Abstract
Protein Misfolding Disorders (PMDs) are a group of diseases characterized by the accumulation of abnormally folded proteins. Despite the wide range of proteins and tissues involved, PMDs share similar molecular and pathogenic mechanisms. Several epidemiological, clinical and experimental reports have described the co-existence of PMDs, suggesting a possible cross-talk between them. A better knowledge of the molecular basis of PMDs could have important implications for understanding the mechanism by which the diseases appear and progress and ultimately to develop novel strategies for treatment. Due to their similar molecular mechanisms, common therapeutic strategies could be applied for the diseases in this group.
MOLECULAR BASIS OF PROTEIN MISFOLDING DISORDERS
For a protein, the amino acid sequence is the blueprint that dictates the biologically active conformation. However, throughout the life of the protein there are many factors that lead to its unfolding and refolding, opening the door for a misfolding event to occur. This misfolding event can lead to the aggregation of the misfolded protein into amyloids which then wreak havoc on the body, leading to a variety of debilitating diseases. PMDs are a group of diverse, fatal diseases resulting from the failure of a protein to fold into the correct conformation, followed by its subsequent aggregation and deposition in tissues [1, 2]. This group of diseases comprises disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), transmissible spongiform encephalopathies (TSEs) and type 2 diabetes among many others [2]. The contribution of these aggregates to pathological decline is better understood in some of these diseases; however their presence is a hallmark in all cases.
Transmissible Spongiform Encephalopathies
TSEs, also known as prion diseases, consist of a group of fatal neurodegenerative disorders that are found in a variety of mammals. TSEs are the only PMDs known to be transmissible by infection and the infectious agent (termed prion) is most likely composed exclusively of the misfolded prion protein. During prion replication, the disease associated isoform of the prion protein (PrPSc) imposes its three dimensional structure on the normal cellular form of the protein (PrPC) resulting in the exponential accumulation and aggregation of PrPSc (Fig. 1) [3, 4]. A remarkable feature of these diseases is the prolonged incubation period followed by a very rapid clinical phase that is invariably fatal. TSEs are unique diseases due to the tripartite epidemiological appearance (inherited, sporadic, and acquired), and while rare in humans, with a sporadic Creutzfeldt Jacob Disease (sCJD) incidence of 1–2 cases per million persons per year [5], they are more common in animals. TSEs can occur naturally or as a direct result of the consumption of contaminated food. The recently described variant CJD (vCJD) resulted from the consumption of meat infected with the bovine spongiform encephalopathy (BSE) agent [6–8].
Fig. 1. Generation of misfolded prions in TSEs.
PrPSc, the disease-associated form of the prion protein, is able to impose its structure to a normally produced protein, termed PrPC. As result, an exponential generation of misfolded prions is obtained. PrPSc has been associated with several cytotoxic events in vitro and in vivo. The accumulation of the abnormal protein in the brain leads to clinical decline, which invariably ends in the death of the affected individuals.
Prions, as other more conventional type of infectious agents, have two important properties: prion strains and the species barrier phenomenon [9, 10]. Prion strains are characterized classically by their stable pathological and biochemical characteristics, including incubation time in susceptible animals, clinical symptoms, and by their lesion profile in the central nervous system [9]. While it is not entirely clear how strain characteristics are encoded, most data indicates that the folding of PrPSc is responsible [11–13]. However, recent evidence suggests that quaternary structure also likely has an influence on strain properties [14].
The species barrier phenomenon in TSEs describes the ability of prions from one species to cause disease in another [15, 16]. Initial passage of prions from one species to another can be associated with complete resistance or long incubation times with 100% attack rate or less followed by a large drop in the incubation period on subsequent passages with all animals developing disease [17]. A clear example can be seen in the difficulty of hamster prions to cause disease in wild type mice [18]. However, expression of hamster PrPC in transgenic mice was shown to abolish this resistance [19]. Further experiments then demonstrated that the most important determinant of the species barrier is the degree of homology between PrPC and PrPSc [20]. Although this is thought to result in optimal prion transmission, prion strains have been found to overcome the influence of primary structure [21]. One example of this is BSE, which has been found to transmit to multiple species while having a primary structure that is not identical to PrPC in the host [22–26]. This demonstrates that the factors controlling prion transmission barriers likely work in combination rather than one factor dominating over the others.
Mechanisms of Protein Misfolding and Seeded Aggregation
In each PMD, protein aggregates are composed of a misfolded protein unique to the disease, such as amyloid β (Aβ peptide in AD or PrPSc in TSEs. The misfolded protein aggregates in PMDs have similar characteristics, but vary in distribution and composition leading to different deposition profiles and pathologies [2]. In addition, there are no sequence similarities between the various proteins that are implicated in these disorders [2]. Protein aggregation is not a random process, but occurs slowly through an ordered mechanism termed the nucleation-dependent polymerization model [27, 28].
The nucleation-dependent polymerization model features a slow and thermodynamically unfavorable nucleation phase followed by a rapid elongation phase [28, 29]. In the nucleation phase, the rate-determining step is the formation of a stable seed or nucleus of polymerized protein (Fig. 2). In vivo, the first seeds in this process can be formed spontaneously (as it is mainly observed in TSEs, AD and PD), favored by mutations (as in Huntington’s disease) or exogenously acquired (so far only accepted for TSEs). The seed can then simultaneously bind to and convert multiple molecules of the normal protein, generating a thread of aggregated small oligomers which subsequently will lead to the formation of amyloid fibrils and in some cases, plaques [30] (Fig. 2). The dynamic distribution of these toxic species is characteristic for each disease, but interestingly, it is also dependent on the specific conformation that the proteins can acquire (i.e. as it is observed in different prion strains) [2, 9]. Amyloid aggregates are thought to be the triggering factors in various PMDs [30]. Amyloidogenic-specific dyes such as Thioflavin T and Congo Red can be used to observe the kinetics of fibril formation and have demonstrated that the length of the nucleation phase (or lag phase) and extension phase are highly dependent on the protein concentration [2, 30].
