Abstract
Objective
The anticipation of adverse outcomes, or worry, is a cardinal symptom of generalized anxiety disorder. Prior work with healthy subjects has shown that anticipating aversive events recruits a network of brain regions, including the amygdala and anterior cingulate cortex. This study tested whether patients with generalized anxiety disorder have alterations in anticipatory amygdala function and whether anticipatory activity in the anterior cingulate cortex predicts treatment response.
Method
Functional magnetic resonance imaging (fMRI) was employed with 14 generalized anxiety disorder patients and 12 healthy comparison subjects matched for age, sex, and education. The event-related fMRI paradigm was composed of one warning cue that preceded aversive pictures and a second cue that preceded neutral pictures. Following the fMRI session, patients received 8 weeks of treatment with extended-release venlafaxine.
Results
Patients with generalized anxiety disorder showed greater anticipatory activity than healthy comparison subjects in the bilateral dorsal amygdala preceding both aversive and neutral pictures. Building on prior reports of pretreatment anterior cingulate cortex activity predicting treatment response, anticipatory activity in that area was associated with clinical outcome 8 weeks later following treatment with venlafaxine. Higher levels of pretreatment anterior cingulate cortex activity in anticipation of both aversive and neutral pictures were associated with greater reductions in anxiety and worry symptoms.
Conclusions
These findings of heightened and indiscriminate amygdala responses to anticipatory signals in generalized anxiety disorder and of anterior cingulate cortex associations with treatment response provide neurobiological support for the role of anticipatory processes in the pathophysiology of generalized anxiety disorder.
Generalized anxiety disorder affects 5.7% of the English-speaking population in the United States (1) and can have dramatic effects on one’s social relations, occupational functioning, and well-being. Worry is a cardinal feature of generalized anxiety disorder and is often observed in other anxiety and mood disorders, which may in part explain the high rates of other illnesses that are comorbid with generalized anxiety disorder (2). Despite the high prevalence and frequent comorbidity of generalized anxiety disorder and the suffering it causes, its pathophysiology has been relatively understudied in comparison to other anxiety disorders (3, 4).
A theoretically sound starting place for investigating the pathophysiology of generalized anxiety disorder is to focus on worry and the anticipation of negative outcomes (4–8). Research on the neural circuitry of the anticipation of aversive stimuli has implicated a number of brain regions, including the amygdala, insula, anterior cingulate cortex, and prefrontal cortex (8–14). To facilitate work in this area, we developed a paradigm for investigating brain activity associated with the anticipation of aversive pictures (8, 14, 15). The paradigm involves one warning cue (e.g., a minus sign) that is followed by aversive pictures and a second warning cue (e.g., a circle) that is followed by neutral pictures. Subjects are instructed about the cue-picture pairings at the outset of the experiment. Anticipatory responses in this paradigm have been characterized in nonpsychiatric populations using both eye-blink startle (15) and functional magnetic resonance imaging (fMRI) (8, 14).
We hypothesized that patients with generalized anxiety disorder would show abnormalities in the circuitry normatively activated by the anticipation of aversion (8, 10, 16), particularly in the amygdala (8, 11, 14, 17–19). Findings for amygdala function in generalized anxiety disorder have been mixed (20–23), but no study with patients with generalized anxiety disorder has disentangled stimulus anticipation and stimulus response processes (8, 14). Based on the research reviewed above and related cognitive findings (4), this study was designed to test the prediction that amygdala activation in anticipation of aversive pictures would be greater in patients with generalized anxiety disorder than nonpsychiatric comparison subjects, whereas no group differences were expected in anticipation of neutral pictures. Theoretical and empirical work implicating hyperresponsivity to both unpleasant and neutral stimuli in patients with generalized anxiety disorder (7, 21, 24) suggested the testable alternative hypothesis that patients with generalized anxiety disorder would show greater anticipatory amygdala activation than comparison subjects preceding both aversive and neutral pictures. Group differences were also examined in other brain regions implicated in the anticipation of aversion (8, 10, 12, 14, 16, 25). Finally, based on prior reports of anterior cingulate cortex activity predicting treatment response (26–29), we tested whether pretreatment anticipatory activity in the anterior cingulate cortex was associated with outcome following an 8-week trial of venlafaxine.
Method
Subjects
The 26 subjects participating in this study were recruited through newspaper and e-mail advertisements. All subjects were right-handed (Edinburgh Handedness Inventory). The 14 adult subjects (two men) with generalized anxiety disorder met DSM-IV criteria for generalized anxiety disorder and no other current disorder, as determined by the Structured Clinical Interview for DSM-IV (SCID) and verified by physician interview. Multiple individuals meeting criteria for generalized anxiety disorder were excluded from participation because of axis I comorbidity: two major depressive disorder, two dysthymia, two bipolar disorder, three obsessive-compulsive disorder, one social anxiety disorder, one panic disorder, and one posttraumatic stress disorder. Matched for sex, age, and education (Table 1), the 12 healthy comparison subjects (two men) reported no history of psychopathology according to SCID. In addition, patients with generalized anxiety disorder were required to score at least 18 on the Hamilton Rating Scale for Anxiety (HAM-A) (30) with scores of 2 or more on item 1 (anxious mood) and item 2 (tension).
TABLE 1.
