Abstract
Background
Hypothalamic-pituitary-adrenal (HPA) system activation is adaptive in response to stress, and HPA dysregulation occurs in stress-related psychopathology. It is important to understand the mechanisms that modulate HPA output; yet, few studies have addressed the neural circuitry associated with HPA regulation in primates and humans. Using high-resolution [F-18]-fluoro-deoxyglucose (FDG) positron emission tomography (PET) in rhesus monkeys, we assessed the relation between individual differences in brain activity and HPA function across multiple contexts that varied in stressfulness.
Methods
Using a logical AND conjunctions analysis, we assessed cortisol and brain metabolic activity with FDG-PET in 35 adolescent rhesus monkeys exposed to two threat and two home-cage conditions. To test the robustness of our findings, we used similar methods in an archival data set. In this data set, brain metabolic activity and cortisol were assessed in 17 adolescent male rhesus monkeys that were exposed to three different stress-related contexts.
Results
Results from the two studies, revealed that subgenual PFC metabolism (Area 25/24) consistently predicted individual differences in plasma cortisol concentrations regardless of the context in which brain activity and cortisol were assessed.
Conclusions
These findings suggest that activation in subgenual PFC may be related to HPA output across a variety of contexts (including familiar settings and novel or threatening situations). Individuals prone to elevated subgenual PFC activity across multiple contexts may be individuals who consistently show heightened cortisol, and may be at risk for stress-related HPA dysregulation.
Keywords: cortisol, subgenual PFC, stress, HPA, monkey, PET
Cortisol, a glucocorticoid released from the adrenal cortex, is critical for survival, reflects activation of the hypothalamic-pituitary-adrenal (HPA) system, and represents a primary mechanism through which emotions influence chronic disease. Persistently increased cortisol occurs in some individuals with depression (1,2) as well as in youth at risk for developing stress-related psychopathology (3). Moreover, in various chronic illnesses, increased cortisol is associated with poorer medical outcomes (4,5). Although rodent studies have examined brain mechanisms involved in HPA regulation, little is known about neural circuitry related to individual differences in primate and human cortisol concentrations.
Evidence from rodent studies points to medial prefrontal cortex (mPFC) as being important in HPA regulation and regulation of autonomic, behavioral, and emotional responses associated with stress (6-8). Of particular relevance to these functions are efferents that project from ventral PFC to subcortical and brain stem structures (9-11). Data from rodent studies indicate that dorsomedial PFC regions (prelimbic cortex) are involved in HPA inhibition, whereas ventral regions (infralimbic cortex) are involved in HPA activation (12,13). Subgenual PFC (Area 25/24) is the primate and human cortical region most analogous to the rat infralimbic cortex (7).
Subgenual PFC is of particular interest in conceptualizing the relation between HPA overactivity and stress-related psychopathology, as this region has been the site of both structural and functional alterations in some depressed patients, as well as a successful target for treatment of severe depression (14-17). In a study in treatment-resistant depressives, deep brain stimulation decreased subgenual PFC hypermetabolism resulting in a marked reduction in depressive symptoms (18). Subgenual PFC’s role as part of an “integrated pathway” for regulation of HPA is hypothesized to partially account for involvement of this region in depression (15-17,19).
To study brain circuitry associated with individual differences in HPA regulation in primates, we used data from a study aimed at establishing the relation between anxious temperament and regional brain metabolic activity (20,21). The availability of cortisol data allowed us to examine the relation between cortisol and metabolic activity in monkeys exposed to different contexts. Rhesus monkeys are an ideal species to provide insights into human HPA regulation as they share similarities with humans in HPA function, brain structure (e.g., prefrontal cortical development), and behavior (10,22). Rhesus monkeys are among the best species to investigate brain mechanisms underlying emotion regulation and psychopathology because multiple scans can be repeatedly obtained in the same individual during exposure to ethologically relevant settings (23).
