Abstract
Evidence is emerging indicating that sphingosine-1-phosphate (S1P) participates in signaling in the retina. To determine whether S1P might be involved in signaling in the inner retina specifically, we examine the effects of this sphingolipid on cultured retinal amacrine cells. Whole cell voltage-clamp recordings reveal that S1P activates a cation current that is dependent on signaling through Gi and phospholipase C. These observations are consistent with the involvement of members of the S1P receptor family of G-protein-coupled receptors in the production of the current. Immunocytochemistry and PCR amplification provide evidence for the expression of S1P1R and S1P3R in amacrine cells. The receptor-mediated channel activity is shown to be highly sensitive to blockade by lanthanides consistent with the behavior of transient receptor potential canonical (TRPC) channels. PCR products amplified from amacrine cells reveal that TRPCs 1 and 3–7 channel subunits have the potential to be expressed. Because TRPC channels provide a Ca2+ entry pathway, we asked whether S1P caused cytosolic Ca2+ elevations in amacrine cells. We show that S1P-dependent Ca2+ elevations do occur in these cells and that they might be mediated by S1P1R and S1P3R. The Ca2+ elevations are partially due to release from internal stores, but the largest contribution is from influx across the plasma membrane. The effect of inhibition of sphingosine kinase suggests that the production of cytosolic S1P underlies the sustained nature of the Ca2+ elevations. Elucidation of the downstream effects of these signals will provide clues to the role of S1P in regulating inner retinal function.
INTRODUCTION
Sphingosine-1-phosphate (S1P) is a sphingosine metabolite that has been linked to numerous cellular functions, including cell signaling, growth, differentiation, and programmed cell death. S1P has also been implicated in neuronal signaling with effects ranging from regulation of neural stem cell proliferation (Harada et al. 2004) and migration (Kimura et al. 2007) to calcium signaling (Giussani et al. 2007; Pollock et al. 2002), increases in excitability (Zhang et al. 2006a,b), and exocytosis (Kajimoto et al. 2007).
S1P is produced through phosphorylation of sphingosine by sphingosine kinase (SphK) (Ghosh et al. 1994; Olivera et al. 1996). There are two forms of SphK (SphK1 and 2); however, most is known about SphK1. SphK1 contains numerous phosphorylation sites and calcium/calmodulin binding sites, suggesting that SphK1 is regulated both by phosphorylation and intracellular Ca2+ (Hla et al. 1999). Although the synthesis of S1P is well understood, the mechanism underlying its release is currently unresolved. It is known that S1P is not released via exocytosis, but there is some evidence that release might occur on an ATP binding cassette transporter (for review, see Kim et al. 2009).
S1P has the potential to act both extracellularly via cell surface receptors and intracellularly as a second messenger (Van Brocklyn et al. 1998). Originally believed to function solely as an intracellular second messenger linked to cell proliferation and survival, it is now well-established that S1P is the endogenous ligand for a family of G-protein-coupled cell surface receptors referred to as S1P receptors (S1PRs). Currently five members of this family (S1P1R–S1P5R) have been identified (see Kluk and Hla 2002 for review). S1P4R, however, has a low affinity for S1P and may have a different endogenous agonist (Candelore et al. 2002).
S1P1R is thought to couple exclusively to the heterotrimeric G protein Gi (Ancellin and Hla 1999; Lee et al. 1998; Windh et al. 1999), consistent with the observation that S1P elicits Ca2+ elevations through a pertussis toxin (PTX)-sensitive (and therefore Gi-dependent) pathway in numerous cell types (An et al. 1999; Ancellin and Hla 1999; Kon et al. 1999; Lee O et al. 1999; Okamoto et al. 1998, 1999; Van Brocklyn et al. 1998; van Coppen et al. 1996). Activation of S1P1R results in inhibition of adenylyl cyclase (Zondag et al. 1998), activation of phospholipase C (Okamoto et al. 1998), and mobilization of intracellular Ca2+ through IP3-sensitive (Formigli et al. 2002; Zhou and Murthy 2004) and IP3-insensitive Ca2+ stores (Ghosh et al. 1994; Mattie et al. 1994; Meyer zu Heringdorf et al. 1998; Tornquist et al. 1997).
S1P2R and S1P3R can couple to Gi, Gq, and G13 (Ancellin and Hla 1999; Ishii et al. 2001; Kon et al. 1999; Okamoto et al. 1999; Sato et al. 1999; Windh et al. 1999; for review, see Sanchez and Hla 2004). Consistent with this promiscuity, activation of these receptors has been linked to both stimulation (Sato et al. 1999) and inhibition of adenylyl cyclase (Okamoto et al. 1999) and stimulation of phospolipase C (An et al. 1999; Ancellin and Hla 1999; Ishii et al. 2001; Okamoto et al. 1999; Sato et al. 1999). There is also evidence for preferential coupling with S1P2R coupling most strongly to G13 and S1P3R most strongly activating Gq and subsequent Ca2+ mobilization (Ishii et al. 2002). Less is known about S1P5R, but signaling through Gi and G12 has been reported (Im et al. 2005; Jaillard et al. 2005; Malek et al. 2001; Novgorodov et al. 2007).
In addition to receptor-mediated pathways, sphingosine derivatives can also act intracellularly. Sphinganine-1-phosphate, which is structurally similar to S1P, activates the entire family of S1P receptors yet does not completely mimic the effects of S1P (Van Brocklyn et al. 1998; Xia et al. 1998), suggesting additional sites of action for S1P. Also S1P mobilization of intracellular Ca2+ has been observed independent of S1PR expression (Van Brocklyn et al. 1998). Furthermore, effects on proliferation and survival can be achieved through microinjection of S1P as well as through release of intracellular caged S1P; providing further evidence for an intracellular interaction site for S1P (Meyer zu Heringdorf et al. 2003a; Van Brocklyn et al. 1998; Xia et al. 1998).
Very little is known about the signaling capabilities of S1P in the vertebrate retina, but a few of its functions are emerging. S1P can mediate the pathological process of neovascularization that follows retinal injury (Skoura et al. 2007; Xie et al. 2009). There are also recent reports that S1P functions during retinal development. S1P can regulate the proliferation and differentiation of photoreceptors in rat retinal cultures (Miranda et al. 2009) and can also be involved in axonal path-finding by retinal ganglion cells (Strochlic et al. 2008). In amacrine cells, it has been demonstrated that S1P promotes transient Ca2+ influx events by a receptor-independent mechanism in store-depleted dendrites (Borges et al. 2008). This indication that S1P can be involved in Ca2+ signaling in amacrine cells has led us to ask whether S1P receptor-dependent Ca2+ signaling might also occur in these cells.
Amacrine cells are interneurons that participate in complex synaptic interactions in the inner plexiform layer of the retina. Synaptic input and output is not typically segregated in these cells. Instead pre- and postsynaptic sites are intermingled on amacrine cell dendrites. This arrangement implies that signaling between an amacrine cell and its synaptic partners (bipolar, ganglion, and other amacrine cells) is highly localized. Consistent with this, localized Ca2+ signals have been demonstrated in amacrine cell dendrites both in the intact retina (Denk and Detweiler 1999; Euler et al. 2002) and in culture (Azuma et al. 2004; Hurtado et al. 2002; Medler and Gleason 2002; Sen et al. 2007). Furthermore local and reciprocal signaling has been established for an inhibitory feedback synapse between amacrine and bipolar cells (Chavez et al. 2006; Vigh and von Gersdorff 2005). This theme of local signaling might also be extended to S1P. Thus far, the only evidence for production of S1P in the inner retina is in amacrine cells (Borges et al. 2008), but given its effects on photoreceptors (Miranda et al. 2009) and ganglion cells (Strochlic et al. 2008), it is likely to be produced elsewhere as well. Nonetheless it is well established that many cells that synthesize and release S1P also respond to it (for review, see Maceyka et al. 2008).
To further elucidate the signaling role for S1P in retinal amacrine cells, we have examined the effects of S1P on amacrine cells cultured from the chick retina. Here we use a combination of electrophysiology, immunocytochemistry, Ca2+ imaging, and molecular biology methods to explore the signaling properties of S1P in these retinal neurons. We demonstrate that S1P activates a receptor-dependent current as well as cytosolic Ca2+ elevations. Evidence is presented suggesting that the channels carrying the S1P-dependent current might be transient receptor potential canonical (TRPC) channels. The signaling pathways that underlie the current and the Ca2+ elevations appear to be complex and interacting. Intriguingly, we find that synthesis of internal S1P might also contribute to the response.
METHODS
Cell culture
Dissociation and culture methods have been previously reported (Hoffpauir et al. 2006). Briefly, retinas from 8-day-old White Leghorn chicken embryos (Gallus gallus domesticus, Animal Sciences Department, Louisiana State University) were dissociated in 0.1% trypsin and plated at 5.0 × 105 cells/35-mm polyornithine-coated (0.1 mg/ml) tissue culture dish. Cells were also plated onto polyornithine-coated glass coverslips for immunocytochemistry. Retinal cultures were maintained in Neurobasal (Invitrogen, Carlsbad, CA) supplemented with B27 (Invitrogen), 1,000 U penicillin/ml, 100 μg streptomycin/ml, and 1 mM l-glutamine (Sigma, St. Louis MO).
Immunocytochemistry
Cells were fixed in 1% paraformaldehyde for 1 h at 4°C. Rabbit polyclonal antibodies raised against sphingosine kinase 1 were purchased from Abgent (San Diego, CA) but produced no labeling in either chicken retinal tissue or cultured cells. Rabbit polyclonal antibodies raised against human S1P1R and S1P3R were purchased from Cayman Chemical (Ann Arbor, MI). Cultures were incubated for 1 h in 5% normal goat serum in dilution solution [1% bovine serum albumin (BSA); 0.1% saponin in PBS]. Anti-S1P1R and S1P3R antibodies were diluted in dilution solution to 1:500 and 1:250, respectively. Cells were incubated in primary antibodies for 1 h at room temperature. After washing, Cy3-conjugated goat anti-rabbit secondary antibodies (Invitrogen) were diluted to 1:1,000 and applied for 1 h at room temperature. Coverslips were mounted in Vectashield (Vector Labs, Burlingame, CA) and viewed under fluorescent optics on an Olympus IX-70 inverted microscope. Digital images were captured using IPLab (Bonn, Germany) version 4.0. Adobe Photoshop 7.0 was used to assemble the figures.
