Abstract
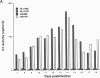
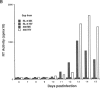
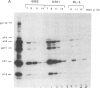
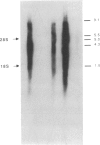
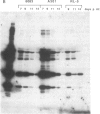
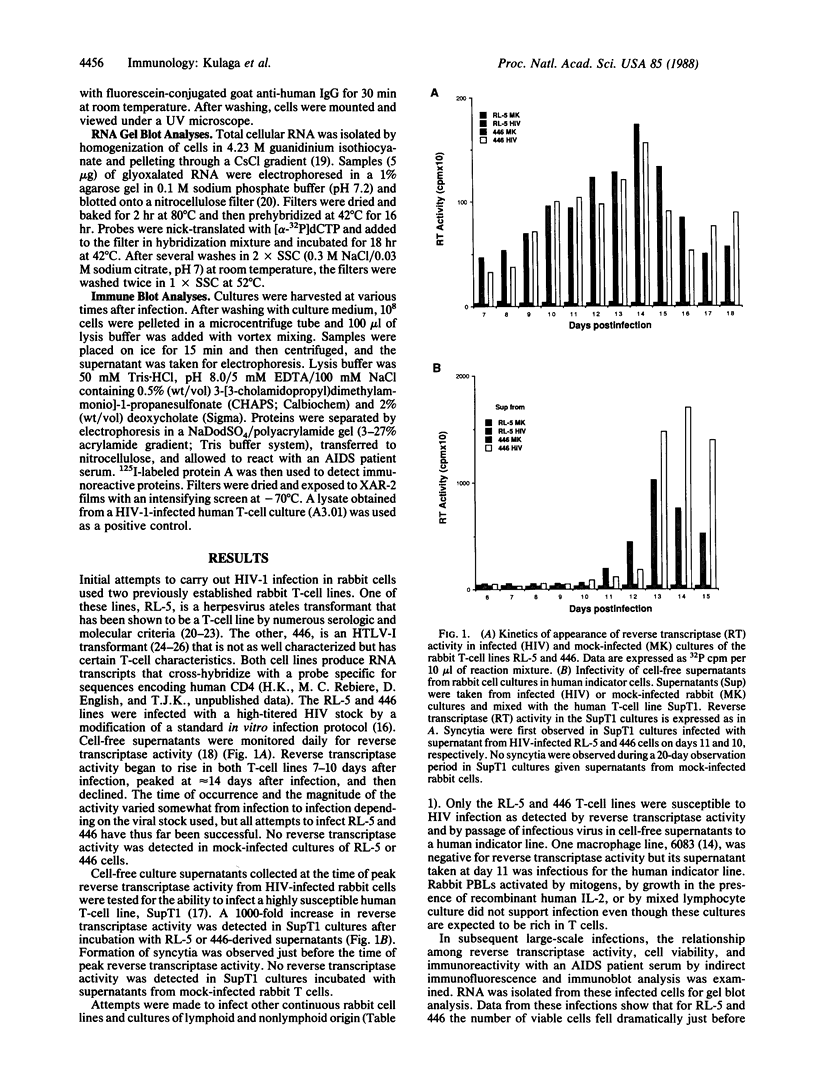
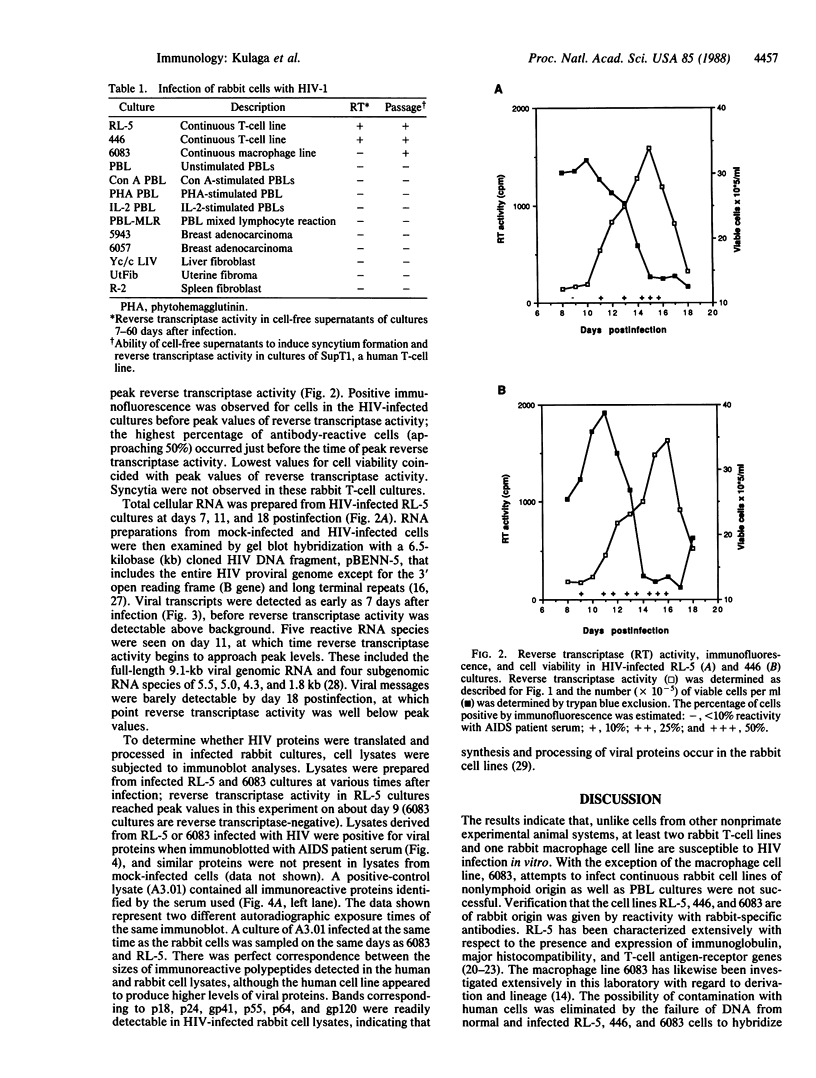
We report the successful infection of two rabbit T-cell lines and one rabbit macrophage line with human immunodeficiency virus 1 (HIV-1). One T-cell line was a herpesvirus ateles transformant, the other T-cell line was a human T-cell leukemia virus I transformant, and the macrophage line was a simian virus 40 transformant. After infection with a high-titered HIV-1 stock, the rabbit cultures exhibited properties that closely mimic those of HIV-1-infected human cells. Productive infection was evident in cultures 7-14 days after infection, as shown by an increase in reverse transcriptase activity, a concomitant increase in positive cells detected by indirect immunofluorescence using serum from a patient with acquired immunodeficiency syndrome, and a decrease in cell viability. RNA gel blot hybridization and protein immunoblot analyses of infected cells indicated that all predicted viral transcripts and proteins were synthesized during the course of the infection. Proof that cell-free culture supernatants of the infected rabbit cell lines contained infectious virus was given by successful passage onto a susceptible human T-cell line. The ability of HIV-1 to infect transformed rabbit cell lines in vitro suggests that, with appropriate manipulation, the rabbit may provide a model for infection with HIV-1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J., Eichberg J. W., Masur H., Saxinger W. C., Gallo R., Macher A. M., Lane H. C., Fauci A. S. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science. 1984 Nov 2;226(4674):549–552. doi: 10.1126/science.6093251. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Benn S., Rutledge R., Folks T., Gold J., Baker L., McCormick J., Feorino P., Piot P., Quinn T., Martin M. Genomic heterogeneity of AIDS retroviral isolates from North America and Zaire. Science. 1985 Nov 22;230(4728):949–951. doi: 10.1126/science.2997922. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Meléndez L. V., Hunt R. D., King N. W., Anver M., Fraser C. E., Barahona H., Baggs R. B. Herpesvirus saimiri: VII. Induction of malignant lymphoma in New Zealand white rabbits. J Natl Cancer Inst. 1974 Dec;53(6):1803–1807. [PubMed] [Google Scholar]
