Abstract
Wnt/β-catenin signaling plays a pivotal role in modulating cellular proliferation, differentiation, tissue organization and embryonic development. Earlier, we found that the endocytic adaptor disabled-2 (Dab2) could attenuate Wnt/β-catenin signaling by stabilizing Axin and preventing its translocation to the membrane. Recently, protein phosphatase 1 (PP1) has been shown to interact with, and dephosphorylate Axin, leading to its destabilization. Here, we show that Dab2 functions upstream of PP1 to block the interaction between Axin and PP1, inhibiting Axin dephosphorylation and thereby stabilizing its expression, ultimately leading to inhibition of Wnt/β-catenin. We show that Dab2 acts as a competitive inhibitor of PP1 by binding to the same C-terminal domain of Axin. Both PP1 and Axin bind to the N-terminus of Dab2 and a Dab2 truncation mutant consisting of the N-terminal phosphotyrosine binding domain blocks PP1–Axin interactions and inhibits Wnt signaling. We confirm the inhibitory effect of Dab2 on Wnt/β-catenin signaling in zebrafish embryos, showing that its ectopic expression phenocopies Axin overexpression resulting in altered dorsoventral patterning. We conclude that Dab2 stabilizes Axin and attenuates Wnt/β-catenin signaling by preventing PP1 from binding Axin.
Keywords: Disabled-2 (Dab2), Axin–protein phosphatase 1 (PP1), Wnt–β-catenin signaling
Wnt signaling is critical for a wide range of developmental and physiological processes, and it is implicated in human disease, including cancer (Bienz and Clevers, 2000). Stabilization and translocation of intracellular β-catenin into the nucleus are essential in ‘canonical’ Wnt signaling. β-catenin stabilization is regulated by a multimeric β-catenin destruction complex that contains, among others, dishevelled proteins (Dvl), Axin, adenomatous polyposis coli, casein kinase 1 and glycogen synthase kinase 3(GSK 3). In this complex, β-catenin is sequentially phosphorylated and earmarked for degradation by the proteasome. Wnt promotes destabilization of the β-catenin destruction complex and inhibition of β-catenin phosphorylation and degradation (van Noort et al., 2002). How β-catenin is stabilized in response to Wnt is not clear. Recent studies show that the intracellular domain of the LRP5/6 co-receptors can bind and recruit Axin to the plasma membrane (He et al., 2004). Wnt stimulation induces the sequential phosphorylation of LRP5/6 co-receptor by a ‘dual kinase’ mechanism involving GSK3and casein kinase 1 at multiple PPPSP sites (Zeng et al., 2005). This phosphorylation of LRP5/6 promotes the recruitment and engagement of LRP5/6 with Axin (Zeng et al., 2005). Dvl aids in the Wnt-induced recruitment of Axin to the membrane and to its interaction with the phosphorylated LRP5/6 co-receptor, which ultimately leads to its dephosphorylation and degradation (Cliffe et al., 2003). Recently, protein phosphatase 1 (PP1) has been shown to interact with and dephosphorylate Axin and that its dephosphorylation is associated with its destabilization (Luo et al., 2007). As the levels of Axin are decreased, the destruction complex is destabilized, and β-catenin levels accumulate. As Axin also serves as a cytoplasmic anchor for β-catenin, when Axin is absent, accumulated β-catenin is free to translocate into the nucleus and activate the transcription of Wnt-responsive genes (Jones and Bejsovec, 2003).
The (disabled-2) Dab2 is a widely expressed endocytic adaptor protein shown to be involved in several receptor-mediated signaling pathways (Prunier et al., 2004). Dab2 contains a conserved N-terminal phosphotyrosine binding (PTB) domain (Yun et al., 2003) that binds to members of the lipoprotein receptor (LDLR) family and with phosphoinositides (Morris and Cooper, 2001). Dab2 binds clathrin, the clathrin adaptor protein, AP2, and myosin VI, facilitating clathrin-coated pit assembly and receptor-mediated endocytosis (Morris and Cooper, 2001; Morris et al., 2002). The endocytic and vesicular trafficking functions of Dab2 are postulated to mediate its effects on cellular signaling (Morris et al., 2002). Earlier, we reported that Dab2 functions as a negative regulator of canonical Wnt signaling by stabilizing the β-catenin destruction complex (Hocevar et al., 2003). We showed that Dab2 associates with Axin and prevents Axin association, and subsequent degradation, with the LRP5 co-receptor, resulting in a stabilization and an increase in the half-life of Axin (Jiang et al., 2008). Here, we report that Dab2 stabilizes Axin by preventing its interaction with and subsequent dephosphorylation by PP1. The PTB domain of Dab2 is shown to bind to both Axin and PP1 in a mutually exclusive manner. In addition, we show that ectopic expression of Dab2 phenocopies Axin overexpression in altering dorsoventral patterning in zebrafish embryos.