Individual Subject Scores for All Administrations of the Hamilton Anxiety and Depression Scalesa
| Hamilton Rating Scale for Anxiety | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Sex | Age | Educationb | Initial Screen |
fMRI Session | Week 1 | Week 2 | Week 4 | Week 6 | Week 8 | |
| Patients | |||||||||||
| 1 | Female | 56 | 3 | 19 | 19 | 13 | 11 | 6 | 6 | 5 | |
| 2 | Female | 23 | 1 | 20 | 19 | 15 | 14 | 7 | 12 | 9 | |
| 3 | Female | 29 | 4 | 18 | 21 | 13 | 12 | 8 | 9 | 12 | |
| 4 | Male | 31 | 3 | 23 | 19 | 16 | 12 | 5 | 7 | 4 | |
| 5 | Female | 30 | 3 | 18 | 19 | 17 | 13 | 14 | 10 | 9 | |
| 6 | Female | 28 | 1 | 18 | 19 | 14 | 8 | 7 | 3 | 5 | |
| 7 | Female | 30 | 4 | 22 | 18 | 13 | 8 | 9 | 8 | 13 | |
| 8 | Female | 31 | 4 | 20 | 19 | 15 | 12 | 9 | 8 | 11 | |
| 9 | Female | 31 | 4 | 21 | 18 | 12 | 12 | 3 | 5 | 5 | |
| 10 | Female | 27 | 1 | 18 | 19 | 11 | 6 | 6 | 2 | 2 | |
| 11 | Female | 41 | 2 | 18 | 16 | 13 | 16 | 13 | 12 | — | |
| 12 | Female | 38 | 4 | 19 | 20 | 18 | 13 | 11 | 6 | 11 | |
| 13 | Male | 54 | 5 | 18 | 19 | 12 | 8 | 9 | 7 | 2 | |
| 14 | Female | 23 | 1 | 21 | 22 | 8 | 5 | 6 | 5 | 3 | |
| Mean | 33.7 | 2.7 | 19.5 | 19.1 | 13.9 | 10.8 | 7.4 | 7.0 | 7.6 | ||
| SD | 10.2 | 1.4 | 1.7 | 1.4 | 1.9 | 2.6 | 3.0 | 3.1 | 3.8 | ||
| Comparison Subjects | |||||||||||
| 1 | Female | 25 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| 2 | Female | 31 | 4 | 0 | 1 | 2 | 5 | 4 | 4 | 3 | |
| 3 | Female | 29 | 4 | 0 | 1 | 2 | 5 | 4 | 0 | 3 | |
| 4 | Male | 34 | 4 | 4 | 1 | 3 | 7 | 6 | 6 | 5 | |
| 5 | Female | 29 | 5 | 2 | 1 | 1 | 2 | 0 | 0 | 3 | |
| 6 | Female | 28 | 5 | 2 | 1 | 4 | 4 | 0 | 0 | 2 | |
| 7 | Female | 27 | 3 | 2 | 3 | 3 | 2 | 6 | 6 | 3 | |
| 8 | Female | 28 | 4 | 1 | 2 | 0 | 0 | 2 | 0 | 0 | |
| 9 | Female | 35 | 3 | 5 | 3 | 1 | 3 | 6 | 6 | 4 | |
| 10 | Female | 53 | 3 | 1 | 2 | 1 | 3 | 2 | 2 | 0 | |
| 11 | Male | 55 | 1 | 0 | 1 | — | 0 | — | — | 3 | |
| 12 | Female | 23 | 1 | 1 | 1 | — | 0 | — | — | 1 | |
| Mean | 33.1 | 3.0 | 1.5 | 1.4 | 1.8 | 3.1 | 3 | 2.4 | 2.3 | ||
| SD | 10.3 | 1.5 | 1.6 | 0.9 | 1.2 | 2.2 | 2.5 | 2.8 | 1.8 | ||
During the last phase of data collection, an attempt was made to decrease the burden on subjects by not administering one or both of these instruments at some of the time points. This accounts for most of the missing data in the last 4 patients with generalized anxiety disorder and the last two comparison subjects above. Week 8 data was only missing from one subject, who did not complete the study due to medication side effects.
Education=education level (1: part college; 2: graduated 2-year college; 3: graduated 4-year college; 4: part professional/graduate school; 5: graduated professional/graduate school).
Efforts were made to minimize the presence of depression in this patient sample by excluding subjects with a major depressive episode in the previous 6 months or with scores above 6 on the Raskin Depression Scale (31). The presence of past depression preceding the 6 months before study entry was assessed in 10 of the 14 patients: five reported no history of depression, three reported a single past major depressive episode, and two reported a subthreshold single past major depressive episode. Healthy comparison subjects scored below 6 on the Hamilton Rating Scale for Anxiety (HAM-A), the Hamilton Rating Scale for Depression (HAM-D) (32), and the Raskin Depression Scale. Subjects were excluded if they had a history of seizures, diabetes, or heart problems. Other exclusion criteria included active neoplastic disease, cancer in the prior 3 years, family history of bipolar disorder, ECT in the prior 3 months, use of any prescription medications or herbal remedies to treat psychiatric symptoms within 14 days of the fMRI session, failure of adequate trial of extended release venlafaxine during the current episode of generalized anxiety disorder, and the failure of two adequate trials of antianxiety or antidepressant treatments.
Subjects with generalized anxiety disorder began an 8-week open-label trial of extended-release venlafaxine immediately following the fMRI session (dosage started at 37.5 mg in the morning as needed and increased to a maximum of 225 mg as needed in the morning per clinical indication). Both Hamilton scales were administered at the initial screening session with the SCID, at the fMRI session that occurred on average 18 days later, and at clinic visits that were weekly for the first 2 weeks of treatment and then biweekly for the remaining 6 weeks of the medication trial (Table 1). One patient withdrew from the study 6 weeks after the start of treatment (due to medication side effects) and therefore was not included in analyses examining treatment response following the 8-week medication trial. Ten of the patients with generalized anxiety disorder and 10 of the healthy comparison subjects in the present study were also included in our recent study on facial expressions (29) because data collection for the two studies overlapped (data collection for that study began and finished earlier than for the present study). After complete description of the study to the subjects, written informed consent was obtained, in accordance with study approval by the Human Subjects Committee of the University of Wisconsin School of Medicine and Public Health. All subjects were paid for their participation.
