Abstract
The purpose of the present study was to determine whether retinal activity can support long-term changes in synaptic strength in the developing dorsal lateral geniculate nucleus (LGN) of thalamus. To test for this we made use of a rodent in vitro explant preparation in which retinal afferents and the intrinsic circuitry of the LGN remain intact. We repetitively stimulated the optic tract with a tetanus protocol that approximated the temporal features of spontaneous retinal waves. We found the amplitude of extracellular field potentials evoked by retinal stimulation changed significantly after tetanus and that the polarity of these alterations was related to postnatal age. At a time when substantial pruning of retinal connections occurs (postnatal day 1 [P1] to P14), high-frequency stimulation led to an immediate and long-term depression (LTD). However, at times when pruning wanes and adultlike patterns of connectivity are stabilizing (P16 to P30), the identical form of stimulation produced a modest form of potentiation (long-term potentiation [LTP]). The LTD was unaffected by the bath application of γ-aminobutyric acid type A and N-methyl-d-aspartate receptor antagonists. However, both LTD and LTP were blocked by L-type Ca2+-channel antagonists. Thus the Ca2+ influx associated with L-type channel activation mediates the induction of synaptic plasticity and may signal the pruning and subsequent stabilization of developing retinogeniculate connections.
INTRODUCTION
The structural and functional composition of the rodent retinogeniculate pathway undergoes extensive remodeling during early postnatal life (Guido 2008; Huberman 2007). Just after birth, retinal projections form a coarse topographic map in the dorsal lateral geniculate nucleus (LGN), although eye-specific domains are not fully developed and the inputs from the two eyes have overlapping terminal fields (Jaubert et al. 2005; Žiburkus and Guido 2006). Functionally, there is also a high degree of retinal convergence (Chen and Regehr 2000; Lo et al. 2002), with many LGN cells receiving direct excitatory input from the two eyes (Jaubert et al. 2005; Žiburkus and Guido 2006). After the first postnatal week, retinal projections recede and there are clear signs of eye-specific segregation. By the second postnatal week, the majority of LGN cells receive monocular input from just a few retinal ganglion cells. Interestingly, there seems to be an additional period of axonal refinement, which by some estimates, persists for up to 3 wk after natural eye-opening (Hooks and Chen 2006).
Remodeling of the retinogeniculate pathway has been attributed to the spontaneous activity of retinal ganglion cells (see Torborg and Feller 2005). Even before photoreceptors are operational, groups of neighboring retinal ganglion cells fire in rhythmic bursts that travel across the retina in a wavelike fashion (Demas et al. 2003; Galli and Maffei 1988; Miester et al. 1991; Wong 1999; Wong et al. 1993). Alterations or the complete elimination of patterned spontaneous activity lead to abnormally widespread retinal arbors or a failure of retinal afferents to segregate into eye-specific domains in the LGN (Feller et al. 1996; Muir-Robinson et al. 2002; Penn et al. 1998; Shatz and Stryker 1988; Sretavan et al. 1988; Stellwagen et al. 2002; Torborg et al. 2005).
Although a great deal is known about the spatiotemporal components of retinal waves needed for retinogeniculate refinement (Butts 2002; Butts and Rokshar 2001; Butts et al. 2007; Torborg and Feller 2005; Torborg et al. 2005) the cellular mechanisms mediating the activity-dependent changes in LGN neurons remain an open question. Perhaps the most plausible model for explaining how neural activity leads to the formation of orderly connections is based on Hebb's postulate. The contemporary version asserts that temporally correlated activity between pre- and postsynaptic elements leads to a strengthening and consolidation of synapses, whereas asynchronous or the absence of activity results in synapse weakening and elimination (Bear et al. 1987; Constantine-Paton et al. 1990; Cramer and Sur 1995; Goodman and Shatz 1993; Katz and Shatz 1996; Stent 1973). A proposed mechanism for the Hebbian postulate is based on forms of synaptic plasticity in which the frequency of pairing between pre- and postsynaptic elements leads to a long-term potentiation (LTP) or depression (LTD) in synaptic strength. Numerous studies indicate that LTP and LTD exist in the developing neocortex and may contribute to the formation of orderly connections (Bear and Kirkwood 1993; Bear and Malenka 1994; Crair and Malenka 1995; Fox 1995; Galaretta and Hestrin 1998; Kirkwood and Bear 1994a,b; Kirkwood et al. 1993, 1996; Seol et al. 2007). Long-term changes in synaptic strength may also be involved in stabilizing retinogeniculate connections (Butts et al. 2007), especially since spontaneous retinal waves are sufficient to generate robust postsynaptic activity among LGN neurons (Mooney et al. 1996; Weliky and Katz 1999). Developing LGN cells also have a strong N-methyl-d-aspartate (NMDA) current (Chen and Regehr 2000; Liu and Chen 2008; Ramoa and McCormick 1994), which seems to participate in the long-term enhancement of excitatory postsynaptic currents (EPSCs) (Mooney et al. 1993).
The aim of the present study was to determine whether the activity of developing retinal ganglion cells can support synaptic plasticity during the period of retinogeniculate axon segregation. We used an in vitro explant preparation in which the innervating retinal afferents and intrinsic circuitry of LGN remain intact (Jaubert et al. 2005; Lo et al. 2002; Žiburkus and Guido 2006; Žiburkus et al. 2003). To determine whether the synaptic responses of LGN cells are modulated by retinal activity we administered a tetanus protocol that approximated the intrinsic firing patterns of developing retinal ganglion cells. Here we present results showing that LGN cells undergo long-term changes in synaptic strength and that the polarity and magnitude of these alterations are related to postnatal age.
METHODS
We used Long–Evans hooded rats that ranged in age from postnatal day 1 (P1) to P30. Animals were obtained from time-pregnant females that were acquired from a commercial vendor (Charles River Laboratories). All surgical procedures were performed in accordance with the IACUC protocol approved by the Louisiana State University Animal Care and Use Committee.