Plasma cortisol and metabolic brain activity were assessed repeatedly in the same monkeys in response to four different situations varying in their degree of stressfulness. Two of the conditions (separation from cagemate and introduction of a human intruder) elicit different threat-related adaptive responses while the other two conditions involved assessment in the monkeys’ home cages (24). Logical AND conjunction analysis (see Nichols et al. 2005 [25]) was used to find clusters of regional metabolic activity that correlated with cortisol concentrations across all four conditions. This logical AND conjunction analysis identifies regions where metabolism and cortisol are significantly correlated during separation from cagemate and introduction of a human intruder and home cage alone and home cage with cagemate; thus, excluding regions that do not show significant correlations across all conditions. To confirm these results, we used the same analytic strategy in a different data set of previously studied animals in which [F-18]-fluoro-deoxyglucose (FDG) positron emission tomography (PET) and cortisol were collected. Based on rodent data suggesting that mPFC acts as a relay for limbic-cortical pathways and integrates various aspects of stress responses, we hypothesized that individual differences in mPFC activity, specifically the subgenual PFC, would predict individual differences in cortisol concentrations across conditions.
Method
Experiment 1
Animals & Procedures
Data include 35 adolescent rhesus monkeys (Macaca mulatta; Mean Age: 2.61 [0.56]; Range: 1.83 - 3.96; Male: 12, mean weights: 4.06 [±0.77] kg); N = 36, 1 animal excluded for refusing to leave its cage. Animals, which were pair-housed at the Wisconsin National Primate Center and Harlow Primate Laboratory, were maintained on 12-hour light-dark cycle (lights on 0600). All experimental procedures were approved by the University of Wisconsin IACUC.
Animals were exposed to 4 conditions prior to FDG-PET scanning, each condition occurring at least one week apart. Because the bulk of FDG uptake occurs within 30 minutes after injection (see e.g. Rilling et al. 2001 [26]), we designed conditions lasting 30 minutes. In the home cagemate (H-CM) condition, animals remained in their home cage with their partner for 30 minutes. In the home alone (H-ALN) condition, animals remained alone in their home cage for 30 minutes. In the no eye contact condition (NEC) animals were placed in a test cage while a human entered the room for 10 minutes, presented his profile, and avoided eye contact with the monkey while standing 2.5m away. The human left the test room for 5 minutes, reentered for 5 minutes, and left again for 5 minutes then reentered for 5 more minutes to avoid habituation. In the alone condition (ALN), animals were placed in a foreign cage and left alone for 30 minutes. Exposure to each condition was counterbalanced: 8 animals received H-ALN first, 9 animals received H-CM, 9 received ALN, and 9 received NEC first. For each animal, all conditions were run at approximately the same time of day (minimum difference in scanning times within an individual: 4 min; maximum difference in scanning times: 2 hrs 48 min). Portions of this dataset have been previously described in Fox et al. 2008 (20) and Kalin et al. 2008 (21).
Cortisol
Blood was sampled by femoral venipuncture at the end of each condition, before the FDG-PET scan. Samples were obtained between 0845 and 1615.
Basal cortisol was evaluated twice at intervals of at least one week. Baseline samples were collected in the morning between 0800 and 0930.
Plasma was immediately extracted from blood by centrifugation at 4°C and frozen at −70°C until assayed. Cortisol was measured in plasma using enzyme immunoassay (Diagnostic Systems Laboratories, Webster, TX) with intraassay variability of 6.1% and interassay variability of 6.3%. The detection limit was 0.125 ng. For analysis, time of day was regressed on cortisol values to remove variance associated with time of day.
Magnetic Resonance Imaging Acquisition
Structural magnetic resonance images (MRI) were used to aid in structural normalization of PET data. Please see the Additional Methods in Supplement 1 for more information.
PET Data Acquisition and Pre-processing
Immediately before each 30-minute condition, animals were injected with <10 milliCuries of FDG through a 19-gauge intravenous catheter in the saphenous vein. After the 30-minute session, blood was drawn for cortisol samples and then monkeys were anesthetized with 15 mg/kg ketamine followed by approximately 1.5% isofluorane gas. The animal’s head was positioned in a sterotaxic apparatus to maintain head positions across conditions in a P4 microPET scanner (Concorde Microsystems, Inc., Knoxville, Tennessee). PET resolution was 2 mm full width at half maximum (FWHM; volumetric resolution of approximately 2 mm3). To facilitate across-subject comparisons, PET images were transformed into a standard space as defined by Paxinos, Huang, and Toga, 2000 (27-29).
We corrected for potential differences in PET scan intensity by applying a global scale factor to each scan. This ensured each PET image volume had the same mean-intensity value. Using standard methods, global scale factors were determined by adjusting the mean based on a whole brain region of interest (30). To compute true regional cerebral metabolic rate of glucose (rCMRglu) values, quantitative arterial blood radioactivity measurements are required; however, obtaining these measures would have interfered with the naturalistic (awake) paradigm. Instead, we performed intensity normalization to obtain a proxy for rCMRglu in order to facilitate intersubject comparisons.