Experimental Paradigm
Each 19-second trial began with a 0.5-second cue followed by a 2.5-second or 4.5-second black screen (pseudo-randomized within valence) and then a 1.0-second picture followed by a 13-second or 15-second black screen (Figure 1 in data supplement available at http://ajp/psychiatryonline.org). For aversive trials, the cue was a minus sign that was followed by an aversive picture (e.g., mutilated bodies, attack scenes). For neutral trials, the cue was a circle that was followed by a neutral picture (e.g., household items). Subjects were instructed about this cue-picture pairing prior to scanning. This trial structure was selected in an attempt to optimize methodological parameters for effectively distinguishing anticipation and picture reactivity periods while keeping subjects engaged (8, 33). Trial order was pseudo-randomized, with the stipulation that neither aversive nor neutral trials were presented more than twice in a row. There were a total of four functional scan runs, each consisting of 12 aversive trials and 12 neutral trials. Subjects performed a task on each trial, pressing one button if there was a match between the cue and picture (a minus sign followed by aversive picture or circle followed by neutral picture) and a second button if there was an obvious mismatch (minus sign followed by pleasant picture or circle followed by aversive picture). This task was employed to help maintain the subjects’ attention to the cue and picture stimuli, which was verified by near-perfect accuracy rates. There were only two mismatch trials in each scan run, and they were excluded from analyses.
Data Acquisition and Analysis
Anatomical and functional data were collected on a General Electric 3.0 Tesla system (Waukesha, Wis.) with a quadrature head coil. Functional images consisted of 30 sagittal echo-planar imaging slices covering the whole brain (slice thickness/gap=4/1 mm; 64×64 in-plane resolution; 240-mm field of vision; TR/TE= 2000/30 msec; flip angle=90°). These data acquisition parameters were selected in part to minimize signal loss in the amygdala and orbitofrontal cortex, areas vulnerable to the differential magnetic susceptibility coefficients of bone/air/tissue boundaries (see data supplement available at http://ajp/psychiatryonline.org). Whole-brain T1-weighted anatomical images were also acquired (three-dimensional spoiled gradient-recalled echo; 256×192 in-plane resolution; 240-mm field of view; 124 1.2-mm axial slices). An Avotec Silent Vision system (Jensen Beach, Fla.) displayed the stimuli by means of a pair of stereoscopic goggles. Head movement was restricted using a customized bite bar, which consisted of dental impression compound affixed to an acrylic plate (see data supplement Methods). Data for two additional fMRI sessions following the start of treatment and for a facial expression paradigm using a different imaging protocol that did not involve whole-brain fMRI acquisition have or will be reported in separate manuscripts that address different theoretical questions (29).
All fMRI data processing was done with AFNI, version 2.41. Data processing included slice-time correction, motion correction, and application of a high-pass temporal Fourier filter (0.0143 Hz). The time series was modeled with a least-squares general linear model (GLM) fit to an ideal hemodynamic response function for the anticipation period and picture period separately, and the resultant beta-weights were converted to percentage signal change. Unlike the area under the curve, these beta-weights are not influenced by baseline differences at the start of an epoch. During the GLM fit, the time-to-onset of response was allowed to vary independently for each voxel from 0 to 4 seconds, and the time lag selected was used for both periods. This variable onset allowed for sensitivity to the varying blood perfusion rates across the brain, while fixing the time lag as the same for both the anticipation and picture periods ensured that the two responses are properly separated and estimated (8, 13). Although the independence of anticipatory activity and picture response activity is difficult to ensure in our paradigm owing to the short interstimulus interval relative to hemodynamic lag (13), the employed statistical modeling procedures minimized the possibility of variance due to the picture being misattributed to the anticipation period and vice versa. Moreover, inspection of the time series plots corroborated the effects reported here. The percentage signal change maps from the GLM were Gaussian-blurred (full width at half maximum=4 mm), resampled to 1 mm3 voxels, and transformed into standardized space using the Talairach atlas.
To examine hypothesized group differences during the anticipation of aversive pictures, we conducted a voxelwise group (patients, comparison subjects) by period (anticipation, picture) by valence (aversive, neutral) repeated-measures analysis of variance (ANOVA). Since the amygdala was the primary focus of study hypotheses, we applied a small volume correction for multiple comparisons using Monte Carlo simulations, as implemented by AlphaSim in AFNI. These simulations were run to correct for multiple comparisons within a region of interest defined by the anatomical boundaries of the amygdala (8, 14). The spatial correlation of the input data and an uncorrected p value threshold of 0.005 resulted in a minimum cluster size of 66 mm3 to achieve a corrected p<0.05. Specificity of group differences to the amygdala were assessed by examining other primary regions—insula, anterior cingulate cortex, prefrontal cortex, and hippocampus—identified in our earlier reports and elsewhere (8, 10, 12, 14, 16, 25) in the voxelwise ANOVA above.