Animals were anesthetized with halothane and killed by decapitation. The brain was excised and placed in a chilled (4°C) solution of artificial cerebrospinal fluid (ACSF, in mM: 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1.0 MgSO4, 26 NaHCO3, 10 dextrose, and 2 CaCl2, saturated with 95% O2-5% CO2; pH 7.4). Surgical preparation of the explant preparation is described elsewhere (Lo et al. 2002; Žiburkus and Guido 2006; Žiburkus et al. 2003). Briefly, the excised brain was cut in half along the midline axis, glued to a silver plate, and then placed into the well of a temperature-controlled recording chamber. The lateral surfaces of the thalamus and midbrain were exposed by removing the forebrain. The preparation was submerged and perfused continuously (4–5 ml/min) with warmed (30–33°C) ACSF. Recordings began 1–3 h after incubation. All electrode penetrations were made along the dorsolateral surface of the LGN and restricted to depths of ≤150 μm. The area we targeted corresponds to the binocular region of LGN, which in the mature rat receives crossed retinal projections (Reese 1988; Žiburkus and Guido 2006; see Fig. 2).
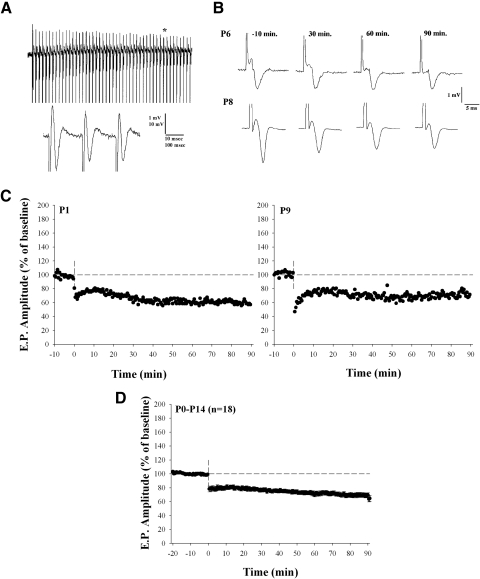
FIG. 2.
Tetanus-induced long-term depression (LTD) at early postnatal ages (P1–P14). A: examples of the field potentials recorded in LGN during high-frequency stimulation (HFS, 1-s, 50-Hz train) of the optic tract. The bottom trace depicts an expanded epoch (see asterisk) of the above response. B: field potentials recorded at P6 and P8 before (−10 min) and after (30, 60, and 90 min) HFS. C: individual plots showing changes in the amplitude of evoked responses as a percentage of the baseline response. Responses were obtained by a single shock given once every 30 s at a stimulus intensity that evoked a response that was between one half and two thirds maximal amplitude. D: summary plot showing the mean ± SE for 18 animals between the ages of P1 and P14 in which HFS led to LTD.
To assess synaptic efficacy we recorded extracellular field potentials evoked by optic tract (OT) stimulation. Recordings were made with tungsten-in-glass (0.5–2 MΩ) electrodes. Neuronal activity was recorded with an Axoclamp 2B amplifier (Axon Instruments), digitized at 5–10 kHz using a DA/AD interface board (Instrutech ITC-16), and stored directly on computer (Heka Elektronik).
To evoke synaptic activity in the LGN, single square-wave pulses (0.1–0.3 ms, 0.1–1.0 mA) were delivered at a rate of 0.03 Hz through a pair of thin-gauged tungsten wires placed on the OT. To determine whether field potentials were postsynaptic in origin, in some experiments we bath applied the glutamate antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 1 mM) or the L-type Ca2+ antagonist nitrendepine (10 μM). To determine whether high-frequency stimulation (HFS) of the OT led to long-term changes in synaptic efficacy, we measured the responses to a single shock (delivered once every 30–60 s) for 10 to 20 min before and 45 to 90 min after tetanus. For all recordings, we used a stimulus intensity that evoked half to two thirds the maximal size in the amplitude of the extracellular field potential (Kirkwood and Bear 1994a,b). The OT was stimulated with a train of high-frequency pulses designed to approximate the temporal patterning of early spontaneous activity of retinal ganglion cells. Prior to eye-opening, developing retinal ganglion cells fire spontaneous bursts of action potentials, 1–6 s in duration, at rates of 7–50 Hz every 30–100 s (Demas et al. 2003, 2006; Meister et al. 1991; Wong 1999; Wong et al. 1993). Based on these estimates we adopted a protocol that consisted of six 1-s trains of 50-Hz pulses delivered at 30-s intervals. In some cases we also used 100-Hz (n = 4, P15–P19) stimulus pulse trains.
To determine the pharmacology underlying any tetanus-induced changes in synaptic strength, the NMDA antagonist d-(−)-2-amino-5-phosphonopentanoic acid (dAP5, 50–100 μM), the γ-aminobutyric acid type A (GABAA) antagonist bicuculline (10 μM) and the L-type Ca2+-channel antagonists nitrendipine (10 μM) or nimodipine (10 μM) were bath applied ≥30 min before HFS of the OT and then throughout the entire posttetanus recording period. To ensure that the drugs had sufficient time to block targeted responses, in each of the antagonist conditions we extended baseline measures in one animal for up to 65 min. Results from these experiments were indistinguishable from others.