Western blots
Western blot analyses were conducted following the method described in Crousillac et al. (2003). Briefly, tissues for Western blots were obtained from Sprague-Dawley rats (Laboratory Animal Medicine, LSU) killed by decapitation and White Leghorn chickens (Animal Sciences, LSU) killed by CO2 exposure followed by decapitation. All methods involving animals were conducted in accord with the National Institutes of Health guidelines and with approval of the Louisiana State University Institutional Animal Care and Use Committee. Whole cell lysates of chicken and rat brains were homogenized in IP buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris pH7.4, 1 mM EDTA, 1 mM EGTA, 0.5% NP40) with a protease inhibitor cocktail (Roche, Indianapolis, IN). Protein content was then quantified using the BioRad (Hercules, CA) protein assay kit. Rat brain homogenate (150 μg) and 50 μg chicken brain homogenates were loaded onto a 7.5% SDS-PAGE gel. Following electrophoresis, proteins were transferred to nitrocellulose membranes. The membranes were then incubated overnight at 4°C with blocking buffer (1% bovine serum albumin, 0.1% Tween 20, and 2% nonfat dry milk in Tris-buffered saline). Membranes were incubated in S1PR1 antibody (1:1,000, Cayman Chemical) at room temperature for 1 h and then incubated with a goat anti-rabbit IgG peroxidase conjugate secondary antibody (1:5,000) for 1h at room temperature. The ECL Western blotting detection reagent kit (Amersham, Piscataway, NJ) was used for visualization of the antibodies.
PCR amplification
Retinal amacrine cells were harvested after 7 days in culture. These cells have been previously characterized and can be identified based on morphological criteria (Gleason et al. 1993). Ten to 20 amacrine cells were collected into patch pipettes. The pipette tip was immediately broken into a lysis buffer containing Tris-HCl, LiCl, EDTA, and LiDS. The lysate was kept frozen at −80°C until it was used for RT-PCR. A basic local alignment search tool (BLAST) search revealed predicted G. gallus gene sequences encoding TRPC protein subunits 1 and 3–7 as well as S1P3R and SphK proteins. Gene-specific primers for transcripts encoding these proteins were designed using Primer3 (Rozen and Skaletsky 2000) and can be found in Table 1. Primers were obtained from Integrated DNA Technologies (Coralville, IA). Messenger RNA isolation was performed using Dynabeads mRNA DIRECT micro kit (Invitrogen,) following the manufacturer's isolation protocol. RT-PCR was conducted using SuperScript III one-step RT-PCR with platinum Taq (Invitrogen) in a PTC-100 thermal cycler (MJ Research, Waltham, MA). See Table 1 for annealing temperatures. Forty cycles were run for each PCR. Actin was used as a positive control and an RT(−) sample was used as a negative control. Samples were then analyzed using 1.5% agarose gel electrophoresis (85 V for 1.25 h) in 0.5× TBE buffer.
Table 1.
Primer sequences for PCR amplifications
| Primer | Sequence | Expected Product Size, bp | Annealing Temperatures |
|---|---|---|---|
| TRPC1 forward | 5′-GGTATTTTCTGTGAGAAG-3′ | 483 | Ramped from 37–45° C, 1° C/30 sec. |
| TRPC1 reverse | 5′-CTTCTGATAATTCTCGTC-3′ | ||
| TRPC3 forward | 5′-TCCTATGACGAAGACGGC-3′ | 600 | 48°C |
| TRPC3 reverse | 5′-ACAGTTTGGATGAGCCAC-3′ | ||
| TRPC4 forward | 5′-GCTGCTCCTAAGTTTTAATG-3′ | 618 | 44°C |
| TRPC4 reverse | 5′-CCAGTTTCAATCTTGCAAG-3′ | ||
| TRPC5 forward | 5′-CGCTTTGGTATGACGGTTTT-3′ | 178 | 48°C |
| TRPC5 reverse | 5′-CCGGTGTGGCAGATAAACTT-3′ | ||
| TRPC6 forward | 5′-GATGAAGATGGAACACGG-3′ | 450 | 45°C |
| TRPC6 reverse | 5′-GCACTTTGGATGTGCTAC-3′ | ||
| TRPC7 forward | 5′-GAGCTCCTCCTCAAAAAGG-3′ | 476 | 47°C |
| TRPC7 reverse | 5′-CTAGTCTGGCCAATTCATTG-3′ | ||
| SphK1 forward | 5′-GAACCATACCACCCATCAG-3′ | 221 | 54°C |
| SphK1 reverse | 5′-TGCAGTTGGTCAGCAGTTTC-3′ | ||
| S1P3R forward | 5′-GCCATTCTGGTAGCCATTGT-3′ | 156 | 53°C |
| S1P3R reverse | 5′-TGACCAACAGGCAATGAAGA-3′ |
TRPC, transient receptor potential canonical; SphK, sphingosine kinase; S1P, sphingosine-1-phosphate.
PCR products were purified using a ChargeSwitch PCR clean-up kit (Invitrogen) then combined with Big Dye 3.1, ABI buffer, and respective primers. Cycle sequencing was performed in a PTC-100 thermal cycler. EtOH dye terminator removal was conducted prior to sequencing. Samples were then re-suspended in formamide and sequenced using a 3130XL genetic analyzer (Applied Biosystems, Foster City, CA). For all work reported here, PCR product sequences corresponded to regions of the predicted coding transcripts for the individual proteins as determined by BLAST queries.
Electrophysiology
Culture dishes were mounted on the stage of an Olympus IX-70 inverted microscope and a Ag/Ag-Cl pellet served as a reference electrode in the culture dish. Data were acquired using an Axopatch-1D amplifier, Digidata 1322 data-acquisition board, and pClamp 9.2 software (Molecular Devices, Sunnyvale, CA). Unless otherwise noted, voltage-clamp recordings were made in the perforated-patch configuration. Patch pipettes were pulled from borosilicate glass (1.5 mm OD, 0.86 mm ID) using a Flaming/Brown puller (Sutter Instruments, Novato, CA) and had tip resistances of 3–5 MΩ. For perforated-patch experiments, amphotericin B stock was made at 40 μg/ml in dimethyl sulfoxide (DMSO). Amphotericin was combined 1:2 with Pluronic F-127 (Invitrogen, 25 mg/ml DMSO) and diluted in internal solution for a final amphotericin concentration of 140 μg/ml. Gigaohm seals were achieved with a brief hyperpolarizing pulse of −140 mV. Then unless otherwise indicated, the voltage was clamped at −70 mV. Voltage values were not corrected for the (unknown) liquid junction potential resulting from the perforated-patch configuration. Recordings were made at room temperature. All current traces shown were leak-subtracted. For experiments in which charge transfers were compared, data from the same time frame were analyzed for each condition, and the mean charge transfer was calculated for five second windows of time.
Fluorescence measurements
Oregon Green bis-(o-aminophenoxy)-N,N,N',N'-tetraacetic acid (BAPTA) 488 was prepared as a 2 mM stock solution in DMSO, combined 1:1 with Pluronic F127 (Invitrogen, 25 mg/ml DMSO), then diluted to 2 μM in Hank's balanced salt solution (Invitrogen). Cells were loaded for 1 h at room temperature in the dark. Cells were then washed with external solution and kept in the dark until the beginning of the experiment, typically ∼20 min. Culture dishes were mounted on the stage of an Olympus IX-70 inverted microscope, and images were acquired using IP Lab image capturing software version 4.0. Shutter interval for imaging experiments was 1 s (2 s for Fig. 11B), and exposure time was 150 ms. Fluorescence intensity data were collected from amacrine cell bodies. Background fluorescence was subtracted from all data. For display, raw fluorescence intensity values were normalized to baseline values and are reported as F/Fo.
Fig. 11.
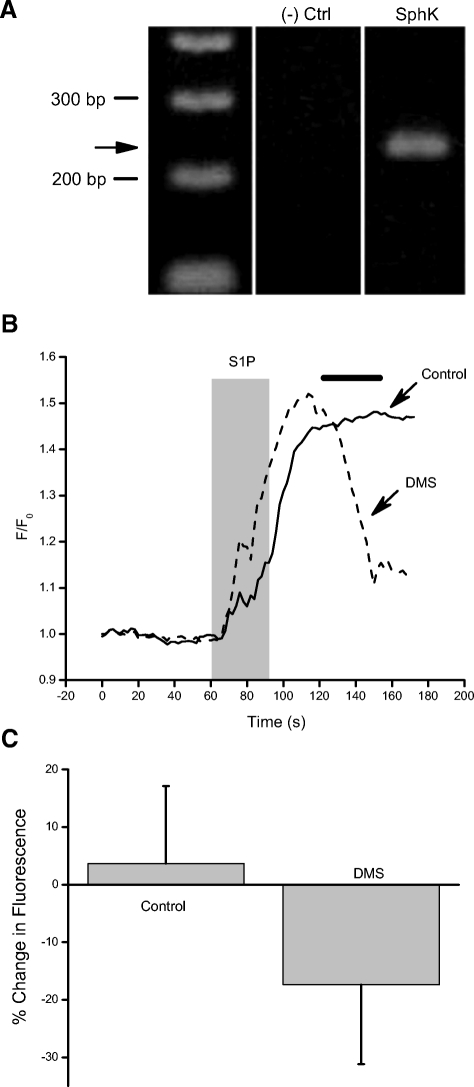
Inhibition of SphK limits the duration of the S1P-dependent Ca2+ elevations. A: SphK1 gene-specific primers were use to amplify mRNA from ∼15 amacrine cells collected from a culture dish. The PCR product produced a single band of the appropriate size (221 bp). The identity of the product as SphK1 was confirmed by sequence analysis. B: control cells were exposed to S1P for 30 s followed by 90 s in normal external. Normalized data from a representative amacrine cell show that the cytosolic Ca2+ level does not recover on this time frame (solid trace). However, when DMS (10 μM) was applied (time indicated by the bar, data from a different cell, dotted trace), a decline in cytosolic Ca2+ was typically observed. C: averaged data are shown from 19 control and 19 DMS-treated cells. To calculate the percent change, raw fluorescence values were obtained for both groups at t = 120 s (the onset of DMS application) and t = 150 s (when DMS was removed). Error bars are SE.