PET images were smoothed using a 4 mm FWHM Gaussian kernel to accommodate small differences in locations of functional regions across subjects (e.g., due to differences in gyral features) and increase signal-to-noise ratio (31).
Gray-matter probability (GMP) maps were created for each subject and used as a regressor in tests of FDG uptake to control for anatomical differences (32,33). Please see Supplement 1 for additional information.
Logical AND Conjunction Analysis
Correlations between cortisol and metabolism were performed within each condition using “fmristat” (34,35). Correlations were performed controlling for age, time of day, and anatomical differences by using the GMP map as a voxelwise covariate in our functional analyses as described in Oakes et al. 2007 (32).
To identify brain regions consistently correlated with cortisol levels across contexts, we performed a logical AND conjunction analysis using a minimum statistic (see Nichols et al. 2005 [25]). This analysis identified brain regions in which metabolism was significantly correlated with cortisol levels in each condition (p < .05, two-tailed uncorrected). We combined the statistical parametric maps from each condition and obtained a map revealing regions where metabolism and cortisol were significantly correlated during the NEC condition and the ALN condition and the H-ALN condition and the H-CM condition. Thus, clusters represent brain regions where metabolism and cortisol are correlated across all 4 conditions.
Follow-up analyses were performed by extracting mean values for each cluster of interest within each condition. Metabolic activity in the threat conditions was calculated by averaging the mean value within each region of interest for ALN and NEC conditions. Metabolic activity in the home conditions was calculated by averaging the mean glucose metabolism value within each region of interest for H-ALN and H-CM. Difference scores were created for each subject by subtracting the mean metabolism value for home conditions from the mean metabolism value for threat conditions for each cluster of interest.
Experiment 2
Animals & Procedures
Data include 17 adolescent male rhesus monkeys (Mean Age: 2.99 [0.48]; range: 2.18 – 3.57, mean weight: 4.78 (±0.88) kg); N = 22, 5 animals excluded from analysis due to problems with alignment in the scanner and missing cortisol data. Housing procedures were identical to those presented in Experiment 1. Experimental procedures were approved by the University of Wisconsin IACUC.
Animals were exposed to three 30-minute conditions: alone (ALN), no eye contact (NEC), and stare (ST). In the ST condition, animals were placed in a test cage while an experimenter entered the room and looked directly at the animal in 10-minute increments from 2.5m away with a 5-minute break where the human left the testing room. Breaks were designed to minimize habituation, thus extending the heightened sensitively to the intruder over the 30-minute FDG uptake. The NEC and ALN conditions were identical to conditions from Experiment 1. Six animals received ALN first, 7 animals received ST first, and 4 animals received NEC first. Each condition was not performed more than once per week. Data from Experiment 2 have been described in Fox et al. 2005 (36) and Kalin et al. 2005 (23).
Cortisol
Collection, storage, and processing were identical to Experiment 1.
MRI Acquisition
Structural images were obtained for 6 of the 17 monkeys. Data collection was identical to Experiment 1.
PET Data Acquisition, Processing, and Analysis
Data collection procedures and analysis of brain metabolic activity were identical to Experiment 1 (with the exception of GMP as a covariate, which was not used). Because anatomical data were obtained for only 6 of the 17 monkeys, procedures for registration and transformation to a standard space (see Paxinos, Huang, and Toga 2000 [27]) differed from Experiment 1. These methods are described in Kalin et al. (2005; 23). GMP maps could not be created because of the absent anatomical data.
Logical AND Conjuction Analysis
Correlations between cortisol and brain activity were performed within each condition using “fmristat” (34,35), and we statistically controlled for age and time of day. We again performed a logical AND conjunction analysis (25) using a minimum statistic (p < .05, one-tailed uncorrected) to determine brain regions that were related to cortisol across contexts. We combined the statistical parametric maps from each condition and obtained a map revealing regions where metabolism and cortisol were significantly correlated during the NEC condition and the ST condition and the ALN condition. Clusters represent brain regions where metabolism and cortisol are correlated across all 3 conditions.