- Desrosiers R. C., Letvin N. L. Animal models for acquired immunodeficiency syndrome. Rev Infect Dis. 1987 May-Jun;9(3):438–446. doi: 10.1093/clinids/9.3.438. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Swenson R. B., McGrath C. R., Brodie A., Getchell J. P., Jensen F. C., Anderson D. C., Broderson J. R., Francis D. P. Persistent infection of chimpanzees with human T-lymphotropic virus type III/lymphadenopathy-associated virus: a potential model for acquired immunodeficiency syndrome. J Virol. 1986 Apr;58(1):116–124. doi: 10.1128/jvi.58.1.116-124.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C. The first human retrovirus. Sci Am. 1986 Dec;255(6):88–98. doi: 10.1038/scientificamerican1286-88. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghrayeb J., Kato I., McKinney S., Huang J. J., Chanda P. K., Ho D. D., Sarangadharan M. G., Chang T. W., Chang N. T. Human T-cell lymphotropic virus type III (HTLV-III) core antigens: synthesis in Escherichia coli and immunoreactivity with human sera. DNA. 1986 Apr;5(2):93–99. doi: 10.1089/dna.1986.5.93. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Sinkovics J. G., Gyorkey P. Retrovirus resembling HTLV in macrophages of patients with AIDS. Lancet. 1985 Jan 12;1(8420):106–106. doi: 10.1016/s0140-6736(85)91995-6. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Alpers J. D., Rackowski J. L., Huebner K., Haggarty B. S., Cedarbaum A. J., Reed J. C. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986 Nov 28;234(4780):1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Harada S., Yamamoto N. Correlation between high susceptibility to AIDS virus and surface expression of OKT-4 antigen in HTLV-I-positive cell lines. Jpn J Cancer Res. 1985 Sep;76(9):799–802. [PubMed] [Google Scholar]
- Kulaga H., Kindt T. J., Sogn J. A. Phagocytic rabbit cell lines expressing class II MHC products: establishment of cell lines by viral transformation. J Leukoc Biol. 1986 Aug;40(2):169–181. doi: 10.1002/jlb.40.2.169. [DOI] [PubMed] [Google Scholar]