Solutions and reagents
Reagents were purchased from Sigma unless otherwise indicated. Normal external solution consisted of the following (in mM): 5.3 KCl, 136.9 NaCl, 3.0 CaCl2, 0.4 MgCl2, 3.0 HEPES, and 5.6 glucose. Tetraethylammonium (TEA) external solution consisted of the following (in mM) 5.3 KCl, 116.7 NaCl, 20.0 TEA Cl, 3.0 CaCl2, 0.4 MgCl2, 5.6 glucose, and 10.0 HEPES. For electrophysiology, external solutions also contained bicuculline methiodide (3.0 μM) to block GABAergic synaptic currents and TTX (300 nM) to block voltage-gated Na+ channels. Cesium internal solution (for ruptured patch) consisted of the following: 100.0 Cs acetate, 10.0 CsCl, 0.1 CaCl2, 2.0 MgCl2, 10.0 HEPES, and 1.1 EGTA. Internal B solution (for perforated patch) consisted of the following: 135 Cs acetate, 10.0 CsCl, 2.0 MgCl2, 0.1 CaCl2, 1.1 EGTA, 10.0 HEPES, and 1.0 NaCl.
S1P (Biomol, Plymouth Meeting, PA) was prepared as a 1 mM stock in methanol then diluted in external solution to a final concentration of either 1 or 10 μM. The PLC inhibitor U73122 and its less active analog U73343 were used at 10 μM and purchased from Biomol. The IP3 receptor antagonist heparin (Calbiochem, San Diego, CA) was included in the patch pipette at a final concentration of 5 mg/ml. The S1P1R selective agonist SEW2871 was purchased from Cayman Chemical (Ann Arbor, MI) and prepared as a 10 mM stock in DMSO. PTX (Calbiochem) was used at a final concentration of 200 ng/ml. Cells were incubated for 18 h in PTX diluted in culture medium. Following the incubation period, cells were washed in normal solution and used immediately for electrophysiology experiments. PTX-treated cells used for Ca2+-imaging experiments were washed in normal external solution and loaded with the dye for 1 h prior to recording. Suramin was dissolved directly in the external solution at a concentration of 100 μM. N,N-dimethyl-d-erythro-sphingosine (DMS, Biomol) was diluted in ethanol at a concentration of 10 mM then diluted into external solution at a final concentration of 10 μM.
Data analysis
Statistical significance was evaluated using the Student's t-test. The data in Figs. 2E and 3, B and D, were evaluated using the paired form of the test. Error bars represent SE.
Fig. 2.
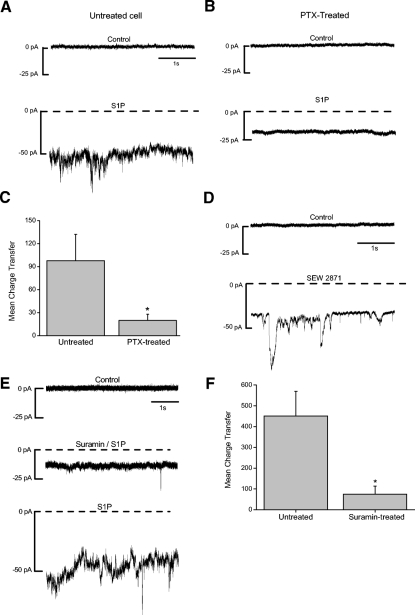
Activation of S1P1R and S1P3R may both contribute to the S1P-dependent current. A: an untreated cell that is voltage-clamped at −70 mV is exposed to S1P (10 μM), and a noisy, inward current is observed (bottom trace). B: cells were pretreated overnight (18–22 h) with pertussis toxin (PTX, 200 ng/ml). A PTX-treated cell that is voltage-clamped at −70 mV exhibits only a small inward shift in baseline current in the presence of S1P. C: comparison of the S1P-dependent charge transfer between treated (n = 21) and untreated cells (n = 17) revealed a significant reduction in the responses of PTX-treated cells (P = 0.02). D: SEW2871 (10 μM), an S1P1R-selective agonist, also elicits a noisy inward current. E: a cell is voltage clamped at −70 mV and exposed to suramin (100 μM), an inhibitor of S1P3R signaling. Suramin reversibly inhibits the S1P-dependent current (middle trace). F: analyses of S1P-dependent charge transfer reveals that suramin significantly suppresses the current (n = 9, P = 0.003). C and F, error bars are SE.
Fig. 3.
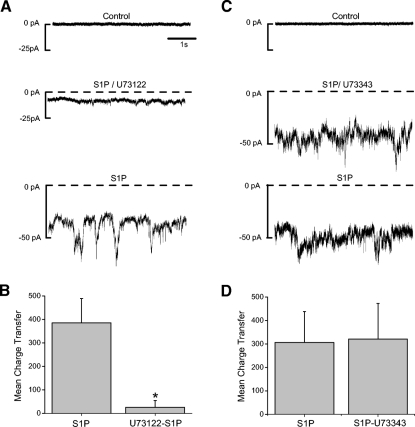
The S1P-induced current is phospholipase C (PLC)-dependent. A: a representative amacrine cell is voltage-clamped at −70 mV. S1P (10 μM) was applied in the presence of the PLC inhibitor U73122 (10 μM, middle trace). After 1 min, the U73122 was removed (bottom trace). B: the mean charge transfer was significantly reduced by the inhibitor (n = 6, P = 0.02). C: a separate cell is voltage-clamped at −70 mV and then exposed to S1P in the presence of U73343 (10 μM), a less active analogue of U73122. The analogue did not suppress the S1P current. D: the mean charge transfer was unaffected by U73343 (n = 6, P = 0.98). B and D, error bars are SE.
RESULTS
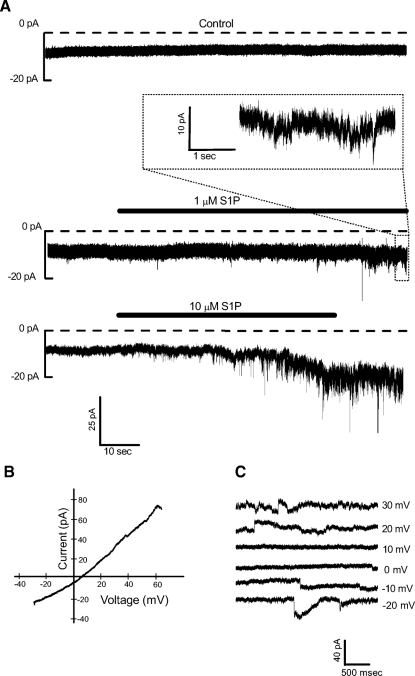
Individual amacrine cells were voltage-clamped at −70 mV. S1P (1 or 10 μM) elicited a noisy inward current that developed after ∼10 s of exposure (Fig. 1A). Once activated, the current usually persisted after the removal of agonist. Although the S1P-dependent current could be elicited with 1 μM S1P, the higher concentration was used for most of the experiments because the responses to 10 μM S1P had a faster onset and were more easily distinguishable. To determine the ionic composition of the S1P-dependent current, voltage ramps were delivered during S1P-dependent current activation to reveal its reversal potential (Fig. 1B). On average, the current reversed at 7.8 ± 8.5 mV (n = 11). The noisy nature of the S1P-dependent current made it difficult to accurately measure its reversal potential and likely contributes to the variability in the values. However, using voltage steps, we found that outward current could only be observed when the voltage was stepped to positive values (Fig. 1C, n = 4) suggesting that the S1P-dependent current is carried by a mixture of cations.
Fig. 1.
Sphingosine-1-phosphate (S1P) elicits a cation current. A, top: a representative amacrine cell is voltage-clamped in the perforated-patch configuration at −70 mV and a control record is obtained. Middle: S1P (1 μM) is applied and a small inward current is elicited. A region of the activated current is shown on an expanded time scale (inset). Bottom: in a recording from another amacrine cell, 10 μM S1P is applied, and after ∼10 s, a noisy inward current begins to develop. B: a voltage ramp is delivered and an S1P-dependent current (leak-subtracted) is measured that reverses at +8 mV, suggesting that the current is carried by a mixture of cations. S1P-dependent currents were also recorded during a series of voltage steps (−70 to +40 mV). Currents recorded in a representative cell from 6 of those steps are shown in C. Outward currents were not observed unless the voltage was stepped positive to 10 mV (n = 4). Currents have been offset vertically for clarity.
S1P-dependent current may involve signaling through S1P1R and S1P3R
Because S1P signals through G-protein-coupled receptors and most commonly through Gi, cells were incubated overnight in PTX (200 ng/ml), an inhibitor of Gi-mediated signaling. In untreated cells, S1P elicited the typical current (Fig. 2A). However, in cells pretreated with PTX, the S1P current was reduced in amplitude (Fig. 2B), suggesting that activation of Gi contributes to the S1P-induced current. Because the S1P-induced current is so noisy, currents were integrated over 5-s windows so that they could be quantified. Comparison of charge transfer revealed a significant reduction in the S1P-dependent current in PTX-treated cells (Fig. 2C). To determine whether the Gi-dependent receptor S1P1R was involved in the response, we used SEW2871, a synthetic agonist selective for the S1P1R receptor (Sanna et al. 2004). SEW2871 (10 μM) elicited a noisy inward current similar to the S1P-induced current (Fig. 2D).
Because PTX treatment resulted in only partial inhibition of the S1P-mediated current, we asked whether another receptor was being activated by S1P. To explore this, we examined the effects of suramin, a polycyclic anionic compound that can disrupt the interactions between G proteins and G-protein-coupled receptors (Freissmuth et al. 1999). Especially relevant here, however, is that suramin has been shown to inhibit the S1P-dependent activation of S1P3R but does not interfere with signaling via S1P1R or S1P2R (Ancellin and Hla 1999). Suramin can also inhibit other receptors (Sim et al. 2008), but it had no effect on the membrane current when applied alone (not shown). In cells exposed to S1P in the presence of suramin, the noisy inward current was reversibly reduced (Fig. 2E). Charge transfer analysis revealed a significant reduction in the S1P-dependent current in the presence of suramin (Fig. 2F), suggesting that signaling through S1P3R contributes to production of the S1P-dependent current. Note that the small S1P-dependent inward shift in the baseline current persists in the presence of either blocker (Fig. 2, B and E, middle trace). Interestingly, PTX and suramin each inhibited the S1P-dependent current by ∼75–80%. This level of inhibition suggests a synergistic relationship between two pathways, such that the total current mediated by S1P and its receptors is greater than the sum of the currents engendered by activation of a single receptor.