Results
Experiment 1 – Relation between cortisol and regional metabolism
Cortisol
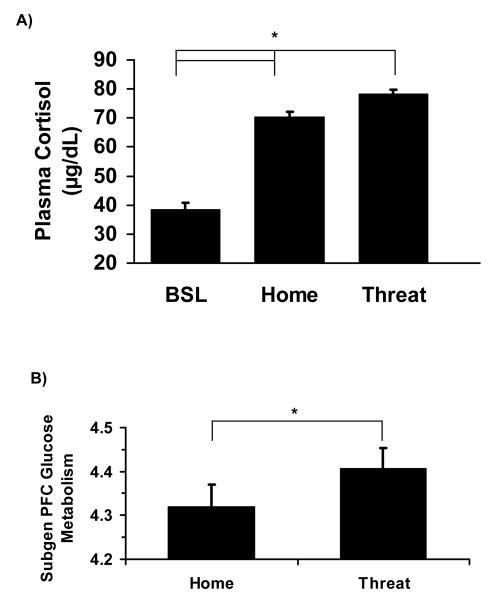
Cortisol values, adjusted for time of day, revealed varying degrees of HPA activation in relation to the different test conditions, F(4, 136) = 37.84, p < .0001 (See Figure 1a). Cortisol values (adjusted for time of day) were greater in the threat conditions (ALN & NEC) compared to the home-cage conditions (H-CM & H-ALN), t(34) = 4.79, p < .001. Cortisol values (adjusted for time of day) were greater in the home-cage conditions compared to baseline, t(34) = 8.68, p < .001. Tests on raw cortisol values also indicate significant differences across conditions (see Figure 1 and Supplement 1 for statistics).
Figure 1.
For Experiment 1, the two threat conditions included: alone in a test cage (ALN) and no eye contact (NEC). The two home-cage conditions included: home alone (H-ALN) and home with cagemate (H-CM). (a) Both threat and home-cage conditions produced a greater cortisol response compared to baseline morning cortisol after controlling for time of day, t’s (34) > 8.68, p’s < .001. Threat conditions produced a greater cortisol response compared to the home-cage conditions after controlling for time of day, t(34) = 4.79, p < .001. Differences between conditions were also significant when using raw cortisol levels, F(4, 136) = 73.97, p < .001; all t’s > 3.10, p’s < .005. Graphically, raw cortisol values are presented. (b) Threat conditions produced higher relative cerebral glucose metabolism in the subgenual prefrontal cortex (PFC) than home conditions, t(34) = 3.10, p < .005. Error bars represent standard error of the mean.
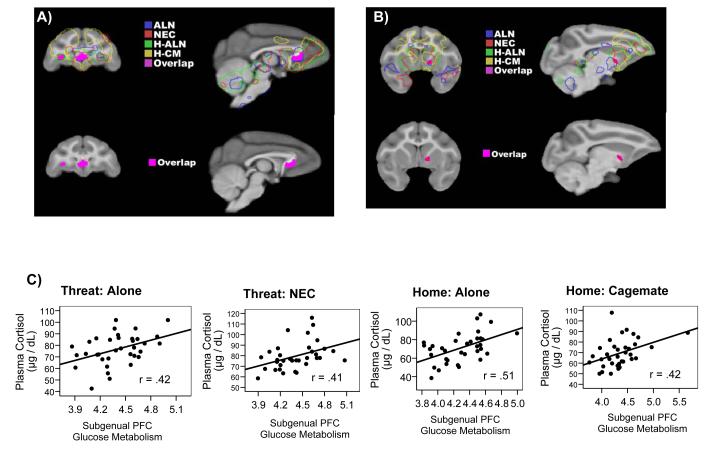
Consistent Correlations between Metabolism and Cortisol
Logical AND conjunction analysis (see Nichols et al. 2005 [25]) revealed that metabolism in subgenual PFC and part of pregenual PFC (Area 25/24) was consistently correlated with individual differences in cortisol (p < .05; Figure 2 and Table 1). A separate prefrontal cluster in which metabolism correlated with cortisol across conditions was identified in left orbtiofrontal cortex (OFC, Area 13; Figure 2a). A region of basal forebrain including the right bed nucleus of the stria terminalis (BNST), ventral striatum, and shell and core of the nucleus accumbens (NAC) was the only other region in which metabolism positively correlated with cortisol across the four conditions (which we will refer to as BNST/NAC; Figure 2b). There were no regions in which metabolism was consistently negatively correlated with cortisol.
Figure 2.