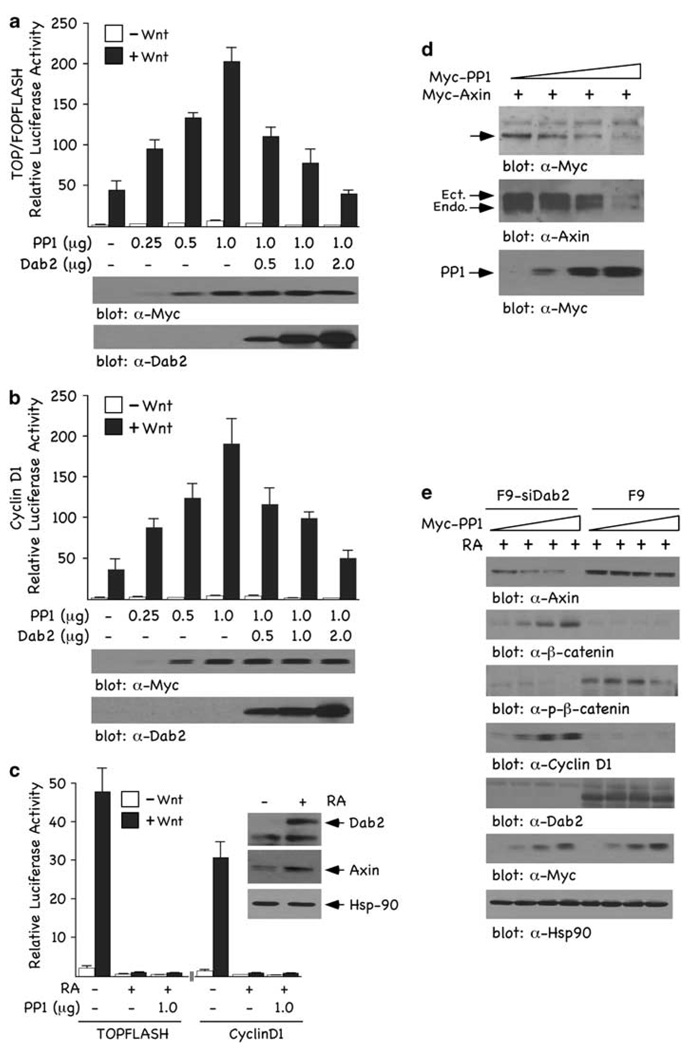
Recently, PP1 has been shown to serve as a positive regulator of Wnt/β-catenin signaling through its ability to interact with, dephosphorylate, and destabilize Axin (Luo et al., 2007). As we have shown that Dab2 stabilizes Axin expression levels, thereby inducing a more active β-catenin destruction complex (Jiang et al., 2008), we postulated that Dab2’s stabilization of Axin may be mediated through PP1. To test this, we investigated the effects of ectopically expressed Dab2 on PP1-modulated Wnt/β-catenin signaling in mouse F9 cells. Titration of increasing concentrations of PP1 (Figure 1a) resulted in a dose-dependent increase in Wnt-induced TOPFLASH (a) and cyclin D1 (b) luciferase activity, confirming the reported positive regulatory role of PP1 on Wnt/β-catenin signaling (Luo et al., 2007). We next titrated PP1 function with increasing concentrations of Dab2, and this led to the downregulation of Wnt3a-induced TOPFLASH and cyclin D1 luciferase activities (Figures 1a and b). Thus, overexpressed Dab2 can block the potentiating effects of PP1 on Wnt/β-catenin signaling. To determine whether endogenous Dab2 could also attenuate PP1-induced Wnt/β-catenin signaling, we treated F9 cells with retinoic acid (RA) for 72 h, conditions that induce visceral endoderm differentiation concomitant with significant induction of Dab2 (Smith et al., 2001). RA-induced, endogenous Dab2 is capable of attenuating the effects of PP1 on Wnt3a-induced TOPFLASH and cyclin D1 luciferase activities (Figure 1c). Inset (Figure 1c) shows that RA induces Dab2, which leads to Axin stabilization. To substantiate Dab2’s inhibitory effects on TOPFLASH and cyclin D1 luciferase, similar experiments were performed in F9 cells with stable small interfering RNA (siRNA)-mediated silencing of Dab2 (F9-siDab2 cells; Prunier and Howe, 2005). The data presented in Supplementary Figure S1a show that RA does not induce Dab2 in these cells and that Axin is not stabilized, nor is TOPFLASH or cyclin D1 luciferase activity attenuated. These data show that PP1 is a positive regulator of Wnt/β-catenin signaling, and that Dab2 can block not only Wnt3a-induced but also PP1’s upregulation of Wnt/β-catenin signaling.
Figure 1.
PP1 activates and Dab2 inhibits Wnt/β-catenin signaling. (a and b) F9 cells were co-transfected with 1 µg TOP/FOPFLASH (a) or cyclin D1 (b) luciferase reporters and increasing concentrations of PP1 and Dab2, alone or in combination as indicated. Luciferase activity was measured as described in Supplementary ‘Materials and methods’ and is expressed as the ratio of TOP/FOPFLASH and cyclin D1 activity in control and Wnt-3A-treated cells. Immunoblots depict the relative levels of Flag-tagged Dab2 and Myc-tagged PP1 after transfection of F9 cells. (c) Endogenous Dab2 inhibits Wnt/β-catenin signaling and PP1 expression is not able to rescue this attenuation. F9 cells treated (+) or non-treated (−) with RA (100 nm) for 72 h were co-transfected with TOP/FOPFLASH or cyclin D1 luciferase reporters ± 1 µg PP1. Luciferase activity was assayed and expressed as above in control and Wnt-3A-treated cells. Inset depicts the expression of Dab2 and Axin after a 72 h RA treatment of F9 cells. (d) PP1 expression attenuates Axin expression levels. F9 cells were co-transfected with Myc-tagged Axin (8 µg) and increasing concentrations of Myc-tagged PP1 (2, 4 and 6 µg) and whole cell lysates (WCLs) were analysed by immunoblotting with α-Myc and α-Axin antibodies to measure exogenously and endogenously expressed Axin. This experiment was carried out three independent times and the quantitated data are shown in Supplementary Figure S1b. (e) PP1 expression upregulates β-catenin and cyclin D1 expression levels and this upregulation is blocked by Dab2. F9 and F9-siDab2 cells were treated with RA for 72 h and subsequently transfected with increasing concentrations of PP1 (2, 4 and 8 µg). WCLs were prepared and analysed by immunoblotting using α-Axin, α-β-catenin, α-p-β-catenin and α-cyclin D1 antibodies. Hsp-90 immunoblot analysis of lysates was used as loading controls.