To assess whether pretreatment anticipatory activity was associated with treatment response following the 8-week open-label trial of venlafaxine, voxelwise regressions were implemented by regressing pretreatment anticipatory activation on scores on the HAM-A, HAM-D, and Penn State Worry Questionnaire (34). In addition to conducting regressions for each instrument independently, we were particularly interested in examining the relationship of pretreatment brain activity to the improvement of anxiety and worry symptoms in the patients with generalized anxiety disorder. This was implemented by calculating residualized HAM-A and Penn State Worry Questionnaire scores for each subject by partialing out depression as measured by the HAM-D, consistent with our earlier work carefully measuring and accounting for co-occurring anxiety and depression symptoms (4, 6, 15, 35). Intercorrelations among these clinical measures are provided in the data supplement Table 1. Treatment response was operationalized by calculating a second residualized score for each subject by partialing pretreatment scores from the posttreatment scores obtained at the end of the 8-week medication trial. Based on prior studies implicating pregenual anterior cingulate cortex in predicting treatment response (26, 27), applying the aforementioned procedure to correct for multiple comparisons resulted in a minimum cluster size of 126 mm3 for the anterior cortex anterior to the genu of the corpus collosum at y=29, well within the boundaries of the pregenual anterior cingulate cortex (36). For clusters meeting the corrected p value criterion identified in the voxelwise ANOVAs and regressions above, post hoc analyses of the extracted image data were conducted in SPSS 14.0 (Chicago). All reported statistics are two-tailed.
| Hamilton Rating Scale for Depression | ||||||
|---|---|---|---|---|---|---|
| Initial Screen |
fMRI Session | Week 1 | Week 2 | Week 4 | Week 6 | Week 8 |
| 13 | 14 | 12 | 9 | 6 | 5 | 4 |
| 12 | 12 | 14 | 13 | 7 | 9 | 7 |
| 15 | 15 | 12 | 11 | 8 | 9 | 6 |
| 16 | 12 | 11 | 9 | 3 | 5 | 2 |
| 10 | 9 | 10 | 9 | 9 | 9 | 6 |
| 10 | 9 | 7 | 4 | 5 | 1 | 1 |
| 11 | 6 | 8 | 5 | 6 | 5 | 5 |
| 7 | 6 | 9 | 7 | 8 | 1 | 7 |
| 10 | 9 | 5 | 4 | 3 | 3 | 4 |
| 7 | 8 | 6 | 5 | 3 | 4 | 1 |
| 10 | — | — | — | — | — | — |
| 14 | 12 | 13 | 8 | — | — | 8 |
| 8 | 7 | — | — | — | — | 1 |
| 16 | 13 | — | 9 | — | — | 7 |
| 11.4 | 10.2 | 9.4 | 7.6 | 5.8 | 5.1 | 4.3 |
| 3.0 | 3.0 | 2.9 | 3.1 | 2.3 | 3.1 | 2.3 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 2 | 1 | 2 | 2 | 2 | 1 |
| 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| 3 | 1 | 1 | 4 | 5 | 5 | 4 |
| 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| 1 | 0 | 2 | 2 | 0 | 0 | 1 |
| 2 | 1 | 0 | 1 | 2 | 2 | 0 |
| 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| 5 | 1 | 0 | 1 | 3 | 3 | 3 |
| 1 | 0 | 0 | 0 | 2 | 2 | 1 |
| 0 | 2 | — | 1 | — | — | 3 |
| 1 | 0 | — | 0 | — | — | 1 |
| 1.1 | 0.7 | 0.6 | 1.1 | 1.5 | 1.5 | 1.3 |
| 1.6 | 0.8 | 0.7 | 1.3 | 1.7 | 1.7 | 1.4 |
Results
Anticipatory Amygdala Activity Differentiating Patients With Generalized Anxiety Disorder and Healthy Comparison Subjects
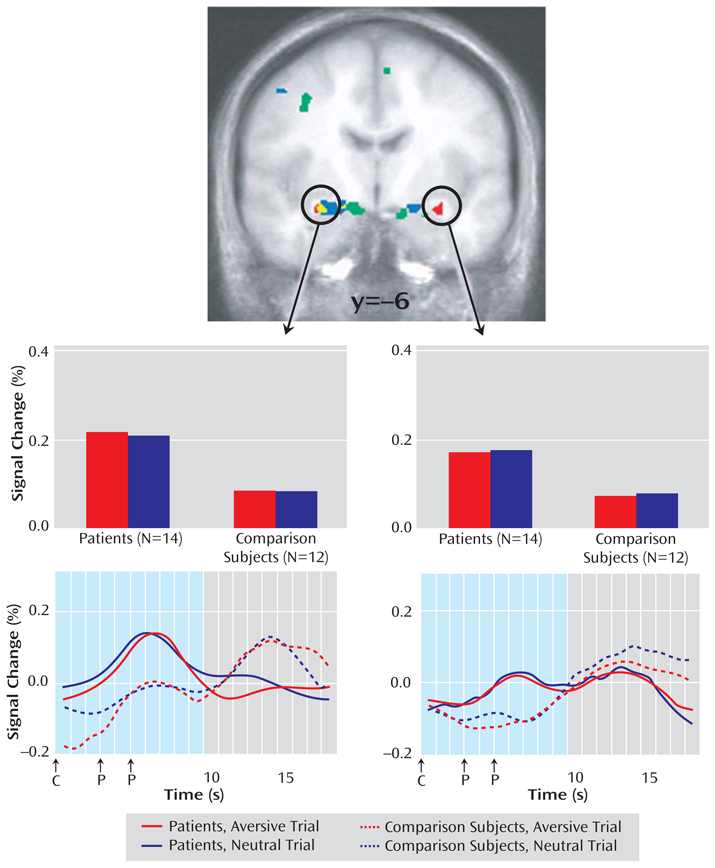
Hypothesized group differences in amygdala activation to the anticipatory cues were assessed with a voxelwise group by period by valence ANOVA (p<0.05, corrected). The absence of a group by period by valence interaction in the amygdala fails to confirm the prediction of group differences in anticipation of aversive but not neutral pictures. However, a group by period interaction was observed for bilateral dorsal amygdala areas extending into the substantia innominata (Figure 1).