Although we used field potentials to assess synaptic efficacy, in some instances whole cell intracellular recordings were made in thalamic slices so that we could determine whether HFS-induced changes in synaptic strength were accompanied by a change in single-fiber excitability. Slice preparation and whole cell recording techniques have been described in detail elsewhere (Bickford et al. 2009; Lo et al. 2002). Patch electrodes were made of borosilicate glass and filled with a solution containing (in mM) 140 K-gluconate, 10 HEPES, 0.3 NaCl, 2 ATP-Mg, and 0.1 GTP-Na (pH 7.25, 260 Osm). The final tip resistance of filled electrodes was 3–5 MΩ. Whole cell recordings were done in current-clamp mode using an Axoclamp 2B amplifier. Pipette capacitance, series resistance, and whole cell capacitance were carefully monitored and compensated electronically during the recording. To evoke synaptic activity in the dorsal LGN, square-wave pulses (0.3 ms, 10–50 μA) were delivered at a rate of 0.1 Hz through a pair of thin-gauged tungsten wires (0.5 MΩ) positioned in the OT. To examine single-fiber activation, the OT was initially stimulated at below-threshold stimulus levels for evoking a synaptic response, after which the stimulus intensity was increased in small increments (0.5 or 1.0 μA) until a reliable response was evoked. Typically, once a reliable response (>90%) was evoked, further increases in stimulus intensity led to a stepwise increase in excitatory postsynaptic potential (EPSP) amplitude, thereby reflecting the activation of additional retinal inputs (Chen and Regehr 2000; Jaubert-Miazza et al. 2005; Lo et al. 2002). Thus to ensure our stimulation protocol activated a single fiber we generated stimulus intensity × EPSP amplitude plots (Fig. 6B). Each cell was tested before and after tetanus and each stimulus level was repeated 10 times to generate pre- and posttetanus response probability functions (Fig. 6C).
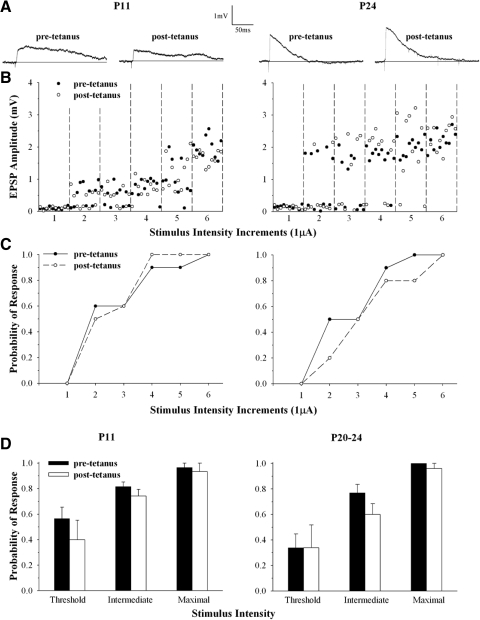
FIG. 6.
Single-fiber activation of LGN cells before and after HFS. A: averaged synaptic responses of a single retinal fiber evoked by applying minimal stimulus intensity levels before and after HFS for single LGN cells recorded at P11 (left) and P24 (right). B: amplitude × stimulus intensity plots for the responses in A. Each point depicts a synaptic response and black and white symbols show responses recorded before and after tetanus. The optic tract (OT) was stimulated at below-threshold stimulus levels (1) and then systematically increased (2–6) in small increments (1.0 μA) until reliable responses for single-fiber activation were evoked. Each stimulus increment was tested 10 times. Note that at P11 further increases in stimulus intensity (5–6) led to the activation of an additional input. Below in C, the amplitude × stimulus plots are the corresponding probability functions. Pre- and posttetanus response probability functions were similar. D: summary histograms showing the mean and SE response probability measured before (pretetanus) and after HFS (posttetanus) at different stimulus levels for single-fiber activation. Within the range of stimulus intensities that evoked a single-fiber response (threshold, intermediate, and maximal), response probability was unaffected by HFS. A total of 3 cells (42 stimulus intervals: 21 pretetanus and 21 posttetanus) at P11 and 4 cells (50 stimulus intervals: 25 pretetanus and 25 posttetanus) at P20–P24 were tested. All cells were recorded at −60 mV.
RESULTS
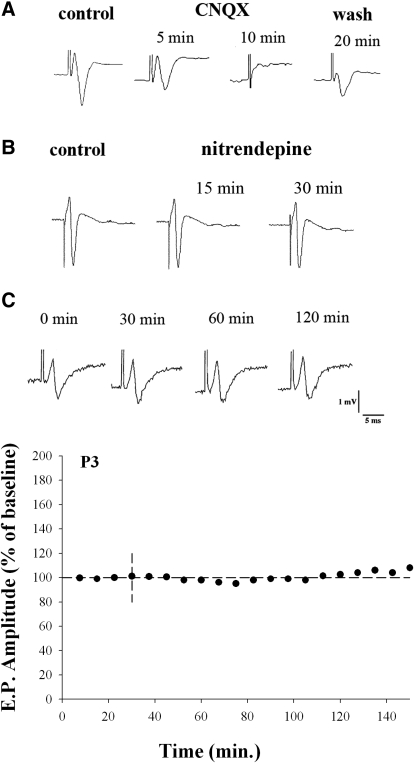
The explant preparation affords an excellent opportunity to record synaptic responses in the form of extracellular field potentials since retinal projections and intrinsic circuitry remain intact. Examples of field potentials evoked by OT stimulation are shown in Figs. 1–4. The responses recorded in vitro looked similar to those reported in vivo (Sefton and Swinburn 1964). At all ages tested (P1–P30), LGN field potentials had fast rising peaks that were followed by a large negative trough that had a variable, but relatively slow, decay. The field potentials were postsynaptic in origin and showed a strong dependence on glutamatergic transmission. As shown in Fig. 1A, the application of CNQX abolished the field potential (n = 4). Evoked field responses were also insensitive to L-type Ca2+-channel antagonists (n = 9), confirming that these channels are not involved in the presynaptic release of glutamate at developing retinal terminals (Lo et al. 2002). Figure 1B shows the bath application of the L-type Ca2+-channel antagonist nitrendipine did not affect the amplitude or the wave form of the evoked response (see also Figs. 3A and 4A). Finally, field potentials proved to be a reliable and stable indicator of retinogeniculate transmission. Figure 1C shows that stable responses could be evoked by a single shock delivered to the OT (once every 30 s) for as long as 2–3 h.
FIG. 1.
