Abstract
Areas of human posterior parietal cortex (PPC) specialized for processing sensorimotor information associated with visually locating an object, reaching to grasp, and manually exploring that object were examined using functional MRI. Cortical activation was observed in response to three tasks: 1) saccadic eye movements, 2) visually guided reaching to grasp, and 3) manual shape discrimination. During saccadic eye movements, cortical fields within the lateral and rostral superior parietal lobe (SPL) and the caudal SPL and parieto-occipital boundary were active. During visually guided reaching to grasp, regions of cortex within the postcentral sulcus (PoCS) and rostral intraparietal sulcus (IPS) were active, as well as the caudal SPL of the left hemisphere and the medial and caudal IPS of the right hemisphere. Cortical regions at the junction of the IPS and PoCS and an area in the medial SPL were active bilaterally during shape manipulation. Only a few regions were most active during a single motor behavior, whereas several areas were highly active during two or more tasks. Hemispheric asymmetries in activation patterns were observed during visually guided reaching to grasp. The gross areal organization of human PPC is likely similar to the pattern previously described in nonhuman primates, including multifunctional regions and asymmetric processing of some manual abilities.
INTRODUCTION
Visual-manual exploration of the environment is a hallmark of primate behavior. This ability requires tactile and proprioceptive inputs from the digits, hands, and forelimbs to be combined within and across cerebral hemispheres and these inputs may direct and are certainly coordinated with eye, limb, and hand movements. This synergistic activity between somatic, visual, and motor systems is necessary to make accurate judgments regarding the physical attributes of an object, the sensory context in which the object is located, and its relation to some internal representation of the body (Castiello 2005; Krubitzer and Disbrow 2008). Such judgments are used to locate an object in space, reach for and grasp the object, and manually manipulate and explore it.
One region of the brain known to integrate somatic and visual inputs and to coordinate these inputs with motor output is posterior parietal cortex (PPC; for reviews see Buneo and Andersen 2006; Colby and Goldberg 1999). Although it has been appreciated for over a century that lesions of this area in humans produce gross visuomotor deficits (Goodale and Milner 1992), the variability in lesion location and resulting range of behavioral impairment has made it difficult to infer functional organization in humans based on this information alone.
Detailed studies in macaque monkeys have revealed functional properties of this region that support the hypothesis that PPC is a site of integration of sensorimotor information necessary for visual-manual exploration. For example, neurons in PPC are multimodal and have large, complex receptive fields (Blatt et al. 1990; Breveglieri et al. 2008; Colby and Duhamel 1991; Padberg et al. 2005). In many of the fields, there is a magnification of representation of body structures central to the animal's natural behavior, such as the arm or hand, and the response properties of these neurons are modified based on the task the animal is performing (Graziano et al. 2000; Iriki et al. 1996; Sakata et al. 1973). Furthermore, neurons in multiple parietal fields are responsive during behaviors where information is incorporated across sensorimotor domains, including self-feeding behaviors, ballistic eye movements, reaching with grasping, and coordinated tasks involving the hands (Fogassi et al. 2005; Hyvärinen 1981; Rozzi et al. 2008).
Many attempts have been made by researchers, using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), to identify regions of the human parietal lobe involved in sensorimotor integration, using the known organization of macaque parietal cortex as a model (for reviews see Culham and Valyear 2006; Grefkes and Fink 2005). Although similarities between human and macaque monkey PPC have been identified using this approach, most often only a single behavior (such as saccadic eye movements) was examined in the same group of subjects (Culham and Valyear 2006). Existing studies in which multiple behaviors were investigated within the same scan session (Astafiev et al. 2003; Connolly et al. 2000; Hagler Jr et al. 2007; Levy et al. 2007; Shikata et al. 2008; Simon et al. 2002, 2004) often found slight differences in activity between two conditions and sometimes used tasks (such as pointing a finger to isolate reach-related areas of PPC) that were somewhat artificial, in that they did not reliably reproduce the complex behaviors used to map these regions in macaques or the behavior of the animal in a natural setting (for a discussion of this issue see Culham et al. 2008). No study to date has examined the role of human PPC in visually locating an object, reaching to grasp, and manually exploring that object in the same group of subjects. Furthermore, given the high intersubject variability in intraparietal sulcus (IPS) morphology and the variability in location of cortical areas with respect to sulcal landmarks (Caspers et al. 2006; Krubitzer et al. 2004; Roland and Zilles 1998; Scheperjans et al. 2008), it has been difficult to obtain a comprehensive map of the many cortical fields in this complex region.
The goal of the present study was to identify regions of human PPC involved in visuomanual exploration, specifically in visually locating an object in space, reaching to and grasping the object, and manually manipulating and exploring it. These data will be used to provide a map of the relative location of multiple cortical fields in human PPC.
METHODS
Subjects
Fourteen right-handed volunteers from the University of California, Davis participated in these experiments. Participants were screened for contraindications for magnetic resonance imaging (MRI) and written consent from each participant was obtained prior to the experiment. All procedures were approved in advance by the Institutional Review Board on Human Subjects Research at the University of California, Davis.
MRI acquisition
Scanning was done on a 1.5-T GE Signa scanner at the University of California, Davis Imaging Research Center. Functional MRI data were collected using a gradient echo planar sequence (repetition time [TR] = 2,000 ms, time to echo [TE] = 50 ms) from 18 5-mm-thick (1-mm gap) axial slices (64 × 64 matrix, field of view [FOV] = 220 mm) using a whole-head radiofrequency (RF) coil. To aid in visualization of statistical maps, coplanar T1-weighted images were also acquired within the same session [18 5-mm slices (1-mm gap); FOV = 260 mm, matrix = 256 × 256, TE = 6 ms, TR = 35 ms, flip angle = 30°]. Task conditions were presented using a block design interleaving experimental, control, and resting epochs of 20 s each.
Tasks
We broke visual-manual exploration into three components: 1) visually locating an object, 2) reaching to and grasping an object, and 3) manually exploring that object. Subjects participated in three corresponding conditions in a single scan session: 1) visually directed saccadic eye movements, 2) visually guided reaching to grasp, and 3) manual shape discrimination. Subjects received training on these tasks prior to data collection in the scanner to ensure familiarity with the experimental design. The order in which conditions were presented was randomized across subjects.
1) Visually directed saccadic eye movement condition.
During the experimental block, targets (crosshairs) appeared at one of eight locations 9.3° peripheral to a central fixation point. Subjects were instructed to saccade to this target and return to fixation at a steady pace. Targets appeared once every 2,000 ms during this experimental block, requiring subjects to saccade and return to fixation at a rate of around 1 Hz. Target locations were pseudorandomized within a block and counterbalanced within a single session. Visual stimuli were displayed on a PC running Presentation software (Neurobehavioral Systems; http://www.neurobs.com/) through a liquid crystal display projector onto a rear projection screen located at the feet of the subjects and were viewed with angled mirrors. During the control period, subjects maintained fixation while preparing for the target stimuli to appear. Subjects were instructed to keep their eyes open throughout the duration of the run. Twenty-second visually directed saccade and control blocks were interleaved and repeated four times within each run.
2) Visually guided reaching to grasp condition.
During the experimental block, subjects were instructed to reach to a target object (tennis ball) suspended above their midline and grasp it with their right hand. Arm movements started at a resting point at the right side of the subject and ended once contact was made with the object. The arm was then slowly returned to the starting point beside the subject. Subjects were instructed to perform this task with their eyes open and were able to view the location of the target ball through angled mirrors mounted on the RF coil. The purpose of this design was to evoke activity in regions of parietal cortex involved in the forearm transporting the hand toward a visual location for the purpose of object interaction. Subjects also performed a motor-control task in which they held the target object with the right hand, lifted the object to their midline, and squeezed it while their eyes were closed. This control task was designed to be matched for muscle stretch during reaching and cutaneous contact with the object during grasping. A comparison between this condition and the experimental condition corrects for the tactile and motor components of the task, leaving regions active only during visual guidance of the hand for grasping.