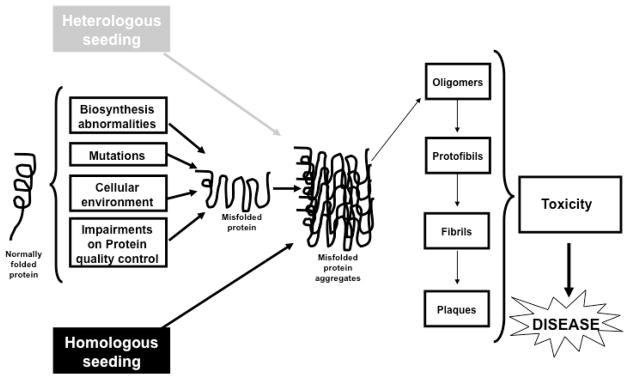
Fig. 2. Seeding and cross-seeding mechanisms in Protein Misfolding Disorders.
A variety of factors, including mutations, abnormal biosynthesis, changes in cellular environment or impairments on the quality control machinery, induce the initial misfolding of normally folded proteins. Misfolded proteins become stabilized upon oligomerization and formation of long fibrillar amyloid aggregates. Preformed seeds have been described to speed this process in vitro and in vivo. In addition, the presence of misfolded nuclei could enhance the aggregation of other amyloids, a phenomenon known as cross-seeding.
The infectious nature of prions can be rationalized by the seeding-nucleation model [28]. While the lag phase is normally a slow process, this phase can be reduced or eliminated by the addition of a preformed seed or nucleus to serve as a template [31]. This would mean that in the case of acquired prion diseases, such as vCJD or iatrogenic CJD, a PrPSc seed is introduced into the body and used as a template to seed the polymerization of PrPSc aggregates, reducing the lag phase and accelerating the elongation phase (Fig. 2). This provides a plausible explanation for why vCJD appeared in much younger individuals, which is very different from the appearance of disease late in life that occurs with the sporadic and inherited TSEs in which there is no preformed seed introduced [6]. Since in vitro and in vivo evidence suggest that in all PMDs the process of protein misfolding and aggregation also follow a seeding-nucleation mechanism [29, 32], it is possible that other PMDs might be transmissible following a similar route [29].
INTERACTION OF PMDS: FACT OR CHANCE?
The co-existence of various PMDs in the same individual has been extensively described [33–35]. Moreover, the presence of two misfolded proteins has also been described in the same amyloidogenic structure (see below). On this basis, it could be hypothesized that PMDs might interact at the protein level through a process termed heterologous seeding or cross-seeding, suggesting that one PMD could be an important risk factor for the development of a second one.
Cross-Seeding
While it is well established that fibril formation is enhanced by adding preformed homologous or heterologous seeds, it has also been shown in vitro that sequence similarity could have an effect on seeding efficiency. By cross-seeding hen lysozyme with a series of proteins, it was demonstrated that differing sequences had a lower efficiency of seeding [36]. In vivo studies revealed that non-mammalian protein fibrils can cross-seed amyloid A protein in a murine experimental amyloid A amyloidosis model [37]. In addition, several reports showing the co-existence of different amyloid pathologies in the same tissue further support the existence of cross-seeding as a disease mechanism [34, 35, 38–42]. This data suggests that one PMD may be capable of influencing the development of another.
The demonstration that seeding with proteins of differing sequences has a lower efficiency is reminiscent of the species barrier described earlier for TSEs. This could be a plausible explanation for the low attack rate and long incubation periods typically observed during the primary transmission of prions from one species to another [9, 12, 17, 18]. However, there are many other factors that could influence this phenomenon, such as the strain of the agent and the genetic background of the host [43, 44]. Nevertheless, recent experiments showing that the species barrier phenomenon is reproduced in transgenic mice expressing PrP from different species support the hypothesis that the sequence is the most important factor responsible for this phenomenon [19, 45–47].
CO-EXISTENCE OF PMDS
The study of a putative interaction of PMDs at the protein level is not only important from a scientific point of view, but also for public health. A better understanding of this phenomenon could explain the origin and prevalence of several PMDs. On the one hand, several of the proteins related to these diseases are located in different cell compartments or cell types, making a protein-protein interaction unlikely. On the other hand, the fact that some of these proteins can increase their levels under certain pathological conditions enhances the possibility of interaction between them. These events could be caused by several mechanisms which include inflammation, overload of the proteasome and protein-chaperone mechanisms (responsible for the elimination of misfolded proteins) and signaling cascades, among others. Such processes could interact, become synergic and enhance disease hallmarks (as misfolded protein deposition) which could increase the chance of misfolded protein-protein interactions. Next, we will discuss epidemiological and experimental data related to misfolded protein cross-seeding and its putative role in the pathological progression.
Epidemiological Evidence
Probably the clearest evidence for the interaction of PMDs comes from the link between AD and diabetes type-2, characterized by Aβ and amylin deposition, respectively. Clinical studies had shown that a high percentage of patients affected with AD are also positive for diabetes type-2 [48]. In 2004, Janson and co-workers showed that in a cohort of AD patients, 81% of them had either diabetes type-2 or Impaired Fasting Glucose (IFG) [48]. In addition, and comparing with age matched non-demented individuals, AD patients have a higher incidence of islet amyloidosis than healthy individuals. However, this study described that Aβ deposition is not increased in patients affected with diabetes type-2, suggesting that this phenomenon could be unidirectional. Other reports have been published supporting or contradicting this conclusion [49–54]. However, the location of amyloid proteins in different tissues and the lack of reports showing the co-existence of both misfolded aggregates in the same organ question the idea that the interaction between AD and diabetes type 2 is due to cross-seeding mechanisms.
Other clinical examples are found in patients affected with PD and AD where the co-existence of Aβ and α-synuclein has been described [33, 55, 56]. In this case, pathological features of both diseases co-exist in the brain, increasing the probabilities that misfolded protein aggregates can interact. Recently, Tsigelni et al. showed that α-synuclein and Aβ directly interact in the brain of patients with Lewy body disease [55]. This report also describes patients clinically diagnosed with AD presenting higher accumulations of α-synuclein compared to healthy individuals. In addition, co-inmmunoprecipitation experiments using anti-Aβ antibodies showed that brains from Lewy body disease patients presented strong anti-Aβ immunoreactivity after analysis by Western blot [55].