Correlations for Experiment 1 between glucose metabolism and cortisol across multiple contexts. (a & b) Upper images: Correlational brain maps where traced outlines indicate brain regions where cortisol is positively correlated with metabolism for each condition. Each color represents one of the four conditions. The solid pink areas represent the brain regions of overlap where cortisol and glucose metabolism are correlated across all 4 conditions as identified using the logical AND conjunction analysis. Lower images: Isolated image of brain regions of overlap (solid pink). (a) Anterior sagital and coronal slices depicting subgenual PFC and the left OFC where cortisol correlates with glucose metabolism across all 4 conditions. (b) A more lateral, sagital slice and more posterior, coronal slice showing the region encompassing the BNST/NAC where cortisol correlates with metabolism in all 4 conditions. (c) Subgenual PFC: Scatter plots of the relationship between metabolism in the subgenual PFC and cortisol in each of the 4 conditions. (Threat Conditions: alone [ALN] and no eye contact [NEC]); Home-cage Conditions: home alone [H-ALN] and home with cagemate [H-CM]).
Table 1.
Experiment 1: Brain Regions that Consistently Predict Cortisol Responses Across Contexts
| Distance Relative To Anterior Commissure | |||||
|---|---|---|---|---|---|
| Local Maxima | Hemisphere | Volume, mm3 | x | y | z |
| Subgenual PFC | Midline | 110 | −1 | 10 | 3 |
| Area 24/25 | |||||
| OFC | Left | 14 | −14 | 11 | 3 |
| BNST/NAC | Right | 31 | 6 | 2 | 1 |
We examined the correlation between glucose metabolism during the four test conditions with baseline cortisol sampled in the morning on non-testing days. Individual differences in baseline cortisol concentrations were significantly positively correlated with glucose metabolism in the subgenual PFC cluster during the ALN, NEC, and H-ALN conditions, r’s(33) > .35; p’s < .05; OFC during the ALN and NEC conditions, r’s(33) > .40; p’s < .05; and BNST/NAC only during the ALN condition, r(33) > .40; p < .05.
Condition-Related Metabolism
We compared metabolism in the threat conditions versus home-cage conditions for brain regions identified in the logical AND conjunction analysis,. Compairing across conditions, metabolism in subgenual PFC, OFC, and BNST/NAC (see Figure 2a for the location of these regions) revealed a similar pattern to that of cortisol. Regional metabolism during the threat conditions (NEC & ALN) was significantly higher than metabolism in the home-cage conditions (H-CM & H-ALN) in subgenual PFC, t(34) = 3.10, p < .005; Figure 1b; OFC, t(34) = 2.96, p < .01; and BNST/NAC, t(34) = 4.82, p < .001.
Follow-up correlational analyses examined the relations among subgenual PFC, OFC, and BNST/NAC metabolism. Metabolic activity was significantly correlated between subgenual PFC and OFC in all 4 conditions (r’s = .47-.76, p < .01). Metabolic activity was significantly correlated between subgenual PFC and BNST/NAC in all 4 conditions (r’s = .52-.65, p < .01). Metabolic activity was significantly correlated between OFC and BNST/NAC in 1 of the 4 conditions (H-CM: r = .45, p < .01).
Individual Differences between Threat and Home-Cage Conditions
To further assess the extent to which individual differences in metabolic activity predict cortisol concentrations, we computed difference scores for each subject between threat and home-cage cortisol levels, as well as difference scores between threat and home-cage regional metabolism. We correlated difference scores for metabolism for each brain region of interest and with difference scores for cortisol. This analysis demonstrated that the difference in cortisol levels between threat and home-cage conditions was significantly correlated with the difference in metabolism in the subgenual PFC, r(33) = .53, p = .001, Figure S1A; OFC, r(33) = .38, p < .05, Figure S1B, and BNST/NAC, r(33) = .55, p = .001, Figure S1C (see Supplement 1).
Experiment 2 - Analysis of archival data
Cortisol Data
Cortisol levels (in μg/dL) following each of the stressful conditions were greater than the baseline concentrations assessed on a different day (M: 41.94 [11.61]; F(3, 48) = 39.09, p < .001) and did not significantly differ across ALN (M: 75.79 [18.10]), NEC (M: 74.10 [11.78]), and ST (M: 75.39 [16.98]) conditions (t’s(16) < .54, p’s > .60).