S1P-induced current is PLC-dependent
The activation of either S1P1R or S1P3R can lead to activation of PLC (Fig. 4A). To determine whether S1P is activating the current through a PLC-dependent pathway, we used the PLC inhibitor U73122 in voltage-clamp experiments. The S1P-dependent current was measured in the presence of U73122. The S1P-induced current was inhibited in the presence of U73122 (Fig. 3A), suggesting that the current is PLC dependent. Charge transfer analysis indicated that U73122 caused a significant reduction in the S1P-dependent current (Fig. 3B). In control experiments, the less active analog U73343 was applied along with S1P and had no effect on the S1P-dependent current (Fig. 3, C and D).
Fig. 4.
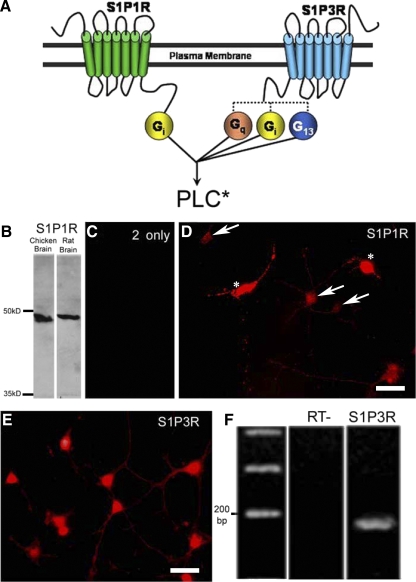
S1P receptors are expressed by amacrine cells. A: S1P1R is thought to couple to Gi exclusively, whereas S1P3R can couple to Gi, Gq, or G13. Signaling through these G proteins converges at the level of PLC activation. B: Western blot analysis of the S1P1R antibody reveals a single band present for both chicken and rat brain homogenates just below the 50-kDa marker, consistent with the predicted molecular weight for the S1P1R (47 kDa). C: secondary-only controls did not show any nonspecific labeling in cultured cells. D: the S1P1R antibody labels all amacrine cell bodies (arrows) and their processes in culture. Cone photoreceptors label intensely with this antibody (asterisks) and have been overexposed in this image so that the amacrine cell labeling can be observed. E: in a pattern similar to the S1P1R antibody, the S1P3R antibody labels all amacrine cell bodies and their processes in cell culture. All of the cells in this image are amacrine cells. Scale bars are 25 μm. F: S1P3R gene-specific primers were use to amplify mRNA from ∼15 amacrine cells collected from a culture dish. The PCR product produced a single band of the appropriate size (156 bp). The identity of the product as a component of the transcript encoding S1P3R was confirmed by sequence analysis.
S1P receptor expression
Because we had physiological evidence that S1P1R and S1P3R might be involved in the S1P response, the binding of polyclonal antibodies raised to these receptors was examined. Western blot analysis was used to confirm the specificity of the S1P1R antibody. Antibodies were incubated with a blot of proteins from chicken brain and rat brain homogenates, respectively. A single band was present just below the 50-kDa marker in both lanes (Fig. 4B) consistent with the predicted molecular weight of S1P1R (47 kDa). For immunocytochemistry, secondary-only control experiments revealed no nonspecific binding of the secondary antibodies (Fig. 4C). S1P1R antibodies labeled all amacrine cell bodies as well as their processes in cell culture (Fig. 4D, arrows). Interestingly, another identified cell type in these cultures, cone photoreceptors, showed very strong anti-S1P1R labeling (Fig. 4D, asterisks), suggesting a possible signaling role for S1P in the outer retina.
The antibody raised against S1P3R labeled all amacrine cells in culture (Fig. 4E). Unfortunately we were not able to identify a S1P3R band in Western blots of proteins from either chicken or rat brain. As such, the labeling of amacrine cells is considered S1P3R-like. To support this labeling, we took an alternative approach of RT-PCR amplification of S1P3R mRNA collected from a population of amacrine cells using gene-specific primers (Fig. 4F). A 156-bp PCR product was amplified, which was consistent with the predicted product size. The PCR product was sequenced to confirm its identity as a component of the S1P3R coding transcript. These results are consistent with the S1P3R-like antibody labeling and our physiological results and suggest that amacrine cells express both S1P1R and S1P3R. Nonetheless, complete confirmation of the expression of S1P3R must await a demonstration that the protein is expressed.
S1P-induced current is IP3 receptor-independent
Activation of phospholipase C results in the hydrolysis of phosphatidylinositol 4,5 bisphosphate (PIP2) into diacylglycerol (DAG) and inositol trisphosphate (IP3), which then typically activates IP3 receptors. To test the involvement of IP3 receptors specifically, we included the IP3 receptor antagonist heparin (5 μg/ml) in the patch pipette and recorded from amacrine cells in the ruptured patch configuration. The S1P-induced current was not blocked by the inclusion of heparin in the recording pipette (n = 7; Fig. 5), even after >20 min of recording time (post-rupture, Fig. 5B), suggesting that the current activation is independent of IP3 receptor activation.
Fig. 5.
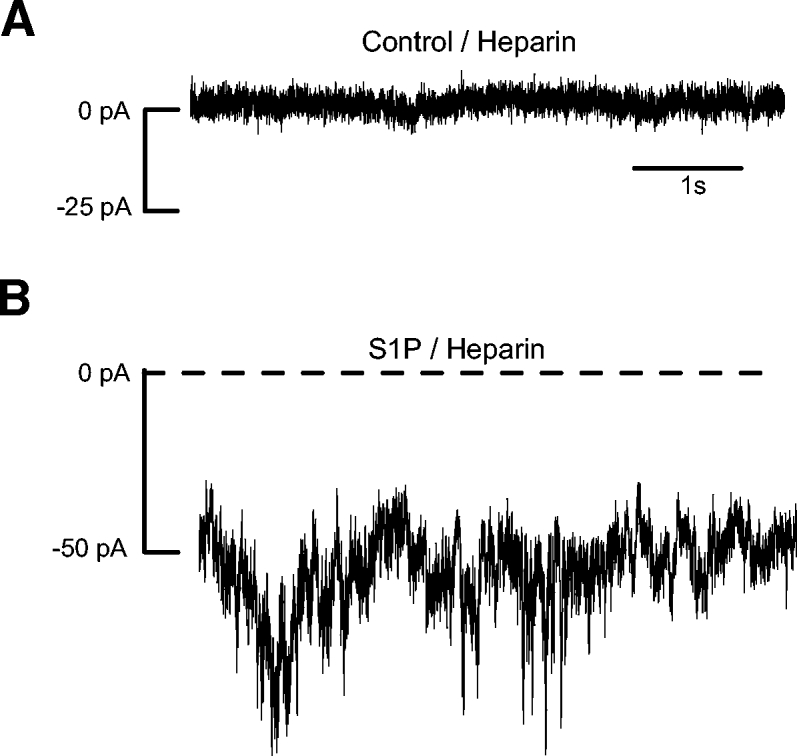
The S1P current is IP3 receptor-independent. A: the IP3 receptor blocker heparin (5 μg/ml) was included in the patch pipette for ruptured-patch voltage-clamp recordings. Before S1P application, the holding current in the heparin-perfused cells was similar to that seen in perforated patch recordings. In S1P, noisy currents develop that are similar in amplitude and appearance to S1P-induced currents recorded in the perforated-patch configuration.
S1P-induced current is lanthanide-sensitive
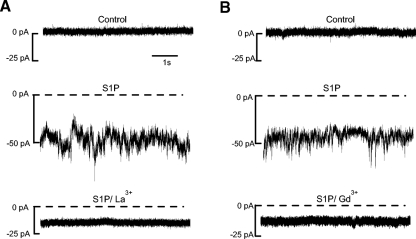
In considering the possible mediators of the S1P-dependent current, we were able to rule out activation of GABAA receptors because the Erev for the S1P-dependent current was too positive (ECl- = −58 mV) and because external recording solutions contained bicuculline methiodide to block these receptors. Cyclic-nucleotide-gated channels were also ruled out because 8 Br-cAMP and 8 Br-cGMP do not elicit a current in amacrine cells (Sosa et al. 2002; B. K. Hoffpauir and E. Gleason unpublished observations). We were also able to rule out activation of L-type voltage-gated Ca2+ channels based on three considerations. First, the voltage was clamped at −70 mV, well outside the activation range of these L-type voltage-gated Ca2+ channels (approximately −40 mV). Second, nifedipine (10 μm) was routinely included in external recording solutions to block L-type Ca2+ channels. And third, the Erev for the S1P-dependent current was not positive enough to represent Ca2+ flux alone. As nonselective cation channels linked to G-protein-coupled receptor activation, TRPC channels were good candidates for the mediators of this current. TRPC channels are sensitive to low micromolar concentrations of the lanthanides La3+ and Gd3+. To address the possibility that TRPC channels mediate the S1P-induced current, we examined the effects of La3+ (10 μm) and Gd3+ (10 μm) on the S1P (10 μM) current. Both La3+ (Fig. 6A) and Gd3+ (B) consistently inhibited the S1P current. Note that with lanthanides, as for the G protein inhibitors and PLC inhibitor, a sustained S1P-dependent current persists. Although the origin of this current component is not known, its resistance to all of these agents suggests that it might be due to a direct, nonreceptor-mediated effect of S1P. As for the larger component of the current, however, its regulation, selectivity, and lanthanide sensitivity are all consistent with the possibility that it is mediated by TRPC channels.
Fig. 6.
The S1P-dependent current is lanthanide-sensitive. A and B, top traces: 2 separate cells are voltage-clamped at −70 mV in the perforated-patch configuration. S1P activates a noisy inward current (middle traces). Most of the S1P-dependent current is eliminated in the presence of either La3+ (10 μM) or Gd3+ (10 μM, bottom traces).