FIGURE 1. Anticipatory Amygdala Activity Differentiating Patients With Generalized Anxiety Disorder and Healthy Comparison Subjects a.
aPatients with generalized anxiety disorder showed greater bilateral amygdala activation than healthy comparison subjects during the anticipation of both aversive and neutral pictures, as indicated by a group main effect (red) for a voxelwise group by valence analysis of variance (ANOVA) for the anticipation period only (N=26, p<0.05, corrected, Table 2). Depicted in blue are bilateral amygdala areas showing a group by period interaction for a voxelwise group by valence by period ANOVA (N=26, p<0.05, corrected, Table 2). In a more medial area of the amygdala, all subjects showed greater bilateral amygdala activation on aversive than neutral trials across both anticipation and picture periods, as indicated by a valence main effect (green) for a voxelwise group by valence by period ANOVA (N=26, p<0.05, corrected; Table 2, data supplement Figure 3). Bar graphs of the circled clusters for the group main effect illustrate average percentage signal change for the anticipation period. The data depicted in the brain images and bar graphs are beta-weights indicating fit to an ideal hemodynamic response. These beta-weights were used for analyses on the anticipation period indicated by the shaded area for the time series, which were derived from deconvolved estimates for display purposes only. Time series plots of the circled clusters illustrate average percentage signal change across all time points of the aversive (red) and neutral (blue) trials for patients with generalized anxiety disorder (solid lines) and healthy comparison subjects (dotted lines) separately. The onset of the 1-second picture (P) occurred 3 seconds after cue (C) onset on half of the trials and 5 seconds after cue onset on the other half.
A follow-up voxelwise group by valence ANOVA for the anticipation period resulted in a group main effect also in the bilateral dorsal amygdala, with 72% of the right amygdala area overlapping with the corresponding group by period cluster above (Table 2). Patients showed greater activation than healthy comparison subjects in anticipation of both aversive and neutral pictures (Figures 1 and 2 in the data supplement), consistent with findings of hyperresponsivity to both unpleasant and neutral stimuli (7, 21, 24). The location of this amygdala group effect was lateral to the dorsal amygdala regions showing a main effect of valence across periods both here (Figure 1 and in the data supplement Figure 2) and in previous reports (8, 14). A group by period by valence by hemisphere ANOVA for percent signal change values extracted from the entire amygdala using Talairach-defined anatomical boundaries (8) resulted in a valence effect (p<0.03) but no effects involving group (all p>0.19), suggesting the specificity of group effects to the dorsal subregions illustrated in Figure 1.
TABLE 2.
Heightened Anticipatory Activation in Generalized Anxiety Disorder and Prediction of Treatment Responsea
| Talairach Coordinates | |||||
|---|---|---|---|---|---|
| Predictive Effect | x | y | z | Size (mm3) | Statistic |
| ANOVA group main effectb | |||||
| Right dorsal amygdala | 26 | −4 | −8 | 213 | F=11.46 |
| Left dorsal amygdala | −28 | −6 | −12 | 118 | F=11.25 |
| ANOVA group by period effectc | |||||
| Right dorsal amygdala | 22 | −5 | −11 | 659 | F=12.73 |
| Left dorsal amygdala | −17 | −8 | −11 | 210 | F=11.16 |
| ANOVA valence main effectd | |||||
| Right dorsal amygdala | 14 | −6 | −10 | 558 | F=12.34 |
| Left dorsal amygdala | −14 | −2 | −14 | 328 | F=11.75 |
| Left dorsal amygdala | −22 | −9 | −14 | 166 | F=19.23 |
| Response per posttreatment measuree | |||||
| HAM-A | −1 | 32 | 19 | 367 | r=−0.82 |
| Penn State Worry Questionaire | −3 | 32 | 20 | 207 | r=−0.84 |
All listed clusters significant at p<0.05 (corrected). F and r values are for entire cluster.
Amygdala regions circled in Figure 1 that showed a group main effect for a voxelwise group (generalized anxiety disorder, comparison subjects) by valence (aversive, neutral) ANOVA on the anticipation period only (N=26). Patients with generalized anxiety disorder showed greater bilateral amygdala activation than healthy comparison subjects in anticipation of both aversive and neutral pictures.
Amygdala regions depicted in blue in Figure 1 that showed a group by period interaction effect for a voxelwise group (generalized anxiety disorder, comparison subjects) by valence (aversive, neutral) by period (anticipation, picture) ANOVA (N=26).
Amygdala regions depicted in green in Figure 1 (see also data supplement Figure S3) that showed a valence main effect for a voxelwise group (generalized anxiety disorder, comparison subjects) by valence (aversive, neutral) by period (anticipation, picture) ANOVA (N=26). These dorsal amygdala regions showed greater activation for aversive than neutral trials across anticipation and picture periods.