We next examined the effects of PP1 and Dab2 expression on Axin levels. Expression of increasing concentrations of PP1 resulted in a dose-dependent reduction in the expression of exogenous and endogenous Axin (Figure 1d and Supplementary Figure S1b). Co-transfection of increasing concentrations of PP1, in RA-treated F9-siDab2 and F9 cells, shows that Dab2 prevents PP1-mediated destabilization of Axin (Figure 1e). In the presence of Dab2, Axin levels are stabilized and are not attenuated by increased expression of PP1. Similarly, overexpressed PP1 could not attenuate Axin levels in the presence of overexpressed Dab2 in F9 cells (Supplementary Figure S1c). Examination of downstream Wnt-signaling responses shows that phospho-β-catenin levels, attenuated by increasing concentrations of PP1, are restored on RA-induced Dab2 (Figure 1e). Concomitantly, total β-catenin and cyclin D1 levels induced by increasing concentrations of PP1 are attenuated on RA-induced Dab2 (Figure 1e). Controls showing expression of RA-induced endogenous Dab2, PP1 and Hsp-90, used as a loading control, are depicted in the lower panels of Figure 1e. These immunoblot analyses confirm the transient transfection reporter assays and show that PP1-mediated destabilization of Axin and stimulation of Wnt/β-catenin signaling is blocked by Dab2 expression. In the presence of Dab2, Axin expression is stabilized and insensitive to the destabilizing effects of PP1.
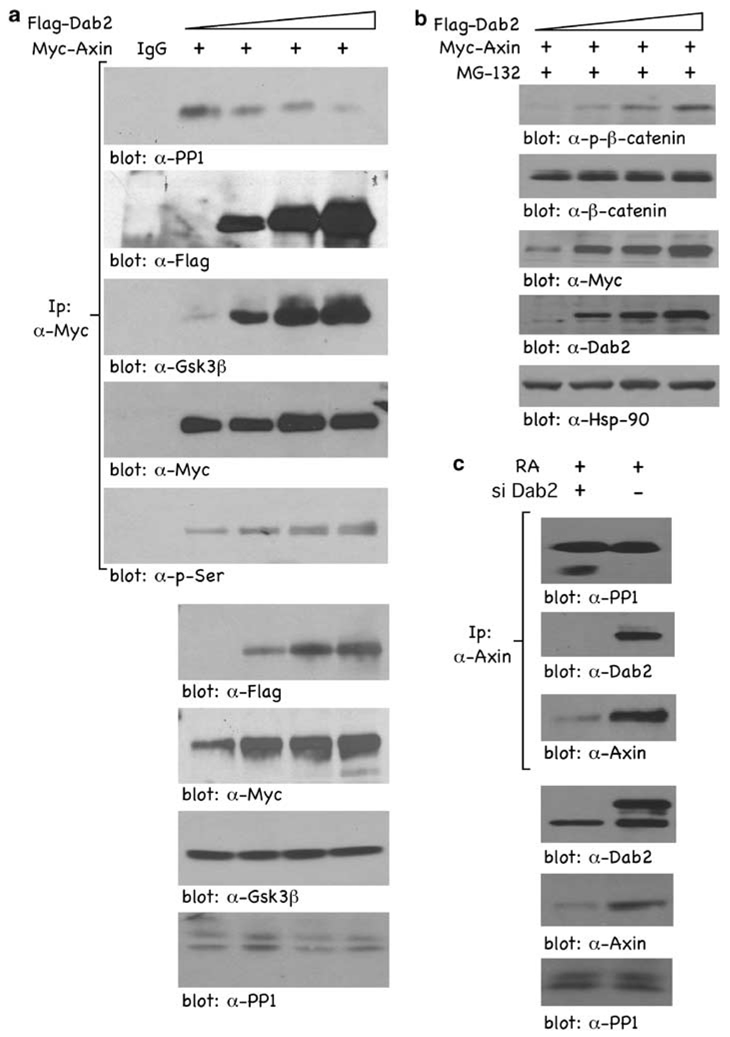
Given that PP1 interacts with and mediates dephosphorylation of Axin (Luo et al., 2007), we tested whether Dab2 could prevent PP1–Axin interactions and thus lead to Axin stabilization. F9 cells were co-transfected with Myc-tagged Axin and increasing concentrations of Flag-tagged Dab2, and α-Myc co-precipitating proteins were analysed by immunoblotting. In the absence of Dab2, endogenous PP1 is co-precipitated in an Axin immunoprecipitate (IP) showing an interaction between Axin and PP1 (Figure 2a; blot: α-PP1). However, in the presence of increasing concentrations of Dab2, there is a dose-dependent decrease in the amount of PP1 co-precipitated in an Axin IP. Dab2 is also co-precipitated in an Axin IP (Figure 2a; blot: α-Flag), showing Axin–Dab2 interactions. Further, in the presence of Dab2, the interaction between Axin and Gsk3β is increased, indicative of a more active β-catenin destruction complex (Figure 2a; blot: α-Gsk3β). Finally, in the presence of increasing concentrations of Dab2, Axin phosphorylation on Ser is significantly increased (Figure 2a; blot: α-p-Ser), suggestive of a block in the ability of PP1 to dephosphorylate Axin. Lower panels in Figure 2a show the relative expression levels of transfected Flag-tagged Dab2, Myc-tagged Axin, and endogenous Gsk3β and PP1. Under identical experimental conditions, except in the presence of MG-132 to block proteasomal degradation of β-catenin, Dab2 overexpression mediates increased phosphorylation of β-catenin, indicative of a stabilized Axin and β-catenin