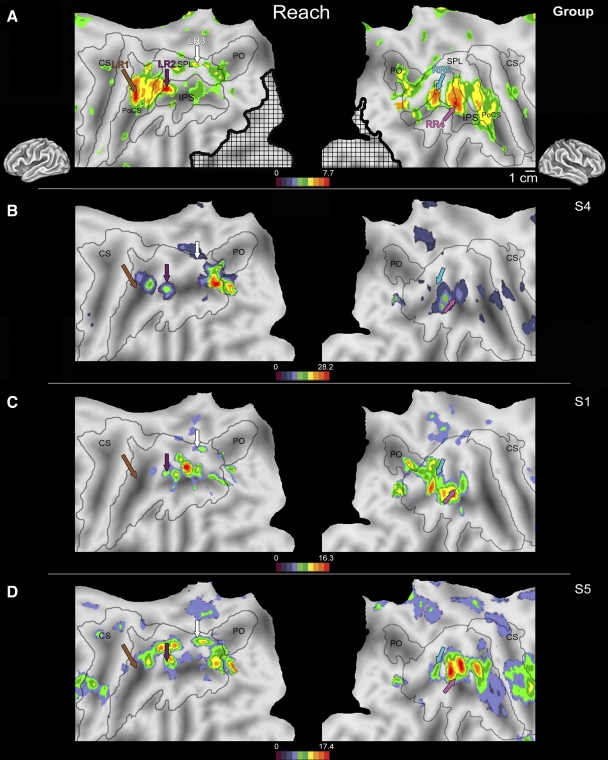
Subjects were trained prior to data collection to ensure that fixation was maintained during these tasks and that limb movements included only the forearm, minor rotations of the wrist, and flexion of the digits. No shoulder movements were made and padding placed around the subject within the bore of the scanner minimized rotation of the body during reaching. Subjects were also trained to maintain a steady pace during reaching in both tasks, with a complete reach and return taking about 2 s. The target remained stationary during the experimental block to eliminate any eye movements that might be generated from tracking a moving target. Twenty-second experimental and control blocks were interleaved with a resting block where subjects fixated on the target without responding. To control for activity in visual cortex during the experimental condition, data were also collected from four 20-s blocks of passive viewing of a flowing field of moving dots (aperture = 13°, rate = 1 Hz) and contrasted against a 20-s fixation block (data not shown). This comparison produced a visual cortex mask (hatched region in Fig. 2A) that was excluded from the analysis using the MARSBAR Matlab toolbox (http://marsbar.sourceforge.net).
FIG. 2.
Flattened left (left column) and right (right column) hemispheres of a human brain taken from a stereotactic atlas (colin.27), with activity overlays of statistical maps showing areas active during visually guided reaching to grasp vs. the lifting and squeezing motor control from a second-level (random effects) group analysis (A) and from 3 individual subjects (B–D). To correct for visual confounds produced during the experimental period, striate and extrastriate cortical fields active during the passive viewing of a flowfield were masked out from all analyses (black crosshatch area). Brown, purple, white, light blue, and pink arrows in B–D indicate locations of peak activations shown in the group analysis in A. Although intersubject variability in activation across the rostral, medial, and caudal IPS is present when the arm is used to guide the hand to grasp, clusters in the left hemisphere within the medial post central sulcus (LR1, brown arrow), rostral IPS (LR2, purple arrow), and caudal SPL (LR3, white arrow) and in the right hemisphere in the medial IPS (RR4, pink arrow) and caudal IPS (RR5, light blue arrow) are active across all subjects. The rostral activity in LR1 in each subject (B–D, brown arrow) was located near the peak of activity in LR1 in the group analysis (A, brown arrow), illustrating variations in peak location across subjects. This pattern was also evident for the LR2 (purple arrow) and LR3 (white arrow) regions of the left hemisphere as well as areas RR4 (pink arrow) and RR5 (light blue arrow). Unlike the relatively symmetric pattern identified during saccadic eye movements, the regions of PPC active during visually guided reaching to grasp differ between the left and right hemispheres. Conventions as in previous figures. LR1, -2, -3, left reach activation 1, 2, 3; SPL, superior parietal lobule; RR4, -5, right reach activation 4, 5; PPC, posterior parietal cortex.
3) Manual shape-discrimination condition.
During the experimental block, subjects actively manipulated one of eight hand-sized geometric plastic blocks with their right hand only. These blocks were approximately the same weight and texture and differed from each other only in surface configuration (interior angles of the shape and number of sides). The use of novel shapes (i.e., not nameable or recognizable) required the subjects to incorporate tactile and motor information from object characteristics such as edge length and the relative position and orientation of corners to form shape percepts. Subjects were presented with similarly sized amounts of clay that had no well-defined structure during motor control blocks and were asked to manipulate this clay with the same hand in a manner similar to that in the experimental block. The act of manipulating the clay during the control block changed its structure, greatly diminishing any sense of permanent surface structure, but maintained many of the other features of the geometric blocks (e.g., perceived volume) and the tactile and proprioceptive inputs and motor outputs. Objects were manipulated during both experimental and control blocks by the right hand as it lay next to the subject in the bore of the MRI machine. The experimental and control blocks were interleaved with a resting block during which subjects had their eyes closed and did not engage in any task.
Control stimuli were designed to correct for both volume and weight relative to the experimental objects. Subjects kept their eyes closed and were instructed to maintain eye position during scanning and at no time before or during the experiment did subjects have visual experience with the objects. Subjects were given practice before the scanning session exploring the clay and a shape (not used during the scans). It was determined in these behavioral sessions that the block length (20 s) was sufficient for manual shape discrimination. An experimenter observed each subject throughout the scanning procedure to confirm compliance with instructions. To ensure that subjects were paying attention and properly discriminating each shape during scanning, at the end of the scan subjects were given a single shape and asked whether it was one of the shapes presented during the scan.
Data analysis
Functional data from 14 individual subjects were spatially realigned, corrected for head motion, and smoothed with an 8-mm3 full-width at half-maximum kernel using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/software/spm2). Statistical maps were generated for each subject using SPM2 and displayed on T1-weighted images coregistered to an average of the subject's echo-planar scans. For a group analysis, individual subjects' realigned functional data were spatially normalized to a standard EPI template, resliced into 2-mm isotropic voxels, and spatially smoothed using SPM2. To evaluate areas active across subjects during a single task, results from single-subject analyses for each condition were entered into separate second-level group analyses (random effects) as a one-sample t-test, treating subjects as a random variable.
Statistical maps were overlaid on inflated and flattened anatomical data from the colin.27 atlas (http://sumsdb.wustl.edu:8081/sums/directory.do?id=636032; Van Essen 2002) using CARET (http://brainmap.wustl.edu/caret; Van Essen et al. 2001). Coordinates in Montreal Neurological Institute (MNI) space were converted to Talairach coordinates using nonlinear transforms (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html) and locations of brain activity relative to the atlas of Brodmann were verified using the Talairach Daemon client (http://ric.uthscsa.edu/projects/talairachdaemon.html).
Regions of interest and nomenclature
Although we describe active regions in the group analysis with respect to major sulcal and gyral landmarks in a standardized atlas, tying activation solely to these locations can be problematic, given the known intra- and interindividual variability in cortical field location in the primate brain (Caspers et al. 2006; Krubitzer et al. 2004). Furthermore, hemispheric specializations that result in functional asymmetries can be blurred when activation is assigned to anatomical locations on a normalized atlas. Instead, we identify areas of activation in each subject using functional labels (see Tables 1 and 2 for abbreviations) that are not limited to a specific anatomical location along the IPS. The usage of functional definitions is a common approach when describing a region of activity, particularly in nonprimary cortical fields such as PPC (Filimon et al. 2009; Levy et al. 2007; Schluppeck et al. 2005). Congruent patterns of activity between subjects were confirmed in the spatially normalized data set based on standardized stereotactic coordinates (Talairach and Tournoux 1988) and labeled with respect to the task that resulted in the activation.
Table 1.
List of abbreviations
| Abbreviation | Definition |
|---|---|
| Parietal sulci and gyri | |
| CS | Central sulcus |
| IPS | Intraparietal sulcus |
| PoCS | Postcentral sulcus |
| SPL | Superior parietal lobule |
| PO | Parietooccipital sulcus |
| Cortical areas and regions | |
| AIP | Anterior intraparietal area |
| BA5 | Broadmann's Area 5 |
| CIP | Caudal intraparietal area |
| LIP | Lateral intraparietal area |
| MIP | Medial intraparietal area |
| PMd | Dorsal premotor area |
| PMv | Ventral premotor area |
| PRR | Parietal reach region |
Table 2.