TRPC transcripts are present in cultured amacrine cells
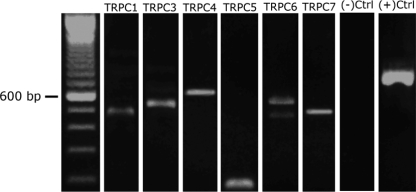
To address the possibility that TRPC channels are involved in the S1P-induced current, gene-specific primers were designed for TRPC subunits 1 and 3–7 using predicted sequences in the chicken genome. PCR-amplification of TRPC subunits was performed on small populations (10–20) of amacrine cells collected in a patch pipette. Amplifications produced bands at the appropriate molecular weights (Fig. 7 and Table 1). Individual TRPC subunit PCR product identity was confirmed by sequencing. Because we used mRNA isolated from multiple amacrine cells in our amplifications, we cannot resolve whether an individual amacrine cell can express all of the subunits.
Fig. 7.
Transient receptor potential canonical (TRPC) subunit mRNAs are expressed in cultured amacrine cells. A: gene-specific primers were designed against TRPC subunits 1 and 3–7. Transcripts were PCR-amplified from 10 to 20 amacrine cells collected in patch pipettes. All of these PCR products were run on the same gel. Expected sizes of the PCR products are in Table 1. Actin was amplified as a positive control.
S1P causes a cytosolic Ca2+ increase
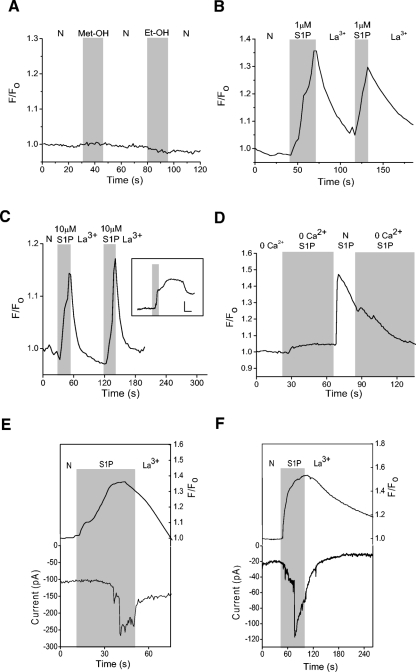
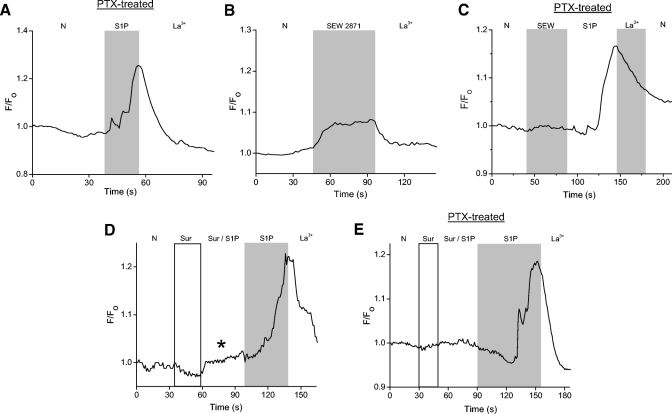
One of the primary functions of TRPC channels is to mediate Ca2+ influx. Thus if TRPC channels (or other nonselective, Ca2+-permeable cation channels) are involved, S1P should elicit Ca2+ elevations that are dependent on external Ca2+. To determine the effects of S1P on cytosolic Ca2+, Ca2+-imaging experiments were done on Oregon Green BAPTA 488-loaded amacrine cells. Control data show that the vehicles for S1P (methanol) and DMS (see following text, ethanol) do not elicit a Ca2+ elevation (Fig. 8A). S1P at either 1 or 10 μM produced La3+-sensitive increases in cytosolic Ca2+ (B and C). In the absence of La3+, the time course of the Ca2+ elevations was more variable and could outlast the exposure to S1P, sometimes for minutes (Fig. 8C, inset). To examine the source of the Ca2+ increase, Ca2+ imaging experiments were repeated in the absence of external Ca2+. In 0-Ca2+, S1P elicited small Ca2+ increases that were presumably due to release of Ca2+ from internal stores (Fig. 8D). When external Ca2+ was re-introduced, substantial cytosolic Ca2+ increases were observed, further supporting the idea that S1P stimulates Ca2+ entry across the plasma membrane.
Fig. 8.
S1P generates La3+-sensitive Ca2+ elevations in amacrine cells. Cells were loaded for 1 h with the Ca2+- sensitive dye Oregon Green BAPTA 488. A: a representative example of a fluorescence recording testing the effects of the vehicle for S1P (1% Met-OH) and N,N-dimethyl-d-erythro-sphingosine (DMS, 1% Et-OH), a reagent used in the experiments depicted in Fig. 11. No Ca2+ elevations were seen in response to these compounds. B: a representative recording shows that 1 μM S1P can elicit La3+-sensitive Ca2+ elevations. C: data from a different cell showing a La3+-sensitive cytosolic Ca2+ increase to 10 μM S1P. Inset: data from a different cell showing that in the absence of La3+, the Ca2+ elevations can persist well after the removal of S1P (scale bars are 1.1 F/F0 and 30 s). D: in 0-Ca2+, a small S1P-dependent Ca2+ increase was observed, likely representing release of Ca2+ from internal stores. Re-introduction of external Ca2+ produced a dramatic increase in the S1P-dependent Ca2+ elevation. E: an amacrine cell preloaded with Oregon Green Bapta 488 is voltage-clamped at −70 mV in the perforated-patch configuration. S1P causes a cytosolic Ca2+ increase that precedes the inward S1P-dependent current. F: in a different cell, S1P-induces a cytosolic Ca2+ increase that occurs simultaneously with the S1P-dependent current.
To examine the temporal relationship between the S1P-dependent Ca2+ elevations and the S1P-dependent current, simultaneous Ca2+-imaging and voltage-clamp experiments were done. Amacrine cells were preloaded with Oregon Green BAPTA 488 AM then voltage-clamped in the perforated-patch recording configuration. In some cells (5 of 13), S1P produced cytosolic Ca2+ increases that preceded development of the S1P-dependent current (Fig. 8E). In other cells, (8 of 13), S1P-dependent Ca2+ elevations and the S1P-dependent current had a similar onset (Fig. 8F). The S1P-induced membrane current was never observed to precede elevations in cytosolic Ca2+, suggesting that release of internal Ca2+ is a prerequisite for the activation of the current.
S1P-induced Ca2+ increase is receptor-mediated
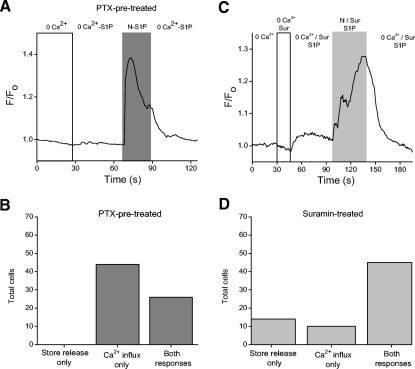
To further explore the link between the S1P-dependent Ca2+ elevation and the S1P-dependent current, we used PTX to inhibit Gi-mediated signaling. In PTX-treated cells, S1P induced increases in cytosolic Ca2+ in 50 of 99 cells tested (Fig. 9A), suggesting that the S1P-dependent Ca2+ increases could be activated through a Gi-independent mechanism. However, in a separate experiment, the S1P1R-selective agonist SEW2871 consistently produced Ca2+ elevations, indicating that activation of S1P1R alone was sufficient to elicit a Ca2+ increase (Fig. 9B). If SEW2871 is activating S1P1R alone and S1P1R is the primary receptor linked to Gi, then we would expect that PTX pretreatment would inhibit SEW-dependent Ca2+ elevations. In 65% of PTX-treated cells examined (n = 46), SEW2871 did not elicit Ca2+ elevations, but Ca2+ elevations were produced by subsequent exposure to S1P (Fig. 9C). The remaining cells (16/46) had very small SEW responses in comparison to the responses engendered by S1P. These small SEW responses might be due to incomplete inhibition of Gi by PTX. These results suggest that in control cells, SEW2871 is eliciting Ca2+ elevations by activating S1P1R and Gi.
Fig. 9.
The S1P-induced cytosolic Ca2+ increase may be mediated by both S1P1R and S1P3R. A: cells were pretreated overnight (18–22 h) in pertussis toxin (200 ng/ml) to inhibit receptor signaling through Gi. A cell is loaded with Oregon Green BAPTA 488 and then exposed to S1P. S1P still induces a Ca2+ increase despite the pretreatment with PTX. B: to determine if activation of S1P1R alone is sufficient to elicit a Ca2+ increase, SEW2871 (10 μM) is applied. In the presence of SEW2871, a small cytosolic Ca2+ increase is observed. C: in a representative PTX-treated cell, SEW2871 produces little or no Ca2+ elevation. S1P, however, elicited a La3+-sensitive response. D: a cell is exposed to suramin (100 μM) to inhibit signaling through S1P3R. In suramin and S1P, a small cytosolic Ca2+ increase was observed (asterisk). When suramin was removed, the cell responded with a substantially larger Ca2+ increase. E: cells were pretreated overnight in PTX and exposed to suramin to inhibit signaling through Gi and S1P3R. The majority of PTX-treated cells (27/44) showed no S1P-dependent Ca2+ increase in the presence of suramin.
To test the possible involvement of S1P3R in the S1P-dependent Ca2+ elevations, we examined the effects of suramin. Suramin alone had no effect on cytosolic Ca2+. S1P in the presence of suramin, typically produced small elevations in cytosolic Ca2+ (Fig. 9D, asterisk) relative to those elicited in the same cell after suramin had been removed (D), suggesting that a substantial fraction of the S1P-induced Ca2+ increases occurs through activation of S1P3R.
Because our fluorescence measurements are nonquantitative, we do not know whether S1P responses in cells pretreated with PTX are smaller than those in untreated cells. To address whether S1P-dependent Ca2+ elevations typically occurred by signaling via both S1P1R and S1P3R, we examined the effects of blocking signaling through both receptors. In 72% (n = 61) of PTX- and suramin-treated cells, S1P had no effect on cytosolic Ca2+ (Fig. 9E). The balance of the PTX-treated cells had small but detectable Ca2+ increases in response to S1P in the presence of suramin, again suggesting that the inhibition of Gi by PTX might be incomplete. Alternatively, it is possible that another receptor is involved or that there is a direct (nonreceptor-mediated) effect of S1P as observed by Van Brocklyn et al. (1998).