The phenomenon of amyloids’ co-existence has been described for several other amyloidogenic proteins. Among them, the simultaneous presence of Aβ and prions has been extensively documented in patients affected with sCJD, Gerstmann-Sträussler-Scheinker (GSS) disease and AD [34, 35, 39, 57–60]. In some cases of AD, accumulation of PrP within Aβ deposits occurs as diffuse plaques [57, 60]. These aggregates are easily eliminated after proteinase K treatment on brain slides, suggesting that prion aggregates are composed in part or totally by PrPC or by protease-sensitive PrPSc. These findings are similar to what is observed for double transgenic mice over-expressing amyloid precursor protein with Swedish and Indiana mutations (associated with strong Aβ deposition) and hamster PrP [61]. However, due to the high density of PrP in these areas, the possibility of de novo PrPSc generation should be considered. In addition, it is important to mention that many cases of TSEs are associated with proteinase K sensitive PrPSc [62–64], which could be mainly composed of oligomeric protease-sensitive species.
Several cases of sCJD and GSS patients with plaques composed of misfolded PrP and Aβ have been reported [34, 35, 39,58, 59]. A recent study shows that a subgroup of sCJD patients show higher levels of Aβ42 in their brains [58]. As a consequence, Aβ plaques were found in the brain of these sCJD affected individuals. As expected, little or no senile plaques were identified in sCJD patients harboring lower levels of Aβ. Interestingly, sCJD patients harboring higher amounts of Aβ have lower levels of PrPSc accumulation. This data can be interpreted in two different ways. The first explanation is that the presence of Aβ inhibits misfolding and accumulation of PrPSc. The second one is that part of the PrPSc molecules are used to induce misfolding and aggregation of Aβ instead of further accumulation of PrP. The presence of both amyloidogenic species could have enhanced toxic effects, triggering clinical disease without substantial accumulation of PrPSc. Due to the specific characteristics of these diseases, it is possible that some clinical and pathological features of AD may camouflage sCJD [58,65]. In a more extreme case, it could be possible to imagine that PrPSc formation, produced for example by an infectious exposure may trigger Aβ misfolding and accumulation and lead to AD clinical disease, instead of CJD. Thus, it could be interesting to study whether in countries with high exposure to BSE (such as UK or France) there is an increased number of cases of AD or other neurodegenerative diseases associated to protein misfolding and aggregation. It would also be important to carry out more experiments in animal models to properly assess this important issue.
Evidence In Vitro
The easiest way to test the hypothesis of cross-seeding is by in vitro aggregation assays. Classical seeding assays show that the addition of a preformed seed reduced the extent of the lag phase of aggregation in amyloidogenic proteins [27, 29, 66]. In addition, several reports show that the extent of the nucleation phase of a specific protein could be reduced after the addition of a heterologous seed [55, 67]. This cross-seeding effect can be different according to the protein/protein pair studied.
Subsequent to the clinical evidence described earlier in diabetes type-2 and AD patients [48], in vitro seeding assays using amylin and Aβ were performed [67]. Interestingly, there is some degree of sequence similarity between amylin and Aβ. If we add this information to the previously mentioned reports that link AD and diabetes type-2 [48], it would be rational to look for a putative interaction between both proteins. Seeding assays show that Aβ amyloids are good seeds for amylin aggregation. However, the same report described that globular and fibrillar aggregates of amylin have inert or very low effects on Aβ aggregation [67]. These results are interesting if we consider the clinical data reported by Janson et al., where the interaction between diabetes Type-2 in AD seems to work only in one direction [48].
Another important example of in vitro cross-seeding comes from studies involving α-synuclein and Aβ [55, 68]. The evidence in this case goes further, explaining a possible association between different oligomeric aggregates [55]. Additionally, it is suggested that both proteins could form annular structures able to generate pores in the cell membrane [55]. It is hypothesized that these structures could produce cationic imbalance between intracellular and extracellular spaces, leading to cell death events observed in this disorders.
By coupling seeding assays with other in vitro and in silico techniques, we could enormously improve our knowledge regarding this important issue. However, in order to understand the contribution of other events such us inflammation, signaling cascades and protein clearance mechanisms in the progression of the disease, experimentation in animal models is absolutely necessary.
Studies in Animal Models
The best way to analyze if two PMDs can cross-talk is by using animal models. Animal models allow us to measure many pathological features in a controlled way and at different stages of the disease. Several reports studying PMDs interactions in mouse models are available. Again, the Aβ/α-synuclein pair is one of the best studied cases [55, 69]. The results indicate that Aβ enhances α-synuclein accumulation and neuronal deficits in a double transgenic mouse model of AD and PD [69]. Considering their cellular distribution, interaction between Aβ extra-cellular and α-synuclein (intra-cellular) seems unlikely. Nevertheless, immunoprecipitation assays demonstrated the co-existence of both proteins in the mouse brain [55]. These data open new questions regarding the mechanisms of interaction for these particular proteins and their consequences in pathological events.
The presence of Aβ aggregates and Tau neurofibrillary tangles is the major hallmark in AD. It has been proposed for a long time that a putative interaction between both proteins could enhance the clinical decline observed in AD patients. Two interesting pieces of evidence were supplied in 2001 by Lewis et al. [70] and Gotz et al. [71]. These studies show that the simultaneous presence of Aβ and Tau enhanced neurofribillary tangle formation in mouse models. In the first approach, researchers crossed Tg2576 mice with transgenic mice expressing a mutant form of Tau protein. They observed that Tg2576 and double transgenic mice develop Aβ deposit at the same age, however neurofibrillary tangle deposition was significantly enhanced compared to the single mutant Tau transgenic [70]. Interestingly, the increase in Tau deposition was not correlated with areas rich in Aβ deposits. The second study consisted in the inoculation of pre-formed Aβ42 fibrils in the brain of P301L Tau transgenic mice. As in the previous report, the presence of misfolded Aβ in the brain of these mice increased Tau deposition [71].
Considering the findings suggesting the co-existence of AD and sCJD, the study of a possible link between both diseases is important. As suggested by Debatin et al. [58], the fact that the AD phenotype could mask some sCJD cases makes this issue even more relevant. Since Aβ and PrPSc have similar aggregation mechanisms and are located in the same tissue (brain) and subcellular location (extracellular space), it is likely that these misfolded proteins may interact. However, two recent reports suggest that PrPC biological function may be related to Aβ. First, it was shown that PrPC could be a protective molecule in AD via β-secretase inhibition [72]. Second, it was shown that PrPC may be a receptor for Aβ oligomers, contributing to AD neurodegeneration [73]. These somehow contradictory findings need to be further explored. The stable characteristic of Aβ accumulation in transgenic mice [74–76] and the constant incubation periods for prions [13] should make these studies easy to follow.