Extracellular field potentials recorded in LGN evoked by optic tract stimulation. A and B: examples of field potentials recorded before and after the bath application of AMPA antagonist CNQX (A) or the L-type channel blocker nitrendipine (B). After 5 min, CNQX reduced the field potential to 61% of its baseline amplitude and after 10 min the response was completely abolished. By 20 min after washout, the field response was restored to 46% of its baseline amplitude. The application of nitrendipine had no effect on synaptic responses in LGN (see also Fig. 3). After 15–30 min responses were similar (97–99%) to baseline measures. C: examples of field responses recorded in normal ACSF from a single animal at P3. The corresponding amplitude × time plot (below) reflects an averaged (mean ± SE) response measured every 30 s over a 7.5-min time interval. Amplitude measurements are depicted as a percentage of the response measured during the first 7.5 min. LGN, lateral geniculate nucleus; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; ACSF, artificial cerebrospinal fluid; P3, postnatal day 3.
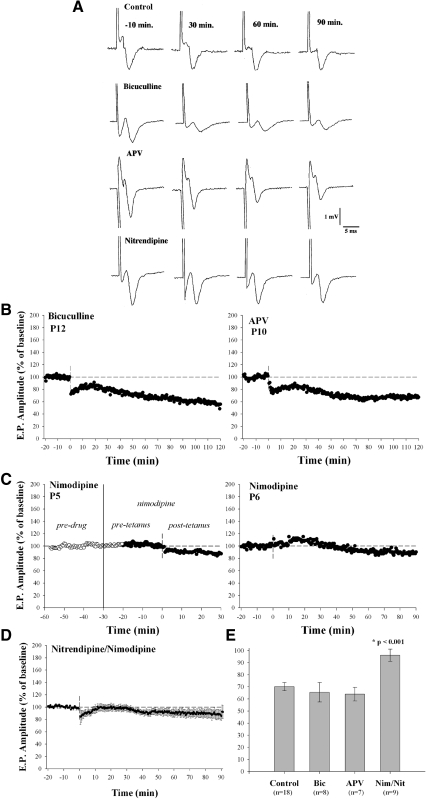
FIG. 3.
Pharmacology of LTD. A: examples of the field responses recorded in the presence of control ACSF, the GABAA antagonist bicuculline, the NMDA antagonist d-AP5, or the L-type Ca2+ channel antagonist nitrendipine, before (−10 min) and after (30, 60, and 90 min) HFS. B and C: individual plots at P12 and P10 showing amplitude changes before and after HFS. Bicuculline and d-AP5 failed to block tetanus-induced depression. C: additional cases showing the L-type Ca2+ antagonist nimodipine had no effect on the responses evoked by a single shock but blocked the tetanus-induced depression. D: amplitude × time plot for 9 cases showing mean (±SE) values for animals treated with either nimodipine or nitrendipine. E: bar graph summarizing pharmacology experiments. Each bar depicts mean (±SE) amplitude changes at 60 min posttetanus. The numbers of animals tested in each condition are listed below each bar. Neither bicuculline nor d-AP5 was capable of blocking the tetanus-induced LTD; however, LTD was blocked in the presence of the L-type Ca channel antagonists. GABAA, γ-aminobutyric acid type A; NMDA, N-methyl-d-aspartate; d-AP5, d-(−)-2-amino-5-phosphonopentanoic acid.
FIG. 4.
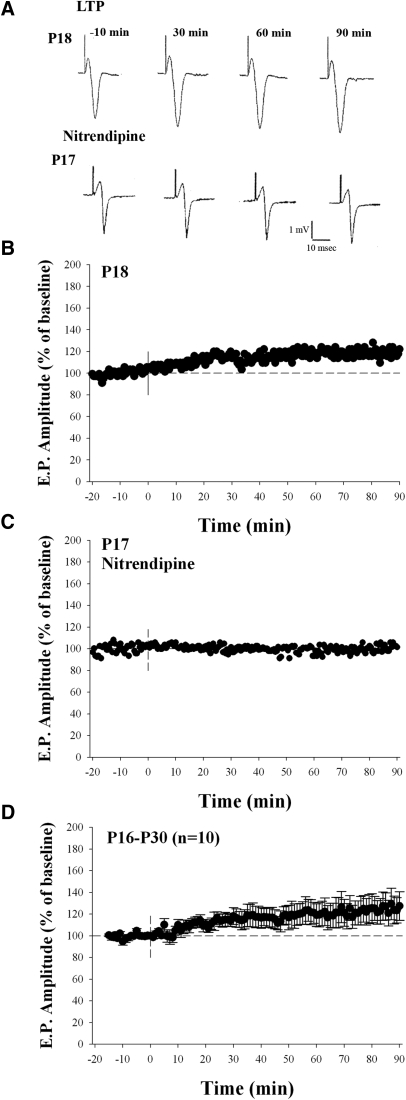
Tetanus-induced long-term potentiation (LTP) at late postnatal ages (P16–P30). A: examples of field potentials recorded at P18 and P17 before (−10 min) and after (30, 60, and 90 min) after HFS. The case at P17 was recorded in the presence of nitrendipine. B: amplitude × time plot for the case shown at P18. HFS led to a modest form of LTP. C: amplitude × time plot for the case shown at P17. The application of nitrendipine blocked the tetanus-induced potentiation. D: summary plot showing mean (±SE) amplitude changes and tetanus-induced LTP for 10 animals.
To determine whether high-frequency stimulation (HFS) of the OT led to long-term changes in synaptic efficacy, we measured the responses to a single shock (delivered once every 30 or 60 s) before and after tetanus (six 1-s trains of 50-Hz pulses delivered at 30-s intervals). An example of one such train is shown in Fig. 2A . Figure 2B shows examples of field potentials recorded at P6 and P8 before (−10 min) and after (30, 60, and 90 min) HFS. The amplitude × time plots in Fig. 2C from two additional animals (P1 and P9) show changes in the field potential expressed as a percentage of the baseline response. At early ages (≤P14), HFS consistently led to a long-term reduction (30–40%) in amplitude. Figure 2D plots mean changes in the amplitude of the field potential for 18 animals. Prior to eye-opening (P1–P14), we found that HFS led to a significant and sustained reduction in amplitude of the field response compared with baseline values (Wilcoxon matched-pairs signed-rank test, P < 0.001).
