatively, PTK activation might occur upstream from PKC and regulate activation of PKC.
At present, our results do not permit a decisive choice as to which, if any, of these models might be relevant to Hermissenda type B photoreceptors. The partial block of cumulative depolarization by the introduction into B cells of inhibitors or activators of either PTK or PKC (with the block by PTK inhibitors being stronger than that of PKC inhibitors) and complete inhibition of cumulative depolarization by their combination are consistent with a parallel/independent model of PKC–PTK interaction in B cells. In addition, our finding that activators of PTKs versus PKC had different effects on IA and IDelayed voltage dependence and IA kinetics also argues for a parallel/independent model, as does our observation that PKC activators can still change excitability and inhibit voltage-dependent K+ currents in the face of PTK inhibition. These results are difficult to reconcile with any model in which PKC activation occurred upstream from, and was obligatory for, PTK activation, or vice versa. On the other hand, the nearly complete block of cumulative depolarization by genistein seems most consistent with the serial/interdependent class of models. However, since genistein affected the basal excitability and K+ currents of type B photoreceptors, implying that these are affected by constitutive (as well as conditioning-stimulated) PTK activity, and the PTK(s) that are affected by conditioning have not yet been identified, interpretation of how PTK and PKC signal-transduction cascades interact in B cells is complicated.
Nevertheless, our results imply that tyrosine kinase activity is involved in the induction of short-term learning-related excitability changes in type B cells. PTK activity may also be important for long-term changes in B cells. One potentially relevant ubiquitous signal-transduction pathway in which PKC and PTK converge is that involving mitogen-activated protein kinase (MAPK family) activation. The serine/threonine kinase (MAP kinase kinase kinase; i.e., Raf) activator of this pathway is activated by both PKC phosphorylation as well as by Ras proteins that are associated with receptor-type PTKs (Sun and Tonks 1994). Crow and colleagues (1998; see also Ormond et al. 2004; Purcell et al. 2003) reported that MAPK is involved in persistent, protein-synthesis–dependent changes in type B cell excitability, suggesting a potential role for PTK activity in long-term memory in Hermissenda.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grants NS-26108, NS-30950, and NS-48156.
ACKNOWLEDGMENTS
We thank the staff at the Friday Harbor Laboratories, University of Washington (Friday Harbor, WA) for providing accommodations that greatly facilitated preparation of the manuscript.
Present address of I. Jin: Department of Neuroscience, Columbia University, 40 Haven Ave., Kolb Building Room 160, New York, NY 10032.
REFERENCES
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem 75: 2277–2287, 2000 [DOI] [PubMed] [Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262: 5592–5595, 1987 [PubMed] [Google Scholar]
- Alkon DL. Sensory interactions in the nudibranch mollusk Hermissenda crassicornis. Fed Proc 33: 1083–1090, 1974 [PubMed] [Google Scholar]
- Alkon DL. Membrane depolarization accumulates during acquisition of an associative behavioral change. Science (Wash DC) 210: 1375–1376, 1980 [DOI] [PubMed] [Google Scholar]
- Alkon DL, Anderson MJ, Kuzirian AM, Rogers DF, Fass DM, Collin C, Nelson TJ, Kapetanovic IM, Matzel LD. GABA-mediated synaptic interaction between the visual and vestibular pathways of Hermissenda. J Neurochem 61: 556–566, 1993 [DOI] [PubMed] [Google Scholar]