STRATEGIES FOR PMDs TREATMENT
It is well accepted that the misfolding process is the key event in the development of PMDs. Therefore, therapeutic strategies targeted to the prevention of misfolding are today the most promising for delaying the disease progression.
Currently, therapies for most PMDs are just palliative. It is possible that attacking the central event in the disease (the misfolding and aggregation of the protein) could lead to more effective disease-modifying therapies. In order to attack misfolding processes, different approaches have been followed. One approach is attack specifically abnormally folded proteins that can later on act as aggregation nuclei. Another strategy consists of inhibiting the misfolding process itself. The former may allow depletion of putative seeds, whereas the latter might inhibit the formation of newly converted units. Both approaches could help the endogenous protein clearance systems, resulting in the total depletion of disease associated structures.
β-Sheet Breakers and Small Molecule Amyloid Inhibitors
One approach that has been used to discover small chemical inhibitors of protein misfolding and aggregation has been the screening of large libraries of compounds using simple in vitro assays [77–79]. The development of high throughput screening requires a robust, simple and relevant in vitro assay to monitor compound activity. In the case of the search for compounds able to prevent or reverse the protein misfolding cascade, the development of a screening assay with these characteristics has been challenging. Nevertheless, there have been many small chemical compounds that have been reported to inhibit protein misfolding and aggregation. Some of these compounds came from screening, but several others were identified serendipitously or based on epidemiological studies which have suggested they may be active. Some small molecules that that have been reported to prevent the misfolding and aggregation of proteins involved in PMDs include Congo red and derivatives, curcumin and quinacrine, among others (a more comprehensive list of compounds, including the specific references, can be found in [77–80]).
Even though many molecules have been identified as strong inhibitors, the usefulness of these small molecules is compromised by their lack of specificity, toxicity and their unclear mechanism of action in most of the cases.
A strategy that has been more successful in identifying potential hit compounds is the rational development of specific inhibitors based on the use of short peptides targeting the protein region needed for protein-protein interaction [80, 81]. The approach consists of synthesizing short peptides combining a self-recognition motif with a β-sheet disrupting element. The self-recognition domain is typically the region of the protein implicated in early misfolding and protein-protein interaction. As disrupting elements, different groups have used distinct strategies, including: the use of a bulky group (e.g cholyl) that sterically inhibit protein aggregation; N-methylations (or N-alkylations) to generate peptides having a blocking face; β-sheet breaker amino acids to disrupt beta-sheet conformation; and addition of charged residues to reduce the hydrophobic interaction which trigger protein aggregation [80, 81]. The use of peptides as a therapy struggles with three main problems, namely transport across biological membranes (i.e. the blood brain barrier and intestinal barrier), their rapid degradation in the body and generation of an immune response. However, these problems can be minimized by shortening the length of the peptide or by introducing chemical modifications [82]. The usefulness of these types of molecules has been clearly shown by β-sheet breaker peptides in models of AD and TSEs. In these studies it was shown that β-sheet breaker peptides decrease the burden of misfolded protein aggregates in brain [83–85] and reduce the toxicity associated to these proteins in cell cultures [83].
However, the recent identification of oligomers as the most toxic species in both diseases [86–88] opens a new window to consider in the exploration and use of these therapeutic agents. Following these findings it could be possible that the disruption of large aggregates into small oligomeric units by these molecules could even increase the problem by releasing new toxic species. These possibilities need to be experimentally evaluated in order to choose the right therapeutic concentrations for the chosen molecule.
Vaccines
Several attempts for immunization against PMDs have been tried. Probably the best known is the one tried by Elan pharmaceuticals against Aβ deposition [89]. The experimental strategy included synthetic Aβ42 as immunogen in PDAPP transgenic mice, which spontaneously generate AD plaques with age. Results showed an almost complete prevention of Aβ aggregates and significant reduction of astrocytosis in mice immunized before plaque generation. Animals treated with the Aβ vaccine at late stages of Aβ deposition showed a significant reduction in amyloid burden compared to PBS treated controls. As expected, high levels of antibodies against Aβ were found in the blood of Aβ42 treated mice. Unfortunately, clinical trials using this vaccine in humans were not successful since some of the patients involved in the trial developed meningeoencephalitis, leading in some of the cases to the death of the individuals [90, 91]. Currently, several approaches looking to reduce these adverse effects are under development. They include, passive immunization or vaccination with non-fibrillary Aβ derivatives and DNA immunization, among others [92–94].
Similar strategies have been tried for α-synuclein in Parkinson’s disease [95] and PrP in TSEs [96]. For the latter we can mention the studies of Sigurdsson et al. [97] and Magri et al. [98], where the treatment with recombinant PrP or synthetic prion derived peptides increase the incubation periods of mice and hamsters inoculated intraperitoneally with the 139A and 263K prion strains, respectively. These results expand the concept applied for AD and confirm that immunization using sequence specific peptides can decrease clinical features of PMDs. Recently, an interesting approach using an attenuated Salmonella vaccine expressing mouse PrP showed promising results in mice infected with 139A prions [99].
Conformational Antibodies
Current evidence supports the hypothesis that smaller and soluble oligomeric aggregates along the pathway to form large fibrillar deposits could be the molecules mostly responsible for the toxic effects observed in these maladies [86–88]. In this scenario, fibers could be acting as a protective mechanism in order to trap these particles and encapsulate them in tissue. Recent studies pioneered by the group of Charles Glabe showed the generation of antibodies specific to diverse misfolded aggregated forms of amyloidogenic proteins, including oligomers and fibrils [100]. Surprisingly, these antibodies are able to recognize oligomeric or fibrillar species regardless of the sequence. The use of such antibodies may represent a universal treatment for all amyloidogenic proteins and has the additional advantage that it targets exclusively the structure that is thought to be the toxic one.