List of region of interest (ROI) abbreviations
| Abbreviation | Hemisphere | ROI |
|---|---|---|
| LS1 | Left | Left Saccade Activation 1 |
| LS2 | Left | Left Saccade Activation 2 |
| LS3 | Left | Left Saccade Activation 3 |
| RS1 | Right | Right Saccade Activation 1 |
| RS2 | Right | Right Saccade Activation 2 |
| RS3 | Right | Right Saccade Activation 3 |
| LR1 | Left | Left Reach Activation 1 |
| LR2 | Left | Left Reach Activation 2 |
| LR3 | Left | Left Reach Activation 3 |
| RR4 | Right | Right Reach Activation 4 |
| RR5 | Right | Right Reach Activation 5 |
| LMS1 | Left | Left Manual Shape Activation 1 |
| LMS2 | Left | Left Manual Shape Activation 2 |
| RMS1 | Right | Right Manual Shape Activation 1 |
| RMS2 | Right | Right Manual Shape Activation 2 |
To compare activity across conditions in a single area, regions of interest (ROIs) were drawn from peaks of activity (P < 0.05, familywise error [FWE] corrected) in PPC during the three experimental comparisons from single-subject analyses (Table 3). ROIs were selected only if 1) a spatially distinct local maxima was seen in the group statistics and 2) a similar region could be identified across all 14 participants. Data from 15 ROIs (sphere with a 4-mm radius centered on the peak in activity, total volume = 268 mm3 or ∼33 voxels), with eight in the left hemisphere and seven in the right hemisphere, were extracted from individual subjects' spatially normalized data using the MARSBAR toolbox (http://marsbar.sourceforge.net). The size of the ROI sphere was small to avoid confounding the data by extracting the signal from cortical fields neighboring the region of interest.
Table 3.
Mean (SD) Talairach coordinates for regions of interest (ROIs)
| Left |
Right |
|||||
|---|---|---|---|---|---|---|
| ROI | x | y | z | x | y | z |
| LS1/RS1 | −40.1 (10.2) | −44.0 (9.7) | 41.6 (6.9) | 37.6 (6.6) | −46.4 (4.7) | 49.4 (11.12) |
| LS2/RS2 | −25.6 (6.3) | −63.1 (6.5) | 50.9 (7.6) | 28.7 (6.5) | −59.9 (7.6) | 50.1 (9.4) |
| LS3/RS3 | −14.7 (7.4) | −70.3 (10.3) | 48.1 (8.9) | 17.9 (4.3) | −72.8 (8.0) | 46.0 (10.3) |
| LR1 | −30.4 (9.7) | −43.6 (5.5) | 52.4 (8.0) | |||
| LR2 | −28.3 (8.1) | −57.3 (8.0) | 49.0 (6.8) | |||
| LR3 | −6.4 (5.6) | −61.5 (11.8) | 55.7 (8.5) | |||
| RR4 | 34.3 (11.5) | −39.7 (7.6) | 51.6 (5.9) | |||
| RR5 | 27.1 (8.3) | −58.7 (6.0) | 51.1 (4.2) | |||
| LMS1/RMS1 | −40.6 (4.3) | −40.5 (7.2) | 42.6 (7.5) | 42.2 (6.7) | −41.9 (6.0) | 42.9 (4.5) |
| LMS2/RMS2 | −22.1 (9.6) | −58.6 (9.4) | 55.0 (9.4) | 24.0 (8.3) | −62.8 (6.6) | 53.5 (6.6) |
To verify that the regions selected in each subject were similar to the locations active in the group analysis, the voxel coordinates (x, y, z) of each ROI for all subjects were entered into a one-sample t-test and compared against the location of peak activity in the group statistics (P > 0.05, Bonferroni corrected). Intensity values were normalized to percentage signal change from baseline (mean signal intensity during the resting block) prior to statistical analysis. Differences in percentage signal change between two conditions (saccade vs. reach, saccade vs. shape, reach vs. shape) in a given ROI were evaluated using two-tailed paired t-test.
RESULTS
We describe the pattern of activation that was elicited in posterior parietal cortex during visual-manual exploration, specifically in response to three separate components of this behavior: 1) visually directed saccadic eye movements, 2) visually guided reaching to grasp, and 3) manual shape discrimination.
Visually directed saccadic eye movements
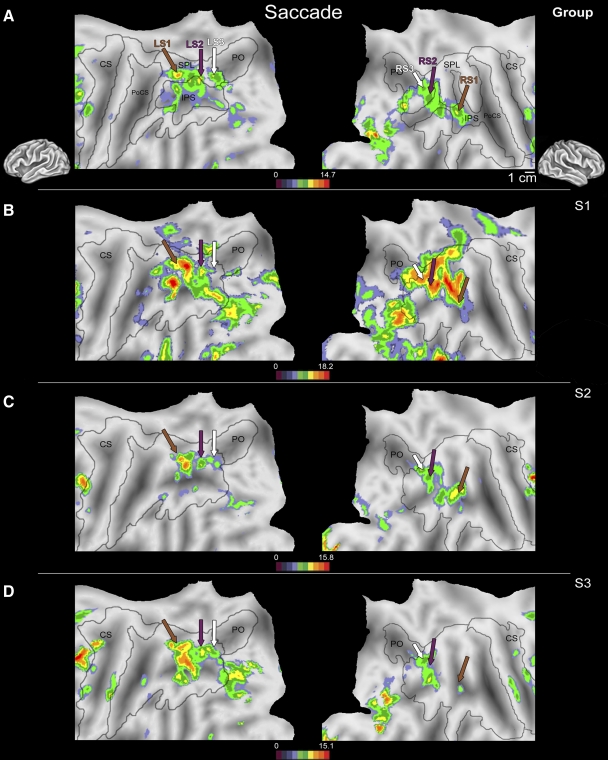
In all subjects, three locations in posterior parietal cortex were consistently active in both hemispheres during visually directed saccadic eye movements when compared with a fixation control period (Table 3; Fig. 1). Although there was some variability in the location of this activity with respect to sulcal and gyral landmarks as well as the amplitude of the blood oxygen level–dependent (BOLD) signal change across subjects (this variability is shown in representative subjects S1, S2, and S3; Fig. 1, B–D), a second-level group analysis that included data from all 14 subjects (Fig. 1A) demonstrated that this pattern of activity was consistent across subjects.
Fig. 1.
Flattened left (left column) and right (right column) hemispheres of a human brain taken from a stereotactic atlas (colin.27), with activity overlays of statistical maps showing areas more active during visually directed saccades to peripheral targets vs. a fixation period from a second-level (random effects) group analysis (A) and from 3 individual subjects (B–D). Brown, purple, and white arrows in B–D indicate locations of peak activations shown in the group analysis in A. Although intersubject variability in activation across medial and caudal cortex can be found in the IPS during visually directed saccades, clusters in the rostral superior parietal lobe of the left hemisphere and rostral IPS of the right hemisphere (LS1/RS1; brown arrow), lateral superior parietal lobe of both hemispheres (LS2/RS2; purple arrow), and the junction of the caudal IPS + PO bilaterally (LS3/RS3; white arrow) were commonly active across the entire group. Variability in the location and extent of activation can be appreciated by comparing the activity around a given arrow across individual subjects (B–D). For example, activation in LS2 (purple arrows) is relatively uniform, with only small shifts in location across subjects. However, the variability in location and extent of activation were slightly greater for LS1 (brown arrows), with somewhat larger shifts in location and amplitude. IPS, intraparietal sulcus; LS1, -2, -3, left saccade activation 1, 2, 3; RS1, -2, -3, right saccade activation 1, 2, 3; PO, parietooccipital sulcus.
In the group analysis, one focus of activity was located in the rostral portion of the superior parietal lobe (SPL) of the left hemisphere (left hemisphere saccade region 1, or LS1; Fig. 1A, brown arrow, left column; x = −36, y = −45, z = 41). In the right hemisphere activation was slightly more medial in the rostral intraparietal sulcus (IPS; right hemisphere saccade region 1, or RS1; Fig. 1A, brown arrow, right column; x = 42, y = −50, z = 54). Although the location of activity with respect to gyral landmarks were slightly different across hemispheres, the Talaraich coordinates indicate that this active area was in a similar location in each hemisphere. In single subjects, a rostral region of PPC was active in both hemispheres near the peak identified in the group analysis (Fig. 1, B–D, brown arrow; location verified by one-sample t-test on the Talairach coordinates). The volume of activity and center of this active region varied relative to major sulci and gyri between subjects. This intersubject variability can be appreciated by examining the large foci of activation near the brown arrow (LS1/RS1) in Fig. 1, B–D in three individual subjects and comparing them with the location of LS1 and RS1 from our group analysis depicted in Fig. 1A.
A second focus of activity was observed in the group analysis in the lateral portion of the SPL of the left (LS2) and right (RS2) hemispheres (Fig. 1A, purple arrow; LH: x = −26, y = −67, z = 49; RH: x = 28, y = −63, z = 53). A third focus was located bilaterally in the left (LS3) and right (RS3) caudal SPL near the parietooccipital sulcus (Fig. 1A; white arrow, LH: x = −18, y = −69, z = 51; RH: x = 20, y = −71, z = 50). In the single-subject data, the locations of LS2/RS2 and LS3/RS3 were similar to the corresponding regions active in the group analysis (Fig. 1, B–D). Again, intersubject variability can be appreciated by examining the large foci of activation neighboring the purple (LS2/RS2) or white (LS3/RS3) arrows in Fig. 1, B–D and comparing them with the location of group activation depicted in Fig. 1A.