To examine the pharmacology underlying these long-term changes, we bath applied specific antagonists to selectively block either NMDA (d-AP5, 50–100 μM), GABAA (bicuculline, 10 μM), or L-type Ca2+-channel (nimodipine or nitrendipine, 10 μM) activity. In each case, application of all drugs started ≥30 min before HFS and continued throughout the entire posttetanus recording period. Figure 3A shows examples of the synaptic responses recorded before and after HFS in the presence of normal ACSF or during the bath application of bicuculline, d-AP5, or nitrendipine. Examples of individual amplitude plots are shown in Fig. 3, B and C. Neither bicuculline nor d-AP5 influenced the tetanus-induced depression. However, as shown in Fig. 3C and the averaged (n = 9) amplitude × time plot in Fig. 3D, L-type Ca2+-channel antagonists nimodipine or nitrendipine blocked the depression.
Figure 3E summarizes the results of our pharmacological experiments. Each bar shows the mean and SE values of amplitude measured at 60 min posttetanus. Animals treated with bicuculline (n = 8) and d-AP5 (n = 7) were no different from controls, whereas those treated with L-type Ca2+-channel antagonists (n = 9) failed to show depression. Thus animals treated with nimodipine or nitrendipine were significantly different from control or those treated with bicuculline and d-AP5 (Mann–Whitney U test, P < 0.001). These experiments indicate that neither NMDA nor GABAA activity played a role in the induction of LTD. However, the activation of L-type Ca2+ channels was necessary and sufficient for such changes.
We tested whether long-term changes in synaptic strength persisted past the period of natural eye-opening, when spontaneous retinal activity subsides (Demas et al. 2003) and eye-specific patterning of retinal projections is complete (Žiburkus and Guido 2006). Figure 4 summarizes recordings done between P16 and P30. At these ages, the identical form of HFS led to a modest (10–30%, n = 10), long-lasting increase in the amplitude of the field potential (Wilcoxon matched-pairs signed-rank test, P < 0.01, Fig. 4D). A similar form of potentiation was also observed between P15 and P19 (n = 4), when the HFS consisted of a 100-Hz stimulus train. Finally, tetanus-induced changes were blocked by L-type Ca2+ antagonists (n = 4, Fig. 4C). At 60 min posttetanus the amplitude of the field potential was similar to baseline (n = 4, 99.7 ± 4.5% [mean ± SE]).
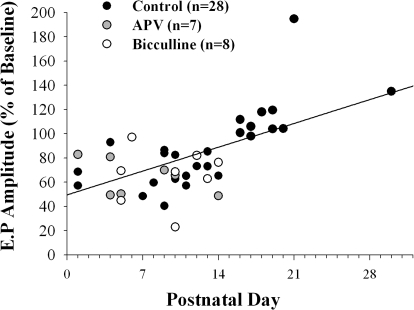
Figure 5 plots posttetanus values obtained at 60 min for 43 animals. When data from all ages are considered (P1–P30), including cases in which d-AP5 or bicuculline was added to the bath, postnatal age and the polarity of tetanus-induced changes were significantly correlated, with the best fit showing a linear relation (n = 43, r2 = 0.358, P < 0.0001). However, at ages when LTD (P1–P14) or when LTP (P16–P30) occurred, neither group by itself showed a significant change in amplitude with age (LTD: P1–P14, n = 33, r2 = 0.009, P < 0.59; LTP: P16–P30, n = 10, r2 = 0.18, P < 0.21). That is, within these groups, the magnitude of change did not vary systematically with age. Thus these results indicate that the retinogeniculate synapse can support bidirectional, long-term changes in synaptic efficacy. LTD prevails during the first 2 postnatal weeks, but between P16 and P30, the identical form of tetanus leads to a modest form of LTP.
FIG. 5.
A scatterplot showing the magnitude and polarity of tetanus-induced changes in the amplitude of field potentials measured at 60 min at different postnatal ages. Each point depicts a single case and the different symbols represent animals recorded under control conditions (n = 28) or when d-AP5 (n = 7) or bicuculline (n = 8) were added to the bath. The best-fit line shows a significant linear correlation between postnatal age and the polarity of tetanus-induced changes (n = 43, r2 = 0.358, P < 0.0001). At ages when LTD (P1–P14) or LTP (P16–P30) prevailed, neither group by itself showed a significant change in amplitude with age (LTD: P1–P14, n = 33, r2 = 0.009, P < 0.59; LTP: P16–P30, r2 = 0.18, P < 0.21).
To examine whether HFS leads to changes in single-fiber excitability, we recorded the synaptic responses of individual LGN cells by stimulating the OT at minimal stimulus intensities before and after HFS. Examples of whole cell intracellular recordings at P11 and P24 are shown in Fig. 6. We began by stimulating the OT at below-threshold stimulus levels and then systematically increased the intensity in small increments until reliable responses for single-fiber activation were evoked. We examined whether the probability of response evoked at a given stimulus intensity was altered by HFS stimulation. Figure 6, B and C shows amplitude × stimulus intensity plots and their corresponding probability functions before and after HFS for the responses shown in Fig. 6A. In each case, an increase in stimulus intensity led to a progressive increase in response probability. Indeed, the response probability functions generated before and after HFS were similar, suggesting that tetanus did not lead to a substantial change in single-fiber excitability. The results of seven cells (n = 3 at P11 and n = 4 at P20–P24) are summarized in Fig. 6D. Shown are histograms that plot response probability for single-fiber activation at threshold, intermediate, and maximal intensity values. At each stimulus level (n = 42 for 3 cells at P11, n = 50 for 4 cells at P20–P24), pre- and posttetanus response probabilities were similar (t-test values ranged from P < 0.13 to P < 0.70). Thus these data indicate that the tetanus-induced changes in synaptic strength were not due to a change in fiber excitability at ages when LTD or LTP was observed.