One of the main problems with using antibodies for treatment is their stability and delivery into the CNS. Improvements in their delivery and stability could increase the use of these molecules for the treatment of PMDs affecting the brain. However, this approach could be very interesting in treating peripheral amyloidoses such as diabetes type-2. Additionally, the use of these antibodies may contribute to increase our understanding of the amyloid biology and the nature of the toxic species.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
This article describes common mechanisms associated with a variety of human diseases, collectively called PMDs. Compelling evidence accumulated over the years indicates that the key event in these diseases is the misfolding, aggregation and tissue accumulation of a protein. The available evidence suggests that these proteins misfold and aggregate by a seeding - nucleation mechanism. The formation of a stable seed, corresponding to an oligomer of the protein adopting the misfolded structure, is the key event in the process. The oligomeric seed is not only the most likely candidate for the toxic species, but also is able to accelerate the polymerization of the aggregates. As such, seeds have the intrinsic ability to propagate the misfolding and aggregation process. This provides a plausible model to explain the infectious nature of TSEs, which is so far the only PMD considered transmissible. Importantly, the ability of seeds composed of one protein to nucleate the aggregation of a different protein, through a phenomenon termed cross-seeding, provides a mechanistic explanation for the relatively common co-existence of more than one PMD in the same patient. In this article we also discussed epidemiological and experimental evidence for the interaction of PMDs and their implications for public health.
These topics open new issues in public health. The interaction between PMDs and the effect that some of them can have as risk factors for more prevalent PMDs need to be considered. In addition, the information gathered so far suggests that in all PMDs amiloidogenic structures could be transmissible. Considering the high incidence of sporadic cases in some PMDs, it is possible that a fraction of sporadic PMDs are originally generated by seeding or cross-seeding mechanisms. Therefore, disease propagation by seeding and cross-seeding is an important topic that needs to be further addressed in the years to come. A better understanding of these mechanisms could help for rationale design of novel therapeutic and diagnostic methodologies targeting these disorders.
Acknowledgments
This work was supported in part by the NIH grant R01AG028821 and by funding from the Mitchell Foundation and CART Foundation.
ABBREVIATIONS
- AD
Alzheimer’s disease
- Aβ
Amyloid β
- BSE
Bovine spongiform encephalopathy
- GSS
Gerstmann-Straüssler-Scheinker
- PMDs
Protein misfolding disorders
- PD
Parkinson’s disease
- PrPC
Cellular form of the prion protein
- PrPSc
Disease-associated form of the prion protein
- sCJD
sporadic Creutzfeldt Jacob Disease
- TSEs
Transmissible spongiform encephalopathies
- vCJD
Variant Creutzfeldt Jacob Disease
References
- 1.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 2.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nature Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 3.Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Ann Rev Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 4.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadsworth JD, Collinge J. Update on human prion disease. Biochim Biophys Acta. 2007;1772:598–609. doi: 10.1016/j.bbadis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Collinge J. Variant Creutzfeldt-Jakob disease. Lancet. 1999;354:317–323. doi: 10.1016/S0140-6736(99)05128-4. [DOI] [PubMed] [Google Scholar]
- 7.Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey LJ, Lantos P. The same prion strain causes vCJD and BSE. Nature. 1997;389:448–450. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 8.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature. 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 9.Morales R, Abid K, Soto C. The prion strain phenomenon; molecular basis and unprecedented features. Biochim Biophys Acta. 2007;1772:681–691. doi: 10.1016/j.bbadis.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill AF, Collinge J. Prion strains and species barriers. Contrib Microbiol. 2004;11:33–49. doi: 10.1159/000077061. [DOI] [PubMed] [Google Scholar]
- 11.Peretz D, Scott MR, Groth D, Williamson RA, Burton DR, Cohen FE, Prusiner SB. Strain-specified relative conformational stability of the scrapie prion protein. Protein Sci. 2001;10:854–863. doi: 10.1110/ps.39201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartz JC, Bessen RA, McKenzie D, Marsh RF, Aiken JM. Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy. J Virol. 2000;74:5542–5547. doi: 10.1128/jvi.74.12.5542-5547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castilla J, Morales R, Saa P, Barria M, Gambetti P, Soto C. Cell-free propagation of prion strains. EMBO J. 2008;27:2557–2566. doi: 10.1038/emboj.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sim VL, Caughey B. Ultrastructures and strain comparison of under-glycosylated scrapie prion fibrils. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.02.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Hill AF, Collinge J. Subclinical prion infection. Trends Microbiol. 2003;11:578–584. doi: 10.1016/j.tim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Pattison IH. Experiments with scrapie with special reference to the nature of the agent and the pathology of the disease. In: Gajdusek CJ, Gibbs CJ, Alpers MP, editors. Slow, Latent and Temperate Virus Infections; NINDB Monograph. Vol. 2. U.S. Goberment Printing Office; Washington, DC: 1965. pp. 249–257. [Google Scholar]