Activation of either S1P1R or S1P3R can elicit release of Ca2+ from internal stores
To further investigate the source of the receptor-mediated Ca2+ increases, cells were either incubated overnight in PTX or exposed to suramin to inhibit signaling through Gi (S1P1R) and S1P3R, respectively, and to isolate signaling through the two receptors. Ca2+-free external solution was used to isolate the source of the Ca2+ increase. In PTX-treated cells, S1P resulted in either release of Ca2+ from internal stores, followed by external Ca2+ influx, or Ca2+ influx without detectable store release. Store release alone was never observed in PTX-treated cells (n = 70; Fig. 10, A and B). In suramin-treated cells, S1P resulted in release of Ca2+ from stores, Ca2+ influx, or in most cases a combination of both mechanisms (n = 69; Fig. 10, C and D). These results suggest that S1P1R-mediated Ca2+ elevations (suramin-treated) can result from either Ca2+ influx or store release or both. They also suggest that Ca2+ influx dominates S1P3R-dependent Ca2+ elevations (PTX pretreated) and that the responses via this receptor are not generated by store release alone.
Fig. 10.
Activation of either receptor can elicit release of Ca2+ from internal stores. A: an amacrine cell has been pretreated with PTX. In this cell, no S1P-dependent Ca2+ elevation is observed in the absence of external Ca2+. Re-introduction of extracellular Ca2+ results in a cytosolic Ca2+ elevation. B: cells treated with PTX responded with either release of Ca2+ from internal stores followed by external Ca2+ influx, or Ca2+ influx without internal store release (as in A, n = 70). C: data from an amacrine cell exposed to suramin. In 0 external Ca2+, a small S1P-dependent calcium elevation is observed. Re-introduction of extracellular Ca2+ increased the magnitude of the S1P-dependent Ca2+ elevation. D: cells in suramin responded to S1P with either store release only, Ca2+ influx only, or most often, a combination of both responses (n = 69).
Synthesis of S1P in amacrine cells
These studies provide multiple pieces of evidence for receptor-mediated S1P signaling in retinal amacrine cells. There is physiological evidence suggesting that cultured amacrine cells express the synthetic enzyme for S1P, sphingosine kinase (SphK) (Borges et al. 2008). We attempted to use polyclonal antibodies raised against SphK1 (see methods) but were unsuccessful with this approach. To further explore the expression of SphK in amacrine cells, we designed gene-specific primers for SphK 1. A PCR product of the appropriate size was amplified from mRNA isolated from a collection of 10–20 amacrine cells. Sequencing confirmed that the PCR product was from the mRNA encoding SphK 1, consistent with the findings of Borges et al. (2008) and suggesting that it is possible for the enzyme to be expressed in amacrine cells (Fig. 11A).
Because SphK is Ca2+-calmodulin-sensitive (Alemany et al. 2000; Sutherland et al. 2006), it is possible that the receptor-mediated, S1P-dependent Ca2+ elevations lead to further production of internal S1P. To address the possibility that cytosolic S1P was being generated, we asked whether a competitive inhibitor of SphK, DMS (Edsall et al. 1998; Yatomi et al. 1996) would have any effect on the duration of S1P-dependent Ca2+ elevations. DMS is reported to also inhibit protein kinase C in vitro (Igarashi and Hakomori 1989), but this effect was not observed in intact cells from three different cell lines (Edsall et al. 1998). Additionally, results reported for human neutrophils (Igataki and Hauser 2003) and cultured chick amacrine cells (Borges et al. 2008) indicate that the target of DMS is SphK rather than the influx channels themselves. It is important to note here that in the absence of inhibition by lanthanides, both the S1P-dependent current and the S1P-dependent Ca2+ elevations often persist after removal of the agonist and that Ca2+ influx (as opposed to release from stores) is the primary source of the Ca2+ elevation. Here we test the hypothesis that the persistent nature of the response is due to generation of cytosolic S1P subsequent to receptor activation. Control cells (n = 19) were exposed to S1P for 30 s then returned to normal external. After removal of S1P, Ca2+ levels tended to remain elevated for the remainder of the recording period (90 s, Fig. 11B, solid trace). We tested for the involvement of internal S1P in a separate set of cells (n = 19) by applying DMS after washout of S1P. With DMS, cytosolic Ca2+ levels typically declined more rapidly than in control (Fig. 11B, dotted trace). Overall, DMS significantly accelerated the recovery of the Ca2+ elevations (Fig. 11C, P = 0.0002). This suggests that extracellular S1P binding to receptors might lead to activation of SphK, synthesis of S1P, and a relatively sustained activation of a Ca2+ influx pathway.
DISCUSSION
In this study, we demonstrate that S1P activates a receptor-mediated cation current in retinal amacrine cells. S1P-dependent currents have been observed in other cell types, including human umbilical vein endothelial cells (Muraki et al. 2001). This current is a PTX-sensitive, nonselective cation current similar to what we have found in amacrine cells. It is well-established that S1P can induce increases in cytosolic Ca2+. However, the source of the S1P-mediated Ca2+ increase varies from one cell type to another. In Swiss 3T3 fibroblasts, Mattie et al. (1994) observed a transient increase in intracellular Ca2+ in response to S1P. The Ca2+ increase was independent of extracellular Ca2+ and was abolished when internal Ca2+ stores were depleted with thapsigargin. In contrast, Formigli et al. (2002) demonstrated in myoblast cells that S1P elicits a transient Ca2+ increase propagating as a wave throughout the cell and that the Ca2+ increase required both intra- and extracellular Ca2+ mobilization. Here in amacrine cells, S1P elicits a cytosolic Ca2+ increase that is dominated by Ca2+ influx but also involves release of Ca2+ from internal stores. The influx pathway, however, appears to be different for myoblasts and amacrine cells. In myoblast cells, the S1P-mediated Ca2+ response was significantly reduced through pretreatment of cells with the L-type calcium channel blocker nifedipine (Formigli et al. 2002), indicating the involvement of these voltage-dependent Ca2+ channels. In the current study, however, we rule out the involvement of these channels on several criteria. Thus S1P is capable of activating an inward cation current in amacrine cells independent of voltage-gated ion channel activation.
Our data suggest that the S1P-dependent current and Ca2+ influx are initiated by activation of both S1P1R and S1P3R in amacrine cells. For S1P1R, this suggestion is supported in part by antibody labeling. Although there can be specificity problems with antibodies against G-protein-coupled receptors (Michel et al. 2009), the ability of SEW2871 to activate both the current and Ca2+ influx at a concentration demonstrated to be ineffective on the other S1P receptors (Sanna et al. 2004) tempers this concern.
The evidence for the expression of S1P3R in amacrine cells is somewhat more tenuous. We have amplified a PCR product from amacrine cell mRNA that corresponds to a coding region of the S1P3R gene, and we observe S1P3R-like immunoreactivity but we are not able to confirm the expression of the protein. Several of our physiological results, however, suggest that this receptor is expressed in amacrine cells. First, we find that suramin is an effective inhibitor of S1P-dependent current and Ca2+ influx. The effects of suramin must be interpreted with caution because it can have wide ranging effects on G proteins and G-protein-coupled receptors (Freissmuth et al. 1999). Nonetheless in the context of S1P receptors, suramin has been shown to be effective in inhibiting signaling through S1P3R but ineffective in inhibiting signaling via S1P1R or S1P2R into the millimolar range (Ancellin and Hla 1999). Another indication that S1P is signaling through an additional receptor in amacrine cells comes from data in Fig. 10, B and D. The distinctive Ca2+ response patterns with respect to store release versus influx produced in the presence of each inhibitor (PTX or suramin) provide additional physiological evidence for signaling through both S1P1R and S1P3R in amacrine cells.
Intriguingly, there is evidence for an obligate collaboration between S1P1R and S1P3R in some signaling pathways. S1P1R and S1P3R are co-expressed and are both required for endothelial cell proliferation (Kimura et al. 2000), migration (Kimura et al. 2000; Paik et al. 2001), and morphogenesis (Lee et al. 1999). Especially relevant to our results, S1P1R has been shown to actually suppress Ca2+ signaling unless it is co-expressed with S1P3R specifically (Meyer zu Heringdorf et al. 2003b). Interestingly, heterodimers of S1P1R and S1P3R have been detected in expressions studies (Van Brocklyn et al. 2002), so it may be that dimerization is a requirement for interactions between these receptors.
Much debate exists as to whether S1P couples to release of Ca2+ from internal stores through activation of IP3Rs on the endoplasmic reticulum. In Swiss 3T3 fibroblasts, S1P stimulated an increase in cellular IP3 levels, but the S1P-induced Ca2+ increase was unaffected by blocking IP3 receptors with heparin (Mattie et al. 1994). The IP3R-independent but S1P-dependent Ca2+ increase has been observed in other cell types as well (Ghosh et al. 1994; Im et al. 2005; Spiegel and Milstein 2002; Tornquist et al. 1997). In the present study, we observed S1P activation of a PLC-dependent but IP3R-independent current.
In addition to activation of receptors at the plasma membrane, S1P can also signal internally. There is good evidence that S1P can act as a Ca2+ influx factor (Borges et al. 2008; Igataki and Hauser 2003). A potentially relevant intracellular interaction has been shown between S1P and TRPC5 (Xu et al. 2006). It was demonstrated that intracellular S1P acts directly as an internal ligand for the natively expressed TRPC1-5 heteromultimers in the plasma membrane of smooth muscle cells. Furthermore, this interaction was shown to lead to channel activity and Ca2+ influx. These observations fit well with the Ca2+/calmodulin-dependence of SphK (Hla et al. 1999), and the idea that receptor-dependent Ca2+ elevations could activate SphK, and produce S1P that triggers gating of a Ca2+ influx channel. An important link was established when it was demonstrated that Ca2+ influx was eliminated in mast cells from SphK2 knockout mice (Olivera et al. 2007). Interestingly, it is emerging that it may not be the activity of SphK that is Ca2+/calmodulin sensitive but the localization. In expression systems, a Ca2+-dependent translocation of SphK1 to the plasma membrane leads to generation of S1P (Alemany et al. 2000, 2001; Sutherland et al. 2006). Although we have not yet confirmed the involvement of TRPC channels specifically, the effects of inhibiting SphK on the receptor-mediated Ca2+ elevations provides further evidence that internally generated S1P can gate a Ca2+ influx channel in amacrine cells.