DISCUSSION
Our results indicate that the developing retinogeniculate synapse can support long-term changes in synaptic strength. When the OT is stimulated in a manner that approximates early spontaneous retinal activity, long-term changes in the amplitude of the extracellular field potential occur. Such modifications are believed to reflect a change in synaptic strength and/or efficacy (Bear and Malenka 1994; Bear et al. 1987; Kirkwood and Bear 1994a,b; Kirkwood et al. 1993, 1996). A decrease in amplitude reflects synaptic weakening, whereas an increase in amplitude reflects synaptic strengthening. Additionally, we found the changes in synaptic strength are bidirectional and related to postnatal age. Prior to natural eye-opening (≤P14) LTD is observed, but after eye-opening (P16–P30) the identical form of stimulation yields LTP. Recently it was shown that bidirectional changes in synaptic strength can also occur in a single LGN cell rather than across many, as shown in our recorded extracellular field potentials (Butts et al. 2007). In these perforated patch recordings, the relative timing between presynaptic tetantus stimulation of OT and postsynaptic LGN cell depolarization proved to be the defining feature. A single LGN cell exhibited LTP (21%) when OT stimulation and LGN cell depolarization were overlapping, but LTD (6%) occurred when pairings were nonoverlapping. Although it may be difficult to cast the present results into such a Hebbian scheme, our age-related bidirectional changes in the field potential may represent a more generalized state of synaptic efficacy. For example, LTD may reflect the weakening of highly labile convergent inputs. In rodents there is significant pruning of retinal projections and a loss of functional inputs during early postnatal life. Initially, LGN cells are binocularly innervated and receive weak synaptic input from as many as 12–20 retinal ganglion cells (Chen and Regehr 2000; Jaubert et al. 2005; Lo et al. 2002; Žiburkus and Guido 2006). The most intense period of pruning seems to occur during the first 2 postnatal weeks (Žiburkus et al. 2006), a time when retinal waves prevail (Demas et al. 2003) and provide the primary excitatory drive for LGN cells (Mooney et al. 1996). In rats our estimates reveal that by natural eye-opening (P14–P15) LGN cells can lose as many as four to eight inputs from each eye (Žiburkus and Guido 2006). Thus the LTD we note prior to eye-opening may herald the structural and functional refinement of retinogeniculate connections. In contrast to the observed LTD, the LTP that occurs after eye-opening (P16–P30) may reflect a strengthening and subsequent stabilization of remaining inputs that are assuming adultlike patterns of connectivity. Between P16 and P30, pruning and synapse elimination subside along with the few remaining monocular inputs (Žiburkus and Guido 2006).
It is also interesting to examine the developmental switch from LTD to LTP in the context of the reported changes in synapse strength that occur with age. According to estimates of Chen and Regehr (2000) both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and NMDA receptor currents are relatively weak between P10 and P14, compared with P16–P22 and P23–P31. For example, there seems to be a 27-fold increase in AMPAR single-fiber amplitude and a 2.5-fold increase in maximum current amplitude between P10–P14 and P16–P22 (Chen and Regehr 2000). In a more recent report, both single-fiber and maximum current amplitudes show a four- to fivefold increase between P9 and P11 and P15 and P16 and a two- to threefold increase between P16 and P22 and P23 and P31 (Hooks and Chen 2006; see also Supplemental Fig. S3 in Liu and Chen 2008). Taken together, these estimates reveal an age-related increase in synaptic strength with cells at older ages (P23–P31) exhibiting the strongest currents and cells at the youngest ages (P9–P11) exhibiting the weakest. Although there is certainly some overlap between the times when some synapses are weakening and others are strengthening, overall it appears the LTD coincides with a weakening of synapses and LTP, a strengthening of synapses. This view is further bolstered when one considers that extracellular field potentials are summed responses that reflect the activity of several cells. Thus changes in the amplitude of the field potential reflect the net responses of several neurons rather than a single one.
Unfortunately, we are not able to fully address whether the degree of tetanus-induced changes in synaptic strength varies by LGN location (e.g., monocular or binocular portions of LGN) or among cells that initially receive binocular or monocular inputs. However, our previous studies using an explant preparation suggest that recordings target regions that correspond to the binocular region in LGN, an area dominated by crossed retinal projections (Žiburkus and Guido 2006). Intracellular recordings of synaptic responses in these regions also reveal that both monocular and binocular cells undergo synaptic pruning. This would suggest that a mechanism that supports synapse weakening and elimination (LTD) as well as the eventual strengthening and consolidation (LTP) of remaining inputs are present in both groups of cells and perhaps throughout all regions of the LGN.
Our pharmacological experiments reveal that LTD is not caused by a selective enhancement of inhibitory activity. The application of GABA antagonists has no effect on these tetanus-induced changes. The blockade of excitatory NMDA activity also fails to alter LTD. This latter result seems at odds with an earlier report in developing LGN in which HFS of OT led to NMDA receptor-dependent potentiation (Mooney et al. 2003). There are a number of differences between that study and the present one. It was done in ferrets between the ages of P6 and P21 using perforated-patch recordings. Moreover, the LTP was reported in 14 of 35 cells (40%) and there is little information about how the magnitude or polarity of posttetanus responses varied with age. It is also not clear whether the enhancement of NMDA responses plays a major role in the remodeling of retinogeniculate connections. In the mouse, correlated firing between retinal ganglion and LGN cells persists in the absence of NMDA receptors (Mooney et al. 1996). Although not yet tested in the rodent, in ferret LGN NMDA receptor blockade does not interfere with eye-specific segregation (Smetters et al. 1994), although it does seem to disrupt on-off segregation (Hahm et al. 1991). In mouse, developing LGN cells exhibit robust NMDA responses. In fact many aspects of these responses, including overall current amplitude, decay time kinetics, subunit composition, and affinity for Mg2+ contribute to the overall excitability of LGN cells, increasing the probability that postsynaptic membrane depolarization leads to action potential firing (Chen and Regehr 2000; Liu and Chen 2008).