- 17.Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- 18.Race R, Meade-White K, Raines A, Raymond GJ, Caughey B, Chesebro B. Subclinical scrapie infection in a resistant species; persistence, replication, and adaptation of infectivity during four passages. J Infect Dis. 2002;186(Suppl 2):S166–S170. doi: 10.1086/344267. [DOI] [PubMed] [Google Scholar]
- 19.Scott M, Foster D, Mirenda C, Serban D, Coufal F, Walchli M, Torchia M, Groth D, Carlson G, DeArmond SJ. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989;59:847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- 20.Prusiner SB, Scott M, Foster D, Pan KM, Groth D, Mirenda C, Torchia M, Yang SL, Serban D, Carlson GA. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990;63:673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- 21.Moore RA, Vorberg I, Priola SA. Species barriers in prion diseases--brief review. Arch Virol. 2005;(Suppl):187–202. doi: 10.1007/3-211-29981-5_15. [DOI] [PubMed] [Google Scholar]
- 22.Collee JG, Bradley R. BSE: a decade on--Part I. Lancet. 1997;349:636–641. doi: 10.1016/S0140-6736(96)01310-4. [DOI] [PubMed] [Google Scholar]
- 23.Collee JG, Bradley R. BSE: a decade on--Part 2. Lancet. 1997;349:715–721. doi: 10.1016/S0140-6736(96)08496-6. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood JK, Cunningham AA. Epidemiological observations on spongiform encephalopathies in captive wild animals in the British Isles. Vet Rec. 1994;135:296–303. doi: 10.1136/vr.135.13.296. [DOI] [PubMed] [Google Scholar]
- 25.Pearson GR, Wyatt JM, Gruffydd-Jones TJ, Hope J, Chong A, Higgins RJ, Scott AC, Wells GA. Feline spongiform encephalopathy: fibril and PrP studies. Vet Rec. 1992;131:307–310. doi: 10.1136/vr.131.14.307. [DOI] [PubMed] [Google Scholar]
- 26.Bons N, Mestre-Frances N, Belli P, Cathala F, Gajdusek DC, Brown P. Natural and experimental oral infection of nonhuman primates by bovine spongiform encephalopathy agents. Proc Natl Acad Sci USA. 1999;96:4046–4051. doi: 10.1073/pnas.96.7.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid; a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 28.Kocisko DA, Come JH, Priola SA, Chesebro B, Raymond GJ, Lansbury PT, Caughey B. Cell-free formation of protease-resistant prion protein. Nature. 1994;370:471–474. doi: 10.1038/370471a0. [DOI] [PubMed] [Google Scholar]
- 29.Soto C, Estrada L, Castilla J. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci. 2006;31:150–155. doi: 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Soto C. Protein misfolding and disease; protein refolding and therapy. FEBS Lett. 2001;498:204–207. doi: 10.1016/s0014-5793(01)02486-3. [DOI] [PubMed] [Google Scholar]
- 31.Jarrett JT, Lansbury PT., Jr Amyloid fibril formation requires a chemically discriminating nucleation event; studies of an amyloidogenic sequence from the bacterial protein OsmB. Biochemistry. 1992;31:12345–12352. doi: 10.1021/bi00164a008. [DOI] [PubMed] [Google Scholar]
- 32.Gajdusek DC. Nucleation of Amyloidogenesis in Infections and Noninfectious Amyloidoses of Brain. Ann NY Acad Sci. 1994;724:173–190. doi: 10.1111/j.1749-6632.1994.tb38909.x. [DOI] [PubMed] [Google Scholar]
- 33.Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, Brooks DJ, Dickson DW, Dubois B, Emre M, Fahn S, Farmer JM, Galasko D, Galvin JE, Goetz CG, Growdon JH, Gwinn-Hardy KA, Hardy J, Heutink P, Iwatsubo T, Kosaka K, Lee VM, Leverenz JB, Masliah E, McKeith IG, Nussbaum RL, Olanow CW, Ravina BM, Singleton AB, Tanner CM, Trojanowski JQ, Wszolek ZK. DLB and PDD boundary issues; diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 34.Hainfellner JA, Wanschitz J, Jellinger K, Liberski PP, Gullotta F, Budka H. Coexistence of Alzheimer-type neuropathology in Creutzfeldt-Jakob disease. Acta Neuropathol (Berl) 1998;96:116–122. doi: 10.1007/s004010050870. [DOI] [PubMed] [Google Scholar]
- 35.Tsuchiya K, Yagishita S, Ikeda K, Sano M, Taki K, Hashimoto K, Watabiki S, Mizusawa H. Coexistence of CJD and Alzheimer’s disease; an autopsy case showing typical clinical features of CJD. Neuropathology. 2004;24:46–55. doi: 10.1111/j.1440-1789.2003.00513.x. [DOI] [PubMed] [Google Scholar]
- 36.Krebs MR, Morozova-Roche LA, Daniel K, Robinson CV, Dobson CM. Observation of sequence specificity in the seeding of protein amyloid fibrils. Protein Sci. 2004;13:1933–1938. doi: 10.1110/ps.04707004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundmark K, Westermark GT, Olsen A, Westermark P. Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: cross-seeding as a disease mechanism. Proc Natl Acad Sci USA. 2005;102:6098–6102. doi: 10.1073/pnas.0501814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown P, Jannotta F, Gibbs CJ, Jr, Baron H, Guiroy DC, Gajdusek DC. Coexistence of Creutzfeldt-Jakob disease and Alzheimer’s disease in the same patient. Neurology. 1990;40:226–228. doi: 10.1212/wnl.40.2.226. [DOI] [PubMed] [Google Scholar]
- 39.Miyazono M, Kitamoto T, Iwaki T, Tateishi J. Colocalization of prion protein and beta protein in the same amyloid plaques in patients with Gerstmann-Straussler syndrome. Acta Neuropathol (Berl) 1992;83:333–339. doi: 10.1007/BF00713522. [DOI] [PubMed] [Google Scholar]
- 40.Muramoto T, Kitamoto T, Koga H, Tateishi J. The coexistence of Alzheimer’s disease and Creutzfeldt-Jakob disease in a patient with dementia of long duration. Acta Neuropathol (Berl) 1992;84:686–689. doi: 10.1007/BF00227747. [DOI] [PubMed] [Google Scholar]
- 41.Iida T, Doh-Ura K, Kawashima T, Abe H, Iwaki T. An atypical case of sporadic Creutzfeldt-Jakob disease with Parkinson’s disease. Neuropathology. 2001;21:294–297. doi: 10.1046/j.1440-1789.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- 42.Preusser M, Strobel T, Gelpi E, Eiler M, Broessner G, Schmutzhard E, Budka H. Alzheimer-type neuropathology in a 28 year old patient with iatrogenic Creutzfeldt-Jakob disease after dural grafting. J Neurol Neurosurg Psychiatry. 2006;77:413–416. doi: 10.1136/jnnp.2005.070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bessen RA, Marsh RF. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J Gen Virol. 1992;73(Pt 2):329–334. doi: 10.1099/0022-1317-73-2-329. [DOI] [PubMed] [Google Scholar]