- Alkon DL, Farley J, Sakakibara M, Hay B. Voltage-dependent calcium and calcium-activated potassium conductances of a molluscan photoreceptor. Biophys J 46: 605–614, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon DL, Lederhendler I, Shoukimas JJ. Primary changes of membrane currents during retention of associative learning. Science (Wash DC) 215: 693–695, 1982 [DOI] [PubMed] [Google Scholar]
- Alkon DL, Sakakibara M, Forman R, Harrigan J, Lederhendler I, Farley J. Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behav Neural Biol 44: 278–300, 1985 [DOI] [PubMed] [Google Scholar]
- Bernier M, Laird DM, Lane MD. Effects of vanadate on the cellular accumulation of pp15, an apparent product of insulin receptor tyrosine kinase action. J Biol Chem 263: 13626–13634, 1988 [PubMed] [Google Scholar]
- Boixel C, Tessier S, Pansard Y, Lang-Lazdunski L, Mercadier J-J, Hatem SN. Tyrosine kinase and protein kinase C regulate L-type Ca2+ current cooperatively in human atrial myocytes. Am J Physiol Heart Circ Physiol 278: H670–H676, 2000 [DOI] [PubMed] [Google Scholar]
- Boxall AR, Lancaster B, Garthwaite J. Tyrosine kinase is required for long-term depression in the cerebellum. Neuron 16: 805–813, 1996 [DOI] [PubMed] [Google Scholar]
- Brown DJ, Gordon JA. The stimulation of pp60v-src kinase activity by vanadate in intact cells accompanies a new phosphorylation state of the enzyme. J Biol Chem 259: 9580–9586, 1984 [PubMed] [Google Scholar]
- Collin C, Ikeno H, Harrigan JF, Lederhendler I, Alkon DL. Sequential modification of membrane currents with classical conditioning. Biophys J 54: 955–960, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Alkon DL. Associative behavioral modification in Hermissenda: cellular correlates. Science (Wash DC) 209: 412–414, 1980 [DOI] [PubMed] [Google Scholar]
- Crow T, Forrester J. Down-regulation of protein kinase C and kinase inhibitors dissociate short- and long-term enhancement produced by one-trial conditioning of Hermissenda. J Neurophysiol 69: 636–641, 1993 [DOI] [PubMed] [Google Scholar]
- Crow T, Forrester J, Williams M, Waxham MN, Neary JT. Down-regulation of protein kinase C blocks 5-HT-induced enhancement in Hermissenda B photoreceptors. Neurosci Lett 121: 107–110, 1991 [DOI] [PubMed] [Google Scholar]
- Crow T, Xue-Bian JJ, Siddiqi V, Kang Y, Neary JT. Phosphorylation of mitogen-activated protein kinase by one-trial and multi-trial classical conditioning. J Neurosci 18: 3480–3487, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci 66: 852–875, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantus IG, Kadota S, Deragon G, Foster BM, Posner BI. Pervanadate [peroxides of vanadate] mimics insulin action in rat adipocytes via activation of insulin receptor tyrosine kinase. Biochemistry 28: 8864–8871, 1989 [DOI] [PubMed] [Google Scholar]
- Farley J. Contingency-sensitivity in Hermissenda: II. Cellular mechanisms. Behav Neurosci 101: 28–56, 1987 [DOI] [PubMed] [Google Scholar]
- Farley J. Associative training results in persistent reduction in a calcium-activated potassium current in Hermissenda type B photoreceptors. Behav Neurosci 102: 784–802, 1988 [Google Scholar]
- Farley J, Alkon DL. Associative neural and behavioral change in Hermissenda: consequences of nervous system orientation for light- and pairing-specificity. J Neurophysiol 48: 785–807, 1982 [DOI] [PubMed] [Google Scholar]
- Farley J, Alkon DL. In vitro associative conditioning of Hermissenda: cumulative depolarization of type B photoreceptors and short-term associative behavioral changes. J Neurophysiol 57: 1639–1668, 1987 [DOI] [PubMed] [Google Scholar]
- Farley J, Auerbach S. Protein kinase C activation induces conductance changes in Hermissenda photoreceptors like those seen in associative learning. Nature 319: 220–223, 1986 [DOI] [PubMed] [Google Scholar]