In our experiments the critical element responsible for the induction of long-term changes in synaptic strength is the activation of L-type Ca2+ channels. The application of L-type Ca2+-channel antagonists leads to a complete blockade of the tetanus-induced changes in synaptic strength. A role for L-type Ca2+ channels and synaptic plasticity is well established and has been reported in a number of developing structures, including the hippocampus (Bolshakov and Siegelbaum 1994), superior colliculus (Lo and Mize 2000; Mize and Salt 2004), and brain stem trigeminal complex (Guido et al. 2001). In LGN, L-type activity figures prominently during the period of retinogeniculate axon segregation. Strong and/or repetitive stimulation of retinal fibers evokes EPSPs of sufficient amplitude to activate voltage-gated L-type Ca2+ activity. These responses take the form of a plateau potential, a high-amplitude (25–60 mV), long-lasting (300–1,500 ms), slow-decaying depolarization (Jaubert et al. 2005; Liu and Chen 2008; Lo et al. 2002). Synaptically evoked plateau potentials are encountered far more frequently during the period of retinogeniculate refinement. This has been attributed to the high degree of retinal convergence and heightened NMDA activity, which in turn favors the spatial and temporal summation of EPSPs (Lo et al. 2002). These summed synaptic events lead to sustained levels of depolarization, thereby greatly increasing the likelihood of activating the voltage-gated high-threshold L-type channels. The periodic barrages of retinal EPSCs associated with the spontaneous retinal waves (Mooney et al. 1996) are also well suited to activate L-type-mediated plateau potentials. In fact, tetanus protocols designed to mimic retinal waves trigger robust plateau-like activity (Jaubert et al. 2005; Lo et al. 2002; see also Butts et al. 2007). In rodent LGN, L-type channels are located primarily on somata and proximal dendrites (Budde et al. 1998) and their expression seems particularly high at early postnatal ages (Jaubert-Miazza et al. 2005). Thus these channels reside in the same vicinity as retinal terminals (Bickford et al. 2009; Rafols and Valverde 1973) and are well positioned to ensure coincident activation with retinally mediated events.
Finally, the synaptically evoked Ca2+ influx through L-type channels may be particularly effective at activating transcription factors that lead to the expression of genes linked to synaptic plasticity (Lonze and Ginty 2002; West et al. 2002). In the developing LGN, a likely candidate involves the cyclic adenosine monophosphate (cAMP) response element (CRE/CREB) transcription pathway (Dolmetsch et al. 2001; Mermelstein et al. 2000). The CRE binding protein (CREB) is a Ca2+- and cAMP-regulated transcriptional activating protein, shown to be important for thalamic circuit development and retinogeniculate axon segregation. In the mouse LGN, CRE-mediated gene expression peaks during early postnatal life (Pham et al. 2001) and mutant mice that show reduced levels of L-type Ca2+-channel activity or decreased levels of CRE expression fail to undergo normal retinogeniculate axon segregation (Cork et al. 2001; Green et al. 2004; Pham et al. 2001). Thus the Ca2+ influx through L-type channels, which mediates the induction of synaptic plasticity, may provide the basis for the pruning and stabilization of developing retinogeniculate connections.
GRANTS
This work was supported by National Eye Institute Grant EY-12716 and the Whitehall Foundation.
ACKNOWLEDGMENTS
We thank E. Green for technical assistance, Dr. T. Krahe for critical feedback and statistical advice, and Louisiana State University Health Sciences Center, where the majority of studies were conducted.
REFERENCES
- Bear MF, Cooper LN, Ebner FF. A physiological basis for a theory of synapse modification. Science 237: 42–48, 1987 [DOI] [PubMed] [Google Scholar]
- Bear MF, Kirkwood A. Neocortical long-term potentiation. Curr Opin Neurobiol 3: 197–202, 1993 [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4: 389–399, 1994 [DOI] [PubMed] [Google Scholar]
- Bickford ME, Slusarczyk A, Dilger EK, Krahe TE, Kucuk C, Guido W. Synaptic development of the mouse dorsal lateral geniculate nucleus. J Comp Neurol (September16, 2009). doi:10.1002/cne.2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science 264: 1148–1152, 1994 [DOI] [PubMed] [Google Scholar]
- Budde T, Munsch T, Pape HC. Distribution of L-type calcium channels in rat thalamic neurones. Eur J Neurosci 10: 586–597, 1998 [DOI] [PubMed] [Google Scholar]
- Butts DA. Retinal waves: implications for synaptic learning rules during development. Neuroscientist 8: 243–253, 2002 [DOI] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based “hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol 5: e61, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Rokhsar DS. The information content of spontaneous retinal waves. J Neurosci 21: 961–973, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron 28: 955–966, 2000 [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci 13: 129–154, 1990 [DOI] [PubMed] [Google Scholar]
- Cork RJ, Namkung Y, Shin HS, Mize RR. Development of the visual pathway is disrupted in mice with a targeted disruption of the calcium channel beta(3)-subunit gene. J Comp Neurol 440: 177–191, 2001 [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature 375: 325–328, 1995 [DOI] [PubMed] [Google Scholar]
- Cramer KS, Sur M. Activity-dependent remodeling of connections in the mammalian visual system. Curr Opin Neurobiol 5: 106–111, 1995 [DOI] [PubMed] [Google Scholar]
- Demas J, Eglen SJ, Wong RO. Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience. J Neurosci 23: 2851–2860, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas J, Sagdullaev BT, Green E, Jaubert-Miazza L, McCall MA, Gregg RG, Wong RO, Guido W. Failure to maintain eye-specific segregation in nob, a mutant with abnormally patterned retinal activity. Neuron 50: 247–259, 2006 [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 294: 333–339, 2001 [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science 272: 1182–1187, 1996 [DOI] [PubMed] [Google Scholar]