Consistent Correlations between Metabolism and Cortisol
To further test the reliability of the relation between cortisol and PFC activity, we used similar methods to examine an archival data set. Based on the prior analyses, we hypothesized that across each of the 3 contexts that metabolic activity in the subgenual PFC, OFC, and BNST/NAC regions would predict individual differences in cortisol concentrations in response to the conditions. A one-tailed p < .05 threshold for the predicted regions was used based on the a priori directional hypothesis. Consistent with the earlier results, across the 3 conditions, positive correlations were found between cortisol levels and metabolic rate in a cluster overlapping with the subgenual PFC (Areas 25/24; local maxima, x: −2, y: 11, z: 6; cluster volume = 65 mm3). In contrast to the initial study, significant correlations between cortisol and metabolic activity were not found in the BNST/NAC or OFC regions (see Table S1 in Supplement 1 for all clusters). We did not observe significant correlations between baseline plasma cortisol levels and subgenual PFC glucose metabolism in any of the conditions in this archival data set, r’s(15) < .31 , p’s > .22.
DISCUSSION
This study provides novel data suggesting that in primates subgenual PFC is a brain area that is critically related to HPA output. By using a logical AND conjunction analysis (25) we were able to identify brain regions in which glucose metabolic rate significantly predicts cortisol in each condition and thus is consistently related to individual differences across multiple contexts. This analytic strategy allowed us to pinpoint brain regions that may act as trait-like neural regulators. The data demonstrate that individual differences in subgenual PFC metabolic rate predict cortisol levels across different contexts that vary in their degree of stressfulness. The data also show that both subgenual PFC metabolic activity and cortisol concentrations during threat conditions were greater than in the less stressful home conditions. Furthermore, analysis of the archival data confirmed the context-independent relation between subgenual PFC activity and cortisol concentrations. Taken together, these data suggest that activity in the subgenual PFC has a trait-like relationship with individual variation in cortisol levels that remains stable across threatening and nonthreatening contexts.
In primates, the subgenual PFC is linked to many brain regions that are involved in HPA regulation as well as visceral and emotional responses to stress (amygdala, bed nucleus of the stria terminalis, hypothalamus, and periaqueductal gray) (11,37,38). Furthermore, using a similar analytic strategy in the same animals used in Experiment 1, we recently identified a network consisting of many of these regions (amygdala, hippocampus, and periaqueductal gray) that is predictive of individual differences in anxious temperament (20). In rodent studies it has been demonstrated that during stress the infralimbic cortex (IL; region in rodents that is most analogous to subgenual PFC in primates [7]) provides input to the basolateral amygdala (BLA; 39). Based on rodent data, IL is thought to influence behavior during extinction learning through indirect input (projections to intercalated amygdala neurons) to the central nucleus of the amygdala (40,41). Other studies show that lesions to the infralimbic cortex suppress stress-related corticosterone responses (13,42), elevate basal levels of cortisol (42), prolong extinction learning to fearful stimuli (43), and alter social behavior (42). The observed relationship, across contexts, between the subgenual PFC metabolic rate and cortisol is consistent with the idea that this region is critically involved in the regulation of the HPA axis and the stress response. In conjunction with other primate and rodent data, our findings suggest that the primate subgenual PFC might represent the core of an integrated pathway for both initiation and regulation of a cortisol response.
Data from our primary study also suggest that individual differences in activity in the OFC as well as an area encompassing the BNST/NAC are associated with HPA output. While metabolic activity between the BNST/NAC and OFC was correlated only in 1 of the 4 conditions, metabolic activity in the subgenual PFC was correlated with BNST/NAC and OFC across all four conditions. This further suggests that the subgenual PFC is part of a network of structures whose activity is related to the HPA axis. The BNST/NAC has dense projections from the mPFC which includes BA 25 (10,44). Importantly, BNST/NAC have direct connections to the paraventricular nucleus (PVN) of the hypothalamus (9,23,45). This region of the basal forebrain is thought to be part of a network that integrates endocrine, autonomic, and emotional states (46). The OFC also plays a role in regulating emotion and anxiety and is bidirectionally linked to the amygdala (47). While the relations between cortisol and metabolism in these regions were evident in Experiment 1, they were not replicated in the analysis of the archival data, and thus should be regarded with caution.