Additional regions outside of PPC were identified in the group analysis as active during saccadic eye movements, including regions of primary and extrastriate visual cortex (Fig. 1), because during the experimental block subjects were generating eye movements to visual targets that were being flashed on the screen. In the frontal lobe, two foci of activity were consistently identified in all subjects during visually directed saccades. A region along the caudalmost portion of the superior frontal sulcus (LH: x = −46, y = −1, z = 53; RH: x = 37, y = −3, z = 43; data not shown) as well as a region on the inferior frontal gyrus (LH: x = −57, y = 4, z = 23; RH: x = 50, y = 0, z = 27; data not shown) were active. Activity anterior to premotor cortex in the superior frontal sulcus and inferior frontal gyrus measured using fMRI is commonly reported during saccades as part of a frontal oculomotor system (which includes the frontal eye fields; see Berman et al. 1999; Kastner et al. 2007; Petit et al. 1997).
Visually guided reaching to grasp
In the visually guided reaching to grasp versus motor control comparison, three regions of left posterior parietal cortex and two regions of right posterior parietal cortex were active in all subjects (Table 3; Fig. 2) . As during visually directed saccades, although both the extent of cortex active and the magnitude of the BOLD signal change were variable across subjects (spatial variability is demonstrated in representative subjects S1, S4, and S5; Fig. 2, B–D), similar locations in PPC were significantly active across subjects, as demonstrated by a more powerful second-level group analysis (Fig. 2A). Regions active during passive viewing of a visual flowfield were excluded from the analysis (Fig. 2A, crosshatch area). In the left hemisphere, activity was identified in a region within the medial postcentral sulcus (LR1; Fig. 2, brown arrow; LH: x = −36, y = −42, z = 57), cortex along the rostral IPS (LR2; Fig. 2, purple arrow; LH: x = −34, y = −52, z = 52), and a region of the caudal SPL (LR3; Fig. 2, white arrow; LH: x = −6, y = −69, z = 55). Two different regions of PPC were active in the right hemisphere: a region of the medial IPS (RR4; Fig. 2, pink arrow; RH: x = 34, y = −38, z = 52) and a more caudal region of the IPS (RR5; Fig. 2, light blue arrow; RH: x = 22, y = −62, z = 51). The location of each peak in each subject did not differ from peaks of activity isolated in the group analysis (Fig. 2A).
For the visually guided reaching-to-grasp condition we observed asymmetry of activation across hemispheres, with activity in the left hemisphere in three regions of the most rostral and caudal regions of PPC and activity in two regions of the right hemisphere in the middle IPS (Fig. 2). Although this finding may be due to the unimanual nature of the task (e.g., only the right hand was used), the fact that such asymmetries were not observed for the third task (which was also a unimanual task) suggests that these hemispheric differences represent real differences in cortical processing for this task.
Several regions outside of PPC were active during visually guided reaching to grasp. In the frontal lobe regions of cortex within the superior frontal sulcus (SFS; LH: x = −36, y = −2, z = 52; RH: x = 33, y = 2, z = 52; data not shown) and on the medial wall including the dorsal posterior cingulate (LH: x = −2, y = −20, z = 45; RH: x = 17, y = −23, z = 42; data not shown) were also active. This activity corresponds well with the known organization of human premotor cortex, where cortical areas in the SFS representing the arm and hand are active during movements of human forelimbs (Kertzman et al. 1997; Prado et al. 2005).
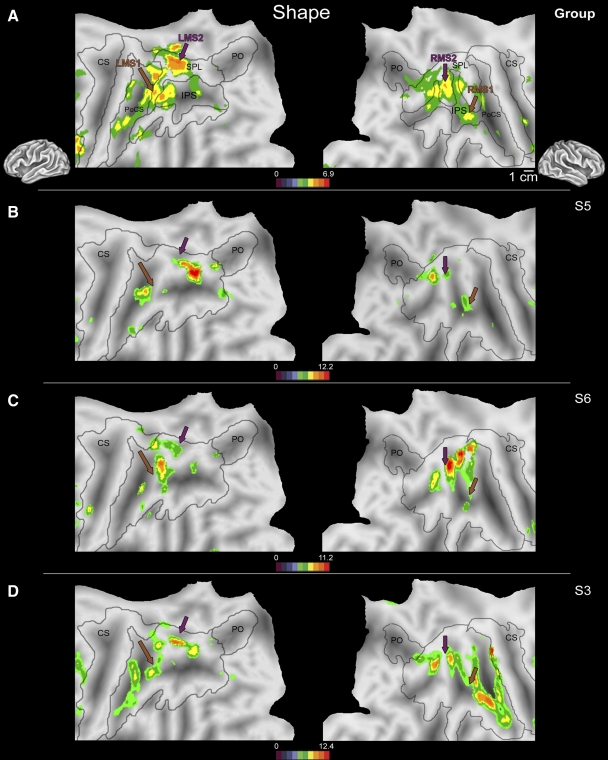
Manual shape discrimination
Two regions of PPC were consistently active in both hemispheres in all subjects during manual shape discrimination versus motor control (Table 3; Fig. 3). This pattern of activity was bilateral in both single-subject (Fig. 3, B–D) and group analysis (Fig. 3A). As in the previous conditions, although the location of the peak of activity varied from subject to subject (this variability is shown in representative subjects 3, 5, and 6; Fig. 3, B–D), the general pattern of activity was consistent across subjects, as confirmed by the group analysis (Fig. 3A). The most rostral region active during manual shape discrimination (LMS1/RMS1) was located at the junction of the intraparietal sulcus and postcentral sulcus (Fig. 3, brown arrow; LH: x = −38, y = −44, z = 48; RH: x = 38, y = −41, z = 41). A second peak of activity (LMS2/RMS2) was centered on the medial SPL in both hemispheres in the group analysis (Fig. 3, purple arrow; LH: x = −24, y = −54, z = 51; RH: x = 20, y = −63, z = 58). The exact location of activity in each hemisphere relative to anatomical landmarks was variable; however, the activation fell within a similar range of Talairach coordinates for each subject (Table 3) and was comparable to the peak in the group analysis (Fig. 3A), as evaluated using a one-sample t-test (P > 0.05).
Fig. 3.
Flattened left (left column) and right (right column) hemispheres of a human brain taken from a stereotactic atlas (colin.27), with activity overlays of statistical maps showing areas active during nonvisual manual shape discrimination vs. the shapeless motor control in the second-level (random effects) group analysis (A) and from 3 individual subjects (B–D). Brown and purple arrows indicate locations of activation peaks shown in A. Although intersubject variability in activation across PPC is present during manual shape discrimination at the single-subject level, common clusters of activity at the junction of the IPS + PoCS (LMS1/RMS1, brown arrows) as well as a region of the medial SPL (LMS2/RMS2, purple arrows) are active in all subjects. The region of rostral PPC active during manual shape discrimination in each subject (B–D, brown arrow) was located near area LMS1/RMS1 in the group analysis (A, brown arrow). Likewise, there was a close proximity between the second focus of activity (LMS2/RMS2) in each subject and the location of activity in the group analysis, indicated by the purple arrow in B–D. Conventions as in previous figures. PoCS, postcentral sulcus; LMS1, -2, left manual shape activation; RMS1, -2, right manual shape activation 1, 2.
Areas outside of PPC active during manual shape discrimination included cortex within the superior frontal sulcus (LH: x = −30, y = −1, z = 57; RH: x = 28, y = 2, z = 55; data not shown) and in the inferior frontal gyrus (LH: x = −38, y = 4, z = 26; RH: x = 40, y = 10, z = 26; data not shown) bilaterally, near where other investigators have reported the hand representation in ventral premotor (PMv) and dorsal premotor (PMd) cortex in humans (Fink et al. 1997).
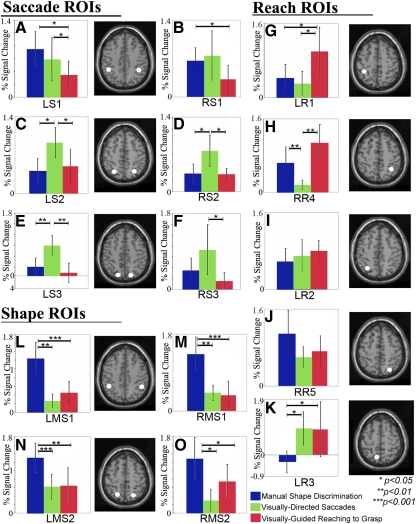
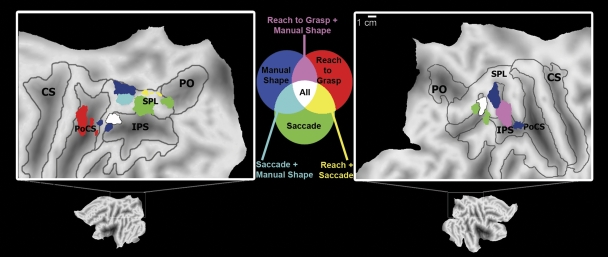
Task-preferred and multifunctional fields (ROI analysis)
Data were extracted from each ROI during the visually directed saccade, visually guided reaching-to-grasp, and manual shape-discrimination conditions (Fig. 4). Although elevated BOLD signal change (above baseline) was present in nearly all of these areas across all conditions (Fig. 4) in some of these regions cortical activity was significantly greater during one behavior than in the other two. Areas that show significantly greater activity in one condition were identified as being task-preferred, whereas regions that were significantly active during two or all three were labeled multifunctional.