Is S1P activating store-operated Ca2+ entry (SOCE) in amacrine cells? SOCE is defined as the influx of Ca2+ that is dependent on depletion of Ca2+ from internal stores (for review, see Smyth et al. 2006). A corollary of this definition is that the activation of receptors is not involved. The expression of SOCE is variable among neurons (for review, see Putney 2003), but there is strong evidence that SOCE exists in amacrine cell dendrites (Borges et al. 2008). Our current recordings are from the whole cell and our fluorescent measurements have been made from cell bodies but there are some consistencies with SOCE in the results reported here. First, we show that the S1P-dependent Ca2+ elevation typically involves release of Ca2+ from stores and that the S1P-dependent current does not precede the elevation of cytosolic Ca2+. We also show that the S1P-dependent current is not dependent on the activity of the IP3 receptor, and we demonstrate that both the S1P-dependent current and the S1P-dependent Ca2+ elevations are sensitive to μM concentrations of lanthanides. Nonetheless, our results fail a primary test of SOCE: independence from receptor activation. The inhibitory effects of PTX, the requirement for PLC activity, and the ability for SEW2871 to partially substitute for S1P all point to the involvement of a receptor (or receptors) in the mediation of the effects of S1P reported here. Perhaps the answer lies somewhere in the middle in that receptor-mediated S1P signaling might somehow co-opt the SOCE mechanism expressed by amacrine cells.
The evidence presented in this study points to S1P activation of a Ca2+-permeable cation channel at the plasma membrane. One potential function of this sort of channel is to mediate localized receptor-dependent Ca2+ entry and allow for neurotransmitter release without the involvement of voltage-gated Ca2+ channels. Indeed Chavez et al. (2006) demonstrated GABAergic feedback in amacrine cells through activation of postsynaptic ionotropic glutamate receptors and Ca2+-induced-Ca2+ release. It has also been demonstrated that release from internal stores can elicit GABA release in cultured amacrine cells (Warrier et al. 2005). It will be important to determine whether S1P, through the complex lipid signaling pathway proposed in this study, is capable of mediating synaptic signaling in the inner retina.
GRANTS
This work was supported by National Eye Institute Grant R01EY-12204 to E. Gleason.
ACKNOWLEDGMENTS
We thank S. Prasad for her assistance in preparing the manuscript and M. Tekmen and V. Krishnan for their critical reading of the manuscript. We also thank R. Broch and M. Hellberg for expert advice.
Present addresses: J. Colonna; The Institute for Neuroscience, The University of Texas, Austin, TX 78712; Dewey, Department of Biochemistry and Biophysics, Texas A & M University College Station, TX 77843.
REFERENCES
- Alemany R, Kleuser B, Ruwisch L, Danneberg K, Lass H, Hashemi R, Spiegel S, Jakobs KH, Meyer zu Heringdorf D. Depolarization induces rapid and transient formation of intracellular sphingosine-1-phosphate. FEBS Lett 509: 239–244, 2001 [DOI] [PubMed] [Google Scholar]
- Alemany R, Sichelschmidt B, Meyer zu Heringdorf D, Lass H, Van Koppen CJ, Jakobs KH. Stimulation of sphingosine-1-phosphate formation by the P2Y2 receptor in HL-60 cells: Ca2+ requirement and implication in receptor-mediated Ca2+ mobilization, but not MAP kinase activation. Mol Pharmacol 58: 491–497, 2000 [DOI] [PubMed] [Google Scholar]
- An S, Bleu T, Zheng Y. Transduction of intracellular calcium signals through g protein-mediated activation of phospholipase C by recombinant sphingosine 1-phosphate receptors. Mol Pharmacol 55: 787–794, 1999 [PubMed] [Google Scholar]
- Ancellin N, Hla T. Differential pharmacological properties and signal transduction of the sphingosine-1-phosphate receptors EDG-1, EDG-3, and EDG-5. J Biol Chem 274: 18997–19002, 1999 [DOI] [PubMed] [Google Scholar]
- Azuma T, Enoki R, Iwamuro K, Kaneko A, Koizumi A. Multiple spatiotemporal patterns of dendritic Ca2+ signals in goldfish retinal amacrine cells. Brain Res 1023: 64–73, 2004 [DOI] [PubMed] [Google Scholar]
- Borges S, Lindstrom S, Walters C, Warrier A, Wilson M. Discrete influx events refill depleted Ca2+ stores in a chick retinal neuron. J Physiol 586: 605–622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelore MR, Wright MJ, Tota LM, Milligan J, Shei G, Bergstorm JD, Mandala SM. Phytosphingosine 1-phosphate: a high affinity ligand for the S1P4/Edg-6 receptor. Biochem Biophys Res Comm 297: 600–606, 2002 [DOI] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca2+ influx through AMPA-type glutamate receptors. Nature 443: 705–708, 2006 [DOI] [PubMed] [Google Scholar]
- Crousillac S, LeRouge M, Rankin M, Gleason E. Immunolocalization of TRPC channel subunits 1 and 4 in the chicken retina. Vis Neurosci 20: 253–263, 2003 [DOI] [PubMed] [Google Scholar]
- Denk W, Detwiler PB. Optical recording of light-evoked calcium signals in the functionality intact retina. Proc Natl Acad Sci USA 96: 7035–7040, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine Kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry 37: 12892–12898, 1998 [DOI] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418: 845–852, 2002 [DOI] [PubMed] [Google Scholar]
- Formigli L, Francini F, Meacci E, Vassalli M, Nosi D, Quercioli F, Tiribilli B, Bencini C, Pipiero C, Bruni P, Orlandini SZ. Sphingosine-1-phosphate induces Ca2+ transients and cytoskeletal rearrangement in C2C12 myoblastic cells. Am J Physiol Cell Physiol 282: C1361–C1373, 2002 [DOI] [PubMed] [Google Scholar]
- Freissmuth M, Waldhoer M, Bofill-Cardona E, Nanoff C. G protein antagonists. Trends Pharmacol Sci 20: 237–244, 1999 [DOI] [PubMed] [Google Scholar]
- Ghosh TK, Bian J, Gill D. Sphingosine-1-phosphate generated in the endoplasmic reticulum membrane activates release of stored calcium. J Biol Chem 269: 22628–22635, 1994 [PubMed] [Google Scholar]
- Giussani P, Ferraretto A, Gravaghi C, Bassi R, Tettamanti G, Riboni L, Viani P. Sphingosine-1-phosphate and calcium signaling in cerebellar astrocytes and differentiated granule cells. Neurochem Res 32: 27–37, 2007 [DOI] [PubMed] [Google Scholar]
- Gleason E, Borges S, Wilson M. Synaptic transmission between pairs of retinal amacrine cells in culture. J Neurosci 13: 2359–2370, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada J, Foley M, Moskowitz MA, Waeber C. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem 88: 1026–1039, 2004 [DOI] [PubMed] [Google Scholar]
- Hla T, Lee M, Ancellin M, Liu C, Thangada S, Thompson BD, Kluk M. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem Pharmacol 58: 201–207, 1999 [DOI] [PubMed] [Google Scholar]
- Hoffpauir B, McMains E, Gleason E. Nitric oxide transiently converts synaptic inhibition to excitation in retinal amacrine cells. J Neurophysiol 95: 2866–77, 2006 [DOI] [PubMed] [Google Scholar]
- Hurtado J, Borges S, Wilson M. Na+-Ca+ exchanger controls the gain of the Ca2+ amplifier in the dendrites of amacrine cells. J Neurophysiol 88: 2765–2777, 2002 [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Hakomori S. Enzymatic synthesis of N,N-dimethyl-sphingosine: demonstration of the sphingosine: N-methyltransferase in mouse brain. Biochem Biophys Res Comm 164: 1411–1416, 1989 [DOI] [PubMed] [Google Scholar]
- Im Y, Im D, Lee Y, Lee E, Sato K, Tomura H, Katada T, Ui M, Okajima F. Study on action mode of sphingosine-1-phosphate in rat hepatocytes. J Pharmacol Sci 97: 443–446, 2005 [DOI] [PubMed] [Google Scholar]
- Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJA, Kingsbury MA, Zhang G, Brown JH, Chun J. Selective loss of sphingosine-1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its g protein coupled receptor LPB3/Edg-3. J Biol Chem 276: 33697–33704, 2001 [DOI] [PubMed] [Google Scholar]
- Ishii I, Ye X, Friedman B, Kawamura S, Contos JJA, Kingsbury MA, Yang AH, Zhang G, Brown JH, Chun J. Marked perinatal lethality and cellular signaling deficits in Mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P2/LPB2/EDG-5 and S1P3/LPB3/EDG-3. J Biol Chem 277: 25152–25159, 2002 [DOI] [PubMed] [Google Scholar]
- Itagaki K, Hauser CJ. Sphingosine-1-Phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J Biol Chem 278: 27540–27547, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, Pangalos MN, Arimura N, Kaibuchi K, Zalc B, Lubetzki C. Edg8/S1P5: an oligodentroglial receptor with dual function on process retraction and cell survival. J Neurosci 25: 1459–1469, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto T, Okada T, Yu H, Goparaju SK, Jahangeer S, Nakamura S. Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol Cell Biol 27: 3429–3440, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Takabe K, Milstein S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta 1792: 692–696, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Mimuro J, Murakami T, Kobayashi E, Hoshino Y, Yatomi Y, Sakata Y. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells 25: 115–124, 2007 [DOI] [PubMed] [Google Scholar]
- Kimura T, Watanabe T, Sato K, Kon J, Tomura H, Tamama K, Kuwabara A, Kanda T, Kobayashi I, Ohta H, Ui M, Okajima F. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem J 348: 71–76, 2000 [PMC free article] [PubMed] [Google Scholar]
- Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of g protein-coupled receptors. Biochim Biophys Acta 1582: 72–80, 2002 [DOI] [PubMed] [Google Scholar]
- Kon J, Sato K, Watanabe T, Tomura H, Kuwabara A, Kimura T, Tamama K, Ishizuka T, Murata N, Kanda T, Kobayashi I, Ohta H, Ui M, Okajima F. Comparison of intrinsic activities of the putative sphingosine-1-phosphate receptor subtypes to regulate several signaling pathways in their cDNA-transfected Chinese hamster ovary cells. J Biol Chem 274: 23940–2394, 1999 [DOI] [PubMed] [Google Scholar]