- Farley J, Han Y. Learning-correlated changes in a delayed K+ current in Hermissenda type A photoreceptors, J Neurophysiol 77: 1861–1888, 1997 [DOI] [PubMed] [Google Scholar]
- Farley J, Richards WG, Grover LM. Associative learning changes intrinsic to Hermissenda type A photoreceptors. Behav Neurosci 104: 135–152, 1990 [DOI] [PubMed] [Google Scholar]
- Farley J, Richards WG, Ling LJ, Liman E, Alkon DL. Membrane changes in a single photoreceptor cause associative learning in Hermissenda. Science (Wash DC) 221: 1201–1203, 1983 [DOI] [PubMed] [Google Scholar]
- Farley J, Schuman EM. Protein kinase C inhibitors prevent induction and continued expression of cell memory in Hermissenda type B photoreceptors. Proc Natl Acad Sci USA 88: 2016–2020, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley J, Wu R. Serotonin modulation of Hermissenda type B-photoreceptor light responses and ionic currents: implications for mechanisms underlying associative learning. Brain Res Bull 22: 335–351, 1989 [DOI] [PubMed] [Google Scholar]
- Fohr KJ, Scott J, Ahnert-Hilger G, Gratzl M. Characterization of the inositol 1,4,5-trisphosphate-induced calcium release from permeabilized endocrine cells and its inhibition by decavanadate and p-hydroxymercuribenzoate. Biochem J 262: 83–89, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh Y, Lederhendler I, Alkon DL. Input and output changes of an identified neural pathway are correlated with associative learning in Hermissenda. J Neurosci 5: 536–543, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SGN, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science (Wash DC) 258: 1903–1909, 1992 [DOI] [PubMed] [Google Scholar]
- Grover LM, Farley J, Auerbach S. Serotonin involvement during in vitro conditioning of Hermissenda. Brain Res Bull 22: 363–372, 1989 [DOI] [PubMed] [Google Scholar]
- Heffetz D, Bushkin I, Dror R, Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem 265: 2896–2902, 1990 [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- Holmes TC, Fadool DA, Levitan IB. Tyrosine phosphorylation of the Kv1.3 potassium channel. J Neurosci 16: 1581–1590, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JF, Tank DW, Greengard P, Huganir RL. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature 336: 677–680, 1988 [DOI] [PubMed] [Google Scholar]
- Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+-binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol 120: 267–305, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototype in its regulatory domain. Science (Wash DC) 238: 1726–1728, 1988 [DOI] [PubMed] [Google Scholar]
- Huang H, Farley J. PP1 inhibitors depolarize Hermissenda photoreceptors and reduce K+ currents. J Neurophysiol 86: 1297–1311, 2001 [DOI] [PubMed] [Google Scholar]
- Huang X, Morielli AD, Peralta EG. Tyrosine kinase-dependent suppression of a potassium channel by the G-protein-coupled m1 muscarinic acetylcholine receptor. Cell 75: 1145–1156, 1993 [DOI] [PubMed] [Google Scholar]
- Inazu T, Takanobu T, Yanagi S, Yamamura H. Protein-tyrosine phosphorylation and aggregation of intact human platelets by vanadate with H2O2. Biochem Biophys Res Commun 170: 259–263, 1990 [DOI] [PubMed] [Google Scholar]
- Ito E, Oka K, Collin C, Schreurs BG, Sakakibara M, Alkon DL. Intracellular calcium signals are enhanced for days after Pavlovian conditioning. J Neurochem 62: 1337–1344, 1994 [PubMed] [Google Scholar]
- Jonas EA, Kaczmarek LK. Regulation of potassium channels by protein kinases. Curr Opin Neurobiol 6: 318–323, 1996 [DOI] [PubMed] [Google Scholar]