destruction complex (Figure 2b). RA-induced, endogenous Dab2 similarly prevents endogenous Axin–PP1 interactions and also co-precipitates in an Axin IP, indicating interaction between Dab2 and Axin (Figure 2c). Endogenous Dab2 also inhibits the amount of endogenous PP1 co-precipitating in an Axin IP (Figure 1c). These results show that Dab2 inhibits PP1–Axin interactions, preventing PP1-mediated dephosphorylation of Axin, stabilizing Axin and its association with Gsk3β, resulting in increased β-catenin phosphorylation and degradation.
Figure 2.
Dab2 expression blocks Axin–PP1 interactions. (a) F9 cells were co-transfected with Myc-tagged Axin (8 µg) and increasing concentrations of Flag-tagged Dab2 (2, 4 and 6 µg). After a 24 h tranfection, WCLs were subjected to immunoprecipitation with control IgG or α-Myc antibody. The immunocomplexes were separated by SDS–PAGE, and subjected to immunoblot analysis using α-PP1, α-Flag, α-Gsk3β and α-Myc antibodies. To blot for phosphorylated Axin, the α-Myc (Axin) blot was stripped and re-probed with α-p-Ser antibody. (b) Increasing concentrations of Flag-tagged Dab2 (2, 4 and 6 µg) and Myc-tagged Axin (5 µg) were co-transfected in F9 cells, and after a 24 h transfection, cells were treated with 30 nm MG-132 for 4 h. WCLs were collected and subjected to immunoblotting using α-p-β-catenin, α-β-catenin, α-Myc, α-Dab2 and α-Hsp90 antibodies. (c) RA-induced endogenous Dab2 blocks Axin–PP1 interactions. F9 cells and F9-siDab2 cells were treated with RA (100 nm) for 72 h, and 6 mg WCLs were prepared and immunoprecipitated with α-Axin antisera. Immunocomplexes were analysed by SDS–PAGE and subjected to immunoblot analysis using α-PP1, α-Dab2 and α-Axin antibodies.
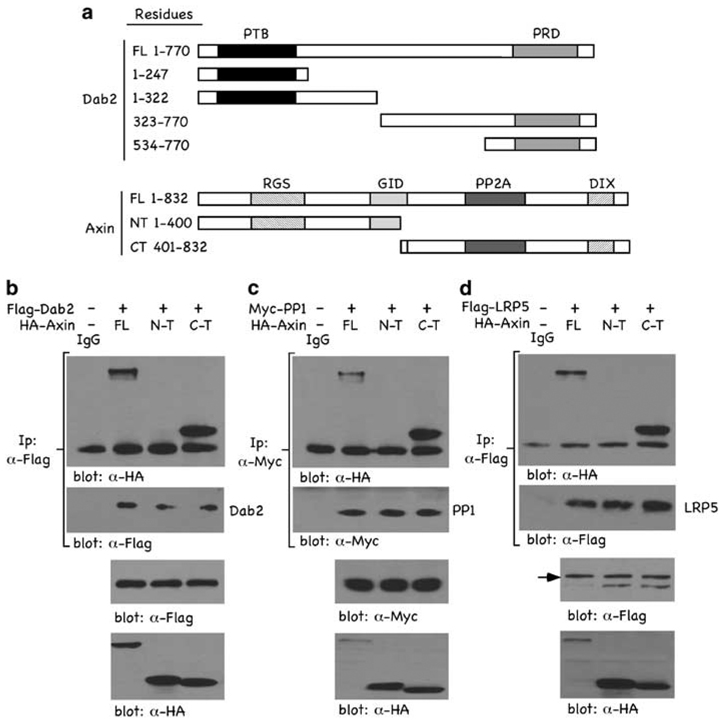
To gain insight into the mechanism through which Dab2 inhibits Axin–PP1 interactions, we assessed the contribution of individual Dab2 and Axin domains. We performed reciprocal co-immunoprecipitation (co-IP) assays with epitope-tagged versions of either full length or a series of Dab2 and Axin truncation mutants as schematically depicted in Figure 3a. The data indicate that full length and the C-terminal domain of Axin co-IP with Dab2 and PP1, whereas the N-terminal domain of Axin does not (Figures 3b and c). In addition, we found that the C-terminal of Axin is responsible for interacting with the Wnt co-receptor, LRP5 (Figure 3d). These data show that the C-terminal of Axin is responsible for binding LRP5, PP1 and Dab2 and suggests that these may compete with one another and/or physically displace each other in binding to Axin. To further delineate the domain of Dab2 required for binding to Axin and PP1, we co-transfected Myc-tagged Axin (Figure 3e) or Myc-tagged PP1 (Figure 3f) with either full-length or truncation mutants of Dab2 and performed reciprocal co-IPs. The data show that the N-terminal of Dab2, residues 1–247, encompassing the PTB domain, is required for binding to both Axin and PP1. To determine whether a functional