- Lee M, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99: 301–312, 1999 [DOI] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu C, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the g protein-coupled receptor EDG-1. Science 279: 1552–1555, 1998 [DOI] [PubMed] [Google Scholar]
- Lee O, Kim Y, Lee YM, Moon EJ, Lee D, Kim J, Kim K, Kwon Y. Sphingosine-1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Comm 294: 743–750, 1999 [DOI] [PubMed] [Google Scholar]
- Maceyka M, Milstien S, Spiegel S. Spinghosine 1-phosphate: the Swiss army knife of sphingolipid signaling. J Lipid Res 50: S272–S276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek RL, Toman RE, Edsall LC, Wong S, Chiu J, Letterle CA, Van Brocklyn JR, Milstein S, Spiegel S, Lee NH. Nrg-1 belongs to the endothelial differentiation gene family of g protein-coupled sphingosine-1-phosphate receptors. J Biol Chem 276: 5692–5699, 2001 [DOI] [PubMed] [Google Scholar]
- Mattie M, Brooker G, Spiegel S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol-trisphosphate-independent pathway. J Biol Chem 269, 5: 3181–3188, 1994 [PubMed] [Google Scholar]
- Medler K, Gleason EL. Mitochondrial Ca2+ buffering regulates synaptic transmission between retinal amacrine cells. J Neurophysiol 87: 1426–1439, 2002 [DOI] [PubMed] [Google Scholar]
- Meyer zu Heringdorf DM, Lass H, Alemany R, Laser KT, Neumann E, Zhang C, Schmidt M, Rauen U, Jakobs KH, van Koppen CJ. Sphingosine kinase-mediated Ca2+ signaling by g protein-coupled receptors. EMBO J 17: 2830–2837, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu Heringdorf DM, Liliom K, Schaefer M, Danneberg K, Jaggar JH, Tigyi G, Jakobs JH. Photolysis of intracellular caged sphingosine-1-phosphate causes Ca2+ mobilization independently of g protein-coupled receptors. FEBS Lett 554: 443–449, 2003a [DOI] [PubMed] [Google Scholar]
- Meyer zu Heringdorf D, Vincent MEM, Lipinski M, Danneberg K, Stropp U, Wang D, Tigyi G, Jakobs KH. Inhibition of Ca2+ signaling by the spinghosine 1-phosphate receptor S1P1. Cellr Signal 15: 677–687, 2003b [DOI] [PubMed] [Google Scholar]
- Michel MC, Wieland T, Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn-Schmied Arch Pharmacol 379: 385–388, 2009 [DOI] [PubMed] [Google Scholar]
- Miranda GE, Abrahan CE, Politi LE, Rotstein NP. Sphingosine-1-phosphate is a key regulator of proliferation and differentiation in retina photoreceptors. Invest Opthalmol Vis Sci 50: 4416–4428, 2009 [DOI] [PubMed] [Google Scholar]
- Muraki K, Imaizumi Y. A novel function of sphingosine-1-phosphate to activate a non-selective cation channel in human endothelial cells. J Physiol 537: 431–441, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novgorodov AS, El-Alwani M, Bielawski J, Obeid LM, Gudz TI. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte migration. FASEB J 21: 1503–1514, 2007 [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y, Shigematsu H, Takuwa Y. Edg-1 is a functional sphingosine-1-phosphate receptor that is linked via Gi/o to multiple signaling pathways including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem 273: 27104–27110, 1998 [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takuwa N, Yatomi Y, Shigematsue H, Takuwa Y. EDG-3 is a functional receptor specific for sphingosine-1-phosphate and sphingosylphosphorylcholine with signaling characteristics distinct from EDG-1 and AGR16. Biochem Biophys Res Comm 260: 203–208, 1999 [DOI] [PubMed] [Google Scholar]
- Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase- sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity 26: 287–297, 2007 [DOI] [PubMed] [Google Scholar]
- Olivera A, Rosenthal J, Spiegel S. Effect of acidic phospholipids on sphingosine kinase. J Cell Biochem 60: 529–537, 1996 [DOI] [PubMed] [Google Scholar]
- Paik JH, Chae S, Lee M, Thangada S, Hla T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of αvβ3- and β1- containing integrins. J Biol Chem 15: 11830–11837, 2001 [DOI] [PubMed] [Google Scholar]
- Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, Scott RH. TNF-α receptors simultaneously activates Ca2+ mobilization and stress kinases in cultured sensory neurons. Neuropharmacology 42: 93–106, 2002 [DOI] [PubMed] [Google Scholar]
- Putney JW. Capacitative calcium entry in the nerous system. Cell Calcium 34: 339–344, 2003 [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Bioinformatics Methods and Protocols: Methods in Molecular Biology, edited by Krawetz S, Misener S. Totowa, NJ: Humanna, 132: 365–386, 2000 [DOI] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biol 92: 913–922, 2004 [DOI] [PubMed] [Google Scholar]
- Sanna MG, Liao J, Jo E, Alfonso C, Ahn M, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 279: 13839–13848, 2004 [DOI] [PubMed] [Google Scholar]
- Sato K, Tomura H, Igarashi Y, Ui M, Okajima F. Possible involvement of cell surface receptors in sphingosine-1-phosphate induced activation of extracellular signal-regulated kinase in C6 glioma cells. Mol Pharmacol 55: 126–133, 1999 [DOI] [PubMed] [Google Scholar]
- Sen M, McMains E, Gleason E. Local influence of mitochondrial calcium transport in retinal amacrine cells. Vis Neurosci 24: 1–16, 2007 [DOI] [PubMed] [Google Scholar]
- Sim JA, Broomhead HE, North RA. Ectodomain lysines and suramin block of P2X1 receptors. J Biol Chem 283: 29841–29846, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest 117: 2506–2516, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, DeHaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta 1763: 1147–1160, 2006 [DOI] [PubMed] [Google Scholar]
- Sosa R, Hoffpauir B, Rankin ML, Bruch RC, Gleason EL. Metabotropic glutamate receptor 5 and calcium signaling in retinal amacrine cells. J Neurochem 81: 973–83, 2002 [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstein S. Sphingosine-1-phosphate, a key cell signaling molecule. J Biol Chem 277: 25851–25854, 2002 [DOI] [PubMed] [Google Scholar]
- Strochlic L, Dwivedy A, van Horck FPG, Falk J, Holt CE. A role for S1P signaling in axon guidance in the Xenopus visual system. Development 135: 333–342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland CM, Moretti PAB, Hewitt NM, Bagley CJ, Vadas MA, Pitson SM. The calmodulin-binding site of sphingosine kinase and its role in agonist-dependent translocation of sphingosine kinase 1 to the plasma membrane. J Biol Chem 281: 11693–11701, 2006 [DOI] [PubMed] [Google Scholar]
- Tornquist K, Saarinen P, Vainio M, Ahlstrom M. Sphingosine-1-phosphate mobilizes sequestered calcium, activates calcium entry, and stimulates deoxyribonucleic acid synthesis in thyroid FRTL-5 cells. Endocrinology 138: 4049–4057, 1997 [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Behbahani B, Lee NH. Homodimerization and heterodimerization of S1P/EDG sphingosine-1-phosphate receptors. Biochim Biophys Acta 1582: 89–93, 2002 [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJP, Thangada S, Liu CH, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate, extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulation of proliferation and survival. J Cell Biol 142: 229–240, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Coppen CJ, Meyer zu Heringdorf D, Laser KT, Zhang C, Jakobs KH. Activation of a high-affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J Biol Chem 271: 2082–2087, 1996 [DOI] [PubMed] [Google Scholar]
- Vigh J, von Gersdorff H. Prolonged reciprocal signaling via NMDA and GABA receptors at a retinal synapse. J Neurosci 25: 11412–11423, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier A, Borges S, Dalcino D, Walters C, Wilson M. Calcium from internal stores triggers GABA release from retinal amacrine cells. J Neurophysiol 94: 4196–4208, 2005 [DOI] [PubMed] [Google Scholar]
- Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine-1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the Gi, Gq, and G12 families of heterotrimeric g proteins. J Biol Chem 274: 27351–27358, 1999 [DOI] [PubMed] [Google Scholar]
- Xia P, Gamble JR, Rye K, Wang L, Hii CST, Cockerill P, Khew-Goodall Y, Bert AG, Barter PJ, Vadas MA. Tumor necrosis factor-α induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA 95: 14196–14201, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B, Shen J, Dong A, Rashid A, Stoller G, Campochiaro PA. Blockade of sphingosine-1-phosphate reduces macrophage influx and retinal and choroidal neovascularization. J Cell Physiol 218: 192–198, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S-Z, Katsuhiko M, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, Beech DJ. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res 98: 1381–1389, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Megidish T, Toyokuni T, Hakomori S, Igarashi Y. N,N-Dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry 35: 626–633, 1996 [DOI] [PubMed] [Google Scholar]
- Zhang YH, Fehrenbacher JC, Vasko MR, Nicol GD. Sphingosine-1-phosphate via activation of a G-protein-coupled receptor(s) enhances the excitability of rat sensory neurons. J Neurophysiol 96: 1042–1052, 2006a [DOI] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Intracellular sphingosine-1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J Physiol 575: 101–113, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Murthy KS. Distinctive g protein signaling in smooth muscle by sphingosine-1-phosphate receptors S1P1 and S1P2. Am J Physiol Cell Physiol 286: C1130–C1138, 2004 [DOI] [PubMed] [Google Scholar]
- Zondag GCM, Postma FR, van Etten I, Verlaan I, Moolenaar WH. Sphingosine-1-phosphate signaling through the G protein-coupled receptor Edg-1. Biochem J 330: 605–609, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]