While our findings demonstrate that greater subgenual PFC activation is related to greater cortisol secretion, the 30 minute time frame of FDG uptake (see e.g. Rilling et al. 2001 [26]), along with our use of a single cortisol sample per condition, makes it difficult to interpret the specific mechanisms involved in this relationship. Because subgenual PFC provides input to the hypothalamus, amygdala, and BNST/NAC and because it is also implicated in negative feedback of the HPA system (12), it is likely that the positive relation between subgenual PFC activity and cortisol reflects the combination of stimulatory and inhibitory processes within the HPA axis. Because of the correlational nature of our data, the possibility exists that cortisol and subgenual regional metabolism show a simultaneous increase during these contexts but share no mechanistic relationship with one another. However, rodent data provide clear evidence for a mechanistic relationship between infralimbic cortex and HPA regulation, which suggest that our correlational findings may identify in primates a region that is mechanistically related to variation in HPA output. In addition, it is notable that the primate PFC contains a wealth of glucocorticoid receptors (48) and the distribution of receptors in the PFC is affected by stress (22) further suggesting a functional relationship between these systems. The possibility also exists that the relation we observed between subgenual PFC activity and cortisol could be due to the effects of cortisol on brain metabolic activity. Future work should continue to address the causal nature of this relationship and provide data with increased sensitivity to detect time profiles of these responses.
Another potential limitation is our inability to confidently address sex-related differences in these samples. Our sample from Experiment 2 contained only male monkeys, and our sample for Experiment 1 contained an insufficient number of males (N = 12) and females (N = 23) to address sex-related differences in these findings.
It has been suggested that depression is partly due to alterations in stress-related systems (16). Studies in some depressed patients demonstrate altered regulation of the HPA axis (49). Other studies point to increased subgenual PFC activity associated with the pathophysiology of depression (18), and recent human neuroimaging studies support the involvement of the subgenual PFC in HPA regulation during stressful contexts (50). The findings presented in the current study provide a link between individual differences in subgenual PFC and HPA activity and may be important in understanding the basis of psychopathology related to dysregulation of the stress response. Future work should examine whether individuals with elevations in subgenual PFC activity across multiple contexts are at risk for stress-related HPA dysregulation and psychopathology.
In summary, the current study used multiple contexts, varying in their degree of stressfulness, to elucidate relations between regional brain metabolism and HPA activity in primates. Heightened subgenual PFC glucose metabolism consistently predicted higher cortisol levels across contexts that varied in their degree of stressfulness. The findings point to a role for the primate subgenual PFC as a possible trait-like neural regulator of HPA activity. These data linking subgenual PFC to HPA function may be particularly relevant to understanding mechanisms associated with the pathophysiology of stress-related psychopathology in which both subgenual function and HPA function can be dysregulated.
Supplementary Material
Acknowledgements
We are grateful to H. Van Valkenberg, T. Johnson, K. Meyer, E. Zao, R. Monticelli, and the staff at the Harlow Center for Biological Psychology and the Wisconsin National Primate Research Center (RR000167), for their technical support. This work was supported by grants MH46729, MH69315, MH84051, the HealthEmotions Research Institute, Meriter Hospital, a National Science Foundation Graduate Student Fellowship awarded to A. Jahn, and an NIMH K08 award (K08 MH074715) to H. Abercrombie.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures Allison Jahn, Andrew Fox, Dr. Heather Abercrombie, Dr. Terry Oakes, and Dr. Richard Davidson reported no biomedical financial interests or potential conflicts of interest. Dr. Shelton reports owning stock options in General Electric Corp (GE). Dr. Kalin reports serving on the scientific advisory board for AstraZeneca, Bristol-Myers-Squibb, CeNeRx Biopharma, Corcept Therapeutics, Cyberonics, Elsevier, Eli Lilly and Company, Forest Laboratories, General Electric Corp (GE Healthcare), GlaxoSmithKline, Jazz Pharmaceutical, Neuronetics, Novartis, Otsuka American Pharmaceuticals, Takeda International, and Wyeth Pharmaceutical. He reports stock options in Corcept Therapeutics and CeNeRx Biopharma. He is the owner of Promoter Neurosciences, LLC. He holds a patent for the promoter sequences for corticotropin-releasing factor CRF2alpha and for a method of identifying agents that alter the activity of the promoter sequences: U.S. Patent issued on 07-04-06; patent #7071323, divisional patent applied for on 9/26/2005; patent application #11/234916. He holds a patent for the promoter sequences for urocortin II and the use thereof: U.S. Patent issued on08-08-06; patent #7087385. He holds a patent for the promoter sequences for corticotropin-releasing factor binding protein and use thereof: U.S. Patent issued on10-17-06; patent #7122650. He holds a patent for the method for reducing CRF receptor mRNA: Patent applied for on 07-22-04 patent application #20050042212.