Fig. 4.
Mean percentage signal change (vs. baseline; error bars = 95% confidence intervals) for the regions of interest (ROIs) active during the manual shape discrimination, visually directed saccade, and visually guided reaching to grasp conditions. Regions with the greatest activity during a single condition included area LS2/RS2 and LS3 for saccades (C–E); LR1 for reaching to grasp (G); and both LMS1/RMS1 and LMS2/RMS2 for manual shape discrimination (L–O). Regions that were the most active during 2 conditions included area LS1 (greater activity during saccade and manual shape-discrimination conditions than reaching, A), RR4 (greater activity during reaching to grasp and manual shape-discrimination conditions, vs. saccade, H), and LR3 (greater activity during saccade and reaching to grasp conditions than during manual shape discrimination, K). In regions LR2 and RR5 no significant difference in activity was identified between the 3 experimental conditions, suggesting that those 2 areas are active during all 3 conditions (I and J).
1) Task-preferred regions.
For saccadic eye movements, three of the six identified regions had a task-preferred pattern of activation, where activity in these regions was the highest during saccades. In areas LS2 and RS2 (Fig. 4, C and D) and area LS3 of the left hemisphere (Fig. 4E) activity was greater during visually directed saccades than that during either the visually guided reaching-to-grasp or manual shape-discrimination conditions. Previous human neuroimaging studies of ballistic eye movements have reported activity in a region of cortex near LS2/RS2 along the lateral SPL (Fig. 5, in green), which is thought to correspond to macaque area LIP, a region of parietal cortex that contains neuronal populations involved in oculomotor behaviors (Hagler Jr et al. 2007; Koyama et al. 2004; Sereno et al. 2001).
Fig. 5.
Group analyses from the 3 experimental conditions (visually guided saccades, visually guided reaching to grasp, manual shape discrimination) color coded and superimposed on flattened left (left column) and right (right column) hemispheres of the colin.27 stereotactic atlas. Robust activity in more than one condition was identified in 3 areas, in LS1 (in light blue), LR3 (in yellow), and RR4 (in pink). In LR2 and RR5 (in white), activity increased during all 3 conditions. Areas that are significantly active in 2 or 3 conditions might process information from more than one structure in the body. Conventions as in Fig. 1.
Only one of the five regions active during visually guided reaching to grasp (LR1) was task-preferred, with activity significantly greater during this task than that during visually directed saccades or manual shape discrimination (Fig. 4G). The location of this large region of the medial postcentral sulcus overlaps the Talairach coordinates for the representation of the hand and arm in human Brodmann's Area 5 (BA5; Table 3). Further, this region overlaps an area active in fMRI studies during reaching behaviors that has been proposed as a homologue to macaque medial intraparietal (MIP) area (Cavina-Pratesi et al. 2007; Prado et al. 2005). This region is likely to play a role in computing the location of the hand in personal space and coordinating the movements of the contralateral limb for the purposes of interacting with an object (Cavina-Pratesi et al. 2007).
The four fields active during manual shape discrimination were task-preferred, with signal change in these areas significantly greater than activity during either saccades or reaching to grasp (Fig. 4, L–O). The coordinates for the activity in LMS1 and RMS1 at the junction of the IPS + postcentral sulcus (PoCS) are similar to those areas active in fMRI and PET during the performance of complex movements of the hand to explore and identify graspable objects. This ROI has been identified as a putative homologue to macaque anterior intraparietal (AIP) area, known to be involved in grasping behaviors (Begliomini et al. 2007a,b; Binkofski et al. 1999; Cavina-Pratesi et al. 2007; Culham et al. 2003; Frey et al. 2005; Króliczak et al. 2007). Activity near region LMS2 and RMS2 has been reported in other neuroimaging studies when surface orientation is used to aid grasping, much like the neurons in macaque caudal intraparietal (CIP) area (Shikata et al. 2003). During manual shape discrimination, it was necessary for subjects to detect the orientation of the object across the surface of the hand for recognition, a parameter absent during the shapeless motor control.
2) Multifunctional regions.
We identified three regions in posterior parietal cortex that were the most active during two tasks: LS1 (Fig. 4A), LR3 (Fig. 4K), and RR4 (Fig. 4J). In these regions, an increase in BOLD signal change was not significantly different between two conditions, although activity during either preferred task was significantly greater than activity during the nonpreferred third task (Fig. 4). For example, area LS1, which was active during saccades, was also significantly active during manual shape discrimination (Fig. 4A) and activity in LS1 during either saccades or shape discrimination was greater than activity during visually guided reaching to grasp (P < 0.05). LS1 overlaps with regions of cortex adjacent to LMS1 active during manual shape discrimination (Fig. 5, in light blue). The pattern of activation in RS1 was slightly different. There were no significant differences in activation between the saccadic eye movement condition and the other two conditions; however, there was significantly greater activation during the shape-discrimination condition than that during the reaching-to-grasp condition (Fig. 4B). Activity in RS3 was greater during saccadic eye movements than that during visually guided reaching to grasp, although there was no significant difference in activity between the saccade condition and manual shape discrimination or between manual shape discrimination and visually guided reaching to grasp (Fig. 4F).
The other two areas with the greatest BOLD signal change during two conditions (LR3 and RR4) were most active during visually guided reaching-to-grasp as well as another condition. First, in the caudal SPL, area LR3 was also significantly active during visually directed saccades and activity during reaching to grasp and saccades was greater than activity during manual shape discrimination (P < 0.05; Fig. 4K). The peak of activity in LR3 overlaps with regions of cortex medial to LS3 active during visually directed saccades (Fig. 5, in yellow). Although it could be argued that activity in LR3 is purely due to visual stimulation (given that visual stimulation was present during the visually directed saccade and visually guided reaching-to-grasp conditions but not manual shape discrimination), this area was not significantly active during passive viewing of a visual flowfield (Fig. 2, crosshatch area). The location of LR3 (Table 3) in the present data set is similar to previous reports of a region active during reaching preparation and execution believed to be homologous to either reach-selective macaque parietal reach region (PRR) area (Astafiev et al. 2003; Connolly et al. 2003) or V6A (Pitzalis et al. 2006) in the human brain (motion-selective areas of human PPC with magnocellular inputs; see Galletti et al. 2003; Portin et al. 1998), although the cortical boundaries of areas like PRR remain undefined and may include portions of MIP and V6A (see Andersen and Buneo 2002; Gail and Andersen 2006).
Second, in the right hemisphere, the ROI analysis for area RR4 (Fig. 5, in pink) showed no significant difference in activity between visually guided reaching to grasp and manual shape discrimination (P = 0.14), although both tasks resulted in increased activity compared with visually directed saccades (P < 0.005; Fig. 4H). Activity in this region overlaps with activity in the right SPL during manual shape discrimination in RMS2 (Fig. 5, in pink). Low activity in RR4 during saccades could be due to a difference in experimental design, since eye movements were made along two-dimensional axes (vs. three in reaching to grasp and manual shape discrimination). It seems more likely that region RR4 instead plays a role in behaviors that require manipulating an object with the ipsilateral hand because in both the visually guided reaching-to-grasp and manual shape-discrimination conditions subjects were grasping with the right hand.
We identified two regions of PPC that were significantly active during all three conditions with no difference in percentage signal change between the tasks—region LR2 of the left hemisphere (Fig. 4I) and region RR5 of the right hemisphere (Fig. 4J). These cortical areas, although active during all three tasks, were located in different regions of left and right PPC, with region LR2 in the rostral IPS of the left hemisphere and region RR5 in the caudal IPS of the right hemisphere (Fig. 5, in white).