- 44.Stephenson DA, Chiotti K, Ebeling C, Groth D, DeArmond SJ, Prusiner SB, Carlson GA. Quantitative trait loci affecting prion incubation time in mice. Genomics. 2000;69:47–53. doi: 10.1006/geno.2000.6320. [DOI] [PubMed] [Google Scholar]
- 45.Browning SR, Mason GL, Seward T, Green M, Eliason GA, Mathiason C, Miller MW, Williams ES, Hoover E, Telling GC. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol. 2004;78:13345–13350. doi: 10.1128/JVI.78.23.13345-13350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groschup MH, Buschmann A. Rodent models for prion diseases. Vet Res. 2008;39:32. doi: 10.1051/vetres:2008008. [DOI] [PubMed] [Google Scholar]
- 47.Windl O, Buchholz M, Neubauer A, Schulz-Schaeffer W, Groschup M, Walter S, Arendt S, Neumann M, Voss AK, Kretzschmar HA. Breaking an absolute species barrier; transgenic mice expressing the mink PrP gene are susceptible to transmissible mink encephalopathy. J Virol. 2005;79:14971–14975. doi: 10.1128/JVI.79.23.14971-14975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 49.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia; The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 50.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 51.Landin K, Blennow K, Wallin A, Gottfries CG. Low blood pressure and blood glucose levels in Alzheimer’s disease. Evidence for a hypometabolic disorder? J Intern Med. 1993;233:357–363. doi: 10.1111/j.1365-2796.1993.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 52.Nielson KA, Nolan JH, Berchtold NC, Sandman CA, Mulnard RA, Cotman CW. Apolipoprotein-E genotyping of diabetic dementia patients; is diabetes rare in Alzheimer’s disease? J Am Geriatr Soc. 1996;44:897–904. doi: 10.1111/j.1532-5415.1996.tb01857.x. [DOI] [PubMed] [Google Scholar]
- 53.Kokmen E, Beard CM, Chandra V, Offord KP, Schoenberg BS, Ballard DJ. Clinical risk factors for Alzheimer’s disease; a population-based case-control study. Neurology. 1991;41:1393–1397. doi: 10.1212/wnl.41.9.1393. [DOI] [PubMed] [Google Scholar]
- 54.Curb JD, Rodriguez BL, Abbott RD, Petrovitch H, Ross GW, Masaki KH, Foley D, Blanchette PL, Harris T, Chen R, White LR. Longitudinal association of vascular and Alzheimer’s dementias, diabetes, and glucose tolerance. Neurology. 1999;52:971–975. doi: 10.1212/wnl.52.5.971. [DOI] [PubMed] [Google Scholar]
- 55.Tsigelny IF, Crews L, Desplats P, Shaked GM, Sharikov Y, Mizuno H, Spencer B, Rockenstein E, Trejo M, Platoshyn O, Yuan JX, Masliah E. Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer’s and Parkinson’s diseases. PLoS ONE. 2008;3:e3135. doi: 10.1371/journal.pone.0003135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, De Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB); report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 57.Ferrer I, Blanco R, Carmona M, Puig B, Ribera R, Rey MJ, Ribalta T. Prion protein expression in senile plaques in Alzheimer’s disease. Acta Neuropathol (Berl) 2001;101:49–56. doi: 10.1007/s004010000271. [DOI] [PubMed] [Google Scholar]
- 58.Debatin L, Streffer J, Geissen M, Matschke J, Aguzzi A, Glatzel M. Association between deposition of beta-amyloid and pathological prion protein in sporadic Creutzfeldt-Jakob disease. Neurodegener Dis. 2008;5:347–354. doi: 10.1159/000121389. [DOI] [PubMed] [Google Scholar]
- 59.Paquet C, Privat N, Kaci R, Polivka M, Dupont O, Haik S, Laplanche JL, Hauw JJ, Gray F. Cerebral amyloid angiopathy with co-localization of prion protein and beta-amyloid in an 85-year-old patient with sporadic Creutzfeldt-Jakob disease. Acta Neuropathol. 2008;116:567–573. doi: 10.1007/s00401-008-0394-y. [DOI] [PubMed] [Google Scholar]
- 60.Leuba G, Saini K, Savioz A, Charnay Y. Early-onset familial Alzheimer disease with coexisting beta-amyloid and prion pathology. JAMA. 2000;283:1689–1691. doi: 10.1001/jama.283.13.1689-a. [DOI] [PubMed] [Google Scholar]
- 61.Schwarze-Eicker K, Keyvani K, Gortz N, Westaway D, Sachser N, Paulus W. Prion protein (PrP(c)) promotes beta-amyloid plaque formation. Neurobiol Aging. 2005;26:1177–1182. doi: 10.1016/j.neurobiolaging.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Lasmezas CI, Deslys JP, Robain O, Jaegly A, Beringue V, Peyrin JM, Fournier JG, Hauw JJ, Rossier J, Dormont D. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science. 1997;275:402–405. doi: 10.1126/science.275.5298.402. [DOI] [PubMed] [Google Scholar]
- 63.Manuelidis L, Sklaviadis T, Manuelidis EE. Evidence suggesting that PrP is not the infectious agent in Creutzfeldt-Jakob disease. EMBO J. 1987;6:341–347. doi: 10.1002/j.1460-2075.1987.tb04760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collinge J, Owen F, Poulter M, Leach M, Crow TJ, Rossor MN, Hardy J, Mullan MJ, Janota I, Lantos PL. Prion dementia without characteristic pathology. Lancet. 1990;336:7–9. doi: 10.1016/0140-6736(90)91518-f. [DOI] [PubMed] [Google Scholar]
- 65.Manuelidis EE, Manuelidis L. A transmissible Creutzfeldt-Jakob disease-like agent is prevalent in the human population. Proc Natl Acad Sci, USA. 1993;90:7724–7728. doi: 10.1073/pnas.90.16.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lomakin A, Chung DS, Benedek GB, Kirschner DA, Teplow DB. On the nucleation and growth of amyloid beta-protein fibrils; detection of nuclei and quantitation of rate constants. Proc Natl Acad Sci USA. 1996;93:1125–1129. doi: 10.1073/pnas.93.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Nuallain B, Williams AD, Westermark P, Wetzel R. Seeding specificity in amyloid growth induced by heterologous fibrils. J Biol Chem. 2004;279:17490–17499. doi: 10.1074/jbc.M311300200. [DOI] [PubMed] [Google Scholar]
- 68.Mandal PK, Pettegrew JW, Masliah E, Hamilton RL, Mandal R. Interaction between Abeta peptide and alpha synuclein; molecular mechanisms in overlapping pathology of Alzheimer’s and Parkinson’s in dementia with Lewy body disease. Neurochem Res. 2006;31:1153–1162. doi: 10.1007/s11064-006-9140-9. [DOI] [PubMed] [Google Scholar]