- Jonas EA, Knox RJ, Kaczmarek LK, Shwartz JH, Solomon DH. Insulin receptor in Aplysia neurons: characterization, molecular cloning and modulation of currents. J Neurosci 16: 1645–1658, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Schuman EM. Long lasting neurotrophin-induced enhancement of synaptic transmission in adult hippocampus. Science (Wash DC) 267: 1658–1662, 1995 [DOI] [PubMed] [Google Scholar]
- Kang J, Huguenard JR, Prince DA. Development of BK channels in neocortical pyramidal neurons. J Neurophysiol 76: 188–198, 1996 [DOI] [PubMed] [Google Scholar]
- Kawai R, Horikoshi T, Yasuoka T, Sakakibara M. In vitro conditioning induces morphological changes in Hermissenda type B photoreceptor. Neurosci Res 43: 363–372, 2002 [DOI] [PubMed] [Google Scholar]
- Koshio O, Akanuma Y, Kasuga M. Hydrogen peroxide stimulates tyrosine phosphorylation of the insulin receptor and its tyrosine kinase activity in intact cells. Biochem J 250: 95–101, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R, Brauchi S. Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res 39: 385–401, 2006 [DOI] [PubMed] [Google Scholar]
- Lederhendler II, Etcheberrigaray R, Yamoah EN, Matzel LD, Alkon DL. Outgrowths from Hermissenda photoreceptor somata are associated with activation of protein kinase C. Brain Res 534: 195–200, 1990 [DOI] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Mussacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature 376: 737–744, 1995 [DOI] [PubMed] [Google Scholar]
- Magleby KL. Gating mechanism of BK (Slo1) channels: so near, yet so far. J Gen Physiol 121: 81–96, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Lederhendler II, Alkon DL. Regulation of short-term associative memory by calcium-dependent protein kinase. J Neurosci 10: 2300–2307, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Rogers R. Postsynaptic calcium, but not cumulative depolarization, is necessary for the induction of associative plasticity in Hermissenda. J Neurosci 13: 5029–5040, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 14: 645–650, 1995 [DOI] [PubMed] [Google Scholar]
- Meisenhelder J, Suh PG, Rhee SG, Hunter T. Phospholipase C-γ is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell 57: 1109–1122, 1989 [DOI] [PubMed] [Google Scholar]
- Missan S, Linsdell P, Mcdonald TF. Tyrosine kinase and phosphatase regulation of slow delayed-rectifier K+ current in guinea-pig ventricular myocytes. J Physiol 573: 469–482, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Gorrie GH, Amato A, Smart TG. Modulation of GABAA receptors by tyrosine phosphorylation. Nature 377: 344–348, 1995 [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Silva AJ, Yamaguchi T, Hayashi T, Yamamoto T, Nawa H. Growth factor-mediated Fyn signaling regulates alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression in rodent neocortical neurons. Proc Natl Acad Sci USA 96: 2461–2466, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell TJ, Kandel ER, Grant SGN. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature 353: 558–560, 1991 [DOI] [PubMed] [Google Scholar]
- Onoda T, Iinuma H, Sasaki Y, Hamada M, Isshiki K, Naganawa H, Takeuchi T. Isolation of a novel tyrosine kinase inhibitor, lavendustin A, from Streptomyces griseolavendus. J Natl Prod 52: 1252–1257, 1989 [DOI] [PubMed] [Google Scholar]
- Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: maxiK channel β-subunits. News Physiol Sci 17: 156–161, 2002 [DOI] [PubMed] [Google Scholar]
- Ormond J, Hislop J, Zhao Y, Webb N, Vaillaincourt F, Dyer JR, Ferraro G, Baker P, Martin KC, Sossin WS. ApTrkI, a Trk-like receptor, mediates serotonin-dependent ERK activation and long-term facilitation in Aplysia sensory neurons. Neuron 44: 715–728, 2004 [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increase in BDNF and NT-3 mRNAs. Neuron 9: 1081–1088, 1992 [DOI] [PubMed] [Google Scholar]