DISCUSSION
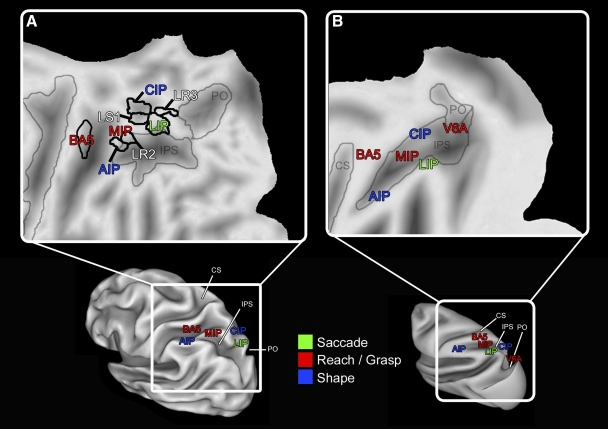
In the present investigation we identified multiple regions of the PPC active during visual-manual behaviors, specifically directing the eyes toward a visual target, reaching with the arm to grasp a target, and manually exploring an object. Response patterns were complex, with some areas most active in response to a single condition, whereas the majority of areas were active during multiple conditions. A pattern of hemispheric laterality for visually guided reaching to grasp was also observed. Based on anatomical location and response properties, several of these areas are plausible homologues to well-described fields in macaque PPC (Fig. 6).
Fig. 6.
Summary of the organization of PPC in humans (A) and macaque monkeys (B) illustrated on a flattened (top figures) and inflated (bottom figures) cortical atlas for either species. Lateral views of the left hemisphere show the layout of regions hypothesized to be homologous across the 2 species and areas that were multifunctional in the present study (white letters). The general layout of areas relative to each other is similar across species. Color represents the body structure(s) from which these regions process information. Faint gray lines denote borders between sulci and, in the human, black lines denote boundaries between functional areas. Conventions as in previous figures. See Tables 1 and 2 for abbreviations. Human and macaque atlas courtesy of the Surface Management Systems Database (http://sumsdb.wustl.edu:8081/sums/directory.do?id=636032). Part B was adapted from Colby (1998) and Andersen and Buneo (2002).
Parietal cortical areas active during visually guided behavior and task-preferred regions of human PPC
In accordance with previous imaging studies, visual-manual behaviors drive activity across multiple regions of the human parietal lobe bilaterally. Interestingly, several identified fields showed task-preferred activation consistent with the idea that PPC has an “effector-specific” organization, where certain areas are specialized for behaviors that require the use of a single body structure or “effector,” such as the eye or the hand (for a review see Andersen and Buneo 2002; Colby and Goldberg 1999). However, as in previous fMRI studies of human PPC (Astafiev et al. 2003; Levy et al. 2007; Simon et al. 2002) many (8/15) of these areas were active during more than one task. These findings are in agreement with the multifunctional fields identified in nonhuman primate PPC (Hyvärinen 1981; Rozzi et al. 2008), supporting the idea that PPC is a site of integration of sensorimotor information necessary for visual-manual exploration.
OCULOMOTOR AREAS.
Three regions were active bilaterally across the intraparietal sulcus during saccadic eye movements. This pattern of activity was relatively symmetric between the two hemispheres and has been reported previously in human imaging studies of saccadic eye movements (Hagler Jr et al. 2007; Schluppeck et al. 2005). However, in only one of these areas (LS2/RS2) was BOLD signal change during saccades greater than activity in the same region during shape discrimination and reaching to grasp (Fig. 4). Although multiple regions of the macaque inferior parietal lobule are involved in ballistic movements of the eyes (Barash et al. 1991) neurons in area LIP respond preferentially during saccadic eye movements because microstimulation of neighboring cortex produces complementary facial and shoulder movements in addition to a saccade (Thier and Andersen 1998). However, evidence from previous human fMRI studies of the task specificity of this region for the generation of an eye movement toward a visual location is contentious (see Astafiev et al. 2003; Levy et al. 2007).
The location of LS2/RS2 (Figs. 1, 4, and 5) is in agreement with a putative LIP homologue in humans, based on fMRI data from saccade preparation and generation tasks (Astafiev et al. 2003; Hagler Jr et al. 2007; Sereno et al. 2001). The most compelling evidence for homology between LIP in the macaque and human areas LS2/RS2 comes from an fMRI study by Koyama and colleagues (2004), in which similar regions of macaque and human PPC (including area LIP) were active during the same oculomotor tasks. In agreement with the present study, both Koyama et al. (2004) and others (Hagler Jr et al. 2007) have reported that the location of peak activity in LIP varied significantly from subject to subject.
VISUALLY GUIDED REACH TO GRASP.
Three regions of left PPC and two regions of right PPC were active during visually guided reach to grasp (Fig. 2). In only one of these regions (LR1; Figs. 2, 4, and 5) was an increase in activity during reaching to grasp greater than activity during saccades and shape discrimination. This region (LR1) was in the medial portion of the postcentral sulcus in the hemisphere contralateral to the limb being used and, based on Talairach coordinates, overlaps the location of the arm and hand in Brodmann's Area 5 (BA5) in human cortex (Talairach and Tournoux 1998). In macaques, neural activity in BA5 is also modulated during behavioral components of reaching, including movement execution (Kalaska 1996; MacKay and Mendonça 1995), and by arm position (Buneo et al. 2002; Mountcastle et al. 1975; Sakata 1973). However, the relationship between neural activity in BA5 and hand position is complex because neurons in this area are known to be active when visual feedback regarding arm and hand position is available, even when the limb is stationary (Graziano et al. 2000; Hasson et al. 2004).
LR1 and other areas in the human IPS (including LR2) have been shown to be active in fMRI studies of visually guided reaching (Chapman et al. 2002; Prado et al. 2005) and tasks in which a joystick was used to guide a cursor toward a visual target (Grefkes et al. 2004). Reaching behaviors with visual feedback have been shown to drive activity in neurons caudal to BA5 in macaque area MIP during both neuroimaging studies of reaching (Gregoriou and Savaki 2001; Nishimura et al. 2007) and electrophysiological recordings from single neurons when monkeys move a joystick toward a visual target (Eskandar and Assad 1999). Filimon and colleagues (2007) used fMRI to demonstrate that medial intraparietal cortical fields (like LR2) are active not only during visually guided reaching to grasp but also during mental imagery of the movement and the passive viewing of a reach toward an object. These findings suggest that areas like LR2/MIP may be a component of a mirror-neuron network involving PPC and premotor cortex serving reaching for the purpose of grasping (Filimon et al. 2007).
Activity overlapping LR3 has been observed in fMRI studies of both visually guided and nonvisually guided reaching (Filimon et al. 2009). In contrast, our data show that this region was significantly more active during both visually guided reaching and saccadic eye movements than during shape discrimination (Fig. 4). Although neurons active during movements of the arm and hand or the eyes alone have been described in macaque PPC in the caudal SPL (Calton et al. 2002), data are sparse. Based on location, LR3 may be a homologue of V6A (Pitzalis et al. 2006). Previous human fMRI studies (Filimon et al. 2007, 2009) have shown reach-related activity in two primary regions of the caudal IPS believed to be homologous to V6A, one of which overlaps LR3 and another (in the parieto-occipital sulcus) that was inconsistently active in our own study (PO; Fig. 2).
SHAPE-DISCRIMINATION AREAS.
During manual shape discrimination, two regions of PPC (LMS1/RMS1, LMS2/RMS2) were active bilaterally in all subjects (Figs. 3 and 5). These areas were most active during the manual shape-discrimination task compared with either visually directed saccades or reach to grasp (Fig. 4). Activity in LMS1/RMS1 is consistent with existing human fMRI data from grasping in a region thought to be a homologue to macaque area AIP (Begliomini et al. 2007a,b; Binkofski et al. 1999; Culham et al. 2003; Frey et al. 2005). In the macaque, neurons in AIP process information on somesthetic feedback from the hand during object contact (Iwamura et al. 1995) and are selective for the visual and motor representations of a specific object to be grasped (Murata et al. 2000), both essential components in shaping the hand prior to and during object contact or prehension (Gardner 1988, 2007a,b).
Activity in the LMS2/RMS2 region of human cortex (a possible homologue of macaque area CIP; Tsutsui et al. 2001) has been reported during object manipulation, particularly when visual information about surface orientation is available to guide grasping (Shikata et al. 2003). Although no visual information was available in the present study to guide grasping, tactile and motor information about object orientation was constantly updated during shape manipulation, suggesting that object surface information may be processed in LMS1/RMS1 and LMS2/RMS2. Our findings support emerging ideas about the functional relationship between areas AIP and CIP, where CIP relays information about object orientation to AIP, which is involved in shaping the hand during object manipulation (Shikata et al. 2003) when the position and configuration of the hand are constantly changing.