- Fox K. The critical period for long-term potentiation in primary sensory cortex. Neuron 15: 485–488, 1995 [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat Neurosci 1: 587–594, 1998 [DOI] [PubMed] [Google Scholar]
- Galli L, Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science 242: 90–91, 1988 [DOI] [PubMed] [Google Scholar]
- Green E, Bui K, Mills J, Shin H, Gregg RG, Guido W. Anomalous retinal projections and altered L-type calcium channel expression in the LGN of calcium channel B3 subunit deficient mice. Soc Neurosci Abstr 61312, 2004 [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell Suppl 72: 77–98, 1993 [DOI] [PubMed] [Google Scholar]
- Guido W. Refinement of the retinogeniculate pathway. J Physiol 586: 4357–4362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W, Lo F-S, Erzurumlu RS. Synaptic plasticity in the trigeminal principal nucleus during the period of barrelette formation and consolidation. Brain Res Dev Brain Res 132: 97–102, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm JO, Langdon RB, Sur M. Disruption of retinogeniculate afferent segregation by antagonists to NMDA receptors. Nature 351: 568–570, 1991 [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52: 281–291, 2006 [DOI] [PubMed] [Google Scholar]
- Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol 17: 73–80, 2007 [DOI] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo F-S, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci 22: 661–676, 2005 [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science 274: 1133–1138, 1996 [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci 14: 1634–1645, 1994a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. J Neurosci 14: 3404–3412, 1994b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science 260: 1518–1521, 1993 [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature 381: 526–528, 1996 [DOI] [PubMed] [Google Scholar]
- Lo F-S, Mize RR. Synaptic regulation of L-type Ca2+ channel activity and long-term depression during refinement of the retinocollicular pathway in developing rodent superior colliculus. J Neurosci 20: RC58 (1–6), 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo F-S, Žiburkus J, Guido W. Synaptic mechanisms regulating the activation of a Ca2+-mediated plateau potential in developing relay cells of the LGN. J Neurophysiol 87: 1175–1185, 2002 [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35: 605–623, 2002 [DOI] [PubMed] [Google Scholar]
- Liu X, Chen C. Different roles for AMPA and NMDA receptors in transmission at the immature retinogeniculate synapse. J Neurophysiol 99: 629–643, 2008 [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252: 939–943, 1991 [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci 20: 266–273, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mize RR, Salt TE. Contribution of GABAergic inhibition to synaptic responses and LTD early in postnatal development in the rat superior colliculus. Eur J Neurosci 20: 1331–1340, 2004 [DOI] [PubMed] [Google Scholar]
- Mooney R, Madison DV, Shatz CJ. Enhancement of transmission at the developing retinogeniculate synapse. Neuron 10: 815–825, 1993 [DOI] [PubMed] [Google Scholar]
- Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron 17: 863–874, 1996 [DOI] [PubMed] [Google Scholar]
- Muir-Robinson G, Hwang BJ, Feller MB. Retinogeniculate axons undergo eye-specific segregation in the absence of eye specific layers. J Neurosci 22: 5259–5264, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science 279: 2108–2112, 1998 [DOI] [PubMed] [Google Scholar]
- Pham TA, Rubenstein JL, Silva AJ, Storm DR, Stryker MP. The CRE/CREB pathway is transiently expressed in thalamic circuit development and contributes to refinement of retinogeniculate axons. Neuron 31: 409–420, 2001 [DOI] [PubMed] [Google Scholar]
- Rafols JA, Valverde F. The structure of the dorsal lateral geniculate nucleus in the mouse. A Golgi and electron microscopic study. J Comp Neurol 150: 303–332, 1973 [DOI] [PubMed] [Google Scholar]
- Ramoa AS, McCormick DA. Enhanced activation of NMDA receptor responses at the immature retinogeniculate synapse. J Neurosci 14: 2098–2105, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton AJ, Swinburn M. Electrical activity of lateral geniculate nucleus and optic tract of the rat. Vision Res 4: 315–328, 1964 [DOI] [PubMed] [Google Scholar]
- Seol GH, Žiburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron 55: 919–929, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science 242: 87–89, 1988 [DOI] [PubMed] [Google Scholar]
- Smetters DK, Hahm J, Sur M. An N-methyl-d-aspartate receptor antagonist does not prevent eye-specific segregation in the ferret retinogeniculate pathway. Brain Res 658: 168–178, 1994 [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ, Stryker MP. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature 336: 468–471, 1988 [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron 33: 357–367, 2002 [DOI] [PubMed] [Google Scholar]
- Stent GS. A physiological mechanism for Hebb's postulate of learning. Proc Natl Acad Sci USA 70: 997–1001, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol 76: 213–235, 2005 [DOI] [PubMed] [Google Scholar]
- Torborg CL, Hansen KA, Feller MB. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat Neurosci 8: 72–78, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science 285: 599–604, 1999 [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci 3: 921–931, 2002 [DOI] [PubMed] [Google Scholar]
- Wong RO. Retinal waves and visual system development. Annu Rev Neurosci 22: 29–47, 1999 [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron 11: 923–938, 1993 [DOI] [PubMed] [Google Scholar]
- Žiburkus J, Guido W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J Neurophysiol 96: 2775–2784, 2006 [DOI] [PubMed] [Google Scholar]
- Žiburkus J, Lo F-S, Guido W. Nature of inhibitory postsynaptic activity in developing relay cells of the lateral geniculate nucleus. J Neurophysiol 90: 1063–1070, 2003 [DOI] [PubMed] [Google Scholar]