- Pazin MJ, Williams LT. Triggering signaling cascades by receptor tyrosine kinases. Trends Biochem Sci 17: 374–378, 1992 [DOI] [PubMed] [Google Scholar]
- Petterson L, Hedman B, Anderson I, Ingri N. Multicomponent polyanions. Chem Scripta 22: 254–264, 1983 [Google Scholar]
- Prevarskaya NB, Skryma RN, Vacher P, Daniel N, Djiane J, Dufy B. Role of tyrosine phosphorylation in potassium channel activation-functional association with prolactin receptor and JAK2 tyrosine kinase. J Biol Chem 270: 24292–24299, 1995 [DOI] [PubMed] [Google Scholar]
- Purcell AL, Sharma SK, Bagnall MW, Sutton MA, Carew TJ. Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron 37: 473–484, 2003 [DOI] [PubMed] [Google Scholar]
- Purves R. Ionophoresis: progress and pitfalls. Trends Neurosci 118: 245–247, 1980 [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci 24: 4796–4806, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Stein PL, Park CR, Bernstein IL. Taste aversion learning in fyn mutant mice. Behav Neurosci 110: 845–848, 1996 [PubMed] [Google Scholar]
- Scharader LA, Birnbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, Sweatt JD. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of pore-forming subunit. Am J Physiol Cell Physiol 290: C852–C861, 2006 [DOI] [PubMed] [Google Scholar]
- Sun H, Tonks NK. The coordinated action of protein tyrosine phosphatase and kinases in cell signaling. Trends Biochem Sci 19: 480–485, 1994 [DOI] [PubMed] [Google Scholar]
- Swarup G, Cohen S, Garbers DL. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun 107: 1104–1109, 1982 [DOI] [PubMed] [Google Scholar]
- Tabata M, Alkon DL. Positive synaptic feedback in visual system of nudibranch mollusk Hermissenda crassicornis. J Neurophysiol 48: 174–191, 1982 [DOI] [PubMed] [Google Scholar]
- Terlau M, Seifert W. Influence of epidermal growth factor on long-term potentiation in the hippocampal slice. Brain Res 484: 352–356, 1989 [DOI] [PubMed] [Google Scholar]
- Thornton C, Yaka R, Dinh S, Ron D. H-Ras modulates N-methyl-D-aspartate receptor function via inhibition of Src tyrosine kinase activity. J Biol Chem 26: 23823–23829, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiran Z, Peretz A, Attali B, Elson A. Phosphorylation-dependent regulation of Kv2.1 channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase epsilon. J Biol Chem 278: 17509–17514, 2003 [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Stitt TN, DiStefano PS, Rojas E, Mattson K, Compton DL, Nunez L, Park JS, Stark JL, Geis DR, Thomas S, LeBeau MM, Fernald AA, Copeland NG, Jenkins NA, Burden SJ, Glass DJ, Yancopoulous GD. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic mouse, at the neuromuscular junction, and after injury. Neuron 15: 573–584, 1995 [DOI] [PubMed] [Google Scholar]
- Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science 285: 1929–1931, 1999 [DOI] [PubMed] [Google Scholar]
- Wahl MI, Daniel TO, Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science (Wash DC) 241: 968–970, 1988 [DOI] [PubMed] [Google Scholar]
- Wallace BG. Regulation of interaction of nicotinic acetylcholine receptors with cytoskeleton by agrin-activated protein tyrosine kinase. J Cell Biol 128: 1121–1129, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinase and phosphatases. Nature 369: 233–235, 1994 [DOI] [PubMed] [Google Scholar]
- Wann KT, Richards CD. Properties of single calcium-activated potassium channels of large conductance in rat hippocampal neurons in culture. Eur J Neurosci 6: 607–617, 1994 [DOI] [PubMed] [Google Scholar]
- Yamoah EN, Crow T. Protein kinase and G-protein regulation of Ca2+ currents in Hermissenda photoreceptors by 5-HT and GABA. J Neurosci 16: 4799–4809, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]