Multifunctional activity in primate PPC
It is important to note that, even in areas with a “task-preferred” response pattern, an increase in BOLD signal was present to some degree during all of the three behaviors. Classic investigations of the functional organization of macaque PPC have also demonstrated a mixture of responses within a single cortical field (Fleming and Crosby 1955; Leinonen et al. 1979; Lynch 1977; Mountcastle et al. 1975). More recent studies, in which the number of neurons responsive during a behavior with a single body structure were quantified (Calton et al. 2002; Hyvärinen et al. 1981; Rozzi et al. 2008; Snyder et al. 2000), reveal a mixture of response properties within some cortical fields, a finding consistent with the notion that PPC is a site of sensorimotor integration.
It is also possible (particularly within areas active during all three tasks) that these regions play a generalized cognitive role, for example, in the control of nonspatial attention (Russ et al. 2006; Wojciulik and Kanwisher 1999) irrespective of task, sensory modality, or effector. No attempt was made to match attentional demands across tasks and, although outside the scope of the present study, many regions of the human SPL and IPS fall within the dorsal network of Corbetta and Shulman (2002), believed to be involved in endogenous attentional control.
It could be argued that the comparison of the BOLD effect across conditions (Fig. 4) is confounded by how the ROIs were defined because they were selected from data that are nonindependent from the ROI analysis itself (for a detailed discussion on this issue, see Baker et al. 2007; Vul and Kanwisher 2009; Vul et al. 2009). Although this approach may bias the results toward stronger activation in one condition, BOLD signal change in many of these areas was quite robust and, in some cases, significant effects were independent from voxel selection (Fig. 4, B, H, and L). Although ROI analyses are commonly used in neuroimaging, it is critical to minimize selection bias, either through simulations or through the incorporation of independent data sets (Vul and Kanwisher 2009).
Hemispheric asymmetry during manual behavior
In the reaching-to-grasp condition, the Talairach coordinates (Table 3) indicated hemispheric asymmetry in the location of the peak response for LR1 (putative BA5 homologue in the left hemisphere). It is possible that this asymmetry in location is due to the performance of a unimanual task. However, such asymmetry was not observed for the haptic shape task, which was also unimanual, suggesting that there may be asymmetry in processing and thus hemispheric specializations associated with reaching to grasp an object under visual guidance. Although asymmetries associated with visuospatial attention (e.g., hemispatial neglect; see Nachev and Husain 2006) have been well described in human parietal cortex, hemispheric asymmetries associated with visually guided reaching to grasp have not been described in human or macaque area 5.
We also found that in the left hemisphere (contralateral to manual task performance), there were three locations that were active during two or more tasks, whereas in the right hemisphere there were two multifunctional regions. Interestingly, processing was sometimes asymmetric, with unilateral activity in three multifunctional areas (LS1, RR4, and LR3). In contrast, one region in each hemisphere (LR2, RR5) was highly active during all three tasks, independent of the behavior being conducted. Thus we observed a pattern of bilateral and asymmetrical (Fig. 5) processing across conditions.
Although further investigation is necessary, it is possible that these cortical fields are unique to our lineage. The concept of derived cortical areas in human posterior parietal cortex is consistent with current models in evolutionary neurobiology that predict that cortical fields specialized for species-specific behavior, such as reaching to grasp, emerge from a common plan of organization (Krubitzer and Kaas 2005; Krubitzer and Kahn 2003) and are interspersed between evolutionarily older fields. However, at this stage comparisons between our results and existing macaque monkey data are speculative because of the differences in the number and design of tasks studied as well as the techniques used.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS-044590-03 to E. Disbrow and R01 NS-035103-11 to L. Krubitzer.
ACKNOWLEDGMENTS
We thank M. Lowenthal for assistance in data collection and S. Murray for helpful comments and assistance in stimulus design and data analysis.
REFERENCES
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25: 189–220, 2002 [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and functional cortex for attending, looking and pointing. J Neurosci 23: 4689–4699, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CI, Hutchison TL, Kanwisher N. Does the fusiform face area contain subregions highly selective for nonfaces? Nat Neurosci 10: 3–4, 2007 [DOI] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a.. J Neurophysiol 66: 1095–1108, 1991 [DOI] [PubMed] [Google Scholar]
- Begliomini C, Caria A, Grodd W, Castiello U. Comparing natural and constrained movements: new insights into the visuomotor control of grasping. PLoS ONE 2: e1108, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begliomini C, Wall MB, Smith AT, Castiello U. Differential cortical activity for precision and whole-hand visually guided grasping in humans. Eur J Neurosci 25: 1245–1252, 2007 [DOI] [PubMed] [Google Scholar]
- Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA. Cortical networks subserving pursuit and saccadic eye movements in humans: an fMRI study. Hum Brain Mapp 8: 209–225, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci 11: 3276–3286, 1999 [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol 299: 421–425, 1990 [DOI] [PubMed] [Google Scholar]
- Breveglieri R, Galletti C, Monaco S, Fattori P. Visual, somatosensory and bimodal activities in the macaque parietal area PEc. Cereb Cortex 18: 806–816, 2008 [DOI] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44: 2594–2606, 2006 [DOI] [PubMed] [Google Scholar]
- Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature 416: 632–636, 2002 [DOI] [PubMed] [Google Scholar]
- Calton JL, Dickinson AR, Snyder LH. Non-spatial, motor-specific activation in posterior parietal cortex. Nat Neurosci 5: 580–588, 2002 [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage 33: 430–448, 2006 [DOI] [PubMed] [Google Scholar]
- Castiello U. The neuroscience of grasping. Nat Rev Neurosci 6: 726–736, 2005 [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Goodale MA, Culham JC. FMRI reveals a dissociation between grasping and perceiving the size of real 3D objects. PLoS ONE 2: e424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H, Gavrilescu M, Wang H, Kean M, Egan G, Castiello U. Posterior parietal cortex control of reach-to-grasp movements in humans. Eur J Neurosci 15: 2037–2042, 2002 [DOI] [PubMed] [Google Scholar]
- Colby CL. Action-oriented spatial reference frames in cortex. Neuron 20: 15–24, 1998 [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia 29: 517–537, 1991 [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci 22: 319–349, 1999 [DOI] [PubMed] [Google Scholar]
- Connolly JD, Andersen RA, Goodale MA. FMRI evidence for a “parietal reach region” in the human brain. Exp Brain Res 153: 140–145, 2003 [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Desouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol 84: 1645–1655, 2000 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215, 2002 [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res 153: 180–189, 2003 [DOI] [PubMed] [Google Scholar]
- Culham JC, Gallivan J, Cavina-Pratesi C, Quinlan DJ. fMRI investigations of reaching and ego space in human superior parieto-occipital cortex. In: Embodiment, Ego-space, and Action, edited by Klatzky RL, Behrmann M, MacWhinney B. New York: Psychology Press, 2008, p. 247–274 [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol 16: 205–212, 2006 [DOI] [PubMed] [Google Scholar]
- Eskandar EN, Assad JA. Dissociation of visual, motor and predictive signals in parietal cortex during visual guidance. Nat Neurosci 2: 88–93, 1999 [DOI] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Hagler DJ, Sereno MI. Human cortical representations for reaching: mirror neurons for execution, observation and imagery. Neuroimage 37: 1315–1328, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Huang RS, Sereno MI. Multiple parietal reach regions in humans: cortical representations for visual and proprioceptive feedback during on-line reaching. J Neurosci 29: 2961–2971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RSJ, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol 77: 2164–2174, 1997 [DOI] [PubMed] [Google Scholar]
- Fleming JFR, Crosby EC. The parietal lobe as an additional motor area. The motor effects of electrical stimulation and ablation of cortical areas 5 and 7 in monkeys. J Comp Neurol 103: 485–512, 1955 [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science 308: 662–667, 2005 [DOI] [PubMed] [Google Scholar]
- Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Brain Res Cogn Brain Res 23: 397–405, 2005 [DOI] [PubMed] [Google Scholar]
- Gail A, Andersen RA. Neural dynamics in monkey parietal reach region reflect context-specific sensorimotor transformations. J Neurosci 26: 9376–9384, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti C, Kutz DF, Gamberini M, Breveglieri R, Fattori P. Role of the medial parieto-occipital cortex in the control of reaching and grasping movements. Exp Brain Res 153: 158–170, 2003 [DOI] [PubMed] [Google Scholar]