PTB domain is necessary for Dab2–Axin interaction, increasing concentrations of a non-functional PTB domain mutant F166V (Hocevar et al., 2001) was co-transfected with Myc-Axin. The results (Figure 3h) indicate that increasing concentrations of the F166V Dab2 mutant neither upregulates Axin levels nor interacts with Axin when compared with wild-type Dab2. To establish the functional relevance of the Dab2 truncation mutants in mediating inhibition of Wnt/β-catenin signaling, we examined their effects on Wnt-mediated TOPFLASH luciferase activity (Figure 3g). Full-length Dab2 and N-terminal constructs, containing residues 1–247, were active in inhibiting TOPFLASH activity, whereas C-terminal constructs of Dab2 were without effect. The results show that the C-terminal of Axin binds to both PP1 and Dab2, and that the N-terminal, functional PTB domain of Dab2 is required for binding to Axin and PP1. Further, the PTB domain of Dab2 that binds to Axin and PP1 is functionally active in mediating inhibition of Wnt/β-catenin signaling. Coupled to the mutually exclusive binding data of Figure 2, where it is shown that Dab2 attenuates Axin–PP1 interactions, these results suggest that Dab2 and PP1 compete for binding to Axin.
Figure 3.
Mapping of interaction domains among Dab2, Axin and PP1. (a) Schematic of the various Dab2 and Axin constructs employed. Depicted are the full length (FL) and deletion constructs of Dab2 and Axin. (b–d) F9 cells were co-transfected with HA-Axin (8 µg) and either Flag-tagged Dab2 (8 µg) (b), Myc-tagged PP1 (5 µg) (c) or Flag-tagged LRP5 (8 µg) (d). After a 24 h transfection, WCLs were prepared and subjected to immunoprecipitation using α-Flag (b and d) or α-Myc (c) antibodies. Immunocomplexes were resolved by SDS–PAGE and immunoblot analysis was performed using α-HA antibody to identify co-precipitating Axin or α-Flag or α-Myc antibodies to show levels of Dab2, PP1 and LRP5 in the immunoprecipitate. (e and f) F9 cells were co-transfected with Myc-Axin (6 µg) (e) or Myc-PP1 (6 µg) (f) together with either Flag-tagged FL-Dab2 or the various Dab2 truncation mutants (8 µg) depicted in (a). α-Myc immunocomplexes were resolved by SDS–PAGE and immunoblot analysis with α-Flag antibody was performed to detect Dab2 interactions. (g) F9 cells were co-transfected with 1 µg TOP/FOPFLASH and 2 µg of the indicated Dab2 constructs. The pRK5 vector (backbone for the Dab2 plasmids) was used as control. Luciferase activity was measured as described above and is expressed as the ratio of TOP/FOPFLASH activity in control and Wnt-3A-treated cells. The lower panel shows the relative expression levels of the various Dab2 constructs after transfection in F9 cells. (h) F9 cells were transfected with Flag-tagged wild-type Dab2 or increasing concentrations of Flag-tagged F166V Dab2 mutant as indicated. WCLs were subjected to immunoprecipitation with control IgG or α-Flag antibody. The immunocomplexes were separated by SDS–PAGE and subjected to immunoblot analysis using α-Myc.