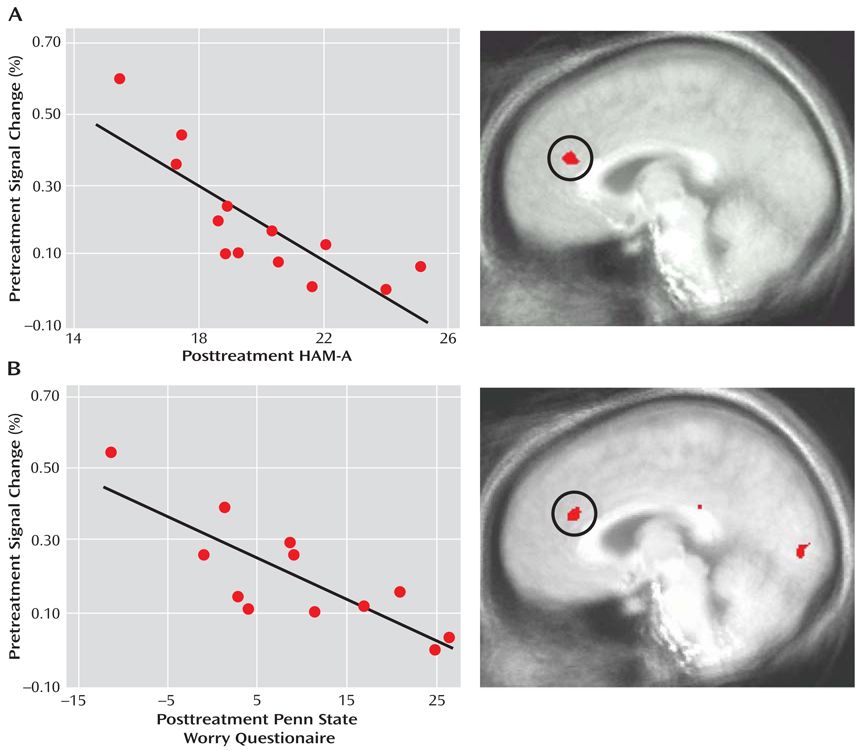
Anterior cingulate cortex regions depicted in Figure 2 where greater anticipatory activation preceding aversive and neutral pictures predicted treatment response after statistically controlling for the HAM-D (N=13).
The voxelwise group by valence ANOVA for the picture period (p<0.05, corrected) revealed no amygdala effects in response to the pictures. Significant effects involving group were not observed for any of the above ANOVAs in the insula, the anterior cingulate cortex, the prefrontal cortex, or the hippocampus using a liberal threshold of 50 mm3 at p<0.005 (uncorrected), confirmed by analyses on regions previously identified with this anticipation paradigm (Table 3 in data supplement) (8).
Anticipatory Anterior Cingulate Cortex Activity Associated With Treatment Response in Patients With Generalized Anxiety Disorder
Pretreatment anticipatory activity in the anterior cingulate cortex was associated with response to venlafaxine 8 weeks later. A voxelwise regression on anticipatory brain activity preceding both aversive and neutral pictures (p<0.05, corrected) revealed a pregenual anterior cingulate cortex area that was strongly associated with the reduction of posttreatment HAM-A scores when statistically controlling for pretreatment HAM-A and for depression as measured by the HAM-D (Figure 2A, Table 2). Consistent with prior reports (26–29), increased pretreatment anterior cingulate cortex activity was associated with better treatment response. As shown in data supplement Figure 2B, the same anterior cingulate cortex area was observed for the analogous voxelwise regression for posttreatment Penn State Worry Questionnaire scores (when there was control for pretreatment Penn State Worry Questionnaire and depression as measured by the HAM-D). No anterior cingulate cortex areas were observed for voxelwise regressions on pretreatment anticipatory activity for posttreatment HAM-A or Penn State Worry Questionnaire without statistically accounting for the HAM-D. There were no anterior cingulate cortex associations with the HAM-D.
FIGURE 2. Pretreatment Anticipatory Anterior Cingulate Cortex Activity in Patients With Generalized Anxiety Disorder Predicting Treatment Response 8 Weeks Latera.
aFor patients with generalized anxiety disorder, greater pretreatment anterior cingulate cortex activation during the anticipation of both aversive and neutral trials predicted better treatment response on the HAM-A (A) and Penn State Worry Questionnaire (B), as indicated by voxelwise regression analysis for the anticipation period only (N=13, p<0.05, corrected,Table 2). Values on the×axis indicate posttreatment scores after statistical control was added for pretreatment scores and for depression as measured by the HAM-D, with lower values indicative of better treatment response. The magnitude of the correlations for the HAM-A (r=−0.82) and the Penn State Worry Questionnaire (r=−0.84) was not appreciably affected by excluding the individual with the highest pretreatment anterior cingulate cortex activity and best treatment response in each scatterplot (r=−0.76 and r=−0.74, respectively).
The association between posttreatment HAM-A and the anterior cingulate cortex remained significant after also partialing out variance associated with posttreatment Penn State Worry Questionnaire and activity during the picture period in that same anterior cingulate cortex area (rp=−0.65, p<0.03). The analogous partial correlation for posttreatment Penn State Worry Questionnaire and the anterior cingulate cortex was also significant (rp=−0.66, p<0.03). Furthermore, voxelwise regressions on activity during the picture period revealed no anterior cingulate cortex areas associated with treatment response (see data supplement Results).
No amygdala areas met statistical thresholds for analyses testing relations with treatment response, as determined by voxelwise regressions or correlations for the activation values extracted from the amygdala areas identified in the ANOVAs. Consistent with the results of the voxelwise ANOVAs above, there were no significant effects involving group for ANOVAs on the activation values extracted from the anterior cingulate cortex areas identified in the voxelwise regressions.
Discussion
Anticipatory amygdala responses differentiated patients with generalized anxiety disorder and healthy comparison subjects. Specifically, the subjects with generalized anxiety disorder showed greater anticipatory activity in the bilateral dorsal amygdala preceding both aversive and neutral pictures than did the comparison subjects. These group differences for the right amygdala during anticipation were not present during picture viewing. This increased and indiscriminate response to anticipatory signals in the amygdala of subjects with generalized anxiety disorder may reflect a pathophysiological mechanism associated with the anticipatory anxiety and worry that are cardinal features of the disorder. In addition, anticipatory anterior cingulate cortex activity prior to the start of treatment was associated with response to venlafaxine.