- 69.Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98:12245–12250. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 71.Gotz J, Chen F, Van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 72.Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, Manson JC, Baybutt HN, Turner AJ, Hooper NM. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer’s amyloid precursor protein. Proc Natl Acad Sci USA. 2007;104:11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 75.Van DD, De Deyn PP. Drug discovery in dementia; the role of rodent models. Nat Rev Drug Discov. 2006;5:956–970. doi: 10.1038/nrd2075. [DOI] [PubMed] [Google Scholar]
- 76.Dodart JC, May P. Overview on rodent models of Alzheimer’s disease. Curr Protoc Neurosci. 2005;Chapter 9 doi: 10.1002/0471142301.ns0922s33. [DOI] [PubMed] [Google Scholar]
- 77.De LE, Giorgetti S, Grossi S, Merlini G, Caccialanza G, Bellotti V. Pharmaceutical strategies against amyloidosis; old and new drugs in targeting a “protein misfolding disease”. Curr Med Chem. 2004;11:1065–1084. doi: 10.2174/0929867043455549. [DOI] [PubMed] [Google Scholar]
- 78.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 79.Soto C, Martin Z. Therapeutic strategies against protein misfolding in neurodegenerative diseases. Expert Opin Drug Discov. 2009;4:71–84. doi: 10.1517/13543770802630455. [DOI] [PubMed] [Google Scholar]
- 80.Mason JM, Kokkoni N, Stott K, Doig AJ. Design strategies for anti-amyloid agents. Curr Opin Struct Biol. 2003;13:526–532. doi: 10.1016/s0959-440x(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 81.Estrada LD, Soto C. Peptide inhibitors of protein misfolding and aggregation. Curr Pharm Des. 2006;12:2557–2568. doi: 10.2174/138161206777698792. [DOI] [PubMed] [Google Scholar]
- 82.Adessi C, Soto C. Converting a peptide into a drug; Strategies to improve stability and bioavailability. Curr Med Chem. 2002;9:963–978. doi: 10.2174/0929867024606731. [DOI] [PubMed] [Google Scholar]
- 83.Soto C, Sigurdsson EM, Morelli L, Kumar RA, Castano EM, Frangione B. Beta-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis; Implications for Alzheimer’s therapy. Nat Med. 1998;4:822–826. doi: 10.1038/nm0798-822. [DOI] [PubMed] [Google Scholar]
- 84.Permanne B, Adessi C, Saborio GP, Fraga S, Frossard MJ, Van Dorpe J, Dewachter I, Banks WA, Van Leuven F, Soto C. Reduction of amyloid load and cerebral damage in a transgenic mouse model of Alzheimer’s disease by treatment with a beta-sheet breaker peptide. FASEB J. 2002;16:860–862. doi: 10.1096/fj.01-0841fje. [DOI] [PubMed] [Google Scholar]
- 85.Soto C, Kascsak RJ, Saborio GP, Aucouturier P, Wisniewski T, Prelli F, Kascsak R, Mendez E, Harris DA, Ironside J, Tagliavini F, Carp RI, Frangione B. Reversion of prion protein conformational changes by synthetic beta-sheet breaker peptides. Lancet. 2000;355:192–197. doi: 10.1016/s0140-6736(99)11419-3. [DOI] [PubMed] [Google Scholar]
- 86.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration; separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 87.Glabe CG, Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. 2006;66:S74–S78. doi: 10.1212/01.wnl.0000192103.24796.42. [DOI] [PubMed] [Google Scholar]
- 88.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration; lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Molec Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 89.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang JP, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao ZM, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 90.Spinney L. Update on Elan vaccine for Alzheimer’s disease. Lancet Neurol. 2004;3:5. doi: 10.1016/s1474-4422(03)00634-3. [DOI] [PubMed] [Google Scholar]
- 91.Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease; the end of the beginning. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 92.Sigurdsson EM, Knudsen E, Asuni A, Fitzer-Attas C, Sage D, Quartermain D, Goni F, Frangione B, Wisniewski T. An attenuated immune response is sufficient to enhance cognition in an Alzheimer’s disease mouse model immunized with amyloid-beta derivatives. J Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schiltz JG, Salzer U, Mohajeri MH, Franke D, Heinrich J, Pavlovic J, Wollmer MA, Nitsch RM, Moelling K. Antibodies from a DNA peptide vaccination decrease the brain amyloid burden in a mouse model of Alzheimer’s disease. J Mol Med. 2004;82:706–714. doi: 10.1007/s00109-004-0570-z. [DOI] [PubMed] [Google Scholar]
- 94.Okura Y, Matsumoto Y. DNA vaccine therapy for Alzheimer’s disease; present status and future direction. Rejuvenation Res. 2008;11:301–308. doi: 10.1089/rej.2007.0638. [DOI] [PubMed] [Google Scholar]
- 95.Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 96.Sigurdsson EM, Wisniewski T. Promising developments in prion immunotherapy. Expert Rev Vaccines. 2005;4:607–610. doi: 10.1586/14760584.4.5.607. [DOI] [PubMed] [Google Scholar]
- 97.Sigurdsson EM, Brown DR, Daniels M, Kascsak RJ, Kascsak R, Carp R, Meeker HC, Frangione B, Wisniewski T. Immunization delays the onset of prion disease in mice. Am J Pathol. 2002;161:13–17. doi: 10.1016/S0002-9440(10)64151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Magri G, Clerici M, Dall’Ara P, Biasin M, Caramelli M, Casalone C, Giannino ML, Longhi R, Piacentini L, Della Bella S, Gazzuola P, Martino PA, Della Bella S, Pollera C, Puricelli M, Servida F, Crescio I, Boasso A, Ponti W, Poli G. Decrease in pathology and progression of scrapie after immunisation with synthetic prion protein peptides in hamsters. Vaccine. 2005;23:2862–2868. doi: 10.1016/j.vaccine.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 99.Goni F, Knudsen E, Schreiber F, Scholtzova H, Pankiewicz J, Carp R, Meeker HC, Rubenstein R, Brown DR, Sy MS, Chabalgoity JA, Sigurdsson EM, Wisniewski T. Mucosal vaccination delays or prevents prion infection via an oral route. Neuroscience. 2005 doi: 10.1016/j.neuroscience.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 100.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]