- Gardner EP. Somatosensory cortical mechanisms of feature detection in tactile and kinesthetic discrimination. Can J Physiol Pharmacol 66: 439–452, 1988 [DOI] [PubMed] [Google Scholar]
- Gardner EP, Babu KS, Ghosh S, Sherwood A, Chen J. Neurophysiology of prehension. III. Representation of object features in posterior parietal cortex of the macaque monkey. J Neurophysiol 98: 3708–3730, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EP, Babu KS, Reitzen SD, Ghosh S, Brown AS, Chen J, Hall AL, Herzlinger MD, Kohlenstein JB, Ro JY. Neurophysiology of prehension. I. Posterior parietal cortex and object-oriented hand behaviors. J Neurophysiol 97: 387–406, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci 15: 20–25, 1992 [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF, Taylor CS. Coding the location of the arm by sight. Science 290: 1782–1786, 2000 [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat 207: 3–17, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Ritzl A, Zilles K, Fink GR. Human medial intraparietal cortex subserves visuomotor coordinate transformation. Neuroimage 23: 1494–1506, 2004 [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Savaki HE. The intraparietal cortex: subregions involved in fixation, saccades, and in the visual and somatosensory guidance of reaching. J Cereb Blood Flow Metab 21: 671–682, 2001 [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Riecke L, Sereno MI. Parietal and superior frontal visuospatial maps activated by pointing and saccades. Neuroimage 35: 1562–1577, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science 303: 1634–1640, 2004 [DOI] [PubMed] [Google Scholar]
- Hyvärinen J. Regional distribution of functions in parietal association area 7 of the monkey. Brain Res 206: 287–303, 1981 [DOI] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport 7: 2325–2330, 1996 [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Tanaka M, Hikosaka O, Sakamoto M. Postcentral neurons of alert monkeys activated by the contact of the hand with objects other than the monkey's own body. Neurosci Lett 186: 127–130, 1995 [DOI] [PubMed] [Google Scholar]
- Kalaska JF. Parietal cortex area 5 and visuomotor behavior. Can J Physiol Pharmacol 74: 483–498, 1996 [PubMed] [Google Scholar]
- Kastner S, DeSimone K, Konen CS, Szczepanski SM, Weiner KS, Schneider KA. Topographic maps in human frontal cortex revealed in memory-guided saccade and spatial working-memory tasks. J Neurophysiol 97: 3494–3507, 2007 [DOI] [PubMed] [Google Scholar]
- Kertzman C, Schwarz U, Zeffiro TA, Hallett M. The role of posterior parietal cortex in visually guided reaching in humans. Exp Brain Res 114: 170–183, 1997 [DOI] [PubMed] [Google Scholar]
- Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron 41: 795–807, 2004 [DOI] [PubMed] [Google Scholar]
- Króliczak G, Cavina-Pratesi C, Goodman DA, Culham JC. What does the brain do when you fake it? An fMRI study of pantomimed and real grasping. J Neurophysiol 97: 2410–2422, 2007 [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Disbrow E. The evolution of parietal areas involved in hand use in primates. In: The Senses: A Comprehensive Reference. Volume 6, Somatosensation, edited by Kaas JH, Gardner EP. Oxford, UK: Elsevier, 2008, p. 183–214 [Google Scholar]
- Krubitzer L, Huffman KJ, Disbrow E, Recanzone G. Organization of area 3a in macaque monkeys: contributions to the cortical phenotype. J Comp Neurol 471: 97–111, 2004 [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Kaas J. The evolution of the neocortex in mammals: how is phenotypic diversity generated? Curr Opin Neurobiol 15: 444–453, 2005 [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Kahn DM. Nature versus nurture revisited: an old idea with a new twist. Prog Neurobiol 70: 33–52, 2003 [DOI] [PubMed] [Google Scholar]
- Leinonen L, Hyvärinen J, Nyman G, Linnankoski I. Functional properties of neurons in lateral part of associative area 7 in awake monkeys. Exp Brain Res 34: 299–320, 1979 [DOI] [PubMed] [Google Scholar]
- Levy I, Schluppeck D, Heeger DJ, Glimcher PW. Specificity of human cortical areas for reaches and saccades. J Neurosci 27: 4687–4696, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JC, Mountcastle VB, Talbot WH, Yin TC. Parietal lobe mechanisms for directed visual attention. J Neurophysiol 40: 362–389, 1977 [DOI] [PubMed] [Google Scholar]
- MacKay WA, Mendonça AJ. Field potential oscillatory bursts in parietal cortex before and during reach. Brain Res 704: 167–174, 1995 [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol 38: 871–908, 1975 [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol 83: 2580–2601, 2000 [DOI] [PubMed] [Google Scholar]
- Nachev P, Husain M. Disorders of visual attention and the posterior parietal cortex. Cortex 42: 766–773, 2006 [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Onoe H, Morichika Y, Tsukada H, Isa T. Activation of parieto-frontal stream during reaching and grasping studied by positron emission tomography in monkeys. Neurosci Res 59: 243–250, 2007 [DOI] [PubMed] [Google Scholar]
- Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in Titi monkeys: do new world monkeys have an area 2? Cereb Cortex 15: 1938–1963, 2005 [DOI] [PubMed] [Google Scholar]
- Petit L, Clark VP, Ingeholm J, Haxby JV. Dissociation of saccade-related and pursuit-related activation in human frontal eye fields as revealed by fMRI. J Neurophysiol 77: 3386–3390, 1997 [DOI] [PubMed] [Google Scholar]
- Pitzalis S, Galletti C, Huang RS, Patria F, Committeri G, Galati G, Fattori P, Sereno MI. Wide-field retinotopy defines human cortical visual area v6. J Neurosci 26: 7962–7973, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portin K, Salenius S, Salmelin R, Hari R. Activation of the human occipital and parietal cortex by pattern and luminance stimuli: neuromagnetic measurements. Cereb Cortex 8: 253–260, 1998 [DOI] [PubMed] [Google Scholar]
- Prado J, Clavagnier S, Otzenberger H, Scheiber C, Kennedy H, Perenin MT. Two cortical systems for reaching in central and peripheral vision. Neuron 48: 849–858, 2005 [DOI] [PubMed] [Google Scholar]
- Roland PE, Zilles K. Structural divisions and functional fields in the human cerebral cortex. Brain Res Rev 26: 87–105, 1998 [DOI] [PubMed] [Google Scholar]
- Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur J Neurosci 28: 1569–1588, 2008 [DOI] [PubMed] [Google Scholar]
- Russ BE, Kim AM, Abrahamsen KL, Kiringoda R, Cohen YE. Responses of neurons in the lateral intraparietal area to central visual cues. Exp Brain Res 174: 712–727, 2006 [DOI] [PubMed] [Google Scholar]
- Sakata H, Takaoka Y, Kawarasaki A, Shibutani H. Somatosensory properties of neurons in the superior parietal cortex (area 5) of the rhesus monkey. Brain Res 64: 85–102, 1973 [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickoff SB, Homke L, Mohlberg H, Hermann K, Amunts K, Zilles K. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18: 2141–2157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol 94: 1372–1384, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science 294: 1350–1354, 2001 [DOI] [PubMed] [Google Scholar]
- Shikata E, Hamzei F, Glauche V, Koch M, Weiller C, Binkofski F, Buchel C. Functional properties and interaction of the anterior and posterior intraparietal areas in humans. Eur J Neurosci 17: 1105–1110, 2003 [DOI] [PubMed] [Google Scholar]
- Shikata E, McNamara A, Sprenger A, Hamzei F, Glauche V, Buchel C, Binkofski F. Localization of human intraparietal areas AIP, CIP, and LIP using surface orientation and saccadic eye movement tasks. Hum Brain Mapp 29: 411–421, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon O, Kherif F, Flandin G, Poline JB, Riviere D, Mangin JF, Le Bihan D, Dehaene S. Automatized clustering and functional geometry of human parietofrontal networks for language, space and number. Neuroimage 23: 1192–1202, 2004 [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation and language-related areas in the human parietal lobe. Neuron 33: 475–487, 2002 [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Saccade-related activity in the parietal reach region. J Neurophysiol 83: 1099–1102, 2000 [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme, 1988 [Google Scholar]
- Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. J Neurophysiol 80: 1713–1735, 1998 [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Jiang M, Yara K, Sakata H, Taira M. Integration of perspective and disparity cues in surface-orientation-selective neurons of area CIP. J Neurophysiol 86: 2856–2867, 2001 [DOI] [PubMed] [Google Scholar]
- Van Essen DC. Windows on the brain. The emerging role of atlases and databases in neuroscience. Curr Opin Neurobiol 12: 574–579, 2002 [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An integrated software system for surface-based analyses of cerebral cortex. J Am Med Inform Assoc 41: 1359–1378, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci 4: 274–290, 2009 [DOI] [PubMed] [Google Scholar]
- Vul E, Kanwisher N. Begging the question: the non-independence error in fMRI data analysis. In: Foundational Issues for Human Brain Mapping, edited by Hanson S, Bunzl M. Cambridge, MA: MIT Press, 2009In press [Google Scholar]
- Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron 23: 747–7641999 [DOI] [PubMed] [Google Scholar]