- Kulaga H., Sogn J. A., Weissman J. D., Marche P. N., LeGuern C., Long E. O., Kindt T. J. Expression patterns of MHC class II genes in rabbit tissues indicate close homology to human counterparts. J Immunol. 1987 Jul 15;139(2):587–592. [PubMed] [Google Scholar]
- LeGuern C., Marche P. N., Kindt T. J. Molecular evidence for five distinct MHC class II alpha genes in the rabbit. Immunogenetics. 1985;22(2):141–148. doi: 10.1007/BF00563511. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., McHugh T., Casavant C., Stites D., Oshiro L. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology. 1985 Dec;147(2):441–448. doi: 10.1016/0042-6822(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Marche P. N., Tykocinski M. L., Max E. E., Kindt T. J. Structure of a functional rabbit class I MHC gene: similarity to human class I genes. Immunogenetics. 1985;21(1):71–82. doi: 10.1007/BF00372243. [DOI] [PubMed] [Google Scholar]
- McClure M. O., Sattentau Q. J., Beverley P. C., Hearn J. P., Fitzgerald A. K., Zuckerman A. J., Weiss R. A. HIV infection of primate lymphocytes and conservation of the CD4 receptor. Nature. 1987 Dec 3;330(6147):487–489. doi: 10.1038/330487a0. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Yoshimoto S., Taguchi H., Kubonishi I., Fujishita M., Ohtsuki Y., Shiraishi Y., Akagi T. Transformation of rabbit lymphocytes with T-cell leukemia virus. Gan. 1983 Feb;74(1):1–4. [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Pitha P. M. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rabson A. B., Daugherty D. F., Venkatesan S., Boulukos K. E., Benn S. I., Folks T. M., Feorino P., Martin M. A. Transcription of novel open reading frames of AIDS retrovirus during infection of lymphocytes. Science. 1985 Sep 27;229(4720):1388–1390. doi: 10.1126/science.2994220. [DOI] [PubMed] [Google Scholar]
- Rebiere M. C., Marche P. N., Kindt T. J. A rabbit class I major histocompatibility complex gene with a T cell-specific expression pattern. J Immunol. 1987 Sep 15;139(6):2066–2074. [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]