Preclinical and clinical research has consistently shown that the amygdala preferentially responds to aversion across a variety of paradigms (8, 11, 14, 17–19). We did not find support for the hypothesis that amygdala activity in patients with generalized anxiety disorder was exclusively heightened in anticipation of aversion. Instead, subjects with generalized anxiety disorder showed anticipatory hyperresponsivity preceding both aversive and neutral pictures in amygdala regions lateral to the dorsal amygdala areas that respond preferentially to aversion (Figure 1) (8, 14). These findings lend neurobiological support to the conclusion drawn by Hoehn-Saric et al. (21) that “GAD patients overreact to both pathology-specific and non-specific cues.” The mere onset of any anticipatory cue presented in the context of cues that signal aversion may result in an initial indiscriminate amygdala response that alerts the GAD patient to the possibility of a negative outcome. Future research could address whether this putative pathophysiological mechanism in generalized anxiety disorder also extends to positive stimuli.
Patients with generalized anxiety disorder with increased anticipatory activity in the pregenual anterior cingulate cortex had better responses to venlafaxine 8 weeks later. Based on evidence pointing to the importance of the pregenual anterior cingulate cortex for detecting conflict in the emotional domain and recruiting cognitive control processes to resolve the conflict (37, 38), we believe that heightened activity in the anterior cingulate cortex is indicative of preserved top-down regulation and volition in patients with better outcomes (27, 28, 38). This finding is consistent with multiple other reports of pretreatment activity in the anterior cingulate cortex predicting better clinical outcome in depressed patients (26–29). Our previous report on generalized anxiety disorder (29) found that larger pretreatment anterior cingulate cortex responses to facial expressions were associated with better clinical outcome in a region that overlaps with the anterior cingulate cortex areas shown in Figure 2. With 10 of the same patients participating in both studies (see Method), this marks the first time that the association between pretreatment anterior cingular cortex activity and treatment response has been demonstrated using different paradigms in the same subjects. The similar findings for generalized anxiety disorder and depression suggest commonalities in the neural predictors of treatment response. Further research is needed to determine the extent to which the relation between treatment response and anterior cingulate cortex activity is shared with other anxiety disorders, as has been found for other sources of overlap between generalized anxiety disorder and depression (39, 40).
The present study further adds to this literature by demonstrating that anticipatory brain processes are associated with treatment response. This association was found for both a general measure of anxiety (HAM-A) and a specific measure of worry (the Penn State Worry Questionnaire), but only after control was added for depression as measured by the HAM-D. Indeed, the analytic tools employed to statistically account for co-occurring depression symptoms represent a methodological contribution to this literature (35). Our findings suggest that research on neural predictors of treatment response will benefit from efforts to carefully address co-occurring depression and anxiety symptoms in sample recruitment and statistical testing. Moreover, the anterior cingulate cortex association with posttreatment HAM-A remained even after control was added for the Penn State Worry Questionnaire, and the anterior cingulate cortex association with posttreatment Penn State Worry Questionnaire remained even after control was added for the HAM-A, suggesting that anticipatory anterior cingulate cortex activity is independently associated with decreases in worry and other anxiety symptoms. Finally, the pregenual anterior cortex area found here and in previous studies (28, 29) is dorsal to the area implicated in other works (26, 27). Anatomic specificity deserves attention in future research investigating the anterior cingulate cortex in treatment response, especially in light of recently reported cytoarchitecture data for pregenual anterior cingulate cortex (36).
In contrast to the amygdala findings, group differences were not observed for other key brain areas activated by our anticipation paradigm in healthy volunteers in this study (data supplement Tables 2 and 3) and previously (8, 14). In a recent report, “anxiety-prone” subjects (nine of 13 met DSM-IV criteria for generalized anxiety disorder) showed greater insula responses than “anxiety-normative” subjects (no DSM-IV disorders) during the anticipation of snake and spider pictures (25). The differences in sample and stimuli may explain why we found no group differences anywhere in the insula at p<0.05 corrected (or at p<0.005 uncorrected) for the voxelwise analyses or for analyses using the anterior insula regions previously identified with this anticipation paradigm (data supplement Table 2) (8). To address concerns about type II error, larger samples are needed to further assess group effects. Another limitation is the absence of online behavioral or autonomic data for examining relations with the neural findings. Of relevance, however, are the associations of brain data with treatment response, a valid and important behavioral criterion.
Using a paradigm that capitalized on anticipatory abnormalities as a key feature of anxiety disorders, we found that anticipatory amygdala hypersensitivity in a lateral region of the dorsal amygdala is a pathological signature of generalized anxiety disorder, whereas greater anticipatory activity in the pregenual anterior cingulate cortex may have adaptive benefits. This association of anterior cingulate cortex activity with treatment response to venlafaxine builds on prior reports indicating promise for neuroimaging as a prognostic tool (26–29). Crossover studies and double-blind designs involving placebo controls are important next steps for extending the findings from this initial open-label study that implicated amygdala-based anticipatory processes in the pathophysiology of generalized anxiety disorder and anterior cingulate cortex-based anticipatory processes in treatment response.
Supplementary Material
Acknowledgments
Supported by National Institute of Mental Health grants to Dr. Nitschke (R01-MH74847, K08-MH63984) and to Dr. Davidson, Dr. Kalin, and Dr. Whalen (P50-MH069315) and by a research grant from Wyeth Pharmaceuticals. This research was also supported by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30-HD03352).
Footnotes
Presented in part at the 35th and 37th annual meetings of the Society for Neuroscience, Washington, DC, Nov. 12–16, 2005, and San Diego, Nov. 3–7, 2007, and at the 21st annual meeting of the Society for Research in Psychopathology, Iowa City, Oct. 4–7, 2007.