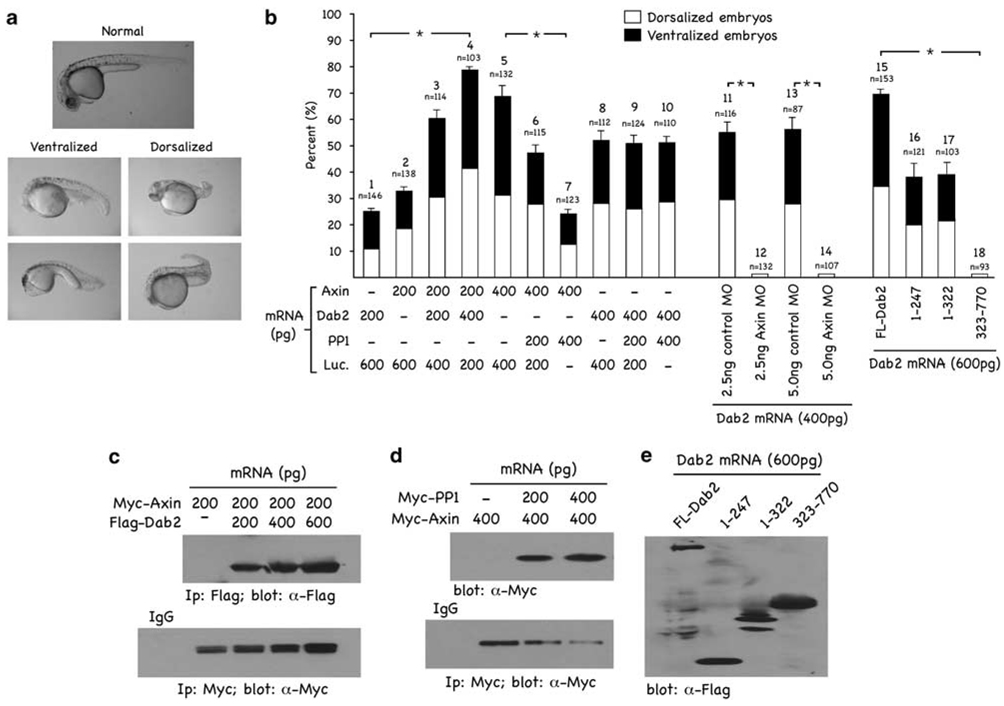
Earlier studies have shown that Axin plays an important role in the establishment of the dorsoventral axis during zebrafish embryogenesis (Rui et al., 2007). As our data suggest that Dab2 expression regulates Axin levels, we investigated the effect of ectopic expression of Dab2 on embryonic development in zebrafish embryos. We injected Dab2 mRNA (600 pg) into one-cell stage embryos, and 24-h post-fertilization (hpf) we observed two different populations of abnormal embryos. Shown in Figure 4a are the lateral views of two representatives, live embryos exhibiting either a ventralized or dorsalized phenotype. The dorsalized phenotype exhibits a shortened tail and lack of the ventral tail fin. The ventralized phenotype lacks a head and notochord and has a large blood island compared with the normal phenotype. These two phenotypes have earlier been observed after Axin mRNA microinjection (Rui et al., 2007). Thus, ectopic expression of Dab2 seems to phenocopy Axin in modulation of dorsoventral patterning. To further characterize Dab2’s dorsoventral patterning activities, we injected Dab2, Axin and PP1 mRNAs singly or in various combinations and determined phenotypic effects 24 hpf. (Luciferase mRNA was used as control for equal (800 pg total) mRNA injection.) The results (Figure 4b) show that both injected Axin and Dab2 mRNA induced altered phenotypes. A dose of 200 pg/embryo of injected Dab2, induced an altered phenotype in ~25.4% of the embryos (n = 146), with ~14.4% being ventralized and ~11% being dorsalized (column 1). At a dose of 200 pg/embryo, injected Axin mRNA induced an altered phenotype in 32.6% of the injected embryos (n = 138), with ~17.4% of these exhibiting a dorsalized and ~15.2% exhibiting a ventralized phenotype, respectively (column 2). At a dose of 400 pg/embryo, injected Axin mRNA induced an altered phenotype in 68% of the injected embryos (n = 132), with ~31.8% of these exhibiting a dorsalized and ~37.8% exhibiting a ventralized phenotype, respectively (column 5). A dose of 400 pg/embryo of injected Dab2 induced an altered phenotype in ~56.3% of the embryos (n = 112), with ~28.6% being ventralized and ~27.7% being dorsalized (column 8). No phenotypic alterations were observed with injected PP1 mRNA (200 and 400 pg/embryo; data not shown). When mRNAs were co-injected, we observed that Dab2 could potentiate in a dose-dependent manner the phenotypic effects of low doses of injected Axin mRNA (columns 2, 3 and 4). Further, although PP1 mRNA was without effect when injected alone, it was capable of attenuating, in a dose-dependent manner, the Axin phenotype (columns 5, 6 and 7). When co-injected with Dab2, however, PP1 could not reverse the phenotype induced by Dab2 mRNA injection (columns 8, 9 and 10). We also injected Dab2 mRNA together with the standard control and Axin morphilinos (columns 11–14) and observed that Dab2’s phenotypic effects are neutralized by Axin, but not control, morpholinos. These data show that Dab2 requires Axin function to induce effects on dorsoventral patterning. Finally, the relative dorsoventral patterning activities of the various Dab2 truncation mutants (columns 15–18) were examined. Both N-terminal constructs, containing the PTB domain of Dab2, induce an abnormal phenotype in ~38% of the injected embryos (columns 16 and 17) compared with full-length Dab2 mRNA (column J), which elicits an altered phenotype in ~68.5% of the injected embryos at 600 pg/embryo. The C-terminal domain of Dab2 does not induce an abnormal phenotype when injected (column 18). Thus, in this in vivo assay, the PTB domain of Dab2 is only ~50% as effective as full-length Dab2.
Figure 4.