References
- 1.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 3.Mannie ZN, Harmer CJ, Cowen PJ. Increased waking salivary cortisol levels in young people at familial risk of depression. Am J Psychiatry. 2007;164:617–621. doi: 10.1176/ajp.2007.164.4.617. [DOI] [PubMed] [Google Scholar]
- 4.Leserman J, Petitto JM, Golden RN, Gaynes BN, Gu H, Perkins DO, et al. Impact of Stressful Life Events, Depression, Social Support, Coping, and Cortisol on Progression to AIDS. Am J Psychiatry. 2000;157:1221–1228. doi: 10.1176/appi.ajp.157.8.1221. [DOI] [PubMed] [Google Scholar]
- 5.Brown ES, Varghese FP, McEwen BS. Association of depression with medical illness: does cortisol play a role? Biol Psychiatry. 2004;55:1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- 6.Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex; a substrate for emotional behavior? Prog Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- 7.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Schneider M, Koch M. Behavioral and morphological alterations following neonatal excitotoxic lesions of the medial prefrontal cortex in rats. Experimental Neurology. 2005;195:185–198. doi: 10.1016/j.expneurol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 11.Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- 12.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 16.Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9:325, 393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- 17.Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61:729–730. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- 20.Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 25.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Rilling JK, Winslow JT, O’Brien D, Gutman DA, Hoffman JM, Kilts CD. Neural correlates of maternal separation in rhesus monkeys. Biol Psychiatry. 2001;49:146–157. doi: 10.1016/s0006-3223(00)00977-x. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Academic Press; San Diego, CA: 2000. [Google Scholar]
- 28.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 29.Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Camargo EE, Szabo Z, Links JM, Sostre S, Dannals RF, Wagner HN. The influence of biological and technical factors on the variability of global and regional brain metabolism of 2-[18F]fluoro-2-deoxy-D-glucose. J Cereb Blood Flow Metab. 1992;12:281–290. doi: 10.1038/jcbfm.1992.38. [DOI] [PubMed] [Google Scholar]
- 31.Worsley KJ, Marret S, Neelin P, Evans AC. Searching scale space for activation in PET images. Hum Brain Mapp. 1996;4:74–16. doi: 10.1002/(SICI)1097-0193(1996)4:1<74::AID-HBM5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 32.Oakes TR, Fox AS, Johnstone T, Chung MK, Kalin N, Davidson RJ. Integrating VBM into the General Linear Model with voxelwise anatomical covariates. Neuroimage. 2007;34:500–508. doi: 10.1016/j.neuroimage.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 34.Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- 35.Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J. Unified univariate and multivariate random field theory. Neuroimage. 2004;23(Suppl 1):S189–95. doi: 10.1016/j.neuroimage.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Fox AS, Oakes TR, Shelton SE, Converse AK, Davidson RJ, Kalin NH. Calling for help is independently modulated by brain systems underlying goal-directed behavior and threat perception. Proc Natl Acad Sci U S A. 2005;102:4176–4179. doi: 10.1073/pnas.0409470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 38.Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- 39.Maroun M. Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. Eur J Neurosci. 2006;24:2917–2922. doi: 10.1111/j.1460-9568.2006.05169.x. [DOI] [PubMed] [Google Scholar]
- 40.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rangel A, Gonzalez LE, Villarroel V, Hernandez L. Anxiolysis followed by anxiogenesis relates to coping and corticosterone after medial prefrontal cortical damage in rats. Brain Research. 2003;992:96–103. doi: 10.1016/j.brainres.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 43.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 44.Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- 45.Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- 46.Heimer L, Harlan RE, Alheid GF, Garcia MM, de Olmos J. Substantia innominata: a notion which impedes clinical–anatomical correlations in neuropsychiatric disorders. Neuroscience. 1997;76:957–1006. doi: 10.1016/s0306-4522(96)00405-8. [DOI] [PubMed] [Google Scholar]
- 47.Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of Corticosteroid Receptors in the Rhesus Brain: Relative Absence of Glucocorticoid Receptors in the Hippocampal Formation. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young EA, Kotun J, Haskett RF, Grunhaus L, Greden JF, Watson SJ, et al. Dissociation between pituitary and adrenal suppression to dexamethasone in depression. Arch Gen Psychiatry. 1993;50:395–403. doi: 10.1001/archpsyc.1993.01820170073010. [DOI] [PubMed] [Google Scholar]
- 50.Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.