Dr. Kalin is a consultant to Wyeth Pharmaceuticals, Bristol-Myers-Squibb, Corcept Therapeutics, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutical, Neuronetics, Pfizer Pharmaceuticals, Ce-NeRx BioPharma, AstraZeneca, Cyberonics, General Electric Corporation, Jazz Pharmaceuticals, Eli Lilly, Novartis, Takeda International, and Sanofi-Syntholabo. Dr. Kalin is owner of Promoter Neurosciences LLC and a stockholder of Corcept, CeNeRx BioPharma, and Neurocrine Inc. Study drug was provided by Wyeth Pharmaceuticals. The remaining authors report no competing interests.
The authors thank Andrew Alexander, Michael Anderle, Krista Bluske, Krystal Cleven, Ron Fisher, Jay Freidland, Thomas Ihde-Scholl, Hyejeen Lee, Mai Youa Lor, Kristen Mackiewicz, Terrence Oakes, Adrian Pederson, Sara Polis, Hillary Schaefer, Lorene Semen, Leah Somerville, Eric Steege, and Lesley Tarleton for their contributions to this project.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, Merkangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37:8–25. [PubMed] [Google Scholar]
- 4.Nitschke JB, Heller W. Distinguishing neural substrates of heterogeneity among anxiety disorders. Int Rev Neurobiol. 2005;67:1–42. doi: 10.1016/S0074-7742(05)67001-8. [DOI] [PubMed] [Google Scholar]
- 5.Borkovec TD, Robinson E, Pruzinsky T, DePree JA. Preliminary exploration of worry: some characteristics and processes. Behav Res Ther. 1983;21:9–16. doi: 10.1016/0005-7967(83)90121-3. [DOI] [PubMed] [Google Scholar]
- 6.Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cognit Ther Res. 2001;25:1–22. [Google Scholar]
- 7.Borkovec TD. Psychological Aspects and Treatment of Generalized Anxiety Disorder. London: Martine Dunitz; 2002. [Google Scholar]
- 8.Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 9.Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- 10.Plognaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 11.LeDoux JE. The Synaptic Self. New York: Viking; 2002. [Google Scholar]
- 12.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 13.Sarinopoulos I, Dixon GE, Short SJ, Davidson RJ, Nitschke JB. Brain mechanisms of expectation associated with insula and amydala response to aversive taste: implications for placebo. Brain Behav Immun. 2006;20:120–132. doi: 10.1016/j.bbi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc Natl Acad Sci USA. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitschke JB, Larson CL, Smoller JJ, Navin SD, Pederson AJC, Ruffalo D, Mackiewicz KL, Gray SM, Victor E, Davidson RJ. Startle potentiation in aversive anticipation: evidence for state but not trait effects. Psychophysiology. 2002;39:254–258. doi: 10.1017/S0048577202010156. [DOI] [PubMed] [Google Scholar]
- 16.Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: expectation, hypothesis, placebo. Trends Cogn Sci. 2003;7:197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 17.Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychology. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- 18.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 19.Kalin NH, Shelton SE, Davidson R. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PF, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 21.Hoehn-Saric R, Schlund MW, Wong SH. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res. 2004;131:11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJ, Chen G, Charney DS, Ernst M, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 23.Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, Mc-Caffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney DS, Blair RJ, Drevets WC, Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thayer JF, Friedman BH, Borkovec TD, Johnsen BH, Molina S. Phasic heart period reactions to cued threat and nonthreat stimuli in generalized anxiety disorder. Psychophysiology. 2000;37:361–368. [PubMed] [Google Scholar]
- 25.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 26.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 27.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ. Anterior cingulate activity as a predictor of degree of treatment in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;58:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 28.Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- 29.Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Ihde-Scholl T, Tarleton L, Alexander AL, Davidson RJ, Kalin NH. A functional MRI predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 31.Raskin A, Schulterbrandt J, Reatig N, McKeon JJ. Replication of factor of psychopathology in interview, ward behavior and self-report ratings of hospitalized depressives. J Nerv Ment Dis. 1969;148:87–98. doi: 10.1097/00005053-196901000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI: I. The Method. Neuroimage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- 34.Meyer TJ, Miller ML, Metzger RL, Borkovec TC. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 35.Keller J, Nitschke JB, Bhargava T, Deldin PJ, Gergen JA, Miller GA, Heller W. Neuropsychological differentiation of depression and anxiety. J Abnormal Psychol. 2000;109:3–10. [PubMed] [Google Scholar]
- 36.Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508:906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etkin A, Pittenger C, Polan HG, Kandel ER. Toward a neurobiology of psychotherapy: basic science and clinical applications. J Neuropsychiatry Clin Neurosci. 2005;17:145–158. doi: 10.1176/jnp.17.2.145. [DOI] [PubMed] [Google Scholar]
- 38.Nitschke JB, Mackiewicz KL. Prefrontal and anterior cingulate contributions to volition in depression. Int Rev Neurobiol. 2005;67:73–94. doi: 10.1016/S0074-7742(05)67003-1. [DOI] [PubMed] [Google Scholar]
- 39.Hettema JM. The nosologic relationship between generalized anxiety disorder and major depression. Depress Anxiety. 2008;25:300–316. doi: 10.1002/da.20491. [DOI] [PubMed] [Google Scholar]
- 40.Mennin DS, Heimberg RG, Fresco DM, Ritter MR. Is generalized anxiety disorder an anxiety or a mood disorder? considering multiple factor as we ponder the fate of GAD. Depress Anxiety. 2008;25:289–299. doi: 10.1002/da.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.