Dab2 alters dorsoventral patterning in zebrafish embryos. (a) Dab2 mRNA (600 pg) was injected into one-cell embryos. Shown are the lateral views of two representative, live embryos at 24 hpf displaying either a ventralized or dorsalized phenotype. (b) Dab2, Axin, PP1 or luciferase (control) mRNAs were injected, into one-cell stage embryos, alone or in various combinations and at the doses indicated. Live embryos were collected 24 hpf and observed under a microscope. The percentage of embryos displaying an altered phenotype was quantitated based on the total number of injected embryos (n) and these were further categorized based on displaying either a dorsalized or ventralized phenotype. Data are presented as mean ± s.d. and show significant alterations in dorsalventral phenotypes (* significant) across injection groups. Pairwise comparison was performed by t-test, multiple comparisons by ANOVA using SigmaStat 3.5 software. The differences among injections 1, 2, 3 and 4 are significant, P < 0.01. The differences among 5, 6 and 7 are significant, P < 0.01. The differences among 8, 9 and 10 are not significant, P > 0.05. The difference between 11 and 12 is significant, P < 0.001, so is the difference between 13 and 14. The differences among 15, 16/17 and 18 are significant, P < 0.01. (c–e) Expression of injected mRNAs in embryos. (c) Myc-tagged Axin mRNA (200 pg) and increasing concentrations of Flag-tagged Dab2 were co-injected into one-cell embryos. After a 10 h incubation, embryos lysates were processed for immunoprecipitations using α-Flag and α-Myc antibodies. The immunocomplexes were analysed by SDS–PAGE and immunoblot analysis to detect ectopic Dab2 (α-Flag) and Axin (α-Myc) protein expression levels. (d) Myc-tagged Axin mRNA (400 pg) and increasing concentrations of Myc-tagged PP1 were co-injected into one-cell embryos. Embryos were processed and analysed as in (c) to detect ectopically expressed PP1 (α-Myc) and Axin (α-Myc). (e) FL-Flag-tagged or truncated Dab2 deletion constructs were injected into one-cell embryos. Embryos were processed and analysed as in (c and d) to detect ectopically expressed FL-Dab2 and its indicated truncation mutants (α-Flag).
The data presented in Figure 4c–e show the protein levels of the various injected mRNAs prepared from embryo lysates subjected to immunoprecipitation and immunoblotting analyses. As predicted, ectopic expression of Dab2 enhanced ectopic Axin expression levels (Figure 4c), whereas overexpression of PP1 results in significant downregulation of Axin (Figure 4d). Comparable expression levels of full-length Dab2 and its truncation mutants are also shown (Figure 4e). In summary, these data show that Dab2 alters dorsoventral patterning during zebrafish development, resulting in a morphology that is indistinguishable from that mediated by ectopic expression of Axin. Although injection of PP1 mRNA alone did not have any effect, it attenuated the Axin-induced, but not the Dab2-induced, phenotype, suggesting that PP1 function is required downstream of Axin but not of Dab2. The data also show that the functional PTB domain of Dab2, responsible for binding Axin and PP1 (Figure 3), is only ~50% as effective in mediating the observed effects of full-length Dab2. This suggests that full-length Dab2, similarly to Axin, blocks other Wnt-induced dorsoventral patterning pathways, and that its PTB domain is effective in inhibiting solely the Wnt/β-catenin branch.
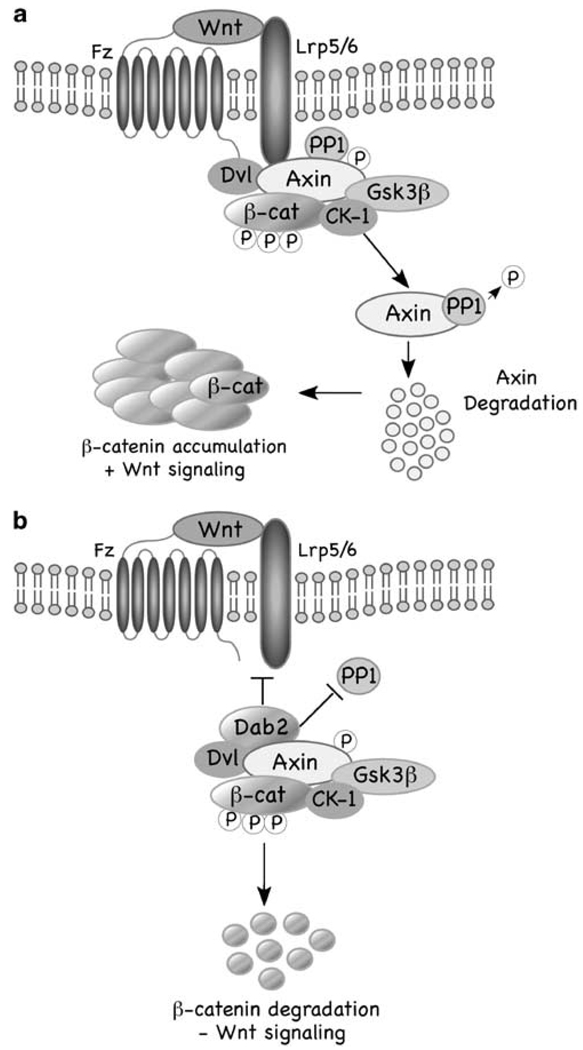
In summary, our results suggest a model (Figure 5) whereby in the absence of elevated levels of Dab2 (a), the β-catenin destruction complex is recruited to the Fz/LRP5/6 receptor complex, which promotes Axin–PP1 interactions, leading to the dephosphorylation and destabilization of Axin, disassembly of the complex, resulting in increased β-catenin and Wnt signaling. In the presence of Dab2 (b), Dab2 prevents recruitment of the β-catenin destruction complex to the receptors, Axin–PP1 interactions do not occur, thereby Axin levels are maintained, and β-catenin is degraded and Wnt signaling is attenuated. How and where in the cell Dab2 blocks Axin–PP1 interactions is not clear. Recently, it has been shown that Wnt induces co-clustering of receptors and Dvl in ‘LRP6 signalosomes’ promoting LRP6 phosphorylation and recruitment of Axin leading to β-catenin stabilization (Bilic et al., 2007). It has also been suggested that this clustering occurs in caveolin-containing vesicles and that endocytosis through these lipid rafts ultimately leads to Axin destabilization and β-catenin accumulation (Yamamoto et al., 2008). Interestingly, clathrin-mediated endocytosis of the ‘LRP6 signalosome’ was shown not to lead to β-catenin accumulation and induction of Wnt signaling (Yamamoto et al., 2008). As Dab2 has been shown earlier to bind to the clathrin adaptor AP2, as has Dvl2 (Yu et al., 2007), and be involved in clathrin-mediated endocytosis (Morris and Cooper, 2001), it is tempting to speculate that Dab2 may recruit the ‘LRP6 signalosome’ to this endocytic route to attenuate Wnt-mediated signaling.
Figure 5.
Model of how Dab2 regulates Wnt/β-catenin signaling. (a) In the absence of Dab2, Axin is recruited to the LRP5/6 co-receptor, which is facilitated by dishevelled (Dvl), resulting in Axin dephosphorylation by protein phosphatase 1 (PP1) and, ultimately its degradation. Axin degradation leads to disassociation of the β-catenin destruction complex, an increase in β-catenin levels and in Wnt target gene transcription. (b) In the presence of Dab2, Axin recruitment to the LRP5–6 co-receptor is inhibited, Axin–PP1 interactions do not occur, leading to Axin and β-catenin destruction complex stabilization, and attenuated Wnt signaling.
Supplementary Material
Acknowledgements
We thank Dr S Takada for the generous provision of control Wnt-3A-producing mouse L cells and Drs Furuhashi, Wu, Zeng and He for generous provision of the Myc-Axin, Flag-tagged LRP5 plasmids and LRP5/6 antibody, respectively. We also thank Dr David Virshup and Dr Sheng-Cai Lin for generous provision of PP1 and Axin reagents. We are also grateful to Drs Sanjay Pimplikar and Ping Song for all their help with the zebrafish studies. This work was supported by Grant CA55536 and CA80095 from the National Cancer Institute to PHH.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M. A role of dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol. 2003;13:960–966. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Smine A, Xu XX, Howe PH. The adaptor molecule Disabled-2 links the transforming growth factor beta receptors to the Smad pathway. EMBO J. 2001;20:2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Mou F, Rennolds JL, Morris SM, Cooper JA, Howe PH. Regulation of the Wnt signaling pathway by Disabled-2 (Dab2) EMBO J. 2003;22:3084–3094. doi: 10.1093/emboj/cdg286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Jones WM, Bejsovec A. Wingless signaling: an axin to grind. Curr Biol. 2003;13:R479–R481. doi: 10.1016/s0960-9822(03)00407-x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Prunier C, Howe PH. The inhibitory effects of Disabled-2 (Dab2) on Wnt signaling are mediated through Axin. Oncogene. 2008;27:1865–1875. doi: 10.1038/sj.onc.1210829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, et al. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. EMBO J. 2007;26:1511–1521. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM, Cooper JA. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2001;2:111–123. doi: 10.1034/j.1600-0854.2001.020206.x. [DOI] [PubMed] [Google Scholar]
- Morris SM, Arden SD, Roberts RC, Kendrick-Jones J, Cooper JA, Luzio JP, et al. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic. 2002;3:331–341. doi: 10.1034/j.1600-0854.2002.30503.x. [DOI] [PubMed] [Google Scholar]
- Prunier C, Hocevar BA, Howe PH. Wnt signaling: physiology and pathology. Growth Factors. 2004;22:141–150. doi: 10.1080/08977190410001720860. [DOI] [PubMed] [Google Scholar]
- Prunier C, Howe PH. Disabled-2 (Dab2) is required for TGFβ-induced epithelial to mesenchymal transition (EMT) J. Biol Chem. 2005;280:17540–17548. doi: 10.1074/jbc.M500974200. [DOI] [PubMed] [Google Scholar]
- Rui Y, Xu Z, Xiong B, Cao Y, Lin S, Zhang M, et al. A beta-catenin-independent dorsalization pathway activated by Axin/JNK signaling and antagonized by aida. Dev Cell. 2007;13:268–282. doi: 10.1016/j.devcel.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Smith ER, Capo-chichi CD, He J, Smedberg JL, Yang DH, Prowse AH, et al. Disabled-2 mediates c-Fos suppression and the cell growth regulatory activity of retinoic acid in embryonic carcinoma cells. J Biol Chem. 2001;276:47303–47310. doi: 10.1074/jbc.M106158200. [DOI] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev Cell. 2008;15:37–48. doi: 10.1016/j.devcel.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Yun M, Keshvara L, Park CG, Zhang YM, Dickerson JB, Zheng J, et al. Crystal structures of the Dab homology domains of mouse disabled 1 and 2. J Biol Chem. 2003;278:36572–36581. doi: 10.1074/jbc.M304384200. [DOI] [PubMed] [Google Scholar]
- Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, Kirchhausen T. Association of Dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev Cell. 2007;12:129–141. doi: 10.1016/j.devcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.